Serotonin Transporter Genetic Variation and Antidepressant Response and Tolerability: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
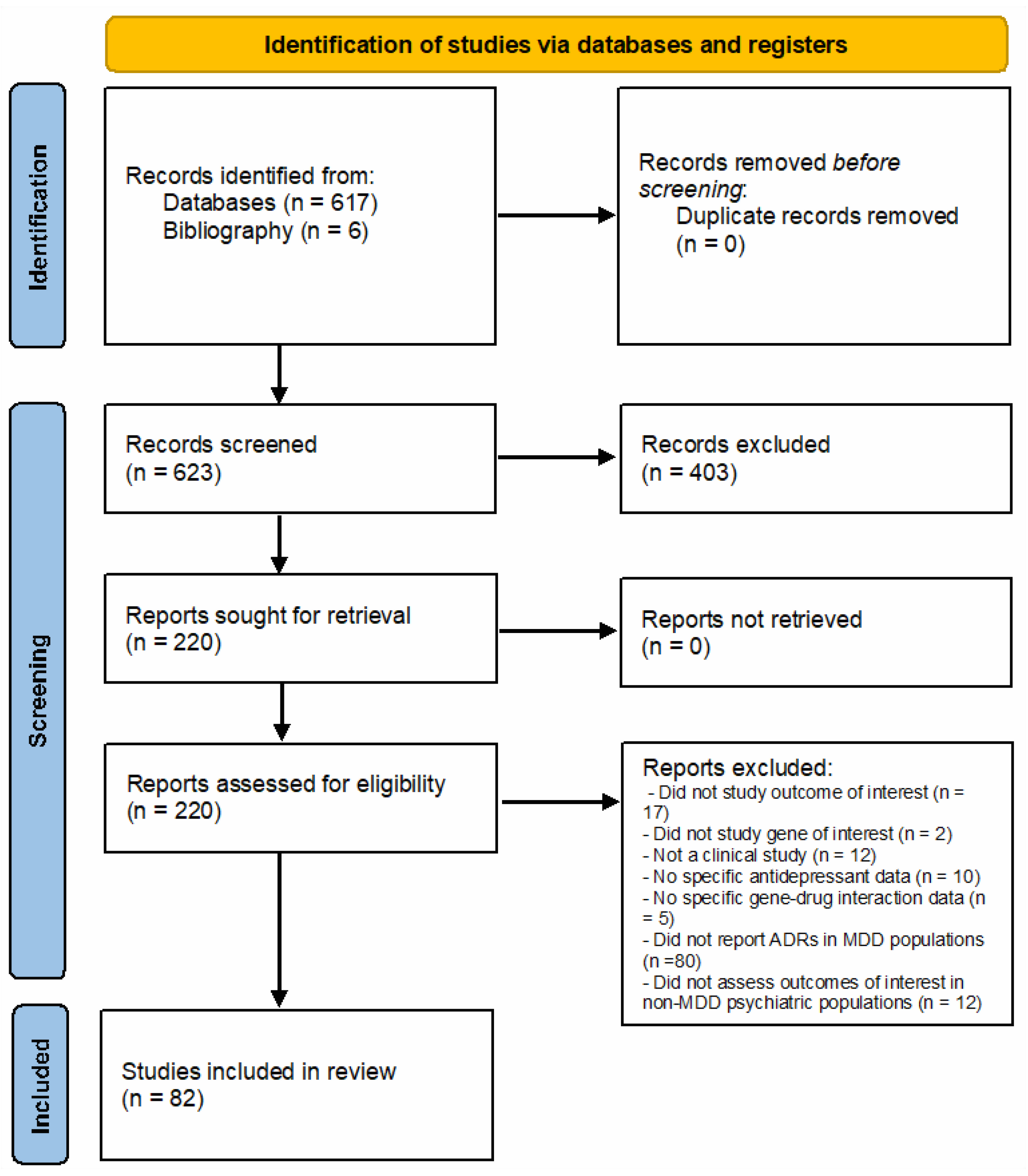
2.1. Search Strategy and Selection Criteria
2.2. Quality Review
2.3. Data Analysis
3. Results
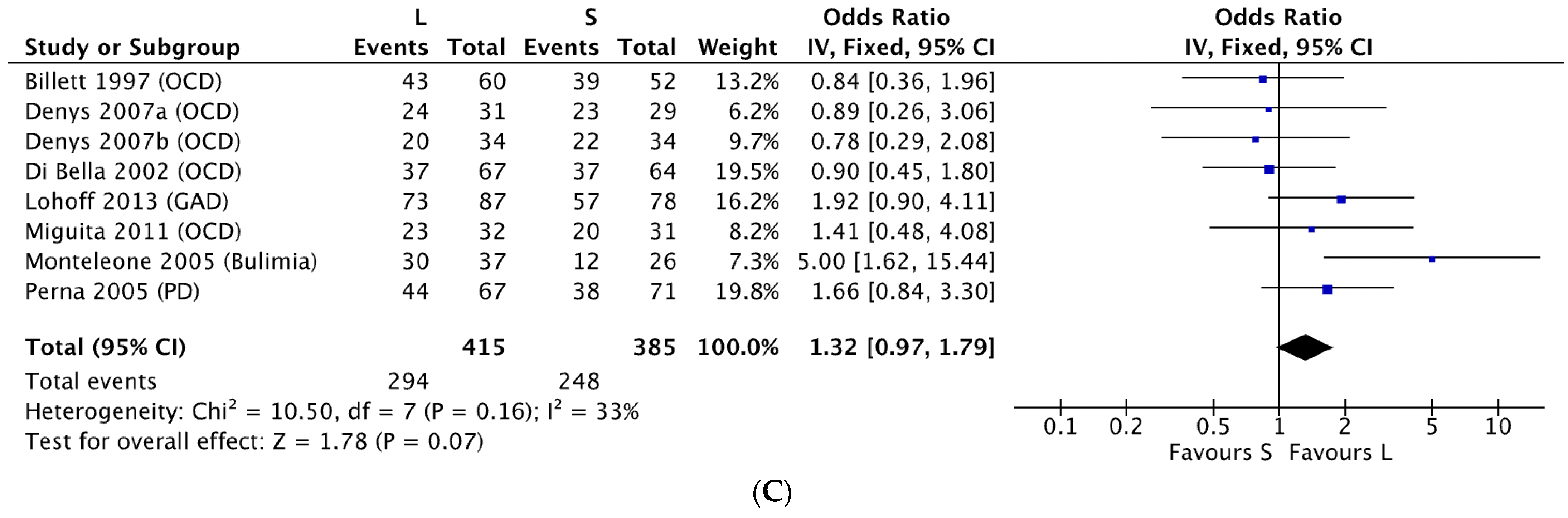
3.1. 5-HTTLPR and Antidepressant Response in Non-MDD Patients
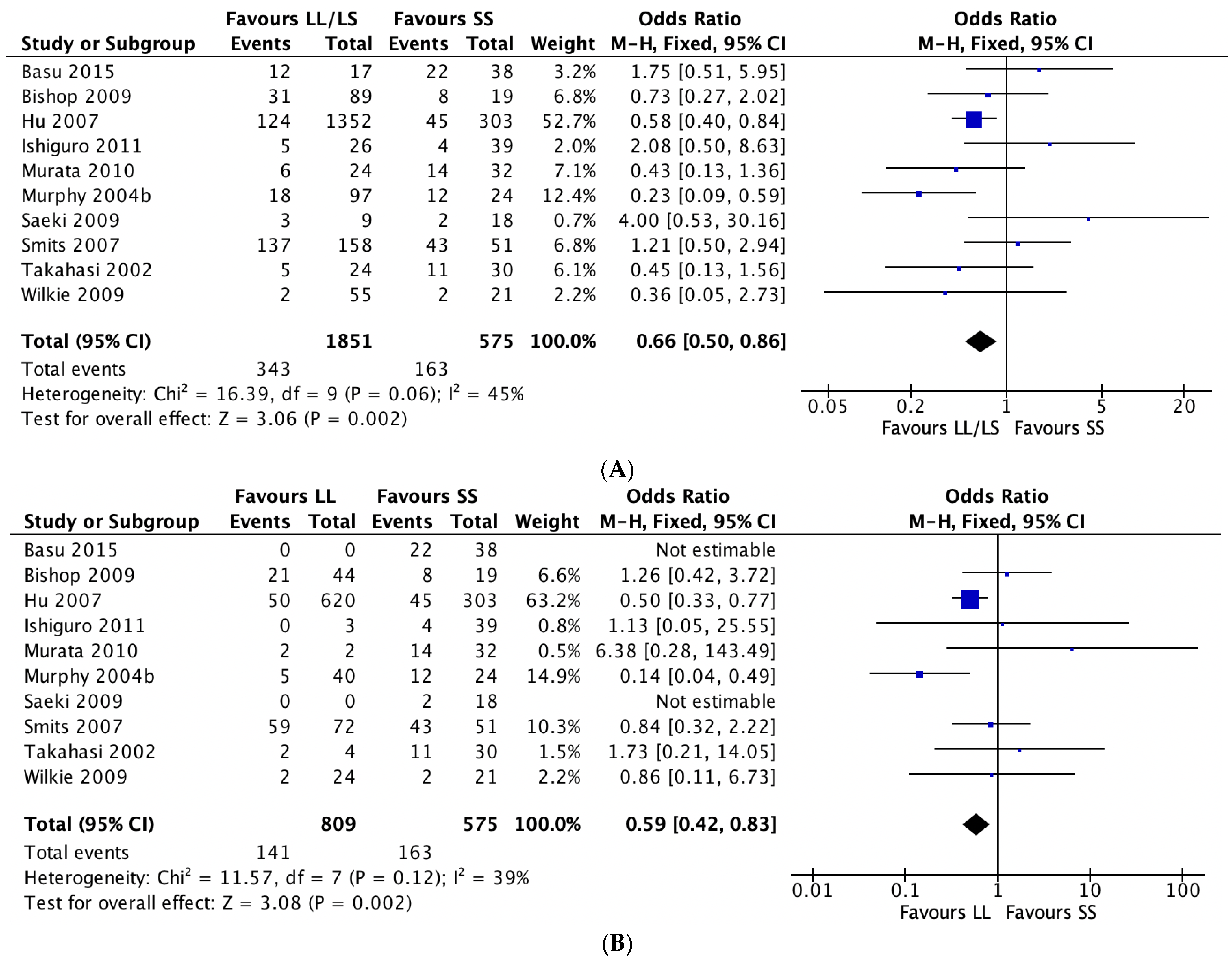
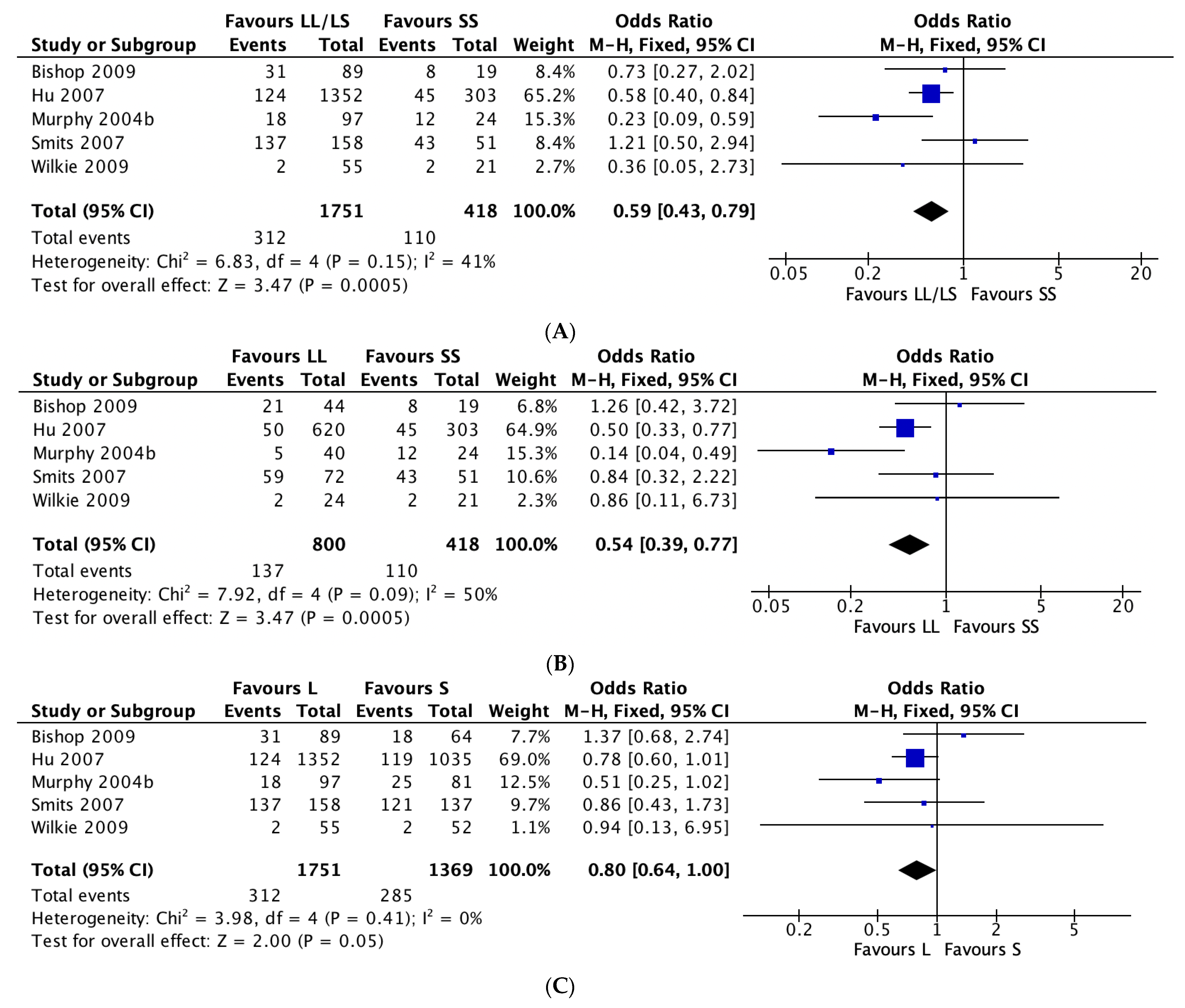
3.2. 5-HTTLPR and Antidepressant Tolerability
4. Discussion
4.1. 5-HTTLPR and Antidepressant Response
4.2. 5-HTTLPR and Antidepressant Tolerability
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cascade, E.; Kalali, A.H.; Kennedy, S.H. Real-world data on SSRI antidepressant side effects. Psychiatry 2009, 6, 16. [Google Scholar]
- Papakostas, G.I.; Fava, M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur. Neuropsychopharmacol. 2008, 19, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tansey, K.E.; Guipponi, M.; Hu, X.; Domenici, E.; Lewis, G.; Malafosse, A.; Wendland, J.R.; Lewis, C.M.; McGuffin, P.; Uher, R. Contribution of Common Genetic Variants to Antidepressant Response. Biol. Psychiatry 2013, 73, 679–682. [Google Scholar] [CrossRef]
- Bousman, C.A.; Hopwood, M. Commercial pharmacogenetic-based decision-support tools in psychiatry. Lancet Psychiatry 2016, 3, 585–590. [Google Scholar] [CrossRef]
- Hicks, J.; Sangkuhl, K.; Swen, J.; Ellingrod, V.; Müller, D.; Shimoda, K.; Bishop, J.; Kharasch, E.; Skaar, T.; Gaedigk, A.; et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef]
- Hicks, J.; Bishop, J.; Sangkuhl, K.; Müller, D.; Ji, Y.; Leckband, S.; Leeder, J.; Graham, R.; Chiulli, D.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef]
- Bousman, C.A.; Bengesser, S.A.; Aitchison, K.J.; Amare, A.T.; Aschauer, H.; Baune, B.T.; Asl, B.B.; Bishop, J.R.; Burmeister, M.; Chaumette, B.; et al. Review and Consensus on Pharmacogenomic Testing in Psychiatry. Pharmacopsychiatry 2021, 54, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Z.; Rush, A.J.; Charney, D.; Wilson, A.F.; Sorant, A.J.; Papanicolaou, G.J.; Fava, M.; Trivedi, M.H.; Wisniewski, S.R.; Laje, G.; et al. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch. Gen. Psychiatry 2007, 64, 783–792. [Google Scholar] [CrossRef]
