Abstract
The characteristics of interhemispheric resting-state functional connectivity (FC) in Parkinson’s disease (PD) with fatigue remain unclear; therefore, we aimed to explore the changes in interhemispheric FC in PD patients with fatigue. Sixteen PD patients with fatigue (PDF), 16 PD patients without fatigue (PDNF) and 15 matched healthy controls (HCs) were enrolled in the retrospective cross-sectional study. We used voxel-mirrored homotopic connectivity (VMHC) to analyze the resting-state functional magnetic resonance imaging (fMRI) data of these subjects. Compared to PDNF, PDF patients had decreased VMHC values in the supramarginal gyri (SMG). Furthermore, the mean VMHC values of the SMG were negatively correlated with the mean fatigue severity scale (FSS/9) scores (r = −0.754, p = 0.001). Compared to HCs, PDF patients had decreased VMHC in the SMG and in the opercular parts of the inferior frontal gyri (IFG operc). The VMHC values in the IFG operc and middle frontal gyri (MFG) were notably decreased in PDNF patients compared with HCs. Our findings suggest that the reduced VMHC values within the bilateral SMG may be the unique imaging features of fatigue in PD, and may illuminate the neural mechanisms of fatigue in PD.
1. Introduction
Fatigue is one of the most frequent non-motor symptoms of Parkinson’s disease (PD), which is described as a significantly diminished energy level or an increased perception of effort that is disproportionate to the attempted activities. It affects about 33–58% of PD patients [1,2,3] and impairs quality of life [4]. While the pathophysiology of fatigue in PD patients is not clear, many studies have attempted to probe the potential mechanisms within the last decade. An early study found a correlation between fatigue and frontal lobe hypoperfusion in PD patients [5]. Subsequently, neuroimaging studies demonstrated abnormal regional cerebral blood flow (rCBF) and glucose metabolism in the frontal lobe, caudate, insula, middle temporal gyrus, precuneus and middle occipital gyrus in PD-related fatigue [6,7]. Furthermore, functional magnetic resonance imaging (fMRI) studies reported abnormal local activities in cognitive regions, including the left anterior cingulate cortex (ACC), right superior frontal gyrus (dorsolateral part), left postcentral gyrus and right inferior frontal gyrus (orbital and triangular part) for chronic fatigue [8,9], which may have similar pathological mechanisms with fatigue in PD. Thereafter, Cho et al. considered that impaired activation of the salience network, which mainly comprises bilateral anterior insulas and the anterior cingulate cortex, could lead to a persistent broad and unfocused mental state, resulting in distracting, internally focused information, which could contribute to fatigue in PD patients [10]. Recently, a group-level independent component analysis demonstrated the increased connectivity of DMN in PD patients with fatigue, which may suggest that a higher attention level could represent an initial cognitive compensatory response, as a manifestation of cognitive cortical plasticity [11]. These prior outcomes suggest that fatigue is associated with the dysfunctional regions implicated in cognition function, such as the regulation of attention.
However, little research pointed to the changes in functional connectivity between two hemispheres. In fact, PD is clinically manifested as unilateral onset, and is asymmetrical during the progression of the disease on both sides of the body, indicating the discordancy in the pathological damage to the two hemispheres [12,13]. Meanwhile, previous studies revealed that PD patients with fatigue had abnormal motor and cognitive network connectivity [14], which was mainly regulated by the right hemisphere due to its dominance for attention and arousal [15]. So, the laterality of cognitive networks might have an impact on fatigue in PD. Therefore, it would be meaningful to pay close attention to the interhemispheric resting-state FC in fatigue, which can identify the characteristics of intrinsic functional architecture between geometrically corresponding regions in each hemisphere and reflect interhemispheric communication to the integrated brain function underlying coherent cognition and behavior [16]. Voxel-mirrored homotopic connectivity (VMHC) is a method of measuring the interhemispheric resting-state FC [16]. It has not been thoroughly applied to evaluate the neuroimaging features of fatigue in PD patients. Here, we explored the hypothesis that PD patients with fatigue (PDF) would exhibit abnormal interhemispheric FC in the brain regions associated with cognition, compared with non-fatigued PD patients (PDNF) and healthy controls (HCs) by VMHC. We expected that the cognitive regions would be distinctively influenced in PDF patients, except for motor-related areas, which would be affected in both PDF and PDNF patients.
