Abstract
Background/Objectives: In patients with metastatic castration-resistant prostate cancer (mCRPC) and osseous metastases only, 223Radium therapy represents a valuable therapeutic option. Bone scintigraphy (BS) is typically performed to assess metastasis load, with the BS-derived automated bone scan index (aBSI) used for response assessment. This study aimed to evaluate the prognostic value of aBSI in patients receiving three or six cycles of 223Ra therapy. Methods: We included patients that were diagnosed with extensive osseous tumor load on BS, had no visceral or nodal metastases, had undergone 223Ra therapy. The aBSI prior to and following three or six cycles of therapy, total tumor volume (TTV), SUVmax, and overall survival were analyzed. Results: This study included 49 mCRPC patients (mean age: 70 ± 9 years) with 42 (85.7%) receiving six and 7 (14.3%) receiving three cycles. After three cycles, the mean aBSI (p = 0.369), TTV (p = 0.902), and SUVmax (p = 0.149) remained unchanged. After six cycles, the mean aBSI (p = 0.247) and TTV (p = 0.784) were unchanged, while SUVmax decreased significantly (p = 0.001). The aBSI did not significantly correlate with the mean aBSI (six cycles: χ2 = 1.823, p = 0.177; three cycles: χ2 = 0.308, p = 0.579). Conclusions: Although quantitative changes in TTV and aBSI did not significantly correlate with each other, their respective absolute values consistently indicated stable disease burden under therapy. This highlights its potential as a useful tool for monitoring disease burden while indicating that aBSI alone is insufficient for predicting overall survival.
1. Introduction
In advanced prostate cancer, resistance to androgen deprivation therapy (ADT) necessitates alternative treatment options, particularly for patients who progress to metastatic castration-resistant prostate cancer (mCRPC) [1,2]. In this setting, the selection of therapy depends largely on the pattern of metastatic spread [3,4]. Bone scintigraphy (BS) using 99mTc-phosphonate is commonly employed to evaluate the extent of osseous tumor burden [5]. Patients with osseous metastases, but without visceral or nodal metastases, may be eligible for treatment with 223Radium (223Ra) dichloride [3,6]. This alpha particle-emitting radiopharmaceutical selectively targets bones by mimicking calcium and inducing double-strand DNA breaks in tumor cells within the bones [7]. The international phase III ALSYMCA trial demonstrated that 223Ra significantly prolongs overall survival (OS) and reduces the risk of symptomatic skeletal events compared to placebo. Consequently, 223Ra was approved by the FDA for treating patients with mCRPC, symptomatic bone metastases, and no visceral metastases [8]. Automated bone scan index (aBSI), a quantitative parameter derived from BS, has emerged as a promising prognostic biomarker to assess disease burden and monitor therapeutic response in mCRPC patients undergoing 223Ra therapy. Changes in the aBSI have been shown to correlate with overall survival [9,10,11].
The objective of this study was to evaluate the prognostic value of the aBSI in assessing treatment response in patients receiving three or six cycles of 223Ra therapy.
2. Materials and Methods
2.1. Patients
We included patients that were diagnosed with an extensive osseous tumor load on BS, had no visceral or nodal metastases, and had undergone 223Ra therapy. This analysis was performed in compliance with the principles of the Declaration of Helsinki and was approved by the institutional ethics board of the LMU Munich (230-15, approved date: 17 April 2015). General patient characteristics as well as imaging data were collected and compiled in an anonymized data sheet.
2.2. Radiopharmaceutical and Imaging Protocol
Whole-body bone scintigraphy was performed after intravenous administration of 99mTc-phosphonate (TECEOS, CIS bio GmbH, Berlin, Germany) at a mean of 19.3 ± 21.3 days prior to 223Ra therapy at the Department of Nuclear Medicine, LMU Munich. Images were acquired in planar imaging mode at a mean of 3 h after injection using a Siemens Symbia Intevo 16/6/2 2014 (Siemens Healthcare, Erlangen, Germany). Additionally, SPECT/CT images were performed (256 × 1024 matrix; scanning speed 15 cm/min).