- Perlis, R.H.; Mischoulon, D.; Smoller, J.W.; Wan, Y.J.Y.; Lamon-Fava, S.; Lin, K.M.; Rosenbaum, J.F.; Fava, M. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol. Psychiatry 2003, 54, 879–883. [Google Scholar] [CrossRef]
- AlOlaby, R.R.; Sweha, S.R.; Silva, M.; Durbin-Johnson, B.; Yrigollen, C.M.; Pretto, D.; Hagerman, R.J.; Tassone, F. Molecular biomarkers predictive of sertraline treatment response in young children with fragile X syndrome. Brain Dev. 2017, 39, 483–492. [Google Scholar] [CrossRef]
- Kronenberg, S.; Apter, A.; Brent, D.; Schirman, S.; Melhem, N.; Pick, N.; Gothelf, D.; Carmel, M.; Frisch, A.; Weizman, A. Serotonin Transporter Polymorphism (5-HTTLPR) and Citalopram Effectiveness and Side Effects in Children with Depression and/or Anxiety Disorders. J. Child Adolesc. Psychopharmacol. 2007, 17, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Mandelli, L.; Lorenzi, C.; Pirovano, A.; Olgiati, P.; Colombo, C.; Smeraldi, E. Serotonin transporter gene influences the time course of improvement of “core” depressive and somatic anxiety symptoms during treatment with SSRIs for recurrent mood disorders. Psychiatry Res. 2007, 149, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.; Tiger, M.; Landén, M.; Halldin, C.; Farde, L. Serotonin transporter occupancy with TCAs and SSRIs: A PET study in patients with major depressive disorder. Int. J. Neuropsychopharmacol. 2012, 15, 1167–1172. [Google Scholar] [CrossRef]
- Bloch, B.; Reshef, A.; Cohen, T.; Tafla, A.; Gathas, S.; Israel, S.; Gritsenko, I.; Kremer, I.; Ebstein, R.P. Preliminary effects of bupropion and the promoter region (HTTLPR) serotonin transporter (SLC6A4) polymorphism on smoking behavior in schizophrenia. Psychiatry Res. 2010, 175, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Watanabe, T.; Ueda, M.; Saeki, Y.; Hayashi, Y.; Akiyama, K.; Saito, A.; Kato, K.; Inoue, Y.; Shimoda, K. Determinants of pharmacodynamic trajectory of the therapeutic response to paroxetine in Japanese patients with panic disorder. Eur. J. Clin. Pharmacol. 2011, 67, 1213–1221. [Google Scholar] [CrossRef]
