Abstract
Background: Atrial fibrillation is a prevalent arrhythmia with significant morbidity and recurrence challenges. Paroxysmal atrial fibrillation (PAF) is characterized by episodic occurrences and unpredictable recurrences; therefore, it demands innovative diagnostic approaches to predict relapses and guide management. Objectives: This pilot, exploratory study evaluates the feasibility and prognostic value of integrating cardiopulmonary exercise testing (CPET), echocardiographic indices, and plasma biomarkers for predicting PAF recurrence. Methods: The PLACEBO trial is a single-center, prospective observational study of 73 adults with PAF in sinus rhythm at baseline. Comprehensive assessments included CPET, transthoracic echocardiography, 24 h electrocardiographic Holter monitoring with heart rate variability (HRV) metrics, and plasma biomarkers, such as galectin-3 (GAL3). Recurrence was defined as any documented AF episode lasting ≥30 s within 12 months of follow-up. Results: Binary logistic regression revealed that the standard deviation of RR intervals (SDRR) and GAL3 were significant predictors of recurrence. Particularly, higher SDRR [odds ratio (OR): 1.061, p = 0.021] and GAL3 > 10.95 ng/mL (OR: 5.206, p = 0.006) were associated with recurrence. Moreover, lower right ventricular fractional area change (RV FAC) exhibited a marginally significant association with recurrence (OR: 0.927, p = 0.062). CPET parameters demonstrated limited prognostic value in this cohort. Conclusion: This pilot study demonstrates that integrating novel echocardiographic indices, biomarkers, and HRV metrics is feasible and may provide valuable prognostic insights for PAF recurrence. Larger multicenter studies are needed to validate these findings and optimize personalized risk stratification strategies.
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide, contributing significantly to morbidity and mortality [1]. AF leads to rising healthcare costs, whilst posing a major impact on the health-related quality of life of the affected subjects [2,3]. AF presents with various subtypes, including paroxysmal, persistent, and permanent forms, each characterized by unique clinical patterns and challenges in management [4]. Specifically, paroxysmal atrial fibrillation (PAF) is characterized by its episodic nature, unpredictable recurrences, and variable clinical outcomes; even though PAF seems to confer a more favorable prognosis compared to other forms of AF, the effective management and refinement of the prognosis of the disease is crucial [5,6]. Despite advancements in AF management, predicting recurrence remains a major clinical challenge, necessitating a comprehensive approach that integrates advanced diagnostic modalities.
Cardiopulmonary exercise testing (CPET) is a valuable tool for the comprehensive assessment of the function of cardiovascular, respiratory, and musculoskeletal systems, commonly used in clinical practice with the aim of evaluating the multiorgan response to exercise [7]. The role of CPET in the assessment of patients with AF has already been established, while the exact relationship between cardiorespiratory fitness and AF course prognosis is not yet fully identified [8]. In the same line, a series of measurements obtained from transthoracic echocardiography (TTE) is crucial for the evaluation of patients with AF, as they aid in the assessment of structural and functional cardiac changes that influence patient outcomes; novel echocardiographic indices such as right ventricular fractional area change (RV FAC) and left atrial (LA) strain offer detailed cardiac function assessments in patients with AF [9,10,11]. Finally, plasma biomarkers, such as galectin-3 (GAL3) and homocysteine (Hcy), are established tools for evaluating cardiovascular health, as they are directly linked with fibrosis and inflammation [12,13]. The combined prognostic value of CPET, TTE measurements, and biomarker evaluation in PAF is not yet well understood.
Heart rate variability (HRV) metrics, such as the standard deviation of R-R intervals (SDRR) and the standard deviation of normal-to-normal R-R intervals (SDNN), offer valuable insights into the autonomic regulation of the heart and have been increasingly explored in AF [14]. While reduced HRV is well established as a marker of poor prognosis in AF, emerging evidence suggests that increased HRV may also play a role in AF recurrence, possibly through heightened vagal tone, which contributes to arrhythmogenesis [15]. Understanding the complex relationship between HRV and AF may help refine risk stratification and management strategies.
Despite advancements in risk stratification for PAF, the existing models often focus on isolated parameters, such as structural echocardiographic indices or single biomarkers. This fragmented approach may overlook the interplay between hemodynamic, biochemical, and structural changes. Our study bridges this gap by integrating CPET, novel echocardiographic indices, and plasma biomarkers, aiming to create a unified predictive model for PAF recurrence [16,17]. In this context, the PLACEBO exploratory trial aims to integrate the aforementioned diagnostic modalities to enhance our understanding of PAF recurrence. This manuscript presents the exploratory results of the study, as well as their implications for clinical practice.
2. Materials and Methods
2.1. Study Design and Setting
The PLACEBO (ParoxysmaL Atrial fibrillation prognosis based on Cardiopulmonary Exercise test data and novel echocardiographic and plasma BiOchemical indices) trial is a prospective, exploratory, single-center, observational cohort study conducted at Ippokratio General Hospital in Thessaloniki, Greece. The protocol of the trial is registered at ClinicalTrials.gov (Identifier: NCT05246423) and was reviewed and approved by the Institutional Review Board of Ippokratio General Hospital of Thessaloniki, as well as by the Aristotle University of Thessaloniki Ethics Committee. The study was conducted according to the latest version of the Declaration of Helsinki (2013), and all participants provided informed consent prior to study enrollment. Adults diagnosed with PAF and in sinus rhythm at baseline were included. The study was designed to comprehensively evaluate novel diagnostic and prognostic parameters for PAF recurrence through an integrative approach, encompassing advanced cardiac imaging, biomarker analysis, HRV metrics, and functional assessment. The rationale, design, and protocol of the study were previously published [18].
