Abstract
Artificial intelligence (AI) is reshaping radiological practice, with recent advancements in natural language processing (NLP), large language models (LLMs), and chatbot technologies opening new avenues for clinical integration. These AI-driven conversational agents have demonstrated potential in streamlining patient triage, optimizing imaging protocol selection, supporting image interpretation, automating radiology report generation, and improving communication among radiologists, referring physicians, and patients. Emerging evidence also highlights their role in decision-making, clinical data extraction, and structured reporting. While the clinical adoption of chatbots remains limited by concerns related to data privacy, model robustness, and ethical oversight, ongoing developments and regulatory efforts are paving the way for responsible implementation. This review provides a critical overview of the current and emerging applications of chatbots in radiology, evaluating their capabilities, limitations, and future directions for clinical and research integration.
1. Introduction
The integration of artificial intelligence (AI) into radiology has markedly transformed various aspects of clinical workflow, from image acquisition to interpretation [1,2,3,4,5]. While much of the existing research has focused on imaging-based applications, recent advancements in natural language processing (NLP) and large language models (LLMs) have introduced new frontiers. Of particular interest is the emergence of chatbots, intelligent conversational agents capable of generating human-like text, which are increasingly being explored for their utility in radiology [6,7]. Although several reviews have previously addressed this topic, the rapid pace of research and continuous development of new models highlight the need for ongoing updates. This literature review aims to provide an in-depth and current overview of the existing and emerging applications of chatbot technologies, offering a comprehensive understanding of the technological landscape and evolving trends within the domain of radiology.
2. Literature Search Strategy
A narrative review was conducted to explore the use of chatbots and large language models in the field of radiology. Literature searches were performed using four major databases: PubMed, Google Scholar, Scopus, and Web of Science. The search covered publications from 2017 to 2025, using the following keywords: “chatbot” OR “large language model” OR “GPT” AND “radiology” OR “imaging”.
Eligible studies included peer-reviewed articles describing clinical or research applications of chatbots in radiology, encompassing both general discussions on large language models and studies focused on specific subspecialties within imaging. Non-English language articles and those lacking full-text access were excluded from the review.
All selected articles were critically assessed and synthesized to provide a comprehensive overview of current chatbot applications in radiology, their limitations, and potential future directions.
3. Natural Language Processing and Large Language Models
The NLP allows computers to understand and generate human language, forming the foundation for contemporary chatbot technologies [8]. The field has evolved from rule-based systems to neural-network-driven conversational agents.
The journey began in 1966 with ELIZA, a program based on scripted rules that used pattern matching, followed by incremental developments in conversational models such as PARRY, Jabberwacky, and ALICE, which, despite advances, lacked genuine contextual comprehension [9,10].
The emergence of voice-activated virtual assistants such as Apple’s Siri, Amazon Alexa, Microsoft Cortana, and Google Assistant represented a shift towards AI-powered personal assistants capable of addressing more complex queries [11,12]. A major leap occurred in 2017 with the introduction of the transformer architecture in the seminal paper “Attention Is All You Need” [13], leading to the creation of the generative pretrained transformer (GPT) models, which revolutionized the NLP landscape [14].
The public release of ChatGPT (OpenAI) in 2022, based on GPT-3.5, demonstrated the broad applicability of LLMs, from summarizing documents to generating executable code [15]. In 2023, the introduction of GPT-4 further improved performance, with enhancements in reasoning, factual accuracy, and multimodal capabilities (image and text processing), spurring competition and leading to the release of comparable models such as Claude 3 Opus (Anthropic), Gemini 1.5 Pro (Google), and GPT-4o (OpenAI) [16,17].
While these general-purpose models have shown impressive capabilities, there is growing interest in domain-specific LLMs tailored for healthcare. Several models, including BioGPT, PubMedBERT, GatorTron, Med-PaLM, and LLaVA-Med, have been developed to serve clinical and research needs more effectively [18]. Table 1 presents a terminology box summarizing the main LLM models referenced throughout this review.

Table 1.
Summary of key foundation models and tools used in radiology and biomedical domains, highlighting their developers, core capabilities, and clinical relevance.
4. Current Applications in Radiology
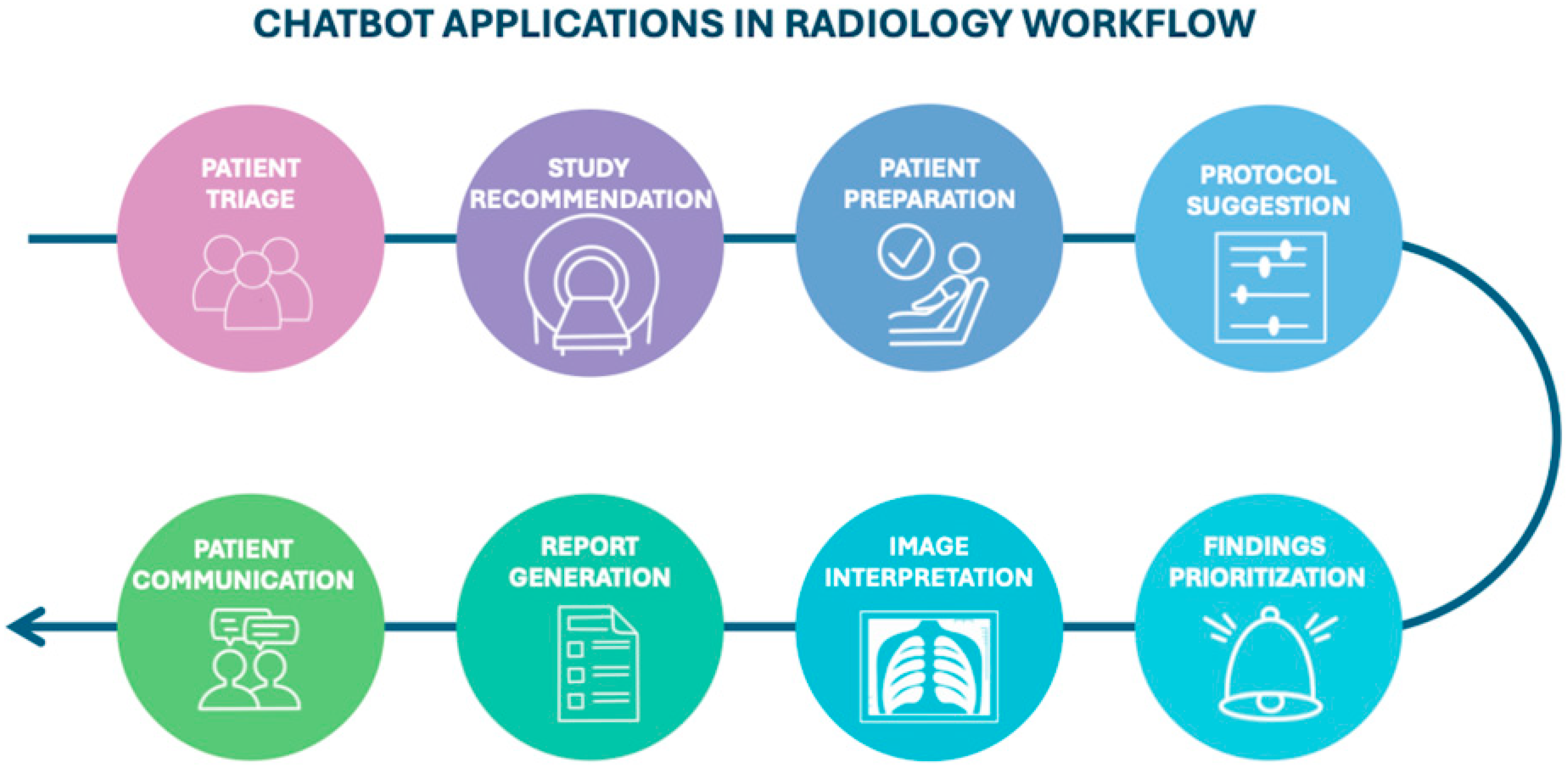
The increasing availability of chatbots and LLMs is accelerating their adoption in clinical radiology (Figure 1), where they can support a range of tasks.
