Deep Learning Algorithms for Screening and Diagnosis of Systemic Diseases Based on Ophthalmic Manifestations: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
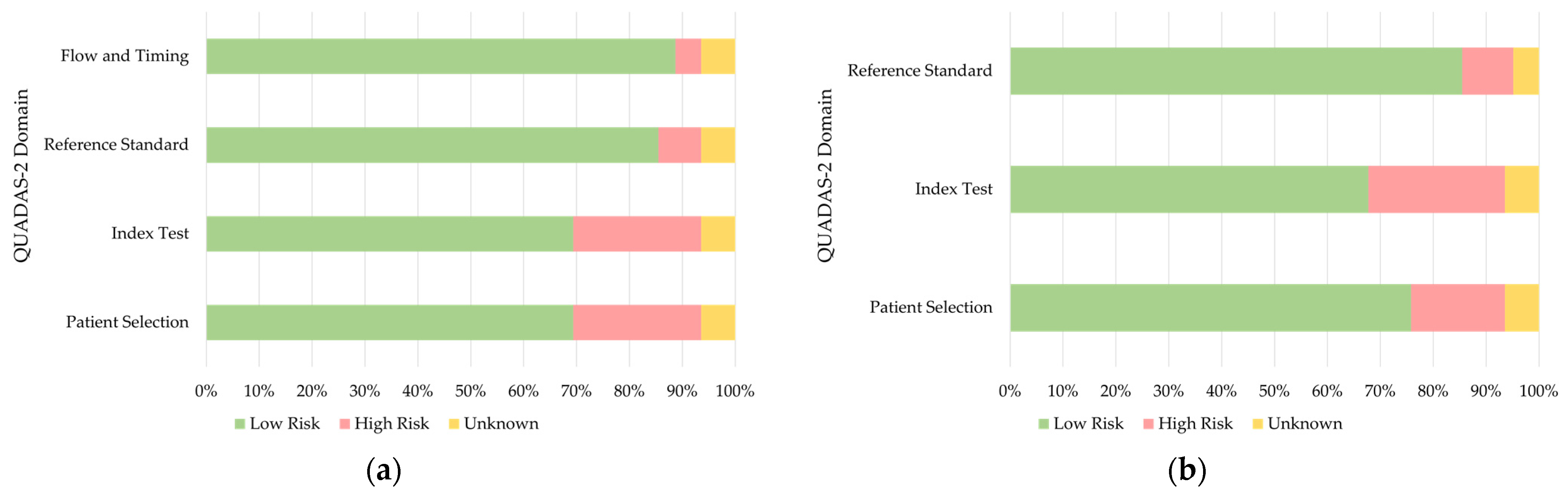
2.2. Data Extraction and Quality Assessment
3. Results
3.1. Study Selection
| Author, Year | Ocular Data | DL Model | Training Dataset | Testing/Validation Dataset | External Validation | Systemic Health Features/Diseases | Outcome | Performance 1 |
|---|---|---|---|---|---|---|---|---|
| Babenko et al., 2022 [13] | External eye images | DLS | EyePACS (CA): 126,066 patients, 290,642 images | 19,766 patients, 41,928 images | Set A: EyePACS (non-CA): 27,415 patients, 53,861 images; Set B: EyePACS (non-CA): 5058 patients, 9853 images; Set C: 10,402 patients, 19,763 images; Set D: 6266 patients, 12,751 images | HbA1c Total cholesterol Triglycerides | Binary Binary Binary | AUC: 73.4% AUC: 62.3% AUC: 67.1% |
| Li et al., 2022 [14] | Conjunctival images | HMT-Net | 68 patients, 405 images; 62 HC, 206 images | 5-fold cross-validation | N/A | T2DM | Binary | Sensitivity: 78.70% Specificity: 69.08% Accuracy: 75.15% AUC: 0.82 |
| Preston et al., 2022 [15] | CCM images | ResNet-50 | 65 HC images, 63 T1DM, 89 T2DM, 28 prediabetes | Test: 15 HC, 11 T1DM, 10 T2DM, 4 prediabetes; Validation: 10 HC, 14 T1DM, 42 T2DM, 18 prediabetes | N/A | DPN | Ternary | 1. Healthy: F1-score: 0.91 2. No DPN: F1-score: 0.88 3. DPN: F1-score: 0.91 |
| Scarpa et al., 2020 [16] | CCM images | CNN | 40 patients, 240 images; 40 HC, 240 images | 10 patients, 60 images; 10 HC, 60 images; 5-fold cross-validation | N/A | DPN | Binary | Sensitivity: 98% Specificity: 94% Accuracy: 96% |
| Althnian et al., 2021 [17] | Scleral images | VGG-16 | 24 images of patients, 44 images of HC | N/M | N/A | Neonatal jaundice | Binary | Accuracy: 79.03% F1-score: 70.73% AUC: 69.67% |
| Lv et al., 2021 [18] | Scleral images | U-Net, Resnet-18, MIL model | 576 participants, 4608 images | 145 participants, 1160 images; 5-fold cross-validation | N/A | PCOS | Binary | AUC: 0.979 Accuracy: 0.929 F1-score: 0.934 |
3.2. Algorithms Based on the Anterior Segment of the Eye
3.3. Algorithms Based on the Posterior Segment of the Eye
| Author, Year | Ocular Data | DL Model | Training Dataset | Testing/Validation Dataset | External Validation | Systemic Health Features/Diseases | Outcome | Performance 1 |
|---|---|---|---|---|---|---|---|---|
| Betzler et al., 2021 [20] | Retinal images | VGG-16 | SEED: 7969 participants, 137,511 images | 1987 participants, 34,659 images | N/A | Gender | Binary | AUC: 0.94 |
| Corbin et al., 2022 [21] | Fundus images | EfficientNet | 14,711 participants for all datasets; 18,000 images for training | Validation: 3860 images; Test: 3877 images | N/A | Age SBP DBP BMI Sex (image) Sex (individual) APOE4 (image) APOE4 (individual) | Regression Regression Regression Regression Binary Binary Binary Binary | R2: 0.778, MAE: 3.24 R2: 0.229, MAE: 10.94 R2: 0.227, MAE: 6.80 R2: 0.032, MAE: 3.99 AUC: 0.84 AUC: 0.85 AUC: 0.47 AUC: 0.50 |
| Gerrits et al., 2020 [22] | Retinal images | MobileNet-V2 | Qatar Biobank: 1800 participants, 7200 images | Validation: 600 participants, 2400 images Test: 600 participants, 2400 images | N/A | Age Sex SBP DBP HbA1c BMI RFM Glucose Insulin SHBG Estradiol Testosterone Tch HDL LDL Tg Smoking status | Regression Binary Regression Regression Regression Regression Regression Regression Regression Regression Regression Regression Regression Regression Regression Regression Binary | MAE: 2.78, R2: 0.89 AUC: 0.97 MAE: 8.96, R2: 0.40 MAE: 6.84, R2: 0.24 MAE: 0.61, R2: 0.34 MAE: 4.31, R2: 0.13 MAE: 5.68, R2: 0.43 MAE: 1.06, R2: 0.12 MAE: 7.15, R2: −0.04 MAE: 21.09, R2: 0.06 MAE: 154.18, R2: −0.03 MAE: 3.76, R2: 0.54 MAE: 0.75, R2: 0.03 MAE: 0.31, R2: 0.05 MAE: 0.72, R2: −0.03 MAE: 0.49, R2: 0.03 AUC: 0.78 |
| Hu et al., 2022 [23] | Retinal images | DL | UK Biobank: 11,052 participants, 19,200 images | 35,834 participants | N/A | Retinal age | Regression | MAE: 3.55 |
| Khan et al., 2022 [24] | Retinal images | DenseNet-201 | 760 participants for all datasets; 1021 images for training | 256 images for testing | N/A | Gender ARB Smoking status ACEi LDL Hypertension HDL Cardiac disease HbA1c Age Aspirin Ethnicity | Binary Binary Binary Binary Binary Binary Binary Binary Binary Binary Binary Binary | AUC: 0.852 AUC: 0.783 AUC: 0.732 AUC: 0.815 AUC: 0.766 AUC: 0.687 AUC: 0.756 AUC: 0.7 AUC: 0.708 AUC: 0.902 AUC: 0.696 AUC: 0.926 |
| Kim et al., 2020 [25] | Retinal images | ResNet-152 | 155,449 participants for all datasets; 216,866 HC images for training | Validation: 2436 HC images; Test: 24,366 HC images, 40,659 hypertension, 14,189 DM, 113,510 smoking | N/A | Age Sex | Regression Binary | MAE: 3.06, R2: 0.92 AUC: 0.969 |
| Korot et al., 2021 [26] | Retinal images | CFDL | UK Biobank: 84,743 patients, 173,819 images | 728 patients, 1287 images | 252 patients, 252 images | Sex | Binary | Sensitivity: 83.9% Specificity: 72.2% Accuracy: 78.6% |