2. Materials and Methods
2.1. Participants and Clinical Assessment
A cross-sectional retrospective study was designed. PD patients were consecutively recruited from the Department of Neurology in the First Affiliated Hospital of Nanjing Medical University between March 2018 and April 2020. Meanwhile, healthy controls (HCs) were consecutively recruited from hospital personnel and society. This study conformed to the standards set by the latest revised version of the Declaration of Helsinki (as revised in 2013) and was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University. All participants signed informed consent before beginning the experiment.
The diagnosis of definite PD met the criteria of the British Parkinson’s Disease Society Brain Bank for PD [17]. The exclusion criteria were as follows: (1) uncertain diagnosis of PD or parkinsonian plus syndromes; (2) diagnosis of severe neurological or psychiatric diseases; (3) contraindications for MRI scan; (4) taking antidepressants or medications that have fatigue as a side effect according to the package insert, or having other diseases that can result in the onset of fatigue; (5) other confounding factors associated with fatigue, such as significant cognitive dysfunction (Mini-Mental State Examination (MMSE) scores < 24), moderate or severe depression (the 24-item Hamilton Depression Rating Scale (HAMD) > 17), apathy (apathy scale (AS) > 14) and excessive daytime sleepiness (Epworth sleepiness scale (ESS) > 10). Finally, 37 idiopathic PD patients and 15 HCs matched with age, sex and education were enrolled and all subjects were right-handed. However, the data of only 32 PD patients and 15 HCs were analyzed because 5 patients (2 fatigued patients and 3 non-fatigued patients) were excluded due to abnormal head motions. The flow chart is shown in Figure 1.
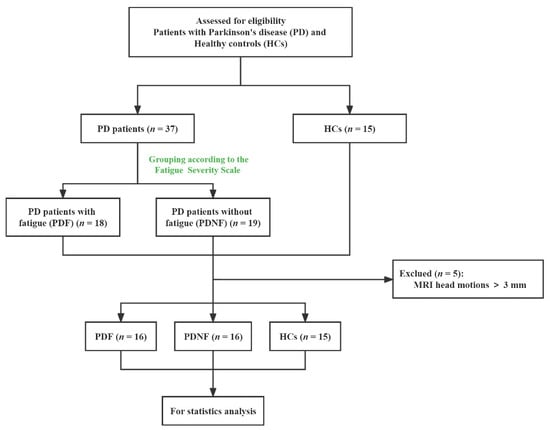
Figure 1.
Study flow diagram. MRI: magnetic resonance imaging.
All subjects underwent the scale evaluation and MRI examinations after more than 12 h withdrawal from antiparkinsonian medications to alleviate the pharmacological effects on neural activity. The presence and severity of fatigue were defined by the fatigue severity scale (FSS), which has 9 items and was widely used in PD, owing to its reliability, validity and sensitivity for detection of fatigue symptoms [18]. Patients were divided into two groups based on the presence (n = 18) or absence (n = 19) of fatigue. PD patients with a mean FSS (FSS/9) score > 4.0 were assigned to the PDF group, while the remaining patients were enrolled in the PDNF group. In addition, the disease duration, severity and non-motor symptoms were evaluated by clinical scales, including Hoehn and Yahr (H&Y) stage, the motor element of Unified Parkinson’s Disease Rating Scale (UPDRS-III), MMSE, ESS, AS, HAMD, and HAMA (Hamilton Anxiety Rating Scale). According to recognized methods [19], we calculated the levodopa equivalent daily dose (LEDD) for each patient.
2.2. Image Acquisition
MRI data from all subjects were obtained using a Siemens 3.0-Tesla signal scanner (Siemens Medical Solutions, Erlangen, Germany). In order to reduce scanner noise and limit head motions, participants were fitted with foam padding and earplugs, then were instructed to close their eyes, remain still, stay awake, and not think of anything during scanning. High-resolution brain structural images were obtained using T1-weighted, sagittal 3D magnetization-prepared rapid gradient echo (MPRAGE) sequences with the following parameters: repetition time (TR) = 1900 ms, echo time (TE) = 2.95 ms, flip angle (FA) = 9°, slice thickness = 1 mm, slices = 160, field of view (FOV) = 230 × 230 mm2, matrix size = 256 × 256, and voxel size = 1 × 1 × 1 mm3. Functional images were acquired using an echo-planar imaging (EPI) sequence (TR = 2000 ms, TE = 21 ms, FA = 90°, FOV = 256 × 256 mm2, in-plane matrix = 64 × 64, slices = 35, slice thickness = 3 mm, no slice gap, voxel size = 3 × 3 × 3 mm3, and total volumes = 240) on each subject.
