The Emerging Biomarkers in Chronic Obstructive Pulmonary Disease: A Narrative Review
Abstract
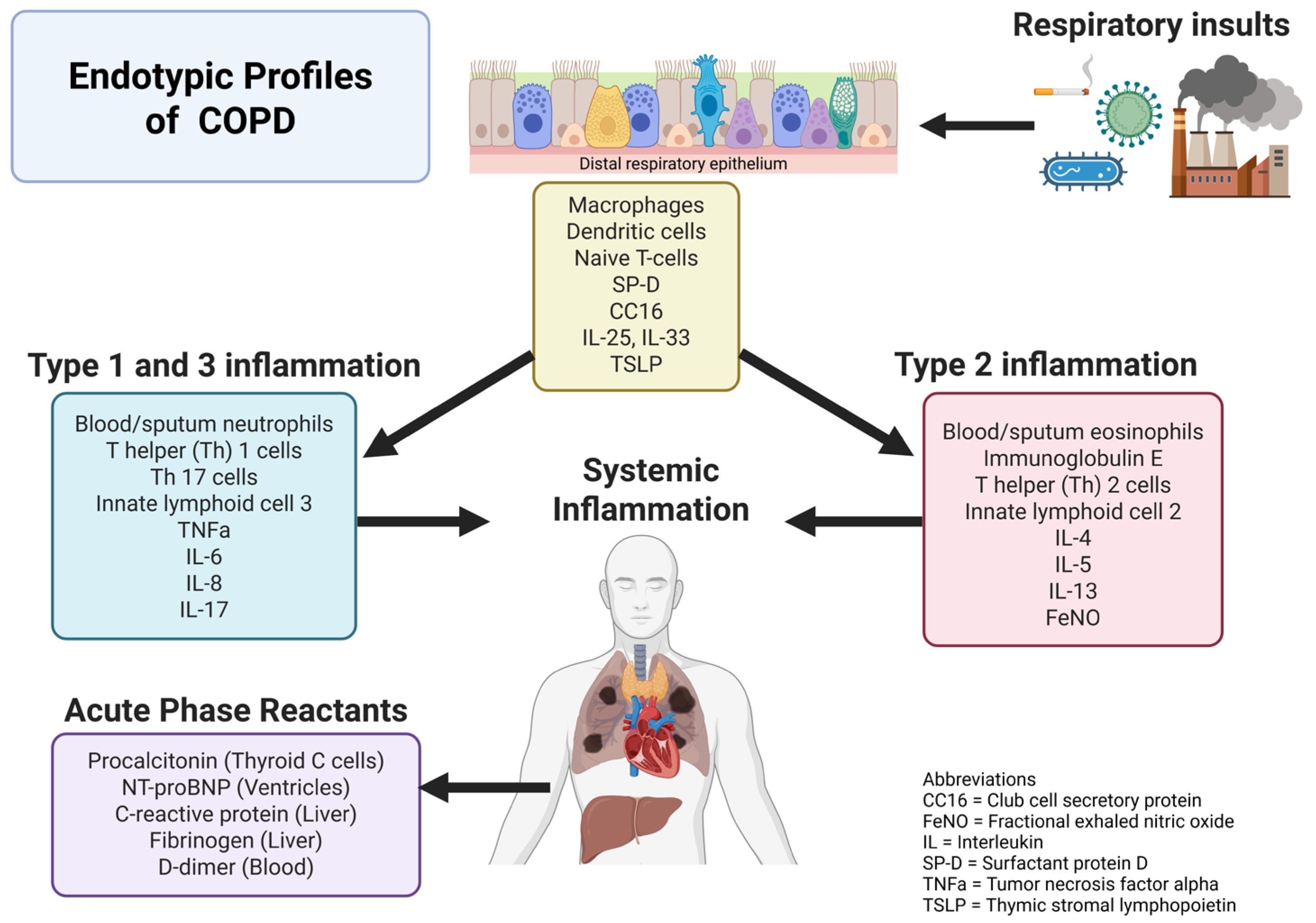
1. Background
2. Overview of Serum Biomarkers in COPD
2.1. Blood Eosinophil Count
2.2. Immunoglobulin E
2.3. C-Reactive Protein
2.4. Fibrinogen
2.5. Procalcitonin
2.6. IL-6 and IL-8
2.7. IL-33/ST-2
2.8. Soluble Receptor for Advanced Glycation End Products
2.9. Club Cell Secretory Protein
2.10. Surfactant Protein-D
3. Overview of Lung Samples in COPD
3.1. Sputum Biomarkers
3.2. Invasive Biomarkers
3.3. FENO in COPD
3.4. Biomarkers of Exhaled Breath Condensate
4. Overview of Imaging Biomarkers for COPD
4.1. Changes in Airway Anatomy
4.2. Developing and Progressing COPD: Emphysema and Lung Function
4.3. Predicting Exacerbations by Imaging
4.4. Pulmonary Vasculature and Other Imaging Markers
4.5. Identifying Undiagnosed COPD by Imaging
5. Clinical Utility of COPD Biomarkers
6. Differential Biomarkers Between Tobacco Smoke and Biomass Burning Smoke Exposure
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; Patel, N.; et al. NOTUS Study Investigators. Dupilumab for COPD with Blood Eosinophil Evidence of Type 2 Inflammation. N. Engl. J. Med. 2024, 390, 2274–2283. [Google Scholar] [CrossRef] [PubMed]
- Stockley, R.A.; Halpin, D.M.G.; Celli, B.R.; Singh, D. Chronic Obstructive Pulmonary Disease Biomarkers and Their Interpretation. Am. J. Respir. Crit. Care Med. 2019, 199, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Vitenberga, Z.; Pilmane, M.; Babjoniševa, A. An Insight into COPD Morphopathogenesis: Chronic Inflammation, Remodeling, and Antimicrobial Defense. Medicina 2019, 55, 496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Celli, B.R.; Anderson, J.A.; Brook, R.; Calverley, P.; Cowans, N.J.; Crim, C.; Dixon, I.; Kim, V.; Martinez, F.J.; Morris, A.; et al. Serum biomarkers and outcomes in patients with moderate COPD: A substudy of the randomised SUMMIT trial. BMJ Open Respir. Res. 2019, 6, e000431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vedel-Krogh, S.; Nielsen, S.F.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2016, 193, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, L.R.; Zhang, S.; Lu, Y.; Chen, Y.Y.; Shi, H.Z.; Lin, Y.X. Blood Eosinophilia and Its Stability in Hospitalized COPD Exacerbations are Associated with Lower Risk of All-Cause Mortality. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1123–1134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prudente, R.; Ferrari, R.; Mesquita, C.B.; Machado, L.H.S.; Franco, E.A.T.; Godoy, I.; Tanni, S.E. Peripheral Blood Eosinophils and Nine Years Mortality in COPD Patients. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 979–985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, W.C.; Bourbeau, J.; Nadeau, G.; Wang, W.; Barnes, N.; Landis, S.H.; Kirby, M.; Hogg, J.C.; Sin, D.D.; CanCOLDCollaborative Research Group. Authors would also like to thank the men women who participated in the study individuals in the CanCOLDCollaborative Research Group not listed as authors: High eosinophil counts predict decline in FEV1: Results from the CanCOLDstudy. Eur. Respir. J. 2021, 57, 2000838. [Google Scholar] [CrossRef] [PubMed]
- Çolak, Y.; Afzal, S.; Marott, J.L.; Vestbo, J.; Nordestgaard, B.G.; Lange, P. Type-2 inflammation and lung function decline in chronic airway disease in the general population. Thorax 2024, 79, 349–358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathioudakis, A.G.; Bate, S.; Sivapalan, P.; Jensen, J.S.; Singh, D.; Vestbo, J. Rethinking Blood Eosinophils for Assessing Inhaled Corticosteroids Response in COPD: A Post Hoc Analysis from the FLAME Trial. Chest 2024, 166, 987–997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pascoe, S.; Locantore, N.; Dransfield, M.T.; Barnes, N.C.; Pavord, I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 2015, 3, 435–442, Erratum in Lancet Respir. Med. 2015, 3, e19. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.H.; Guasconi, A.; Vestbo, J.; Jones, P.; Agusti, A.; Paggiaro, P.; Wedzicha, J.A.; Singh, D. Blood Eosinophils: A Biomarker of Response to Extrafine Beclomethasone/Formoterol in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 523–525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavord, I.D.; Lettis, S.; Locantore, N.; Pascoe, S.; Jones, P.W.; Wedzicha, J.A.; Barnes, N.C. Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax 2016, 71, 118–125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wedzicha, J.A.; Banerji, D.; Chapman, K.R.; Vestbo, J.; Roche, N.; Ayers, R.T.; Thach, C.; Fogel, R.; Patalano, F.; Vogelmeier, C.F.; et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N. Engl. J. Med. 2016, 374, 2222–2234. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; Rabe, K.F.; Martinez, F.J.; Singh, D.; Darken, P.; Jenkins, M.; Aurivillius, M.; Patel, M.; Dorinsky, P. Benefits of Budesonide/Glycopyrronium/Formoterol Fumarate Dihydrate on COPD Exacerbations, Lung Function, Symptoms, and Quality of Life Across Blood Eosinophil Ranges: A Post-Hoc Analysis of Data from ETHOS. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 3061–3073. [Google Scholar] [CrossRef]
- Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2024 Report. Available online: www.goldcopd.org (accessed on 1 March 2025).
