Bioelectrical Impedance Profiling to Estimate Neuropathic and Vascular Risk in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Size Calculation
2.3. Data Collection
2.3.1. Anthropometry
2.3.2. Bioelectrical Impedance Analysis
2.3.3. Assessment of Diabetes-Related Complications
2.4. Covariates
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Baseline Characteristics
3.2. Principal Component Structure
3.3. Variable Contribution and Adjusted Associations
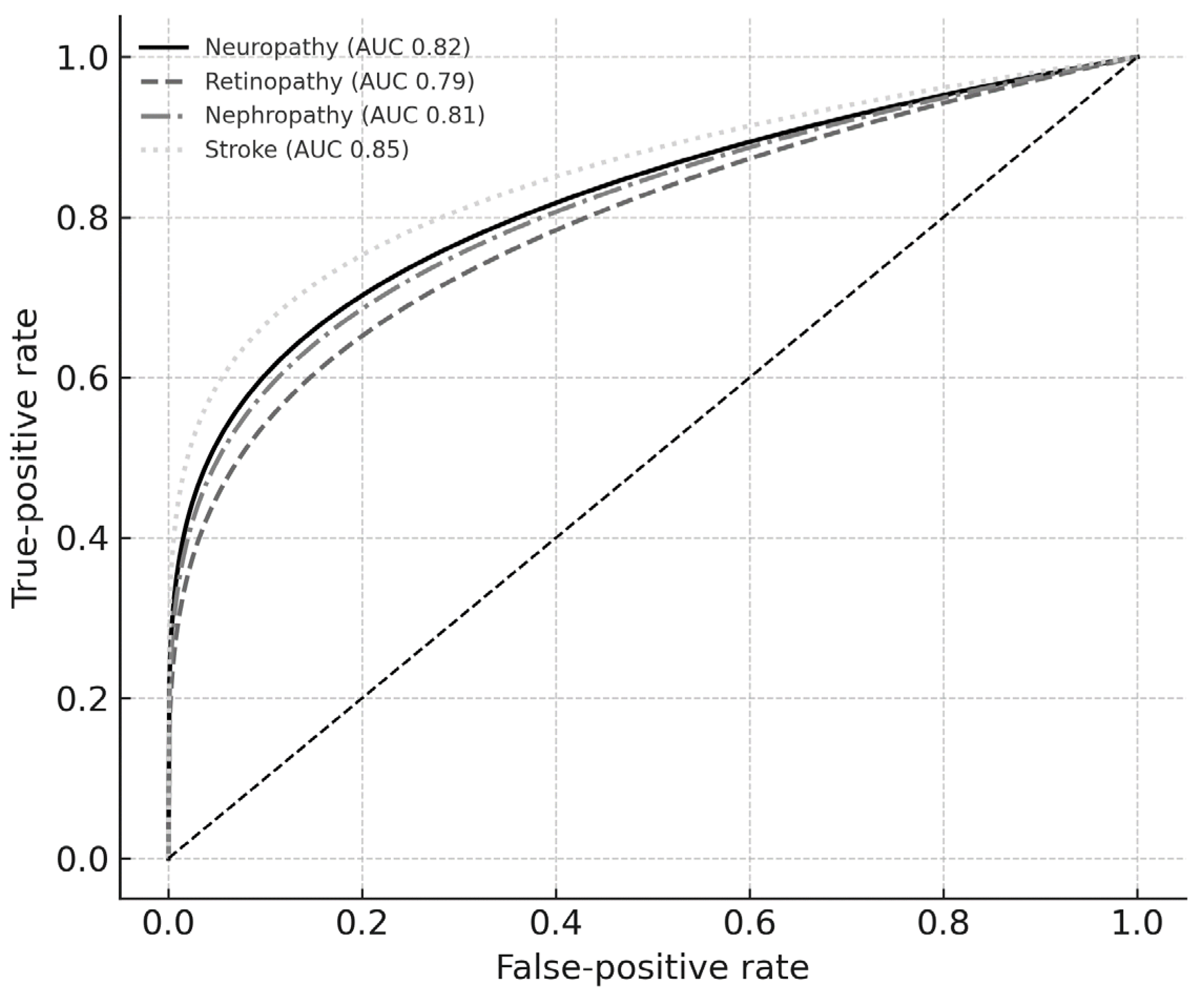
3.4. Discrimination Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AUC | Area under the curve |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| CI | Confidence interval |
| CT | Computed tomography |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| HbA1c | Glycated hemoglobin |
| ICW | Intracellular water |
| IQR | Interquartile range |
| ISAK-II | International Society for the Advancement of Kinanthropometry (level 2) |
| MRI | Magnetic resonance imaging |
| OR | Odds ratio |
| PCA | Principal-component analysis |
| PhA | Phase angle |
| ROC | Receiver-operating characteristic |
| SD | Standard deviation |
| SMM | Skeletal muscle mass |
| SPSS | Statistical Package for the Social Sciences |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| T2DM | Type 2 diabetes mellitus |
| VFA | Visceral fat area |
References
- Ozougwu, O. The Pathogenesis and Pathophysiology of Type 1 and Type 2 Diabetes Mellitus. J. Physiol. Pathophysiol. 2013, 4, 46. [Google Scholar] [CrossRef]
- Hammes, H.-P. Pathophysiological Mechanisms of Diabetic Angiopathy. J. Diabetes Its Complicat. 2003, 17, 16. [Google Scholar] [CrossRef]
- Mima, A. Incretin-Based Therapy for Prevention of Diabetic Vascular Complications. J. Diabetes Res. 2016, 12, 1379274. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M.; et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 707479. [Google Scholar] [CrossRef]
- Galiero, R.; Caturano, A.; Vetrano, E.; Beccia, D.; Brin, C.; Alfano, M.R.; Salvo, J.D.; Epifani, R.; Piacevole, A.; Tagliaferri, G.; et al. Peripheral Neuropathy in Diabetes Mellitus: Pathogenetic Mechanisms and Diagnostic Options. Int. J. Mol. Sci. 2023, 24, 3554. [Google Scholar] [CrossRef]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; Chiarelli, F. Autonomic Neuropathy in Diabetes Mellitus. Front. Endocrinol. 2014, 5, 205. [Google Scholar] [CrossRef]