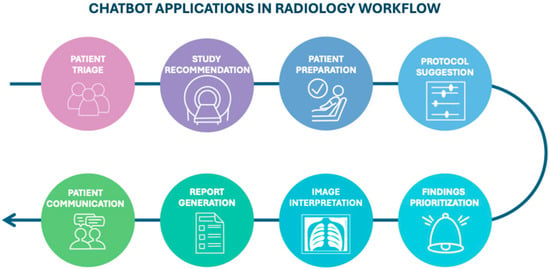
Figure 1.
Diagram illustrating the potential stages of the radiology workflow where chatbots could be integrated, from patient triage to communication.
4.1. Before Imaging: From Study Recommendation to Protocoling
The early phases of radiologic assessment, including study justification and protocol selection, are often time-intensive and vulnerable to variability. Radiologists frequently encounter incomplete or inappropriate referrals, leading to delays and inefficiencies. In this context, LLMs offer a promising tool for supporting imaging decision-making for clinicians [19].
Rau et al. demonstrated that chatbots can accurately recommend imaging modalities, performing comparably to radiologists while reducing both the time (2–8 min vs. 22–73 min) and cost of decision-making (both p ≤ 0.003) [20]. This functionality is particularly valuable in high-throughput settings such as emergency departments, where timely imaging decisions are critical [21].
Chatbots also serve as a patient education resource, offering tailored explanations of imaging indications, risks, and benefits, especially in complex scenarios such as imaging during pregnancy, contrast administration in renal impairment, and interventional procedures. While LLMs effectively convey accurate information, their simplification capabilities are currently limited and may require patients to possess a baseline level of health education [22,23,24,25].
Another emerging application is protocol selection optimization. Gertz et al. reported that GPT-4 achieved 84% agreement (95% CI: 75.3–90.6) with expert radiologists in choosing the correct imaging modality, region, contrast use and phase [26]. Moreover, AI can enhance the completeness of clinical data in requisition forms and assist in automated protocol recommendation, thereby improving the quality of radiologic interpretation [27].
4.2. Interpretation, Data Extraction and Diagnostic Capacity
The application of AI-powered chatbots in radiology has proven beneficial across several domains, including identifying radiological imaging findings, enhancing population screening programs, and reducing diagnostic errors [28,29]. ChatGPT has emerged as a potential tool for clinical decision support, particularly within neuroradiology and oncological imaging.
Horiuchi et al. investigated the use of ChatGPT-4.0 in neuroradiology imaging across various diseases. Their results suggested that integrating ChatGPT-4.0 into clinical workflows could enhance diagnostic accuracy, especially in metabolic, cerebrovascular, and neurodegenerative conditions. These improved outcomes were attributed to the presence of distinctive keywords in patient histories and imaging patterns. In contrast, diagnostic performance was lower in central nervous system tumors, likely due to their histopathological diversity and the rarity of some subtypes [30]. Notably, the study’s limitations included ChatGPT’s inability to directly process medical images and the absence of a direct radiologist comparison.
To address these limitations, Mitsuyama et al. evaluated GPT-4 in real-world clinical scenarios, focusing on brain tumor diagnosis. Their findings indicated that GPT-4 performed comparably to experienced neuroradiologists in providing differential diagnoses based on preoperative MRI reports, suggesting its potential as a supportive diagnostic tool for clinicians [31].
With the advent of GPT-4V, a vision-enabled model capable of both textual and visual analysis, future applications may extend into image-based diagnostics. A recent exploratory study by Kelly et al. examined the zero-shot performance of GPT-4V in detecting multiple sclerosis progression, comparing it to state-of-the-art vision models such as U-Net and Vision Transformer (ViT). Zero-shot learning (ZSL) is a machine learning paradigm that empowers models to identify novel objects, concepts, or diseases without having been explicitly trained on examples of those specific categories [32]. The study focused on identifying new white matter hyperintensities in fluid-attenuated inversion recovery (FLAIR) brain images. However, GPT-4V underperformed compared to the dedicated vision models, due to its inconsistencies and lack of interpretability, including misclassified cases and overly cautious non-answers [33].
Despite its multimodal potential, studies consistently indicate that GPT-4V’s diagnostic sensitivity and specificity remain inferior to domain-specific models [34,35].
Most recently, Koyun et al. evaluated the diagnostic performance of ChatGPT-4o for the detection and classification of intracranial hemorrhages on non-contrast CT. Their study highlights the potential of a general-purpose LLM in a specific medical imaging field, and its ability to classify hemorrhage type, stage, anatomical location, and associated findings. However, the model struggled with detecting certain hemorrhage types, such as subarachnoid hemorrhage [36].
Progress has also been made in exploring LLMs for oncological applications. A study published in Patient Education and Counseling evaluated ChatGPT’s alignment with the United States Preventive Services Task Force (USPSTF) guidelines for breast and prostate cancer screening. While responses generally adhered to evidence-based recommendations, discrepancies emerged in scenarios involving older patients, emphasizing the continued necessity of human oversight and clinical judgement [37].
Beyond screening recommendations, the use of large language models, particularly focusing on transformer-based models as Bidirectional Encoder Representations from Transformers (BERT) have demonstrated robust performance in clinical information extraction [38]. Hu et al. developed a deep learning pipeline for automated extraction of lung cancer staging parameters from CT reports based on TNM classification. Their model showed high accuracy in identifying tumor size, lymph node involvement, and metastatic status, streamlining the staging process and reducing manual workload [39].
Similarly, Zhou et al. [40] developed CancerBERT, a fine-tuned BERT model enriched with oncology-specific vocabulary. It reliably extracted eight key attributes, including tumor grade, histological type, and stage, thereby facilitating structured staging for breast cancer patients [40].
Building on these developments, Fink et al. [41] evaluated GPT-4’s accuracy in extracting oncological information from unstructured CT reports in lung cancer. When guided by predefined prompts, GPT-4 demonstrated improved performance over previous versions of ChatGPT, particularly in identifying metastatic spread, and tracking disease progression [41].
However, persistent challenges with hallucinations and referencing accuracy have limited LLM adoption for high-stakes clinical tasks. Recent developments in retrieval-augmented generation (RAG) models aim to address these concerns. By linking model output to externally validated knowledge sources, RAG-enhanced LLMs offer improved factual accuracy and contextual grounding [42]. Tozuka et al. [42] evaluated NotebookLM, a RAG-based LLM, for lung cancer staging. Using national staging guidelines as the retrieval base, NotebookLM staged 100 hypothetical cases from CT findings, outperforming GPT-4o and underscoring its potential for structured image-based decision-making [42].
4.3. Chatbots and Report Generation
A key application of LLMs in radiology is their potential to enhance clinical workflows by supporting radiology report generation. These reports typically comprise three major components:
- The “indication”, which outlines the clinical context and rationale for the examination.
- The “findings section”, which documents the radiologist’s observations from imaging data.
- The “impression”, which synthetizes key findings to inform potential diagnoses or management recommendations.
Radiologists must integrate imaging findings with clinical information to generate accurate and actionable impressions. Recent studies have demonstrated that LLMs, such as ChatGPT, can generate diagnostic impressions when provided with imaging findings, either in the form of structured data or narrative text.