- Baraldi, F.; Bartlett-Pestle, S.; Allinson, J.P.; Macleod, M.; Mah, J.; Bloom, C.; Brady-Green, A.; Marion, A.; Papi, A.; Wedzicha, J.A.; et al. Blood Eosinophil Count Stability in COPD and the Eosinophilic Exacerbator Phenotype. Am. J. Respir. Crit. Care Med. 2025; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med. 2023, 389, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Karakioulaki, M.; Papakonstantinou, E.; Goulas, A.; Stolz, D. The Role of Atopy in COPD and Asthma. Front. Med. 2021, 8, 674742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lommatzsch, M.; Speer, T.; Herr, C.; Jörres, R.A.; Watz, H.; Müller, A.; Welte, T.; Vogelmeier, C.F.; Bals, R.; COSYCONET Study Group. IgE is associated with exacerbations and lung function decline in COPD. Respir. Res. 2022, 23, 1. [Google Scholar] [CrossRef]
- Hai, C.N.; Ba, T.T.; Duc, T.B.; Xuan, C.H.; Manh, T.V. Serum immunoglobulin levels in group E of chronic obstructive pulmonary disease: Insights for clinical management and immunoglobulin therapy strategies. BMC Pulm. Med. 2024, 24, 381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Çolak, Y.; Ingebrigtsen, T.S.; Nordestgaard, B.G.; Marott, J.L.; Lange, P.; Vestbo, J.; Afzal, S. Plasma immunoglobulin E and risk of exacerbation and mortality in chronic obstructive pulmonary disease: A contemporary population-based cohort. Ann. Allergy Asthma Immunol. 2022, 129, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441843/ (accessed on 25 April 2025).
- Cleland, D.A.; Eranki, A.P. Procalcitonin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539794/ (accessed on 25 April 2025).
- Munuswamy, R.; De Brandt, J.; Burtin, C.; Derave, W.; Aumann, J.; Spruit, M.A.; Michiels, L. Monomeric CRP is Elevated in Patients with COPD Compared to Non-COPD Control Persons. J. Inflamm. Res. 2021, 14, 4503–4507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pantazopoulos, I.; Magounaki, K.; Kotsiou, O.; Rouka, E.; Perlikos, F.; Kakavas, S.; Gourgoulianis, K. Incorporating Biomarkers in COPD Management: The Research Keeps Going. J. Pers. Med. 2022, 12, 379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papi, A.; Magnoni, M.S.; Muzzio, C.C.; Benso, G.; Rizzi, A. Phenomenology of COPD: Interpreting phenotypes with the ECLIPSE study. Monaldi Arch. Chest Dis. 2016, 83, 721. [Google Scholar] [CrossRef] [PubMed]
- Fermont, J.M.; Masconi, K.L.; Jensen, M.T.; Ferrari, R.; Di Lorenzo, V.A.P.; Marott, J.M.; Schuetz, P.; Watz, H.; Waschki, B.; Müllerova, H.; et al. Biomarkers and clinical outcomes in COPD: A systematic review and meta-analysis. Thorax 2019, 74, 439–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zemans, R.L.; Jacobson, S.; Keene, J.; Kechris, K.; Miller, B.E.; Tal-Singer, R.; Bowler, R.P. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir. Res. 2017, 18, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoult, G.; Gillespie, D.; Wilkinson, T.M.A.; Thomas, M.; Francis, N.A. Biomarkers to guide the use of antibiotics for acute exacerbations of COPD (AECOPD): A systematic review and meta-analysis. BMC Pulm. Med. 2022, 22, 194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prins, H.J.; Duijkers, R.; van der Valk, P.; Schoorl, M.; Daniels, J.M.A.; van der Werf, T.S.; Boersma, W.G. CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur. Respir. J. 2019, 53, 1802014. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.E.; Tal-Singer, R.; Rennard, S.I.; Furtwaengler, A.; Leidy, N.; Lowings, M.; Martin, U.J.; Martin, T.R.; Merrill, D.D.; Snyder, J.; et al. Plasma Fibrinogen Qualification as a Drug Development Tool in Chronic Obstructive Pulmonary Disease. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am. J. Respir. Crit. Care Med. 2016, 193, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Gruys, E.; Toussaint, M.J.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, D.; Criner, G.J.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Lange, P.; Lettis, S.; Lipson, D.A.; Mannino, D.; Martin, N.; et al. InforMing the PAthway of COPD Treatment (IMPACT) trial: Fibrinogen levels predict risk of moderate or severe exacerbations. Respir. Res. 2021, 22, 130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivapalan, P.; Jensen, J.U. Biomarkers in Chronic Obstructive Pulmonary Disease: Emerging Roles of Eosinophils and Procalcitonin. J. Innate Immun. 2022, 14, 89–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daubin, C.; Valette, X.; Thiollière, F.; Mira, J.P.; Hazera, P.; Annane, D.; Labbe, V.; Floccard, B.; Fournel, F.; Terzi, N.; et al. Procalcitonin algorithm to guide initial antibiotic therapy in acute exacerbations of COPD admitted to the ICU: A randomized multicenter study. Intensive Care Med. 2018, 44, 428–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, H.; Huang, X.; Zeng, K.; Deng, F.; Lin, C.; Huang, W. Interleukin-6 is a Strong Predictor of the Frequency of COPD Exacerbation Within 1 Year. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2945–2951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wedzicha, J.A.; Seemungal, T.A.; MacCallum, P.K.; Paul, E.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Meade, T.W. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb. Haemost. 2000, 84, 210–215. [Google Scholar] [PubMed]
- Zhang, J.; Bai, C. The Significance of Serum Interleukin-8 in Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Tanaffos 2018, 17, 13–21. [Google Scholar] [PubMed] [PubMed Central]