- Taylor, A.A. Pathophysiology of Hypertension and Endothelial Dysfunction in Patients with Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2001, 30, 983. [Google Scholar] [CrossRef]
- Burgess, J.; Frank, B.; Marshall, A.; Khalil, R.S.; Ponirakis, G.; Petropoulos, I.N.; Cuthbertson, D.J.; Malik, R.A.; Alam, U. Early Detection of Diabetic Peripheral Neuropathy: A Focus on Small Nerve Fibres. Diagnostics 2021, 11, 165. [Google Scholar] [CrossRef]
- Carlson-Newberry, S.J.; Costello, R.B. Bioelectrical Impedance: A History, Research Issues, and Recent Consensus. 1997. Available online: https://www.ncbi.nlm.nih.gov/books/NBK233766/ (accessed on 6 August 2025).
- Marra, M.; Prat, B.D.; Montagnese, C.; Caldara, A.; Sammarco, R.; Pasanisi, F.; Corsetti, R. Segmental Bio-impedance Analysis in Professional Cyclists during a Three Week Stage Race. Physiol. Meas. 2016, 37, 1035. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Garcia-Almeida, J.M. Phase angle in applications of bio-impedance in health and disease. Rev. Endocr. Metab. Disord. 2023, 24, 367–370. [Google Scholar] [CrossRef]
- Catapano, A.; Trinchese, G.; Cimmino, F.; Petrella, L.; D’Angelo, M.; Maio, G.D.; Crispino, M.; Cavaliere, G.; Monda, M.; Mollica, M.P. Impedance Analysis to Evaluate Nutritional Status in Physiological and Pathological Conditions. Nutrients 2023, 15, 2264. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Shi, T.; Li, D.; Du, Z.; Xu, K. Using Skin Impedance to Improve Prediction Accuracy of Continuous Glucose Monitoring System. In Proceedings of the SPIE 2008 the International Society for Optical Engineering, San Jose, CA, USA, 22 February 2008; Volume 6863. [Google Scholar] [CrossRef]
- Norton, K. Standards for Anthropometry Assessment. In Kinanthropometry and Exercise Physiology; Routledge: Abingdon-on-Thames, UK, 2018; Volume 68. [Google Scholar] [CrossRef]
- InBodyS10 User’s Manual. 2024. Available online: https://nl.inbody.com/wp-content/uploads/2019/01/InBodyS10_CDmanual_Eng_E.pdf (accessed on 6 August 2025).