2.3. Data Preprocessing
Rs-fMRI data preprocessing was executed on Data Processing Assistant for Resting-State fMRI (DPARSF, http://www.restfmri.net/forum/dparsf, accessed on 10 November 2021). With reference to this literature [20], we partitioned the preprocessing strategies into the following steps. To begin with, the first 10 time points were disposed of and the remaining 230 images were revised for timing differences between slices and head motion (Friston 24 parameter), taking the middle layer as the reference slice. Subsequently, individual T1 structural images were co-registered to the mean EPI scans and segmented into gray matter and white matter by “New Segment”. Then, the transformations were computed from the native space to the Montreal Neurological Institute (MNI) space by DARTEL normalization and applied to spatially normalize the EPI images. The following steps were implemented: resampling with 3 × 3 × 3 mm3 resolution, spatially smoothing with a 6 mm full-width half-maximum Gaussian kernel to decrease spatial noise, removing the linear trend and temporally filtering (0.01–0.08 Hz). Several sources of spurious variance were regressed out, including the white matter signal, the cerebral spinal fluid signal, and six head motion parameters obtained by head motion correction. Five participants (2 fatigued patients and 3 non-fatigued patients) with head motions more than 3.0 mm of translation or 3.0° of rotation were excluded.
2.4. Voxel-Mirrored Homotopic Connectivity
The values of VMHC were calculated with REST software (http://restfmri.net, accessed on 10 November 2021), and on the basis of the Gan’s article [20]. First, a mean normalized T1 image was established by averaging the spatially normalized T1 images. Afterwards, a group-specific symmetric brain template was created by averaging the above resulting T1 image with its left-right mirrored version, which was used for nonlinear registration of the individual T1 images. The identical transformation was applied to the resting fMRI images. For each subject, the Pearson correlation coefficient was computed between any pair of symmetric interhemispheric voxels and correlation values were then Fisher z-transformed to improve the normality. The resultant values constituted the VMHC and were used for the group analysis.
2.5. Statistical Analysis
Values of demographic and clinical variables were expressed as the mean ± standard deviation (SD). All analyses were processed by SPSS 21.0 (SPSS Inc, Chicago, IL, USA). One-way analysis of variance (ANOVA), Kruskal–Wallis test, the Pearson χ2 test, Student’s t test, and Mann–Whitney U test were used to analyze the differences among the three groups (PDF, PDNF and HCs) regarding demographic and clinical variables, then least significant difference(LSD) was used for post hoc tests, as appropriate. p < 0.05 (two-tailed) was considered statistically significant.
Voxel-based comparisons of the entire VMHC maps were conducted with REST software (http://restfmri.net, accessed on 10 November 2021). Statistical tests were as follows: first, the one-way analysis of covariance (ANCOVA) to identify brain areas with significant differences in VMHC among the three groups with age, sex, and education level as covariates, followed by post hoc two-sample t tests. The ANCOVA result was corrected by AlphaSim correction (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf, accessed on 10 November 2021), with a voxel-level p < 0.01 and cluster size > 58 voxels, corresponding to a corrected p < 0.01. The post hoc two-sample t tests were conducted within a mask showing significant differences obtained from the ANCOVA analysis, with corrections (voxel-level p < 0.01; cluster size > 10 voxels, determined by a Monte Carlo simulation that resulted in a cluster-level significance threshold of p < 0.01).
The brain areas showing significant differences between PDF and PDNF patients were selected as regions of interest (ROIs). Afterwards, Pearson correlation coefficients were computed between the extracted mean VMHC values within the ROIs and the FSS/9 scores of PDF patients. The significance level was set at p < 0.05 (two-tailed).
3. Results
3.1. Demographic and Clinical Characteristics
The demographic and clinical data are summarized in Table 1. There were no significant differences in age, sex, education levels and MMSE among the three groups. Similarly, no significant differences were detected for PDF and PDNF groups, in terms of disease duration, H&Y stage, UPDRS-III, LEDD, ESS, AS, HAMD and HAMA. As expected, the FSS/9 value was significantly higher in the PDF group than the PDNF group (mean difference = 3.06, 95% confidence interval [CI] 2.52 to 3.83, p < 0.001).