2.2. Study Population
The study participants were recruited from the cardiology outpatient clinic and inpatient referrals at Ippokratio General Hospital of Thessaloniki, ensuring a diverse yet representative cohort of patients with PAF.
The inclusion criteria were (a) age ≥ 18 years, (b) diagnosis of PAF, (c) sinus rhythm at the time of baseline evaluation, (d) ability to provide written informed consent, (e) ability to undergo CPET, and (f) ability to comply with this study’s follow-up schedule.
The exclusion criteria were (a) structural cardiomyopathy, (b) congenital heart disease, (c) permanent AF, (d) recent acute coronary syndrome (within the last month), (e) heart failure, (f) end-stage renal disease, (g) autoimmune disease, (h) active malignancy, (i) uncontrolled thyroid disease, (j) recent surgery (within the last 2 months), (k) poor echocardiographic windows, (l) uncontrolled hypertension, (m) pregnancy, and (n) inability to comply with the study protocol or to provide informed consent.
A total of 76 patients were initially screened for participation. Three patients were excluded, including one due to an in-hospital complication, one due to a new diagnosis of hypertrophic cardiomyopathy during screening, and one who failed to complete the follow-up requirements. Thus, 73 patients were included in the final analysis and were followed for one year for AF recurrence. The study flowchart is presented in Figure 1.
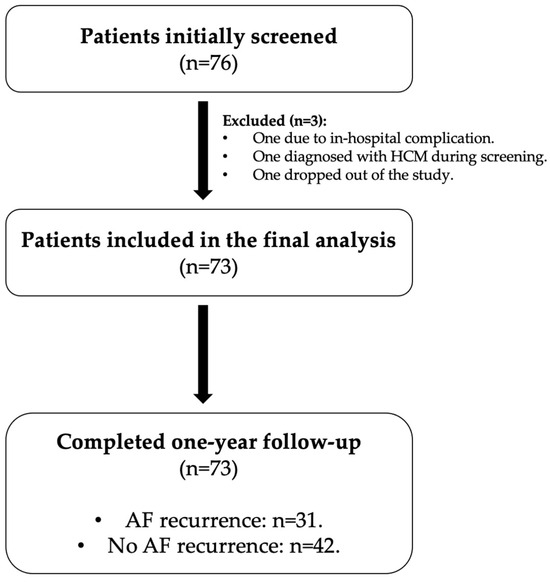
Figure 1.
Study flowchart of patient enrollment and follow-up. Abbreviations: HCM, hypertrophic cardiomyopathy; AF, atrial fibrillation.
2.3. Data Collection
2.3.1. Baseline
At baseline, the participants underwent comprehensive evaluations, including the following:
- Demographics and medical history: Demographic data [e.g., age, sex, body mass index (BMI)] and detailed clinical history, including cardiovascular risk factors, comorbidities, prior PAF episodes, and their characteristics, were recorded.
- CPET: CPET was performed on a cycle ergometer with continuous 12-lead ECG monitoring and respiratory gas analysis. The key parameters obtained included peak oxygen uptake (peak VO2), minute ventilation/carbon dioxide production (VE/VCO2), and other exercise indices with established prognostic value in cardiovascular conditions.
- Transthoracic echocardiography: A comprehensive echocardiographic assessment was conducted under stable hemodynamic conditions. The parameters included left ventricular (LV) ejection fraction (LVEF), global longitudinal strain (GLS), LV diastolic indices, and LA dimensions, volume, and strain. Right ventricular parameters, such as RV FAC and free wall strain, were also evaluated. All echocardiographic examinations were performed by a single experienced cardiologist to ensure consistency. For the echocardiographic measurements, the GE Vivid E95 ultrasound system (GE Healthcare, Chicago, IL, USA) was used. The 2D measurements were performed offline using the commercially available EchoPAC GE software package, version PC (GE Healthcare, Chicago, IL, USA). All measurements adhered to the European Association of Cardiovascular Imaging (EACVI) guidelines [19,20].
- Electrocardiographic (ECG) Holter monitoring (24 h): Continuous ECG data were recorded to assess AF episodes, premature atrial and ventricular contractions, HRV indices, and other rhythm-related metrics. For ambulatory ECG Holter monitoring, we utilized the GE Seer 1000 and GE CardioMem 4000 Holter monitors (GE Healthcare, USA). HRV analysis was performed using GE CardioDay ECG Holter software, v2.6 (GE Healthcare, Chicago, IL, USA).
- Plasma biomarker analysis: Blood samples were collected upon study enrollment, after at least 12 h of overnight fasting. Basic biochemical measurements, including brain natriuretic peptide (BNP) and high-sensitivity cardiac troponin I (hs-cTnI), were performed immediately after collection. Additional blood samples were obtained in vacuum collection tubes without heparin, EDTA, or gel (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and transferred to the laboratory within 1 h of vein puncture while maintained on ice. The samples were preserved on ice until centrifugation to minimize biochemical changes, as certain biomarkers, including Hcy, can continue to be produced by red blood cells post-collection. After a minimum of 45 min to allow clot formation, the blood samples were centrifuged at 4000 rpm for 10 min in a refrigerated centrifuge. The serum samples that were not analyzed immediately were stored at −80 °C. Lipemic or hemolyzed samples were excluded.
At the conclusion of this study, the stored serum samples were removed from the −80 °C freezer and allowed to thaw gradually in a refrigerated environment (2–8 °C) to maintain sample integrity. Once thawed, the samples were inspected for any signs of lipemia or hemolysis, and those that failed quality checks were excluded from further analysis. The remaining samples were centrifuged again at 4000 rpm for 10 min in a refrigerated centrifuge to ensure the removal of any residual particulate matter. Following this step, the supernatant was carefully collected and used for the analysis of Hcy and GAL3. Hcy and GAL3 were measured using the immunoassay analyzer Alinity-i (Abbott) by chemiluminescence (CMIA). These markers were chosen for their roles in inflammation, fibrosis, and myocardial stress, which are pivotal in AF pathophysiology.