LLMs can potentially reduce the cognitive workload associated with drafting diagnostic impressions, particularly in complex cases involving ambiguous or overlapping imaging findings. Impression generation is a crucial step in radiologic interpretation, guiding downstream clinical decision-making. Conventionally, this task is completed manually by the radiologist, which is time-intensive and prone to variability.
Although fine-tuned models can automate impression drafting, they typically require extensive annotated datasets—resources that are often scarce in subspecialties such as interventional, oncological, or neuroradiology. To address this, Sun et al. [43] systematically evaluated GPT-4’s performance in zero-shot impression generation. The model’s outputs were compared with radiologist-authored impressions using four key criteria: coherence, comprehensiveness, factual accuracy, and potential for harm. Results indicated that while GPT-4 exhibits promising capabilities, it does not yet achieve parity with human-generated impressions, underscoring the need for further model refinement and clinical validation before routine use [43].
Another active area of investigation concerns the capacity of LLMs to deliver tailored clinical recommendations. A study published in European Radiology compared ChatGPT’s performance in imaging appropriateness with that of a validated clinical decision support system (CDSS), the ESR iGuide. While ChatGPT demonstrated reasonable accuracy in recommending imaging tests, it lacked integration with electronic health records and could not provide fully personalized advice. Its reliance on statistical pattern recognition, rather than individualized reasoning, limits its current clinical applicability in this domain [44].
Similarly, Truhn et al. [45] explored GPT-4’s performance in recommending orthopedic treatments based on MRI findings. While the model generated largely appropriate recommendations, it occasionally failed to grasp the urgency or clinical context, instead producing generalized suggestions of limited utility in nuanced cases [45]. In pediatric radiology, LLMs like ChatGPT have been considered as adjunct tools to support decision-making, particularly where imaging guidelines are complex or age-specific. In oncology, a recent study in npj Breast Cancer evaluated ChatGPT-3.5 as a virtual participant in breast tumor board discussions. While the model was effective at summarizing case information and explaining the rationale, it was less proficient in issuing clinical recommendations, reinforcing the current role of LLMs as supportive, rather than autonomous tools [46].
Structured Reporting
Structured reporting represents another promising application for LLMs in radiology. It enhances clarity, facilitates data retrieval, and promotes standardization of terminology across institutions. However, transitioning large volumes of free-text reports into structured templates remains a logistical challenge.
Adams et al. [47] explored the feasibility of using GPT-4 to automate this process across multiple imaging modalities (CT, MRI, chest radiography) and languages. Their findings suggest that GPT-4 can reliably extract relevant content and reformat it according to predefined templates, although challenges related to language variability and contextual nuance require further investigation [47].
In a complementary study, Grewal et al. [48] evaluated GPT-4’s ability to generate radiology report templates through NLP. The model successfully extracted demographic data, clinical history, anatomical details, and imaging findings from narrative reports and translated them into structured formats. This capability has implications not only for standardizing reporting practices but also for supporting educational initiatives and decision-making, provided that ethical and legal considerations are carefully addressed [48].
4.4. Role of Chatbots in Patient Communication
Radiology reports are typically written using technical and specialized terminology intended for healthcare professionals, which can create a significant communication barrier for patients. This complexity often leads to confusion, anxiety, and misinterpretation, particularly when patients access their imaging reports through electronic health record portals prior to consultation with their referring physician.
LLMs have demonstrated the potential to address this challenge by translating radiological findings into layperson-friendly summaries. These AI-generated interpretations can enhance patient understanding by conveying key information with high levels of factual accuracy and completeness [49,50]. By improving health literacy and reducing ambiguity, LLMs may facilitate more effective communication between patients and healthcare providers and foster greater patient engagement in clinical decision-making.
Nevertheless, significant concerns remain regarding the reliability of AI-generated explanations. Inaccurate or misleading simplifications can have serious consequences, potentially leading to inappropriate patient actions, emotional distress, or the erosion of trust in the healthcare system. Therefore, ensuring that AI-generated content maintains clinical accuracy and conveys nuance appropriately is essential for safe integration into patient-facing communication tools [51,52,53].
A study by Jeblick et al. underscores these risks, revealing that approximately one-third of simplified radiology report summaries contained factual inaccuracies or misrepresentations [54].
These findings highlight the critical need for careful validation, human oversight, and transparent risk communication when deploying LLMs for patient education purposes.
Table 2 provides a comprehensive overview of the role of advanced LLMs across the various stages of the radiology workflow, detailing their potential applications while also critically addressing the limitations associated with each specific use case.

Table 2.
Benefits and limitations of advanced LLMs across key radiology workflow stages.
5. Performance of Contemporary Chatbots
Developing a dedicated specialist AI model for each clinical task is often impractical due to the wide-ranging complexity of healthcare environments and the resource-intensive nature of model development. Although relatively few comparative studies of LLMs have been conducted to date, early evidence reveals important performance distinctions across platforms.
In a study evaluating responses to non-expert-level questions on lung cancer, including topics such as prevention, screening, and basic radiology terminology, ChatGPT-3.5 demonstrated superior accuracy compared to Google’s Bard. However, neither model achieved full consistency or reliability across all tasks, underscoring ongoing limitations in generalist chatbot performance [55]. Similarly, in tasks involving radiology report simplification, both ChatGPT-3.5 and ChatGPT-4 outperformed Bard and Bing Chat, particularly in producing summaries that were more coherent, accurate, and accessible to lay readers [56].
In cardiovascular imaging, Silbergleit et al. [57] conducted a comparative analysis of four general-purpose LLMs—ChatGPT-3.5, ChatGPT-4, Google Gemini, and Gemini Advanced—in the automated generation of coronary artery disease reporting and data system (CAD-RADS) scores and recommendations based on coronary CT angiography (CCTA) reports. CAD-RADS scoring is essential for standardized risk assessment and clinical decision-making in cardiac imaging. Among the evaluated models, ChatGPT-4 demonstrated the highest accuracy and concordance with radiologist-generated scores, followed by Gemini Advanced. Although ChatGPT-3.5 showed faster response times, its accuracy was inferior to newer models. These findings reinforce the clinical potential of LLMs while emphasizing the continued need for large, high-quality annotated datasets and significant computational resources for optimal performance [57].
In neuroimaging, Koyun et al. [28] evaluated Claude 3.5 Sonnet in the detection of acute ischemic stroke using diffusion-weighted imaging (DWI). Claude 3.5 outperformed ChatGPT-4 in both lesion localization and agreement with expert radiologists, highlighting the rapid advancements and nuanced capabilities of emerging LLMs in image interpretation tasks [28].
In oncology, a recent study explored the use of GPT-4 to automate TNM staging for lung cancer based on unstructured CT reports. Using advanced prompt engineering to guide GPT-4 according to formal staging criteria, the model significantly outperformed GPT-3.5 Turbo, demonstrating higher accuracy and consistency. These findings illustrate the expanding role of LLMs in streamlining complex oncological workflows and supporting data abstraction for clinical decision-making [58].
It is important to acknowledge the pace at which LLMs are evolving, making it difficult to predict which platform may emerge as the most suitable or dominant in a clinical setting [39]. Given the heterogeneity of radiological tasks and their varying degrees of complexity, radiologists must critically assess whether to deploy general-purpose LLMs, domain-adapted generalist models, or bespoke specialist systems. Such strategic selection is essential to ensure optimal model performance, safety, and clinical relevance.
6. Limitations
LLMs have demonstrated strong performance when guided by structured prompts, serving as virtual assistants for radiologists and other healthcare professionals in a variety of tasks. However, their integration into clinical decision-making continues to be limited by several critical shortcomings.
