Association of HLA-B*51:01, HLA-B*55:01, CYP2C9*3, and Phenytoin-Induced Cutaneous Adverse Drug Reactions in the South Indian Tamil Population
Abstract
1. Introduction
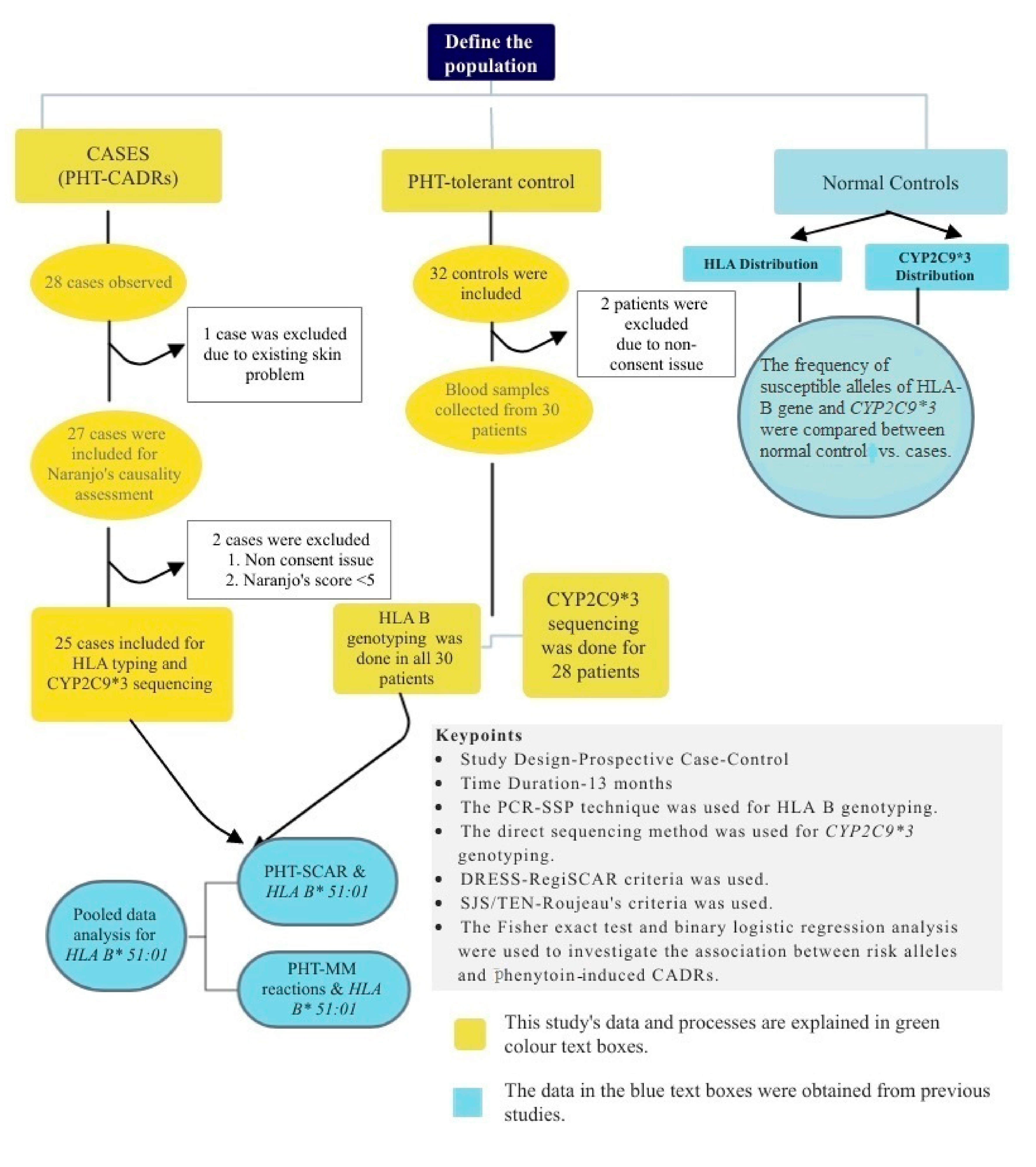
2. Methodology
2.1. Study Design and Settings
2.2. Participants
2.3. Causality Assessment
2.4. Genotyping
2.4.1. DNA Extraction/HLA-B Genotyping
2.4.2. CYP2C9*3 Sequencing
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Clinical Features of PHT-CADRs
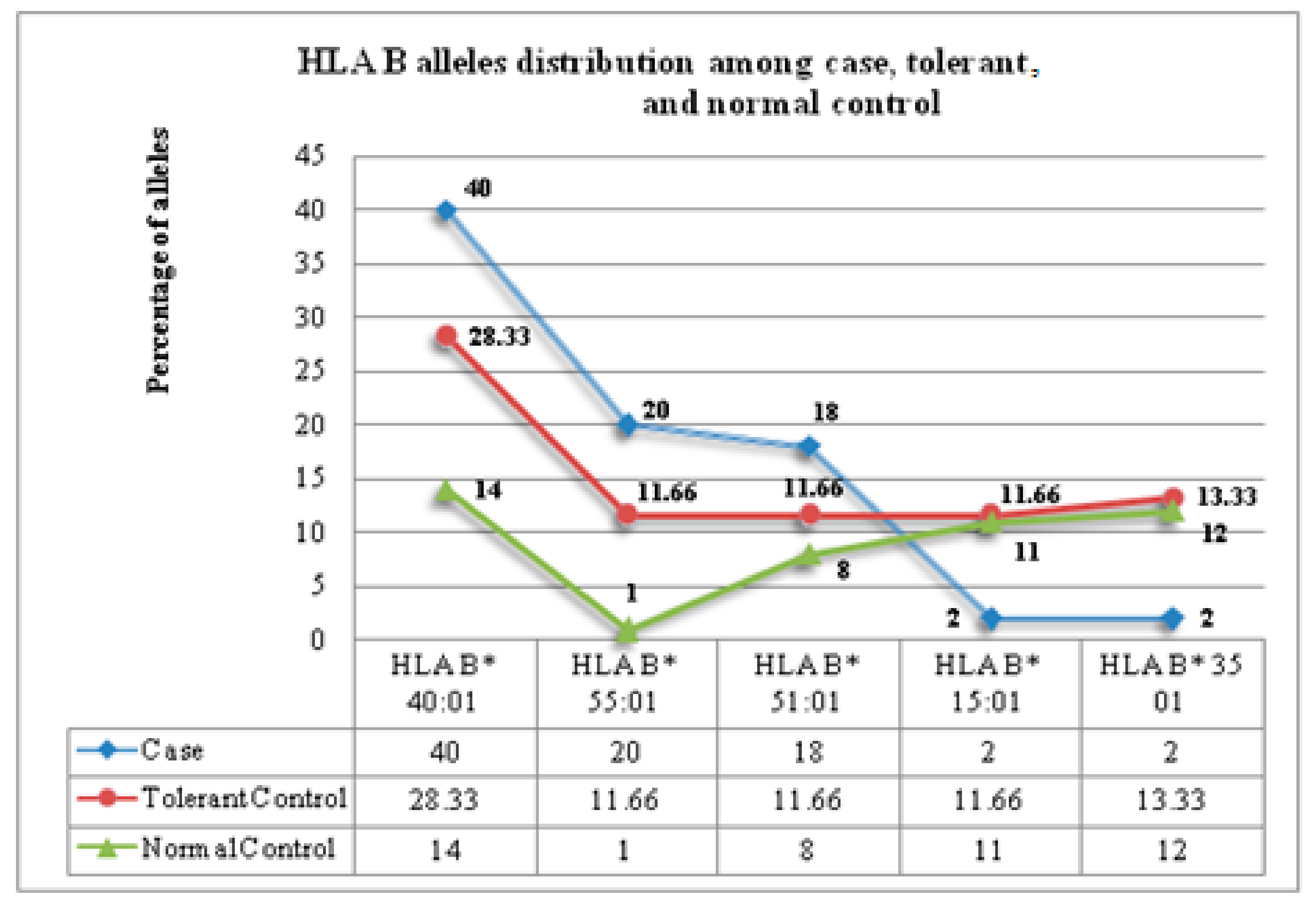
3.3. Frequency of HLA-B Alleles in PHT-Cases and Tolerant Controls
3.4. Bivariate Analysis of HLA B Alleles and PHT-CADRs
3.4.1. SCARs
3.4.2. Mild-Moderate Reactions
3.4.3. Overall PHT-Induced CADRs
3.4.4. CYP2C9*3
3.5. Pooled Data Analysis
3.6. Multivariate Binary Logistic Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Ethics Approval:
Abbreviations
| ALDEN | Algorithm of drug causality for epidermal necrolysis |
| AEDs | Anti-epileptic drugs |
| CBZ | Carbamazepine |
| PHT | Phenytoin |
| DRESS | Drug reaction with eosinophilia and systemic symptoms |
| HLA | Human leukocyte antigen |
| MPE | Maculopapular exanthema |
| NPV | Negative predictive values |
| SCARs | Severe cutaneous adverse drug reactions |
| ED | Exfoliative dermatitis |
| AFDE | Acneiform drug eruption |
| FDE | Fixed drug eruption |
| LDE | Lichenoid drug eruption |
| CADRs | Cutaneous adverse drug reactions |
| VPA | Valproic acid |
| TEN | Toxic epidermal necrolysis |
References
- Twardowschy, C.A.; Germiniani, F.; Werneck, L.C.; Silvado, C.; De Paola, L. Pearls & Oysters: Soft-tissue necrosis as a result of intravenous leakage of phenytoin. Neurology 2009, 73, e94–e95. [Google Scholar] [PubMed]
- Glick, T.H.; Workman, T.P.; Gaufberg, S.V. Preventing phenytoin intoxication: Safer use of a familiar anticonvulsant. J. Fam. Pr. 2004, 53, 197–202. [Google Scholar]
- Błaszczyk, B.; Szpringer, M.; Czuczwar, S.J.; Lasoń, W. Single centre 20 years survey of antiepileptic drug-induced hypersensitivity reactions. Pharmacol. Rep. 2013, 65, 399–409. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ghosh, A.; Barbhuiya, J.; Dey, S. Adverse cutaneous drug reactions: A one year survey at a dermatology outpatient clinic of a tertiary care hospital. Indian J. Pharmacol. 2006, 38, 429–431. [Google Scholar] [CrossRef]
- Patel, T.; Barvaliya, M.; Sharma, D.; Tripathi, C. A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 389–398. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Hong, H.S. Medical genetics: A marker for Stevens-Johnson syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef] [PubMed]
- Karnes, J.H.; Rettie, A.E.; Somogyi, A.A.; Huddart, R.; Fohner, A.E.; Formea, C.M.; Lee, M.T.M.; Llerena, A.; Whirl-Carrillo, M.; Klein, T.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2C9 and HLA-B genotypes and phenytoin dosing: 2020 Update. Clin. Pharmacol. Ther. 2020, 109, 302–309. [Google Scholar] [CrossRef]