2.3.2. Follow-Up
The participants were followed for 12 months. During this period, comprehensive records were maintained for each patient to document the occurrence of new AF episodes. Recurrence was defined as any episode of AF lasting ≥30 s, in accordance with ESC Guidelines [4], and was identified through one of the following: (a) 24 h ECG Holter monitoring, (b) documentation in medical records, including arrhythmic events managed at external centers, or (c) patient-reported episodes consistent with prior confirmed AF, typically managed with self-administered antiarrhythmic drugs. Only objectively verified events based on ECGs, medical documentation, or consistent symptomatic episodes managed as AF were considered valid recurrence events.
2.3.3. Outcomes
The primary outcome of this study was AF recurrence within 12 months of enrollment, while the time to first AF recurrence was identified as a secondary outcome. Even though the biomarkers were measured only at baseline, their prognostic significance for AF recurrence was evaluated.
2.4. Statistical Analysis
The statistical analysis included descriptive statistics to assess the quantitative and qualitative variables. For the quantitative variables, the mean value and the standard deviation were computed. For the qualitative variables, the frequency of the categories and the corresponding percentages were employed. The differences in each variable between the two AF states (recurrence/no recurrence) were assessed using either the independent samples t-test or the chi-squared test (as appropriate). Next, univariable binary logistic regression was employed to assess the impact of each variable of interest on the binary form of the variable recurrence after one year (1: at least one event in one year, 0: no events in one year). Particularly for GAL3, receiver operating characteristic (ROC) analysis was used to determine a binary threshold related to the recurrence after one year (using the Youden Index criterion), and, if appropriate, transform the quantitative GAL3 into a binary one. All variables that exhibited a p-value less than 0.05 in the univariable binary logistic regression analysis were then included in a multivariable binary logistic regression model. The estimated odds ratio (OR) for each independent quantitative variable reflected the ratio of the odds of recurrence when the independent variable increased by one unit to the odds of recurrence before this increase. The odds of recurrence were the ratio of the probability of recurrence occurring to the probability of it not occurring. For independent qualitative binary variables (Yes/No), the OR reflected the ratio of the odds with the “Yes” category to the odds with the “No” category (reference). For independent qualitative variables with more than two categories, the first category was used as the reference. Here, OR > 1 implied that increasing values of the predictor variable were related to an increasing probability of recurrence, while OR < 1 implied that increasing values of the predictor variable were related to a decreasing probability of recurrence.
Similarly, survival analysis was also conducted to evaluate the impact of each variable on the time that the recurrence occurred during the 1-year follow-up period. More specifically, the Cox regression model was employed. The estimated hazard ratio (HR) for each independent variable reflected the ratio of the hazards for recurrence when the independent variable increased by one unit (quantitative variables) or when each category was compared to the reference category (qualitative variables). Here, HR > 1 implied that increasing values of the predictor variable were related to a higher risk of recurrence, while HR < 1 implied that increasing values of the predictor variable were related to a lower risk of recurrence. The proportional hazards assumption for the Cox regression model was assessed by employing the “cox.zph” R function, which uses Schoenfeld residuals to test whether the effect of a covariate changes over time. The purpose of the Cox regression analysis was to assess whether the specific time that the recurrence occurred during the 1-year follow-up period (for example, early or late during this time interval) differentiated the results compared to the binary logistic regression.
The level of statistical significance was set at 0.05 in all cases, and that of marginal statistical significance was set at 0.10. All analyses were performed with IBM SPSS Statistics, version 22 (IBM Corp., Armonk, NY, USA), and the R programming language, version 4.4.1.
3. Results
3.1. Demographic and Lifestyle Characteristics
The baseline characteristics of the study population, stratified by AF recurrence during the 1-year follow-up (No/Yes), are presented in Table 1. The total cohort included 73 patients, with 42 patients in the no recurrence group and 31 in the recurrence group. The mean age of the cohort was 60 years (standard deviation [SD] 11.6), with no significant difference between the no recurrence and recurrence groups (p = 0.424). The sex distribution was similar between the two groups, with 47.9% male participants in the total cohort [42.9% in the no recurrence group vs. 54.8% in the recurrence group (p = 0.311)]. Smoking, alcohol consumption, and physical activity rates were comparable between the no recurrence and recurrence groups (p = 0.695, p = 0.542, and p = 0.368, respectively). These findings suggest no significant differences in lifestyle habits between the groups.

Table 1.
Baseline characteristics are displayed for the total cases and, separately, within the two categories of the variable representing recurrence in 1 year (No/Yes). For quantitative variables, the mean value and the standard deviation (in parentheses) are displayed. For qualitative variables, the frequency of the stated category (in parentheses) is presented along with the corresponding percentage (in parentheses). All data refer to the total study population, including both male and female participants.
3.2. Atrial Fibrillation Characteristics
The total disease duration for AF was 40 months (SD 49.4), with no significant difference between groups (38.6 [SD 54.3] vs. 41.9 [SD 42.5], p = 0.781). Across both groups, the prevalence of PAF episodes in the last 6 months was comparable, with 81% of the participants in the no recurrence group and 77.4% in the recurrence group reporting episodes (p = 0.712). Similarly, the proportion of individuals experiencing PAF episodes in the last 1 month was almost identical, with 38.1% in the no recurrence group and 38.7% in the recurrence group (p = 0.957). These findings suggest a consistent burden of PAF episodes in our study cohort. Most of the patients reported symptoms (93.2% in the total cohort, 92.9% in the no recurrence group, and 93.5% in the recurrence group), with no significant difference between the groups (p = 0.908). The distribution of EHRA scores was similar between the groups: 23.3% of the total cohort had an EHRA score of 1, 65.8% had a score of 2, and 11.0% had a score of 3, with no significant differences between the groups (p = 0.540).