| Mendoza et al., 2021 [27] | OCT | DL | 1772 patients, 52,552 circle B-scans; 730 patients, 111,456 radial B-scans; 85% for training | 5% for validation, 10% for testing | N/A | Age Axial length Sex Race Diabetes Hypertension CVD | Regression Regression Binary Binary Binary Binary Binary | MAE: 5.4, R2: 0.73 MAE: 0.7, R2: 0.3 AUC: 0.72 AUC: 0.96 AUC: 0.65 AUC: 0.71 AUC: 0.56 |
| Munk et al., 2021 [28] | Fundus images, OCT | ResNet-152 | 16,196 participants, 135,667 fundus images; 5578 participants, 85,536 OCT scans; 80% for training | 10% for validation, 10% for testing | N/A | Age Sex | Binary Regression | 1. Fundus images: MAE: 6.328, AUC: 0.80 2. OCT cross sections: MAE: 5.625, AUC: 0.84 3. OCT volumes: MAE: 4.541, AUC: 0.90 |
| Nusinovici et al., 2022 [29] | Retinal images | RetiAGE | 36,432 participants, 116,312 images | Validation: 4048 participants, 12,924 images; Test: 10,171 participants, 32,318 images | UK Biobank: 56,301 participants | Age | Binary | AUC: 0.756 |
| Poplin et al., 2018 [30] | Retinal images | Inception-v3 | UK Biobank: 48,101 patients, 96,082 images; EyePACS: 236,234 patients, 1,682,938 images | UK Biobank: 12,026 patients, 24,008 images; EyePACS-2K: 999 patients, 1958 images | N/A | Age Gender Smoking status HbA1c BMI SBP DBP | Regression Binary Binary Regression Regression Regression Regression | MAE: 3.26, R2: 0.74 AUC: 0.97 AUC: 0.71 MAE: 1.39, R2: 0.09 MAE: 3.29, R2: 0.13 MAE: 11.35, R2: 0.36 MAE: 6.42, R2: 0.32 |
| Rim et al., 2020 [31] | Retinal images | VGG-16 | 27,516 participants, 86,994 images | 6879 participants, 21,698 images | Set 1: 4343 participants, 9324 images; Set 2: BES: 1060 participants, 4234 images; Set 3: SEED: 7726 participants, 63,275 images; Set 4: UK Biobank: 25,366 participants, 50,732 images | Sex Age BMM Height Bodyweight PBF BMI Creatinine DBP SBP Hematocrit Hemoglobin RBC count | Binary Regression Regression Regression Regression Regression Regression Regression Regression Regression Regression Regression Regression | AUC: 0.91, Accuracy: 0.85 MAE: 3.78, R2: 0.36 MAE: N/A, R2: N/A MAE: 5.48, R2: 0.23 MAE: 8.28, R2: 0.17 MAE: N/A, R2: N/A MAE: 2.90, R2: 0.06 MAE: 0.11, R2: 0.12 MAE: 8.09, R2: 0.2 3MAE: 13.20, R2: 0.19 MAE: N/A, R2: N/A MAE: N/A, R2: N/A MAE: N/A, R2: N/A |
| Tham et al., 2019 [32] | Fundus images | ResNet, DenseNet | 13,937 participants, 25,637 images | 3485 participants, 6830 images | N/A | HbA1c | Regression | MAE: 0.87% |
| Vaghefi et al., 2019 [33] | Retinal images | CNN | 81,711 participants, 165,104 images; 60% for training | 20% for validation, 20% for testing | N/A | Smoking | Binary | Accuracy: 88.88% Specificity: 93.87% Sensitivity: 62.62% AUC: 0.86 |
| Yang et al., 2020 [34] | Retinal images | VGG-16 | SEED: 9748 participants, 110,099 images; 80% for training | 20% for testing | N/A | Race | Ternary | Accuracy: 95.1% |
| Zhang et al., 2020 [35] | Retinal images | Inception-v3 | 625 participants, 1222 images; 80% for training | 10% for validation, 10% for testing | N/A | Hyperglycemia Hypertension Dyslipidemia Age Gender Drinking status Salty taste Smoking status BMI WHR HCT MCHC T-BIL D-BIL | Binary Binary Binary Binary Binary Binary Binary Binary Binary Binary Binary Binary Binary Binary | AUC: 0.880 AUC: 0.766 AUC: 0.703 AUC: 0.850 AUC: 0.704 AUC: 0.948 AUC: 0.809 AUC: 0.794 AUC: 0.731 AUC: 0.704 AUC: 0.759 AUC: 0.686 AUC: 0.764 AUC: 0.703 |
| Al-Absi et al., 2022 [36] | Retinal images | ResNet-34 | Qatar Biobank: 233 patients, 874 images; 250 HC, 931 images | 5-fold cross-validation | N/A | CVD | Binary | Accuracy: 75.6% |
| Mellor et al., 2019 [37] | Fundus images | ResNet | 4782 participants | 5-fold cross-validation | N/A | CVD | Binary | AUC: 0.77 |
| Chang et al., 2019 [38] | Retinal images | NASNet-Large | 33,025 participants, 96,968 images | 6597 participants, 13,373 images | N/A | FAD | Regression | MAE: 2.74 |
| Ng et al., 2022 [39] | Retinal images | DLPPC | 58 patients, 116 images; 80% for training | 20% for testing | N/A | SpO2 LICUS PC OT CBT ABT | Binary Binary Binary Binary Binary Binary | AUC: 0.712 AUC: 0.731 AUC: 0.722 AUC: 0.581 AUC: 0.800 AUC: 0.767 |
| Mueller et al., 2022 [40] | Fundus images | MIL | 97 patients, 34 HC; 83,126 images for training | 9237 images for validation | N/A | PAD | Binary | Accuracy: 0.837 F1-score: 0.883 AUC: 0.89 |
| Chang et al., 2020 [41] | Retinal images | DL-FAS | 5296 participants, 12,362 images | Validation: 647 participants, 1526 images; Test: 654 participants, 1520 images | N/A | Atherosclerosis | Binary | AUC: 0.713 Accuracy: 0.583 Sensitivity: 0.891 Specificity: 0.404 |
| Barriada et al., 2022 [42] | Retinal images | VGG-16 | 76 patients, 152 images | 5-fold cross-validation | N/A | CACS | Binary | Accuracy: 0.72 F1-score: 0.62 |
| Rim et al., 2021 [43] | Retinal images | RetiCAC | 15,911 participants, 36,034 images | 3965 participants, 8930 images | Set 1: 8707 participants, 18,920 images; Set 2: 527 participants, 1054 images | CACS | Binary | AUC: 0.742 |
| Son et al., 2020 [44] | Retinal images | Inception-v3 | 20,130 participants, 44,184 images; 80% for training | 20% for training; 5-fold cross-validation | N/A | CACS | Binary | AUC: 83.2% |