- Pratte, K.A.; Curtis, J.L.; Kechris, K.; Couper, D.; Cho, M.H.; Silverman, E.K.; DeMeo, D.L.; Sciurba, F.C.; Zhang, Y.; Ortega, V.E.; et al. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COPD. Respir. Res. 2021, 22, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serban, K.A.; Pratte, K.A.; Bowler, R.P. Protein Biomarkers for COPD Outcomes. Chest 2021, 159, 2244–2253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, H.Y.; Churg, A.; Wright, J.L.; Li, Y.; Tam, S.; Man, S.P.; Tashkin, D.; Wise, R.A.; Connett, J.E.; Sin, D.D. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1413–1419. [Google Scholar] [CrossRef]
- Lock-Johansson, S.; Vestbo, J.; Sorensen, G.L. Surfactant protein D, club cell protein 16, pulmonary and activation-regulated chemokine, C-reactive protein, and fibrinogen biomarker variation in chronic obstructive lung disease. Respir. Res. 2014, 15, 147. [Google Scholar] [CrossRef]
- Loewenthal, L.; Menzies-Gow, A. FeNO in Asthma. Semin. Respir. Crit. Care Med. 2022, 43, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, T.; He, R.; Li, A.; Dang, W.; Liu, X.; Chen, M. The Value of Inflammatory Biomarkers in Differentiating Asthma-COPD Overlap from COPD. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 3025–3037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donohue, J.F.; Herje, N.; Crater, G.; Rickard, K. Characterization of airway inflammation in patients with COPD using fractional exhaled nitric oxide levels: A pilot study. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 745–751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alcázar-Navarrete, B.; Romero-Palacios, P.J.; Ruiz-Sancho, A.; Ruiz-Rodriguez, O. Diagnostic performance of the measurement of nitric oxide in exhaled air in the diagnosis of COPD phenotypes. Nitric Oxide 2016, 54, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hong, C.; Liu, Y.; Chen, H.; Huang, X.; Hong, M. Diagnostic value of fractional exhaled nitric oxide for asthma-chronic obstructive pulmonary disease overlap syndrome. Medicine 2018, 97, e10857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddel, H.K.; Vestbo, J.; Agustí, A.; Anderson, G.P.; Bansal, A.T.; Beasley, R.; Bel, E.H.; Janson, C.; Make, B.; Pavord, I.D.; et al. NOVELTY study investigators. Heterogeneity within and between physician-diagnosed asthma and/or COPD: NOVELTY cohort. Eur. Respir. J. 2021, 58, 2003927. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alcázar-Navarrete, B.; Díaz-Lopez, J.M.; García-Flores, P.; Ortega-Antelo, M.; Aguilar-Cruz, I.; Ruiz-Rodríguez, O.; Santiago-Diaz, P.; Romero Palacios, P.J. T2 Biomarkers as Predictors of Exacerbations of Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2022, 58, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Río Ramírez, M.T.; Juretschke Moragues, M.A.; Fernández González, R.; Álvarez Rodríguez, V.; Aznar Andrés, E.; Zabaleta Camino, J.P.; Romero Pareja, R.; Esteban de la Torre, A. Value of Exhaled Nitric Oxide (FeNO) And Eosinophilia During the Exacerbations of Chronic Obstructive Pulmonary Disease Requiring Hospital Admission. COPD 2018, 15, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Alcázar-Navarrete, B.; Ruiz Rodríguez, O.; Conde Baena, P.; Romero Palacios, P.J.; Agusti, A. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur. Respir. J. 2018, 51, 1701457. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Su, W.L.; Huang, C.Y.; Yang, M.C.; Chen, S.Y.; Lan, C.C. Treatment of chronic obstructive pulmonary disease in patients with different fractional exhaled nitric oxide levels. Medicine 2018, 97, e11922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zietkowski, Z.; Kucharewicz, I.; Bodzenta-Lukaszyk, A. The influence of inhaled corticosteroids on exhaled nitric oxide in stable chronic obstructive pulmonary disease. Respir. Med. 2005, 99, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Rice, K.L.; Janoff, E.N.; Rector, T.S.; Niewoehner, D.E. Exhaled nitric oxide, systemic inflammation, and the spirometric response to inhaled fluticasone propionate in severe chronic obstructive pulmonary disease: A prospective study. Ther. Adv. Respir. Dis. 2008, 2, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Cowan, J.; Gray, A.; Brockway, B.; Dummer, J. Effect of Inhaled β2-Agonist on Exhaled Nitric Oxide in Chronic Obstructive Pulmonary Disease. PLoS ONE 2016, 11, e0157019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva-Gallardo, J.; Sansores, R.H.; Ramírez-Venegas, A.; Robles Hernández, R.E.; Centeno-Saenz, G.I.; Hernández-Zenteno, R.J. Fractional Exhaled Nitric Oxide (FeNO) in Biomass Smoke-Associated Chronic Obstructive Pulmonary Disease. Med. Sci. 2024, 12, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Riera-Martínez, L.; Cànaves-Gómez, L.; Iglesias, A.; Martin-Medina, A.; Cosío, B.G. The Role of IL-33/ST2 in COPD and Its Future as an Antibody Therapy. Int. J. Mol. Sci. 2023, 24, 8702. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, J.; Shang, J.; Li, M.; Zeng, Z.; Zhao, J.; Wang, J.; Xu, Y.; Xie, J. Increased IL-33 Expression in Chronic Obstructive Pulmonary Disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L619–L627. [Google Scholar] [CrossRef]
- Rabe, K.F.; Celli, B.R.; Wechsler, M.E.; Abdulai, R.M.; Luo, X.; Boomsma, M.M.; Staudinger, H.; Horowitz, J.E.; Baras, A.; Ferreira, M.A.; et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: A genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir. Med. 2021, 9, 1288–1298. [Google Scholar] [CrossRef]
