Abstract
Background: Basal cell carcinoma (BCC) of the ocular and periocular region is characterized by a painless progressive extension. An early diagnosis can limit the extent of facial tissue involvement and subsequent resection resulting in better cosmetic and functional results. Objectives: The aim is to provide the largest and most up-to-date overview of ocular and periocular BCCs. We also reported the first case of caruncle BCC investigated by dermoscopy and reflectance confocal microscopy (RCM). Methods: A systematic review and meta-analysis (Prospero ID CRD583032) were carried out by searching PUBMED–MEDLINE, including all articles with a full-text English version and with BCCs in eyelids, medial and lateral canthus, caruncle, conjunctiva, and orbit. The following data were collected: authors, year, title and type of publication, medical specialization, number, sex, age and comorbidities of the patients, anatomic localization of the disease, clinical and dermoscopic aspect, histological examination, and treatment. Results: We identified 731 articles through a database search, of which 236 articles matched our inclusion criteria. A total of 71.730 patients with ocular and periocular BCCs were included in the present study, and all data collected were reported in a dataset. Most of the articles included were described by ophthalmologists (67.5%), dermatologists (11.2%), or plastic surgeons (5.6%). The proportional meta-analysis revealed varying significance and heterogeneity for each type of study included. Conclusions: BCC more frequently affects the lower eyelid. The most common BCC subtype of ocular and periocular area is the nodular form. Limited data are available concerning the application of dermoscopy and RCM in this area. RCM may be particularly useful for early diagnosis, mapping, and treatment monitoring of ocular and periocular BCCs. Surgery still remains the first-choice treatment.
Keywords:
neoplasms; tumor; carcinoma; basal cell; eye; ocular; periocular; eyelid; caruncle; dermoscopy; microscopy; confocal 1. Introduction
Basal cell carcinoma (BCC) is a skin carcinoma that originates from epidermal cells. It is the most common malignant tumor in white populations, accounting for 75% of cases [1]. The incidence varies geographically; it also increases with age and is slightly more common in men. Several risk factors are involved in the pathogenesis, and ultraviolet radiation (UVR) is the most important cause [1]. BCC typically affects sun exposure areas, especially on the head and neck region, of which 20% occurs on the eyelids [2]. BCC is a malignant cancer that exceptionally metastasizes. The risk of recurrence may depend on the location of the tumor (H zone of the face), the histological characteristics, and immunosuppression [3]. The precise and early identification of these tumors can limit the extent of facial tissue involvement and subsequent resection resulting in better cosmetic and functional results. The diagnosis of BCC often requires a biopsy, especially in the case of ambiguous lesions. Dermoscopy usually allows for the early identification of BCC and preoperatively detects its subtype [1]. Additional non-invasive skin-imaging technology that has proven its high diagnostic value is in vivo reflectance confocal microscopy (RCM), which is often only accessible in specialized skin cancer centers [4].
RCM permits the capture of in vivo, cellular-resolution images of lesions, parallel to the skin surface, from the stratum corneum to the superficial dermis [5]. Acquisition of high-quality images with RCM can be impeded by technical difficulties in curved, or relatively inaccessible, surfaces such as ocular and periocular structures. Herein, we describe a rare case of caruncle BCC investigated with dermoscopy and RCM, after performing a systematic review and meta-analysis of all the available literature concerning ocular and periocular BCC. Currently, this is the first report on the application of non-invasive imaging tools in this anatomical location.
2. Materials and Methods
2.1. Search Strategy
A systematic review and meta-analysis of ocular and periocular BCCs reported in the literature were carried out by searching PUBMED–MEDLINE. Keywords used were “ocular basal cell carcinoma”, “periocular basal cell carcinoma”, “caruncle basal cell carcinoma”, “lacrimal caruncle basal cell carcinoma”, “ocular basal cell carcinoma AND confocal microscopy”, and “ocular basal cell carcinoma AND reflectance confocal microscopy”. PUBMED–MEDLINE was searched for studies published up to 31 December 2023.
2.2. Study Registration
This systematic review with meta-analysis was registered in PROSPERO (Prospero ID CRD583032) and conducted following PRISMA guidelines (Figure 1).
Figure 1.
Flow diagram of the study selection process.
2.3. Data Collection
Data were independently extracted by two authors (MC and MT) and disagreement was resolved by a consensus or a third author (VDM) who acted as a referee. We included published articles with a full-text English version available and with specific BCCs’ localization in eyelids, medial and lateral canthus, caruncle, conjunctiva, and orbit. The search was restricted to studies on humans, while systematic and literature reviews were excluded. The following data were collected: authors, year, title and type of publication, medical specialization, number, sex, age and comorbidities of the patients, anatomic localization of the disease, clinical and dermoscopic aspect, histological examination, and treatment. Moreover, we reported the first case of caruncle BCC investigated with dermoscopy (VivaCam®: Mavig GmbH, Munich, Germany) and RCM (VivaScope® 3000: Mavig GmbH, Munich, Germany). Instruments and the acquisition procedure have been described elsewhere [5,6].
2.4. Statistical Analysis
We performed a proportional meta-analysis using MedCalc 14.8.1 software (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014, accessed on 1 September 2024) applying the Freeman–Tukey transformation (arcsine square root transformation) to calculate the weighted overall proportion (DerSimonian and Laird, 1986). Symptom proportions (expressed in percentage), within the 95% confidence interval (CI), from each study were included in the meta-analysis. The overall proportion within the 95% CI was calculated using both the random-effects model and the fixed-effects model. The fixed-effects model assumes that all included studies have a similar effect, so the summary effect is an estimate of the weight of similar effects in the studies. The random-effects model assumes that effects vary among studies, and the summary analysis is a weighted average reported in different studies.
A Forest plot was performed for each study included in the meta-analysis; values related to the effect size and CI were presented. The Forest plot also included the weighted effect of the prevalence of BCC by study type, with a 95% CI. The size of the marker (square) represents the weight of each study; studies with a smaller patient sample will have less weight. The overall effect is represented in the plot by a diamond: Its width represents precision, and its position represents the estimate of effects.
Heterogeneity among studies was estimated using Cochran’s Q statistic test and the I2 index. Heterogeneity, defined as whether observed variance exceeded expected variance, was considered significant when p < 0.01 for the Q statistic. The I2 index of given heterogeneity [I2 = 100% × (Q − df)/Q] was defined as I2 = 0–25%, homogeneous; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 = 75–100%, extreme heterogeneity.
3. Results
3.1. Study Selection and Characteristics
We identified 731 articles through database search. Duplicates were omitted and 428 records were excluded (Table S1) because they did not meet the inclusion criteria as mentioned above. A total of 236 articles were considered in our study (235 papers matched our criteria, while one was added after screening references of all selected studies) [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242]. All data collected are reported in a dataset (Table 1), while the characteristics of the studies are summarized in Table 2. The articles selected were mainly retrospective (32.6%), case series (32.6%), and reports (22.8%). Most of the articles included were described by ophthalmologists (67.5%), dermatologists (11.2%), and plastic surgeons (5.6%) but also less frequently by other specialists. A total of 71.730 patients with ocular and periocular BCCs are included in the present systematic review with meta-analysis and their characteristics are described in Table 3. Different anatomical locations are summarized in Table 4. “Eyelid and periocular area” (48.7%) and “eyelid” (29.9%) were the two more commonly involved sites, while “periocular”, “ocular”, and “caruncle” areas were only rarely interested. Clinic, dermoscopy, RCM, and histopathology data are included in Table 5, while treatments are given in Table 6.

Table 1.
Dataset.

Table 2.
Characteristics of the 236 studies included.

Table 3.
Characteristics of the 71.730 patients.

Table 4.
Different anatomic localization described in the 236 articles included.

Table 5.
Assessment performed in the 236 studies included.

Table 6.
Treatment selected in the 236 studies included.
3.2. Case
In September 2022, a 38-year-old lady was referred to San Gallicano Dermatological Institute-IRCCS, for evaluation of a caruncle pigmented lesion on the left eye. Her family and medical history were unremarkable. The lesion, which appeared approximately 7 months ago, was indolent. Clinical examination revealed a millimetric flat lesion, with faded and irregular margins, black-blue in color (Figure 2a). After applying oil immersion fluid, dermoscopic examination with VivaCam® showed the presence of blue–black structureless areas (Figure 2b,c). Sharply demarcated lobular nests, outlined by dark peritumoral clefting, were detected with the RCM handheld probe and corresponded to basaloid islands (Figure 2). Polarized elongated keratinocytes (streaming) characterized the overlying skin (Figure 2d). Hyper-refractile thin dendrites and bright oval structures were observed within and around the tumor island (Figure 2e,f), corresponding to melanophages, while peripheral palisading of nuclei can also be detected at higher magnification. The adjacent dermal stroma contained small bright dots compatible with smaller inflammatory cells. Convoluted and dilated blood vessels coursing in the horizontal plane of imaging were seen in real-time or video-mode RCM imaging, juxtaposed to the tumor islands (Video S1). Based on RCM findings, a diagnosis of pigmented BCC was made, and the lesion was subsequently surgically removed by an ophthalmologist. Histopathologic examination confirmed the diagnostic suspicion and the tumor was completely excised with a 2 mm of free margins. The patient did not complain of any discomfort or functional impairment. At a two-year follow-up, no signs of local recurrence were observed.
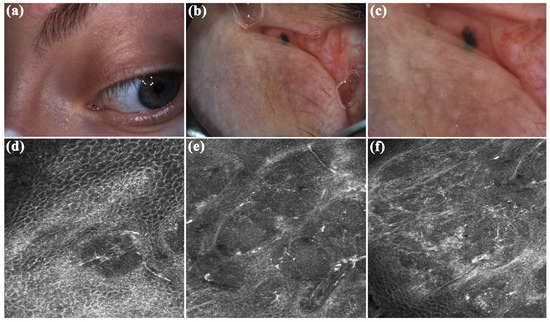
Figure 2.
Caruncle basal cell carcinoma. (a) Clinically appeared as a millimetric flat lesion, with faded and irregular margins, blue–black in color. (b,c) Dermoscopic assessment revealed black–blue structureless areas. (d–f) Reflectance confocal microscopy (RCM) showed the presence of sharply demarcated lobular nests, outlined by dark peritumoral clefting. Streaming, peripheral palisading, hyper-refractile thin dendrites and bright oval structures, and convoluted and dilated blood vessels are other RCM criteria detected.
3.3. Results of Meta-Analysis
The results of the proportional meta-analysis, including the combined proportion (95% CI), are summarized in Table 7, with estimates of the overall proportion shown in the Forest Plot (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8). The overall proportion for “clinical trial” was 97.6% (91.6–99.9), demonstrating significant and extreme heterogeneity (Q = 122.7, df = 3, I2 = 75.6%, p = 0.006). For “prospective study” and “prospective case series”, the overall proportion was 93.0% (79.2–99.6), indicating significant and extreme heterogeneity (Q = 40.6, df = 20, I2 = 99.5%, p < 0.001). “Retrospective study” showed an overall proportion of 61.1% (48.8–72.8), with significant and extreme heterogeneity (Q = 346.3, df = 75, I2 = 99.8%, p < 0.001). “Case reports” and “case report with review of the literature” studies showed an overall proportion of 85.3% (77.7–91.0), with non-significant and homogeneous (Q = 77.8, df = 53, I2 = 0.0%, p = 1.000). “Case series” studies showed an overall proportion of 73.3% (62.1–83.3), with significant and extreme heterogeneity (Q = 199.1, df = 66, I2 = 99.6%, p < 0.001) and “letter” showed an overall proportion of 89.9% (72.8 to 99.0), with significant and moderate heterogeneity (Q = 233.3, df = 8, I2 = 65.7%, p = 0.003).

Table 7.
Meta-analysis of the aggregated proportion of included studies for the prevalence of BCC.
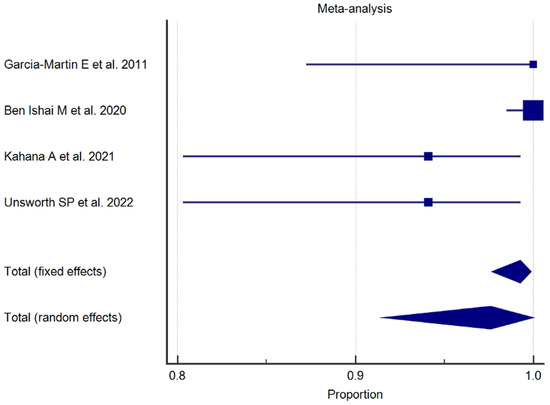
Figure 3.
Proportional meta-analysis of the included “clinical trial” studies.