| Dai et al., 2020 [45] | Retinal images | CNN | 735 patients, 684 HC; 60% for training | 20% for validation, 20% for testing; 5-fold cross-validation | N/A | Hypertension | Binary | Accuracy: 60.94% Specificity: 63.80% AUC: 0.6506 |
| Lo et al., 2021 [46] | Fundus images | AML-Net | 200 patient images, 200 HC; 70% for training | 30% for validation | N/A | Mild hypertension | Binary | Accuracy: 93.75% |
| Islam et al., 2021 [47] | Retinal images | DiaNet | EyePACS: over 80,000 images; Qatar Biobank: 246 patients, 246 controls, total 1852 images | 5-fold cross-validation | N/A | Diabetes | Binary | Accuracy: 84.47% Specificity: 83.06% AUC: 84.46% |
| Wang et al., 2022 [48] | Retinal images | CNN | 10,766 images | N/M | N/A | Short-term readmission risk in diabetes | Binary | Specificity: 0.79 Accuracy: 0.837 |
| Zhang et al., 2018 [49] | Fundus images | ResNet | 79 patients, 79 HC; 80% for training | 20% for testing | N/A | Diabetes | Binary | Accuracy: 84.7% |
| Abbasi-Sureshjani et al., 2018 [50] | Retinal images | ResNet | 5791 HC images, 3133 T2DM; 80% for training | 20% for validation | N/A | T2DM | Binary | F1-score: 0.758 |
| Heslinga et al., 2020 [51] | Retinal images | VGG-19 | 1376 participants, 5222 images | Validation: 464 participants, 1802 images; Test: 496 participants, 1900 images; | N/A | T2DM | Binary | AUC: 0.746 |
| Yun et al., 2022 [52] | Retinal images | ResNet-18 | UK Biobank: 37,904 patients, 69,639 images | Test: 12,173 patients, 22,342 images; Validation: 12,185 patients, 22,394 images | 6575 images | T2DM | Binary | AUC: 0.731 Sensitivity: 0.662 Specificity: 0.662 |
| Cervera et al., 2021 [53] | Retinal images | Squeezenet v1.0 | 1081 patients, 17,028 images | 121 patients, 1892 images; 5-fold cross-validation | N/A | DPN | Binary | AUC: 0.8013 |
| Mitani et al., 2020 [54] | Retinal images | Inception-v4 | UK Biobank: 40,041 participants, 80,006 images | Validation: 11,388 participants, 22,742 images; Test: 5734 participants, 11,457 images | N/A | Hemoglobin Anemia | Regression Binary | MAE: 0.67 AUC: 0.87 |
| Wei et al., 2021 [55] | OCT | AneNet | 17 patients, 221 images; 13 HC, 207 images | 5-fold cross-validation | N/A | Anemia | Binary | Accuracy: 0.9865 Sensitivity: 0.9838 Specificity: 0.9594 AUC: 0.9983 |
| Zhao et al., 2022 [56] | UWF Fundus images | ASModel_ UWF, ASModel_ CroppedUWF | 2445 participants, 9221 images | Validation: 213 participants, 577 images; Test: 565 participants, 1730 images | N/A | Hemoglobin Anemia | Regression Binary | MAE: 0.83 AUC: 0.93 Sensitivity: 91.2% Specificity: 80.00% |
| Kang et al., 2020 [57] | Retinal images | VGG-19 | 4970 patients, 20,787 images | Validation: 621 patients, 2189 images; Test: 621 patients, 2730 images | N/A | Early renal function impairment | Binary | AUC: 0.81 Sensitivity: 0.83 Specificity: 0.62 Accuracy: 0.73 |
| Sabanayagam et al., 2020 [58] | Retinal images | CondenseNet | SEED: 5188 participants, 10,376 images | 1297 participants, 2594 images; 5-fold cross-validation | 1. 3735 participants, 7470 images; 2. BES: 1538 participants, 3076 images | CKD | Binary | AUC: 0.835 Sensitivity: 0.75 Specificity: 0.75 |
| Zhang et al., 2021 [59] | Retinal images | ResNet-50 | 30,122 participants, 60,244 images | Validation: 4307 participants, 8614 images; Test: 8727 participants, 17,454 images | 1. 8059 participants, 16,118 images; 2. 3081 participants, 6162 images | CKD Early CKD T2DM | Binary Binary Binary | AUC: 0.885 AUC: 0.834 AUC: 0.854 |
| Xiao et al., 2021 [19] | Retinal imagesSlit-lamp images | ResNet-101 | 1252 participants, 2481 slit-lamp images, 1989 retinal images; 75% for training | 25% for tuning | 537 participants, 1069 slit-lamp images, 800 retinal images | Liver cancer Liver cirrhosis Chronic viral hepatitis Non-alcoholic fatty liver disease Cholelithiasis Hepatic cyst | Binary Binary Binary Binary Binary Binary | Slit-lamp; Retinal images: AUC: 0.93; 0.84 AUC: 0.90; 0.83 AUC: 0.69; 0.62 AUC: 0.63; 0.70 AUC: 0.58; 0.68 AUC: 0.66; 0.69 |
| Cho et al., 2022 [60] | Retinal images | DenseNet-201, EfficientNet-B7 | 1703 patients, 3353 images | 189 patients, 373 images; 10-fold cross-validation | N/A | WMH | Binary | Sensitivity: 66.1 Specificity: 71.3 AUC: 0.736 |
| Appaji et al., 2022 [61] | Retinal images | CNN | 116 patients, 82 HC | Validation: 33 patients, 23 HC; Test: 17 patients, 13 HC; Confirmatory: 21 patients, 22 HC | N/A | SCZ | Binary | Accuracy: 95% AUC: 0.98Sensitivity: 91.66% Specificity: 95% |
| Lai et al., 2020 [62] | Retinal images | ResNet-50 | 46 patients, 24 HC | 10-fold cross-validation | N/A | ASD | Binary | Sensitivity: 82.6% Specificity: 91.3% AUC: 0.907 |
| Wisely et al., 2019 [63] | Retinal images | ResNet-18 | 36 patients, 117 HC for all datasets; 57 patient eyes, 198 HC eyes for training | 6 patient eyes, 24 control eyes for testing; 9-fold cross validation | N/A | AD | Binary | AUC: 0.74 Accuracy: 0.79 |
| Huang et al., 2020 [64] | Retinal images | EfficientNet-B1 | 144 patients, 74 HC, total 342 images; Training and validation: 187 participants | Training and validation: 187 participants Testing: 31 participants | N/A | Axial spondyloarthritis | Binary | AUC: 0.735 Sensitivity: 87% Specificity: 62.5% |