- Sukasem, C.; Sririttha, S.; Chaichan, C.; Nakkrut, T.; Satapornpong, P.; Jaruthamsophon, K.; Jantararoungtong, T.; Koomdee, N.; Medhasi, S.; Oo-Puthinan, S.; et al. Spectrum of cutaneous adverse reactions to aromatic antiepileptic drugs and human leukocyte antigen genotypes in Thai patients and meta-analysis. Pharm. J. 2021, 1–9. [Google Scholar] [CrossRef]
- Locharernkul, C.; Loplumlert, J.; Limotai, C.; Korkij, W.; Desudchit, T.; Tongkobpetch, S.; Kangwanshiratada, O.; Hirankarn, N.; Suphapeetiporn, K.; Shotelersuk, V. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia 2008, 49, 2087–2091. [Google Scholar] [CrossRef]
- Chang, C.-C.; Ng, C.-C.; Too, C.-L.; Choon, S.-E.; Lee, C.-K.; Chung, W.-H.; Hussein, S.H.; Lim, K.-S.; Murad, S. Association of HLA-B*15:13 and HLA-B*15:02 with phenytoin-induced severe cutaneous adverse reactions in a Malay population. Pharm. J. 2016, 17, 170–173. [Google Scholar] [CrossRef]
- Thorn, C.F.; Whirl-Carrillo, M.; Leeder, J.S.; Klein, T.E.; Altman, R.B. PharmGKB summary: Phenytoin pathway. Pharm. Genom. 2012, 22, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-H.; Chang, W.-C.; Lee, Y.-S.; Wu, Y.-Y.; Yang, C.-H.; Ho, H.-C.; Chen, M.-J.; Lin, J.-Y.; Hui, R.C.-Y.; Ho, J.-C.; et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. J. Am. Med. Assoc. 2014, 312, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Rosemary, J.; Surendiran, A.; Rajan, S.; Shashindran, C.H.; Adithan, C. Influence of the CYP2C9 AND CYP2C19 polymorphisms on phenytoin hydroxylation in healthy individuals from south India. IndianJ. Med. Res. 2006, 123, 665–670. [Google Scholar]
- Agrawal, S.; Srivastava, S.K.; Borkar, M.; Chaudhuri, T.K. Genetic affinities of north and northeastern populations of India: Inference from HLA-based study. Tissue Antigens 2008, 72, 120–130. [Google Scholar] [CrossRef]
- Joshi, C.G.; Patel, J.S.; Patel, M.M.; Koringa, P.G.; Shah, T.M.; Patel, A.K.; Tripathi, A.K.; Mathew, A.; Rajapurkar, M.M. Human leukocyte antigen alleles, genotypes and haplotypes frequencies in renal transplant donors and recipients from West Central India. Indian J. Hum. Genet. 2013, 19, 219–232. [Google Scholar] [CrossRef][Green Version]
- Coon, C.S. Human populations. The New Encyclopedia Britannica, 15th ed.; Encyclopaedia Britannica: Chicago, IL, USA, 1983; Volume 14, pp. 839–848. [Google Scholar]
- Mittal, K.K.; Naik, S.; Sansonetti, N.; Cowherd, R.; Kumar, R.; Wong, D.M. The HLA antigens in Indian Hindus. Tissue Antigens 1982, 20, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Shankarkumar, U.; Hla, A.B.C. Allele frequencies in a Parsi population from Western India. Hum. Immunol. 2004, 65, 992–993. [Google Scholar] [CrossRef]
- Shankarkumar, U.; Hla, A.B.C. Allele frequencies in a Pawra population from Khandesh, India. Hum. Immunol. 2004, 65, 958. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Dubey, S.; Singh, S.; Sharma, S.; Mishra, A.; Harish, K.; Joshi, J.; Thangaraj, K. Genetic polymorphism of Cytochrome-P450-2C9 (CYP2C9) in Indian populations. bioRxiv 2017. [Google Scholar] [CrossRef]