- Yousuf, A.J.; Mohammed, S.; Carr, L.; Ramsheh, M.Y.; Micieli, C.; Mistry, V.; Haldar, K.; Wright, A.; Novotny, P.; Parker, S.; et al. Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): A phase 2a, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 469–477. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; García-Carmona, S.; Alanis-Ponce, J.; Pérez-Rubio, G.; Ramírez-Venegas, A.; Montiel-Lopez, F.; Robles-Hernández, R.; de Jesús Hernández-Zenteno, R.; Rea, D.V.P.; Bautista-Becerril, B.; et al. sRAGE levels are decreased in plasma and sputum of COPD secondary to biomass-burning smoke and tobacco smoking: Differences according to the rs3134940 AGER variant. Heliyon 2024, 10, e28675. [Google Scholar] [CrossRef]
- Hastie, A.T.; Martinez, F.J.; Curtis, J.L.; Doerschuk, C.M.; Hansel, N.N.; Christenson, S.; Putcha, N.; Ortega, V.E.; Li, X.; Barr, R.G.; et al. SPIROMICS investigators. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2017, 5, 956–967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giraldo-Montoya, Á.M.; Torres-Duque, C.A.; Giraldo-Cadavid, L.F.; Laucho-Contreras, M.E.; González-Flórez, A.; Santos, A.M.; Tuta-Quintero, E.A.; Celli, B.R.; González-García, M. Sputum Biomarkers in Wood and Tobacco Smoke Etiotypes of Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Asai, K.; Hirata, K.; Yoshikawa, J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am. J. Med. 2003, 114, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Bartel, S.; Wolters, J.C.; Noor, H.; Rafie, K.; Fang, J.; Kirchner, B.; Nolte-THoen, E.; Pfaffl, M.W.; Rutgers, S.; Timens, W.; et al. Altered Extracellular Vesicle-Derived Protein and microRNA Signatures in Bronchoalveolar Lavage Fluid from Patients with Chronic Obstructive Pulmonary Disease. Cells 2024, 13, 945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DiLillo, K.M.; Norman, K.C.; Freeman, C.M.; Christenson, S.A.; Alexis, N.E.; Anderson, W.H.; Barjaktarevic, I.Z.; Barr, R.G.; Comellas, A.P.; Bleecker, E.R.; et al. A blood and bronchoalveolar lavage protein signature of rapid FEV1 decline in smoking-associated COPD. Sci. Rep. 2023, 13, 8228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borrill, Z.L.; Roy, K.; Singh, D. Exhaled breath condensate biomarkers in COPD. Eur. Respir. J. 2008, 32, 472–486. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.; Sauvain, J.J.; Suarez, G.; Wild, P.; Charreau, T.; Debatisse, A.; Sakthithasan, K.; Jouannique, V.; Pralong, J.A.; Guseva Canu, I. Discriminative potential of exhaled breath condensate biomarkers with respect to chronic obstructive pulmonary disease. J. Occup. Med. Toxicol. 2024, 19, 10. [Google Scholar] [CrossRef]
- Maniscalco, M.; Paris, D.; Melck, D.J.; Molino, A.; Carone, M.; Ruggeri, P.; Caramori, G.; Motta, A. Differential diagnosis between newly diagnosed asthma and COPD using exhaled breath condensate metabolomics: A pilot study. Eur. Respir. J. 2018, 51, 1701825. [Google Scholar] [CrossRef]
- Maniscalco, M.; Paris, D.; Cuomo, P.; Fuschillo, S.; Ambrosino, P.; Tramice, A.; Palomba, L.; Motta, A. Metabolomics of COPD Pulmonary Rehabilitation Outcomes via Exhaled Breath Condensate. Cells 2022, 11, 344. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Loukides, S.; Minas, M.; Kontogianni, K.; Bakakos, P.; Gourgoulianis, K.I.; Alchanatis, M.; Papiris, S.; Kostikas, K. Exhaled breath condensate pH as a biomarker of COPD severity in ex-smokers. Respir. Res. 2011, 12, 67. [Google Scholar] [CrossRef]
- Inonu, H.; Doruk, S.; Sahin, S.; Erkorkmaz, U.; Celik, D.; Celikel, S.; Seyfikli, Z. Oxidative stress levels in exhaled breath condensate associated with COPD and smoking. Respir. Care 2012, 57, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Corhay, J.L.; Moermans, C.; Henket, M.; Nguyen Dang, D.; Duysinx, B.; Louis, R. Increased of exhaled breath condensate neutrophil chemotaxis in acute exacerbation of COPD. Respir. Res. 2014, 15, 115. [Google Scholar] [CrossRef]
- Zakharkina, T.; Koczulla, A.R.; Mardanova, O.; Hattesohl, A.; Bals, R. Detection of microorganisms in exhaled breath condensate during acute exacerbations of COPD. Respirology 2011, 16, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Van Velzen, P.; Brinkman, P.; Knobel, H.H.; Van den Berg, J.W.K.; Jonkers, R.E.; Loijmans, R.J.; Prins, J.M.; Sterk, P.J. Exhaled Breath Profiles Before, During and After Exacerbation of COPD: A Prospective Follow-Up Study. COPD 2019, 16, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Koutsokera, A.; Kostikas, K.; Nicod, L.P.; Fitting, J.W. Pulmonary biomarkers in COPD exacerbations: A systematic review. Respir. Res. 2013, 14, 111. [Google Scholar] [CrossRef]
- Brinkman, P.; Wilde, M.; Ahmed, W.; Wang, R.; van der Schee, M.; Abuhelal, S.; Schaber, C.; Cunoosamy, D.; Clarke, G.W.; Maitland-van der Zee, A.H.; et al. Fulfilling the Promise of Breathomics: Considerations for the Discovery and Validation of Exhaled Volatile Biomarkers. Am. J. Respir. Crit. Care Med. 2024, 210, 1079–1090. [Google Scholar] [CrossRef]
- Galbán, C.J.; Han, M.K.; Boes, J.L.; Chughtai, K.A.; Meyer, C.R.; Johnson, T.D.; Galbán, S.; Rehemtulla, A.; Kazerooni, E.A.; Martinez, F.J.; et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat. Med. 2012, 18, 1711–1715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pompe, E.; van Rikxoort, E.M.; Schmidt, M.; Rühaak, J.; Estrella, L.G.; Vliegenthart, R.; Oudkerk, M.; de Koning, H.J.; van Ginneken, B.; de Jong, P.A.; et al. Parametric response mapping adds value to current computed tomography biomarkers in diagnosing chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Pompe, E.; Galbán, C.J.; Ross, B.D.; Koenderman, L.; Ten Hacken, N.H.; Postma, D.S.; van den Berge, M.; de Jong, P.A.; Lammers, J.J.; Mohamed Hoesein, F.A. Parametric response mapping on chest computed tomography associates with clinical and