3.3.1. Systemic Health Features
3.3.2. CVD
3.3.3. Hypertension
3.3.4. Diabetes Mellitus
3.3.5. Anemia
3.3.6. Hepatobiliary Diseases and Kidney Diseases
3.3.7. Neurological Disorders
3.4. Algorithms Based on the Movements of the Eye
3.4.1. Dementia and Parkinson’s Disease
| Author, Year | Ocular Data | DL Model | Training Dataset | Testing/Validation Dataset | External Validation | Systemic Health Features/Diseases | Outcome | Performance 1 |
|---|---|---|---|---|---|---|---|---|
| Li et al., 2022 [74] | Gaze estimation videos | AttentionGazeNet, LSTM | 50 participants, 64,000 images | 1. 15 participants, about 1500 images; 2. 16 participants | 405 participants, 405 videos | ASD | Binary | Accuracy: 94.8% Sensitivity: 91.1% Specificity: 96.7% |
| Li et al., 2020 [75] | Eye movement videos | LSTM | 136 patients, 136 videos; 136 HC, 136 videos | 10-fold cross-validation | N/A | ASD | Binary | Accuracy: 92.7% Sensitivity: 91.9% Specificity: 93.4% |
| Varma et al., 2022 [76] | Eye movement videos | LSTM | 68 patients and 27 HC in all datasets; 324 videos for training | Validation: 71 videos; Test: 54 videos | N/A | ASD | Binary | Recall: 0.656 Precision: 0.661 |
| Xie et al., 2022 [77] | Eye movement data | VGG-16 | 20 patients, 19 HC | Leave-one-out and 13-fold cross-validation | N/A | ASD | Binary | Accuracy: 0.95 Sensitivity: 1.00 Specificity: 0.89 AUC: 0.93 |
| Jiang et al., 2017 [78] | Eye movement data | VGG-16 | 39 participants, 100 images | Leave-one-subject-out cross-validation | N/A | ASD | Binary | Accuracy: 0.92 Sensitivity: 0.93 Specificity: 0.92 AUC: 0.92 |
| Mengoudi et al., 2020 [71] | Eye movement data | Self-Supervised Learning, SVM | 432 HC | 30 patients, 144 HC | N/A | Dementia | Binary | Accuracy: 78.3% Sensitivity: 89.7% Specificity: 67.6% |
| Biondi et al., 2018 [72] | Eye movement data | Sparse-Autoencoders | 22 patients, 39 HC, total 2922 trials | 4 patients, 4 HC, total 313 trials | N/A | AD | Binary | Accuracy: 89.78% |
| Archila et al., 2021 [73] | Eye movement videos | LSTM | 12 patients, 144 videos; 13 HC, 156 videos | Leave-one-patient-out cross-validation | N/A | PD | Ternary | Specificity: Control: 1, Stage2: 0.87, Stage3: 0.86 F1-score: Control: 0.81, Stage2: 0.57, Stage3: 0.72 |
| Mao et al., 2020 [79] | Eye movement data | LSTM | 34 HC, 34 patients with brain injury, and 30 patients with vertigo; 64 subjects for training | 34 subjects for testing | N/A | Brain injury and vertigo | Ternary | Accuracy: 0.9412 |
| Ahmadi et al., 2020 [80] | Eye movement data | RF, ANN, SingleGMC, MultiGMC | 40 patients with vestibular stroke, 68 patients with peripheral AVS; 90% for training | 10% for testing | N/A | AVS | Binary | Accuracy: 82% AUC: 0.96 |
3.4.2. Autism Spectrum Disorders
3.4.3. Other Disorders
4. Discussion
4.1. Present and Prospects
4.2. Advantages and Drawbacks of AI in Clinical Settings
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- van der Heijden, A.A.; Abramoff, M.D.; Verbraak, F.; van Hecke, M.V.; Liem, A.; Nijpels, G. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol. 2018, 96, 63–68. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175. [Google Scholar] [CrossRef]
- Gargeya, R.; Leng, T. Automated Identification of Diabetic Retinopathy Using Deep Learning. Ophthalmology 2017, 124, 962–969. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Keel, S.; Meng, W.; Chang, R.T.; He, M. Efficacy of a Deep Learning System for Detecting Glaucomatous Optic Neuropathy Based on Color Fundus Photographs. Ophthalmology 2018, 125, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Burlina, P.M.; Joshi, N.; Pekala, M.; Pacheco, K.D.; Freund, D.E.; Bressler, N.M. Automated Grading of Age-Related Macular Degeneration From Color Fundus Images Using Deep Convolutional Neural Networks. JAMA Ophthalmol. 2017, 135, 1170–1176. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.; Iselin, K.C.; Borroni, D.; Romano, D.; Gokul, A.; McGhee, C.N.J.; Zhao, Y.; Sedaghat, M.R.; Momeni-Moghaddam, H.; et al. Keratoconus detection of changes using deep learning of colour-coded maps. BMJ Open Ophthalmol. 2021, 6, e000824. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: A development and validation study. Diabetologia 2020, 63, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Xie, J.; Kawasaki, R.; Kramer, H.; Shlipak, M.; Klein, R.; Klein, B.; Cotch, M.F.; Wong, T.Y. Retinal arteriolar narrowing and subsequent development of CKD Stage 3: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. 2011, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, W.; Wang, C. Relationship between retinal vascular occlusions and incident cerebrovascular diseases: A systematic review and meta-analysis. Med. (Baltim.) 2016, 95, e4075. [Google Scholar] [CrossRef]