- Ihtisham, K.; Ramanujam, B.; Srivastava, S.; Mehra, N.K.; Kaur, G.; Khanna, N.; Jain, S.; Kumar, S.; Kaul, B.; Samudrala, R.; et al. Association of cutaneous adverse drug reactions due to antiepileptic drugs with HLA alleles in a North Indian population. Seizure 2019, 66, 99–103. [Google Scholar] [CrossRef]
- Devi, K. The association of HLA B*15:02 allele and Stevens-Johnson syndrome/toxic epidermal necrolysis induced by aromatic anticonvulsant drugs in a South Indian population. Int. J. Dermatol. 2017, 57, 70–73. [Google Scholar] [CrossRef]
- Ramanujam, B.; Ihtisham, K.; Kaur, G.; Srivastava, S.; Mehra, N.K.; Khanna, N.; Singh, M.; Tripathi, M. Spectrum of cutaneous adverse reactions to Levetiracetam and human Leukocyte antigen typing in North-Indian patients. J. Epilepsy Res. 2016, 6, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Sharma, M.; Modi, M.; Garg, V.K.; Salaria, M. HLA-B*15 02 is associated with carbamazepine induced Stevens-Johnson syndrome in North Indian population. Hum. Immunol. 2014, 75, 1120–1122. [Google Scholar] [CrossRef]
- Patel, T.K.; Thakkar, S.H.; Sharma, D.C. Cutaneous adverse drug reactions in Indian population: A systematic review. Indian Dermatol. Online J. 2014, 5, S76–S86. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.W.; Min, F.L.; Zhou, D.; Qin, B.; Wang, J.; Hu, F.Y.; Cheung, Y.K.; Zhou, J.H.; Hu, X.S.; Zhou, J.Q.; et al. HLA-A*24:02 as a common risk factor for antiepileptic drug-induced cutaneous adverse reactions. Neurology 2017, 88, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Du-Thanh, A.; Kluger, N.; Bensalleh, H.; Guillot, B. Drug-induced acneiform eruption. Am. J. Clin. Dermatology 2011, 12, 233. [Google Scholar] [CrossRef]
- Rita, V.; Nilofar, D.; Nidhi, J.; Rochit, S. A study of adverse cutaneous drug reactions (ACDR) owing to antiepileptics at a rural-based tertiary-care center, Gujarat. Natl. J. Physiol. Pharm.Pharmacol. 2016, 6, 140. [Google Scholar]
- Roji, M.; Sebastian, M.; Lucca, J.M.; Pss, R. Phenytoin induced oral Lichenoid eruption and melasma: A case report. Indian J. Pharm. Pr. 2018, 11, 55–57. [Google Scholar] [CrossRef]
- Roujeau, J.C. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: A clinical classification. J.Invest. Dermatol. 1994, 102, 28S–30S. [Google Scholar] [CrossRef]
- Voora, L.; Shastry, C.; Bhandari, R.; Sukeerthi, D.; Rawal, K.B.; Chand, S. Phenytoin-induced Erythroderma. J. Young Pharm. 2019, 11, 320–321. [Google Scholar] [CrossRef]
- Dedhia, L.; Gadekar, S.; Mehta, P.; Parekh, S. HLA haplotype diversity in the South Indian population and its relevance. Indian J. Transplant. 2015, 9, 138–143. [Google Scholar] [CrossRef]
- Nahar, R.; Deb, R.; Saxena, R.; Puri, R.D.; Verma, I.C. Variability in CYP2C9 allele frequency: A pilot study of its predicted impact on warfarin response among healthy South and North Indians. Pharmacol. Rep. 2013, 65, 187–194. [Google Scholar] [CrossRef]