3.3. Comorbidities
Arterial hypertension was the most common comorbidity, present in 45.2% of the cohort, with no significant difference between the groups (p = 0.639). Obstructive sleep apnea (OSA) was more prevalent in the recurrence group (19.4% vs. 4.8%, p = 0.049). The prevalence of other comorbidities, including diabetes, dyslipidemia, coronary artery disease, stroke, valvular disease, and thyroid disease, did not differ significantly between the groups.
3.4. Echocardiographic Characteristics
The results regarding the echocardiographic parameters are displayed in Table 2. LVEF was similar between the no recurrence and recurrence groups (p = 0.126). There were no significant differences in left ventricular mass index (LVMI), GLS, or the other echocardiographic parameters, including E/E’, LA volume index (LAVI), and LA strain parameters. However, the RV FAC was significantly lower in the recurrence group (40.1% vs. 44.6%, p = 0.014), indicating worse right ventricular function.

Table 2.
Echocardiographic, CPET, and 24 h ECG Holter monitoring values and biomarker measurements are displayed for the total cases and, separately, within the two categories of the variable representing recurrence in 1 year (No/Yes). For quantitative variables, the mean value and the standard deviation (in parentheses) are displayed. For qualitative variables, the frequency of the stated category (in parentheses) is presented along with the corresponding percentage (in parentheses).
3.5. CPET Results
Peak VO2 was comparable between the groups (18.3 vs. 19.0 mL/kg/min, p = 0.585), as were the other CPET parameters, including anaerobic threshold (VO2 AT), total work at VO2 max, and VO2/HR at VO2 max (Table 2). No significant differences were observed in VE/VCO2 or in partial pressure of end-tidal carbon dioxide (PETCO2), which remained similar across the groups (Table 2).
3.6. Twenty-Four-Hour ECG Holter Monitoring
Baseline 24 h ambulatory ECG Holter monitoring revealed a significantly higher incidence of AF episodes in the recurrence group at the time of recording (seven vs. zero episodes, p = 0.001), while no significant differences were demonstrated in the occurrence of other arrhythmias, such as atrial flutter. It is important to note that this finding reflects only the baseline Holter recordings and does not correspond to the total number of patients who experienced AF recurrence during the one-year follow-up (n = 31). The minimum 24 h heart rate was similar between the groups (p = 0.758), while the maximum heart rate was higher in the recurrence group (p = 0.031). Significant differences in HRV were also noted, with the recurrence group showing a higher SDNN (p = 0.078) and a higher SDRR (p = 0.015).
3.7. Regression
The univariable binary logistic regression (Table 3) revealed that three variables exhibited a statistically significant impact on the probability of recurrence after one year (1: at least one event in one year, 0: no events in one year). In particular, RV FAC was a positive factor with an odds ratio (OR) of 0.917 (p = 0.020), while the SDRR (OR = 1.053, p = 0.015) was a negative factor. Namely, increasing values of RV FAC were related to a decreasing probability of recurrence, while increasing values of the SDRR were related to an increasing probability of recurrence. On the other hand, GAL3 only marginally did not exhibit statistical significance (OR = 1.149, p = 0.051) when treated as a continuous variable. However, since GAL3 was of particular interest in this study as an established biomarker in AF, known for its potential role in fibrosis and remodeling processes, ROC analysis was employed to assess whether it would be appropriate to dichotomize GAL3. It was found that the area under the ROC curve was 0.663 (p = 0.019), indicating that the dichotomization of GAL3 would be beneficial. By employing the Youden Index criterion, the binary threshold was estimated to be 10.95, and GAL3 was transformed into a binary variable (1: GAL3 > 10.95, 0: GAL3 < 10.95). GAL3 > 10.95 ng/mL exhibited statistically significant negative impact on the probability of recurrence after one year with an OR of 6.127 (p = 0.001); namely, the odds of the patients with a GAL3 value higher than 10.95 were 6.127 times the odds of the patients with a GAL3 value less than 10.95. Of note, OSA exhibited a marginal statistically significant negative impact on the probability of recurrence after one year, with an OR of 4.800 (p = 0.067). Antiarrhythmic and beta-blocker use were also included in the univariate analysis and showed no significant association with AF recurrence. Specifically, the use of antiarrhythmic drugs was associated with an OR of 1.422 (95% CI: 0.560–3.614, p = 0.459), while the combination of beta-blockers and antiarrhythmic drugs showed an OR of 1.174 (95% CI: 0.455–3.025, p = 0.740).

Table 3.
The results of the univariable binary logistic regression are displayed for all the variables considered. The dependent variable in the analysis is AF recurrence within 1 year of follow-up. The odds ratio (OR) for each independent quantitative variable reflects the ratio of the odds of recurrence when the independent variable increases by one unit to the odds of recurrence before this increase (the odds of recurrence are the ratio of the probability of recurrence occurring to the probability of it not occurring). For independent qualitative variables, the OR reflects the ratio of the odds of each category to the odds of the reference category (as appropriately denoted). An estimated OR > 1 implies that increasing values of the predictor variable are related to an increasing probability of recurrence, while an OR < 1 implies that increasing values of the predictor variable are related to a decreasing probability of recurrence.
