Inversion of Left Atrial Appendage Will Cause Compressive Stresses in the Tissue: Simulation Study of Potential Therapy
Abstract
:1. Introduction
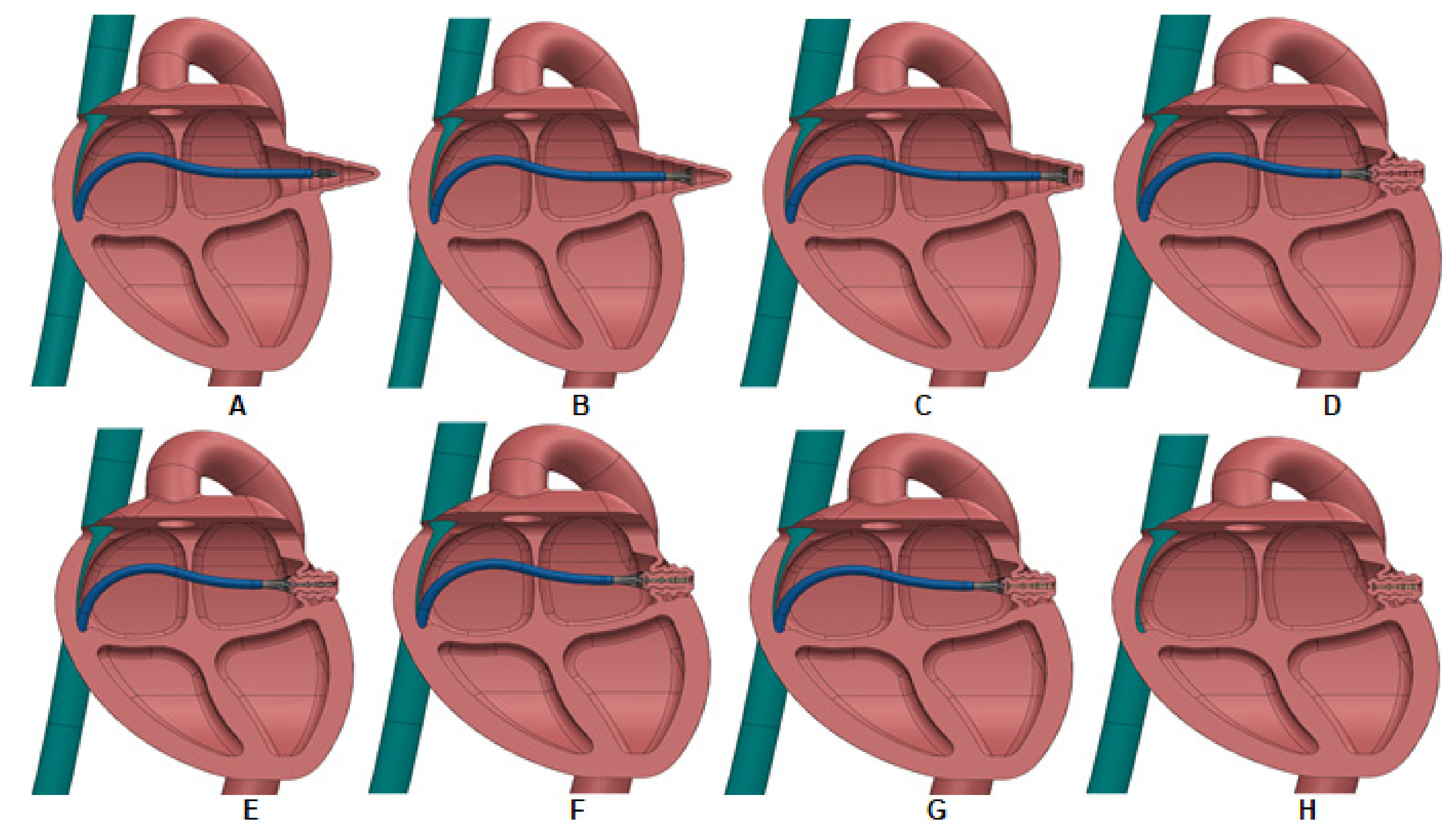
2. Materials and Methods
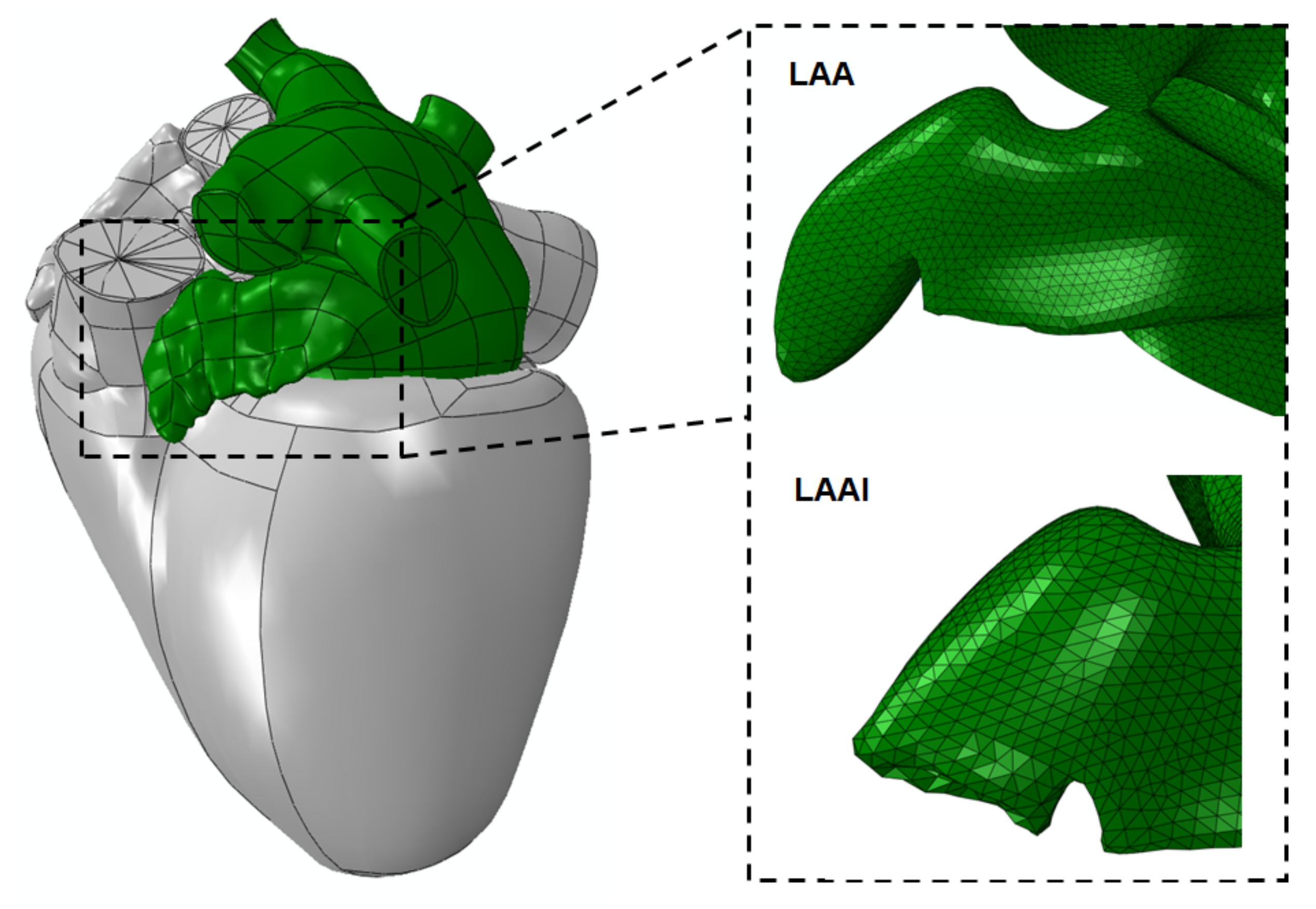
2.1. LAAI Structural Analysis
2.2. LAAI Fluid Dynamic Analysis
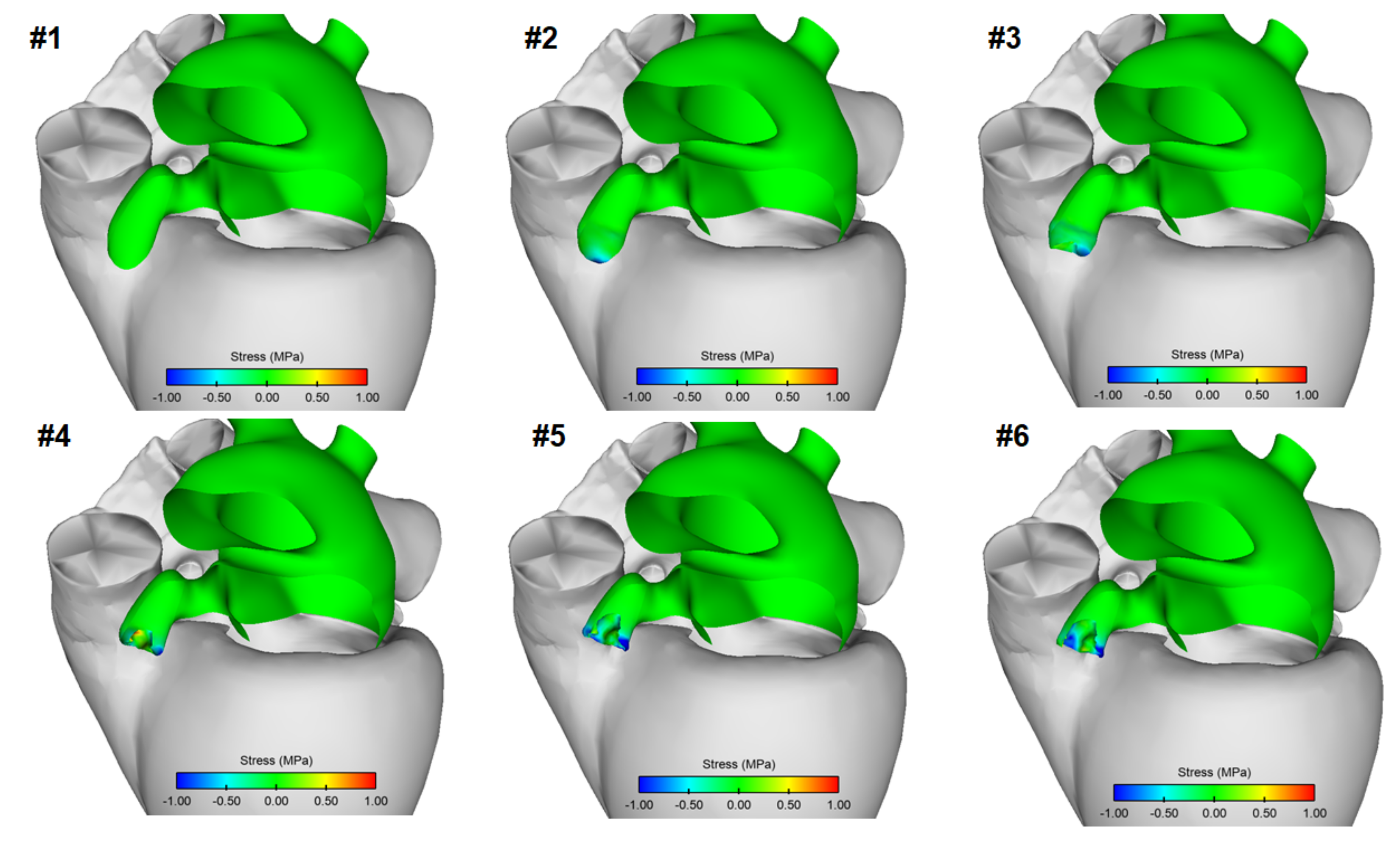
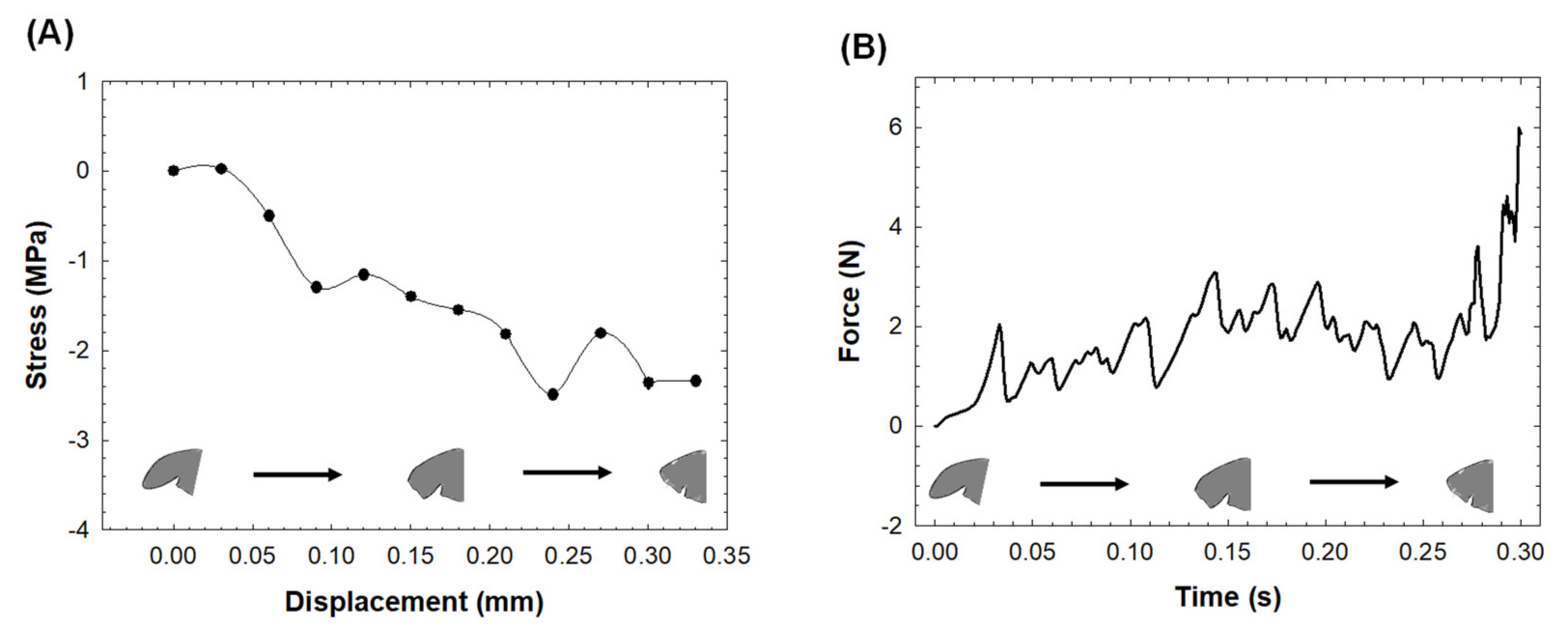
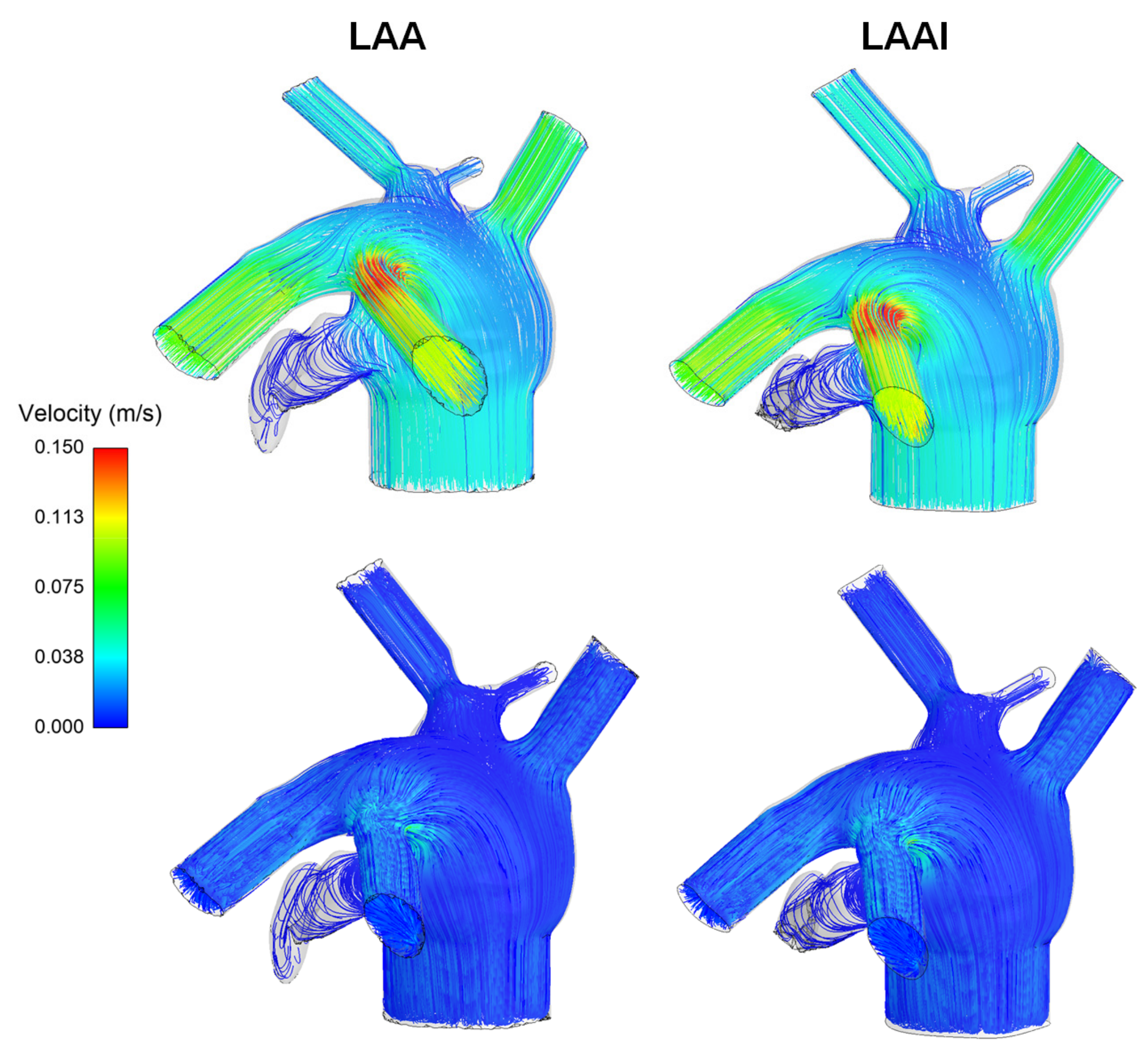
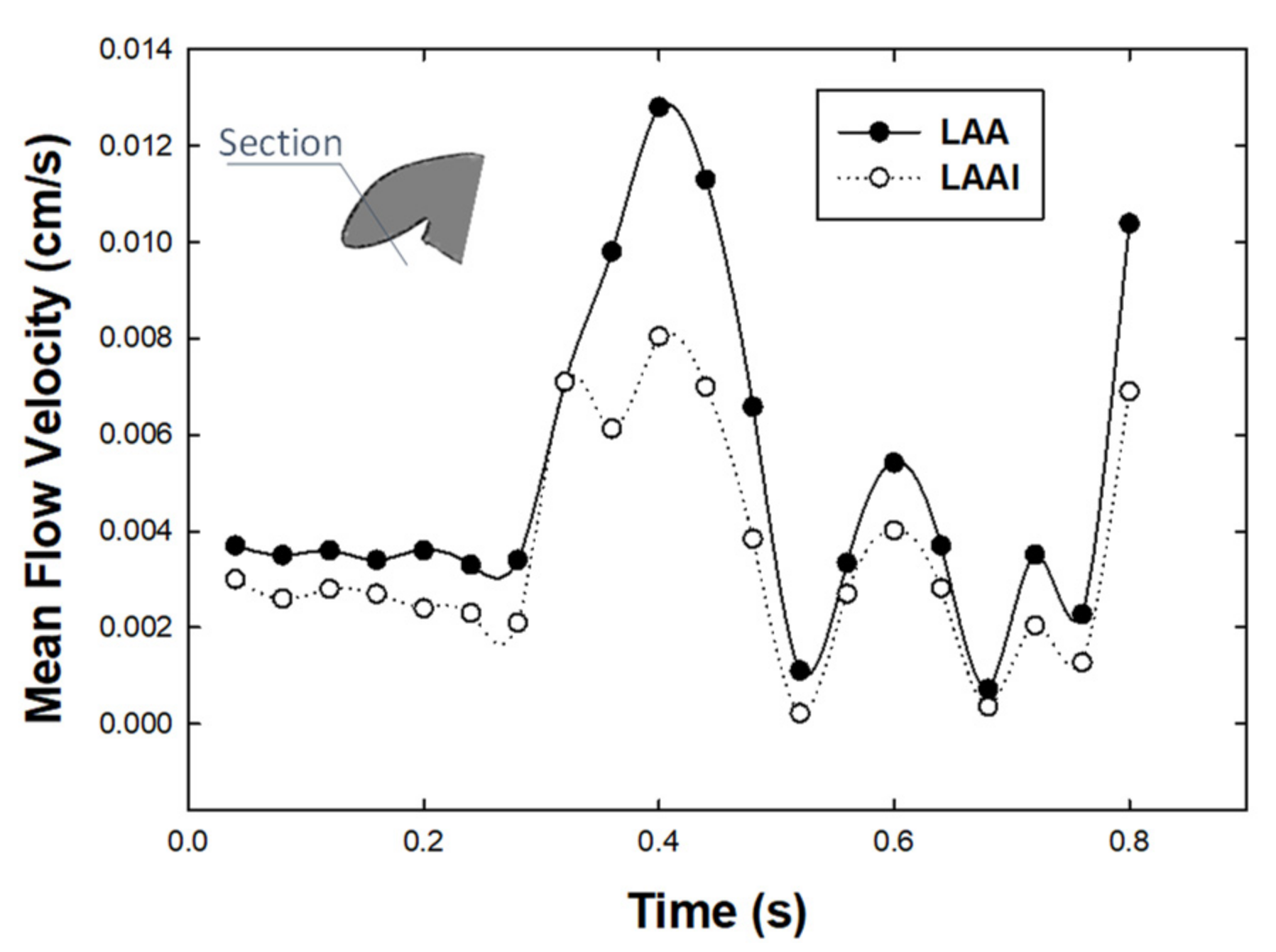
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Rasekh, A.; Shuraih, M.; Chelu, M.G.; Bartlett, T.; Mathuria, N.; Naeini, P.; Strickland, J.; Massumi, A.; Razavi, M.; et al. Safety and effectiveness of compassionate use of LARIAT(R) device for epicardial ligation of anatomically complex left atrial appendages. J. Interv. Card. Electrophysiol. 2015, 42, 11–19. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Johnston, S.S.; Chu, B.C.; Dalal, M.R.; Schulman, K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camm, A.J.; Lip, G.Y.; De Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; Hindricks, G.; Kirchhof, P.; Bax, J.J.; Baumgartner, H.; et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012, 33, 2719–2747. [Google Scholar] [CrossRef] [Green Version]