- Naranjo, C.; Busto, U.; Sellers, E.; Sandor, P.; Ruiz, I.; Roberts, E.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sidoroff, A.; Valeyrie-Allanore, L.; Halevy, S.; Davidovici, B.B.; Mockenhaupt, M.; Roujeau, J.C. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br.J. Dermatol. 2007, 156, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, B.; Haddad, C.; Mockenhaupt, M.; Dunant, A.; Liss, Y.; Bork, K.; Haustein, U.F.; Vieluf, D.; Roujeau, J.C.; Le Louet, H. ALDEN, an algorithm for assessment of drug causality in Stevens—Johnson syndrome and toxic epidermal necrolysis: Comparison with case-control analysis. Clin. Pharmacol. Ther. 2010, 88, 60–68. [Google Scholar] [CrossRef]
- Welsh, K.; Bunce, M. Molecular typing for the MHC with PCR-SSP. Rev. Immunogenet. 1999, 1, 157–176. [Google Scholar]
- Bunce, M.; O’neill, C.M.; Barnardo, M.C.N.M.; Krausa, P.; Browning, M.J.; Morris, P.J.; Welsh, K.I. Phototyping: Comprehensive DNA Typing for HLA A, B, C, DRB1, DRB3, DRB4, and DRB5 & DQB1 by PCR with 144 primers mixes utilizing sequence specific primers (PCR-SSP). Tissue Antigens 1995, 46, 355–367. [Google Scholar]
- Tonks, S.; Marsh, S.G.E.; Bunce, M.; Bodmer, J.G. Molecular typing for HLA class I using ARMS-PCR: Further developments following the 12th international histocompatibility workshop. Tissue Antigens 1999, 53, 175–183. [Google Scholar] [CrossRef]
- Kaniwa, N.; Sugiyama, E.; Saito, Y.; Kurose, K.; Maekawa, K.; Hasegawa, R.; Furuya, H.; Ikeda, H.; Takahashi, Y.; Muramatsu, M.; et al. Specific HLA types are associated with antiepileptic drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese subjects. Pharmacogenomics 2013, 14, 1821–1831. [Google Scholar] [CrossRef]
- Manuyakorn, W.; Likkasittipan, P.; Wattanapokayakit, S.; Suvichapanich, S.; Inunchot, W.; Wichukchinda, N.; Khongkhatithuml, C.; Thampratankul, L.; Kamchaisatian, W.; Benjaponpitak, S.; et al. Association of HLA genotypes with phenytoin induced severe cutaneous adverse drug reactions in Thai children. Epilepsy Res. 2020, 162, 106321. [Google Scholar] [CrossRef]
- Su, S.-C.; Chen, C.-B.; Chang, W.-C.; Wang, C.-W.; Fan, W.-L.; Lu, L.-Y.; Nakamura, R.; Saito, Y.; Ueta, M.; Kinoshita, S.; et al. HLA alleles and CYP2C9*3 as predictors of Phenytoin hypersensitivity in East Asians. Clin. Pharmacol. Ther. 2018, 105, 476–485. [Google Scholar] [CrossRef]
- Shankarkumar, U.; Ghosh, K.; Colah, R.B.; Gorakshakar, A.C.; Gupte, S.C.; Mohanty, D. HLA antigen distribution in selected caste groups from Mumbai, Maharastra, India. J. Hum. Ecol. 2002, 13, 209–215. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Selvaraj, P.; Narayanan, P.R.; Prabhakar, R.; Damodaran, C. Distribution of HLA (class I and class II) antigens in the native Dravidian Hindus of Tamil Nadu, south India. Gene Geogr. Comput. Bull. Hum. Gene Freq. 1995, 9, 15–24. [Google Scholar]