- Anderson, T.J.; MacAskill, M.R. Eye movements in patients with neurodegenerative disorders. Nat. Rev. Neurol. 2013, 9, 74–85. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; Group, Q. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Babenko, B.; Mitani, A.; Traynis, I.; Kitade, N.; Singh, P.; Maa, A.Y.; Cuadros, J.; Corrado, G.S.; Peng, L.; Webster, D.R.; et al. Detection of signs of disease in external photographs of the eyes via deep learning. Nat. Biomed. Eng. 2022, 6, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, C.; Li, X.; Wei, S.; Zhou, S.; Yu, X.; Gao, J.; Cao, Y.; Zhang, H. Identifying diabetes from conjunctival images using a novel hierarchical multi-task network. Sci. Rep. 2022, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Preston, F.G.; Meng, Y.; Burgess, J.; Ferdousi, M.; Azmi, S.; Petropoulos, I.N.; Kaye, S.; Malik, R.A.; Zheng, Y.; Alam, U. Artificial intelligence utilising corneal confocal microscopy for the diagnosis of peripheral neuropathy in diabetes mellitus and prediabetes. Diabetologia 2022, 65, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, F.; Colonna, A.; Ruggeri, A. Multiple-Image Deep Learning Analysis for Neuropathy Detection in Corneal Nerve Images. Cornea 2020, 39, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Althnian, A.; Almanea, N.; Aloboud, N. Neonatal Jaundice Diagnosis Using a Smartphone Camera Based on Eye, Skin, and Fused Features with Transfer Learning. Sensors 2021, 21, 7038. [Google Scholar] [CrossRef]
- Lv, W.; Song, Y.; Fu, R.; Lin, X.; Su, Y.; Jin, X.; Yang, H.; Shan, X.; Du, W.; Huang, Q.; et al. Deep Learning Algorithm for Automated Detection of Polycystic Ovary Syndrome Using Scleral Images. Front. Endocrinol. 2021, 12, 789878. [Google Scholar] [CrossRef]
- Xiao, W.; Huang, X.; Wang, J.H.; Lin, D.R.; Zhu, Y.; Chen, C.; Yang, Y.H.; Xiao, J.; Zhao, L.Q.; Li, J.O.; et al. Screening and identifying hepatobiliary diseases through deep learning using ocular images: A prospective, multicentre study. Lancet. Digit. Health 2021, 3, e88–e97. [Google Scholar] [CrossRef]
- Betzler, B.K.; Yang, H.H.S.; Thakur, S.; Yu, M.; Quek, T.C.; Soh, Z.D.; Lee, G.; Tham, Y.C.; Wong, T.Y.; Rim, T.H.; et al. Gender Prediction for a Multiethnic Population via Deep Learning Across Different Retinal Fundus Photograph Fields: Retrospective Cross-sectional Study. JMIR Med. Inf. 2021, 9, e25165. [Google Scholar] [CrossRef]
- Corbin, D.; Lesage, F. Assessment of the predictive potential of cognitive scores from retinal images and retinal fundus metadata via deep learning using the CLSA database. Sci. Rep. 2022, 12, 5767. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, N.; Elen, B.; Craenendonck, T.V.; Triantafyllidou, D.; Petropoulos, I.N.; Malik, R.A.; De Boever, P. Age and sex affect deep learning prediction of cardiometabolic risk factors from retinal images. Sci. Rep. 2020, 10, 9432. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, W.; Wang, Y.; Chen, Y.; Shang, X.; Liao, H.; Huang, Y.; Bulloch, G.; Zhang, S.; Kiburg, K.; et al. Retinal age gap as a predictive biomarker of future risk of Parkinson’s disease. Age Ageing 2022, 51, afac062. [Google Scholar] [CrossRef]
- Khan, N.C.; Perera, C.; Dow, E.R.; Chen, K.M.; Mahajan, V.B.; Mruthyunjaya, P.; Do, D.V.; Leng, T.; Myung, D. Predicting Systemic Health Features from Retinal Fundus Images Using Transfer-Learning-Based Artificial Intelligence Models. Diagnostics 2022, 12, 1714. [Google Scholar] [CrossRef]
- Kim, Y.D.; Noh, K.J.; Byun, S.J.; Lee, S.; Kim, T.; Sunwoo, L.; Lee, K.J.; Kang, S.H.; Park, K.H.; Park, S.J. Effects of Hypertension, Diabetes, and Smoking on Age and Sex Prediction from Retinal Fundus Images. Sci. Rep. 2020, 10, 4623. [Google Scholar] [CrossRef]
- Korot, E.; Pontikos, N.; Liu, X.; Wagner, S.K.; Faes, L.; Huemer, J.; Balaskas, K.; Denniston, A.K.; Khawaja, A.; Keane, P.A. Predicting sex from retinal fundus photographs using automated deep learning. Sci. Rep. 2021, 11, 10286. [Google Scholar] [CrossRef]
- Mendoza, L.; Christopher, M.; Brye, N.; Proudfoot, J.A.; Belghith, A.; Bowd, C.; Rezapour, J.; Fazio, M.A.; Goldbaum, M.H.; Weinreb, R.N.; et al. Deep learning predicts demographic and clinical characteristics from optic nerve head OCT circle and radial scans. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2120. [Google Scholar]
- Munk, M.R.; Kurmann, T.; Márquez-Neila, P.; Zinkernagel, M.S.; Wolf, S.; Sznitman, R. Assessment of patient specific information in the wild on fundus photography and optical coherence tomography. Sci. Rep. 2021, 11, 8621. [Google Scholar] [CrossRef]
- Nusinovici, S.; Rim, T.H.; Yu, M.; Lee, G.; Tham, Y.-C.; Cheung, N.; Chong, C.C.Y.; Soh, Z.D.; Thakur, S.; Lee, C.J.; et al. Retinal photograph-based deep learning predicts biological age, and stratifies morbidity and mortality risk. Age Ageing 2022, 51, afac065. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Rim, T.H.; Lee, G.; Kim, Y.; Tham, Y.-C.; Lee, C.J.; Baik, S.J.; Kim, Y.A.; Yu, M.; Deshmukh, M.; Lee, B.K.; et al. Prediction of systemic biomarkers from retinal photographs: Development and validation of deep-learning algorithms. Lancet Digit. Health 2020, 2, E526–E536. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Liu, Y.; Ting, D.; Tjio, G.C.e.; Anees, A.; Tan, G.S.W.; Sabanayagam, C.; Goh, R.; Wong, T.Y.; Cheng, C.-Y. Estimation of Haemoglobin A1c from Retinal photographs via Deep Learning. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1456. [Google Scholar]