- Al-Saady, N.M.; Obel, O.A.; Camm, A.J. Left atrial appendage: Structure, function, and role in thromboembolism. Heart 1999, 82, 547–554. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Lakkireddy, D.R.; Whitlock, R.P.; Waksman, R.; Mack, M.J. Left atrial appendage occlusion: Opportunities and challenges. J. Am. Coll. Cardiol. 2014, 63, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Kakkar, A.K.; Mueller, I.; Bassand, J.P.; Fitzmaurice, D.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; Hacke, W.; Lip, G.Y.; Mantovani, L.G.; et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: Perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE 2013, 8, e63479. [Google Scholar] [CrossRef] [Green Version]
- Molteni, M.; Cimminiello, C. Warfarin and atrial fibrillation: From ideal to real the warfarin affaire. Thromb. J. 2014, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P.; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, I.; Cubeddu, R.J.; Sanchez-Ledesma, M.; Cury, R.C.; Coggins, M.; Maree, A.O.; Palacios, I.F. Left atrial appendage exclusion using an Amplatzer device. Int. J. Cardiol. 2009, 134, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Dukkipati, S.R.; d’Avila, A.; Doshi, S.K.; Reddy, V.Y. Percutaneous left atrial appendage closure with an epicardial suture ligation approach: A prospective randomized pre-clinical feasibility study. Heart Rhythm 2010, 7, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, F.; Kleine, P.; Martens, S.; Dzemali, O.; Dogan, S.; Keller, H.; Ackermann, H.; Zierer, A.; Ozaslan, F.; Wittlinger, T.; et al. Simplified technique for surgical ligation of the left atrial appendage in high-risk patients. J. Thorac. Cardiovasc. Surg. 2008, 135, 430–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzberg, S.P.; Gillinov, A.M.; Anyanwu, A.; Castillo, J.; Filsoufi, F.; Adams, D.H. Surgical left atrial appendage occlusion: Evaluation of a novel device with magnetic resonance imaging. Eur. J. Cardio-Thorac. Surg. 2008, 34, 766–770. [Google Scholar] [CrossRef] [Green Version]
- Turagam, M.K.; Vuddanda, V.; Verberkmoes, N.; Ohtsuka, T.; Akca, F.; Atkins, D.; Bommana, S.; Emmert, M.Y.; Gopinathannair, R.; Dunnington, G.; et al. Epicardial Left Atrial Appendage Exclusion Reduces Blood Pressure in Patients with Atrial Fibrillation and Hypertension. J. Am. Coll. Cardiol. 2018, 72, 1346–1353. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Turagam, M.; Afzal, M.R.; Rajasingh, J.; Atkins, D.; Dawn, B.; Di Biase, L.; Bartus, K.; Kar, S.; Natale, A.; et al. Left Atrial Appendage Closure and Systemic Homeostasis: The LAA HOMEOSTASIS Study. J. Am. Coll. Cardiol. 2018, 71, 135–144. [Google Scholar] [CrossRef]
- Sulkin, M.S.; Berwick, Z.C.; Hermiller, J.B.; Navia, J.A.; Kassab, G.S. Suction catheter for enhanced control and accuracy of transseptal access. EuroIntervention 2016, 12, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Fung, Y.C. Biomechanics: Motion, Flow, Stress and Growth; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Peirlinck, M.; Costabal, F.S.; Yao, J.; Guccione, J.M.; Tripathy, S.; Wang, Y.; Ozturk, D.; Segars, P.; Morrison, T.M.; Levine, S.; et al. Precision medicine in human heart modeling: Perspectives, challenges, and opportunities. Biomech. Model. Mechanobiol. 2021, 20, 803–831. [Google Scholar] [CrossRef]
- Holzapfel, G.A.; Ogden, R.W. Constitutive modelling of passive myocardium: A structurally based framework for material characterization. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 3445–3475. [Google Scholar] [CrossRef]
- Rinaudo, A.; Pasta, S. Regional variation of wall shear stress in ascending thoracic aortic aneurysms. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 627–638. [Google Scholar] [CrossRef]