functional parameters in chronic obstructive pulmonary disease. Respir. Med. 2017, 123, 48–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diaz, A.A.; Orejas, J.L.; Grumley, S.; Nath, H.P.; Wang, W.; Dolliver, W.R.; Yen, A.; Kligerman, S.J.; Jacobs, K.; Manapragada, P.P.; et al. Airway-Occluding Mucus Plugs and Mortality in Patients with Chronic Obstructive Pulmonary Disease. JAMA 2023, 329, 1832–1839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunican, E.M.; Elicker, B.M.; Henry, T.; Gierada, D.S.; Schiebler, M.L.; Anderson, W.; Barjaktarevic, I.; Barr, R.G.; Bleecker, E.R.; Boucher, R.C.; et al. Mucus Plugs and Emphysema in the Pathophysiology of Airflow Obstruction and Hypoxemia in Smokers. Am. J. Respir. Crit. Care Med. 2021, 203, 957–968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mettler, S.K.; Nath, H.P.; Grumley, S.; Orejas, J.L.; Dolliver, W.R.; Nardelli, P.; Yen, A.C.; Kligerman, S.J.; Jacobs, K.; Manapragada, P.P.; et al. Silent Airway Mucus Plugs in COPD and Clinical Implications. Chest 2024, 166, 1010–1019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanabe, N.; Shimizu, K.; Shima, H.; Wakazono, N.; Shiraishi, Y.; Terada, K.; Terada, S.; Oguma, T.; Sakamoto, R.; Suzuki, M.; et al. Computed tomography mucus plugs and airway tree structure in patients with chronic obstructive pulmonary disease: Associations with airflow limitation, health-related independence and mortality. Respirology 2024, 29, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Okajima, Y.; Come, C.E.; Nardelli, P.; Sonavane, S.K.; Yen, A.; Nath, H.P.; Terry, N.; Grumley, S.A.; Ahmed, A.; Kligerman, S.; et al. Luminal Plugging on Chest CT Scan: Association with Lung Function, Quality of Life, and COPD Clinical Phenotypes. Chest 2020, 158, 121–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gietema, H.A.; Edwards, L.D.; Coxson, H.O.; Bakke, P.S.; ECLIPS Einvestigators. Impact of emphysema airway wall thickness on quality of life in smoking-related COPD. Respir. Med. 2013, 107, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Saure, E.W.; Bakke, P.S.; Lind Eagan, T.M.; Aanerud, M.; Jensen, R.L.; Grydeland, T.B.; Johannessen, A.; Nilsen, R.M.; Thorsen, E.; Hardie, J.A. Diffusion capacity and CT measures of emphysema and airway wall thickness—Relation to arterial oxygen tension in COPD patients. Eur. Clin. Respir. J. 2016, 3, 29141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johannessen, A.; Skorge, T.D.; Bottai, M.; Grydeland, T.B.; Nilsen, R.M.; Coxson, H.; Dirksen, A.; Omenaas, E.; Gulsvik, A.; Bakke, P. Mortality by level of emphysema and airway wall thickness. Am. J. Respir. Crit. Care Med. 2013, 187, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Bodduluri, S.; Puliyakote, A.S.K.; Gerard, S.E.; Reinhardt, J.M.; Hoffman, E.A.; Newell, J.D., Jr.; Nath, H.P.; Han, M.K.; Washko, G.R.; San José Estépar, R.; et al. Airway fractal dimension predicts respiratory morbidity mortality in COPD. J. Clin. Investig. 2018, 128, 5374–5382, Erratum in J. Clin. Investig. 2018, 128, 5676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wyszkiewicz, P.V.; Sharma, M.; Desaigoudar, V.; Cunningham, I.A.; McCormack, D.G.; Abdelrazek, M.A.; Kirby, M.; Parraga, G. Reduced Total Airway Count and Airway Wall Tapering after Three-Years in Ex-Smokers. COPD 2023, 20, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.; Tanabe, N.; Tan, W.C.; Zhou, G.; Obeidat, M.; Hague, C.J.; Leipsic, J.; Bourbeau, J.; Sin, D.D.; Hogg, J.C.; et al. Total Airway Count on Computed Tomography the Risk of Chronic Obstructive Pulmonary Disease Progression Findings from a Population-based Study. Am. J. Respir. Crit. Care Med. 2018, 197, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bodduluri, S.; Kizhakke Puliyakote, A.; Nakhmani, A.; Charbonnier, J.P.; Reinhardt, J.M.; Bhatt, S.P. Computed Tomography-based Airway Surface Area-to-Volume Ratio for Phenotyping Airway Remodeling in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 203, 185–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kahnert, K.; Jörres, R.A.; Kauczor, H.U.; Alter, P.; Trudzinski, F.C.; Herth, F.; Jobst, B.; Weinheimer, O.; Nauck, S.; Mertsch, P.; et al. Standardized airway wall thickness Pi10 from routine CT scans of COPD patients as imaging biomarker for disease severity, lung function decline, and mortality. Ther. Adv. Respir. Dis. 2023, 17, 17534666221148663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woodruff, P.G.; Barr, R.G.; Bleecker, E.; Christenson, S.A.; Couper, D.; Curtis, J.L.; Gouskova, N.A.; Hansel, N.N.; Hoffman, E.A.; Kanner, R.E.; et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N. Engl. J. Med. 2016, 374, 1811–1821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lutchmedial, S.M.; Creed, W.G.; Moore, A.J.; Walsh, R.R.; Gentchos, G.E.; Kaminsky, D.A. How Common Is Airflow Limitation in Patients with Emphysema on CT Scan of the Chest? Chest 2015, 148, 176–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, A.S.; Baraghoshi, D.; Lynch, D.A.; Ash, S.Y.; Crapo, J.D.; Humphries, S.M. COPDGene Investigators. Emphysema Progression at CT by Deep Learning Predicts Functional Impairment and Mortality: Results from the COPDGene Study. Radiology 2022, 304, 672–679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schroeder, J.D.; McKenzie, A.S.; Zach, J.A.; Wilson, C.G.; Curran-Everett, D.; Stinson, D.S.; Newell JDJr Lynch, D.A. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am. J. Roentgenol. 