- Vaghefi, E.; Yang, S.; Hill, S.; Humphrey, G.; Walker, N.; Squirrell, D. Detection of smoking status from retinal images; a Convolutional Neural Network study. Sci. Rep. 2019, 9, 7180. [Google Scholar] [CrossRef]
- Yang, H.H.S.; Rim, T.H.; Tham, Y.C.; Yoo, T.K.; Lee, G.; Kim, Y.; Wong, T.Y.; Cheng, C.-Y. Deep learning system differentiates ethnicities from fundus photographs of a multi-ethnic Asian population. Investig. Ophthalmol. Vis. Sci. 2020, 61, 5248. [Google Scholar]
- Zhang, L.; Yuan, M.; An, Z.; Zhao, X.; Wu, H.; Li, H.; Wang, Y.; Sun, B.; Li, H.; Ding, S.; et al. Prediction of hypertension, hyperglycemia and dyslipidemia from retinal fundus photographs via deep learning: A cross-sectional study of chronic diseases in central China. PLoS ONE 2020, 15, e0233166. [Google Scholar] [CrossRef]
- Al-Absi, H.R.H.; Islam, M.T.; Refaee, M.A.; Chowdhury, M.E.H.; Alam, T. Cardiovascular Disease Diagnosis from DXA Scan and Retinal Images Using Deep Learning. Sensors 2022, 22, 4310. [Google Scholar] [CrossRef]
- Mellor, J.; Storkey, A.; Colhoun, H.M.; McKeigue, P.; Investigators, S.B. Predicting cardiovascular disease from fundus images using deep learning. Diabetologia 2019, 62, S37. [Google Scholar]
- Chang, J.; Ko, A.; Park, S.M.; Choi, S.; Kim, K.; Kim, S.M.; Yun, J.M.; Kang, U.; Shin, I.H.; Shin, J.Y.; et al. Association of DeepLearning-Based Fundus Age Difference with Carotid Atherosclerosis and Mortality. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 1179–1181. [Google Scholar]
- Ng, W.W.Y.; Liang, H.; Peng, Q.; Zhong, C.; Dong, X.; Huang, Z.; Zhong, P.; Li, C.; Xu, M.; Sun, Y.; et al. An automatic framework for perioperative risks classification from retinal images of complex congenital heart disease patients. Int. J. Mach. Learn. Cybern. 2022, 13, 471–483. [Google Scholar] [CrossRef]
- Mueller, S.; Wintergerst, M.W.M.; Falahat, P.; Holz, F.G.; Schaefer, C.; Schahab, N.; Finger, R.P.; Schultz, T. Multiple instance learning detects peripheral arterial disease from high-resolution color fundus photography. Sci. Rep. 2022, 12, 1389. [Google Scholar] [CrossRef]
- Chang, J.; Ko, A.; Park, S.M.; Choi, S.; Kim, K.; Kim, S.M.; Yun, J.M.; Kang, U.; Shin, I.H.; Shin, J.Y.; et al. Association of Cardiovascular Mortality and Deep Learning-Funduscopic Atherosclerosis Score derived from Retinal Fundus Images. Am. J. Ophthalmol. 2020, 217, 121–130. [Google Scholar] [CrossRef]
- Barriada, R.G.; Simo-Servat, O.; Planas, A.; Hernandez, C.; Simo, R.; Masip, D. Deep Learning of Retinal Imaging: A Useful Tool for Coronary Artery Calcium Score Prediction in Diabetic Patients. Appl. Sci. 2022, 12, 1401. [Google Scholar] [CrossRef]
- Rim, T.H.; Lee, C.J.; Tham, Y.C.; Cheung, N.; Yu, M.; Lee, G.; Kim, Y.; Ting, D.S.W.; Chong, C.C.Y.; Choi, Y.S.; et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. Lancet. Digit. Health 2021, 3, e306–e316. [Google Scholar] [CrossRef]
- Son, J.; Shin, J.Y.; Chun, E.J.; Jung, K.-H.; Park, K.H.; Park, S.J. Predicting High Coronary Artery Calcium Score From Retinal Fundus Images With Deep Learning Algorithms. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; He, W.; Xu, L.; Pazo, E.E.; Lin, T.; Liu, S.; Zhang, C. Exploring the effect of hypertension on retinal microvasculature using deep learning on East Asian population. PLoS ONE 2020, 15, e0230111. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.; Qu, L.; Li, C.; Yang, C.; Qin, P.; Dong, Y. AML-Net: A Preliminary Screening Model for Mild Hypertension. In Proceedings of the 14th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Shanghai, China, 23–25 October 2021. [Google Scholar]
- Islam, M.T.; Al-Absi, H.R.H.; Ruagh, E.A.; Alam, T. DiaNet: A Deep Learning Based Architecture to Diagnose Diabetes Using Retinal Images Only. IEEE Access 2021, 9, 15686–15695. [Google Scholar] [CrossRef]
- Wang, R.; Li, P.; Yang, Z. Analysis and Recognition of Clinical Features of Diabetes Based on Convolutional Neural Network. Comput. Math. Methods Med. 2022, 2022, 7902786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, K.; Chen, B.; Lan, H.; Zhou, M.; Gao, F. Pathology Study for Blood Vessel of Ocular Fundus Images by Photoacoustic Tomography. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018. [Google Scholar]
- Abbasi-Sureshjani, S.; Dashtbozorg, B.; Romeny, B.M.t.H.; Fleuret, F. Exploratory Study on Direct Prediction of Diabetes Using Deep Residual Networks. In Proceedings of the 6th ECCOMAS Thematic Conference on Computational Vision and Medical Image Processing (VipIMAGE), Porto, Portugal, 18–20 October 2017; pp. 797–802. [Google Scholar]
- Heslinga, F.G.; Pluim, J.P.W.; Houben, A.J.H.M.; Schram, M.T.; Henry, R.M.A.; Stehouwer, C.D.A.; van Greevenbroek, M.J.; Berendschot, T.T.J.M.; Veta, M. Direct Classification of Type 2 Diabetes From Retinal Fundus Images in a Population-based Sample From The Maastricht Study. In Proceedings of the Conference on Medical Imaging—Computer-Aided Diagnosis, Houston, TX, USA, 16–19 February 2020. [Google Scholar]