After including RV FAC, SDRR, and GAL3 > 10.95 ng/mL in a multivariable binary logistic regression model (Table 4), it was found that SDRR and GAL3 > 10.95 ng/mL retained their statistical significance, with an OR of 1.061 (p = 0.021) and 5.206 (p = 0.006), respectively. On the other hand, RV FAC exhibited a marginal impact on recurrence, with an OR of 0.927 (p = 0.062).

Table 4.
Multivariable binary logistic regression analysis for AF recurrence. The dependent variable in the model is AF recurrence within 1 year of follow-up. The odds ratio (OR) for each independent quantitative variable reflects the ratio of the odds of recurrence when the independent variable increases by one unit to the odds of recurrence before this increase (the odds of recurrence are the ratio of the probability of recurrence occurring to the probability of it not occurring). For independent qualitative variables, the OR reflects the ratio of the odds of each category to the odds of the reference category (as appropriately denoted). An estimated OR > 1 implies that increasing values of the predictor variable are related to an increasing probability of recurrence, while an OR < 1 implies that increasing values of the predictor variable are related to a decreasing probability of recurrence.
The Cox regression analysis revealed similar results to the binary logistic regression. In particular, the only variables that exhibited statistical significance in the univariable model were RV FAC, SDRR, and GAL3 > 10.95 ng/mL. In addition to that, the estimates of HR were very similar in value to the corresponding estimates of the OR (HR: 0.948, p = 0.028; HR: 1.030, p = 0.003; and HR: 4.175, p = 0.002, respectively). By including all three predictors in a multivariable Cox regression model, it was found that SDRR and GAL3 > 10.95 ng/mL retained their statistical significance with an HR of 1.023 (p = 0.028, 95% CI-HR: 1.002–1.043) and 3.315 (p = 0.014, 95% CI-HR: 1.274–8.626), respectively. On the contrary, RV FAC was not statistically significant, with an HR of 0.976 (p = 0.365, 95% CI-HR: 0.927–1.028). The results of the proportional hazard assumption assessment for the multivariable Cox regression model showed that the assumption was not violated for any of the three variables (p: 0.36, 0.47, and 0.73, respectively).
4. Discussion
This pilot study emphasizes the feasibility of integrating CPET, echocardiography, and biomarkers for PAF recurrence prediction. The findings of this study underscore the utility of a multifaceted diagnostic approach, highlighting specific parameters such as right ventricular RV FAC, SDRR, and GAL3 levels as key predictors of AF recurrence. These insights have important clinical implications for improving risk stratification and personalizing management strategies in this challenging patient population.
Our analysis identified SDRR and GAL3 as independent predictors of PAF recurrence within one year. Higher SDRR and elevated GAL3 levels were significantly associated with increased recurrence risk, underlining the role of autonomic dysfunction and fibrosis in AF pathophysiology. Additionally, RV FAC demonstrated a trend toward significance, suggesting a possible association between RV function and AF recurrence. While not reaching full statistical significance, this finding aligns with previous studies that emphasize the importance of RV performance in maintaining sinus rhythm, suggesting that slight impairments in right ventricular performance may exacerbate atrial remodeling and electrical instability, thereby promoting AF recurrence. Incorporating RV FAC into routine echocardiographic assessments of patients with PAF may aid in the identification of high-risk individuals.
The consistency observed between the Cox regression and binary logistic regression models validates the robustness of our findings. Both models identified SDRR and GAL3 as statistically significant predictors, while RV FAC, although significant in the univariable analysis, did not retain significance in the multivariable Cox model. These results emphasize the importance of autonomic function and fibrotic pathways in predicting AF recurrence, aligning with prior studies that highlight the prognostic role of HRV and GAL3 levels in arrhythmia pathophysiology.
In the context of HRV analysis, SDRR, derived from 24 h ECG Holter monitoring, has been proven to correlate with SDNN, a commonly used measure of global HRV [21]. Studies have demonstrated that long-term recordings, such as those from 24 h ambulatory ECG Holter monitors, provide a robust and stable representation of autonomic nervous system activity, with SDRR serving as a valid indicator of overall HRV, even when NN intervals are not specifically isolated [22,23]. SDRR has been utilized as a comparable metric in assessing autonomic nervous system function, particularly when preprocessing for NN intervals is unavailable or impractical [22]. Although SDNN is more widely adopted, SDRR offers a practical and reliable alternative for analyzing variability in such datasets. In our study, higher SDRR values were linked with higher AF recurrence possibility, reflecting high sympathetic activity or impaired vagal tone, both of which are known contributors to AF pathogenesis. Although SDNN did not reach statistical significance in our study, the observed trend aligns with the existing literature, which consistently reports a correlation between increased SDNN values and higher AF recurrence risk [24].
GAL3 is a β-galactoside-binding protein that is proven to exacerbate fibrotic activity, whilst, at the same time, AF induces GAL3 production through tissue injury and structural and electrical remodeling [25]. This biomarker seems to play a pivotal role in cardiac fibrosis, as it is highly expressed in fibrotic tissues, such as the LA tissue of patients with AF, and it is upregulated in chronic inflammatory and fibrotic conditions [26,27]. GAL3 emerged as a robust biomarker in AF, as elevated levels have been strongly correlated with AF development, while GAL3 levels were found to be higher among patients with persistent AF, underlining the importance of this biomarker in the maintenance of the arrhythmia [28,29]. Our study is in accordance with the existing literature, identifying GAL3 as an independent and important predictor of AF recurrence. Targeting GAL3 through novel pharmacologic agents or lifestyle interventions may hold promise for modifying the AF trajectory.