Table 1.
Demographic and clinical characteristics of all subjects.
3.2. Voxel-Mirrored Homotopic Connectivity
ANCOVA revealed significant differences in VMHC among PDF, PDNF and HC groups, where age, sex, and education level were included as covariates, followed by a post hoc t-test within the mask obtained from the ANCOVA analysis. Compared to the PDNF group, PDF had decreased VMHC values in the supramarginal gyri (SMG) (p < 0.01) (Table 2; Figure 2A). Compared to HCs, PDF had lower VMHC values in the SMG (p = 0.005) and the opercular parts of inferior frontal gyri (IFG operc) (p < 0.01) (Table 2; Figure 2B). Meanwhile, VMHC in the IFG operc (p = 0.001) and middle frontal gyri (MFG) (p < 0.01) were notably decreased in PDNF patients compared with HCs (Table 2; Figure 2C).

Table 2.
Regions showing significant differences in VMHC between groups.
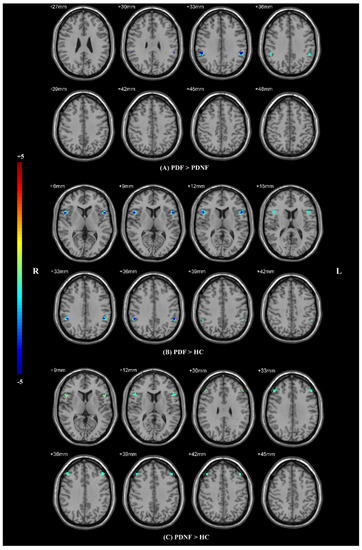
Figure 2.
Statistical maps showing VMHC differences in different brain regions between three groups. The results were corrected by AlphaSim (with a combined threshold of p < 0.01). (A) Differences between PDF patients and PDNF patients; (B) differences between PDF patients and HC group; (C) differences between PDNF patients and HC group. Abbreviations: VMHC: voxel-mirrored homotopic connectivity, HCs: healthy controls, PDF: Parkinson’s disease with fatigue, PDNF: Parkinson’s disease without fatigue, L: left, R: right.
3.3. Correlation Analysis
Based on the VMHC results, correlation analyses between FSS/9 scores and mean VMHC values of SMG were conducted for PDF patients. FSS/9 scores were negatively correlated with the mean VMHC signals within the SMG regions (r = −0.754, p = 0.001) (Figure 3), indicating that with the aggravation of fatigue, the function coordination of SMG would be poorer in PDF patients. However, this correlation was not found in the PDNF group (r = −0.155, p = 0.566).
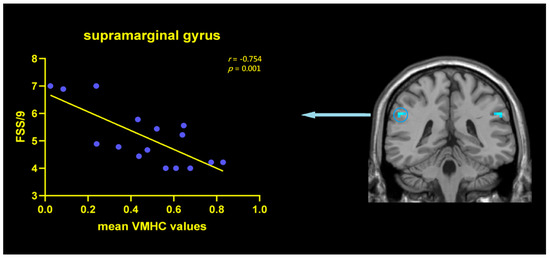
Figure 3.
Correlations between VMHC values in SMG and FSS/9 scores within the PDF patients. Scatterplots demonstrated that there was a significant negative correlation between the mean VMHC values in the SMG (r = −0.754, p = 0.001) and FSS/9 scores in PDF patients. Abbreviations: VMHC: voxel-mirrored homotopic connectivity; FSS: fatigue severity scale; SMG: supramarginal gyrus; PDF: Parkinson’s disease with fatigue.
4. Discussion
Based on the clinical characteristics of the unilateral onset of PD and the asymmetry of functional connectivity and structural changes in the bilateral hemispheres in PD with fatigue [13,14,21,22,23,24], we applied VMHC, which can detect interhemispheric functional asynchronization sensitively, to detect functional coordination between hemispheres in fatigue. Moreover, this study represented the first attempt to characterize fatigue-related interhemispheric brain synchrony abnormalities in patients with PD by the VMHC methodology. Compared to both PDNF and HC groups, we found that the PDF group had lower VMHC values in the bilateral SMG, which makes valuable contributions to cognitive function, such as attentional control and emotional regulation [25]. Additionally, when compared to the HCs, the PDF and PDNF patients both showed reduced VMHC values, mainly in the bilateral IFG operc, and PDNF patients displayed an additional decrease in the VMHC values of bilateral MFG. Furthermore, a significant negative correlation was found between FSS/9 scores and the VMHC values of the SMG in the PDF patients, indicating that the decreased VMHC values of SMG might be associated with the severity of PD-related fatigue.
