Optical Coherence Tomography in Inflammatory and Neoplastic Lesions Deforming the Choroidal Profile
Abstract
1. Introduction
2. Inflammatory Lesions
2.1. Choroidal Inflammatory Lesions
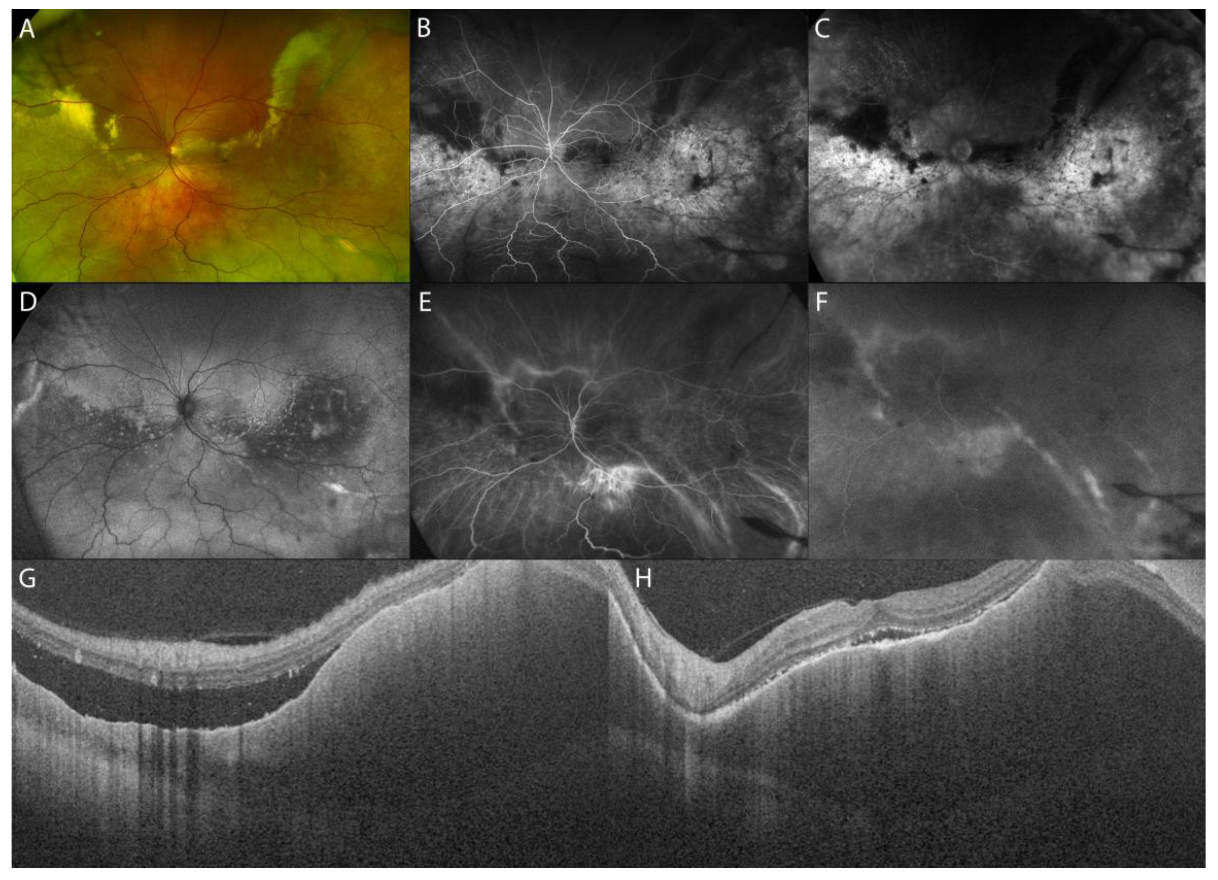
2.1.1. Acute Uveitic Phase of Vogt–Koyanagi–Harada (VKH) Disease
Chronic Recurrent Uveitic Phase of VKH Disease
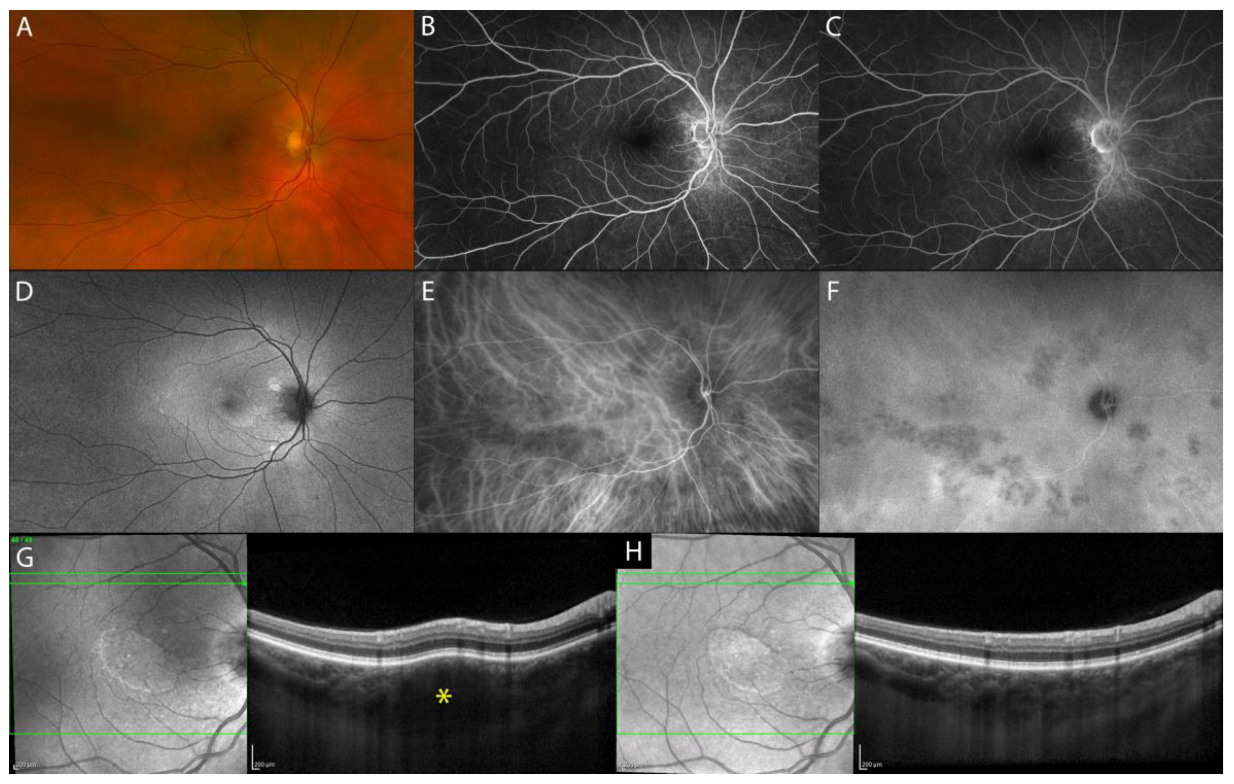