2013, 201, W460–W470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grydeland, T.B.; Thorsen, E.; Dirksen, A.; Jensen, R.; Coxson, H.O.; Pillai, S.G.; Sharma, S.; Eide, G.E.; Gulsvik, A.; Bakke, P.S. Quantitative CT measures of emphysema and airway wall thickness are related to D(L)CO. Respir. Med. 2011, 105, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Pompe, E.; Strand, M.; van Rikxoort, E.M.; Hoffman, E.A.; Barr, R.G.; Charbonnier, J.P.; Humphries, S.; Han, M.K.; Hokanson, J.E.; Make, B.J.; et al. COPDGene Investigators. Five-year Progression of Emphysema and Air Trapping at CT in Smokers with and Those without Chronic Obstructive Pulmonary Disease: Results from the COPDGene Study. Radiology 2020, 295, 218–226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilgus, M.L.; Abtin, F.; Markovic, D.; Tashkin, D.P.; Phillips, J.E.; Buhr, R.G.; Flynn, M.J.; Dembek, M.; Cooper, C.B.; Barjaktarevic, I. Panlobular emphysema is associated with COPD disease severity: A study of emphysema subtype by computed tomography. Respir. Med. 2022, 192, 106717. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Tanabe, N.; Shimizu, K.; Oguma, A.; Shima, H.; Sakamoto, R.; Yamazaki, H.; Oguma, T.; Sato, A.; Suzuki, M.; et al. Stronger Associations of Centrilobular Than Paraseptal Emphysema with Longitudinal Changes in Diffusing Capacity and Mortality in COPD. Chest 2023, 164, 327–338. [Google Scholar] [CrossRef] [PubMed]
- El Kaddouri, B.; Strand, M.J.; Baraghoshi, D.; Humphries, S.M.; Charbonnier, J.P.; van Rikxoort, E.M.; Lynch, D.A. Fleischner Society Visual Emphysema CT Patterns Help Predict Progression of Emphysema in Current and Former Smokers: Results from the COPDGene Study. Radiology 2021, 298, 441–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, A.S.; Strand, M.; Pratte, K.; Regan, E.A.; Humphries, S.; Crapo, J.D.; Lynch, D.A.; Genetic Epidemiology of COPDGene Investigators. Visual Emphysema at Chest CT in GOLD Stage 0 Cigarette Smokers Predicts Disease Progression: Results from the COPDGene Study. Radiology 2020, 296, 641–649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ash, S.Y.; San José Estépar, R.; Fain, S.B.; Tal-Singer, R.; Stockley, R.A.; Nordenmark, L.H.; Rennard, S.; Han, M.K.; Merrill, D.; Humphries, S.M.; et al. Relationship between Emphysema Progression at CT and Mortality in Ever-Smokers: Results from the COPDGene and ECLIPSE Cohorts. Radiology 2021, 299, 222–231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell, A.J.; Pal, R.; Labaki, W.W.; Hoff, B.A.; Wang, J.M.; Murray, S.; Kazerooni, E.A.; Galban, S.; Lynch, D.A.; Humphries, S.M.; et al. Local heterogeneity of normal lung parenchyma and small airways disease are associated with COPD severity and progression. Respir. Res. 2024, 25, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pistenmaa, C.L.; Nardelli, P.; Ash, S.Y.; Come, C.E.; Diaz, A.A.; Rahaghi, F.N.; Barr, R.G.; Young, K.A.; Kinney, G.L.; Simmons, J.P.; et al. Pulmonary Arterial Pruning and Longitudinal Change in Percent Emphysema and Lung Function: The Genetic Epidemiology of COPD Study. Chest 2021, 160, 470–480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Washko, G.R.; Nardelli, P.; Ash, S.Y.; Vegas Sanchez-Ferrero, G.; Rahaghi, F.N.; Come, C.E.; Dransfield, M.T.; Kalhan, R.; Han, M.K.; Bhatt, S.P.; et al. Arterial Vascular Pruning, Right Ventricular Size, and Clinical Outcomes in Chronic Obstructive Pulmonary Disease. A Longitudinal Observational Study. Am. J. Respir. Crit. Care Med. 2019, 200, 454–461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Shu, T.; Wang, L.; Yang, L.; Hu, C.; Du, S.; Wei, H. Pulmonary artery enlargement predicts poor survival in patients with COPD: A meta-analysis. Pulm. Circ. 2022, 12, e12099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatt, S.P.; Soler, X.; Wang, X.; Murray, S.; Anzueto, A.R.; Beaty, T.H.; Boriek, A.M.; Casaburi, R.; Criner, G.J.; Diaz, A.A.; et al. Association between Functional Small Airway Disease and FEV1 Decline in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016, 194, 178–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.M.; Bell, A.J.; Ram, S.; Labaki, W.W.; Hoff, B.A.; Murray, S.; Kazerooni, E.A.; Galban, S.; Hatt, C.R.; Han, M.K.; et al. Topologic Parametric Response Mapping Identifies Tissue Subtypes Associated with Emphysema Progression. Acad. Radiol. 2024, 31, 1148–1159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coxson, H.O.; Dirksen, A.; Edwards, L.D.; Yates, J.C.; Agusti, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Crim, C.; Duvoix, A.; et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: A prospective analysis from the ECLIPSE study. Lancet Respir. Med. 2013, 1, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Klont, F.; Horvatovich, P.; Bowler, R.P.; van Rikxoort, E.; Charbonnier, J.P.; Kwiatkowski, M.; Lynch, D.A.; Humphries, S.; Bischoff, R.; Ten Hacken, N.H.T.; et al. Plasma sRAGE levels strongly associate with centrilobular emphysema assessed by HRCT scans. Respir. Res. 2022, 23, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diaz, A.A.; Strand, M.; Coxson, H.O.; Ross, J.C.; San Jose Estepar, R.; Lynch, D.; van Rikxoort, E.M.; Rosas, I.O.; Hunninghake, G.M.; Putman, R.K.; et al. Disease Severity Dependence of the Longitudinal Association Between CT Lung Density and Lung Function in Smokers. Chest 2018, 153, 638–645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baraghoshi, D.; Strand, M.; Humphries, S.M.; San José Estépar, R.; Vegas Sanchez-Ferrero, G.; Charbonnier, J.P.; Latisenko, R.; Silverman, E.K.; Crapo, J.D.; Lynch, D.A. Quantitative