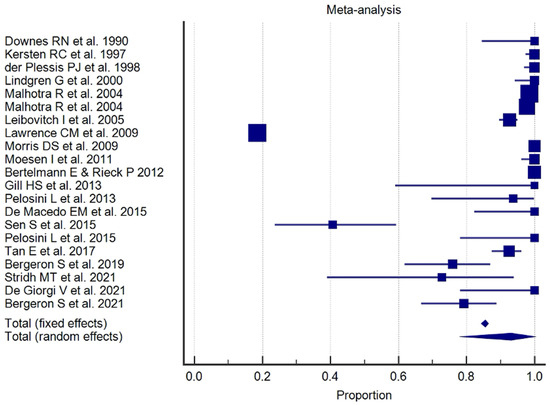
Figure 4.
Proportional meta-analysis of the included “prospective study” and “prospective case series”.
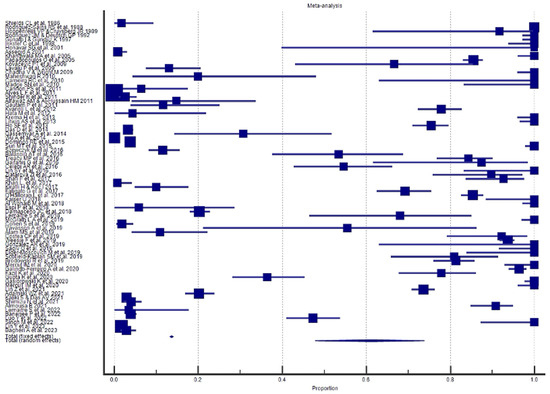
Figure 5.
Proportional meta-analysis of the included “retrospective study”.
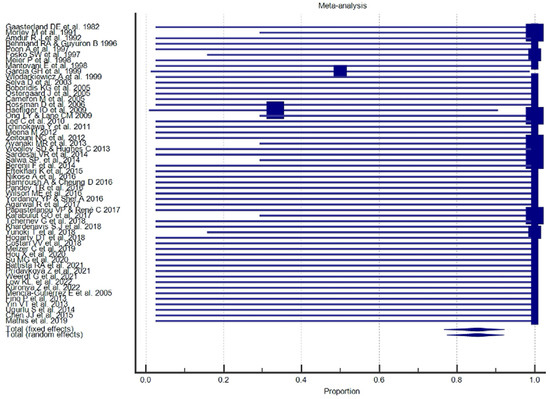
Figure 6.
Proportional meta-analysis of the included “case reports” and “case report with review of the literature”.
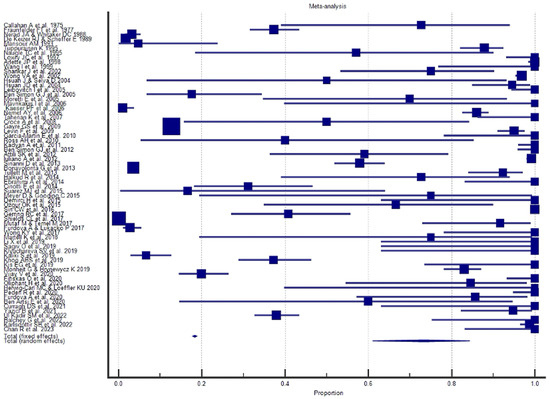
Figure 7.
Proportional meta-analysis of the included “case series”.
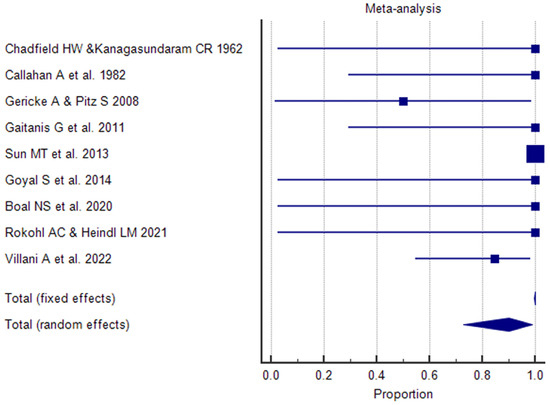
Figure 8.
Proportional meta-analysis of the included “letter”.
4. Discussion
4.1. Epidemiology and Localization
In the development of BCC, the main risk factors include UV radiation exposure, fair pigmentary characteristics, older age, genodermatoses, a family and personal history of BCC, and immunosuppression [1]. UVB radiation (290–320 nm) is believed to play a greater role than UVA radiation (320–400 nm). Different carcinogens can target different stem cell compartments and subsequently give rise to BCCs. Several hypotheses have been formulated regarding the origin of BCCs. Most BCCs seem to derive from hair follicle stem cells, while some authors claim that BCC stem cells are in the interfollicular epidermis. There is generally a latency period of several years between sun damage and the onset of BCC. Thereby, BCC develops in chronically photo-exposed areas most commonly in the head and neck [243] and accounts for 90% of malignant eyelid tumors [2]. Due to changes in sun exposure behavior and the general aging of the population, an increase in the incidence of BCC has been observed in many countries around the world. Wide regional variations in the reported incidence rates of BCC have been found, due to the latitude of the population, the study period, and the methods of recording BCC [244]. Generally, BCC arises in the elderly population. We observed an age range of 9 to 105 years for all cases examined. In 45 reports, the age range is not available. However, some patients can develop this skin cancer at an earlier age (<40 years), and patients with genetic syndromes such as Xeroderma Pigmentosum (XP) or Basal Cell Naevus Syndrome (BCNS or Gorlin–Goltz) can develop BCC earlier, even before 20 years of age [3]. In our study, 21 patients were affected by BCNS with a median age of 43 years and 27 patients suffered from XP with a mean age of 21 years. An increasing trend has been observed for patients aged ≥ 50 years over the next 10 years, and the incidence of BCC is expected to increase by 30–40% (males) and 25–30% (females) [244]. BCC is a tumor that affects both sexes, and no sex predilection has been observed [245]. In the literature, some authors, such as Saleh GM et al. in 2017 [246] in an article on eyelid basal cell carcinoma in England, reported a slight preponderance in males. Dessinioti C et al., in 2010 [247], reported a female-to-male ratio of 2:1. Our analysis showed a mild prevalence of the female sex (53.4%). However, in 91 articles, there were no data regarding the sex of patients. In the case we described, BCC occurred in a woman, who was younger than the average age reported in the literature even with a negative medical history.
The site most frequently affected by BCC is the lower eyelid, followed by the medial canthus, upper eyelid, and lateral canthus [49,63,248]. The involvement of the lower eyelid could be the consequence of light reflection by the cornea, and other irritant chemical or physical insults could be considered [109,249,250,251,252]. The uncommon envelopment of the upper eyelid could be due to the protection of the eyebrow [251].
Our analysis showed that in 193 (81.8%) articles, there was eyelid involvement; in 138 (58.5%), the upper/lower eyelid location was specified; among these, the involvement of both was reported in 89 papers (37.7%); in 38 (16.1%), just the lower eyelid was involved and in 11 (4.7%), just the upper eyelid. In the ocular and periocular regions, the growth of the tumors is characterized by a painless progressive extension; therefore, we often have the involvement of contiguous ocular structures [249].
On the eyelids, the BCC rapidly invades the dermis, followed by the infraorbital extension. On the medial canthus, the tumor can spread on the orbit and then can destroy the globe. Primary BCCs of the mucocutaneous transition region or the conjunctiva are extremely rare [252]. These sites are more frequently affected secondarily by infiltration from the tumor of the eyelid or canthus region [52]. Malignancies of the caruncle are generally very rare with a frequency of 5% among all the caruncle tumors [253,254,255]. In addition to our case of primary caruncle BCC, we identified 11 articles in the literature describing caruncle BCCs in a total of 15 patients with a median age of 61.1 years (range 24–84 years) [12,25,30,51,59,61,62,76,101,113,153].
4.2. Diagnosis
BCCs show polymorphic clinical aspects and different dermoscopical features due to their anatomical location [256,257,258]. The nodular BCC subtype occurs predominantly on the head–neck region and accounts for 60% of all BCCs. The superficial subtype represents 20% of BCCs and mainly involves the trunk, while other subtypes are less frequent. The clinical aspect of BCCs was reported in only 68 articles (28.8%). Palpable lesions resulted in the most common form (73.5%) and were described as “tumors”, “masses”, “papular”, “nodular”, or “exophytic lesions”, followed by ulcerated (36.8%) and pigmented (17.6%) lesions. Instead, sclerosing morphic-like lesions were reported in only two articles. Regarding primary caruncle BCCs, most of the papers (72.7%) mentioned the clinical aspect and described the nodular (33.3%) and pigmented (20%) lesions as the more common type. Dermatoscopic features were reported in only three articles (none regarding caruncle) of our systematic review with meta-analysis. The cause may be associated with the difficulty of exploring this anatomical area with dermatoscopy instruments. However, it is noted that only 11% of the papers were published by dermatologists, while the majority concerned ophthalmologists (67.5%). Non-dermatological assessment is probably the other main reason for the scarcity of dermatoscopic data in literature. Clinical diagnosis confirmed on dermatoscopy without histopathological examination is considered acceptable only for the small nodular form on typical locations such as the head/neck or trunk, and for the superficial BCC located on the trunk and extremities [257]. Classification of BCCs into low and high risks is based on the probability of recurrence. This risk is related to the localization on the H area of the face, aggressive histological features (perineural and/or vascular involvement), and/or immunosuppression. In case of doubtful lesions as well as high-risk BCCs, histological correlation is mandatory [256,257]. In low-risk BCCs, imaging techniques may be sufficient to confirm the diagnostic suspicion [257]. RCM has been arousing great interest in recent years in the diagnosis of skin tumors and particularly, the diagnosis of eyelid margin lesions to minimize the number of unnecessary surgical excisions [123]. Identification of RCM criteria is particularly important in identifying BCC whose clinical and dermoscopical appearance may mimic other malignant or benign lesions [259]. The RCM handheld probe can be applied directly to the skin, and it allows imaging of lesions in less accessible sites, such as the structure of the eye and oral and genital mucosa [260,261,262,263]. Currently, there is only one study published regarding the application of RCM in the eyelid area [123]. Among 47 eyelid margin lesions, the diagnosis of BCC was made in 14 cases and based on the recognition of at least 2 of the following criteria: dark silhouette, lobular nests or trabecular structures of tightly packed cells, peripheral palisading of elongated cells, peritumoral clefts, convoluted and dilated blood vessels, and polarized elongated keratinocytes (streaming) of the overlying skin. RCM sensitivity and specificity in this study resulted in 100% and 60%, respectively. In our caruncle BCC, we observed with RCM the presence of streaming, lobular nests with peripheral palisading, peritumoral clefts, and dilated blood vessels juxtaposed to the tumor islands, confirming the utility of the criteria identified by Cinotti et al. [123]. In our study, histological diagnosis was reported in 234 articles, and among them, the subtype was specified in 40.7% (nodular in 71% of the papers).
Multiple recurrences are linked to aggressive subtype, male gender, large lesion size, perineural invasion, medial canthal localization, and advanced patient age [90,264,265]. A study on 63 patients revealed that medial canthus lesions appear to have a higher risk of orbital invasion in comparison to the other ocular and periocular areas [47]. The cause may be associated with the difficulty of performing a complete surgical excision in this anatomical zone [266,267].
4.3. Treatment
BCCs belong to a special class of tumors characterized by a slow, persistent, and locally invasive growth pattern. If inadequately treated, it may progress into a large and deeply infiltrating locally advanced tumor (laBCC) or rarely (from 0.0028% to 0.55%) in a metastatic BCC (mBCC) [268].
A recent EADO classification introduced the concept of “easy to treat” and “difficult to treat” BCCs [257]. “Difficult to treat” BCCs included mBCC, laBCCs, and other types which, for any reason, pose specific management difficulties. For the treatment of BCC, different modalities can be used, but only surgical excision provides histological confirmation of successful treatment [269]. Moreover, surgeries still have the lowest recurrence rates, but they can cause functional loss or cosmetic disfigurement and have a risk of bleeding and infections [270]. Topical therapy and destructive approaches may be considered in patients with superficial BCC, while photodynamic therapy (PDT) can be an option for superficial and low-risk nodular BCCs [257]. Moreover, combined modalities such as laser CO2 and PDT could be used for selected patients with problematic reconstruction after surgery, aesthetic reasons, for patients with numerous and frequently occurring BCCs like patients affected by Gorlin–Goltz syndrome or patients who have undergone transplantation, for patients for whom anesthesia may be problematic, for patients receiving oral anticoagulants, and finally for patients who refused other treatments [269,270]. Regarding laBCCs and mBCC, treatments included hedgehog inhibitors, immunotherapy with anti-PD1, radiotherapy, and electrochemotherapy [257].
In our study, the only use of surgical techniques is reported in most of the articles (65.3%), confirming to be the first-choice treatment. Combined modality (surgery plus other treatments) found their application in 27.1% of papers, followed by non-surgical therapies in 6.8%. Only two articles did not mention the therapeutic approach. In our case of caruncle BCC, the therapeutic choice was standard surgical excision with 2 mm of free margins. We highlight the importance of RCM for the diagnosis and treatment monitoring of BCC because it allows to control histologic clearance and detect early recurrences [269,270]. Furthermore, RCM facilitates the presurgical and intrasurgical lateral and deep margin assessment of poorly defined BCCs, especially on aesthetically relevant sites such as ocular and periocular areas [271].