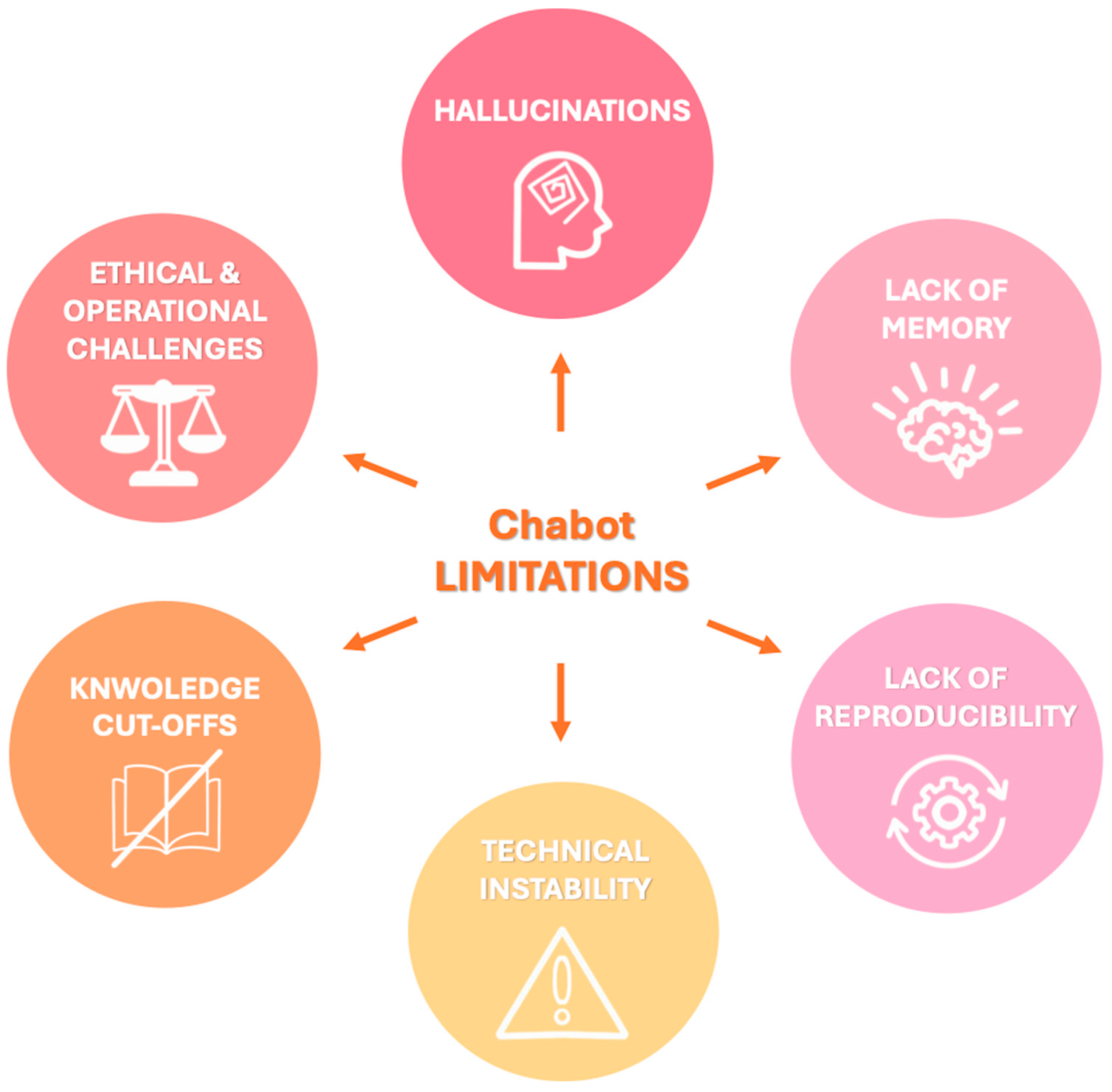
The principal limitations can be summarized as follows and are represented schematically in Figure 2:
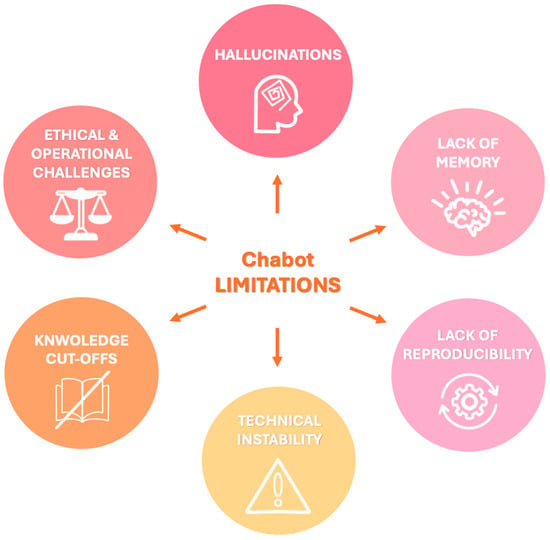
Figure 2.
Visual summary of key limitations associated with chatbot integration in radiology, that should be carefully considered to ensure safe and effective implementation in clinical workflows.
- Hallucinations: LLMs may generate factually incorrect or fabricated content, a phenomenon known as “hallucination” since they generate responses based on patterns learned during training rather than verified data. For example, when asked about non-existent Lung-RADS categories, both ChatGPT and Bard gave confident but incorrect answers rather than indicating the absence of such categories [59]. The dissemination of inaccurate information by chatbots can have serious consequences for patient care, impacting not only clinical decision-making but also the psychological well-being of patients with increased emotional distress which has been linked to an elevated risk of suicidal behavior, especially among adolescents and young adults following a cancer diagnosis [55,59,60]. To mitigate hallucinations and improve factual consistency, recent developments have explored RAG models [48]. These systems combine LLMs with external information retrieval capabilities, enabling them to cite verifiable sources and improve reliability. For instance, a recent proof-of-concept study introduced the Gastrointestinal Imaging-Aware Chatbot (GIA-CB), a GPT-4–based model enhanced with domain-specific retrieval. When applied to abdominal imaging cases, GIA-CB outperformed standard GPT-4 in suggesting accurate differential diagnoses, demonstrating the value of domain-informed RAG systems [61].
- Lack of memory: Current LLMs do not have persistent memory of prior interactions. Each response is generated independently, without continuity, historical awareness, or contextual integration across sessions. Ongoing research is exploring LLMs with memory-enhanced architectures capable of retaining structured interaction histories and implementing a two-tier memory system—modeled after human short-term and long-term memory—to preserve context and ensure continuity throughout conversations [62]. Another promising approach involves developing longitudinal interaction-aware models that support session continuity or interface with electronic health records (EHRs) to maintain contextual coherence across multiple encounters [63].
- Lack of reproducibility: Responses to the same prompt may vary upon repetition due to the models’ stochastic and non-deterministic nature. While this enhances linguistic versatility, it limits credibility and undermines consistency in clinical applications [38]. To enhance the reproducibility of LLM outputs, several strategies have been employed to reduce response variability, including prompt engineering, modifying sampling techniques (e.g., nucleus sampling), and fixing the random seed; notably, setting the temperature parameter to 0.0 in conjunction with a fixed seed has been shown to produce more deterministic responses and minimize outlier benchmark scores, thereby improving the reliability and interpretability of evaluation results [64].
- Technical instability: ChatGPT and similar systems may experience latency or interruptions during periods of high usage, potentially delaying critical information retrieval [65].
- Knowledge cutoffs: LLMs are limited to the data available at the time of their last training update. They are not capable of incorporating newly published medical evidence, evolving guidelines, or real-time clinical data, resulting in potentially outdated or misleading recommendations [48]. One promising solution to this limitation is the integration of lifelong learning capabilities, which aim to enable LLM-based agents to continuously acquire, retain, and apply new knowledge over time, improving their adaptability to evolving medical contexts and enhancing the relevance and accuracy of their outputs in dynamic clinical environments [66].