- Herman, W.H.; Pop-Busui, R.; Braffett, B.H.; Martin, C.; Cleary, P.A.; Albers, J.W.; Feldman, E.L. Use of the Michigan Neuropathy Screening Instrument as a Measure of Distal Symmetrical Peripheral Neuropathy in Type 1 Diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet. Med. 2012, 29, 937. [Google Scholar] [CrossRef]
- Salongcay, R.P.; Jacoba, C.M.P.; Salva, C.M.G.; Rageh, A.; Aquino, L.A.; Saunar, A.V.; Alog, G.P.; Ashraf, M.; Pető, T.; Silva, P.S. One-Field, Two-Field and Five-Field Handheld Retinal Imaging Compared with Standard Seven-Field Early Treatment Diabetic Retinopathy Study Photography for Diabetic Retinopathy Screening. Br. J. Ophthalmol. 2023, 108, 735. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Navarro-Gil, R.; Moreno, A.; Valls, A.; Sagarra, R.; Barrot, J.; Mundet, X. Glomerular Filtration Rate and/or Ratio of Urine Albumin to Creatinine as Markers for Diabetic Retinopathy: A Ten-Year Follow-Up Study. J. Diabetes Res. 2018, 2018, 5637130. [Google Scholar] [CrossRef]
- Wannarong, T.; Sukpornchairak, P.; Naweera, W.; Geiger, C.; Ungprasert, P. Association between Diabetic Peripheral Neuropathy and Sarcopenia: A Systematic Review and Meta-analysis. Geriatr. Gerontol. Int. Geriatr. Gerontol. Int. 2022, 22, 785. [Google Scholar] [CrossRef]
- Low, S.; Pek, S.L.T.; Moh, A.; Khin, C.Y.A.; Lim, C.L.; Ang, S.F.; Wang, J.; Ang, K.; Tang, W.E.; Lim, Z.; et al. Low Muscle Mass Is Associated with Progression of Chronic Kidney Disease and Albuminuria—An 8-Year Longitudinal Study in Asians with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2021, 174, 108777. [Google Scholar] [CrossRef]
- Zong, G.-W.; Wang, W.; Zheng, J.; Zhang, W.; Luo, W.-M.; Fang, Z.; Zhang, Q. A Metabolism-Based Interpretable Machine Learning Prediction Model for Diabetic Retinopathy Risk: A Cross-Sectional Study in Chinese Patients with Type 2 Diabetes. J. Diabetes Res. 2023, 2023, 3990035. [Google Scholar] [CrossRef]
- Prado-Olivarez, J.; Arellano-Olivares, F.; Padilla-Medina, A.; Díaz-Carmona, J.; Ramirez-Agundis, A.; Calderón, A.E.; Garcia-Mesita, M.; Aguilar-Diaz, T. Bio-impedance Phase Angle Analysis of Foot Skin in Diabetic Patients: An Experimental Case Study. IRBM 2015, 36, 233. [Google Scholar] [CrossRef]
- Tronstad, C.; Amini, M.; Olesen, E.; Qvigstad, E.; Pabst, O.; Martinsen, T.; Abie, S.M.; Martinsen, Ø.G.; Hisdal, J.; Jenssen, T.; et al. Diabetic Foot Assessment Using Skin Impedance in a Custom Made Sensor-Sock. J. Electr. Bio-Impedance 2022, 13, 136. [Google Scholar] [CrossRef]
- González, M.C.; Barros, A.J.D. Bioelectrical Impedance Analysis in Clinical Practice: A New Perspective on Its Use beyond Body Composition Equations. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 311. [Google Scholar] [CrossRef]
- Presti, A.L.; Montoya, N.A.; Criscuolo, V.; Khan, G.; Khan, U.; Vecchione, R.; Falconi, C. Fundamentals of Skin Bio-impedances. Adv. Mater. 2023, 35, 2302127. [Google Scholar] [CrossRef]
- Nishibe, T.; Dardik, A.; Akiyama, S.; Kano, M.; Fukuda, S.; Koizumi, J.; Nishibe, M. Reduced Muscle Mass and Muscle Quality in Patients with Intermittent Claudication Due to Peripheral Artery Disease. Ann. Vasc. Surg. 2024, 105, 275. [Google Scholar] [CrossRef] [PubMed]
