Antiplatelet Resumption After Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
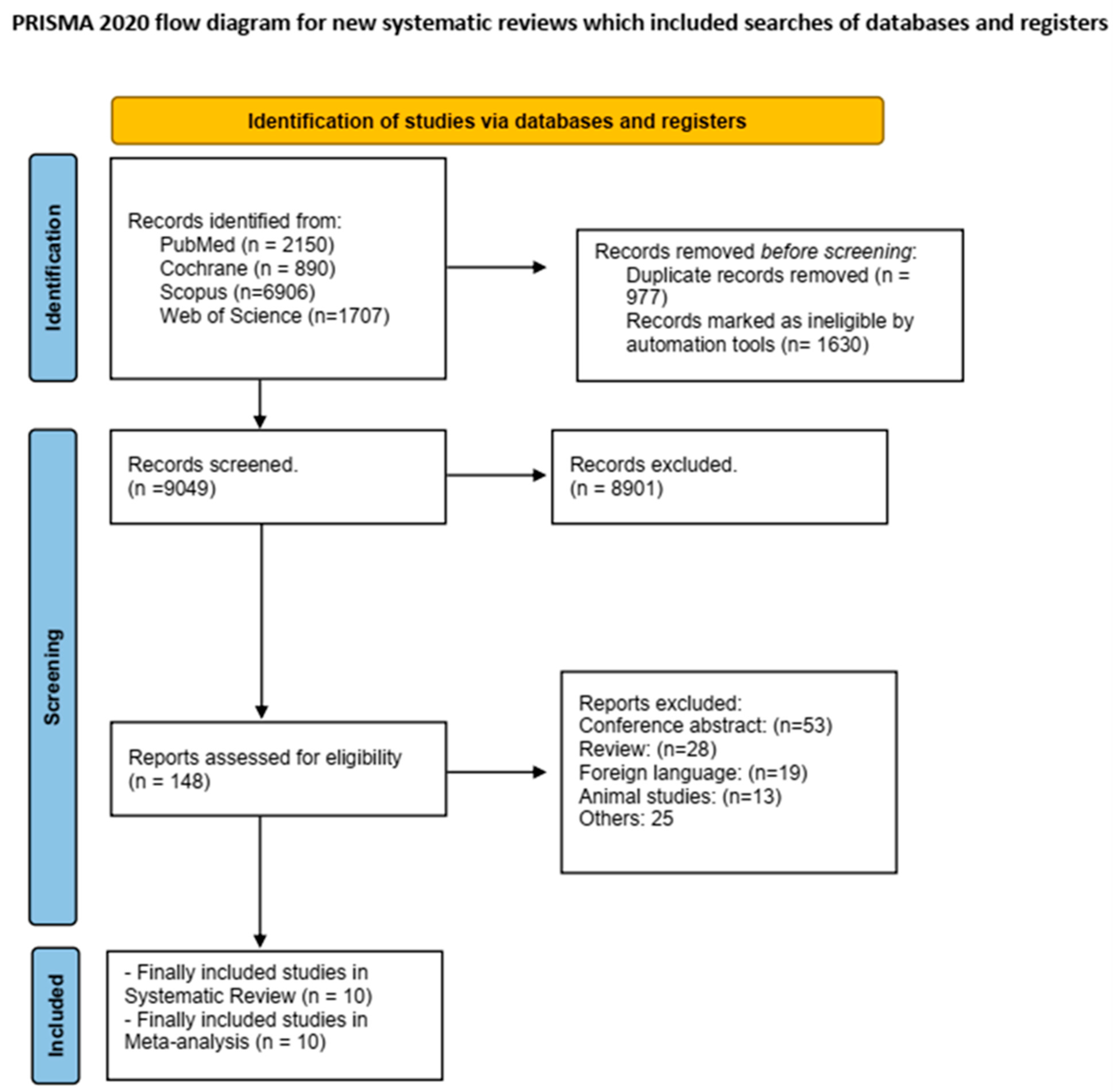
2. Materials and Methods
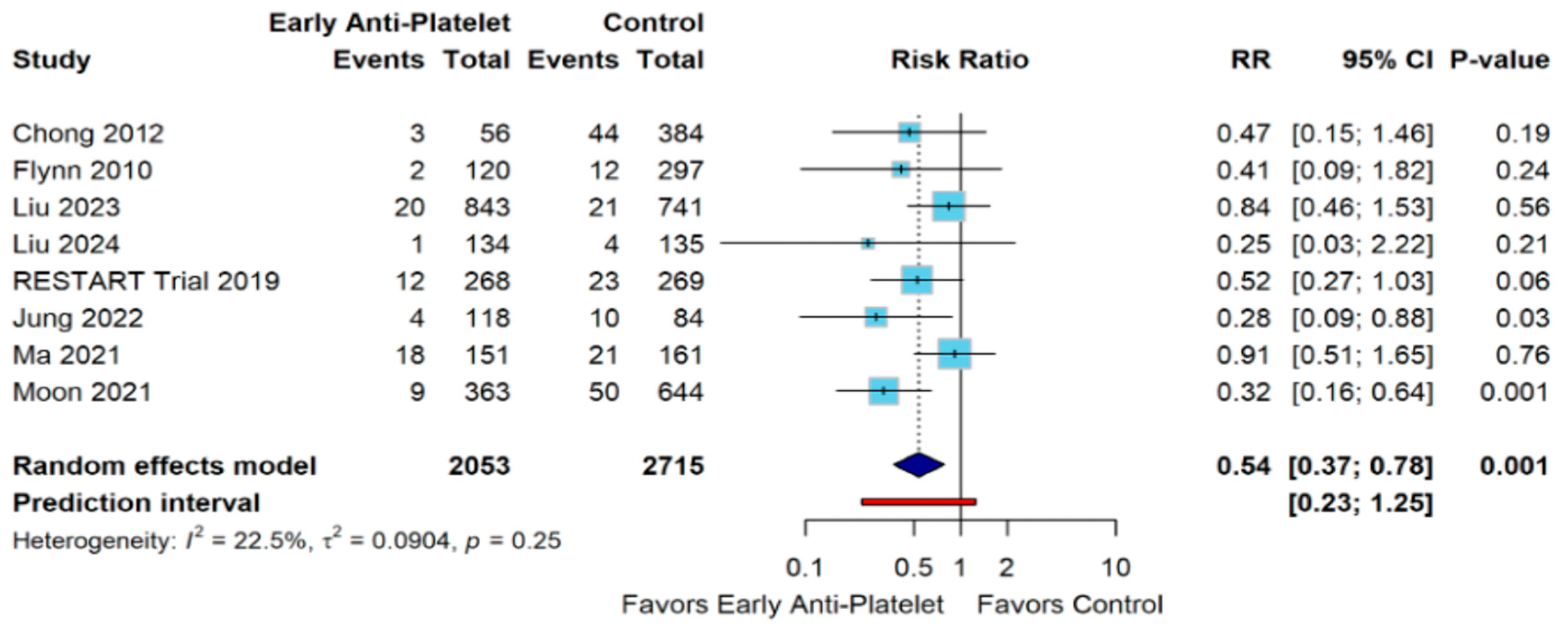
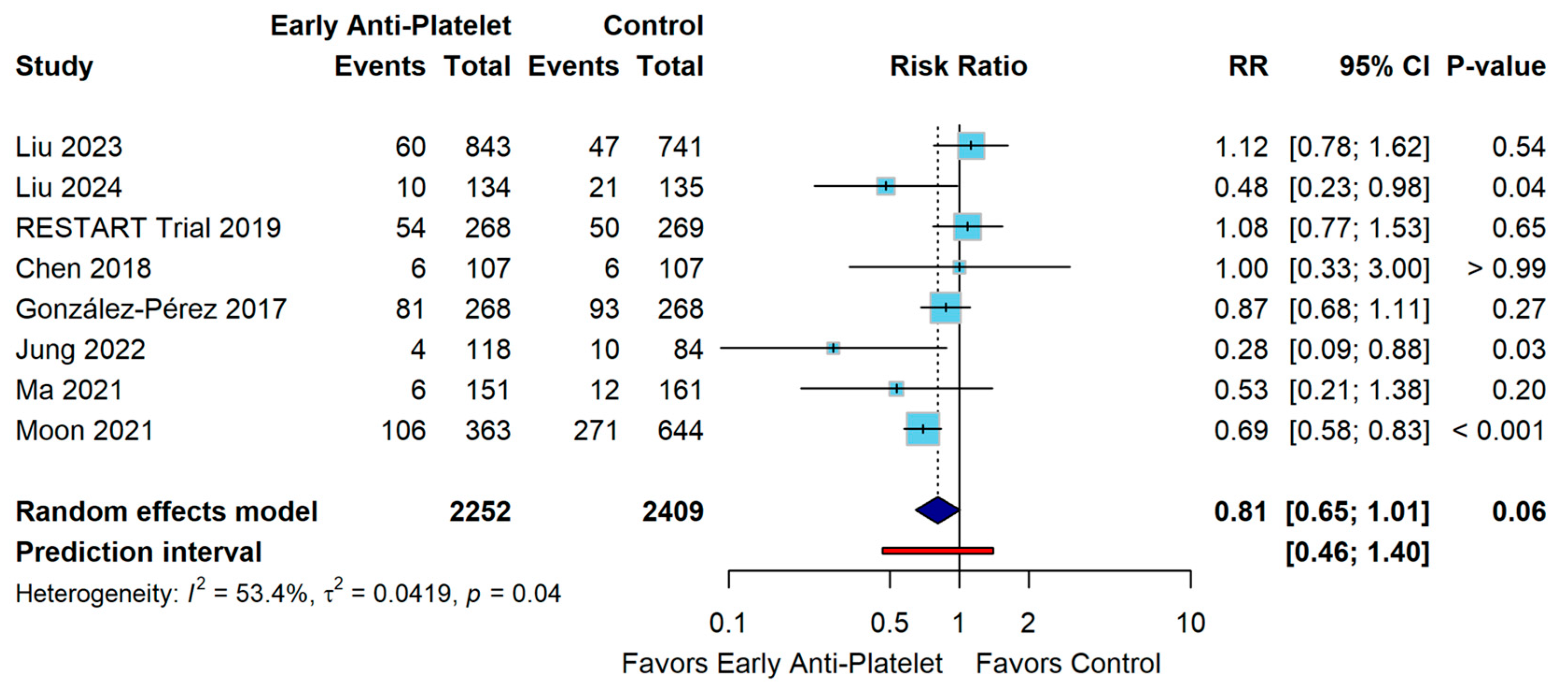
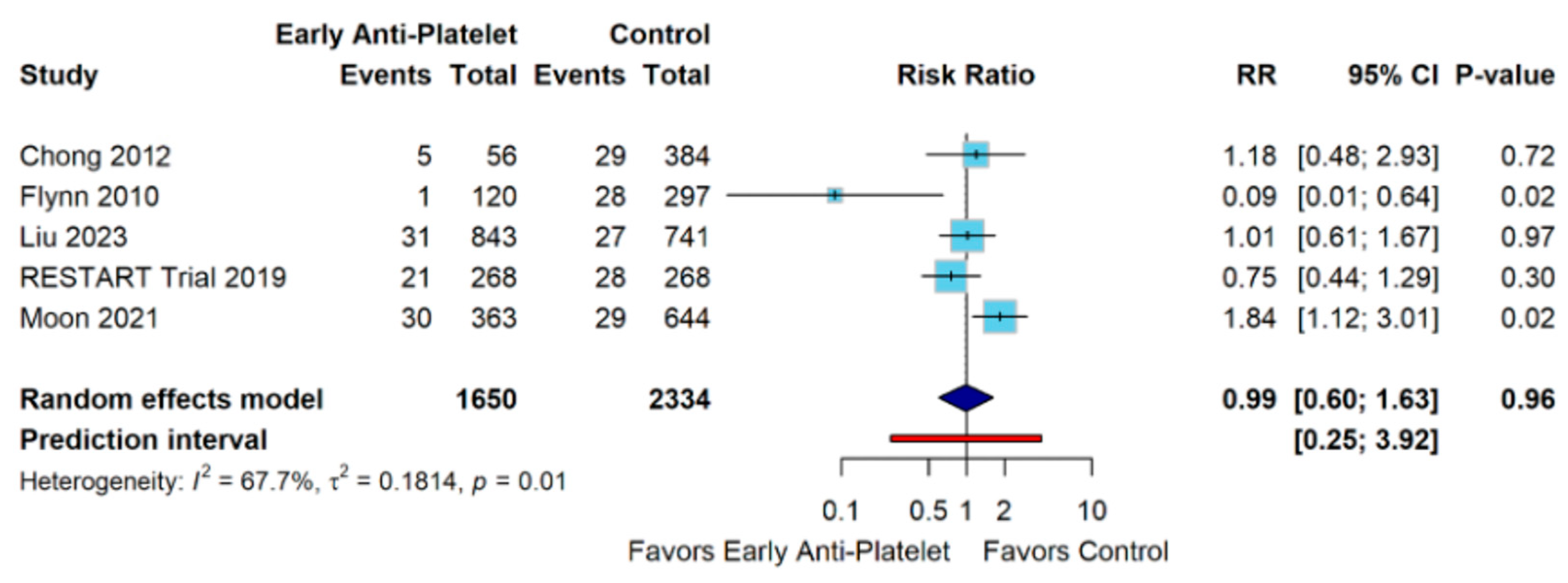
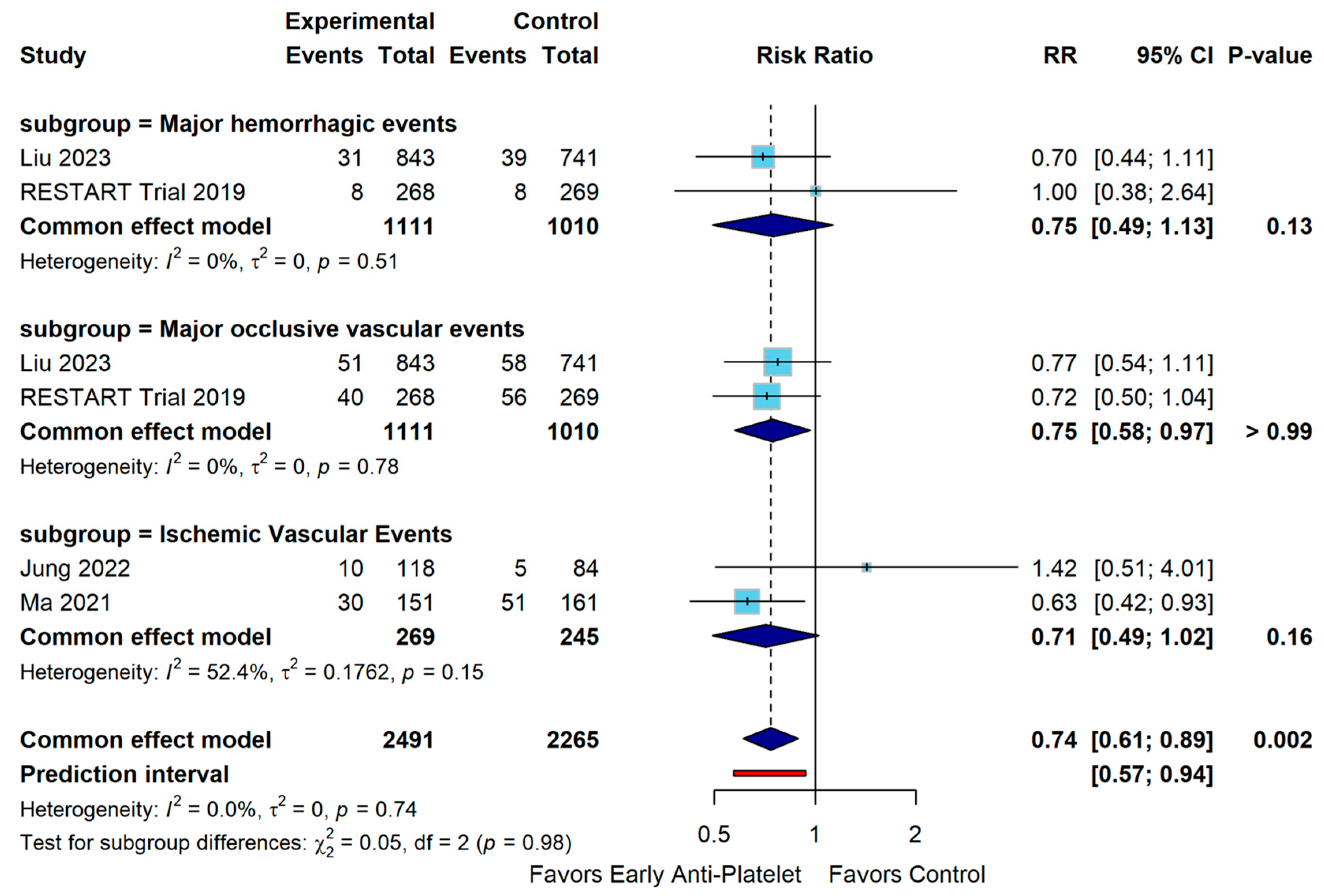
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toyoda, K.; Okada, Y.; Minematsu, K.; Kamouchi, M.; Fujimoto, S.; Ibayashi, S.; Inoue, T. Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology 2005, 65, 1000–1004. [Google Scholar] [CrossRef]
- Frontera, J.A.; Lewin III, J.J.; Rabinstein, A.A.; Aisiku, I.P.; Alexandrov, A.W.; Cook, A.M.; del Zoppo, G.J.; Kumar, M.A.; Peerschke, E.I.B.; Stiefel, M.F.; et al. Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit. Care 2016, 24, 6–46. [Google Scholar] [CrossRef]
- Roquer, J.; Ois, A.; Campello, A.R.; Gomis, M.; Munteis, E.; Conde, J.J.; Martínez-Rodríguez, J.E. Clustering of vascular risk factors and in-hospital death after acute ischemic stroke. J. Neurol. 2007, 254, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Schrag, M.; Kirshner, H. Management of Intracerebral Hemorrhage. J. Am. Coll. Cardiol. 2020, 75, 1819–1831. [Google Scholar] [CrossRef]
- Hill, M.D.; Silver, F.L.; Austin, P.C.; Tu, J.V. Rate of Stroke Recurrence in Patients With Primary Intracerebral Hemorrhage. Stroke 2000, 31, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Zia, E.; Engström, G.; Svensson, P.J.; Norrving, B.; Pessah-Rasmussen, H. Three-Year Survival and Stroke Recurrence Rates in Patients With Primary Intracerebral Hemorrhage. Stroke 2009, 40, 3567–3573. [Google Scholar] [CrossRef]