- Basu, A.; Chadda, R.K.; Sood, M.; Kaur, H.; Kukreti, R. Association of serotonin transporter (SLC6A4) and receptor (5HTR1A, 5HTR2A) polymorphisms with response to treatment with escitalopram in patients with major depressive disorder: A preliminary study. Indian J. Med. Res. 2015, 142, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Oh, M.S.; Kim, J.S.; Chang, D.I.; Park, J.H.; Cha, J.K.; Heo, J.H.; Sohn, S.I.; Kim, D.E.; Kim, H.Y.; et al. Serotonin transporter gene polymorphisms may be associated with poststroke neurological recovery after escitalopram use. J. Neurol. Neurosurg. Psychiatry 2018, 89, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Easteal, S.; Tan, S.; Schweitzer, I.; Ho, B.K.W.; Aziz, S. Serotonin transporter polymorphisms and clinical response to sertraline across ethnicities. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 953–957. [Google Scholar] [CrossRef]
- Iurescia, S.; Seripa, D.; Rinaldi, M. Role of the 5-HTTLPR and SNP Promoter Polymorphisms on Serotonin Transporter Gene Expression: A Closer Look at Genetic Architecture and In Vitro Functional Studies of Common and Uncommon Allelic Variants. Mol. Neurobiol. 2016, 53, 5510–5526. [Google Scholar] [CrossRef]
- Hu, X.Z.; Lipsky, R.H.; Zhu, G.; Akhtar, L.A.; Taubman, J.; Greenberg, B.D.; Xu, K.; Arnold, P.D.; Richter, M.A.; Kennedy, J.L.; et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006, 78, 815–826. [Google Scholar] [CrossRef]
- Martin, J.; Cleak, J.; Willis-Owen, S.A.G.; Flint, J.; Shifman, S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Mol. Psychiatry 2007, 12, 421–422. [Google Scholar] [CrossRef]
- Ren, F.; Ma, Y.; Zhu, X.; Guo, R.; Wang, J.; He, L. Pharmacogenetic association of bi- and triallelic polymorphisms of SLC6A4 with antidepressant response in major depressive disorder. J. Affect. Disord. 2020, 273, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.L.; Williamson, P.R. Methodological quality of pharmacogenetic studies: Issues of concern. Stat. Med. 2008, 27, 6547–6569. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project. Jamovi (Version 1.2) [Computer Software]; Jamovi: Sydney, Australia, 2020. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Dalton, J.E.; Bolen, S.D.; Mascha, E.J. Publication Bias: The Elephant in the Review. Anesth. Analg. 2016, 123, 812. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training. Available online: https://training.cochrane.org/handbook (accessed on 17 September 2021).