OSA has been increasingly recognized as a significant comorbidity in patients with AF [30]. This condition is characterized by intermittent cessation of airflow during sleep, leading to repeated episodes of hypoxia and arousal; these events can trigger sympathetic activation, inflammation, and changes in autonomic tone, all of which can contribute to the development and recurrence of AF [31,32]. In our study, OSA exhibited a marginally significant negative impact on the probability of AF recurrence (OR 4.800, p = 0.067), suggesting that its presence may increase the risk of recurrence in patients with PAF. The association between OSA and AF recurrence has already been established, and several studies have shown that effective management, such as through continuous positive airway pressure (CPAP) therapy, may reduce AF burden and improve outcomes in affected patients [33,34,35]. The potential role of OSA in AF recurrence emphasizes the need for comprehensive risk stratification in patients with AF, which should include the evaluation of underlying conditions.
Hcy levels, along with BNP and hs-cTnI, did not demonstrate any significant association with AF relapse in our study. Despite the well-established roles of BNP and hs-cTnI as markers of cardiac stress and injury, and the recognized involvement of Hcy in vascular and thrombotic processes, none of these biomarkers appeared to provide additional prognostic value for predicting AF recurrence in the patient population studied [36,37,38]. This may reflect the relatively preserved cardiac function in our study population, reducing the sensitivity of these markers. Additionally, the dynamic nature of BNP in response to acute hemodynamic changes may limit its utility in stable outpatients. Future studies should explore whether combining BNP with markers of fibrosis, such as GAL3, could enhance predictive accuracy.
Antiarrhythmic drug therapy is a well-established determinant of AF recurrence and plays a central role in rhythm control strategies. In our study, although treatment with antiarrhythmic agents or their combination with beta-blockers was not a primary focus, we included these variables in the univariate regression analysis to account for potential confounding. Notably, neither antiarrhythmic drug use (OR: 1.422, 95% CI: 0.560–3.614, p = 0.459) nor the combined use of beta-blockers and antiarrhythmic therapy (OR: 1.174, 95% CI: 0.455–3.025, p = 0.740) was significantly associated with AF recurrence in our cohort. These findings may reflect the real-world heterogeneity in treatment choices and the fact that all patients were managed according to the ESC guidelines [4]. Nonetheless, the prognostic value of antiarrhythmic therapy warrants further investigation in larger, treatment-stratified cohorts.
Although CPET was central to the study design, its parameters, including peak VO2 and VE/VCO2, did not independently predict PAF recurrence. This finding contrasts with the existing literature that often highlights the utility of CPET in evaluating exercise tolerance and predicting cardiovascular outcomes in patients with AF and other cardiac conditions [8]. A possible explanation lies in the characteristics of our study population; the participants in the PLACEBO trial were relatively healthy individuals without significant comorbidities, as evidenced by preserved LVEF and the absence of structural cardiomyopathies. Previous studies have shown that the predictive value of CPET parameters is more pronounced in populations with advanced cardiac dysfunction or significant exercise intolerance; the apparent disconnect between CPET metrics and recurrence risk contrasts with some reports linking improved cardiorespiratory fitness to reduced AF burden [39,40]. This discrepancy may reflect differences in population characteristics or the interplay of exercise-induced atrial remodeling. Consequently, the limited variability in CPET metrics among our cohort may have diminished their prognostic utility. Furthermore, our findings align with reports suggesting that the role of CPET in AF prognosis may be more nuanced, dependent on the presence of additional comorbidities or the specific subtype of AF being studied [41]. While CPET remains a valuable tool for assessing overall cardiovascular health and exercise capacity, its application as an isolated prognostic marker for PAF recurrence warrants further investigation in diverse populations.
Our findings are consistent with previous studies that have examined individual components of the PLACEBO model. For instance, the prognostic value of right ventricular function and GAL3 has been validated in broader AF cohorts [42,43,44]. However, the combination of these markers with HRV metrics represents a novel approach that integrates the individual strengths of these diverse diagnostic modalities. In the same line, even though CPET variables such as peak VO2 and VE/VCO2 were not independently predictive in our cohort, they provide critical context for interpreting overall cardiovascular health and may enhance the predictive capacity of other markers in upcoming studies. Future studies with larger multicenter cohorts and extended follow-up periods are needed to validate these preliminary findings and refine the proposed diagnostic approach.
Study Limitations
The most important limitation of this study is that, by design, it is a pilot study; therefore, the results should be interpreted with caution. The sample size, although relatively small, is appropriate for an exploratory analysis. Additionally, the follow-up period of 12 months may not fully capture the long-term recurrence patterns of AF; some patients may have developed recurrence beyond the study period, potentially influencing the interpretation of the predictive markers. Furthermore, while all patients underwent 24 h ECG Holter monitoring and recurrence was documented based on medical records or verified symptomatic episodes, continuous rhythm monitoring was not performed, which may have led to the underdetection of asymptomatic episodes. Furthermore, adherence to antiarrhythmic therapy during follow-up was not formally verified, which may have affected recurrence patterns and represents a further limitation of our analysis. Despite these limitations, our study serves as an important first step in evaluating the feasibility of a multimodal approach to predicting AF recurrence. The exploratory insights that have emerged should be further assessed and confirmed in larger and potentially multicenter studies. Longer follow-up durations may contribute to more effectively assessing the chronicity of AF recurrence and its impact on clinical outcomes.
5. Conclusions
The PLACEBO pilot trial demonstrates the feasibility and clinical relevance of a multimodal diagnostic approach for predicting PAF recurrence. Our findings establish GAL3 and SDRR as significant predictors, while RV FAC demonstrates a potential association with recurrence. These results emphasize the value of integrating functional, structural, and biochemical assessments in routine practice for more comprehensive risk stratification. Future research should focus on validating these findings in larger, more diverse populations and investigating their potential role in guiding personalized therapeutic strategies.