The SMG plays an important role in cognitive circuits [26], which is mainly reflected in bottom-up attention [27], motor control [28] and emotional modulation, especially the down-regulation of negative emotion [29]. The latest study found that the right SMG was one of the fronto-parietal connector hubs, interacting functionally with the dorsal attention network and the ventral attention network [30], which showed differences in the processing of attention information in the bilateral SMG. Meanwhile, fatigue complaints were associated with cognitive impairment in a large PD cohort [31]. Pavese N. et al. found that the feeling of fatigue was often caused by the dissociation of motivation from executive motor movement [6], which depended on the mechanisms of evaluating and comparing the costs and benefits of a motor/mental activity by balancing its energetic cost with the volitional and motivational drive of subjects [32]. Moreover, two studies demonstrated that fatigue was related to the inability to process external stimuli correctly, and PDF patients had difficulty in attentional orienting to salient novel stimuli, which correlated with the severity of subjective fatigue [27,33]. Additionally, recent resting-state fMRI studies found that PD-related fatigue was related to altered neural activity in the areas implicated in the attention, salience and default networks [11,34]. Abnormal functional connectivity in the parietal lobe containing SMG was disclosed in chronic fatigue syndrome [8]. According to some perspectives, fatigue in PD is essentially a cognitive impairment, mainly manifested by abnormal regulation of the attention domain [6]. Particularly, SMG is an important part of this loop. Further correlation analyses showed that the decreased VMHC values in SMG were only associated with FSS/9 scores in the PDF patients, indicating that the decreased VMHC values in SMG were specific in PD patients with fatigue. Hence, we speculated that the reduced VMHC within the bilateral SMG may possibly underlie the neural mechanisms of fatigue in PD, by affecting the synergistic function of bilateral attentional regulation related to cognitive function, and such dysfunction could be accompanied by micro injury of white matter tracts in the cerebral hemisphere [35]. Furthermore, we surmised that SMG might be a possible target for neuromodulation strategies (e.g., implemented by transcranial magnetic stimulation or transcranial direct current stimulation techniques) by modulating SMG activity to ameliorate PD fatigue. In the future, rigorous clinical trials are needed to verify our hypothesis.
Additionally, the comparison between the PD patients (PDF or PDNF) and the HC group revealed reduced interhemispheric synchrony within the bilateral IFG operc. Generally, the cardinal symptoms of PD are thought to be attributed to the dysfunction of motor circuits [36,37] and sensory processing [28]. As we know, the IFG operc is a crucial component of the cognitive locomotor control and self-regulation network [38] involved in corticocortical and subcortical pathways during motor and cognitive inhibition [39,40]. Thus, we supposed that the reduced interhemispheric FC within IFG operc might indicate poor coordination of the two hemispheres in motor control and sensory perception in PD, simultaneously. In agreement with previous studies [41,42], we also observed that, compared with HCs, PDNF patients showed decreased VMHC values in the MFG, known as a key node for regulating motor and non-motor symptoms [43].
The present study has several limitations besides the small sample size. First, the use of drugs could be an important confounding factor. However, we evaluated all patients during off state and the doses of dopaminergic drug usage were well matched in both the PDF and PDNF groups. Second, the brain is not exactly structurally symmetrical. Thus, we smoothed the functional data and normalized them to a symmetric template for resolving this issue as much as possible [16]. Third, the sample size of our PD patients could limit the generalizability of the current results, and further research is needed to verify whether the present results can be applied to individuals, groups or populations with different ages and severities of disease.
5. Conclusions
To sum up, the decreased VMHC values within the bilateral SMG may be the unique imaging features of fatigue in PD. This discovery also suggests that the uncoordinated function of bilateral SMG may have participated in the pathophysiological mechanisms of fatigue in PD, possibly via affecting cognitive function involving attention. We hope that this study can provide some theoretical accumulation for the diagnosis and clinical treatment of fatigue in PD patients.