- Narayan, S.; Maiers, M.; Halagan, M.; Sathishkannan, A.; Naganathan, C.; Madbouly, A.; Periathiruvadi, S. Human leukocyte antigen (HLA)-A, -B, -C, -DRB1 and -DQB1 haplotype frequencies from 2491 cord blood units from Tamil speaking population from Tamil Nadu, India. Mol. Biol. Rep. 2018, 45, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Sukasem, C.; Sririttha, S.; Tempark, T.; Klaewsongkram, J.; Rerkpattanapipat, T.; Puangpetch, A.; Boongird, A.; Chulavatnatol, S. Genetic and clinical risk factors associated with phenytoin-induced cutaneous adverse drug reactions in Thai population. Pharmacoepidemiol. Drug Saf. 2020, 29, 565–574. [Google Scholar] [CrossRef]
- Shi, Y.-W.; Min, F.-L.; Liu, X.-R.; Zan, L.-X.; Gao, M.-M.; Yu, M.-J.; Liao, W.-P. Hla-B alleles and lamotrigine-induced cutaneous adverse drug reactions in the Han Chinese population. Basic Clin. Pharmacol. Toxicol. 2011, 109, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Krebs, K.; Bovijn, J.; Lepamets, M.; Censin, J.C.; Jürgenson, T.; Särg, D.; Abner, E.; Laisk, T.; Luo, L.; Skotee, L.; et al. Genome-wide study identifies association between HLA-B*55:01 and penicillin allergy. Am. J. Hum. Genet. 2020, 107, 612. [Google Scholar] [CrossRef] [PubMed]
- Pavlos, R.; McKinnon, E.J.; Ostrov, D.A.; Peters, B.; Buus, S.; Koelle, D.; Chopra, A.; Schutte, R.; Rive, C.; Redwood, A.; et al. Shared peptide binding of HLA Class I and II alleles associate with cutaneous nevirapine hypersensitivity and identify novel risk alleles. Sci. Rep. 2017, 7, 8653. [Google Scholar] [CrossRef]
- Peña-Balderas, A.M.; López-Revilla, R. Pharmacogenetics of adverse cutaneous reactions to lamotrigine. Rev. Mex. Neuroci. 2019, 20, 200–206. [Google Scholar] [CrossRef]
- Tassaneeyakul, W.; Prabmeechai, N.; Sukasem, C.; Kongpan, T.; Konyoung, P.; Chumworathayi, P.; Tiamkao, S.; Khunarkornsiri, U.; Kulkantrakorn, K.; Saksit, N.; et al. Associations between HLA class I and cytochrome P450 2C9 genetic polymorphisms and phenytoin-related severe cutaneous adverse reactions in a Thai population. Pharmacogenet. Genomics. 2016, 26, 225–234. [Google Scholar] [CrossRef]
- Yampayon, K.; Sukasem, C.; Limwongse, C.; Chinvarun, Y.; Tempark, T.; Rerkpattanapipat, T.; Kijsanayotin, P. Influence of genetic and non-genetic factors on phenytoin-induced severe cutaneous adverse drug reactions. Eur. J. Clin. Pharmacol. 2017, 73, 855–865. [Google Scholar] [CrossRef] [PubMed]
| Parameters | PHT-Induced CADRsN (%) | Total (25) | Tolerant (30) | p Value | ||
|---|---|---|---|---|---|---|
| Severe (6) | MPE and other Mild-Moderate Reactions c (19) | |||||
| Age | <20 | 02 (33.33) | 1 (5.26) | 3 (12) | 5 (16.66) | 0.71 |
| 21–40 | 01 (16.66) | 8 (42.10) | 9 (36) | 11 (36.66) | 0.80 | |
| 41–60 | 2 (33.33) | 6 (31.57) | 8 (32) | 12 (40) | 0.26 | |
| >60 | 2 (33.33) | 3 (15.78) | 5 (20) | 02 (6.66) | 0.12 | |
| Mean age ± SD | 44.5 ± 21.77 | 39.75 ± 1.414 | 42.15 ± 3.35 | 36.21 ± 14.71 | 0.36 a | |
| No of comorbidities (Mean) | 2 ± 0.00 | 1 ± 1.05 | 1.54 ± 0.82 | 037 ± 0.75 | 0.01 | |