- Nielsen, P.B.; Larsen, T.B.; Skjøth, F.; Gorst-Rasmussen, A.; Rasmussen, L.H.; Lip, G.Y.H. Restarting Anticoagulant Treatment After Intracranial Hemorrhage in Patients with Atrial Fibrillation and the Impact on Recurrent Stroke, Mortality, and Bleeding: A Nationwide Cohort Study. Circulation 2015, 132, 517–525. [Google Scholar] [CrossRef]
- Parasram, M.; Parikh, N.S.; Merkler, A.E.; Falcone, G.J.; Sheth, K.N.; Navi, B.B.; Kamel, H.; Zhang, C.; Murthy, S.B. Risk of Mortality After an Arterial Ischemic Event Among Intracerebral Hemorrhage Survivors. Neurohospitalist 2022, 12, 19–23. [Google Scholar] [CrossRef]
- Viswanathan, A.; Rakich, S.M.; Engel, C.; Snider, R.; Rosand, J.; Greenberg, S.M.; Smith, E.E. Antiplatelet use after intracerebral hemorrhage. Neurology 2006, 66, 206–209. [Google Scholar] [CrossRef]
- Salman, R.A.-S.; Dennis, M.S.; Sandercock, P.A.G.; Sudlow, C.; Wardlaw, J.; Whiteley, W.; Murray, G.; Stephen, J.; Newby, D.; Sprigg, N.; et al. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomised, open-label trial. Lancet 2019, 393, 2613–2623. [Google Scholar] [CrossRef]
- Salman, R.A.-S.; Dennis, M.S.; Sandercock, P.A.G.; Sudlow, C.L.M.; Wardlaw, J.M.; Whiteley, W.N.; Murray, G.D.; Stephen, J.; Rodriguez, A.; Lewis, S.; et al. Effects of Antiplatelet Therapy After Stroke Caused by Intracerebral Hemorrhage: Extended Follow-up of the RESTART Randomized Clinical Trial. JAMA Neurol. 2021, 78, 1179. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.B.; Diaz, I.; Wu, X.; Merkler, A.E.; Iadecola, C.; Safford, M.M.; Sheth, K.N.; Navi, B.B.; Kamel, H. Risk of Arterial Ischemic Events After Intracerebral Hemorrhage. Stroke 2020, 51, 137–142. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, J.-G.; Kim, J.H. Antiplatelet Therapy after Intracerebral Hemorrhage and Subsequent Clinical Events: A 12-Year South Korean Cohort Study. Eur. Neurol. 2021, 84, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.T.C.; Fonville, A.F.; Al-Shahi Salman, R. Long-term prognosis after intracerebral haemorrhage: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.A.; Berge, E.; Bowditch, J.; Forfang, E.; Rønning, O.M.; Hankey, G.J.; Villanueva, E.; Al-Shahi Salman, R. Antithrombotic treatment after stroke due to intracerebral haemorrhage. Cochrane Database Syst. Rev. 2017, 5, CD012144. [Google Scholar] [CrossRef]
- Liberati, A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, 2700. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Chen, C.-J.; Ding, D.; Buell, T.J.; Testai, F.D.; Koch, S.; Woo, D.; Worrall, B.B. Restarting antiplatelet therapy after spontaneous intracerebral hemorrhage: Functional outcomes. Neurology 2018, 91, e26–e36. [Google Scholar] [CrossRef]
- Chong, B.-H.; Chan, K.-H.; Pong, V.; Lau, K.-K.; Chan, Y.-H.; Zuo, M.-L.; Lui, W.-M.; Leung, G.K.-K.; Lau, C.-P.; Tse, H.-F.; et al. Use of aspirin in Chinese after recovery from primary intracranial haemorrhage. Thromb. Haemost. 2012, 107, 241–247. [Google Scholar] [CrossRef]