CT Evaluation of Emphysema Progression over 10 Years in the COPDGene Study. Radiology 2023, 307, e222786, Erratum in Radiology 2023, 309, e239028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohamed Hoesein, F.A.; de Jong, P.A.; Lammers, J.W.; Mali, W.P.; Mets, O.M.; Schmidt, M.; de Koning, H.J.; Aalst Cv Oudkerk, M.; Vliegenthart, R.; Ginneken Bv van Rikxoort, E.M.; et al. Contribution of CT quantified emphysema, air trapping and airway wall thickness on pulmonary function in male smokers with and without COPD. COPD 2014, 11, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Arjomandi, M.; Zeng, S.; Barjaktarevic, I.; Barr, R.G.; Bleecker, E.R.; Bowler, R.P.; Buhr, R.G.; Criner, G.J.; Comellas, A.P.; Cooper, C.B.; et al. Radiographic lung volumes predict progression to COPD in smokers with preserved spirometry in SPIROMICS. Eur. Respir. J. 2019, 54, 1802214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, A.L.; Bragman, F.J.S.; Rangelov, B.; Han, M.K.; Galbán, C.J.; Lynch, D.A.; Hawkes, D.J.; Alexander, D.C.; Hurst, J.R.; COPDGene Investigators. Disease Progression Modeling in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 294–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, S.; Luo, G.; Lynch, D.A.; Bowler, R.P.; Arjomandi, M. Lung volumes differentiate the predominance of emphysema versus airway disease phenotype in early COPD: An observational study of the COPDGene cohort. ERJ Open Res. 2023, 9, 00289–02023, Erratum in ERJ Open Res. 2023, 9, 50289–52023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaudhary, M.F.A.; Hoffman, E.A.; Guo, J.; Comellas, A.P.; Newell, J.D., Jr.; Nagpal, P.; Fortis, S.; Christensen, G.E.; Gerard, S.E.; Pan, Y.; et al. Predicting severe chronic obstructive pulmonary disease exacerbations using quantitative CT: A retrospective model development and external validation study. Lancet Digit. Health 2023, 5, e83–e92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.; Sang, L.; Wang, J.; Chen, Y.; Lai, J.; Zhu, X.; Yang, Y.; Zhang, Z.; Liu, Y.; Wen, S.; et al. Analysis of Airway Thickening and Serum Cytokines in COPD Patients with Frequent Exacerbations: A Heart of the Matter. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2353–2364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jairam, P.M.; van der Graaf, Y.; Lammers, J.W.; Mali, W.P.; de Jong, P.A.; PROVIDI Study Group. Incidental findings on chest CT imaging are associated with increased COPD exacerbations and mortality. Thorax 2015, 70, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Hueper, K.; Vogel-Claussen, J.; Parikh, M.A.; Austin, J.H.; Bluemke, D.A.; Carr, J.; Choi, J.; Goldstein, T.A.; Gomes, A.S.; Hoffman, E.A.; et al. Pulmonary Microvascular Blood Flow in Mild Chronic Obstructive Pulmonary Disease and Emphysema. The MESA COPD Study. Am. J. Respir. Crit. Care Med. 2015, 192, 570–580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.Y.; Parikh, M.; Bluemke, D.A.; Balte, P.; Carr, J.; Dashnaw, S.; Poor, H.D.; Gomes, A.S.; Hoffman, E.A.; Kawut, S.M.; et al. Pulmonary artery stiffness in chronic obstructive pulmonary disease (COPD) and emphysema: The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study. J. Magn. Reson. Imaging 2018, 47, 262–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poor, H.D.; Kawut, S.M.; Liu, C.Y.; Smith, B.M.; Hoffman, E.A.; Lima, J.A.; Ambale-Venkatesh, B.; Michos, E.D.; Prince, M.R.; Barr, R.G. Pulmonary hyperinflation due to gas trapping and pulmonary artery size: The MESA COPD Study. PLoS ONE 2017, 12, e0176812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuoka, S.; Washko, G.R.; Dransfield, M.T.; Yamashiro, T.; San Jose Estepar, R.; Diaz, A.; Silverman, E.K.; Patz, S.; Hatabu, H. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: Correlations with emphysema and airflow limitation. Acad. Radiol. 2010, 17, 93–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandra, D.; Gupta, A.; Kinney, G.L.; Fuhrman, C.R.; Leader, J.K.; Diaz, A.A.; Bon, J.; Barr, R.G.; Washko, G.; Budoff, M.; et al. The Association Between Lung Hyperinflation and Coronary Artery Disease in Smokers. Chest 2021, 160, 858–871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goffin, J.R.; Pond, G.R.; Puksa, S.; Tremblay, A.; Johnston, M.; Goss, G.; Nicholas, G.; Martel, S.; Bhatia, R.; Liu, G.; et al. Chronic obstructive pulmonary disease prevalence and prediction in a high-risk lung cancer screening population. BMC Pulm. Med. 2020, 20, 300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruparel, M.; Quaife, S.L.; Dickson, J.L.; Horst, C.; Tisi, S.; Hall, H.; Taylor, M.N.; Ahmed, A.; Shaw, P.J.; Burke, S.; et al. Prevalence, Symptom Burden, and Underdiagnosis of Chronic Obstructive Pulmonary Disease in a Lung Cancer Screening Cohort. Ann. Am. Thorac. Soc. 2020, 17, 869–878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tisi, S.; Dickson, J.L.; Horst, C.; Quaife, S.L.; Hall, H.; Verghese, P.; Gyertson, K.; Bowyer, V.; Levermore, C.; Mullin, A.M.; et al. Detection of COPD in the SUMMIT Study lung cancer screening cohort using symptoms and spirometry. Eur. Respir. J. 2022, 60, 2200795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mets, O.M.; Buckens, C.F.; Zanen, P.; Isgum, I.; van Ginneken, B.; Prokop, M.; Gietema, H.A.; Lammers, J.W.; Vliegenthart, R.; Oudkerk, M.; et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA 2011, 306, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Criner, G.J.; Delage, A.; Voelker, K.; Hogarth, D.K.; Majid, A.; Zgoda, M.; Lazarus, D.R.; Casal, R.; Benzaquen, S.B.; Holladay, R.C.; et al. Improving Lung Function in Severe Heterogenous Emphysema with the Spiration Valve System (EMPROVE). A Multicenter, Open-Label Randomized Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1354–1362. [Google Scholar] [CrossRef]