In addition to these technical limitations, several ethical and operational challenges must be addressed:
- Data privacy: The use of AI tools in radiology often involves handling sensitive patient data. Reliance on cloud-based public platforms introduces risks of data breaches and unauthorized access. A potential solution is to use open-source LLMs within hospital networks. Since their source code is publicly available, they can be downloaded and implemented locally, helping to improve data security, ensure system availability, and promote transparency [67].
- Transparency and traceability: Most LLMs do not provide source references for their responses, complicating validation and undermining clinical trust. Transparency in AI systems requires clear communication about what the system can and cannot do, its purpose, the conditions under which it performs reliably, and its expected accuracy. This information is vital for healthcare providers, but also for patients, who should be informed when AI is involved in their care. Dealing with these ethical challenges in AI will require a multidisciplinary approach: combining technical safeguards, government regulation, oversight mechanisms, and collaborative ethical standards developed with input from clinicians, patients, AI developers, and ethicists [68,69].
- Monopolization of knowledge: The rapid development of LLMs has been dominated by a few large commercial entities (e.g., OpenAI/Microsoft, Google, Meta). To avoid the centralization of medical knowledge and preserve equitable access to AI technologies, support for non-commercial, open-source medical LLMs is crucial [14].
- Regulatory oversight: The absence of standardized policies regarding AI deployment, accountability, and error management poses risks to patient safety. Robust regulatory frameworks are necessary to guide safe and ethical AI integration into radiological workflows [48].
The integration of AI-driven chatbot technologies into radiology workflows presents a range of complex challenges, spanning technical infrastructure, interoperability, workforce training, change management, and financial investment. Technologically, many radiology departments rely on legacy Information Technology (IT) systems that are not readily compatible with modern AI tools, often requiring substantial upgrades or reconfiguration. Achieving seamless interoperability between chatbots and existing systems such as Picture Archiving and Communication Systems (PACS) and Radiology Information Systems (RIS) demands strict adherence to healthcare data standards, including DICOM and HL7, and often involves addressing vendor-specific integration issues. A comprehensive review by the Radiological Society of North America (RSNA) underscores the critical importance of standards-based interoperability to facilitate scalable AI adoption within radiology workflows [70].
From an organizational standpoint, the successful deployment of AI tools hinges on robust training initiatives. Radiologists and allied healthcare professionals must be equipped not only to operate these models but also to critically assess and interpret their outputs. This necessitates customized training programs and continuous professional development. Furthermore, effective-change management is essential to mitigate resistance and support staff as they adapt to AI-enhanced workflows [69].
Financially, the upfront investment required to implement chatbot systems, including infrastructure upgrades, software integration, and personnel training, must be carefully weighed against potential long-term gains. However, the realization of these benefits is highly context-dependent and should be evaluated through rigorous, institution-specific cost-benefit analyses [71,72].
It is worth highlighting that this review does not specifically investigate the underlying technical biases of LLMs, a topic that lies predominantly within the fields of computer science and engineering, although such limitations may nonetheless exert a significant influence on their performance and reliability in radiological applications.
7. Future Directions
As LLMs continue to evolve, their integration into radiology will increasingly span both clinical practice and research (Table 3).

Table 3.
Clinical and research applications of LLMs in radiology, grouped by common functional domains to illustrate parallel use across practice and investigation.
The development of domain-specific medical LLMs, with enhanced contextual comprehension, domain-adapted vocabulary, and integration into electronic health record systems, could significantly enhance their clinical utility. Such integration may also address persistent concerns related to data privacy and regulatory compliance, provided that robust data governance and security protocols are implemented.
In subspecialties like interventional radiology, chatbots could be developed to assist with real-time procedural guidance and patient management. For example, a chatbot integrated with clinical decision support systems could provide instant recommendations during image-guided interventions—such as suggesting optimal access routes or alerting about potential complications based on patient data and imaging [73].
Embedding LLMs within PACS or RIS systems may enable automated alerting for critical findings or recommend next steps based on guidelines.
Beyond clinical utility, LLMs also hold transformative potential in the domain of medical research and education. These tools can democratize access to scientific literature, streamline scientific writing, and assist in complex computational analyses. By enabling researchers to efficiently navigate large datasets, extract relevant information, and generate preliminary drafts of scientific content, LLMs may reduce administrative burden and enhance research productivity [14]. However, the risk of misinformation propagation, the potential for overreliance on AI-generated content, and the necessity for human oversight in scientific authorship and interpretation remain significant. Furthermore, the importance of reproducibility, proper source attribution, and methodological transparency must not be overlooked [74].
LLMs such as ChatGPT have demonstrated near-passing performance on radiology board-style exams without image inputs, particularly excelling in lower-order reasoning and clinical management tasks. However, their performance declines on higher-order questions requiring image interpretation, complex reasoning, or conceptual application. As these models are increasingly integrated into radiology education to simulate board scenarios, generate differential diagnoses, or quizzes, it is essential to leverage their capabilities to support learning, while preserving the development of critical thinking, clinical judgment, and communication skills [75,76].
Although precise timelines remain difficult to predict, initial uses of LLMs in radiology, particularly in supportive or administrative contexts, may emerge gradually in the near future. Wider clinical adoption is likely to follow over time, as technical and regulatory challenges are gradually addressed, with the pace and cost-effectiveness of implementation varying depending on institutional resources, potentially leading to significant discrepancies between large and small centers.
8. Conclusions
Large language models such as ChatGPT demonstrate considerable promise in transforming radiological practice by enhancing workflow efficiency, supporting diagnostic reasoning, and facilitating research innovation. Nonetheless, their clinical deployment must be guided by ethical oversight, awareness of model limitations, and a clear framework for human supervision.
As patient-facing applications such as ChatGPT become more accessible, new challenges may emerge, including the risk of self-interpretation of radiological findings and potential miscommunication. These concerns underscore the importance of maintaining the interpretative role of radiologists and the necessity of professional oversight.
With ongoing model refinements and deeper integration into radiology-specific workflows, ChatGPT and similar tools may play a pivotal role in augmenting radiological services and improving patient outcomes [65]. Realizing this potential will require sustained interdisciplinary collaboration among AI developers, radiologists, and healthcare policymakers to ensure responsible implementation, regulatory alignment, and clinical relevance. At present, these tools should be regarded as supportive adjuncts, designed to assist rather than replace the clinical expertise, contextual reasoning, and ethical judgment of radiology professionals [44].