2.3. Image Analysis
The aBSI was determined automatically by using EXINI Diagnostics AB (Lund, Sweden). The response to treatment was analyzed by investigating changes in aBSI as well as osseous total tumor volume (TTV) and SUVmax on SPECT/CT. A dedicated software package was used (Hermes Hybrid Viewer, version 2.0; Hermes Medical Solutions, Stockholm, Sweden).
2.4. 223Ra Therapy
223Ra dichloride (Xofigo®, Bayer AG, Leverkusen, Germany) was from Bayer. A mean dose of 4.3 ± 0.8 MBq per cycle was administered intravenously as described elsewhere [3,12]. The therapy was conducted in the outpatient department of the Department of Nuclear Medicine, LMU Munich. Patients underwent 223Ra therapy every three weeks for a total of three or six cycles. After three cycles, treatment was either continued up to six cycles or paused, depending on clinical assessment, tolerability, evidence of disease progression, or patient performance status. After three cycles, all patients underwent a bone scan to assess response. For those who continued and completed six cycles of 223Ra therapy, an additional bone scan was performed after the sixth cycle. Changes between baseline parameters and those obtained after either three or six therapy cycles—depending on the total number of cycles administered—were systematically analyzed.
Patients underwent laboratory analyses of hemoglobin level, white blood cell (WBC) counts, platelets, neutrophils, prostate-specific antigen (PSA), and alkaline phosphatase (AP) prior to every therapy cycle as well as 6–8 weeks following the last cycle.
2.5. Statistical Analysis
Data analysis was performed using Microsoft Excel (Excel 2019, Microsoft, Redmond, WA, USA) and GraphPad Prism (Version 9.5.0 (730)). The descriptive statistics are displayed as mean ± standard deviation (STD). Shapiro–Wilk test was used to assess data normality before applying parametric or non-parametric tests. Group comparisons were performed using a parametric or non-parametric unpaired t-test. Correlation analyses were conducted using Spearman or Pearson correlation analyses. Survival data were calculated using Kaplan–Meier-curves and Log rank test. A two-tailed p-value < 0.05 was considered statistically significant.
3. Results
3.1. Bone Scintigraphy, SPECT/CT and Baseline Lab Analysis
In total, 49 patients at a mean age of 70 ± 9 years diagnosed with mCRPC and extensive osseous tumor load on BS were included.
The mean baseline aBSI was 2.9 ± 3.4%, the mean TTV was 237.2 ± 217.1 mL, and the mean SUVmax was 878.7 ± 517.4. The baseline PSA levels were 99.1 ± 273.1 ng/mL, and the AP levels were 173.6 ± 207.0 U/L. The aBSI, the mean total tumor volume (r = 0.629; r2 = 0.396; p < 0.001), and the mean SUVmax (r = 0.301; r2 = 0.091; p = 0.035) correlated significantly (Table 1).

Table 1.
Patient characteristics.
3.2. Changes in Clinical and Imaging Parameters Following 223Ra Therapy
Overall, 7 out of 49 (14.3%) patients underwent three cycles, while 42 out of 49 (85.7%) completed six cycles of 223Ra therapy.
In patients who underwent three cycles of treatment, there was no statistically significant change in the mean aBSI (6.7 ± 5.2%; before: 4.5 ± 3.8%; p = 0.369), mean TTV (353.2 ± 369.2 mL; before: 356.1 ± 382.9 mL; p = 0.902), or mean SUVmax (562.3 ± 156.2; before: 801.7 ± 379.3; p = 0.149). The imaging of a representative patient case is presented in Figure 1.