Author Contributions
Conceptualization, Y.-S.Y. and K.-Z.Z.; methodology, Y.-S.Y., M.J. and C.-T.G.; software, Y.-S.Y., M.J. and C.-T.G.; validation, Y.-S.Y., M.J. and C.-T.G.; formal analysis, Y.-S.Y.; investigation, Y.-S.Y., M.J. and C.-T.G.; resources, K.-Z.Z.; data curation, Y.-S.Y., M.J., C.-T.G., H.-M.S., L.-N.W. and K.-Z.Z.; writing—original draft preparation, Y.-S.Y.; writing—review and editing, Y.-S.Y., M.J., C.-T.G., H.-M.S., L.-N.W. and K.-Z.Z.; visualization, Y.-S.Y.; supervision, K.-Z.Z.; project administration, K.-Z.Z.; funding acquisition, Y.-S.Y. and K.-Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China, grant number 81901297 and grant number 81671258.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by ethics board of the First Affiliated Hospital of Nanjing Medical University (Approval No. 2016-SRFA-094).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting reported results are available from the corresponding author upon reasonable request. Computer Software for VMHC analysis were the Data Processing Assistant for Resting-State fMRI (DPARSF, http://www.restfmri.net/forum/dparsf, accessed on 10 November 2021) and REST (http://restfmri.net, accessed on 10 November 2021).
Acknowledgments
We are grateful to all the participants for their patience and understanding, as well as all members of the neurology and radiology staff for their cooperation and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siciliano, M.; Trojano, L.; Santangelo, G.; De Micco, R.; Tedeschi, G.; Tessitore, A. Fatigue in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2018, 33, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.A.; Lees, A.J.; Schrag, A. What Are the Most Important Nonmotor Symptoms in Patients with Parkinson’s Disease and Are We Missing Them? Mov. Disord. 2010, 25, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Beiske, A.G.; Loge, J.H.; Hjermstad, M.J.; Svensson, E. Fatigue in Parkinson’s disease: Prevalence and associated factors. Mov. Disord. 2010, 25, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Miwa, T. Fatigue in patients with Parkinson’s disease: Impact on quality of life. Intern. Med. 2011, 50, 1553–1558. [Google Scholar] [CrossRef][Green Version]
- Abe, K.; Takanashi, M.; Yanagihara, T. Fatigue in patients with Parkinson’s disease. Behav. Neurol. 2000, 12, 103–106. [Google Scholar] [CrossRef]
- Pavese, N.; Metta, V.; Bose, S.K.; Chaudhuri, K.R.; Brooks, D.J. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain 2010, 133, 3434–3443. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.; Yuan, Y.; Tong, Q.; Jiang, S.; Wang, M.; Wang, J.; Ding, J.; Xu, Q.; Zhang, K. Brain metabolic correlates of fatigue in Parkinson’s disease: A PET study. Int. J. Neurosci. 2018, 128, 330–336. [Google Scholar] [CrossRef]
- Boissoneault, J.; Letzen, J.; Lai, S.; O’Shea, A.; Craggs, J.; Robinson, M.E.; Staud, R. Abnormal resting state functional connectivity in patients with chronic fatigue syndrome: An arterial spin-labeling fMRI study. Magn. Reson. Imaging 2016, 34, 603–608. [Google Scholar] [CrossRef]
- Li, J.; Yuan, Y.; Wang, M.; Zhang, J.; Zhang, L.; Jiang, S.; Ding, J.; Zhang, K. Alterations in regional homogeneity of resting-state brain activity in fatigue of Parkinson’s disease. J. Neural Transm. 2017, 124, 1187–1195. [Google Scholar] [CrossRef]
- Cho, S.S.; Aminian, K.; Li, C.; Lang, A.E.; Houle, S.; Strafella, A.P. Fatigue in Parkinson’s disease: The contribution of cerebral metabolic changes. Hum. Brain Mapp. 2017, 38, 283–292. [Google Scholar] [CrossRef]