Author Contributions
Conceptualization, T.D.; methodology, S.M.; validation, G.A.; formal analysis, C.B. and I.Y.; investigation, V.K. and L.D.G.; data curation, C.G. and G.M.; writing—original draft preparation, L.R.M.L. and C.G.; writing—review and editing, L.R.M.L. and T.D.; visualization, M.G.; supervision, T.J.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial intelligence |
| BERT | Bidirectional encoder representations from transformers |
| CAD-RADS | Coronary artery disease reporting and data system |
| CCTA | Coronary CT angiography |
| DWI | Diffusion-weighted imaging |
| GIA-CB | Gastrointestinal imaging-aware chatbot |
| GPT | Generative pretrained transformer |
| LLMs | Large language models |
| NLP | Natural language processing |
| RAG | Retrieval-augmented generation |
| USPSTF | United States Preventive Services Task Force |
| ViT | Vision transformer |
| ZSL | Zero-shot learning |
References
- Lanzafame, L.R.M.; Bucolo, G.M.; Muscogiuri, G.; Sironi, S.; Gaeta, M.; Ascenti, G.; Booz, C.; Vogl, T.J.; Blandino, A.; Mazziotti, S.; et al. Artificial intelligence in cardiovascular ct and mr imaging. Life 2023, 13, 507. [Google Scholar] [CrossRef]
- Lanzafame, L.R.M.; Gulli, C.; Booz, C.; Vogl, T.J.; Saba, L.; Cau, R.; Toia, P.; Ascenti, G.; Gaeta, M.; Mazziotti, S.; et al. Advancements in computed tomography angiography for pulmonary embolism assessment. Echocardiography 2025, 42, e70116. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Caudo, D.; Blandino, A.; Albrecht, M.H.; Vogl, T.J.; Gruenewald, L.D.; Gaeta, M.; Yel, I.; Koch, V.; Martin, S.S.; et al. Artificial intelligence, machine learning and deep learning in musculoskeletal imaging: Current applications. J. Clin. Ultrasound 2022, 50, 1414–1431. [Google Scholar] [CrossRef]
- D’Angelo, T.; Bucolo, G.M.; Kamareddine, T.; Yel, I.; Koch, V.; Gruenewald, L.D.; Martin, S.; Alizadeh, L.S.; Mazziotti, S.; Blandino, A.; et al. Accuracy and time efficiency of a novel deep learning algorithm for intracranial hemorrhage detection in ct scans. Radiol. Med. 2024, 129, 1499–1506. [Google Scholar] [CrossRef]
- Reschke, P.; Gotta, J.; Gruenewald, L.D.; Bachir, A.A.; Strecker, R.; Nickel, D.; Booz, C.; Martin, S.S.; Scholtz, J.E.; D’Angelo, T.; et al. Deep learning in knee mri: A prospective study to enhance efficiency, diagnostic confidence and sustainability. Acad. Radiol. 2025, 32, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Laymouna, M.; Ma, Y.; Lessard, D.; Schuster, T.; Engler, K.; Lebouché, B. Roles, users, benefits, and limitations of chatbots in health care: Rapid review. J. Med. Internet Res. 2024, 26, e56930. [Google Scholar] [CrossRef] [PubMed]
- Hindelang, M.; Sitaru, S.; Zink, A. Transforming health care through chatbots for medical history-taking and future directions: Comprehensive systematic review. JMIR Med. Inform. 2024, 12, e56628. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; Pirrone, G.; Drigo, A.; Retico, A. The evolution of artificial intelligence in medical imaging: From computer science to machine and deep learning. Cancers 2024, 16, 3702. [Google Scholar] [CrossRef]
- Adamopoulou, E.; Moussiades, L. Chatbots: History, technology, and applications. Mach. Learn. Appl. 2020, 2, 100006. [Google Scholar] [CrossRef]
- Giansanti, D. The chatbots are invading us: A map point on the evolution, applications, opportunities, and emerging problems in the health domain. Life 2023, 13, 1130. [Google Scholar] [CrossRef]
- Adamopoulou, E.; Moussiades, L. An overview of chatbot technology. In Artificial Intelligence Applications and Innovations; Springer International Publishing: Cham, Switzerland, 2020; pp. 373–383. [Google Scholar]
- Caldarini, G.; Jaf, S.; McGarry, K. A literature survey of recent advances in chatbots. Information 2022, 13, 41. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, I. Attention is all you need. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Curran Associates Inc.: Red Hook, NY, USA, 2017; pp. 6000–6010. [Google Scholar]
- Clusmann, J.; Kolbinger, F.R.; Muti, H.S.; Carrero, Z.I.; Eckardt, J.-N.; Laleh, N.G.; Löffler, C.M.L.; Schwarzkopf, S.-C.; Unger, M.; Veldhuizen, G.P.; et al. The future landscape of large language models in medicine. Commun. Med. 2023, 3, 141. [Google Scholar] [CrossRef]
- Bhayana, R. Chatbots and large language models in radiology: A practical primer for clinical and research applications. Radiology 2024, 310, e232756. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukarasu, A.J.; Ting, D.S.J.; Elangovan, K.; Gutierrez, L.; Tan, T.F.; Ting, D.S.W. Large language models in medicine. Nat. Med. 2023, 29, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, S.; Baradwaj, J. Advancing radiology with gpt-4: Innovations in clinical applications, patient engagement, research, and learning. Eur. J. Radiol. Open 2024, 13, 100589. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Lei, J.; Liu, X.; Shen, Y.; Zhang, Z.; Wei, P.; Gu, J.; Chu, Z.; Qin, Z. A survey on medical large language models: Technology, application, trustworthiness, and future directions. arXiv 2024, arXiv:2406.03712. [Google Scholar]
- Bautista, A.B.; Burgos, A.; Nickel, B.J.; Yoon, J.J.; Tilara, A.A.; Amorosa, J.K. Do clinicians use the american college of radiology appropriateness criteria in the management of their patients? AJR Am. J. Roentgenol. 2009, 192, 1581–1585. [Google Scholar] [CrossRef]
- Rau, A.; Rau, S.; Zöller, D.; Fink, A.; Tran, H.; Wilpert, C.; Nattenmüller, J.; Neubauer, J.; Bamberg, F.; Reisert, M.; et al. A context-based chatbot surpasses radiologists and generic chatgpt in following the acr appropriateness guidelines. Radiology 2023, 308, e230970. [Google Scholar] [CrossRef]
- Barash, Y.; Klang, E.; Konen, E.; Sorin, V. Chatgpt-4 assistance in optimizing emergency department radiology referrals and imaging selection. J. Am. Coll. Radiol. 2023, 20, 998–1003. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, P.; Ho, C.; Wood, J.; Guleria, S.; Virostko, J. Can generative ai improve the readability of patient education materials at a radiology practice? Clin. Radiol. 2024, 79, e1366–e1371. [Google Scholar] [CrossRef]
- Kaba, E.; Beyazal, M.; Çeliker, F.B.; Yel, İ.; Vogl, T.J. Accuracy and readability of chatgpt on potential complications of interventional radiology procedures: Ai-powered patient interviewing. Acad. Radiol. 2025, 32, 1547–1553. [Google Scholar] [CrossRef]