- Yun, J.S.; Kim, J.; Jung, S.H.; Cha, S.A.; Ko, S.H.; Ahn, Y.B.; Won, H.H.; Sohn, K.A.; Kim, D. A deep learning model for screening type 2 diabetes from retinal photographs. Nutr. Metab. Cardiovasc. Dis. NMCD 2022, 32, 1218–1226. [Google Scholar] [CrossRef]
- Cervera, D.R.; Smith, L.; Diaz-Santana, L.; Kumar, M.; Raman, R.; Sivaprasad, S. Identifying peripheral neuropathy in colour fundus photographs based on deep learning. Diagnostics 2021, 11, 1943. [Google Scholar] [CrossRef]
- Mitani, A.; Huang, A.; Venugopalan, S.; Corrado, G.S.; Peng, L.; Webster, D.R.; Hammel, N.; Liu, Y.; Varadarajan, A.V. Detection of anaemia from retinal fundus images via deep learning. Nat. Biomed. Eng. 2020, 4, 18–27. [Google Scholar] [CrossRef]
- Wei, H.; Shen, H.; Li, J.; Zhao, R.; Chen, Z. AneNet: A lightweight network for the real-time anemia screening from retinal vessel optical coherence tomography images. Opt. Laser Technol. 2021, 136, 106773. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, L.; Su, H.; Lv, B.; Lv, C.; Xie, G.; Chen, Y. Deep-Learning-Based Hemoglobin Concentration Prediction and Anemia Screening Using Ultra-Wide Field Fundus Images. Front. Cell Dev. Biol. 2022, 10, 888268. [Google Scholar] [CrossRef]
- Kang, E.Y.-C.; Hsieh, Y.-T.; Li, C.-H.; Huang, Y.-J.; Kuo, C.-F.; Kang, J.-H.; Chen, K.-J.; Lai, C.-C.; Wu, W.-C.; Hwang, Y.-S. Deep Learning-Based Detection of Early Renal Function Impairment Using Retinal Fundus Images: Model Development and Validation. JMIR Med. Inform. 2020, 8, e23472. [Google Scholar] [CrossRef] [PubMed]
- Sabanayagam, C.; Xu, D.; Ting, D.S.W.; Nusinovici, S.; Banu, R.; Hamzah, H.; Lim, C.; Tham, Y.C.; Cheung, C.Y.; Tai, E.S.; et al. A deep learning algorithm to detect chronic kidney disease from retinal photographs in community-based populations. Lancet. Digit. Health 2020, 2, e295–e302. [Google Scholar]
- Zhang, K.; Liu, X.; Xu, J.; Yuan, J.; Cai, W.; Chen, T.; Wang, K.; Gao, Y.; Nie, S.; Xu, X.; et al. Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images. Nat. Biomed. Eng. 2021, 5, 533–545. [Google Scholar] [CrossRef]
- Cho, B.-J.; Lee, M.; Han, J.; Kwon, S.; Oh, M.S.; Yu, K.-H.; Lee, B.-C.; Kim, J.H.; Kim, C. Prediction of White Matter Hyperintensity in Brain MRI Using Fundus Photographs via Deep Learning. J. Clin. Med. 2022, 11, 3309. [Google Scholar]
- Appaji, A.; Harish, V.; Korann, V.; Devi, P.; Jacob, A.; Padmanabha, A.; Kumar, V.; Varambally, S.; Venkatasubramanian, G.; Rao, S.V.; et al. Deep learning model using retinal vascular images for classifying schizophrenia. Schizophr. Res. 2022, 241, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Lee, J.; Chiu, S.; Charm, J.; So, W.Y.; Yuen, F.P.; Kwok, C.; Tsoi, J.; Lin, Y.; Zee, B. A machine learning approach for retinal images analysis as an objective screening method for children with autism spectrum disorder. EClinicalMedicine 2020, 28, 100588. [Google Scholar] [CrossRef]
- Wisely, C.E.; Wang, D.; Henao, R.; Grewal, D.S.; Yoon, S.P.; Polascik, B.; Thompson, A.C.; Burke, J.R.; Carin, L.; Fekrat, S. Deep learning algorithm for diagnosis of Alzheimer’s disease using multimodal retinal imaging. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1461. [Google Scholar]
- Huang, Y.J.; Kuo, C.F.; Huang, Y.H.; Hwang, Y.S.; Lin, C. Detecting Subtle Changes in Fundoscopic Retinal Images in Patients with Axial Spondyloarthritis with Deep Learning. Arthritis Rheumatol. 2020, 72 (Suppl. S10), 2629–2630. [Google Scholar]
- Esen, A.M.; Barutcu, I.; Acar, M.; Degirmenci, B.; Kaya, D.; Turkmen, M.; Melek, M.; Onrat, E.; Esen, O.B.; Kirma, C. Effect of smoking on endothelial function and wall thickness of brachial artery. Circ. J. 2004, 68, 1123–1126. [Google Scholar]
- Wang, S.B.; Mitchell, P.; Liew, G.; Wong, T.Y.; Phan, K.; Thiagalingam, A.; Joachim, N.; Burlutsky, G.; Gopinath, B. A spectrum of retinal vasculature measures and coronary artery disease. Atherosclerosis 2018, 268, 215–224. [Google Scholar] [CrossRef]
- Polonsky, T.S.; McClelland, R.L.; Jorgensen, N.W.; Bild, D.E.; Burke, G.L.; Guerci, A.D.; Greenland, P. Coronary artery calcium score and risk classification for coronary heart disease prediction. Jama 2010, 303, 1610–1616. [Google Scholar] [CrossRef]
- Ding, J.; Wai, K.L.; McGeechan, K.; Ikram, M.K.; Kawasaki, R.; Xie, J.; Klein, R.; Klein, B.B.; Cotch, M.F.; Wang, J.J.; et al. Retinal vascular caliber and the development of hypertension: A meta-analysis of individual participant data. J. Hypertens. 2014, 32, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.H.; Thevarajah, M.; Alias, Y.; Khor, S.M. Current aspects in hemoglobin A1c detection: A review. Clin. Chim. Acta 2015, 439, 202–211. [Google Scholar] [CrossRef]
- Eckstein, M.K.; Guerra-Carrillo, B.; Miller Singley, A.T.; Bunge, S.A. Beyond eye gaze: What else can eyetracking reveal about cognition and cognitive development? Dev. Cogn. Neurosci. 2017, 25, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Mengoudi, K.; Ravi, D.; Yong, K.X.X.; Primativo, S.; Pavisic, I.M.; Brotherhood, E.; Lu, K.; Schott, J.M.; Crutch, S.J.; Alexander, D.C. Augmenting dementia cognitive assessment with instruction-less eye-tracking tests. IEEE J. Biomed. Health Inform. 2020, 24, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Biondi, J.; Fernandez, G.; Castro, S.; Agamennoni, O. Eye movement behavior identification for Alzheimer’s disease diagnosis. J. Integr. Neurosci. 2018, 17, 349–354. [Google Scholar]