- Denys, D.; Van Nieuwerburgh, F.; Deforce, D.; Westenberg, H.G. Prediction of response to paroxetine and venlafaxine by serotonin-related genes in obsessive-compulsive disorder in a randomized, double-blind trial. J. Clin. Psychiatry 2007, 68, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Billett, E.A.; Richter, M.A.; King, N.; Heils, A.; Lesch, K.P.; Kennedy, J.L. Obsessive compulsive disorder, response to serotonin reuptake inhibitors and the serotonin transporter gene. Mol. Psychiatry 1997, 2, 403–406. [Google Scholar] [CrossRef]
- Di Bella, D.; Erzegovesi, S.; Cavallini, M.C.; Bellodi, L. Obsessive-Compulsive Disorder, 5-HTTLPR polymorphism and treatment response. Pharm. J. 2002, 2, 176–181. [Google Scholar] [CrossRef][Green Version]
- Lohoff, F.W.; Narasimhan, S.; Rickels, K. Interaction between polymorphisms in serotonin transporter (SLC6A4) and serotonin receptor 2A (HTR2A) genes predict treatment response to venlafaxine XR in generalized anxiety disorder. Pharm. J. 2013, 13, 464–469. [Google Scholar] [CrossRef]
- Monteleone, P.; Santonastaso, P.; Tortorella, A.; Favaro, A.; Fabrazzo, M.; Castaldo, E.; Caregaro, L.; Fuschino, A.; Maj, M. Serotonin transporter polymorphism and potential response to SSRIs in bulimia nervosa. Mol. Psychiatry 2005, 10, 716–718. [Google Scholar] [CrossRef][Green Version]
- Perna, G.; Favaron, E.; Di Bella, D.; Bussi, R.; Bellodi, L. Antipanic efficacy of paroxetine and polymorphism within the promoter of the serotonin transporter gene. Neuropsychopharmacology 2005, 30, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Miguita, K.; Cordeiro, Q.; Shavitt, R.G.; Miguel, E.C.; Vallada, H. Association study between genetic monoaminergic polymorphisms and OCD response to clomipramine treatment. Arq. De Neuro-Psiquiatr. 2011, 69, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Ellingrod, V.L.; Akroush, M.; Moline, J. The association of serotonin transporter genotypes and selective serotonin reuptake inhibitor (SSRI)-associated sexual side effects: Possible relationship to oral contraceptives. Hum. Psychopharmacol. Clin. Exp. 2009, 24, 207–215. [Google Scholar] [CrossRef]