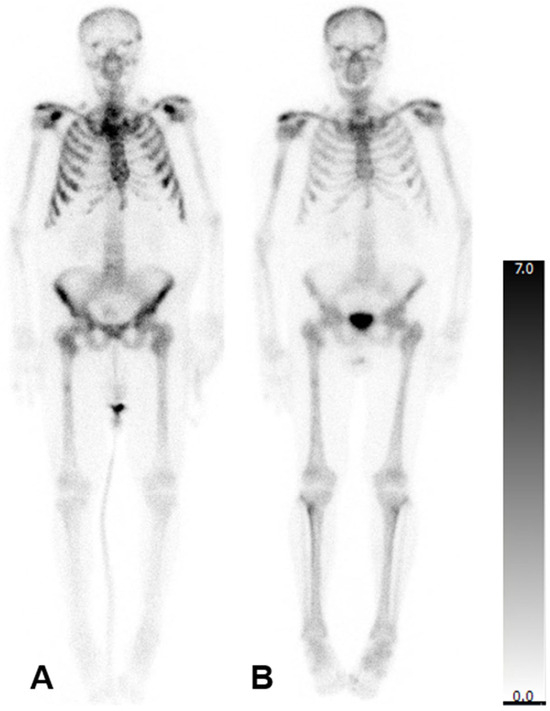
Figure 1.
Bone scintigraphy of a 77-year-old man with mCRPC. aBSI prior to 223Ra therapy was 7.5%, TTV 1112.20 mL and SUVmax 388 (A). After three cycles of 223Ra therapy, aBSI was 6.7%, TTV was 97.03 mL, and SUVmax was 293 (B).
Changes in aBSI were not significantly correlated with changes in TTV (r = 0.559; r2 = 0.312; p = 0.206) or SUVmax (r = −0.400; r2 = 0.160; p = 0.368).
The PSA level (953.4 ± 1884.2 ng/mL; before: 383.8 ± 673.4 ng/mL; p = 0.731) and the AP level (220.6 ± 172.7 U/L; before: 244.4 ± 241.0 U/L; p = 0.949) did not change significantly.
Considering the side effects of a higher grade (CTCAE ≥ 3), lymphopenia was seen in three patients (CTCAE grade 3), whereas anemia (CTCAE grade 3) and thrombopenia (CTCAE grade 4) were present in one patient each.
In patients who were administered six cycles, there was no significant change in the mean aBSI (2.6 ± 4.3%; before: 2.6 ± 3.3%; p = 0.247) or the mean TTV (208.8 ± 185.2 mL; before: 217.4 ± 175.8 mL; p = 0.784). However, the mean SUVmax reduced significantly (548.1 ± 342.2; before: 891.6 ± 539.6; p = 0.001). A representative patient case is illustrated in Figure 2.
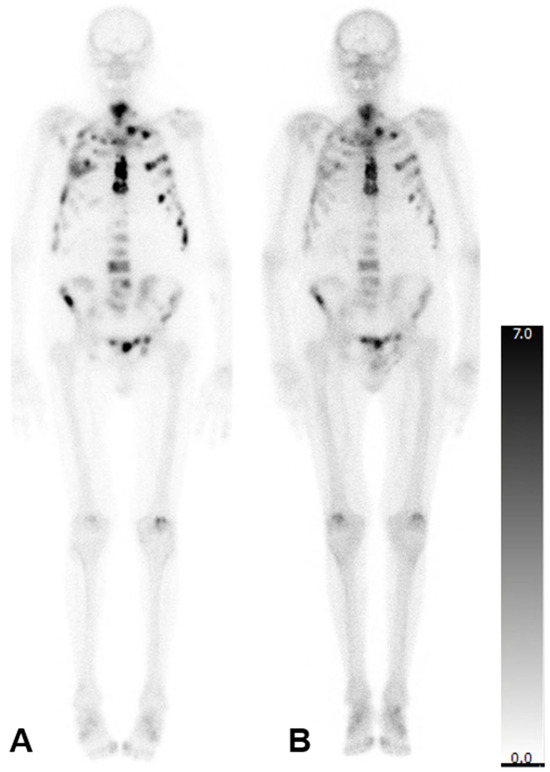
Figure 2.