| Gender | Male | 04 (66.66) | 10 (52.63) | 14 (56) | 18 (60) | 0.59 b |
| Female | 02 (33.33) | 09 | 11 (44) | 12 (40) | ||
| Onset latency (days) | 9–26 | 7–42 | 7–42 | - | - | |
| Indications | Seizure | 3 (50) | 9 (47.36) | 12 (48) | 11 (36.66) | - |
| Epilepsy | 2 (33.33) | 8 (42.10) | 10 (40) | 16 (53.33) | - | |
| CVA | 1 (16.66) | 1 (5.26) | 02 (8) | 03 (10) | - | |
| Others | 0 | 1 (5.26) | 01 (4) | - | - | |
| Social History | Alcohol (Yes) | 1 (16.66) | 6 (31.57) | 7 (28) | 8 (26.66) | >0.99 |
| Smoking (Yes) | 0 | 3 (15.78) | 3 (12) | 1 (3.33) | 0.31 | |
| Causality scores | Naranjo’s | 7 | 5–7 | 5–7 | - | - |
| RegiSCARS | >5 | - | >5 | - | - | |
| ALDEN | 7–8 | 7–8 | - | - | ||
| CM/Skin | Itching | 1 (16.66) | 13 (68.42) | 14 (56) | - | - |
| Pap. Rash/Redness | 6 (100) | 19 (100) | 25 (25) | - | - | |
| Papules/Pustules | 4 (66.66) | 04 (21.05) | 08 (32) | - | - | |
| Blistering/Peeling | 2 (33.33) | 0 | 02 (8) | - | - | |
| Erythema P/P | 3 (50) | 0 | 03 (12) | - | - | |
| CM/Mucosa | Eye | 6 (100) | 2 (10.52) | 08 (32) | - | - |
| Oral Mucosa | 6 (100) | 0 | 06 (24) | - | - | |
| Genital | 4 (66.66) | 0 | 04 (16) | - | - | |
| Anogenital | 2 (33.33) | 0 | 02 (8) | - | - | |
| F.Edema | 4 (66.66) | 2 (10.52) | 06 (24) | - | - | |
| CM/Systemic abnormalities | Fever | 6 (100) | 3 (10.52) | 09 (36) | - | - |
| Liver | ||||||
| AST IU/L (Mean ± SD) | SJS/DRESS/ED | 96.50 ± 9.192/183.7 ± 96.66/185 | - | - | - | - |
| ALT IU/L(Mean ± SD) | SJS/DRESS/ED | 72.00 ± 12.73/241.3 ± 139.9/131 | - | - | - | - |
| Alk.Phosphatase | SJS/DRESS/ED | 117.5 ± 17.68/331.0 ± 128.7/401 | - | - | - | - |
| Haematological abn. | ||||||
| WBC (Mean ± SD) | SJS/DRESS/ED | 5200 ± 424.3/11200 ± 2458/8000/µL | - | - | - | - |
| Lymphocytes (M %) | SJS/DRESS/ED | 17%/56%/- | - | - | - | - |
| Eosinophil (M %) | SJS/DRESS/ED | 09/12/- | - | - | - | - |
| Lymphadnopathy | SJS/DRESS/ED | Absent/3/Absent | - | - | - | - |
| AEDs Combination | PHT+VPA | 2 (33.33) | 2 (10.00) | 4 (15.38) | 6 (18.75) | >0.999 |
| PHT+LEV | 2 (33.33) | 5 (25.00) | 7 (26.92) | 4 (12.50) | 0.1935 | |
| PHT+VPA+LEV | - | 1 (5.00) | 1 (03.84) | - | 0.4576 | |
| Others | 2 (33.33) | 2 (10.00) | 4 (15.38) | 0 | - | |
| HLA B Alleles | Phenotype (no of Cases) | Number of Cases | Case vs. Tolerant | Case vs. Healthy Population | PPV/NPV | Sensitivity/Specificity | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case n = 25 | Tolerant n = 30HLA B/28CYP2C9*3 | General Population R1 & R2 n = 463/82 | OR (95% CI) | p Value a | OR (95% CI) | p Value a | ||||
| N (%) | N (%) | N (%) | ||||||||
| PHT-SCARs | ||||||||||
| B*51/51: 01 | SJS (2) | 2 (100) | 7 (23.33) | 19 (13.86) | 15.67 (0.6743–64.0) | 0.07 | 30.38 (1.405–657.1) | 0.02 | 22/100 | 100/76 |
| DRESS (3) | 3 (100) | 21.93 (1.013–474.9) | 0.02 | 42.54 (2.114–855.8) | 0.003 | 30/100 | 100/76 | |||
| ED (1) | 1 (100) | 9.40 (0.3452–56.0) | 0.25 | 18.23 (0.716–463.8) | 0.14 | 12.5/100 | 100/76 | |||