5. Conclusions
Our systematic review and meta-analysis collected the largest and most up-to-date collection of ocular and periocular BCCs. The site most frequently affected by BCC is the lower eyelid, followed by medial canthus which has a higher risk of orbital invasion, while primary caruncle BCC is extremely rare. Nodular BCC is the most common subtype of ocular and periocular areas. Dermoscopic and RCM studies concerning these areas are few in the literature. RCM may be very useful for early diagnosis, mapping, and treatment monitoring, especially in aesthetically relevant sites. Surgery still remains the first-choice treatment for ocular and periocular BCC, even if studies regarding the use of combined modality are increasing over time.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics15101244/s1, Table S1: List of excluded studies; Video S1: Real-time or video-mode RCM imaging of the caruncle basal cell carcinoma.
Author Contributions
Study conception and design: M.C., and V.D.M.; collection and interpretation of data: M.C., M.T., V.D.M.; manuscript drafting: M.C., and V.D.M.; manuscript editing: V.D.M.; preparation of figures: V.D.M.; preparation of tables and dataset: M.C.; statistical analysis: S.K.; review & editing of the text: P.F., M.C.F., G.P. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, took responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Italian Ministry of Health (Ricerca Corrente). All authors had full access to all the data used in this study and took complete responsibility for the integrity of the data and the accuracy of the data analysis.
Institutional Review Board Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1964 Declaration of Helsinki, as revised in 2013. Ethical review and approval have been waived for this study, as they are not required by our Institutional Ethics Committee for reports on individual cases.
Informed Consent Statement
Patients provided informed written consent to the treatment. Informed consent was also obtained from the patient for publication of this manuscript and accompanying images.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose. Honorarium, grant, or other forms of payment were not given to any of the authors to produce the manuscript.
AI Disclosure
We hereby disclose that no artificial intelligence or assisted technologies were used in the production of this study, including the creation of any figures or illustrations.
References
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Del Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, R.; Fan, X. Ocular basal cell carcinoma: A brief literature review of clinical diagnosis and treatment. OncoTargets Ther. 2017, 10, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Basset-Seguin, N.; Herms, F. Update in the Management of Basal Cell Carcinoma. Acta Derm. Venereol. 2020, 100, adv00140. [Google Scholar] [CrossRef]
- Edwards, S.J.; Osei-Assibey, G.; Patalay, R.; Wakefield, V.; Karner, C. Diagnostic accuracy of reflectance confocal microscopy using VivaScope for detecting and monitoring skin lesions: A systematic review. Clin. Exp. Dermatol. 2017, 42, 266e75. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Mazzaglia, G.; Ciardo, S.; Farnetani, F.; Mandel, V.D.; Longo, C.; Zauli, S.; Bettoli, V.; Virgili, A.; Pellacani, G. Acne: In vivo morphologic study of lesions and surrounding skin by means of reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Mandel, V.D.; Cinotti, E.; Benati, E.; Labeille, B.; Ciardo, S.; Vaschieri, C.; Cambazard, F.; Perrot, J.L.; Pellacani, G. Reflectance confocal microscopy and optical coherence tomography for the diagnosis of bullous pemphigoid and pemphigus and surrounding subclinical lesions. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1562–1569. [Google Scholar] [CrossRef]
- Chadfield, H.W.; Kanagasundaram, C.R. Carcinoma in benign mucous membrane pemphigoid (ocular pemphigus). Br. J. Dermatol. 1962, 74, 458–461. [Google Scholar] [CrossRef]
- Callahan, A.; Wilkins, R.B.; Dowling, E.A. Massive orbital invasion by small malignant lesions. South. Med. J. 1975, 68, 271–278. [Google Scholar] [CrossRef]
- Fraunfelder, F.T.; Farris, H.E., Jr.; Wallace, T.R. Cryosurgery for ocular and periocular lesions. J. Dermatol. Surg. Oncol. 1977, 3, 422–427. [Google Scholar] [CrossRef]
- Gaasterland, D.E.; Rodrigues, M.M.; Moshell, A.N. Ocular involvement in xeroderma pigmentosum. Ophthalmology 1982, 89, 980–986. [Google Scholar] [CrossRef]
- Callahan, A.; Monheit, G.D.; Callahan, M.A. Cancer excision from eyelids and ocular adnexa: The Mohs fresh tissue technique and reconstruction. CA Cancer J. Clin. 1982, 32, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; White, D.; Augsburger, J.J. Types and frequency of lesions of the caruncle. Am. J. Ophthalmol. 1986, 102, 771–778. [Google Scholar] [CrossRef]
- Nerad, J.A.; Whitaker, D.C. Periocular basal cell carcinoma in adults 35 years of age and younger. Am. J. Ophthalmol. 1988, 106, 723–729. [Google Scholar] [CrossRef]
- Rodriguez-Sains, R.S.; Robins, P.; Smith, B.; Bosniak, S.L. Radiotherapy of periocular basal cell carcinomas: Recurrence rates and treatment with special attention to the medical canthus. Br. J. Ophthalmol. 1988, 72, 134–138. [Google Scholar] [CrossRef]
- Hoppenreijs, V.P.; Cruysberg, J.R. Spontaneous repair of lower eyelid after tumour excision. Acta Ophthalmol. 1989, 67, 447–454. [Google Scholar] [CrossRef]
- De Keizer, R.J.; Scheffer, E. Masquerade of eyelid tumours. Doc. Ophthalmol. 1989, 72, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Downes, R.N.; Walker, N.P.; Collin, J.R. Micrographic (MOHS’) surgery in the management of periocular basal cell epitheliomas. Eye 1990, 4, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Morley, M.; Finger, P.T.; Perlin, M.; Weiselberg, L.R.; DeBlasio, D.S. Cis-platinum chemotherapy for ocular basal cell carcinoma. Br. J. Ophthalmol. 1991, 75, 407–410. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Deutsch, G.P. The treatment of periocular basal cell carcinomas by radiotherapy. Br. J. Ophthalmol. 1992, 76, 195–197. [Google Scholar] [CrossRef][Green Version]
- Amdur, R.J.; Kalbaugh, K.J.; Ewald, L.M.; Parsons, J.T.; Mendenhall, W.M.; Bova, F.J.; Million, R.R. Radiation therapy for skin cancer near the eye: Kilovoltage x-rays versus electrons. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 769–779. [Google Scholar] [CrossRef]
- Mansour, A.M. Adnexal findings in AIDS. Ophthalmic Plast. Reconstr. Surg. 1993, 9, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, K. Cryotherapy for eyelid and periocular basal cell carcinomas: Outcome in 166 cases over an 8-year period. Graefes. Arch. Clin. Exp. Ophthalmol. 1995, 233, 205–208. [Google Scholar] [CrossRef]
- Naugle, T.C.; Levine, M.R.; Carroll, G.S. Free graft enhancement using orbicularis muscle mobilization. Ophthalmology 1995, 102, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Behmand, R.A.; Guyuron, B. Resection of bilateral orbital and cranial base basal cell carcinoma with preservation of vision. Ann. Plast. Surg. 1996, 36, 637–640. [Google Scholar] [CrossRef]
- Poon, A.; Sloan, B.; McKelvie, P.; Davies, R. Primary basal cell carcinoma of the caruncle. Arch. Ophthalmol. 1997, 115, 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- Günalp, I.; Gündüz, K. Secondary orbital tumors. Ophthalmic Plast. Reconstr. Surg. 1997, 13, 31–35. [Google Scholar] [CrossRef]
- Fosko, S.W.; Gibney, M.D.; Holds, J.B. Basal cell carcinoma involving the lacrimal canaliculus. A documented mechanism of tumor spread. Dermatol. Surg. 1997, 23, 203–206. [Google Scholar] [CrossRef]
- Lowry, J.C.; Bartley, G.B.; Garrity, J.A. The role of second-intention healing in periocular reconstruction. Ophthalmic Plast. Reconstr. Surg. 1997, 13, 174–188. [Google Scholar] [CrossRef]
- Kersten, R.C.; Ewing-Chow, D.; Kulwin, D.R.; Gallon, M. Accuracy of clinical diagnosis of cutaneous eyelid lesions. Ophthalmology 1997, 104, 479–484. [Google Scholar] [CrossRef]
- Meier, P.; Sterker, I.; Meier, T. Primary basal cell carcinoma of the caruncle. Arch. Ophthalmol. 1998, 116, 1373–1374. [Google Scholar] [CrossRef]
- Mantovani, E.; Doro, D.; Milizia, E.; Steindler, P. Recurrent eyelid basal cell carcinoma with sclerochoroidal infiltration: Echographic findings. Ophthalmologica 1998, 212, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Arlette, J.P.; Carruthers, A.; Threlfall, W.J.; Warshawski, L.M. Basal cell carcinoma of the periocular region. J. Cutan. Med. Surg. 1998, 2, 205–208. [Google Scholar] [CrossRef]
- Der Plessis, P.J.; Dahl, M.G.; Malcolm, A.J.; Sviland, L.; Dickinson, A.J.; Lawrence, C.M. Mohs’ surgery of periocular basal cell carcinoma using formalin-fixed sections and delayed closure. Br. J. Dermatol. 1998, 138, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Inkster, C.; Ashworth, J.; Murdoch, J.R.; Montgomery, P.; Telfer, N.R.; Leatherbarrow, B. Oculoplastic reconstruction following Mohs surgery. Eye 1998, 12, 214–218. [Google Scholar] [CrossRef]
- Garcia, G.H.; Neuburg, M.; Troy, J.L.; Harris, G.J.; Gonnering, R.S. Periocular deep cutaneous basal cell carcinoma. Ophthalmic Plast. Reconstr. Surg. 1999, 15, 393–395. [Google Scholar] [CrossRef]
- Wlodarkiewicz, A.; Staniewicz, J.; Wojszwillo-Geppert, E.; Roszkiewicz, J. Extensive periocular defect reconstruction with local flaps and conchal cartilage graft. Dermatol. Surg. 1999, 25, 904–907. [Google Scholar] [CrossRef]
- Carter, K.D.; Nerad, J.A.; Whitaker, D.C. Clinical factors influencing periocular surgical defects after Mohs micrographic surgery. Ophthalmic Plast. Reconstr. Surg. 1999, 15, 83–91. [Google Scholar] [CrossRef]
- Wang, I.; Bauer, B.; Andersson-Engels, S.; Svanberg, S.; Svanberg, K. Photodynamic therapy utilising topical delta-aminolevulinic acid in non-melanoma skin malignancies of the eyelid and the periocular skin. Acta Ophthalmol. Scand. 1999, 77, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, G.; Lindblom, B.; Larkö, O. Mohs’ micrographic surgery for basal cell carcinomas on the eyelids and medial canthal area. II. Reconstruction and follow-up. Acta Ophthalmol. Scand. 2000, 78, 430–436. [Google Scholar] [CrossRef]
- Honavar, S.G.; Shields, J.A.; Shields, C.L.; Eagle, R.C., Jr.; Demirci, H.; Mahmood, E.Z. Basal cell carcinoma of the eyelid associated with Gorlin-Goltz syndrome. Ophthalmology 2001, 108, 1115–1123. [Google Scholar] [CrossRef]
- Rohrbach, J.M.; Stiemer, R.; Mayer, A.; Riedinger, C.; Duijvestijn, A.; Zierhut, M. Immunology and growth characteristics of ocular basal cell carcinoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2001, 239, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Assegid, A. Pattern of ophthalmic lesions at two histopathology centres in Ethiopia. East Afr. Med. J. 2001, 78, 250–254. [Google Scholar] [CrossRef]