- Wilkie, M.J.; Smith, G.; Day, R.K.; Matthews, K.; Smith, D.; Blackwood, D.; Reid, I.C.; Wolf, C.R. Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharm. J. 2009, 9, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Smits, K.; Smits, L.; Peeters, F.; Schouten, J.; Janssen, R.; Smeets, H.; van Os, J.; Prins, M. Serotonin transporter polymorphisms and the occurrence of adverse events during treatment with selective serotonin reuptake inhibitors. Int. Clin. Psychopharmacol. 2007, 22, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.M.; Hollander, S.B., Jr.; Rodrigues, H.E.; Kremer, C.; Schatzberg, A.F. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch. Gen. Psychiatry 2004, 61, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshida, K.; Ito, K.; Sato, K.; Kamata, M.; Higuchi, H.; Shimizu, T.; Ito, K.; Inoue, K.; Tezuka, T.; et al. No association between the serotonergic polymorphisms and incidence of nausea induced by fluvoxamine treatment. Eur. Neuropsychopharmacol. 2002, 12, 477–481. [Google Scholar] [CrossRef]
- Murata, Y.; Kobayashi, D.; Imuta, N.; Haraguchi, K.; Ieiri, I.; Nishimura, R.; Koyama, S.; Mine, K. Effects of the serotonin 1A, 2A, 2C, 3A, and 3B and serotonin transporter gene polymorphisms on the occurrence of paroxetine discontinuation syndrome. J. Clin. Psychopharmacol. 2010, 30, 11–17. [Google Scholar] [CrossRef]
- Saeki, Y.; Watanabe, T.; Ueda, M.; Saito, A.; Akiyama, K.; Inoue, Y.; Hirokane, G.; Morita, S.; Yamada, N.; Shimoda, K. Genetic and pharmacokinetic factors affecting the initial pharmacotherapeutic effect of paroxetine in Japanese patients with panic disorder. Eur. J. Clin. Pharmacol. 2009, 65, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, H.; Takahashi, H.; Kamata, M.; Yoshida, K. Influence of serotonergic/noradrenergic gene polymorphisms on nausea and sweating induced by milnacipran in the treatment of depression. Neuropsychiatr. Dis. Treat. 2009, 5, 393. [Google Scholar] [CrossRef][Green Version]