Bone scintigraphy of an 83-year-old man with mCRPC. aBSI prior to 223Ra therapy was 7.8%, TTV was 549.32 mL, and SUVmax was 1500 (A). After six cycles of 223Ra therapy aBSI was 4.1%, TTV was 368.05 mL, and SUVmax was 1284 (B).
Changes in aBSI were significantly correlated with changes in TTV (r = 0.360; r2 = 0.130; p = 0.019), but not with changes in SUVmax (r = 0.132; r2 = 0.017; p = 0.405).
Additionally, the PSA level did not change significantly (73.5 ± 110.0 ng/mL; before: 51.7 ± 69.5 ng/mL; p = 0.656), whereas the AP level reduced significantly (101.3 ± 91.1 U/L; before: 161.6 ± 201.5 U/L; p = 0.010).
3.3. Overall Survival
In 5 out of 7 patients that underwent three cycles and in 17/42 patients that underwent six cycles, OS data were available. The mean OS was 809 ± 831 days, with 905 ± 896 days for patients undergoing six cycles and 484 ± 490 days for patients undergoing three cycles.
Log rank test showed no significant difference for patients undergoing six cycles of therapy when investigating the OS for patients with an aBSI above the mean of 2.6% and ≤2.6% (median OS > 2.6%: 431 days; median OS ≤ 2.6% 814 days; χ2 = 1.823, p = 0.177).
There was also no significant difference for patients undergoing three cycles of therapy when investigating the OS for patients with an aBSI above the mean of 4.5% and ≤4.5% (median OS > 4.5%: 129 days; median OS ≤ 4.5% 475 days; χ2 = 0.308, p = 0.579; Figure 3).
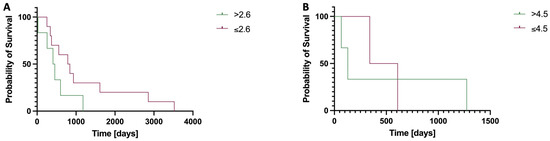
Figure 3.
Kaplan–Meier curves comparing the overall survival (OS) in patients stratified by baseline aBSI values above or below the group mean, following six (A) or three (B) cycles of 223Ra therapy. (A) Median OS > 2.6%: 431 days; median OS ≤ 2.6% 814 days; although a trend toward improved survival was observed in patients with a lower aBSI, this difference did not reach statistical significance (χ2 = 1.823, p = 0.177). (B) Median OS > 4.5%: 129 days; median OS ≤ 4.5% 475 days; however, this difference was also not statistically significant (χ2 = 0.308, p = 0.579).
4. Discussion
With this study, we aimed to investigate the prognostic value of aBSI to assess the response to 223Ra therapy in patients undergoing three or six cycles.
After completing three or six cycles of 223Ra therapy, no significant change in mean aBSI was observed in either group. While TTV remained stable regardless of treatment duration, a significant reduction in mean SUVmax was seen after six cycles but not after three. This suggests that the therapeutic impact on tumor metabolism becomes more evident with prolonged treatment.
Correlation analyses showed a significant association between changes in aBSI and TTV exclusively after six cycles of treatment. In contrast, no significant correlations were found between changes in either aBSI and TTV after three cycles or between changes in SUVmax and aBSI at any time point.