- Shankar, J.; Nair, R.G.; Sullivan, S.C. Management of peri-ocular skin tumours by laissez-faire technique: Analysis of functional and cosmetic results. Eye 2002, 16, 50–53. [Google Scholar] [CrossRef]
- Wong, V.A.; Marshall, J.A.; Whitehead, K.J.; Williamson, R.M.; Sullivan, T.J. Management of periocular basal cell carcinoma with modified en face frozen section-controlled excision. Ophthalmic Plast. Reconstr. Surg. 2002, 18, 430–435. [Google Scholar] [CrossRef]
- Selva, D.; Hale, L.; Bouskill, K.; Huilgol, S.C. Recurrent morphoeic basal cell carcinoma at the lateral canthus with orbitocranial invasion. Australas. J. Dermatol. 2003, 44, 126–128. [Google Scholar] [CrossRef]
- Hsuan, J.; Selva, D. Early division of a modified Cutler-Beard flap with a free tarsal graft. Eye 2004, 18, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Huilgol, S.C.; Huynh, N.T.; Selva, D. The Australian Mohs database, part I: Periocular basal cell carcinoma experience over 7 years. Ophthalmology 2004, 111, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, J.D.; Harrad, R.A.; Potts, M.J.; Collins, C. Small margin excision of periocular basal cell carcinoma: 5-year results. Br. J. Ophthalmol. 2004, 88, 358–360. [Google Scholar] [CrossRef]
- Malhotra, R.; Huilgol, S.C.; Huynh, N.T.; Selva, D. The Australian Mohs database, part II: Periocular basal cell carcinoma outcome at 5-year follow-up. Ophthalmology 2004, 111, 631–636. [Google Scholar] [CrossRef]
- Boboridis, K.G.; Dimitrakos, S.A.; Georgiadis, N.S.; Stangos, N.T. Combination of periocular myocutaneous flaps for one-stage reconstruction of extensive defects of the eyelid. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005, 39, 100–103. [Google Scholar] [CrossRef]
- Østergaard, J.; Boberg-Ans, J.; Prause, J.U.; Heegaard, S. Primary basal cell carcinoma of the caruncle with seeding to the conjunctiva. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, I.; McNab, A.; Sullivan, T.; Davis, G.; Selva, D. Orbital invasion by periocular basal cell carcinoma. Ophthalmology 2005, 112, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Ben Simon, G.J.; Schwarcz, R.M.; Douglas, R.; Fiaschetti, D.; McCann, J.D.; Goldberg, R.A. Orbital exenteration: One size does not fit all. Am. J. Ophthalmol. 2005, 139, 11–17. [Google Scholar] [CrossRef]
- Cameron, M.; Gilbert, P.M.; Mulhern, M.G.; Sneddon, K.J. Synchronous reconstruction of the exenterated orbit with a pericranial flap, skin graft and osseointegrated implants. Orbit 2005, 24, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Ortiz, S.; Gómez García, F.; Barrovechio, J.C.; Valvo, C. Complete mobilization of the cheek zone for orbit exenteration. J. Craniofac. Surg. 2005, 16, 823–828. [Google Scholar] [CrossRef]
- Khandwala, M.A.; Lalchan, S.A.; Chang, B.Y.; Habib, M.; Chakrabarty, A.; Cassells-Brown, A. Outcome of periocular basal cell carcinoma managed by overnight paraffin section. Orbit 2005, 24, 243–247. [Google Scholar] [CrossRef]
- Papadopoulos, O.; Konofaos, P.; Chrisostomidis, C.; Georgiou, P.; Frangoulis, M.; Champsas, G.; Betsi, E.; Zapantis-Fragos, M. Orbitopalpebral repair after 835 excisions of malignant tumours. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005, 39, 353–359. [Google Scholar] [CrossRef]
- Leibovitch, I.; Huilgol, S.C.; Hsuan, J.D.; Selva, D. Incidence of host site complications in periocular full thickness skin grafts. Br. J. Ophthalmol. 2005, 89, 219–222. [Google Scholar] [CrossRef]
- Mencía-Gutiérrez, E.; Gutiérrez-Díaz, E.; Pérez-Martín, M.E. Lacrimal caruncle primary basal cell carcinoma: Case report and review. J. Cutan. Pathol. 2005, 32, 502–505. [Google Scholar] [CrossRef]
- Mavrikakis, I.; Malhotra, R.; Barlow, R.; Huilgol, S.C.; Selva, D. Linear basal cell carcinoma: A distinct clinical entity in the periocular region. Ophthalmology 2006, 113, 338–342. [Google Scholar] [CrossRef]
- Rossman, D.; Arthurs, B.; Odashiro, A.; Saraiva, V.; Burnier, M., Jr. Basal cell carcinoma of the caruncle. Ophthalmic Plast. Reconstr. Surg. 2006, 22, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Kaeser, P.F.; Uffer, S.; Zografos, L.; Hamédani, M. Tumors of the caruncle: A clinicopathologic correlation. Am. J. Ophthalmol. 2006, 142, 448–455. [Google Scholar] [CrossRef]
- Nemet, A.Y.; Deckel, Y.; Martin, P.A.; Kourt, G.; Chilov, M.; Sharma, V.; Benger, R. Management of periocular basal and squamous cell carcinoma: A series of 485 cases. Am. J. Ophthalmol. 2006, 142, 293–297. [Google Scholar] [CrossRef]
- Taherian, K.; Shekarchian, M.; Atkinson, P.L. Surgical excision of periocular basal cell carcinomas. Indian J. Ophthalmol. 2007, 55, 137–138. [Google Scholar] [CrossRef]
- Gericke, A.; Pitz, S. Maggot therapy for periocular skin graft failure in the immunocompromised patient. Br. J. Ophthalmol. 2008, 92, 860–861. [Google Scholar] [CrossRef]
- Croce, A.; Moretti, A.; D’Agostino, L.; Zingariello, P. Orbital exenteration in elderly patients: Personal experience. Acta Otorhinolaryngol. Ital. 2008, 28, 193–199. [Google Scholar]
- Kovacevic, P.T.; Visnjic, M.M.; Kovacevic, T.T.; Radojkovic, M.R.; Stojanovic, M.R. Extended orbital exenteration in the treatment of advanced periocular skin cancer with primary reconstruction with a galeacutaneous flap. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2009, 43, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.M.; Haniffa, M.; Dahl, M.G. Formalin-fixed tissue Mohs surgery (slow Mohs) for basal cell carcinoma: 5-year follow-up data. Br. J. Dermatol. 2009, 160, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Gayre, G.S.; Hybarger, C.P.; Mannor, G.; Meecham, W.; Delfanti, J.B.; Mizono, G.S.; Guerry, T.L.; Chien, J.S.; Sooy, C.D.; Anooshian, R.; et al. Outcomes of excision of 1750 eyelid and periocular skin basal cell and squamous cell carcinomas by modified en face frozen section margin-controlled technique. Int. Ophthalmol. Clin. 2009, 49, 97–110. [Google Scholar] [CrossRef]
- Lavaju, P.; Arya, S.K.; Sinha, A.; Pandey, S.; Adhikari, S.; Shrestha, B.G.; Chetan, S.; Agarwal, T.L. Pattern of ocular tumors in the eastern region of Nepal. Nepal. J. Ophthalmol. 2009, 1, 9–12. [Google Scholar] [CrossRef][Green Version]
- Haefliger, I.O.; Trittibach, P.; Pimentel, A.R.; Piffaretti, J.M. Large upper eyelid full-thickness defects reconstructed only with an anterior lamella. Klin. Monatsblätter Augenheilkd. 2009, 226, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.S.; Elzaridi, E.; Clarke, L.; Dickinson, A.J.; Lawrence, C.M. Periocular basal cell carcinoma: 5-year outcome following Slow Mohs surgery with formalin-fixed paraffin-embedded sections and delayed closure. Br. J. Ophthalmol. 2009, 93, 474–476. [Google Scholar] [CrossRef]
- Levin, F.; Khalil, M.; McCormick, S.A.; Della Rocca, D.; Maher, E.; Della Rocca, R.C. Excision of periocular basal cell carcinoma with stereoscopic microdissection of surgical margins for frozen-section control: Report of 200 cases. Arch. Ophthalmol. 2009, 127, 1011–1015. [Google Scholar] [CrossRef]
- Chadha, V.; Wright, M. Small margin excision of periocular basal cell carcinomas. Br. J. Ophthalmol. 2009, 93, 803–806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ong, L.Y.; Lane, C.M. Eyelid contracture may indicate recurrent basal cell carcinoma, even after Mohs’ micrographic surgery. Orbit 2009, 28, 29–33. [Google Scholar] [CrossRef]
- Lee, C.L.; Hsu, S.L.; Chang, C.H. Primary ocular caruncular basal cell carcinoma in a Chinese patient. Kaohsiung J. Med. Sci. 2010, 26, 562–566. [Google Scholar] [CrossRef]
- Maheshwari, R. Review of orbital exenteration from an eye care centre in Western India. Orbit 2010, 29, 35–38. [Google Scholar] [CrossRef]
- Carneiro, R.C.; de Macedo, E.M.; Matayoshi, S. Imiquimod 5% cream for the treatment of periocular Basal cell carcinoma. Ophthalmic Plast. Reconstr. Surg. 2010, 26, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.; Idoipe, M.; Gil, L.M.; Pueyo, V.; Alfaro, J.; Pablo, L.E.; Zubiri, M.L.; Fernandez, J. Efficacy and tolerability of imiquimod 5% cream to treat periocular basal cell carcinomas. J. Ocul. Pharmacol. Ther. 2010, 26, 373–379. [Google Scholar] [CrossRef]
- Madge, S.N.; Khine, A.A.; Thaller, V.T.; Davis, G.; Malhotra, R.; McNab, A.; O’Donnell, B.; Selva, D. Globe-sparing surgery for medial canthal Basal cell carcinoma with anterior orbital invasion. Ophthalmology 2010, 117, 2222–2228. [Google Scholar] [CrossRef]
- Ross, A.H.; Kennedy, C.T.; Collins, C.; Harrad, R.A. The use of imiquimod in the treatment of periocular tumours. Orbit 2010, 29, 83–87. [Google Scholar] [CrossRef]
- Cannon, P.S.; O’Donnell, B.; Huilgol, S.C.; Selva, D. The ophthalmic side-effects of imiquimod therapy in the management of periocular skin lesions. Br. J. Ophthalmol. 2011, 95, 1682–1685. [Google Scholar] [CrossRef]
- Alves, L.F.; Fernandes, B.F.; Burnier, J.V.; Zoroquiain, P.; Eskenazi, D.T.; Burnier, M.N., Jr. Incidence of epithelial lesions of the conjunctiva in a review of 12,102 specimens in Canada (Quebec). Arq. Bras. Oftalmol. 2011, 74, 21–23. [Google Scholar] [CrossRef]
- Shinder, R.; Al-Zubidi, N.; Esmaeli, B. Survey of orbital tumors at a comprehensive cancer center in the United States. Head Neck 2011, 33, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Alfawaz, A.M.; Al-Hussain, H.M. Ocular manifestations of xeroderma pigmentosum at a tertiary eye care center in Saudi Arabia. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Adhikari, R.K.; Sharma, B.R. A profile of eye-lid conditions requiring reconstruction among the patients attending an oculoplasty clinic in mid-western region of Nepal. Nepal. J. Ophthalmol. 2011, 3, 49–51. [Google Scholar] [CrossRef]
- Moesen, I.; Duncan, M.; Cates, C.; Taylor, A.; Wintle, R.V.; Ismail, A.; Lim, D.K.; Tyers, A.G. Nitrous oxide cryotherapy for primary periocular basal cell carcinoma: Outcome at 5 years follow-up. Br. J. Ophthalmol. 2011, 95, 1679–1681. [Google Scholar] [CrossRef] [PubMed]
- Ichinokawa, Y.; Ohtuki, A.; Hattori, M.; Sadamasa, H.; Hiruma, M.; Matumoto, T. Linear Basal cell carcinoma: A case report. Case Rep. Dermatol. 2011, 3, 142–146. [Google Scholar] [CrossRef]
- Garcia-Martin, E.; Gil-Arribas, L.M.; Idoipe, M.; Alfaro, J.; Pueyo, V.; Pablo, L.E.; Fernandez, F.J. Comparison of imiquimod 5% cream versus radiotherapy as treatment for eyelid basal cell carcinoma. Br. J. Ophthalmol. 2011, 95, 1393–1396. [Google Scholar] [CrossRef]
- Gaitanis, G.; Kalogeropoulos, C.; Bassukas, I.D. Imiquimod can be combined with cryosurgery (immunocryosurgery) for locally advanced periocular basal cell carcinomas. Br. J. Ophthalmol. 2011, 95, 890–892. [Google Scholar] [CrossRef]
- Kadyan, A.; Edmunds, M.R.; Amissah-Arthur, K.N.; Durrani, O.M. High rate of incomplete resection after primary excision of eyelid BCC: Multi-staged resection rarely needs more than two procedures. Orbit 2011, 30, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kvannli, L.; Benger, R.; Gal, A.; Swamy, B. The method of en face frozen section in clearing periocular basal cell carcinoma and squamous cell carcinoma. Orbit 2012, 31, 233–237. [Google Scholar] [CrossRef]