- Pasta, S.; Gentile, G.; Raffa, G.M.; Scardulla, F.; Bellavia, D.; Luca, A.; Pilato, M.; Scardulla, C. Three-dimensional parametric modeling of bicuspid aortopathy and comparison with computational flow predictions. Artif. Organs 2017, 41, E92–E102. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, A.; Raffa, G.M.; Scardulla, F.; Pilato, M.; Scardulla, C.; Pasta, S. Biomechanical implications of excessive endograft protrusion into the aortic arch after thoracic endovascular repair. Comput. Biol. Med. 2015, 66, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Mendez, V.; Di Giuseppe, M.; Pasta, S. Comparison of hemodynamic and structural indices of ascending thoracic aortic aneurysm as predicted by 2-way FSI, CFD rigid wall simulation and patient-specific displacement-based FEA. Comput. Biol. Med. 2018, 100, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, R.; Funamoto, K.; Hayase, T.; Kanke, Y.; Shibata, M.; Shiraishi, Y.; Yambe, T. Numerical analysis of hemodynamic changes in the left atrium due to atrial fibrillation. J. Biomech. 2015, 48, 472–478. [Google Scholar] [CrossRef]
- Lorenzoni, G.; Merella, P.; Pischedda, P.; Casu, G. Percutaneous Management of Left Atrial Appendage Perforation: Keep Calm and Think Fast. J. Invasive Cardiol. 2018, 30, E126–E127. [Google Scholar]
- Katz, E.S.; Tsiamtsiouris, T.; Applebaum, R.M.; Schwartzbard, A.; Tunick, P.A.; Kronzon, I. Surgical left atrial appendage ligation is frequently incomplete: A transesophageal echocardiograhic study. J. Am. Coll. Cardiol. 2000, 36, 468–471. [Google Scholar] [CrossRef] [Green Version]
- Donnino, R.; Tunick, P.A.; Kronzon, I. Left atrial appendage thrombus outside of a ‘successful’ ligation. Eur. J. Echocardiogr. 2008, 9, 397–398. [Google Scholar] [CrossRef] [Green Version]
- Grossman, W. Cardiac hypertrophy: Useful adaptation or pathologic process? Am. J. Med. 1980, 69, 576–584. [Google Scholar] [CrossRef]
- Diakos, N.A.; Selzman, C.H.; Sachse, F.B.; Stehlik, J.; Kfoury, A.G.; Wever-Pinzon, O.; Catino, A.; Alharethi, R.; Reid, B.B.; Miller, D.V.; et al. Myocardial atrophy and chronic mechanical unloading of the failing human heart: Implications for cardiac assist device-induced myocardial recovery. J. Am. Coll. Cardiol. 2014, 64, 1602–1612. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Villalba, M.; Rossini, L.; Gonzalo, A.; Vigneault, D.; Martinez-Legazpi, P.; Duran, E.; Flores, O.; Bermejo, J.; McVeigh, E.; Kahn, A.M.; et al. Demonstration of Patient-Specific Simulations to Assess Left Atrial Appendage Thrombogenesis Risk. Front. Physiol. 