- Schimpfle, L.; Tsilingiris, D.; Mooshage, C.; Κender, Ζ.; Sulaj, A.; von Rauchhaupt, E.; Szendroedi, J.; Herzig, S.; Goepfert, J.C.; Groener, J.B.; et al. Phase Angle of Bioelectrical Impedance Analysis as an Indicator for Diabetic Polyneuropathy in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2024, 109, e2110–e2119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Zhao, S.; Yin, Y.; Zhang, X.; Wang, K. Nomogram for Prediction of Diabetic Retinopathy Among Type 2 Diabetes Population in Xinjiang, China. Diabetes Metab. Syndr. Obes. 2022, 15, 1077–1089. [Google Scholar] [CrossRef]
| Complication | PC1 (%) | PC2 (%) | PC3 (%) |
|---|---|---|---|
| Neuropathy | 64.9 | 26.9 | 6.7 |
| Retinopathy | 66.0 | 26.0 | 6.5 |
| Nephropathy | 67.1 | 22.1 | 9.4 |
| Stroke | 72.3 | 25.9 | 1.8 |
| Complication | Variable | Contribution (%) | Odds Ratio | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|---|---|
| Neuropathy | Skeletal muscle mass | 64.9 | 0.54 | 0.41 | 0.71 | <0.001 |
| Visceral fat area | 26.9 | 1.55 | 1.22 | 1.97 | <0.001 | |
| Phase angle | 6.7 | 0.62 | 0.48 | 0.81 | 0.001 | |
| Body mass index | 1.0 | 1.08 | 0.85 | 1.37 | 0.53 | |
| Retinopathy | Lean mass | 66.0 | 0.58 | 0.44 | 0.77 | <0.001 |
| Body fat % | 26.0 | 1.47 | 1.14 | 1.88 | 0.002 | |
| Phase angle | 6.5 | 0.66 | 0.50 | 0.88 | 0.005 | |
| Body mass index | 1.5 | 1.10 | 0.87 | 1.39 | 0.42 | |
| Nephropathy | Intracellular water | 33.6 | 0.72 | 0.55 | 0.95 | 0.020 |
| Skeletal muscle mass | 33.6 | 0.70 | 0.53 | 0.92 | 0.010 | |
| Body fat % | 22.1 | 1.28 | 1.03 | 1.59 | 0.027 | |
| Phase angle | 9.4 | 0.81 | 0.63 | 1.04 | 0.096 | |
| Body mass index | 1.3 | 1.12 | 0.88 | 1.42 | 0.34 | |
| Stroke | Phase angle | 72.3 | 0.55 | 0.37 | 0.82 | 0.004 |
| Body fat % | 25.9 | 1.41 | 1.02 | 1.95 | 0.037 | |
| Body mass index | 1.8 | 1.05 | 0.78 | 1.40 | 0.73 |
| Complication | AUC | 95% CI Lower | 95% CI Upper | Optimal Cut-Off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Neuropathy | 0.82 | 0.76 | 0.88 | 0.43 | 0.78 | 0.70 |
| Retinopathy | 0.79 | 0.73 | 0.86 | 0.39 | 0.74 | 0.68 |
| Nephropathy | 0.81 | 0.74 | 0.86 | 0.40 | 0.76 | 0.69 |
| Stroke | 0.85 | 0.78 | 0.91 | 0.45 | 0.80 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga-Torres, E.; Marizande, F.; Arteaga, C.; Pilamunga, M.; Reales-Chacón, L.J.; Bonilla, S.; Robayo, D.; Buenaño, S.; Camacho, S.; Galarza, W.; et al. Bioelectrical Impedance Profiling to Estimate Neuropathic and Vascular Risk in Patients with Type 2 Diabetes Mellitus. Diagnostics 2025, 15, 2005. https://doi.org/10.3390/diagnostics15162005
Quiroga-Torres E, Marizande F, Arteaga C, Pilamunga M, Reales-Chacón LJ, Bonilla S, Robayo D, Buenaño S, Camacho S, Galarza W, et al. Bioelectrical Impedance Profiling to Estimate Neuropathic and Vascular Risk in Patients with Type 2 Diabetes Mellitus. Diagnostics. 2025; 15(16):2005. https://doi.org/10.3390/diagnostics15162005
Chicago/Turabian StyleQuiroga-Torres, Elizabeth, Fernanda Marizande, Cristina Arteaga, Marcelo Pilamunga, Lisbeth Josefina Reales-Chacón, Silvia Bonilla, Doménica Robayo, Sara Buenaño, Sebastián Camacho, William Galarza, and et al. 2025. "Bioelectrical Impedance Profiling to Estimate Neuropathic and Vascular Risk in Patients with Type 2 Diabetes Mellitus" Diagnostics 15, no. 16: 2005. https://doi.org/10.3390/diagnostics15162005
APA StyleQuiroga-Torres, E., Marizande, F., Arteaga, C., Pilamunga, M., Reales-Chacón, L. J., Bonilla, S., Robayo, D., Buenaño, S., Camacho, S., Galarza, W., & Bustillos, A. (2025). Bioelectrical Impedance Profiling to Estimate Neuropathic and Vascular Risk in Patients with Type 2 Diabetes Mellitus. Diagnostics, 15(16), 2005. https://doi.org/10.3390/diagnostics15162005