- Ben Simon, G.J.; Lukovetsky, S.; Lavinsky, F.; Rosen, N.; Rosner, M. Histological and clinical features of primary and recurrent periocular Basal cell carcinoma. ISRN Ophthalmol. 2012, 2012, 354829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertelmann, E.; Rieck, P. Relapses after surgical treatment of ocular adnexal basal cell carcinomas: 5-year follow-up at the same university centre. Acta Ophthalmol. 2012, 90, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Koike, I.; Maegawa, J.; Kaneko, A.; Odagiri, K.; Kasuya, T.; Minagawa, Y.; Kaizu, H.; Mukai, Y.; Inoue, T. Radiation therapy for primary carcinoma of the eyelid: Tumor control and visual function. Strahlenther. Onkol. 2012, 188, 1102–1107. [Google Scholar] [CrossRef]
- Meena, M. Triple-Flaps for lateral canthus reconstruction: A novel technique. Oman J. Ophthalmol. 2012, 5, 181–183. [Google Scholar] [CrossRef]
- Attili, S.K.; Ibbotson, S.H.; Fleming, C. Role of non-surgical therapies in the management of periocular basal cell carcinoma and squamous intra-epidermal carcinoma: A case series and review of the literature. Photodermatol. Photoimmunol. Photomed. 2012, 28, 68–79. [Google Scholar] [CrossRef]
- Iuliano, A.; Strianese, D.; Uccello, G.; Diplomatico, A.; Tebaldi, S.; Bonavolontà, G. Risk factors for orbital exenteration in periocular Basal cell carcinoma. Am. J. Ophthalmol. 2012, 153, 238–241. [Google Scholar] [CrossRef]
- Zeitouni, N.C.; Raghu, P.R.; Mansour, T.N. Orbital invasion by periocular infiltrating Basal cell carcinoma. Dermatol. Surg. 2012, 38, 2025–2027. [Google Scholar] [CrossRef]
- Sirianni, D.; Leles, C.R.; Mendonça, E.F. A 12-year retrospective survey of management of patients with malignant neoplasms in the orbital cavity in a brazilian cancer hospital. Open Dent. J. 2013, 7, 140–145. [Google Scholar] [CrossRef]
- Fino, P.; Onesti, M.G.; Fioramonti, P.; Romanzi, A.; Scuderi, N. First reported case of primary basal cell carcinoma of the right caruncle: A case report and review of the literature. In Vivo 2013, 27, 535–539. [Google Scholar]
- Krema, H.; Herrmann, E.; Albert-Green, A.; Payne, D.; Laperriere, N.; Chung, C. Orthovoltage radiotherapy in the management of medial canthal basal cell carcinoma. Br. J. Ophthalmol. 2013, 97, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Bonavolontà, G.; Strianese, D.; Grassi, P.; Comune, C.; Tranfa, F.; Uccello, G.; Iuliano, A. An analysis of 2,480 space-occupying lesions of the orbit from 1976 to 2011. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 79–86. [Google Scholar] [CrossRef]
- Yin, V.T.; Pfeiffer, M.L.; Esmaeli, B. Targeted therapy for orbital and periocular basal cell carcinoma and squamous cell carcinoma. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; Wu, A.; Huilgol, S.C.; Selva, D. Periocular basal cell carcinoma pathological reporting. Br. J. Ophthalmol. 2013, 97, 1612–1613. [Google Scholar] [CrossRef]
- Tullett, M.; Sagili, S.; Barrett, A.; Malhotra, R. Excision of periocular basal cell carcinoma guided by en face frozen section. Br. J. Oral Maxillofac. Surg. 2013, 51, 520–524. [Google Scholar] [CrossRef]
- Gill, H.S.; Moscato, E.E.; Chang, A.L.; Soon, S.; Silkiss, R.Z. Vismodegib for periocular and orbital basal cell carcinoma. JAMA Ophthalmol. 2013, 131, 1591–1594. [Google Scholar] [CrossRef]
- Litwin, A.S.; Rytina, E.; Ha, T.; René, C.; Woodruff, S.A. Management of periocular basal cell carcinoma by Mohs micrographic surgery. J. Dermatol. Treat. 2013, 24, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.F.; Brown, L.; Bamford, M.; Sampath, R.; Burns, J. 5 years review of periocular basal cell carcinoma and proposed follow-up protocol. Eye 2013, 27, 78–83. [Google Scholar] [CrossRef]
- Pelosini, L.; Smith, H.B.; Schofield, J.B.; Meeckings, A.; Dhital, A.; Khandwala, M. In vivo optical coherence tomography (OCT) in periocular basal cell carcinoma: Correlations between in vivo OCT images and postoperative histology. Br. J. Ophthalmol. 2013, 97, 890–894. [Google Scholar] [CrossRef]
- Avanaki, M.R.; Hojjatoleslami, A.; Sira, M.; Schofield, J.B.; Jones, C.; Podoleanu, A.G. Investigation of basal cell carcinoma using dynamic focus optical coherence tomography. Appl. Opt. 2013, 52, 2116–2124. [Google Scholar] [CrossRef]
- Woolley, S.D.; Hughes, C. A young military pilot presents with a periocular Basal Cell Carcinoma: A case report. Travel Med. Infect. Dis. 2013, 11, 435–437. [Google Scholar] [CrossRef]
- Ugurlu, S.; Ekin, M.A.; Altinboga, A.A. Primary basal cell carcinoma of the caruncle: Case report and review of the literature. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 62–64. [Google Scholar] [CrossRef]
- Sardesai, V.R.; Omchery, A.S.; Trasi, S.S. Ocular myiasis with Basal cell carcinoma. Indian J. Dermatol. 2014, 59, 308–309. [Google Scholar] [CrossRef]
- Salwa, S.P.; Bourke, M.G.; Forde, P.F.; O’Shaughnessy, M.; O’Sullivan, S.T.; Kelly, E.J.; Soden, D.M.; Clover, A.J.P. Electrochemotherapy for the treatment of ocular basal cell carcinoma; a novel adjunct in the disease management. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Halkud, R.; Shenoy, A.M.; Naik, S.M.; Chavan, P.; Sidappa, K.T.; Biswas, S. Xeroderma pigmentosum: Clinicopathological review of the multiple oculocutaneous malignancies and complications. Indian J. Surg. Oncol. 2014, 5, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Deka, P.; Ramachandra, V.; Bhattacharjee, K.; Das, J.K.; Kuri, G.C.; Tahiliani, P.S.; Bhattacharyya, P.; Bhattacharjee, H.; Paul, R.; et al. Profile of ocular and adnexal tumours at a tertiary institute of northeast India. Orbit 2014, 33, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Qassemyar, A.; Aljudaibi, N.; Wavreille, O.; Mortier, L.; Martinot-Duquennoy, V.; Guerreschi, P. Orbital exenteration and periorbital skin cancers. J. Oral. Maxillofac. Surg. 2014, 72, 811–816. [Google Scholar] [CrossRef]
- Berenji, F.; Hosseini-Farash, B.R.; Marvi-Moghadam, N. A Case of Secondary Ophthalmomyiasis Caused by Chrysomya bezziana (Diptera: Calliphoridae). J. Arthropod Borne Dis. 2014, 9, 125–130. [Google Scholar]
- Wu, A.; Sun, M.T.; Huilgol, S.C.; Madge, S.; Selva, D. Histological subtypes of periocular basal cell carcinoma. Clin. Exp. Ophthalmol. 2014, 42, 603–607. [Google Scholar] [CrossRef]
- Goyal, S.; Shiver, M.B.; Banda, H.; Brown, H.H.; Pemberton, J.D. Nonhealing trauma masking periocular basal cell carcinoma in a young black male. Can. J. Ophthalmol. 2014, 49, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Rezaei, M.; Kavoussi, R.; Eidizadeh, M.; Madani, S.H.; Kavoussi, H. Superpulsed CO2 Laser with Intraoperative Pathologic Assessment for Treatment of Periorbital Basal Cell Carcinoma Involving Eyelash Line. Dermatol. Res. Pract. 2014, 2014, 931657. [Google Scholar] [CrossRef][Green Version]
- Cinotti, E.; Perrot, J.L.; Campolmi, N.; Labeille, B.; Espinasse, M.; Grivet, D.; Thuret, G.; Gain, P.; Douchet, C.; Forest, F.; et al. The role of in vivo confocal microscopy in the diagnosis of eyelid margin tumors: 47 cases. J. Am. Acad. Dermatol. 2014, 71, 912–918. [Google Scholar] [CrossRef] [PubMed]
- De Macedo, E.M.; Carneiro, R.C.; de Lima, P.P.; Silva, B.G.; Matayoshi, S. Imiquimod cream efficacy in the treatment of periocular nodular basal cell carcinoma: A non-randomized trial. BMC Ophthalmol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, K.; Anderson, R.L.; Suneja, G.; Bowen, A.; Oberg, T.J.; Bowen, G.M. Local Recurrence and Ocular Adnexal Complications Following Electronic Surface Brachytherapy for Basal Cell Carcinoma of the Lower Eyelid. JAMA Dermatol. 2015, 151, 1002–1004. [Google Scholar] [CrossRef][Green Version]
- Sen, S.; Lyngdoh, A.D.; Pushker, N.; Meel, R.; Bajaj, M.S.; Chawla, B. Impression cytology diagnosis of ulcerative eyelid malignancy. Cytopathology 2015, 26, 26–30. [Google Scholar] [CrossRef]
- Suarez, M.J.; Rivera-Michlig, R.; Dubovy, S.; Rodriguez, F.J. Clinicopathological Features of Ophthalmic Neoplasms Arising in the Setting of Xeroderma Pigmentosum. Ocul. Oncol. Pathol. 2015, 2, 112–121. [Google Scholar] [CrossRef]
- Meyer, D.; Gooding, C. Intralesional Bleomycin as an Adjunct Therapeutic Modality in Eyelid and Extraocular Malignancies and Tumors. Middle East Afr. J. Ophthalmol. 2015, 22, 410–414. [Google Scholar] [CrossRef]
- Chen, J.J.; Sartori, J.; Aakalu, V.K.; Setabutr, P. Review of Ocular Manifestations of Nevoid Basal Cell Carcinoma Syndrome: What an Ophthalmologist Needs to Know. Middle East Afr. J. Ophthalmol. 2015, 22, 421–427. [Google Scholar]
- Domingo, R.E.; Manganip, L.E.; Castro, R.M. Tumors of the eye and ocular adnexa at the Philippine Eye Research Institute: A 10-year review. Clin. Ophthalmol. 2015, 9, 1239–1247. [Google Scholar] [CrossRef]
- Demirci, H.; Worden, F.; Nelson, C.C.; Elner, V.M.; Kahana, A. Efficacy of Vismodegib (Erivedge) for Basal Cell Carcinoma Involving the Orbit and Periocular Area. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 463–466. [Google Scholar] [CrossRef]
- Ozgur, O.K.; Yin, V.; Chou, E.; Ball, S.; Kies, M.; William, W.N.; Migden, M.; Thuro, B.A.; Esmaeli, B. Hedgehog Pathway Inhibition for Locally Advanced Periocular Basal Cell Carcinoma and Basal Cell Nevus Syndrome. Am. J. Ophthalmol. 2015, 160, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; Wu, A.; Huilgol, S.C.; Selva, D. Accuracy of Biopsy in Subtyping Periocular Basal Cell Carcinoma. Ophthalmic Plast. Reconstr. Surg. 2015, 31, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Lin, F.P.; Sheck, L.H.; Salmon, P.J.; Ng, S.G. Growth of periocular basal cell carcinomas. Br. J. Dermatol. 2015, 172, 1002–1007. [Google Scholar] [CrossRef]
- Pelosini, L.; Smith, H.B.; Schofield, J.B.; Meeckings, A.; Dithal, A.; Khandwala, M. A novel imaging approach to periocular basal cell carcinoma: In vivo optical coherence tomography and histological correlates. Eye 2015, 29, 1092–1098. [Google Scholar] [CrossRef][Green Version]
- Nikose, A.S.; Laddha, P.M.; Bisen, R.R. Periocular basal cell carcinoma in a young school teacher. Int. J. Appl. Basic Med. Res. 2016, 6, 143–145. [Google Scholar] [CrossRef]
- Hamroush, A.; Cheung, D. Irregularly luscious lashes: Difficult to say but a sinister sign to miss. BMJ Case Rep. 2016, 2016, bcr2016215590. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Pazdrowski, J.; Golusiński, P.; Dańczak-Pazdrowska, A.; Łuczewski, Ł.; Marszałek, S.; Majchrzak, E.; Golusiński, W. Basal cell carcinoma in farmers: An occupation group at high risk. Int. Arch. Occup. Environ. Health 2016, 89, 497–501. [Google Scholar] [CrossRef]