In both treatment groups, PSA levels increased during the initial treatment phase, suggesting the presence of a PSA flare phenomenon [13]. This phenomenon describes a transient rise in PSA levels shortly after treatment initiation, which may not accurately reflect true disease progression but rather a biological response to therapy. The temporary rise in PSA can result in apparent discrepancy between biochemical markers (e.g., PSA) and imaging findings (e.g., TTV) despite stable or even regressing tumor burden, as observed in this study (stable TTV). The PSA flare phenomenon was initially reported in a case report and has since been documented in larger cohorts, particularly in the context of androgen deprivation therapy and other systemic treatments [13,14,15]. Mechanistically, the flare is thought to be caused by increased tumor cell lysis or immune-mediated effects, leading to the release of PSA into the bloodstream [16,17]. Importantly, some studies have found that PSA flares do not negatively impact outcomes and may even be associated with improved prognosis, reflecting effective tumor response despite the rise in transient PSA [14,16,18,19]. However, the flare also complicates early treatment assessment, as rising PSA levels might be misinterpreted as progression, potentially influencing clinical decisions. In contrast, AP levels showed a notable decline only after the completion of six cycles. However, previous studies indicate that alterations of AP levels are not associated with a prolonged overall survival [20]. Given the complex dynamics of PSA and AP levels, additional studies investigating changes in biomarkers and imaging findings are needed to better understand the flare phenomenon. Additionally, our data showed that treatment with 223Ra did not result in a significant reduction in TTV, indicating a stable disease rather than tumor regression.
Baseline aBSI was lower in patients who completed six cycles of treatment (mean aBSI: 2.6%) compared to those who received only three cycles (mean aBSI: 4.5%). Previous studies have demonstrated that a lower baseline aBSI is associated with more favorable outcomes and prolonged OS in patients with mCRPC [21,22,23]. However, in our cohort, no significant difference in OS was observed when comparing patients with an aBSI above versus below the respective group mean. This suggests that although the baseline aBSI reflects overall disease burden, its prognostic utility for predicting overall survival may be limited in this setting. The discrepancy between our findings and those reported in previous studies [9,10,11] may be attributed to differences in baseline patient characteristics or the small, heterogeneous nature of our cohort, which could have influenced the observed outcomes. Nevertheless, aBSI evaluation may serve as a practical and time-efficient method for estimating osseous tumor burden, especially when compared to the more complex and labor-intensive assessment of TTV. In our analysis, neither aBSI nor TTV showed significant changes following 223Ra therapy, irrespective of whether three or six cycles were administered. Although quantitative changes in these two parameters did not significantly correlate, their respective absolute values consistently reflected stable disease burden during treatment. This suggests that both metrics offer a comparable overall assessment of treatment response, despite reflecting different biological aspects of tumor progression.
In this study, adverse events classified as grade 3 or higher according to the CTCAE criteria were observed in five patients (10.2%). This incidence falls within an acceptable range and aligns with the findings of comparable studies [8,24,25]. It has been demonstrated that patients with pre-existing impaired hematopoiesis are particularly vulnerable to developing high-grade hepatotoxicity [26].
This study is limited primarily because of its retrospective nature and small sample size (n = 49). Its retrospective design led to an inherent imbalance between the treatment groups, with notably fewer patients receiving only three cycles. This limitation reduces statistical power and affects the robustness of survival analyses, which limits generalizability and necessiates the inclusion of larger cohorts and long-term follow-ups to validate the findings. The study is intended as a foundational exploration to inform and guide future prospective trials with more balanced cohorts. Additionally, the cohort of patients was heterogeneous due to the administration of various pretreatments (e.g., chemotherapy, ADT, radiation), which may have influenced the observed outcomes and responses. Long-term data regarding safety and outcome are needed, and the data of the REASSURE study are awaited [27]. In patients whose treatment was discontinued after three cycles—due to toxicity, disease progression, or poor clinical performance—it is likely that a more advanced or aggressive disease course was already present at baseline. This introduces a relevant selection bias that may have influenced both the decision to limit treatment duration and the observed clinical outcomes. In order to ensure methodological consistency and valid comparability of the imaging data, all imaging procedures were performed using the same scanners and identical reconstruction protocols.