- Flynn, R.W.V.; MacDonald, T.M.; Murray, G.D.; MacWalter, R.S.; Doney, A.S.F. Prescribing Antiplatelet Medicine and Subsequent Events After Intracerebral Hemorrhage. Stroke 2010, 41, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, A.; Gaist, D.; De Abajo, F.; Sáez, M.; García Rodríguez, L. Low-Dose Aspirin after an Episode of Haemorrhagic Stroke Is Associated with Improved Survival. Thromb. Haemost. 2017, 117, 2396–2405. [Google Scholar] [CrossRef][Green Version]
- Jung, N.Y.; Cho, J. Clinical effects of restarting antiplatelet therapy in patients with intracerebral hemorrhage. Clin. Neurol. Neurosurg. 2022, 220, 107361. [Google Scholar] [CrossRef]
- Liu, C.-H.; Wu, Y.-L.; Hsu, C.-C.; Lee, T.-H. Early Antiplatelet Resumption and the Risks of Major Bleeding After Intracerebral Hemorrhage. Stroke 2023, 54, 537–545. [Google Scholar] [CrossRef]
- Liu, Q.; Mo, S.; Wu, J.; Tong, X.; Wang, K.; Chen, X.; Chen, S.; Guo, S.; Li, X.; Li, M.; et al. Safety and efficacy of early versus delayed acetylsalicylic acid after surgery for spontaneous intracerebral haemorrhage in China (E-start): A prospective, multicentre, open-label, blinded-endpoint, randomised trial. Lancet Neurol. 2024, 23, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, D.; Niu, S.; Zhao, W.; Song, X.; Li, C.; Zhou, L.; Ma, J.; Jia, W. Low-dose antiplatelet therapy survey after intracerebral hemorrhage in China: A retrospective hospital-based study. Neurosurg. Rev. 2021, 44, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Falcone, G.J.; Rosand, J. Aspirin should be discontinued after lobar intracerebral hemorrhage. Stroke 2014, 45, 3151–3152. [Google Scholar] [CrossRef]