- Pandey, T.R.; Shrestha, G.B.; Sitaula, R.K.; Shah, D.N. A Case of Orbital Myiasis in Recurrent Eyelid Basal Cell Carcinoma Invasive into the Orbit. Case Rep. Ophthalmol. Med. 2016, 2016, 2904346. [Google Scholar] [CrossRef]
- Bălăşoiu, A.T.; Ciurea, R.N.; Mănescu, M.R.; Mocanu, C.L.; Stepan, A.E.; Bălăşoiu, M.; Niculescu, M. Assessment of VEGF and EGFR in the study of angiogenesis of eyelid carcinomas. Rom. J. Morphol. Embryol. 2016, 57, 1229–1234. [Google Scholar]
- Treacy, M.P.; Wynne, N.C.; Gale, J.L.; Duignan, E.; Moran, B.; Flynn, A.M.; Ormond, P.; Barry, R.; Khan, R.; Moriarty, P.; et al. Mohs micrographic surgery for periocular skin tumours in Ireland. Ir. J. Med. Sci. 2016, 185, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Pointdujour-Lim, R.; Lally, S.; Shields, C.L.; Rabinowitz, M.P. Acute Charles Bonnet Syndrome following Hughes procedure. Orbit 2016, 35, 292–294. [Google Scholar] [CrossRef]
- Sin, C.W.; Barua, A.; Cook, A. Recurrence rates of periocular basal cell carcinoma following Mohs micrographic surgery: A retrospective study. Int. J. Dermatol. 2016, 55, 1044–1047. [Google Scholar] [CrossRef]
- Gaitanis, G.; Kalogeropoulos, C.D.; Bassukas, I.D. Cryosurgery during Imiquimod (Immunocryosurgery) for Periocular Basal Cell Carcinomas: An Efficacious Minimally Invasive Treatment Alternative. Dermatology 2016, 232, 17–21. [Google Scholar] [CrossRef]
- Celebi, A.R.; Kiratli, H.; Soylemezoglu, F. Evaluation of the ‘Hedgehog’ signaling pathways in squamous and basal cell carcinomas of the eyelids and conjunctiva. Oncol. Lett. 2016, 12, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Liao, S.L.; Hong, J.B.; Chu, C.Y.; Sheen, Y.S.; Jhuang, J.Y.; Tsai, J.H.; Liau, J.Y. TERT promoter mutations in periocular carcinomas: Implications of ultraviolet light in pathogenesis. Br. J. Ophthalmol. 2016, 100, 274–277. [Google Scholar] [CrossRef]
- Yordanov, Y.P.; Shef, A. Synchronous Basal Cell Carcinoma of the Inferior Eyelid-Combined Surgical Approach for Single-Stage Ablation. J. Craniofac. Surg. 2016, 27, e80–e81. [Google Scholar] [CrossRef]
- Zlatarova, Z.I.; Nenkova, B.N.; Softova, E.B. Eyelid Reconstruction with Full Thickness Skin Grafts After Carcinoma Excision. Folia Med. 2016, 58, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Gerring, R.C.; Ott, C.T.; Curry, J.M.; Sargi, Z.B.; Wester, S.T. Orbital exenteration for advanced periorbital non-melanoma skin cancer: Prognostic factors and survival. Eye 2017, 31, 379–388. [Google Scholar] [CrossRef]
- Shafi, F.; Rathore, D.; Johnson, A.; Mehta, P.; Ahluwalia, H.S. Medial canthal defects following tumour excision: To reconstruct or not to reconstruct? Orbit 2017, 36, 64–68. [Google Scholar] [CrossRef]
- Agarwal, R.; Chawla, B.; Asif, M.I.; Pujari, A. Bilateral ocular surface squamous neoplasia with bilateral periocular basal cell carcinoma in a case of xeroderma pigmentosum. BMJ Case Rep. 2017, 2017, bcr2017220882. [Google Scholar] [CrossRef]
- Khan, L.; Malukani, M.; Saxena, A. Conjunctival Lesions: When Should We Perform Biopsy? Nepal. J. Ophthalmol. 2017, 9, 160–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shields, C.L.; Alset, A.E.; Boal, N.S.; Casey, M.G.; Knapp, A.N.; Sugarman, J.A.; Schoen, M.A.; Gordon, P.S.; Douglass, A.M.; Sioufi, K.; et al. Conjunctival Tumors in 5002 Cases. Comparative Analysis of Benign Versus Malignant Counterparts. The 2016 James D. Allen Lecture. Am. J. Ophthalmol. 2017, 173, 106–133. [Google Scholar] [CrossRef] [PubMed]
- Mutaf, M.; Temel, M. A New Technique for Total Reconstruction of the Lower Lid. Ann. Plast. Surg. 2017, 78, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kiratli, H.; Koç, İ. Orbital exenteration: Institutional review of evolving trends in indications and rehabilitation techniques. Orbit 2018, 37, 179–186. [Google Scholar] [CrossRef]
- Furdova, A.; Lukacko, P. Periocular Basal Cell Carcinoma Predictors for Recurrence and Infiltration of the Orbit. J. Craniofac. Surg. 2017, 28, e84–e87. [Google Scholar] [CrossRef]
- Wong, K.Y.; Fife, K.; Lear, J.T.; Price, R.D.; Durrani, A.J. Vismodegib for Locally Advanced Periocular and Orbital Basal Cell Carcinoma: A Review of 15 Consecutive Cases. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1424. [Google Scholar] [CrossRef]
- Tan, E.; Lin, F.; Sheck, L.; Salmon, P.; Ng, S. A practical decision-tree model to predict complexity of reconstructive surgery after periocular basal cell carcinoma excision. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 717–723. [Google Scholar] [CrossRef]
- Papastefanou, V.P.; René, C. Secondary Resistance to Vismodegib After Initial Successful Treatment of Extensive Recurrent Periocular Basal Cell Carcinoma with Orbital Invasion. Ophthalmic Plast. Reconstr. Surg. 2017, 33 (Suppl. S1), S68–S70. [Google Scholar] [CrossRef]
- Karabulut, G.O.; Kaynak, P.; Ozturker, C.; Fazil, K.; Ocak, O.B.; Taskapılı, M. Imiquimod 5% cream for the treatment of large nodular basal cell carcinoma at the medial canthal area. Indian J. Ophthalmol. 2017, 65, 48–51. [Google Scholar] [CrossRef]
- Fatigato, G.; Capitani, S.; Milani, D.; Grassilli, S.; Alameen, A.A.; Candiani, M.; Riberti, C.; Galasso, M.; Previati, M. Risk factors associated with relapse of eyelid basal cell carcinoma: Results from a retrospective study of 142 patients. Eur. J. Dermatol. 2017, 27, 363–368. [Google Scholar] [CrossRef]
- O’Halloran, L.; Smith, H.; Vinciullo, C. Periocular Mohs micrographic surgery in Western Australia 2009–2012: A single centre retrospective review and proposal for practice benchmarks. Australas. J. Dermatol. 2017, 58, 106–110. [Google Scholar] [CrossRef]
- Bladen, J.C.; Moosajee, M.; Tracey-White, D.; Beaconsfield, M.; O’Toole, E.A.; Philpott, M.P. Analysis of hedgehog signaling in periocular sebaceous carcinoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 256, 853–860. [Google Scholar] [CrossRef]
- Tchernev, G.; Lotti, T.; Lozev, I.; Maximov, G.K.; Wollina, U. Locally Advanced Basal Cell Carcinoma with Intraocular Invasion. Open Access Maced. J. Med. Sci. 2018, 6, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, U.; Loeffler, K.U.; Nadal, J.; Holz, F.G.; Herwig-Carl, M.C. Polarization and Distribution of Tumor-Associated Macrophages and COX-2 Expression in Basal Cell Carcinoma of the Ocular Adnexae. Curr. Eye Res. 2018, 43, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Khardenavis, S.J.; Kulkarni, S.; Khardenavis, V.; Deshpande, A. Ophthalmomyiasis in a case of basal cell carcinoma of eyelid. BMJ Case Rep. 2018, 2018, bcr2018225150. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, T.; Tabuchi, Y.; Hirano, T.; Miwa, S.; Imura, J.; Hayashi, A. Gene networks in basal cell carcinoma of the eyelid, analyzed using gene expression profiling. Oncol. Lett. 2018, 16, 6729–6734. [Google Scholar] [CrossRef]
- Al Wohaib, M.; Al Ahmadi, R.; Al Essa, D.; Maktabbi, A.; Khandekar, R.; Al Sharif, E.; Al Katan, H.; Schellini, S.A.; Al Shaikh, O. Characteristics and Factors Related to Eyelid Basal Cell Carcinoma in Saudi Arabia. Middle East Afr. J. Ophthalmol. 2018, 25, 96–102. [Google Scholar]
- Hogarty, D.T.; Dewhurst, N.G.; Burt, B. Vismodegib and orbital excision for treating locally advanced basal cell carcinoma. Int. Med. Case Rep. J. 2018, 11, 177–179. [Google Scholar] [CrossRef]
- Espi, P.; Parajuli, S.; Benfodda, M.; Lebre, A.S.; Paudel, U.; Grange, A.; Grybek, V.; Grange, T.; Soufir, N.; Grange, F. Clinical and genetic characteristics of xeroderma pigmentosum in Nepal. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 832–839. [Google Scholar] [CrossRef]
- Damasceno, J.C.; Isenberg, J.; Lopes, L.R.; Hime, B.; Fernandes, B.F.; Lowen, M.; Camargo, L.M.A.; Belfort, R.N. Largest case series of Latin American eyelid tumors over 13-Years from a single center in Sao Paulo, Brazil. Arq. Bras. Oftalmol. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Lemaître, S.; Lévy-Gabriel, C.; Desjardins, L.; González-Candial, M.; Gardrat, S.; Dendale, R.; Cassoux, N.; Couturaud, B. Outcomes after surgical resection of lower eyelid tumors and reconstruction using a nasal chondromucosal graft and an upper eyelid myocutaneous flap. J. Français Ophtalmol. 2018, 41, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Costan, V.V.; Tamaş, C.; Dobrin, N.; Costache, D.A.; Ciocoiu, M. Mixed (nodular and morpheic) upper eyelid basal cell carcinoma with orbital invasion—Histological and clinical features. Rom. J. Morphol. Embryol. 2018, 59, 977–983. [Google Scholar]
- Martell, K.; Poirier, Y.; Zhang, T.; Hudson, A.; Spencer, D.; Jacso, F.; Hayashi, R.; Banerjee, R.; Khan, R.; Wolfe, N.; et al. Radiation therapy for deep periocular cancer treatments when protons are unavailable: Is combining electrons and orthovoltage therapy beneficial? J. Radiat. Res. 2018, 59, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, L.; Kou, H.; Zhang, J.; Wang, Y.; Li, G.; Lu, Y. Ocular preservation through limited tumor excision combined with ALA-PDT in patients with periocular basal cell carcinoma. Photodiagnosis Photodyn. Ther. 2019, 27, 291–294. [Google Scholar] [CrossRef]
- Sagiv, O.; Nagarajan, P.; Ferrarotto, R.; Kandl, T.J.; Thakar, S.D.; Glisson, B.S.; Altan, M.; Esmaeli, B. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br. J. Ophthalmol. 2019, 103, 775–780. [Google Scholar] [CrossRef] [PubMed]
- McGrath, L.A.; Meeney, A.; Currie, Z.I.; Mudhar, H.S.; Tan, J.H. Staged excision of primary periocular basal cell carcinoma: Absence of residual tumour in re-excised specimens: A 10-year series. Br. J. Ophthalmol. 2019, 103, 976–979. [Google Scholar] [CrossRef]
- Klyuchareva, S.V.; Ponomarev, I.V.; Topchiy, S.B.; Pushkareva, A.E.; Andrusenko, Y.N. Treatment of Basal Cell Cancer With a Pulsed Copper Vapor Laser: A Case Series. J. Lasers Med. Sci. 2019, 10, 350–354. [Google Scholar] [CrossRef]
- Kaliki, S.; Jajapuram, S.D.; Maniar, A.; Mishra, D.K. Ocular and Periocular Tumors in Xeroderma Pigmentosum: A Study of 120 Asian Indian Patients. Am. J. Ophthalmol. 2019, 198, 146–153. [Google Scholar] [CrossRef]
- Cohen, S.; Pretyman, C.S.; Sant’Ana, R.; Singh, N.; Morales, M.; Belfort, R.N. The Amazon Ocular Oncology Center: The first three years. Arq. Bras. Oftalmol. 2019, 82, 107–110. [Google Scholar] [CrossRef]
- Vavassori, A.; Riva, G.; Durante, S.; Fodor, C.; Comi, S.; Cambria, R.; Cattani, F.; Spadola, G.; Orecchia, R.; Jereczek-Fossa, B.A. Mould-based surface high-dose-rate brachytherapy for eyelid carcinoma. J. Contemp. Brachytherapy 2019, 11, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; Sharma, A.; Peters, S.; Aretz, S.; Biswas, A.; Holz, F.G.; Loeffler, K.U.; Herwig-Carl, M.C. Basal cell carcinomas developing independently from BAP1-tumor predisposition syndrome in a patient with bilateral uveal melanoma: Diagnostic challenges to identify patients with BAP1-TPDS. Genes Chromosomes Cancer 2019, 58, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Tongbram, A.; Krishnakumar, S.; Biswas, J.; Mukherjee, B. Sensitivity and specificity of frozen section diagnosis in orbital and adnexal malignancies. Indian J. Ophthalmol. 2019, 67, 1988–1992. [Google Scholar] [CrossRef]