Author Contributions
Conceptualization, A.B. and C.P.; data curation, A.B., T.M. and M.Z.; formal analysis, A.B. and T.M.; investigation, A.B., G.Z. and M.T.; methodology, K.T. and C.P.; software, A.B. and T.M.; supervision, G.G., V.V. and C.P.; validation, A.B., E.T., M.Z. and C.P.; writing—original draft, A.B.; writing—review and editing, G.G., V.V. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Ippokratio General Hospital of Thessaloniki, Greece (375/30-6-20), and the Ethics Committee of Aristotle University of Thessaloniki, Greece (2417/24-11-20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are available on request due to restrictions (personal medical data).
Acknowledgments
The authors would like to thank the Atherosclerosis Society of Northern Greece for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF | atrial fibrillation |
| PAF | paroxysmal atrial fibrillation |
| CPET | cardiopulmonary exercise testing |
| TTE | transthoracic echocardiography |
| RV FAC | right ventricular fractional area change |
| LA | left atrial |
| HCM | hypertrophic cardiomyopathy |
| GAL3 | galectin-3 |
| Hcy | homocysteine |
| HRV | heart rate variability |
| BMI | body mass index |
| Peak VO2 | peak oxygen uptake |
| VE/VCO2 | minute ventilation/carbon dioxide production ratio |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| GLS | global longitudinal strain |
| EACVI | European Association of Cardiovascular Imaging |
| ECG | electrocardiogram |
| BNP | brain natriuretic peptide |
| Hs-cTnI | high-sensitivity cardiac troponin I |
| CMIA | chemiluminescence |
| ROC | receiver operating characteristics |
| SD | standard deviation |
| OSA | obstructive sleep apnea |
| LVMI | left ventricular mass index |
| LAVI | left atrial volume index |
| VO2 AT | anaerobic threshold |
| PETCO2 | partial pressure of end-tidal carbon dioxide |
| OR | odds ratio |
| SDNN | standard deviation of normal to normal R-R intervals |
| SDRR | standard deviation of R-R intervals |
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Thrall, G.; Lane, D.; Carroll, D.; Lip, G.Y. Quality of life in patients with atrial fibrillation: A systematic review. Am. J. Med. 2006, 119, 448.e1–448.e19. [Google Scholar] [CrossRef] [PubMed]
- Peigh, G.; Zhou, J.; Rosemas, S.C.; Roberts, A.I.; Longacre, C.; Nayak, T.; Schwab, G.; Soderlund, D.; Passman, R.S. Impact of Atrial Fibrillation Burden on Health Care Costs and Utilization. JACC Clin. Electrophysiol. 2024, 10, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar]
- Ruigómez, A.; Johansson, S.; Wallander, M.A.; García Rodríguez, L.A. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc. Disord. 2005, 5, 20. [Google Scholar] [CrossRef]
- de Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef]
- Glaab, T.; Taube, C. Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 2022, 23, 9. [Google Scholar] [CrossRef]
- Boulmpou, A.; Teperikidis, E.; Papadopoulos, C.; Patoulias, D.I.; Charalampidis, P.; Mouselimis, D.; Tsarouchas, A.; Boutou, A.; Giannakoulas, G.; Vassilikos, V. The role of cardiopulmonary exercise testing in risk stratification and prognosis of atrial fibrillation: A scoping review of the literature. Acta Cardiol. 2023, 78, 274–287. [Google Scholar] [CrossRef]
- Troughton, R.W.; Asher, C.R.; Klein, A.L. The role of echocardiography in atrial fibrillation and cardioversion. Heart 2003, 89, 1447–1454. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Sparla, S.; Iardino, E.; Mondillo, S. Left atrial strain: A useful index in atrial fibrillation. Int. J. Cardiol. 2016, 220, 208–213. [Google Scholar] [CrossRef]
- Chou, S.H.; Kuo, C.T.; Hsu, L.A.; Ho, W.J.; Wang, C.L. Single-beat determination of right ventricular function in patients with atrial fibrillation. Echocardiograph 2010, 27, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Li, J.; Gao, J. Functions of galectin-3 and its role in fibrotic diseases. J. Pharmacol. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Charalampidis, P.; Teperikidis, E.; Boulmpou, A.; Papadopoulos, C.E.; Potoupni, V.; Tsioni, K.; Rakitzi, P.; Karamitsos, T.; Vassilikos, V. Homocysteine as a Predictor of Paroxysmal Atrial Fibrillation-Related Events: A Scoping Review of the Literature. Diagnostics 2022, 12, 2192. [Google Scholar] [CrossRef] [PubMed]
- Geurts, S.; Tilly, M.J.; Arshi, B.; Stricker, B.H.C.; Kors, J.A.; Deckers, J.W.; de Groot, N.M.S.; Ikram, M.A.; Kavousi, M. Heart rate variability and atrial fibrillation in the general population: A longitudinal and Mendelian randomization study. Clin. Res. Cardiol. 2023, 112, 747–758. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, K.R.; Seo, J.H.; Ryu, D.R.; Lee, B.K.; Cho, B.R.; Chun, K.J. Higher heart rate variability as a predictor of atrial fibrillation in patients with hypertension. Sci. Rep. 2022, 12, 3702. [Google Scholar] [CrossRef]
- Mazaris, S.; Siasos, G.; Oikonomou, E.; Tsigkou, V.; Vavuranakis, M.; Kokkou, E.; Zaromitidou, M.; Papamikroulis, G.A.; Papavassiliou, A.G.; Papaioannou, S.; et al. Atrial Fibrillation: Biomarkers Determining Prognosis. Curr. Med. Chem. 2019, 26, 909–915. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Pueyo, E.; Diez, E.R. Strain Echocardiography to Predict Postoperative Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1355. [Google Scholar] [CrossRef]