Looking ahead, while the conventional use of BS for monitoring response to 223Ra therapy is well-established, the potential advantages of emerging imaging techniques, especially PSMA-PET/CT, should not be overlooked [28,29]. PSMA-PET targets the prostate-specific membrane antigen, providing a more detailed assessment of tumor metabolism [30]. Its superior sensitivity and specificity allow for a more accurate evaluation of metastatic burden and therapeutic response at the molecular level. As these technologies continue to evolve, they may become more suitable for monitoring the efficacy of 223Ra therapy, potentially improving patient management and outcome prediction in mCRPC, as suggested by Shagera et al. [28]. In parallel, novel treatment strategies are being developed to optimize treatment sequences in mCRPC. For instance, the RALU study investigated the outcomes of lutetium radioligand therapy following 223Ra therapy [27].
In light of the retrospectively collected dataset, we recognize that significant advancements have since occurred, including wider adoption of PSMA-PET imaging and increased use of PSMA-targeted radioligand therapies. However, bone scintigraphy remains widely used, especially in settings where PET is not available, and PSMA therapy is not yet universally accessible.
5. Conclusions
In conclusion, while changes in the aBSI did not significantly correlate with changes in TTV, both parameters remained stable during therapy. Although aBSI did not demonstrate prognostic value for overall survival in this cohort, it remains a practicable and readily applicable surrogate for assessing osseous tumor burden, particularly in comparison to TTV. Given the limited sample size, the findings of this study should be interpreted with caution and require further studies in larger, prospective cohorts to clarify the potential role of aBSI in treatment monitoring and decision-making.
Author Contributions
Conceptualization, methodology, validation, visualization: H.I.; writing—original draft preparation: S.C.S., H.I., and M.J.Z.; writing—review and editing: A.G., A.T., M.U., A.D., F.J.G., C.D.A., C.G.S., R.A.W., and L.M.U.; supervision: H.I.; project administration: H.I. All authors have read and agreed to the published version of the manuscript.
Funding
Sophie C. Siegmund was supported by the BZKF.
Institutional Review Board Statement
This analysis was performed in compliance with the principles of the Declaration of Helsinki and was approved by the institutional ethics board of the LMU Munich (230-15, approved date: 17 April 2015).
Informed Consent Statement
Patient consent was waived by the institutional ethics board of the LMU Munich.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
R.A.W. has received speaker honoraria from Novartis/AAA and Pentixapharm, and has served on advisory boards work Novartis/AAA and Bayer. M.J.Z. has received speaker honoraria from Novartis, SIRTEX, and Terumo, and has served on an advisory board for Boston Scientific. C.D.A. has received speaker honoraria from Astellas and Ipsen, as well as travel support from Astellas, BMS, Eisai, Ipsen, Janssen, and MSD. L.M.U. has received funding from Novartis (speaker), Telix (consultant), and Astellas Pharma Inc. (speaker) outside of the submitted work.
Abbreviations
The following abbreviations are used in this manuscript:
| aBSI | Automated Bone Scan Index |
| ADT | Androgen deprivation therapy |
| AP | Alkaline phosphatase |
| BS | Bone Scan |
| CR | Complete response |
| mCRPC | Metastatic castration-resistant prostate cancer |
| PD | Progressive disease |
| PR | Partial Response |
| PSA | Prostate-specific antigen |
| Ra | Radium |
| STD | Standard deviation |
| SD | Stable disease |
| TTV | Total tumor volume |