- Bergeron, S.; Miyamoto, D.; Sanft, D.M.; Burnier, J.V.; Mastromonaco, C.; Romano, A.A.; Arthurs, B.; Burnier, M.N., Jr. Novel application of anterior segment optical coherence tomography for periocular imaging. Can. J. Ophthalmol. 2019, 54, 431–437. [Google Scholar] [CrossRef]
- Mathis, J.; Doerr, T.; Lin, E.; Ibrahim, S.F. Oral Hedgehog Pathway Inhibition as a Means for Ocular Salvage in Locally Advanced Intraorbital Basal Cell Carcinoma. Dermatol. Surg. 2019, 45, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Costea, C.F.; Turliuc, M.D.; Sava, A.; Dimitriu, G.; Dumitrescu, G.F.; Dancă, C.; Cucu, A.I.; Bogdănici, C.M.; Costache, I.I.; Buzdugă, C.M. Periocular basal cell carcinoma: Demographic, clinical, histological and immunohistochemical evaluation of a series of 39 cases. Rom. J. Morphol. Embryol. 2019, 60, 77–86. [Google Scholar]
- Weesie, F.; Naus, N.C.; Vasilic, D.; Hollestein, L.M.; Van den Bos, R.R.; Wakkee, M. Recurrence of periocular basal cell carcinoma and squamous cell carcinoma after Mohs micrographic surgery: A retrospective cohort study. Br. J. Dermatol. 2019, 180, 1176–1182. [Google Scholar] [CrossRef]
- González, A.R.; Etchichury, D.; Gil, M.E.; Del Aguila, R. Neoadjuvant Vismodegib and Mohs Micrographic Surgery for Locally Advanced Periocular Basal Cell Carcinoma. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 56–61. [Google Scholar] [CrossRef]
- Khoo, A.B.S.; Goon, P.K.C.; Sudhoff, H.; Goon, P.K.C. Comparative Analyses of Tumour Volume Doubling Times for Periocular and Non-periocular Head and Neck Basal Cell Carcinomas. Acta Derm. Venereol. 2019, 99, 1266–1269. [Google Scholar] [CrossRef]
- Kis, E.G.; Baltás, E.; Ócsai, H.; Vass, A.; Németh, I.B.; Varga, E.; Oláh, J.; Kemény, L.; Tóth-Molnár, E. Electrochemotherapy in the treatment of locally advanced or recurrent eyelid-periocular basal cell carcinomas. Sci. Rep. 2019, 9, 4285. [Google Scholar] [CrossRef]
- Sagiv, O.; Ding, S.; Ferrarotto, R.; Glisson, B.; Altan, M.; Johnson, F.; Elamin, Y.; Thakar, S.D.; Nagarajan, P.; Esmaeli, B. Impact of Food and Drug Administration Approval of Vismodegib on Prevalence of Orbital Exenteration as a Necessary Surgical Treatment for Locally Advanced Periocular Basal Cell Carcinoma. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Eiger-Moscovich, M.; Reich, E.; Tauber, G.; Berliner, O.; Priel, A.; Ben Simon, G.; Elkader, A.A.; Yassur, I. Efficacy of Vismodegib for the Treatment of Orbital and Advanced Periocular Basal Cell Carcinoma. Am. J. Ophthalmol. 2019, 207, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Monheit, G.; Hrynewycz, K. Mohs Surgery for Periocular Tumors. Dermatol. Surg. 2019, 45 (Suppl. S2), S70–S78. [Google Scholar] [CrossRef]
- Scofield-Kaplan, S.M.; Jackson, C.; Gurney, T.; McDonnell, E.; Mancini, R. Predictive Value of Preoperative Periocular Skin Cancer Measurements for Final Mohs Defect Size. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, R.; Pakla, P.; Dymek, M.; Migut, M.; Ambicki, M.; Stopyra, W.; Ozga, D.; Lewandowski, B. Clinical-pathological characteristics of patients treated for cancers of the eyelid skin and periocular areas. Adv. Clin. Exp. Med. 2019, 28, 325–330. [Google Scholar] [CrossRef]
- Mercuţ, I.M.; Simionescu, C.E.; Stepan, A.E.; Andreiana, B.C.; Ciurea, A.M.; Mercuţ, R.; Ciurea, M.E. The immunoexpression of MMP-1 and MMP-13 in eyelid basal cell carcinoma. Rom. J. Morphol. Embryol. 2020, 61, 1221–1226. [Google Scholar] [CrossRef]
- Galindo-Ferreiro, A.; Sanchez-Tocino, H.; Diez-Montero, C.; Belani-Raju, M.; García-Sanz, R.; Diego-Alonso, M.; Llorente-Gonzalez, I.; Perez, P.C.; Khandekar, R.; Schellini, S. Characteristics and Recurrence of Primary Eyelid Basal Cell Carcinoma in Central Spain. J. Curr. Ophthalmol. 2020, 32, 183–188. [Google Scholar] [CrossRef]
- Hou, X.; Rokohl, A.C.; Ortmann, M.; Heindl, L.M. Effective treatment of locally advanced periocular basal cell carcinoma with oral hedgehog pathway inhibitor? Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2335–2337. [Google Scholar] [CrossRef]
- Vijay, V.; Alam, M.S.; Subramanian, N.; Krishnakumar, S.; Biswas, J.; Mukherjee, B. Periocular Basal Cell Carcinoma: 20-Years Experience at a Tertiary Eye Care Center of South India. Oman. J. Ophthalmol. 2020, 13, 129–135. [Google Scholar]
- Ben Ishai, M.; Tiosano, A.; Fenig, E.; Ben Simon, G.; Yassur, I. Outcomes of Vismodegib for Periocular Locally Advanced Basal Cell Carcinoma From an Open-label Trial. JAMA Ophthalmol. 2020, 138, 749–755. [Google Scholar] [CrossRef]
- Fazil, K.; Karslioglu, S.; Akbaba, M.; Buttanri, I.B.; Serin, D.; Karabulut, G.O.; Bektasoglu, D. Evaluation of Demographic Features of Eyelid Lesions. Beyoglu. Eye J. 2020, 5, 114–117. [Google Scholar] [CrossRef]
- Gupta, R.; Bhaduri, A.; Desai, S.; Das, S.; Menon, V. Malignant tumors of the eyelid in India: A multicenter, multizone study on clinicopathologic features and outcomes. Indian J. Ophthalmol. 2020, 68, 2466–2470. [Google Scholar] [CrossRef] [PubMed]
- Boal, N.S.; Milman, T.; Shields, C.L. A Black-Pigmented Eyelid Nodule in an African American Woman. JAMA Ophthalmol. 2020, 138, 99–100. [Google Scholar] [CrossRef]
- Gąsiorowski, K.; Iwulska, K.; Zapała, J.; Wyszyńska-Pawelec, G. Periocular basal cell carcinoma: Recurrence risk factors/when to reoperate? Postepy Dermatol. Alergol. 2020, 37, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Finskas, O.; Zaar, O.; Svedberg, K. Cryosurgery of Periocular Moderately Aggressive Basal Cell Carcinoma. Acta Derm. Venereol. 2020, 100, adv00336. [Google Scholar] [CrossRef]
- Su, M.G.; Potts, L.B.; Tsai, J.H. Treatment of periocular basal cell carcinoma with neoadjuvant vismodegib. Am. J. Ophthalmol. Case Rep. 2020, 19, 100755. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, H.; Laybourne, J.; Chan, K.; Haridas, A.; Edmunds, M.R.; Morris, D.; Clarke, L.; Althaus, M.; Norris, P.; Cranstoun, M.; et al. Vismodegib for periocular basal cell carcinoma: An international multicentre case series. Eye 2020, 34, 2076–2081. [Google Scholar] [CrossRef]
- Herwig-Carl, M.C.; Loeffler, K.U. Regression of Periocular Basal Cell Carcinoma: A Report of Four Cases with Clinicopathologic Correlation. Ocul. Oncol. Pathol. 2020, 6, 107–114. [Google Scholar] [CrossRef]
- MercuȚ, I.M.; TĂnasie, C.A.; Ilia, L.C.; Simionescu, C.; Stepan, A.; Ciurea, M.; MercuȚ, R. Histopathological Features of the Eyelid Basal Cell Carcinomas. Curr. Health Sci. J. 2020, 46, 167–172. [Google Scholar]
- Peden, R.; Radwan, M.; Wright, M. Small margin (up to 2 mm) excision of periocular basal cell carcinomas in the setting of a one-stop clinic—Long-term outcomes at a minimum of 11 years’ follow-up. Eye 2020, 34, 2036–2040. [Google Scholar] [CrossRef]
- Furdova, A.; Babal, P.; Zahorjanova, P.; Sekac, J. Subtotal Exenteration of the Orbit for Benign Orbital Disease. J. Craniofac. Surg. 2020, 3, 1367–1369. [Google Scholar] [CrossRef]
- Ben Artsi, E.; Ullrich, K.; Malhotra, R. Submental and Anterior Neck Originated Full-Thickness Skin Grafts for Periocular Procedures. Ophthalmic Plast. Reconstr. Surg. 2020, 36, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Qidwai, U.; Igali, L.; Hemmant, B. A multicentre review of the histology of 1012 periocular basal cell carcinomas. Eur. J. Ophthalmol. 2021, 31, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Stridh, M.T.; Hult, J.; Merdasa, A.; Albinsson, J.; Pekar-Lukacs, A.; Gesslein, B.; Scarfì, F.; Maio, V.; Spinelli, G.; Scoccianti, S.; et al. Photoacoustic imaging of periorbital skin cancer ex vivo: Unique spectral signatures of malignant melanoma, basal, and squamous cell carcinoma. Biomed. Opt. Express 2021, 13, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Adamski, W.Z.; Maciejewski, J.; Adamska, K.; Marszałek, A.; Rospond-Kubiak, I. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2021, 38, 804–807. [Google Scholar] [CrossRef]
- De Giorgi, V.; Trane, L.; Pieretti, G.; Santoro, N.; Silvestri, F.; Venturi, F.; Scarfì, F.; Maio, V.; Spinelli, G.; Scoccianti, S.; et al. Treatment of periocular advanced basal cell carcinoma with Hedgehog pathway inhibitors: A single-center study and a new dedicated therapeutic protocol. Dermatol. Rep. 2021, 13, 9240. [Google Scholar] [CrossRef]
- Bergeron, S.; Arthurs, B.; Sanft, D.M.; Mastromonaco, C.; Burnier, M.N., Jr. Optical Coherence Tomography of Peri-Ocular Skin Cancers: An Optical Biopsy. Ocul. Oncol. Pathol. 2021, 7, 149–158. [Google Scholar] [CrossRef]
- Kaliki, S.; Das, A.V. Ocular and periocular tumors in 855 Asian Indian geriatric patients. Oman J. Ophthalmol. 2021, 14, 153–156. [Google Scholar] [CrossRef]
- Shimizu, N.; Oshitari, T.; Yotsukura, J.; Yokouchi, H.; Baba, T.; Yamamoto, S. Ten-year epidemiological study of ocular and orbital tumors in Chiba University Hospital. BMC Ophthalmol. 2021, 21, 344. [Google Scholar] [CrossRef]
- Battista, R.A.; Giordano, L.; Giordano Resti, A.; Bordato, A.; Trimarchi, M.; Familiari, M.; Ferraro, M.; Bandello, F.M.; Bussi, M. Combination of Mustardè cheek advancement flap and paramedian forehead flap as a reconstructive option in orbital exenteration. Eur. J. Ophthalmol. 2021, 31, 1463–1468. [Google Scholar] [CrossRef]
- Prídavková, Z.; Bieliková, A.; Ferková, N.; Lysková, D. Recurrent periocular basal cell carcinoma. Case report. Ceska A Slov. Oftalmol. 2021, 77, 208–213. [Google Scholar] [CrossRef]
- Rokohl, A.C.; Heindl, L.M. Effective systemic treatment of advanced periocular basal cell carcinoma with sonidegib. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3821–3822. [Google Scholar] [CrossRef] [PubMed]
- Almousa, R. Predictors for margin of resection >4 mm in the management of periocular basal cell carcinoma. Korean J. Ophthalmol. 2021, 35, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kahana, A.; Unsworth, S.P.; Andrews, C.A.; Chan, M.P.; Bresler, S.C.; Bichakjian, C.K.; Durham, A.B.; Demirci, H.; Elner, V.M.; Nelson, C.C.; et al. Vismodegib for Preservation of Visual Function in Patients with Advanced Periocular Basal Cell Carcinoma: The VISORB Trial. Oncologist 2021, 26, e1240–e1249. [Google Scholar] [CrossRef] [PubMed]
- Curragh, D.S.; Huilgol, S.C.; Selva, D. Neoadjuvant vismodegib in the management of locally advanced periocular basal cell carcinoma. Eye 2021, 35, 2740–2745. [Google Scholar] [CrossRef]
- Yazici, B.; Yuksel, N.O.; Turgay, T.; Meyer, D.R. Transnasal or transglabellar semicircular flap for medial canthal reconstruction. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3769–3776. [Google Scholar] [CrossRef]
- Weerdt, G.; Luyten, P.; Dubrulle, F.; Loonen, M. Reconstruction of an Extensive Periocular and Bilamellar Defect of the Lower and Upper Eyelid Using Local, Regional and Free Chondral Graft Techniques: A Case Report. World J. Plast. Surg. 2021, 10, 125–131. [Google Scholar] [CrossRef]