- Nogami, T.; Takano, H.; Arakawa, R.; Ichimiya, T.; Fujiwara, H.; Kimura, Y.; Kodaka, F.; Sasaki, T.; Takahata, K.; Suzuki, M.; et al. Occupancy of serotonin and norepinephrine transporter by milnacipran in patients with major depressive disorder: A positron emission tomography study with [(11)C]DASB and (S,S)-[(18)F]FMeNER-D(2). Int. J. Neuropsychopharmacol. 2013, 16, 937–943. [Google Scholar] [CrossRef]
- Bousman, C.A.; Sarris, J.; Won, E.S.; Chang, H.S.; Singh, A.; Lee, H.Y.; Ham, B.J.; Tan, C.H.; Lee, M.S.; Ng, C.H. Escitalopram efficacy in depression: A cross-ethnicity examination of the serotonin transporter promoter polymorphism. J. Clin. Psychopharmacol. 2014, 34, 645–648. [Google Scholar] [CrossRef]
- Kato, M.; Serretti, A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol. Psychiatry 2010, 15, 473–500. [Google Scholar] [CrossRef]
- Marshe, V.S.; Islam, F.; Maciukiewicz, M.; Bousman, C.; Eyre, H.A.; Lavretsky, H.; Mulsant, B.H.; Reynolds, C.F., III; Lenze, E.J.; Müller, D.J. Pharmacogenetic Implications for Antidepressant Pharmacotherapy in Late-Life Depression: A Systematic Review of the Literature for Response, Pharmacokinetics and Adverse Drug Reactions. Am. J. Geriatr. Psychiatry 2020, 28, 609–629. [Google Scholar] [CrossRef]
- Keers, R.; Aitchison, K.J. Gender differences in antidepressant drug response. Int. Rev. Psychiatry 2010, 22, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, O.T.; Martínez-Mota, L.; Herrera-Pérez, J.J.; Jiménez-Rubio, G. Role of Estradiol in the Expression of Genes Involved in Serotonin Neurotransmission: Implications for Female Depression. Curr. Neuropharmacol. 2019, 17, 459–471. [Google Scholar] [CrossRef] [PubMed]
- LeGates, T.A.; Kvarta, M.D.; Thompson, S.M. Sex differences in antidepressant efficacy. Neuropsychopharmacology 2019, 44, 140–154. [Google Scholar] [CrossRef]
- Zhu, J.; Klein-Fedyshin, M.; Stevenson, J.M. Serotonin Transporter Gene Polymorphisms and Selective Serotonin Reuptake Inhibitor Tolerability: Review of Pharmacogenetic Evidence. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 1089–1104. [Google Scholar] [CrossRef]
- rs25531 (SNP)—Population Genetics—Homo_Sapiens—GRCh37 Archive Browser 104. Available online: http://grch37.ensembl.org/Homo_sapiens/Variation/Population?db=core;r=17:28563846-28564846;v=rs25531;vdb=variation;vf=36405919 (accessed on 18 September 2021).