| WBC | White blood cell |
References
- Saad, F.; Hotte, S.J. Guidelines for the management of castrate-resistant prostate cancer. Can. Urol. Assoc. J. 2010, 4, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, S.; Mercinelli, C.; Marandino, L.; Litterio, G.; Marchioni, M.; Schips, L. Metastatic Castration-Resistant Prostate Cancer: Insights on Current Therapy and Promising Experimental Drugs. Res. Rep. Urol. 2023, 15, 243–259. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Handkiewicz-Junak, D.; Andreeff, M.; Becherer, A.; Bockisch, A.; Fricke, E.; Geworski, L.; Heinzel, A.; Krause, B.J.; Krause, T.; et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 824–845. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef]
- Sindhu, K.K.; Nehlsen, A.D.; Stock, R.G. Radium-223 for Metastatic Castrate-Resistant Prostate Cancer. Precis. Radiat. Oncol. 2022, 12, 312–316. [Google Scholar] [CrossRef]
- Brito, A.E.; Etchebehere, E. Radium-223 as an Approved Modality for Treatment of Bone Metastases. Semin. Nucl. Med. 2020, 50, 177–192. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Kitajima, K.; Kuyama, J.; Kawahara, T.; Suga, T.; Otani, T.; Sugawara, S.; Kono, Y.; Tamaki, Y.; Seko-Nitta, A.; Ishiwata, Y.; et al. Assessing Therapeutic Response to Radium-223 with an Automated Bone Scan Index among Metastatic Castration-Resistant Prostate Cancer Patients: Data from Patients in the J-RAP-BSI Trial. Cancers 2023, 15, 2784. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Tsutsumi, S.; Kawahara, T.; Yasui, M.; Uemura, K.; Yoneyama, S.; Yokomizo, Y.; Hayashi, N.; Yao, M.; Uemura, H. Prognostic value of automated bone scan index for predicting overall survival among bone metastatic castration resistant prostate cancer patients treated with radium-223. BJUI Compass 2021, 2, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.H.D.; Osman, S.O.S.; Prise, K.M.; Campfield, C.; Turner, P.G.; Jain, S.F.P.; O’Sullivan, J.M.; Cole, A.J. A novel tool for improving the interpretation of isotope bone scans in metastatic prostate cancer. Br. J. Radiol. 2020, 93, 20200775. [Google Scholar] [CrossRef] [PubMed]
- Bayer. Xofigo® Basiswissen. Available online: https://www.fachinfo.de/fi/pdf/014972 (accessed on 27 July 2024).
- McNamara, M.A.; George, D.J. Pain, PSA flare, and bone scan response in a patient with metastatic castration-resistant prostate cancer treated with radium-223, a case report. BMC Cancer 2015, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.; Khan, N.; Phillips, C.; Briones, J.; Kapoor, A.; Zalewski, P.; Fleshner, N.E.; Chow, E.; Emmenegger, U. Prevalence and Prognostic Implications of PSA Flares during Radium-223 Treatment among Men with Metastatic Castration Resistant Prostate Cancer. J. Clin. Med. 2023, 12, 5604. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Matsubara, N.; Tabata, K.I.; Satoh, T.; Kamiya, N.; Suzuki, H.; Kawahara, T.; Uemura, H. Prostate-Specific Antigen Flare Phenomenon Induced by Abiraterone Acetate in Chemotherapy-Naive Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2017, 15, 320–325. [Google Scholar] [CrossRef]
- Conteduca, V.; Poti, G.; Caroli, P.; Russi, S.; Brighi, N.; Lolli, C.; Schepisi, G.; Romeo, A.; Matteucci, F.; Paganelli, G.; et al. Flare phenomenon in prostate cancer: Recent evidence on new drugs and next generation imaging. Ther. Adv. Med. Oncol. 2021, 13, 1758835920987654. [Google Scholar] [CrossRef]
- Kessler, E.R. What is behind the flare phenomenon? BJU Int. 2016, 118, 845–846. [Google Scholar] [CrossRef]
- Castello, A.; Macapinlac, H.A.; Lopci, E.; Santos, E.B. Prostate-specific antigen flare induced by (223)RaCl(2) in patients with metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2256–2263. [Google Scholar] [CrossRef]