- Low, K.L.; Lai, Y.P.; Alias, R.; Che Hamzah, J. Primary Basal Cell Carcinoma of the Conjunctiva. Cureus 2022, 14, e31516. [Google Scholar] [CrossRef]
- Ul Kadir, S.M.; Rani Mitra, M.; Rashid, R.; Nuruddin, M.; Hassan Khan, M.K.; Haider, G.; Nessa, M.S. Clinicopathological Analysis and Surgical Outcome of Eyelid Malignancies: A Study of 332 Cases. J. Skin Cancer 2022, 2022, 4075668. [Google Scholar] [CrossRef]
- Lemaître, S.; Green, F.; Dendale, R.; Vincent-Salomon, A.; Desjardins, L.; Cassoux, N.; Couturaud, B.; Lévy-Gabriel, C. Total orbital exenteration with temporalis muscle transfer and secondary healing. Can. J. Ophthalmol. 2022, 57, 8–15. [Google Scholar] [CrossRef]
- Baş, Z.; Sharpe, J.; Yaghy, A.; Zhang, Q.; Shields, C.L.; Hyman, L. IRIS Registry Analytic Center Consortium. Prevalence of and Associated Factors for Eyelid Cancer in the American Academy of Ophthalmology Intelligent Research in Sight Registry. Ophthalmol. Sci. 2022, 3, 100227. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Koka, K.; Alam, M.S.; Subramanian, N.; Biswas, J.; Krishnakumar, S.; Mukherjee, B. The spectrum and clinicopathological correlation of eyelid lesions: Twenty years’ experience at a tertiary eye care center in South India. Indian J. Ophthalmol. 2022, 70, 43–50. [Google Scholar] [CrossRef]
- Balchev, G.; Balabanov, C.; Murgova, S. Glabellar flap technique in oculoplastic surgery. Indian J. Ophthalmol. 2022, 70, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, J.; Yang, Y.; Rao, Y.; Chen, X.; Shi, T.; Xu, S.; Jia, R.; Gao, X. Deep learning-based fully automated differential diagnosis of eyelid basal cell and sebaceous carcinoma using whole slide images. Quant. Imaging Med. Surg. 2022, 12, 4166–4175. [Google Scholar] [CrossRef]
- Karlsdóttir, S.B.; Johannessen, S.; Bjerrum, N.C.; Frydkjær-Olsen, U.; Blindbæk, S.L.; Møller, F.; Wellejus, C. Periocular basal cell carcinoma results and surgical outcome during a 5-year period in a larger Danish population. BMC Ophthalmol. 2022, 22, 282. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Costa, C.; Cappello, M.; Fabbrocini, G.; Scalvenzi, M. The effectiveness of vismodegib in patients with advanced periocular basal cell carcinoma: A case series of 13 patients. J. Dermatol. Treat. 2022, 33, 602–603. [Google Scholar] [CrossRef]
- Unsworth, S.P.; Tingle, C.F.; Heisel, C.J.; Eton, E.A.; Andrews, C.A.; Chan, M.P.; Bresler, S.C.; Kahana, A. Analysis of residual disease in periocular basal cell carcinoma following hedgehog pathway inhibition: Follow up to the VISORB trial. PLoS ONE 2022, 17, e0265212. [Google Scholar] [CrossRef]
- Küronya, Z.; Danyi, T.; Balatoni, T.; Liszkay, G.; Tóth, E.; Biró, K.; Géczi, L. Atezolizumab for the treatment of advanced recurrent basal cell carcinoma and urothelial carcinoma of bladder: A case report. J. Med. Case Rep. 2022, 16, 396. [Google Scholar] [CrossRef]
- Singh, M.; Mehta Grewal, A.; Singh, H.; Sharma, M.; Kaur, M.; Gupta, P.; Zadeng, Z. Long-term efficacy and safety of imiquimod 5% and fluorouracil 1% creams in medical monotherapy of complex eyelid basal cell carcinomas. Eur. J. Ophthalmol. 2022, 32, 2093–2100. [Google Scholar] [CrossRef]
- Chan, R.; Li, C.L.; Liu, D.; Luk, N.M.; Young, A.; Choi, P.; Chong, K. Mohs Surgery for Periocular Basal Cell Carcinoma Without a Mohs Surgeon: The First Series in Hong Kong. Cureus 2023, 15, e36235. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, X.; Zhang, Y.; Xie, Z.; Fang, X.; Shi, K.; Zhong, Y.; Su, S.; Cai, M.; Wu, H.; et al. The clinicopathological analysis of ocular and orbit tumors in southeast of China. Front. Oncol. 2023, 13, 1118862. [Google Scholar] [CrossRef]
- Bagheri, A.; Ashtar-Nakhaie, P.; Aletaha, M.; Kheiri, B.; Veisi, A. A Survey on Orbital Space-Occupying Lesions during a Twelve-Year Period from a Referral Center in Iran. J. Ophthalmic Vis. Res. 2023, 18, 202–211. [Google Scholar] [CrossRef]
- Situm, M.; Buljan, M.; Bulat, V.; Lugovic Mihic, L.; Simic, D. The role of UV radiation in the development of basal cell carcinoma. Coll. Antropol. 2008, 32 (Suppl. S2), 167–170. [Google Scholar]
- Wehner, M.R.; Cidre Serrano, W.; Nosrati, A.; Schoen, P.M.; Chren, M.M.; Boscardin, J.; Linos, E. All-cause mortality in patients with basal and squamous cell carcinoma: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2018, 78, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Taylor, W.B.; Falls, H.F.; Davidson, R.T. The nevoid basal cell carcinoma syndrome. Am. J. Hum. Genet. 1967, 19, 12–22. [Google Scholar] [PubMed]
- Saleh, G.M.; Desai, P.; Collin, J.R.; Ives, A.; Jones, T.; Hussain, B. Incidence of eyelid basal cell carcinoma in England: 2000–2010. Br. J. Ophthalmol. 2017, 101, 209–212. [Google Scholar] [CrossRef]
- Dessinioti, C.; Antoniou, C.; Katsambas, A.; Stratigos, A.J. Basal cell carcinoma: What’s new under the sun. Photochem. Photobiol. 2010, 86, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Yin, V.T.; Merritt, H.A.; Sniegowski, M.; Esmaeli, B. Eyelid and ocular surface carcinoma: Diagnosis and management. Clin. Dermatol. 2015, 33, 159–169. [Google Scholar] [CrossRef]
- Allali, J.; D’Hermies, F.; Renard, G. Basal cell carcinomas of the eyelids. Ophthalmologica 2005, 219, 57–71. [Google Scholar] [CrossRef]
- Pfeiffer, M.J.; Pfeiffer, N.; Valor, C. Estudio descriptive sobre el carcinoma basocelular en el párpado [Descriptive study on basal cell eyelid carcinoma]. Arch. Soc. Esp. Oftalmol. 2015, 90, 426–431. [Google Scholar] [CrossRef]
- Echchaoui, A.; Benyachou, M.; Houssa, A.; Kajout, M.; Oufkir, A.A.; Hajji, C.; Daoudi, R.; Hafidi, J.; El Mazouz, S.; Gharib, N.; et al. Prise en charge des carcinomes des paupières: étude bicentrique rétrospective sur 64 cas avec revue de littérature [Management of eyelid carcinomas: Retrospective bicentric study of 64 cases and review of the literature]. J. Fr. Ophtalmol. 2016, 39, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cable, M.M.; Lyon, D.B.; Rupani, M.; Matta, C.S.; Hidayat, A.A. Case report and small case series: Primary basal cell carcinoma of the conjunctiva with intraocular invasion. Arch. Ophthalmol. 2000, 118, 1296. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, N.; Mühl, S.; Eter, N. Primäres Basalzellkarzinom der Karunkel [Primary basal cell carcinoma of the caruncle]. Ophthalmologe 2019, 116, 1034–1037. [Google Scholar] [CrossRef]
- Levy, J.; Ilsar, M.; Deckel, Y.; Maly, A.; Pe’er, J. Lesions of the caruncle: A description of 42 cases and a review of the literature. Eye 2009, 23, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Perucho-Martínez, S.; Mencía-Gutiérrez, E.; Gutiérrez-Díaz, E.; Gόmez-Ledesma, M.I. Neoplasms of the caruncle. Clinicopathologic study of 40 cases. Arch. Soc. Esp. Oftalmol. 2004, 79, 493–499. [Google Scholar]
- Reiter, O.; Mimouni, I.; Gdalevich, M.; Marghoob, A.A.; Levi, A.; Hodak, E.; Leshem, Y.A. The diagnostic accuracy of dermoscopy for basal cell carcinoma: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 1380–1388. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Kaufmann, R.; Arenberger, P.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Brochez, L.; Del Marmol, V.; Dummer, R.; et al. EADO”A, EDF”B, ESTRO”C, UEMS”D and EADV”E. European consensus-based interdisciplinary guideline for diagnosis and treatment of basal cell carcinoma-update 2023. Eur. J. Cancer 2023, 192, 113254. [Google Scholar] [CrossRef]
- Suppa, M.; Micantonio, T.; Di Stefani, A.; Soyer, H.P.; Chimenti, S.; Fargnoli, M.C.; Peris, K. Dermoscopic variability of basal cell car- cinoma according to clinical type and anatomic location. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1732–1741. [Google Scholar] [CrossRef]
- Peccerillo, F.; Mandel, V.D.; Di Tullio, F.; Ciardo, S.; Chester, J.; Kaleci, S.; De Carvalho, N.; Del Duca, E.; Giannetti, L.; Mazzoni, L.; et al. Lesions Mimicking Melanoma at Dermoscopy Confirmed Basal Cell Carcinoma: Evaluation with Reflectance Confocal Microscopy. Dermatology 2019, 235, 35–44. [Google Scholar] [CrossRef]
- Nori, S.; Rius-Dıaz, F.; Cuevas, J.; Goldgeier, M.; Jaen, P.; Torres, A.; González, S. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: A multicenter study. J. Am. Acad. Dermatol. 2004, 51, 923–930. [Google Scholar] [CrossRef]
- Guitera, P.; Menzies, S.W.; Longo, C.; Cesinaro, A.M.; Scolyer, R.A.; Pellacani, G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: Analysis of 710 consecutive clinically equivocal cases. J. Investig. Dermatol. 2012, 132, 2386–2394. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy: Principles, basic terminology, clinical indications, limitations, and practical considerations. J. Am. Acad. Dermatol. 2021, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Que, S.K.; Grant-Kels, J.M.; Rabinovitz, H.S.; Oliviero, M.; Scope, A. Application of Handheld Confocal Microscopy for Skin Cancer Diagnosis: Advantages and Limitations Compared with the Wide-Probe Confocal. Dermatol. Clin. 2016, 34, 469–475. [Google Scholar] [CrossRef]
- Fukumoto, T.; Fukumoto, R.; Oka, M.; Horita, N. Comparing treatments for basal cell carcinoma in terms of longterm treatment-failure: A network meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; Wu, A.; Figueira, E.; Huilgol, S.; Selva, D. Management of periorbital basal cell carcinoma with orbital invasion. Future Oncol. 2015, 11, 3003–3010. [Google Scholar] [CrossRef]
- Pieh, S.; Kuchar, A.; Novak, P.; Kunstfeld, R.; Nagel, G.; Steinkogler, F.J. Long-term results after surgical basal cell carcinoma excision in the eyelid region. Br. J. Ophthalmol. 1999, 83, 85–88. [Google Scholar] [CrossRef]
- Cook, B.E., Jr.; Bartley, G.B. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology 1999, 106, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.I.; Chen, E.H.; Ratner, D. Basal-cell Carcinoma. N. Engl. J. Med. 2005, 353, 2262–2269. [Google Scholar] [CrossRef]
- Mandel, V.D.; Arginelli, F.; Pellacani, G.; Greco, M. Combined carbon dioxide laser with photodynamic therapy for the treatment of nodular and infiltrative basal cell carcinoma. G. Ital. Dermatol. Venereol. 2017, 152, 672–674. [Google Scholar] [CrossRef]
- Mandel, V.D.; Ferrari, F.; Ciardo, S.; Giusti, F.; Pellacani, G. Bowen’s disease of the upper eyelid successfully treated with photodynamic therapy. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e127–e129. [Google Scholar] [CrossRef]
- Michelini, M.; Mandel, V.D.; Ardigò, M.; Ciardo, S.; Cota, C.; Cesinaro, A.M.; Rossi, E.; Ferrari, B.; Kaleci, S.; Di Fraia, M.; et al. Combining Reflectance Confocal Microscopy, Optical Coherence Tomography and Ex-Vivo Fluorescence Confocal Microscopy for Margin Assessment in Basal Cell Carcinoma Excision. Dermatol. Pract. Concept 2024, 14, e2024090. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).