- Charidimou, A.; Imaizumi, T.; Moulin, S.; Biffi, A.; Samarasekera, N.; Yakushiji, Y.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.-C.; et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: A meta-analysis. Neurology 2017, 89, 820–829. [Google Scholar] [CrossRef]
- Biffi, A.; Halpin, A.; Towfighi, A.; Gilson, A.; Busl, K.; Rost, N.; Smith, E.; Greenberg, M.; Rosand, J.; Viswanathan, A. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010, 75, 693–698. [Google Scholar] [CrossRef]
- Teo, K.C.; Lau, G.K.; Mak, R.H.; Leung, H.Y.; Chang, R.S.; Tse, M.Y.; Lee, R.; Leung, G.K.; Ho, S.L.; Cheung, R.T.; et al. Antiplatelet Resumption after Antiplatelet-Related Intracerebral Hemorrhage: A Retrospective Hospital-Based Study. World Neurosurg. 2017, 106, 85–91. [Google Scholar] [CrossRef]
- Li, L.; Murthy, S.B. Cardiovascular Events After Intracerebral Hemorrhage. Stroke 2022, 53, 2131–2141. [Google Scholar] [CrossRef]
- Devereaux, P.; Mrkobrada, M.; Sessler, D.I.; Leslie, K.; Alonso-Coello, P.; Kurz, A.; Villar, J.C.; Sigamani, A.; Biccard, B.M.; Meyhoff, C.S.; et al. Aspirin in patients undergoing noncardiac surgery. N. Engl. J. Med. 2014, 370, 1494–1503. [Google Scholar] [CrossRef]
- Ding, X.; Liu, X.; Tan, C.; Yin, M.; Wang, T.; Liu, Y.; Mo, L.; Wei, X.; Tan, X.; Deng, F.; et al. Resumption of antiplatelet therapy in patients with primary intracranial hemorrhage-benefits and risks: A meta-analysis of cohort studies. J. Neurol. Sci. 2018, 384, 133–138. [Google Scholar] [CrossRef]