- Swen, J.J.; Nijenhuis, M.; De Boer, A.; Grandia, L.; Maitland-Van Der Zee, A.H.; Mulder, H.; Rongen, G.A.P.J.M.; Van Schaik, R.H.N.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From bench to byte an update of guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef] [PubMed]


| Study sample size, Mean (SD) | 176 (303.29) |
| Age, mean (SD) | 41.79 (14.45) |
| Proportion Female, Mean (SD) | 51.96 (21.32) |
| Quality score, Mean (SD) | 12.62 (1.73) |
| Ancestry *, % (N) Studies | |
| American | 1.2 (1) |
| European | 43.9 (36) |
| East Asian | 17.1 (14) |
| Central/South Asian | 1.2 (1) |
| Near Eastern | 4.9 (4) |
| Not specified | 31.7 (26) |
| Diagnosis, % (N) Studies | |
| Mood disorder 1 | 45.1 (37) |
| Mood 1 and/or anxiety disorder 2 | 2.4 (2) |
| Mood 1, anxiety 2 or other 3 disorders | 1.2 (1) |
| Anxiety disorder 2 | 19.5 (16) |
| Chronic tension-type headache | 1.2 (1) |
| Psychotic disorder 4 | 1.2 (1) |
| Substance-related disorder | 6.1 (5) |
| Eating disorder 5 | 1.2 (1) |
| Autism spectrum disorder | 3.7 (3) |
| Healthy | 1.2 (1) |
| Other | 17.1 (14) |
| Antidepressant Used, % (N) Studies | |
| Bupropion | 2.4 (2) |
| Bupropion and TCAs | 1.2 (1) |
| Citalopram | 11.0 (9) |
| Desvenlafaxine | 1.2 (1) |
| Escitalopram | 9.8 (8) |
| Fluoxetine | 2.4 (2) |
| Fluvoxamine | 6.1 (5) |
| Milnacipran | 1.2 (1) |
| Paroxetine | 11.0 (9) |
| Sertraline | 13.4 (11) |
| Venlafaxine | 2.4 (2) |
| Mirtazapine and SSRIs | 1.2 (1) |
| Mirtazapine, MAOAs, SNRIs, SSRIs, and TCAs | 1.2 (1) |
| Mirtazapine, Reboxetine, SNRIs, SSRIs, and TCAs | 1.2 (1) |
| SNRIs and SSRIs | 3.7 (3) |
| SNRIs, SSRIs, and TCAs | 1.2 (1) |
| SNRIs, SSRIs, MAOAs, and TCAs | 2.4 (2) |
| SSRIs | 15.9 (13) |
| SSRIs, MAOAs, and TCAs | 1.2 (1) |
| SSRIs and TCAs | 8.5 (7) |
| Various antidepressants (unspecified) | 1.2 (1) |
| STUDY (Author et al.) | STUDY Design | N | Age [Mean, Years] | Sex [Female (%)] | Ancestry | Diagnosis | Antidepressant (s) Used | Other Drug (s) Used | SLC6A4 rs25531 Tested? | 5-HTTLPR Genotype Frequencies | Phenotype (s) Measurement | Quality Score * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Billett et al. (1997) [31] | Retrospective Case-Control Study | 72 | 36.3 | 53 | European | OCD | SSRIs (Fluoxetine, Clomipramine, Fluvoxamine, Paroxetine, Sertraline) | Not Available | No | SS = 23% SL = 44% LL = 33% | Symptom severity had decreased by at least 25% (Measured with a 3-point scale) | 11 |
| Denys et al. (2007) [30] | Prospective Parallel-group Study | 39 | 33.2 | 61 | European | OCD | Paroxetine | Not Available | No | SS = 20% SL = 54% LL = 26% | YBOCS (≥25% reduction from baseline) | 13 |
| 44 | 33.2 | 61 | European | OCD | Venlafaxine | Not Available | No | SS = 23% SL = 54% LL = 23% | YBOCS (≥25% reduction from baseline) | |||
| Di Bella et al. (2002) [32] | Prospective Case-Control Study | 88 | 33.37 | 50 | European | OCD | Fluvoxamine | Not Available | No | SS = 24% SL = 49% LL = 27% | YBOCS (>35% reduction from baseline) | 16 |
| Lohoff et al. (2013) [33] | Prospective Cohort Study | 112 | >18 years | Not Available | European (72%) | GAD | Venlafaxine | Benzodiazepine Anxiolytics, Hypnotics | Yes | SS = 22% SL = 47% LL = 31% | HAM-A (50% reduction) | 12 |
| Miguita et al. (2011) [36] | Prospective Cohort Study | 41 | 35 | 44 | Not Available | OCD | Clomipramine, Tricyclics, SSRIs | Not Available | No | SS = 22% SL = 54% LL = 24% | Y-BOCS Score (>40% reduction from baseline) | 12 |
| Monteleone et al. (2005) [34] | Prospective Naturalistic Study | 47 | >18 years | 100 | European | Bulimia | SSRIs | Not Available | No | SS = 21% SL = 34% LL = 45% | Bulimia Investigation Test (>50% reduction in binge purging) | 11 |
| Perna et al. (2005) [35] | Prospective Cohort Study | 92 | 34 | 55 | European | PD | Paroxetine | Not Available | No | SS = 26% SL = 53% LL = 21% | PDSS-total scores (50% reduction from baseline) | 13 |
| Study (Author et al.) | Study Design | N | Age [Mean, Years] | Sex [Female (%)] | Ancestry | Diagnosis | Antidepressant (s) Used | Other Drug (s) Used | SLC6A4 rs25531 Tested? | 5-HTTLPR Genotype Frequencies | Phenotype (s) Measurement | Quality Score * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basu et al. (2015) [17] | Prospective Cohort Study | 55 | 35 | 42 | North Indian | MDD | Escitalopram | Anxiolytics, Sedatives, Hypnotics | No | SS = 69% SL = 31% LL = 0% | UKU scores (all side effects recorded irrespective of severity and degree of association) | 14 |