- Patil, N.S.; Huang, R.S.; Caterine, S.; Yao, J.; Larocque, N.; van der Pol, C.B.; Stubbs, E. Artificial intelligence chatbots’ understanding of the risks and benefits of computed tomography and magnetic resonance imaging scenarios. Can. Assoc. Radiol. J. 2024, 75, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Elhakim, T.; Brea, A.R.; Fidelis, W.; Paravastu, S.S.; Malavia, M.; Omer, M.; Mort, A.; Ramasamy, S.K.; Tripathi, S.; Dezube, M.; et al. Enhanced procedural information readability for patient-centered care in interventional radiology with large language models (pro-read ir). J. Am. Coll. Radiol. 2025, 22, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Gertz, R.J.; Bunck, A.C.; Lennartz, S.; Dratsch, T.; Iuga, A.-I.; Maintz, D.; Kottlors, J. Gpt-4 for automated determination of radiologic study and protocol based on radiology request forms: A feasibility study. Radiology 2023, 307, e230877. [Google Scholar] [CrossRef]
- Hallinan, J.; Leow, N.W.; Ong, W.; Lee, A.; Low, Y.X.; Chan, M.D.Z.; Devi, G.K.; Loh, D.D.; He, S.S.; Nor, F.E.M.; et al. Mri spine request form enhancement and auto protocoling using a secure institutional large language model. Spine J. 2025, 25, 505–514. [Google Scholar] [CrossRef]
- Koyun, M.; Taskent, I. Evaluation of advanced artificial intelligence algorithms’ diagnostic efficacy in acute ischemic stroke: A comparative analysis of chatgpt-4o and claude 3.5 sonnet models. J. Clin. Med. 2025, 14, 571. [Google Scholar] [CrossRef] [PubMed]
- Kasalak, Ö.; Alnahwi, H.; Toxopeus, R.; Pennings, J.P.; Yakar, D.; Kwee, T.C. Work overload and diagnostic errors in radiology. Eur. J. Radiol. 2023, 167, 111032. [Google Scholar] [CrossRef]
- Horiuchi, D.; Tatekawa, H.; Shimono, T.; Walston, S.L.; Takita, H.; Matsushita, S.; Oura, T.; Mitsuyama, Y.; Miki, Y.; Ueda, D. Accuracy of chatgpt generated diagnosis from patient’s medical history and imaging findings in neuroradiology cases. Neuroradiology 2024, 66, 73–79. [Google Scholar] [CrossRef]
- Mitsuyama, Y.; Tatekawa, H.; Takita, H.; Sasaki, F.; Tashiro, A.; Oue, S.; Walston, S.L.; Nonomiya, Y.; Shintani, A.; Miki, Y.; et al. Comparative analysis of gpt-4-based chatgpt’s diagnostic performance with radiologists using real-world radiology reports of brain tumors. Eur. Radiol. 2024, 35, 1938–1947. [Google Scholar] [CrossRef]
- Rezaei, M.; Shahidi, M. Zero-shot learning and its applications from autonomous vehicles to covid-19 diagnosis: A review. Intell. Based Med. 2020, 3, 100005. [Google Scholar] [CrossRef]
- Kelly, B.S.; Duignan, S.; Mathur, P.; Dillon, H.; Lee, E.H.; Yeom, K.W.; Keane, P.A.; Lawlor, A.; Killeen, R.P. Can chatgpt4-vision identify radiologic progression of multiple sclerosis on brain mri? Eur. Radiol. Exp. 2025, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Dehdab, R.; Brendlin, A.; Werner, S.; Almansour, H.; Gassenmaier, S.; Brendel, J.M.; Nikolaou, K.; Afat, S. Evaluating chatgpt-4v in chest ct diagnostics: A critical image interpretation assessment. Jpn. J. Radiol. 2024, 42, 1168–1177. [Google Scholar] [CrossRef]
- Haver, H.L.; Bahl, M.; Doo, F.X.; Kamel, P.I.; Parekh, V.S.; Jeudy, J.; Yi, P.H. Evaluation of multimodal chatgpt (gpt-4v) in describing mammography image features. Can. Assoc. Radiol. J. 2024, 75, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Koyun, M.; Cevval, Z.K.; Reis, B.; Ece, B. Detection of intracranial hemorrhage from computed tomography images: Diagnostic role and efficacy of chatgpt-4o. Diagnostics 2025, 15, 143. [Google Scholar] [CrossRef]
- Nickel, B.; Ayre, J.; Marinovich, M.L.; Smith, D.P.; Chiam, K.; Lee, C.I.; Wilt, T.J.; Taba, M.; McCaffery, K.; Houssami, N. Are ai chatbots concordant with evidence-based cancer screening recommendations? Patient Educ. Couns. 2025, 134, 108677. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.-K.; Kim, S.-S. Large language models: A guide for radiologists. Korean J. Radiol. 2024, 25, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhang, H.; Li, S.; Wang, Y.; Wu, N.; Lu, X. Automatic extraction of lung cancer staging information from computed tomography reports: Deep learning approach. JMIR Med. Inform. 2021, 9, e27955. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, N.; Wang, L.; Liu, H.; Zhang, R. Cancerbert: A cancer domain-specific language model for extracting breast cancer phenotypes from electronic health records. J. Am. Med. Inform. Assoc. 2022, 29, 1208–1216. [Google Scholar] [CrossRef]
- Fink, M.A.; Bischoff, A.; Fink, C.A.; Moll, M.; Kroschke, J.; Dulz, L.; Heußel, C.P.; Kauczor, H.U.; Weber, T.F. Potential of chatgpt and gpt-4 for data mining of free-text ct reports on lung cancer. Radiology 2023, 308, e231362. [Google Scholar] [CrossRef]
- Tozuka, R.; Johno, H.; Amakawa, A.; Sato, J.; Muto, M.; Seki, S.; Komaba, A.; Onishi, H. Application of notebooklm, a large language model with retrieval-augmented generation, for lung cancer staging. Jpn. J. Radiol. 2024, 43, 706–712. [Google Scholar] [CrossRef]
- Sun, Z.; Ong, H.; Kennedy, P.; Tang, L.; Chen, S.; Elias, J.; Lucas, E.; Shih, G.; Peng, Y. Evaluating gpt-4 on impressions generation in radiology reports. Radiology 2023, 307, e231259. [Google Scholar] [CrossRef] [PubMed]
- Temperley, H.C.; O’Sullivan, N.J.; Curtain, B.M.M.; Corr, A.; Meaney, J.F.; Kelly, M.E.; Brennan, I. Current applications and future potential of chatgpt in radiology: A systematic review. J. Med. Imaging Radiat. Oncol. 2024, 68, 257–264. [Google Scholar] [CrossRef]
- Truhn, D.; Weber, C.D.; Braun, B.J.; Bressem, K.; Kather, J.N.; Kuhl, C.; Nebelung, S. A pilot study on the efficacy of gpt-4 in providing orthopedic treatment recommendations from mri reports. Sci. Rep. 2023, 13, 20159. [Google Scholar] [CrossRef]
- Sorin, V.; Klang, E.; Sklair-Levy, M.; Cohen, I.; Zippel, D.B.; Lahat, N.B.; Konen, E.; Barash, Y. Large language model (chatgpt) as a support tool for breast tumor board. NPJ Breast Cancer 2023, 9, 44. [Google Scholar] [CrossRef]
- Adams, L.C.; Truhn, D.; Busch, F.; Kader, A.; Niehues, S.M.; Makowski, M.R.; Bressem, K.K. Leveraging gpt-4 for post hoc transformation of free-text radiology reports into structured reporting: A multilingual feasibility study. Radiology 2023, 307, e230725. [Google Scholar] [CrossRef] [PubMed]
- Grewal, H.; Dhillon, G.; Monga, V.; Sharma, P.; Buddhavarapu, V.S.; Sidhu, G.; Kashyap, R. Radiology gets chatty: The chatgpt saga unfolds. Cureus 2023, 15, e40135. [Google Scholar] [CrossRef] [PubMed]
- Maroncelli, R.; Rizzo, V.; Pasculli, M.; Cicciarelli, F.; Macera, M.; Galati, F.; Catalano, C.; Pediconi, F. Probing clarity: Ai-generated simplified breast imaging reports for enhanced patient comprehension powered by chatgpt-4o. Eur. Radiol. Exp. 2024, 8, 124. [Google Scholar] [CrossRef]
- Salam, B.; Kravchenko, D.; Nowak, S.; Sprinkart, A.M.; Weinhold, L.; Odenthal, A.; Mesropyan, N.; Bischoff, L.M.; Attenberger, U.; Kuetting, D.L.; et al. Generative pre-trained transformer 4 makes cardiovascular magnetic resonance reports easy to understand. J. Cardiovasc. Magn. Reson. 2024, 26, 101035. [Google Scholar] [CrossRef]
- Tepe, M.; Emekli, E. Decoding medical jargon: The use of ai language models (chatgpt-4, bard, microsoft copilot) in radiology reports. Patient Educ. Couns. 2024, 126, 108307. [Google Scholar] [CrossRef]