- Cochrane, A.; Chen, C.; Stephen, J.; Rønning, O.M.; Anderson, C.S.; Hankey, G.J.; Salman, R.A.-S. Antithrombotic treatment after stroke due to intracerebral haemorrhage. Cochrane Database Syst. Rev. 2023, 1, CD012144. [Google Scholar] [CrossRef] [PubMed]
- Keir, S.L.; Wardlaw, J.M.; Sandercock, P.A.G.; Chen, Z. Antithrombotic Therapy in Patients with Any Form of Intracranial Haemorrhage: A Systematic Review of the Available Controlled Studies. Cerebrovasc. Dis. 2002, 14, 197–206. [Google Scholar] [CrossRef]
- Al-Shahi Salman, R.; Dennis, M.S. Antiplatelet therapy may be continued after intracerebral hemorrhage. Stroke 2014, 45, 3149–3150. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yu, S.; Zhang, Q.; Yu, T.; Liu, Y.; Sun, Z.; Zhao, M.; Wang, W.; Zhao, J.Z. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of intracerebral haemorrhage. Stroke Vasc. Neurol. 2020, 5, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Intercollegiate Stroke Working Party in collaboration with the Scottish Intercollegiate Guidelines Network (SIGN) and the National Clinical Programme for Stroke Ireland. National Clinical Guideline for Stroke for the United Kingdom and Ireland; Intercollegiate Stroke Working Party: London, UK, 2023. [Google Scholar]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C.; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, E282–E361. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Patrice Lindsay, M.; Castellucci, L.A.; Cayley, A.; Crowther, M.; de Wit, K.; English, S.W.; Hoosein, S.; Huynh, T.; Kelly, M.; et al. Canadian stroke best practice recommendations: Management of Spontaneous Intracerebral Hemorrhage, 7th Edition Update 2020. Int. J. Stroke 2021, 16, 321–341. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, S.Y.; Alsheikh, S.A.; Almousa, D.S.; Alsedrah, S.S.A.; Alshammari, N.M.; Elsayed, M.M.; AlShamrani, R.A.H.; Bellahwal, M.A.Y.; Alnwiji, A.; Albar, R.A.; et al. Antiplatelet Resumption After Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 1780. https://doi.org/10.3390/diagnostics15141780
Alharthi SY, Alsheikh SA, Almousa DS, Alsedrah SSA, Alshammari NM, Elsayed MM, AlShamrani RAH, Bellahwal MAY, Alnwiji A, Albar RA, et al. Antiplatelet Resumption After Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(14):1780. https://doi.org/10.3390/diagnostics15141780
Chicago/Turabian StyleAlharthi, Sarah Yahya, Sarah Abdulaziz Alsheikh, Dawood Salman Almousa, Saud Samer A. Alsedrah, Nouf Mohammed Alshammari, Mariam Mostafa Elsayed, Rahaf Ali Hamed AlShamrani, Mohammed Ahmed Yaslam Bellahwal, Abdulrahman Alnwiji, Raed A. Albar, and et al. 2025. "Antiplatelet Resumption After Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis" Diagnostics 15, no. 14: 1780. https://doi.org/10.3390/diagnostics15141780
APA StyleAlharthi, S. Y., Alsheikh, S. A., Almousa, D. S., Alsedrah, S. S. A., Alshammari, N. M., Elsayed, M. M., AlShamrani, R. A. H., Bellahwal, M. A. Y., Alnwiji, A., Albar, R. A., & Mohamed, A. M. A. (2025). Antiplatelet Resumption After Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Diagnostics, 15(14), 1780. https://doi.org/10.3390/diagnostics15141780