| Bishop et al. (2009) [37] | Prospective Cohort Study | 115 | 29.2 | 76 | European (92%) | MDD | SSRIs (Citalopram, Escitalopram, Fluoxetine, Paroxetine, Sertraline) | Not Available | No | SS = 18% SL = 42% LL = 40% | Changes in sexual functioning questionnaire (CSFQ) (scores lower than 47 for males and 42 for females indicate decreased sexual desire or function) | 13 |
| Higuchi et al. (2009) [44] | Prospective Cohort Study | 80 | 52.4 | 65 | Not Available | MDD | Milnacipran | Brotizolam | No | SS = 65% SL = 34% LL = 1% | UKU scores (nausea) (adverse events were recorded if the score was greater than 1 and were not present before treatment) | 15 |
| SS = 64% SL = 35% LL = 1% | UKU scores (sweating) (adverse events were recorded if the score was greater than 1 and were not present before treatment) | 15 | ||||||||||
| Hu et al. (2007) [8] | Prospective Case-Control Study | 1655 | 42 | 62 | European (79.9%) | MDD | Citalopram | Not Available | No | SS = 18% SL = 44% LL = 38% | Global rating of side effect burden (GRSEB) (score of 4 or greater indicated increased adverse effects) | 11 |
| Ishiguro et al. (2011) [16] | Prospective Cohort Study | 65 | 36 | 65 | Japanese | PD | Paroxetine | Brotizolam, Lorazepam | No | SS = 60% SL = 35% LL = 5% | No. of dropouts due to ADRs | 12 |
| Murata et al. (2010) [42] | Prospective Cohort Study | 56 | 45.9 | 57 | Japanese | MDD, Anxiety Disorder, or others (e.g., pain disorder) | Paroxetine | Tandospirone, Benzodiazepines, Zolpidem, Zopiclone | No | SS = 57% SL = 39% LL = 4% | Paroxetine discontinuation-emergent events (at least 1 qualitatively new symptom within 7 days after stopping medication) | 14 |
| Murphy et al. (2004) [40] | Prospective Cohort Study | 124 | 72 | 50 | European (94%) | MDD | Mirtazapine | Not Available | No | SS = 25% SL = 44% LL = 31% | No. of discontinuations as a result of at least 1 adverse events | 12 |
| 122 | 72 | 52 | European (89%) | MDD | Paroxetine | Not Available | No | SS = 20% SL = 47% LL = 33% | No. of discontinuation as a result of at least 1 adverse events | |||
| Saeki et al. (2009) [43] | Prospective Cohort Study | 27 | 34.3 | 78 | Japanese | PD | Paroxetine | Brotizolam, Lorazepam | No | SS = 67% SL = 33% LL = 0% | Self-report (experienced at least 1 symptom including drowsiness or abnormal sensation) | 12 |
| Smits et al. (2007) [39] | Retrospective Cohort Study | 214 | 48.48 | 70 | European | MDD | SSRIs (Paroxetine, Fluoxetine, Fluvoxamine, Sertraline, Citalopram) | Not Available | No | SS = 24% SL = 41% LL = 33% | Complaints made in face-to-face interview (at least 1 adverse event that began after medication use) | 15 |
| Takahasi et al. (2002) [41] | Prospective Cohort Study | 54 | 51.52 | 59 | Japanese | MDD | Fluvoxamine | Brotizolam | No | SS = 55% SL = 36% LL = 7% | UKU score (recorded patients with nausea according to scale criteria) | 12 |
| Wilkie et al. (2009) [38] | Prospective Cohort Study | 166 | 43.42 | 69 | European | MDD | Paroxetine, Imipramine, Lofepramine, Phenelzine | Not Available | No | SS = 28% SL = 41% LL = 32% | Adverse events (not specifically defined) | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, K.; Maruf, A.A.; Müller, D.J.; Bishop, J.R.; Bousman, C.A. Serotonin Transporter Genetic Variation and Antidepressant Response and Tolerability: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1334. https://doi.org/10.3390/jpm11121334
Stein K, Maruf AA, Müller DJ, Bishop JR, Bousman CA. Serotonin Transporter Genetic Variation and Antidepressant Response and Tolerability: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2021; 11(12):1334. https://doi.org/10.3390/jpm11121334
Chicago/Turabian StyleStein, Kiera, Abdullah Al Maruf, Daniel J. Müller, Jeffrey R. Bishop, and Chad A. Bousman. 2021. "Serotonin Transporter Genetic Variation and Antidepressant Response and Tolerability: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 11, no. 12: 1334. https://doi.org/10.3390/jpm11121334
APA StyleStein, K., Maruf, A. A., Müller, D. J., Bishop, J. R., & Bousman, C. A. (2021). Serotonin Transporter Genetic Variation and Antidepressant Response and Tolerability: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 11(12), 1334. https://doi.org/10.3390/jpm11121334