- Encalada, S.; Gupta, S.; Hunt, C.; Eldrige, J.; Evans, J., 2nd; Mosquera-Moscoso, J.; de Mendonca, L.F.P.; Kanahan-Osman, S.; Bade, S.; Bade, S.; et al. Optimizing patient understanding of spine mri reports using ai: A prospective single center study. Interv. Pain. Med. 2025, 4, 100550. [Google Scholar] [CrossRef]
- Can, E.; Uller, W.; Vogt, K.; Doppler, M.C.; Busch, F.; Bayerl, N.; Ellmann, S.; Kader, A.; Elkilany, A.; Makowski, M.R.; et al. Large language models for simplified interventional radiology reports: A comparative analysis. Acad. Radiol. 2025, 32, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Jeblick, K.; Schachtner, B.; Dexl, J.; Mittermeier, A.; Stüber, A.T.; Topalis, J.; Weber, T.; Wesp, P.; Sabel, B.O.; Ricke, J.; et al. Chatgpt makes medicine easy to swallow: An exploratory case study on simplified radiology reports. Eur. Radiol. 2024, 34, 2817–2825. [Google Scholar] [CrossRef]
- Rahsepar, A.A.; Tavakoli, N.; Kim, G.H.J.; Hassani, C.; Abtin, F.; Bedayat, A. How ai responds to common lung cancer questions: Chatgpt vs google bard. Radiology 2023, 307, e230922. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Amin, K.; Khosla, P.; Bajaj, S.; Chheang, S.; Forman, H.P. Utilizing large language models to simplify radiology reports: A comparative analysis of chatgpt3.5, chatgpt4.0, google bard, and microsoft bing. medRxiv 2023. [Google Scholar] [CrossRef]
- Silbergleit, M.; Tóth, A.; Chamberlin, J.H.; Hamouda, M.; Baruah, D.; Derrick, S.; Schoepf, U.J.; Burt, J.R.; Kabakus, I.M. Chatgpt vs gemini: Comparative accuracy and efficiency in cad-rads score assignment from radiology reports. J. Imaging. Inform. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kikuchi, T.; Yamagishi, Y.; Hanaoka, S.; Nakao, T.; Miki, S.; Yoshikawa, T.; Abe, O. Chatgpt for automating lung cancer staging: Feasibility study on open radiology report dataset. medRxiv 2023, 2023.12.11.23299107. [Google Scholar] [CrossRef]
- Huang, L.; Yu, W.; Ma, W.; Zhong, W.; Feng, Z.; Wang, H.; Chen, Q.; Peng, W.; Feng, X.; Qin, B. A survey on hallucination in large language models: Principles, taxonomy, challenges, and open questions. ACM Trans. Inf. Syst. 2025, 43, 1–55. [Google Scholar] [CrossRef]
- Lu, D.; Fall, K.; Sparén, P.; Ye, W.; Adami, H.O.; Valdimarsdóttir, U.; Fang, F. Suicide and suicide attempt after a cancer diagnosis among young individuals. Ann. Oncol. 2013, 24, 3112–3117. [Google Scholar] [CrossRef]
- Rau, S.; Rau, A.; Nattenmüller, J.; Fink, A.; Bamberg, F.; Reisert, M.; Russe, M.F. A retrieval-augmented chatbot based on gpt-4 provides appropriate differential diagnosis in gastrointestinal radiology: A proof of concept study. Eur. Radiol. Exp. 2024, 8, 60. [Google Scholar] [CrossRef]
- Liu, N.; Chen, L.; Tian, X.; Zou, W.; Chen, K.; Cui, M. From llm to conversational agent: A memory enhanced architecture with fine-tuning of large language models. arXiv 2024, arXiv:2401.02777. [Google Scholar]
- Carrasco-Ribelles, L.A.; Llanes-Jurado, J.; Gallego-Moll, C.; Cabrera-Bean, M.; Monteagudo-Zaragoza, M.; Violán, C.; Zabaleta-del-Olmo, E. Prediction models using artificial intelligence and longitudinal data from electronic health records: A systematic methodological review. J. Am. Med. Inform. Assoc. 2023, 30, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, R.E.; Barry, J.; Cohn, A.G. Towards reproducible llm evaluation: Quantifying uncertainty in llm benchmark scores. arXiv 2024, arXiv:2410.03492. [Google Scholar]
- Lecler, A.; Duron, L.; Soyer, P. Revolutionizing radiology with gpt-based models: Current applications, future possibilities and limitations of chatgpt. Diagn. Interv. Imaging 2023, 104, 269–274. [Google Scholar] [CrossRef]
- Zheng, J.; Shi, C.; Cai, X.; Li, Q.; Zhang, D.; Li, C.; Yu, D.; Ma, Q. Lifelong learning of large language model based agents: A roadmap. arXiv 2025, arXiv:2501.07278. [Google Scholar]
- Bluethgen, C.; Van Veen, D.; Zakka, C.; Link, K.E.; Fanous, A.H.; Daneshjou, R.; Frauenfelder, T.; Langlotz, C.P.; Gatidis, S.; Chaudhari, A. Best practices for large language models in radiology. Radiology 2025, 315, e240528. [Google Scholar] [CrossRef] [PubMed]
- Smuha, N.A. Regulation 2024/1689 of the Eur. Parl. & Council of June 13, 2024 (eu artificial intelligence act). Int. Leg. Mater. 2025, 1–148. [Google Scholar] [CrossRef]
- Brady, A.P.; Allen, B.; Chong, J.; Kotter, E.; Kottler, N.; Mongan, J.; Oakden-Rayner, L.; Santos, D.P.D.; Tang, A.; Wald, C.; et al. Developing, purchasing, implementing and monitoring ai tools in radiology: Practical considerations. A multi-society statement from the acr, car, esr, ranzcr & rsna. Insights Imaging 2024, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Tejani, A.S.; Cook, T.S.; Hussain, M.; Schmidt, T.S.; O’Donnell, K.P. Integrating and adopting ai in the radiology workflow: A primer for standards and integrating the healthcare enterprise (ihe) profiles. Radiology 2024, 311, e232653. [Google Scholar] [CrossRef]
- Barreda, M.; Cantarero-Prieto, D.; Coca, D.; Delgado, A.; Lanza-León, P.; Lera, J.; Montalbán, R.; Pérez, F. Transforming healthcare with chatbots: Uses and applications—A scoping review. Digit. Health 2025, 11, 20552076251319174. [Google Scholar] [CrossRef]
- Fgaier, M.; Zrubka, Z. Cost-effectiveness of using chatbots in healthcare: A systematic review. In Proceedings of the 2022 IEEE 22nd International Symposium on Computational Intelligence and Informatics and 8th IEEE International Conference on Recent Achievements in Mechatronics, Automation, Computer Science and Robotics (CINTI-MACRo), Budapest, Hungary, 21–22 November 2022; p. 000305-10. [Google Scholar] [CrossRef]
- Lastrucci, A.; Iosca, N.; Wandael, Y.; Barra, A.; Lepri, G.; Forini, N.; Ricci, R.; Miele, V.; Giansanti, D. Ai and interventional radiology: A narrative review of reviews on opportunities, challenges, and future directions. Diagnostics 2025, 15, 893. [Google Scholar] [CrossRef]
- Ballard, D.H.; Antigua-Made, A.; Barre, E.; Edney, E.; Gordon, E.B.; Kelahan, L.; Lodhi, T.; Martin, J.G.; Ozkan, M.; Serdynski, K.; et al. Impact of chatgpt and large language models on radiology education: Association of academic radiology—Radiology research alliance task force white paper. Acad. Radiol. 2024, 32, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Bhayana, R.; Krishna, S.; Bleakney, R.R. Performance of chatgpt on a radiology board-style examination: Insights into current strengths and limitations. Radiology 2023, 307, e230582. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, A.P.; Slanetz, P.J.; Baird, G.L. Rise of chatgpt: It may be time to reassess how we teach and test radiology residents. Radiology 2023, 307, e231053. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).