- Tessitore, A.; Giordano, A.; De Micco, R.; Caiazzo, G.; Russo, A.; Cirillo, M.; Esposito, F.; Tedeschi, G. Functional connectivity underpinnings of fatigue in “Drug-Naïve” patients with Parkinson’s disease. Mov. Disord. 2016, 31, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Kempster, P.A.; Gibb, W.R.; Stern, G.M.; Lees, A. Asymmetry of substantia nigra neuronal loss in Parkinson’s disease and its relevance to the mechanism of levodopa related motor fluctuations. J. Neurol. Neurosurg. Psychiatry 1989, 52, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Q.X.; Sun, X.; Vesek, J.; Mosher, Z.; Vasavada, M.; Chu, J.; Kanekar, S.; Shivkumar, V.; Venkiteswaran, K.; et al. MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.; De Micco, R.; Giordano, A.; Di Nardo, F.; Russo, A.; Caiazzo, G.; De Mase, A.; Cirillo, M.; Tedeschi, G.; Trojano, L.; et al. Supplementary motor area functional connectivity in “drug-naïve” Parkinson’s disease patients with fatigue. J. Neural Transm. 2020, 127, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Krall, S.C.; Rottschy, C.; Oberwelland, E.; Bzdok, D.; Fox, P.T.; Eickhoff, S.B.; Fink, G.R.; Konrad, K. The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct. Funct. 2015, 220, 587–604. [Google Scholar] [CrossRef]
- Zuo, X.N.; Kelly, C.; Di Martino, A.; Mennes, M.; Margulies, D.S.; Bangaru, S.; Grzadzinski, R.; Evans, A.C.; Zang, Y.F.; Castellanos, F.X.; et al. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J. Neurosci. 2010, 30, 15034–15043. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Friedman, J.H.; Alves, G.; Hagell, P.; Marinus, J.; Marsh, L.; Martinez-Martin, P.; Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; et al. Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson’s disease. Mov. Disord. 2010, 25, 805–822. [Google Scholar] [CrossRef]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Gan, C.; Wang, M.; Si, Q.; Yuan, Y.; Zhi, Y.; Wang, L.; Ma, K.; Zhang, K. Altered interhemispheric synchrony in Parkinson’s disease patients with levodopa-induced dyskinesias. NPJ Parkinson’s Dis. 2020, 6, 14. [Google Scholar] [CrossRef]
- Cubo, E.; Martinez Martín, P.; Martin-Gonzalez, J.A.; Rodríguez-Blázquez, C.; Kulisevsky, J.; LEP Group Members. Motor laterality asymmetry and nonmotor symptoms in Parkinson’s disease. Mov. Disord. 2010, 25, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Tan, Y.Y.; Liu, D.Q.; Herzallah, M.M.; Lapidow, E.; Wang, Y.; Zang, Y.F.; Gluck, M.A.; Chen, S.D. Motor-symptom laterality affects acquisition in Parkinson’s disease: A cognitive and functional magnetic resonance imaging study. Mov. Disord. 2017, 32, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Merkitch, D.; Karaman, M.M.; Zhang, J.; Sui, Y.; Goldman, J.G.; Zhou, X.J. High-Spatial-Resolution Diffusion MRI in Parkinson Disease: Lateral Asymmetry of the Substantia Nigra. Radiology. 2019, 291, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.M.; Zhao, Q.; Tanner, J.J.; Schwab, N.A.; Levy, S.A.; Burke, S.E.; Huang, H.; Ding, M.; Price, C. Structural brain correlates of fatigue in older adults with and without Parkinson’s disease. NeuroImage Clin. 2019, 22, 101730. [Google Scholar] [CrossRef]
- Bzdok, D.; Schilbach, L.; Vogeley, K.; Schneider, K.; Laird, A.R.; Langner, R.; Eickhoff, S.B. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 2012, 217, 783–796. [Google Scholar] [CrossRef]
- Rubinstein, D.Y.; Camarillo-Rodriguez, L.; Serruya, M.D.; Herweg, N.A.; Waldman, Z.J.; Wanda, P.A.; Sharan, A.D.; Weiss, S.A.; Sperling, M.R. Contribution of left supramarginal and angular gyri to episodic memory encoding: An intracranial EEG study. NeuroImage 2021, 225, 117514. [Google Scholar] [CrossRef]
- Pauletti, C.; Mannarelli, D.; Locuratolo, N.; Currà, A.; Marinelli, L.; Fattapposta, F. Central fatigue and attentional processing in Parkinson’s disease: An event-related potentials study. Clin. Neurophysiol. 2019, 130, 692–700. [Google Scholar] [CrossRef]
- Luo, C.; Guo, X.; Song, W.; Zhao, B.; Cao, B.; Yang, J.; Gong, Q.; Shang, H.F. Decreased Resting-State Interhemispheric Functional Connectivity in Parkinson’s Disease. BioMed Res. Int. 2015, 2015, 692684. [Google Scholar] [CrossRef]