2021, 12, 596596. [Google Scholar] [CrossRef]
- Masci, A.; Barone, L.; Dede, L.; Fedele, M.; Tomasi, C.; Quarteroni, A.; Corsi, C. The Impact of Left Atrium Appendage Morphology on Stroke Risk Assessment in Atrial Fibrillation: A Computational Fluid Dynamics Study. Front. Physiol. 2018, 9, 1938. [Google Scholar] [CrossRef] [PubMed]
- Bosi, G.M.; Cook, A.; Rai, R.; Menezes, L.J.; Schievano, S.; Torii, R.; Burriesci, G.B. Computational Fluid Dynamic Analysis of the Left Atrial Appendage to Predict Thrombosis Risk. Front. Cardiovasc. Med. 2018, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Biase, L.; Santangeli, P.; Anselmino, M.; Mohanty, P.; Salvetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Sack, K.L.; Aliotta, E.; Ennis, D.B.; Choy, J.S.; Kassab, G.S.; Guccione, J.M.; Franz, T. Construction and Validation of Subject-Specific Biventricular Finite-Element Models of Healthy and Failing Swine Hearts from High-Resolution DT-MRI. Front. Physiol. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, K.L.; Aliotta, E.; Choy, J.S.; Ennis, D.B.; Davies, N.H.; Franz, T.; Kassab, G.S.; Guccione, J.M. Intra-myocardial alginate hydrogel injection acts as a left ventricular mid-wall constraint in swine. Acta Biomater. 2020, 111, 170–180. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasta, S.; Guccione, J.M.; Kassab, G.S. Inversion of Left Atrial Appendage Will Cause Compressive Stresses in the Tissue: Simulation Study of Potential Therapy. J. Pers. Med. 2022, 12, 883. https://doi.org/10.3390/jpm12060883
Pasta S, Guccione JM, Kassab GS. Inversion of Left Atrial Appendage Will Cause Compressive Stresses in the Tissue: Simulation Study of Potential Therapy. Journal of Personalized Medicine. 2022; 12(6):883. https://doi.org/10.3390/jpm12060883
Chicago/Turabian StylePasta, Salvatore, Julius M. Guccione, and Ghassan S. Kassab. 2022. "Inversion of Left Atrial Appendage Will Cause Compressive Stresses in the Tissue: Simulation Study of Potential Therapy" Journal of Personalized Medicine 12, no. 6: 883. https://doi.org/10.3390/jpm12060883
APA StylePasta, S., Guccione, J. M., & Kassab, G. S. (2022). Inversion of Left Atrial Appendage Will Cause Compressive Stresses in the Tissue: Simulation Study of Potential Therapy. Journal of Personalized Medicine, 12(6), 883. https://doi.org/10.3390/jpm12060883