| B* 40/40:01 | DRESS (3) | 3 (100) | 14 (46.66) | 22 (16.05) | 7.96 (0.3787–67.5) | 0.22 | 35.93 (1.794–719.9) | 0.005 | 17/100 | 100/53 |
| ED (1) | 1 (100) | 3.41 (0.1288–0.49) | 0.48 | 15.40 (0.607–390.2) | 0.16 | 6.6/100 | 100/53 | |||
| B* 5101 | SCARs (6) | 6 (100) | 7 (23.33) | 19 (13.86) | 40.73 (2.045–811.3) | 0.0009 | 296.3 (16.11–5450) | <0.001 | 46/100 | 100/76 |
| B* 4001 | SCARs (6) | 4 (66.66) | 14 (46.66) | 22 (16.05) | 6.571 (1.157–37.69) | 0.057 | 40.09 (8.70–213.6) | 0.0001 | 36/92 | 66/76 |
| PHT-Mild-Moderate | ||||||||||
| B* 40/40:01 | MPE 15) | 10 (40) | 14 (28.33) | 22 (16.05) | 2.286( 0.6742–7.887) | 0.342 | 10.45 (3.299–29.34) | <0.001 | 41/76 | 66/53 |
| AFDE (2) | 2 (50) | 5.690 (0.2519–128.5) | 0.483 | 25.65 (1.172–552.8) | 0.02 | 12.5/100 | 100/53 | |||
| LDE (1) | 1 (50) | 3.414 (0.1288–90.49) | 0.483 | 15.40 (0.607–390.2) | 0.16 | 6.6/100 | 100/53 | |||
| FDE (1) | 1 (50) | 3.414 (0.1288–90.49) | 0.483 | 15.40 (0.607–390.2) | 0.16 | 6.6/100 | 100/53 | |||
| B* 55/55:01 | MPE (15) | 9 (33.3) | 07 (11.66) | 1 (0.729) | 4.929 (1.322–17.33) | 0.022 | 204.0 (27.72–2229) | <0.001 | 56/79 | 60/76 |
| B* 40/40:01 | Mild-Mode CADRs19 | 14 (73.68) | 14 (46.66) | 22 (4.75) | 3.200 (0. 885–10.34) | 0.080 | 56.13 (18.42–147.5) | <0.001 | 50/70 | 73/53 |
| B* 55/55:01 | Mild-Mode CADRs19 | 9 (47.3) | 07 (23.33) | 1 (0.21) | 2.957 (0.922–10.83) | 0.119 | 415.8 (51.92–454) | <0.001 | 56/69 | 47/76 |
| PHT-Over all CADR | ||||||||||
| B*51/51:01 | CADRs | 9 (36) | 7 (23.33) | 19 (13.8) | 1.848 (0.5871–5.905) | 0.377 | 3.493 (1.422–8.652) | 0.01 | 56/58 | 36/76 |
| B*40/40:01 | CADRs | 18 (72) | 14 (46.66) | 22 (16.05) | 2.939 (0.9870–8.842) | 0.098 | 13.44 (4.849–33.14) | <0.001 | 56/69 | 72/53 |
| B*55/55:01 | CADRs | 10 (40) | 07 (23.33) | 1 (0.72) | 1.848 (0.5871–5.905) | 0.377 | 76.50 (10.40–841.9) | <0.001 | 58/60 | 40/76 |
| B*57/57:01 | CADRs | 02 (8) | 04 (13.33) | 6 (4.37) | 0.5652 (0.1013–2.643) | 0.677 | 1.899 (0.369–8.216) | 0.35 | 33/53 | 8/86 |
| B*52/52:01 | CADRs | 02 (8) | 04 (13.33) | 11 (8.02) | 0.5652 (0.1013–2.643) | 0.677 | 0.996 (0.209–3.973) | >0.99 | 33/53 | 8/86 |
| B*07/07:02 | CADRs | 05 (20) | 04 (13.33) | 26 (18.9) | 1.625 (0.3967–5.800) | 0.716 | 1.067 (0.4067–.909) | >0.99 | 55/56 | 20/86 |
| B*15/15:01 | CADRs | 01 (4) | 07 (23.33) | 11 (8.02) | 0.1369 (0.0117–0.9204) | 0.059 | 0.477(0.042–3.005) | 0.69 | 12.5/48 | 4/76 |
| B*35/35:01 | CADRs | 01 (4) | 08 (26.66) | 22 (16.05) | 0.1146 (0.009–0.7273) | 0.030 | 0.2178 (0.020–.370) | 0.20 | 11/47 | 4/73 |
| CYP2C9*3 carriers | ||||||||||
| CYP2C9*3 | Severe CADRs (6) | 4 (66.6) | 2 (7.14) | 12 (14.63) | 26.00 (2.855–1726) | 0.0006 | 11.69 (2.386–63.63) | 0.009 | 66/92 | 66/92 |
| CYP2C9*3 | DRESS(3) | 3 (100) | 2 (7.14) | 12 (14.63) | 74.20 (2.922–1884) | 0.002 | 39.48 (1.920–811.9) | 0.004 | 60/100 | 100/92 |
| CYP2C9*3 | M-Moder. (19) | 8 (42.2) | 2 (7.14) | 12 (14.63) | 9.455 (1.628–47.25) | 0.008 | 2.917 (0.9178–0.969) | 0.02 | 80/70 | 42/92 |