- Archila, J.; Manzanera, A.; Martinez, F. A recurrent approach for predicting Parkinson stage from multimodal videos. In Proceedings of the 17th International Symposium on Medical Information Processing and Analysis, Campinas, Brazil, 17–19 November 2021. [Google Scholar]
- Li, J.; Chen, Z.; Zhong, Y.; Lam, H.-K.; Han, J.; Ouyang, G.; Li, X.; Liu, H. Appearance-Based Gaze Estimation for ASD Diagnosis. Ieee Trans. Cybern. 2022, 52, 6504–6517. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, Y.; Han, J.; Ouyang, G.; Li, X.; Liu, H. Classifying ASD children with LSTM based on raw videos. Neurocomputing 2020, 390, 226–238. [Google Scholar] [CrossRef]
- Varma, M.; Washington, P.; Chrisman, B.; Kline, A.; Leblanc, E.; Paskov, K.; Stockham, N.; Jung, J.Y.; Sun, M.W.; Wall, D.P. Identification of Social Engagement Indicators Associated With Autism Spectrum Disorder Using a Game-Based Mobile App: Comparative Study of Gaze Fixation and Visual Scanning Methods. J. Med. Internet Res. 2022, 24, e31830. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, L.; Webster, P.; Yao, Y.; Sun, J.; Wang, S.; Zhou, H. Identifying Visual Attention Features Accurately Discerning Between Autism and Typically Developing: A Deep Learning Framework. Interdiscip. Sci. -Comput. Life Sci. 2022, 14, 639–651. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, Q. Learning Visual Attention to Identify People with Autism Spectrum Disorder. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 3287–3296. [Google Scholar]
- Mao, Y.; He, Y.; Liu, L.; Chen, X. Disease Classification Based on Synthesis of Multiple Long Short-Term Memory Classifiers Corresponding to Eye Movement Features. IEEE Access 2020, 8, 151624–151633. [Google Scholar] [CrossRef]
- Ahmadi, S.A.; Vivar, G.; Navab, N.; Möhwald, K.; Maier, A.; Hadzhikolev, H.; Brandt, T.; Grill, E.; Dieterich, M.; Jahn, K.; et al. Modern machine-learning can support diagnostic differentiation of central and peripheral acute vestibular disorders. J. Neurol. 2020, 267, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Nickerson, J.M.; Edelhauser, H.F.; Bergman, L.A.; Berglin, L. Anatomic alterations in aging and age-related diseases of the eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF23–ORSF27. [Google Scholar] [CrossRef]
- Ehrlich, R.; Kheradiya, N.S.; Winston, D.M.; Moore, D.B.; Wirostko, B.; Harris, A. Age-related ocular vascular changes. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2009, 247, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Shiba, T.; Kinoshita, A.; Matsumoto, T.; Hori, Y. The influences of gender and aging on optic nerve head microcirculation in healthy adults. Sci. Rep. 2019, 9, 15636. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ulla, F.; Cutrin, P.; Santos, P.; Fernandez, M.; Abraldes, M.; Abalo-Lojo, J.M.; Gonzalez, F. Age and gender influence on foveal avascular zone in healthy eyes. Exp. Eye Res. 2019, 189, 107856. [Google Scholar] [CrossRef] [PubMed]
- Tariq, Y.M.; Samarawickrama, C.; Pai, A.; Burlutsky, G.; Mitchell, P. Impact of ethnicity on the correlation of retinal parameters with axial length. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4977–4982. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Wang, S.B.; Mitchell, P.; Plant, A.J.; Phan, K.; Liew, G.; Thiagalingam, A.; Burlutsky, G.; Gopinath, B. Metabolic syndrome and retinal microvascular calibre in a high cardiovascular disease risk cohort. Br. J. Ophthalmol. 2016, 100, 1041–1046. [Google Scholar] [CrossRef]
- Dusing, P.; Zietzer, A.; Goody, P.R.; Hosen, M.R.; Kurts, C.; Nickenig, G.; Jansen, F. Vascular pathologies in chronic kidney disease: Pathophysiological mechanisms and novel therapeutic approaches. J. Mol. Med. 2021, 99, 335–348. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iao, W.C.; Zhang, W.; Wang, X.; Wu, Y.; Lin, D.; Lin, H. Deep Learning Algorithms for Screening and Diagnosis of Systemic Diseases Based on Ophthalmic Manifestations: A Systematic Review. Diagnostics 2023, 13, 900. https://doi.org/10.3390/diagnostics13050900
Iao WC, Zhang W, Wang X, Wu Y, Lin D, Lin H. Deep Learning Algorithms for Screening and Diagnosis of Systemic Diseases Based on Ophthalmic Manifestations: A Systematic Review. Diagnostics. 2023; 13(5):900. https://doi.org/10.3390/diagnostics13050900
Chicago/Turabian StyleIao, Wai Cheng, Weixing Zhang, Xun Wang, Yuxuan Wu, Duoru Lin, and Haotian Lin. 2023. "Deep Learning Algorithms for Screening and Diagnosis of Systemic Diseases Based on Ophthalmic Manifestations: A Systematic Review" Diagnostics 13, no. 5: 900. https://doi.org/10.3390/diagnostics13050900
APA StyleIao, W. C., Zhang, W., Wang, X., Wu, Y., Lin, D., & Lin, H. (2023). Deep Learning Algorithms for Screening and Diagnosis of Systemic Diseases Based on Ophthalmic Manifestations: A Systematic Review. Diagnostics, 13(5), 900. https://doi.org/10.3390/diagnostics13050900