- Boulmpou, A.; Moysiadis, T.; Zormpas, G.; Teperikidis, E.; Vassilikos, V.; Giannakoulas, G.; Papadopoulos, C. Exploring the Feasibility of Integrating Cardiopulmonary Exercise Testing, Echocardiography, and Biomarkers for Predicting Atrial Fibrillation Recurrence: Rationale, Design and Protocol for a Prospective Cohort Study (The PLACEBO Trial). J. Clin. Med. 2025, 14, 1690. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.R.; Roach, D.E.; Koshman, M.L.; Sheldon, R.S. Standard Deviation of Sequential Five-Minute R-R Interval Means (SDANN) is a prognostic marker, but not necessarily an autonomic marker. J. Am. Coll. Cardiol. 2006, 48, 1285–1286, author reply 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Zhang, E.; Liang, S.; Sun, T.; Xu, J.; Lu, F.; Wu, D.; Zhang, J.; He, L.; Zhang, F.; Fan, S.; et al. Prognostic value of heart rate variability in atrial fibrillation recurrence following catheter ablation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 1048398. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J.; et al. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation. Hypertension 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Procyk, G.; Czapla, A.; Jałocha, K.; Tymińska, A.; Grabowski, M.; Gąsecka, A. The role of galectin-3 in atrial fibrillation. J. Mol. Med. 2023, 101, 1481–1492. [Google Scholar] [CrossRef]
- Gong, M.; Cheung, A.; Wang, Q.S.; Li, G.; Goudis, C.A.; Bazoukis, G.; Lip, G.Y.H.; Baranchuk, A.; Korantzopoulos, P.; Letsas, K.P.; et al. Galectin-3 and risk of atrial fibrillation: A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23104. [Google Scholar] [CrossRef]
- Linz, D.; McEvoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Lévy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea With Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. 2018, 3, 532–540. [Google Scholar] [CrossRef]
- Trzepizur, W.; Blanchard, M.; Ganem, T.; Balusson, F.; Feuilloy, M.; Girault, J.M.; Meslier, N.; Oger, E.; Paris, A.; Pigeanne, T.; et al. Sleep Apnea-Specific Hypoxic Burden, Symptom Subtypes, and Risk of Cardiovascular Events and All-Cause Mortality. Am. J. Respir. Crit. Care Med. 2022, 205, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003, 177, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Moula, A.I.; Parrini, I.; Tetta, C.; Lucà, F.; Parise, G.; Rao, C.M.; Mauro, E.; Parise, O.; Matteucci, F.; Gulizia, M.M.; et al. Obstructive Sleep Apnea and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Wong, G.R.; Lee, G.; Voskoboinik, A.; Kee, K.; Goldin, J.; Watts, T.; Linz, D.; Parameswaran, R.; Sugumar, H.; et al. Impact of CPAP on the Atrial Fibrillation Substrate in Obstructive Sleep Apnea: The SLEEP-AF Study. Clin. Electrophysiol. 2022, 8, 869–877. [Google Scholar] [CrossRef]
- Konecny, T.; Miles, W.M. Treating Obstructive Sleep Apnea and Atrial Fibrillation: Focus on Substrate, Triggers, and Those Evasive Outcomes. Clin. Electrophysiol. 2022, 8, 878–881. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Hijazi, Z.; Siegbahn, A.; Andersson, U.; Granger, C.B.; Alexander, J.H.; Atar, D.; Gersh, B.J.; Mohan, P.; Harjola, V.P.; Horowitz, J.; et al. High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: Insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 2014, 129, 625–634. [Google Scholar] [CrossRef]
- Ivanov, V.; Smereka, Y.; Rasputin, V.; Dmytriiev, K. Homocysteine and atrial fibrillation: Novel evidences and insights. Monaldi Arch. Chest Dis. 2023, 93. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, S.J.; Kim, J.; Chung, T.W.; Park, S.J.; Park, K.M.; Kim, J.S.; On, Y.K. Exercise capacity and risk of incident atrial fibrillation in healthy adults. Korean J. Intern. Med. 2023, 38, 872–878. [Google Scholar] [CrossRef]
- Tsuneoka, H.; Koike, A.; Nagayama, O.; Sakurada, K.; Kato, J.; Sato, A.; Yamashita, T.; Aonuma, K. Prognostic value of cardiopulmonary exercise testing in cardiac patients with atrial fibrillation. Int. Heart J. 2012, 53, 102–107. [Google Scholar] [CrossRef]
- Terada, T.; Keir, D.A.; Murias, J.M.; Vidal-Almela, S.; Buckley, J.; Reed, J.L. Variability of cardiopulmonary exercise testing in patients with atrial fibrillation and determination of exercise responders to high-intensity interval training and moderate-to-vigorous intensity continuous training. Appl. Physiol. Nutr. Metab. 2024, 49, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Pislaru, S.; Borlaug, B.A. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur. Heart J. 2019, 40, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.F.; Kukin, M.; Javed, F.; Musat, D.; Nader, A.; Pratap, B.; Shah, A.; Enciso, J.S.; Chaudhry, F.A.; Herzog, E. Right ventricular dysfunction is a strong predictor of developing atrial fibrillation in acutely decompensated heart failure patients, ACAP-HF data analysis. J. Card. Fail. 2010, 16, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Yin, X.; Levy, D.; Vasan, R.S.; Magnani, J.W.; Ellinor, P.T.; McManus, D.D.; Lubitz, S.A.; Larson, M.G.; Benjamin, E.J. Galectin 3 and incident atrial fibrillation in the community. Am. Heart J. 2014, 167, 729–734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).