- Gafita, A.; Fendler, W.P.; Hui, W.; Sandhu, S.; Weber, M.; Esfandiari, R.; Calais, J.; Rauscher, I.; Rathke, H.; Tauber, R.; et al. Efficacy and Safety of (177)Lu-labeled Prostate-specific Membrane Antigen Radionuclide Treatment in Patients with Diffuse Bone Marrow Involvement: A Multicenter Retrospective Study. Eur. Urol. 2020, 78, 148–154. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.E.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Vogelzang, N.J.; Bruland, Ø.; Kobina, S.; Wilhelm, S.; et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann. Oncol. 2017, 28, 1090–1097. [Google Scholar] [CrossRef]
- Anand, A.; Trägårdh, E.; Edenbrandt, L.; Beckman, L.; Svensson, J.H.; Thellenberg, C.; Widmark, A.; Kindblom, J.; Ullén, A.; Bjartell, A. Assessing Radiographic Response to (223)Ra with an Automated Bone Scan Index in Metastatic Castration-Resistant Prostate Cancer Patients. J. Nucl. Med. 2020, 61, 671–675. [Google Scholar] [CrossRef]
- Naito, M.; Ukai, R.; Hashimoto, K. Bone scan index can be a useful biomarker of survival outcomes in patients with metastatic castration-resistant prostate cancer treated with radium-223. Cancer Rep. 2019, 2, e1203. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Anand, A.; Edenbrandt, L.; Bondesson, E.; Bjartell, A.; Widmark, A.; Sternberg, C.N.; Pili, R.; Tuvesson, H.; Nordle, Ö.; et al. Phase 3 Assessment of the Automated Bone Scan Index as a Prognostic Imaging Biomarker of Overall Survival in Men with Metastatic Castration-Resistant Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 944–951. [Google Scholar] [CrossRef]
- Raimondi, A.; Sepe, P.; Claps, M.; Maccauro, M.; Aliberti, G.; Pagani, F.; Apollonio, G.; Randon, G.; Peverelli, G.; Seregni, E.; et al. Safety and activity of radium-223 in metastatic castration-resistant prostate cancer: The experience of Istituto Nazionale dei Tumori. Tumori 2020, 106, 406–412. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Coleman, R.E.; Michalski, J.M.; Nilsson, S.; O’Sullivan, J.M.; Parker, C.; Widmark, A.; Thuresson, M.; Xu, L.; Germino, J.; et al. Hematologic Safety of Radium-223 Dichloride: Baseline Prognostic Factors Associated with Myelosuppression in the ALSYMPCA Trial. Clin. Genitourin. Cancer 2017, 15, 42–52.e48. [Google Scholar] [CrossRef] [PubMed]
- Leisser, A.; Nejabat, M.; Hartenbach, M.; Duan, H.; Shariat, S.F.; Kramer, G.; Krainer, M.; Hacker, M.; Haug, A.R. Hematopoiesis is prognostic for toxicity and survival of (223)Radium treatment in patients with metastatic castration-resistant prostate cancer. Hell. J. Nucl. Med. 2017, 20, 157. [Google Scholar]
- Higano, C.S.; George, D.J.; Shore, N.D.; Sartor, O.; Miller, K.; Conti, P.S.; Sternberg, C.N.; Saad, F.; Sade, J.P.; Bellmunt, J.; et al. Clinical outcomes and treatment patterns in REASSURE: Planned interim analysis of a real-world observational study of radium-223 in metastatic castration-resistant prostate cancer. eClinicalMedicine 2023, 60, 101993. [Google Scholar] [CrossRef] [PubMed]
- Shagera, Q.A.; Gil, T.; Barraco, E.; Boegner, P.; Kristanto, P.; El Ali, Z.; Sideris, S.; Martinez Chanza, N.; Roumeguère, T.; Flamen, P.; et al. Evaluating response to radium-223 using 68Ga-PSMA PET/CT imaging in patients with metastatic castration-resistant prostate cancer. Ann. Nucl. Med. 2025, 39, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of Baseline and Post-Therapy 18F-FDG PET in the Prognostic Stratification of Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients Treated with Radium-223. Cancers 2020, 12, 31. [Google Scholar] [CrossRef]
- Unterrainer, L.M.; Calais, J.; Bander, N.H. Prostate-Specific Membrane Antigen: Gateway to Management of Advanced Prostate Cancer. Annu. Rev. Med. 2024, 75, 49–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).