- Favre, P.; Kanske, P.; Engen, H.; Singer, T. Decreased emotional reactivity after 3-month socio-affective but not attention- or meta-cognitive-based mental training: A randomized, controlled, longitudinal fMRI study. NeuroImage 2021, 237, 118132. [Google Scholar] [CrossRef]
- Suo, X.; Ding, H.; Li, X.; Zhang, Y.; Liang, M.; Zhang, Y.; Yu, C.; Qin, W. Anatomical and functional coupling between the dorsal and ventral attention networks. NeuroImage 2021, 232, 117868. [Google Scholar] [CrossRef]
- Song, W.; Guo, X.; Chen, K.; Chen, X.; Cao, B.; Wei, Q.; Huang, R.; Zhao, B.; Wu, Y.; Shang, H.F. The impact of non-motor symptoms on the Health-Related Quality of Life of Parkinson’s disease patients from Southwest China. Parkinsonism Relat. Disord. 2014, 20, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Tinaz, S.; Pillai, A.S.; Hallett, M. Sequence Effect in Parkinson’s Disease Is Related to Motor Energetic Cost. Front. Neurol. 2016, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.; Tamburini, T.; Zis, P.; Rosoklija, G.; Abbruzzese, G.; Ray-Chaudhuri, K.; Pelosin, E.; Avanzino, L. An objective measure combining physical and cognitive fatigability: Correlation with subjective fatigue in Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 32, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ding, J.; Li, J.Y.; Wang, M.; Yuan, Y.S.; Zhang, L.; Jiang, S.M.; Wang, X.X.; Zhu, L.; Zhang, K.Z. Abnormal Resting-State Neural Activity and Connectivity of Fatigue in Parkinson’s Disease. CNS Neurosci. Ther. 2017, 23, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Bang, M.; Hong, J.Y.; Oh, J.; Kim, J.S.; Han, Y.M.; Chang, S.K.; Lee, S.A.; Yoon, U.; Shin, N.Y. Neural and dopaminergic correlates of fatigue in Parkinson’s disease. J. Neural Transm. 2020, 127, 301–309. [Google Scholar] [CrossRef] [PubMed]
- DeLong, M.; Wichmann, T. Changing views of basal ganglia circuits and circuit disorders. Clin. EEG Neurosci. 2010, 41, 61–67. [Google Scholar] [CrossRef]
- Kann, S.J.; Chang, C.; Manza, P.; Leung, H.C. Akinetic rigid symptoms are associated with decline in a cortical motor network in Parkinson’s disease. NPJ Parkinson’s Dis. 2020, 6, 19. [Google Scholar] [CrossRef]
- Huckins, J.F.; Adeyemo, B.; Miezin, F.M.; Power, J.D.; Gordon, E.M.; Laumann, T.O.; Heatherton, T.F.; Petersen, S.E.; Kelley, W.M. Reward-related regions form a preferentially coupled system at rest. Hum. Brain Mapp. 2019, 40, 361–376. [Google Scholar] [CrossRef]
- Wildgruber, D.; Riecker, A.; Hertrich, I.; Erb, M.; Grodd, W.; Ethofer, T.; Ackermann, H. Identification of emotional intonation evaluated by fMRI. NeuroImage 2005, 24, 1233–1241. [Google Scholar] [CrossRef]
- Bernal, B.; Altman, N. Neural networks of motor and cognitive inhibition are dissociated between brain hemispheres: An fMRI study. Int. J. Neurosci. 2009, 119, 1848–1880. [Google Scholar] [CrossRef]
- van Eimeren, T.; Monchi, O.; Ballanger, B.; Strafella, P.A. Dysfunction of the Default Mode Network in Parkinson Disease A Functional Magnetic Resonance Imaging Study. Arch. Neurol. 2009, 66, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Franciotti, R.; Pizzi, S.D.; Russo, M.; Carrarini, C.; Carrozzino, D.; Perfetti, B.; Onofrj, M.; Bonanni, L. Somatic symptoms disorders in Parkinson’s disease are related to default mode and salience network dysfunction. Neuroimage Clin. 2019, 23, 101932. [Google Scholar] [CrossRef] [PubMed]
- Boon, L.I.; Hepp, D.H.; Douw, L.; van Geenen, N.; Broeders, T.A.; Geurts, J.J.; Berendse, H.W.; Schoonheim, M.M. Functional connectivity between resting-state networks reflects decline in executive function in Parkinson’s disease: A longitudinal fMRI study. Neuroimage Clin. 2020, 28, 102468. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).