- Klooster, K.; ten Hacken, N.H.; Hartman, J.E.; Kerstjens, H.A.; van Rikxoort, E.M.; Slebos, D.J. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N. Engl. J. Med. 2015, 373, 2325–2335. [Google Scholar] [CrossRef]
- Caviezel, C.; Froehlich, T.; Schneiter, D.; Muehlematter, U.; Frauenfelder, T.; Guglielmetti, L.C.; Opitz, I.; Weder, W. Identification of target zones for lung volume reduction surgery using three-dimensional computed tomography rendering. ERJ Open Res. 2020, 6, 00305–02020. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, L.J.; Clarke, C.S.; Nazareth, I.; Ambler, G. The value of blood-based measures of liver function and urate in lung cancer risk prediction: A cohort study and health economic analysis. Cancer Epidemiol. 2023, 84, 102354. [Google Scholar] [CrossRef]
- Raoof, S.; Shah, M.; Braman, S.; Agrawal, A.; Allaqaband, H.; Bowler, R.; Castaldi, P.; DeMeo, D.; Fernando, S.; Hall, C.S.; et al. Lung Imaging in COPD Part 2: Emerging Concepts. Chest 2023, 164, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Jenkins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022, 10, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Harch, I.E.; Garcia-Larsen, V.; Benmaamar, S.; Nejjari, C.; Biaze, M.E.; Benjelloun, M.C.; Rhazi, K.E. Association between biomass exposure and COPD occurrence in Fez, Morocco: Results from the BOLD study. BMJ Open Respir. Res. 2024, 11, e002409. [Google Scholar] [CrossRef]
- Ortiz-Quintero, B.; Martínez-Espinosa, I.; Pérez-Padilla, R. Mechanisms of Lung Damage and Development of COPD Due to Household Biomass-Smoke Exposure: Inflammation, Oxidative Stress, MicroRNAs, and Gene Polymorphisms. Cells 2022, 12, 67. [Google Scholar] [CrossRef]
- Moran-Mendoza, O.; Pérez-Padilla, J.R.; Salazar-Flores, M.; Vazquez-Alfaro, F. Wood smoke-associated lung disease: A clinical, functional, radiological and pathological description. Int. J. Tuberc. Lung Dis. 2008, 12, 1092–1098. [Google Scholar]
- González-García, M.; Torres-Duque, C.A.; Bustos, A.; Jaramillo, C.; Maldonado, D. Bronchial hyperresponsiveness in women with chronic obstructive pulmonary disease related to wood smoke. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 367–373. [Google Scholar] [CrossRef]
- Fandiño-Del-Rio, M.; Kephart, J.L.; Williams, K.N.; Malpartida, G.; Boyd Barr, D.; Steenland, K.; Koehler, K.; Checkley, W. Household air pollution and blood markers of inflammation: A cross-sectional analysis. Indoor Air. 2021, 31, 1509–1521. [Google Scholar] [CrossRef]
- Banerjee, A.; Mondal, N.K.; Das, D.; Ray, M.R. Neutrophilic Inflammatory Response and Oxidative Stress in Premenopausal Women Chronically Exposed to Indoor Air Pollution from Biomass Burning. Inflammation 2011, 35, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Ray, M.R.; Banerjee, A. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol. Appl. Pharmacol. 2012, 261, 255–262. [Google Scholar] [CrossRef] [PubMed]

| Serum/Blood Biomarkers | FEV1 Decline | Exacerbations | Emphysema on Imaging | Hospitalization | Mortality | Predicting Response to a Treatment | References |
|---|---|---|---|---|---|---|---|
| Eos | ↑ | ↑ | ↑ * | [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] | |||
| IgE | ↑ | ↑ | ↑ ** | [19,20,21,22] | |||
| CRP | ↔ | ↑ | C | C | ↑ *** | [23,24,25,26,27,28,29,30,31] | |
| Fibrinogen | C | C | ↔ | ↑ | [4,26,27,32,33,34] | ||
| PCT | ↑ | ↔ | C *** | [16,25,30,35,36] | |||
| IL-6 | ↑ | ↔ | [3,27,28,37,38,39] | ||||
| IL-8 | C | ↑ | [3,28,39] | ||||
| sRAGE | ↔ **** | ↔ | ↓ | ↔ | ↔ | [2,40] | |
| CC16 | ↓ | ↔ | [2,41,42] | ||||
| SP-D | C | ↑ | ↑ | [2,43] | |||
| Exhaled Breath Biomarker | |||||||
| FeNO | ↑ | ↑ | ↑ | ↑ | ↑ * | [1,18,44,45,46,47,48,49,50,51,52,53,54,55,56,57] | |
| Imaging Biomarker | Lung Function Decline | Risk for Exacerbation | Functional Status Decline | Mortality | References |
|---|---|---|---|---|---|
| Mucus Plugs | ↑ | ↑ | ↑ | ↑ | [84,85,86,87,88] |
| Airway Wall Thickness | ↑ | ↑ | ↑ | ↑ | [89,90,91,92,93,94,95,96,97] |
| Total Airway Counts | ↑ | [94] | |||
| Emphysema | ↑ | ↑ | ↑ | [98,99,100,101,102,103,104,105,106,107] | |
| fSAD | ↑ | [108] | |||
| Pulmonary Artery Pruning | ↑ | ↑ | ↑ | [109,110] | |
| Enlarging Pulmonary Arteries | ↑ | ↑ | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, K.M.; Lavere, P.F.; Hanania, N.A.; Adrish, M. The Emerging Biomarkers in Chronic Obstructive Pulmonary Disease: A Narrative Review. Diagnostics 2025, 15, 1245. https://doi.org/10.3390/diagnostics15101245
Phillips KM, Lavere PF, Hanania NA, Adrish M. The Emerging Biomarkers in Chronic Obstructive Pulmonary Disease: A Narrative Review. Diagnostics. 2025; 15(10):1245. https://doi.org/10.3390/diagnostics15101245
Chicago/Turabian StylePhillips, Kaitlin M., Philip F. Lavere, Nicola A. Hanania, and Muhammad Adrish. 2025. "The Emerging Biomarkers in Chronic Obstructive Pulmonary Disease: A Narrative Review" Diagnostics 15, no. 10: 1245. https://doi.org/10.3390/diagnostics15101245
APA StylePhillips, K. M., Lavere, P. F., Hanania, N. A., & Adrish, M. (2025). The Emerging Biomarkers in Chronic Obstructive Pulmonary Disease: A Narrative Review. Diagnostics, 15(10), 1245. https://doi.org/10.3390/diagnostics15101245