| CYP2C9*3 | MPE(15) | 5 (33.33) | 2 (7.14) | 12 (14.63) | 6.500 (1.008–35.01) | 0.039 | 5.385 (1.917–13.67) | 0.13 | 71/72 | 33/92 |
| CYP2C9*3 | Overall (25) | 12 (48) | 2 (7.14) | 12 (14.63) | 12.92 (2.777–61.46) | 0.004 | 4.242 (1.378–12.89) | 0.001 | 85/66 | 48/92 |
| Alleles | Phenotype | Present Study | Literatures | Total after Pooling | Before Pooling | After Pooling | PPV/NPV | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | OR (95%CI) | p Value | OR (95%CI) | p Value | |||
| HLA B*51:01R1 | SCARs | 6/6 | 7/30 | 4/15 | 8/100 | 11/21 | 15/130 | 40.73 (2.045–811.3) | 0.0009 | 6.273 (2.24–16.69) | 0.0008 | 46/100 a 47/8 8b |
| MM | 3/19 | 7/30 | 9/35 | 8/100 | 12/54 | 15/130 | 0.6161 (0.1563–2.603) | 0.71 | 2.190 (0.939–4.97) | 0.07 | 30/58 a 44/73 b | |
| Overall CADRs | 9/25 | 7/30 | 13/50 | 8/100 | 22/75 | 15/130 | 0.848 (0.5871–0.377) | 0.37 | 2.323 (1.122–5.899) | 0.03 | 36/76 a 59/68 b | |
| Predictor Variables | β | SE | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| PHT- All types of CADRs/tolerant Control | |||||
| CYP2C9*3 | 2.48 | 0.83 | 12 | 2.759–84.87 | 0.003 |
| HLA B*51:01 | 0.48 | 0.67 | 1.61 | 0.429–6.104 | 0.47 |
| PHT-SCARs/tolerant Control | |||||
| CYP2C9*3 | 2.52 | 1.22 | 12.4 | 1.138–136.2 | 0.003 |
| HLA B*51:01 | 2.04 | 1.45 | 7.70 | 0.447–133.0 | 0.16 |
| HLA B*40:01 | −0.33 | 1.34 | 0.71 | 0.52–9.895 | 0.80 |
| MPE/tolerant Control | |||||
| HLA B*55:01 | 1.39 | 0.66 | 4.04 | 1.125–15.67 | 0.03 |
| HLAB*40:01 | 0.39 | 0.78 | 1.47 | 0.318–6.873 | 0.61 |
| CYP2C9*3 | 1.57 | 1.05 | 4.89 | 0.617–37.79 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, S.; Balakrishnan, K.; Sukasem, C.; Anand, T.C.V.; Canyuk, B.; Pattharachayakul, S. Association of HLA-B*51:01, HLA-B*55:01, CYP2C9*3, and Phenytoin-Induced Cutaneous Adverse Drug Reactions in the South Indian Tamil Population. J. Pers. Med. 2021, 11, 737. https://doi.org/10.3390/jpm11080737
John S, Balakrishnan K, Sukasem C, Anand TCV, Canyuk B, Pattharachayakul S. Association of HLA-B*51:01, HLA-B*55:01, CYP2C9*3, and Phenytoin-Induced Cutaneous Adverse Drug Reactions in the South Indian Tamil Population. Journal of Personalized Medicine. 2021; 11(8):737. https://doi.org/10.3390/jpm11080737
Chicago/Turabian StyleJohn, Shobana, Karuppiah Balakrishnan, Chonlaphat Sukasem, Tharmarajan Chinnathambi Vijay Anand, Bhutorn Canyuk, and Sutthiporn Pattharachayakul. 2021. "Association of HLA-B*51:01, HLA-B*55:01, CYP2C9*3, and Phenytoin-Induced Cutaneous Adverse Drug Reactions in the South Indian Tamil Population" Journal of Personalized Medicine 11, no. 8: 737. https://doi.org/10.3390/jpm11080737
APA StyleJohn, S., Balakrishnan, K., Sukasem, C., Anand, T. C. V., Canyuk, B., & Pattharachayakul, S. (2021). Association of HLA-B*51:01, HLA-B*55:01, CYP2C9*3, and Phenytoin-Induced Cutaneous Adverse Drug Reactions in the South Indian Tamil Population. Journal of Personalized Medicine, 11(8), 737. https://doi.org/10.3390/jpm11080737