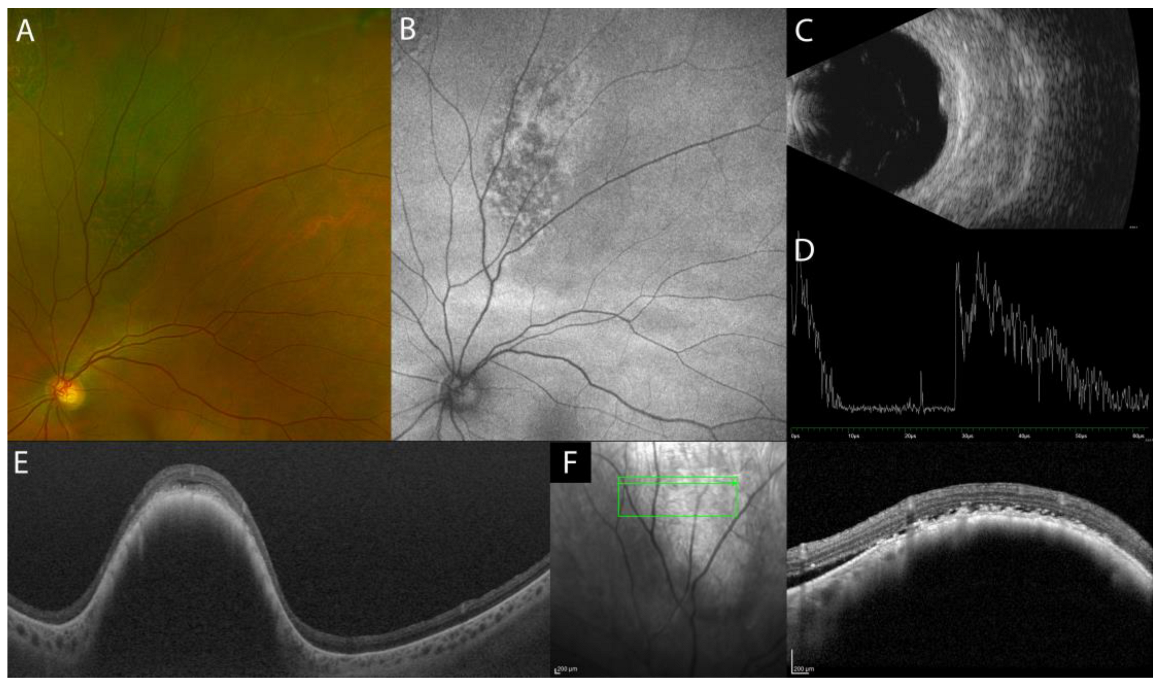
2.1.2. Isolated Tubercular and Sarcoid Choroid Granulomas
2.2. Scleral Inflammatory Lesions
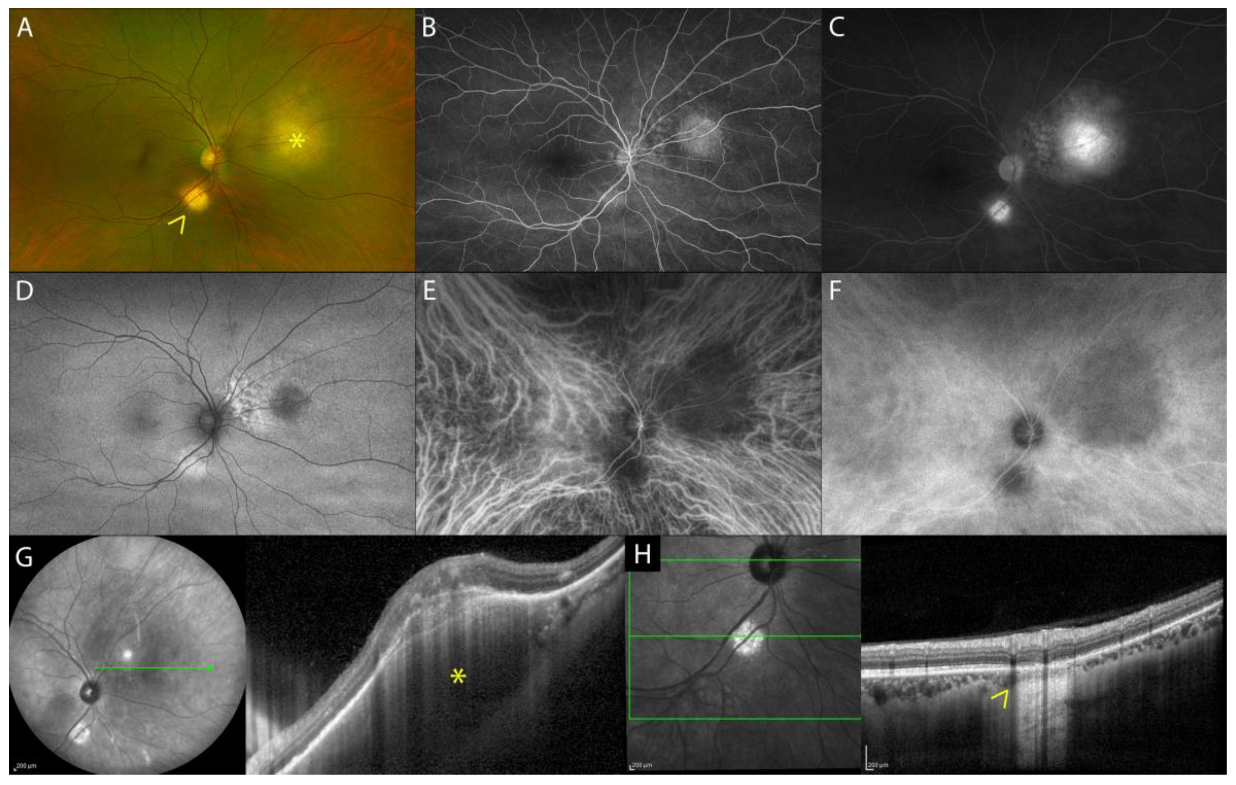
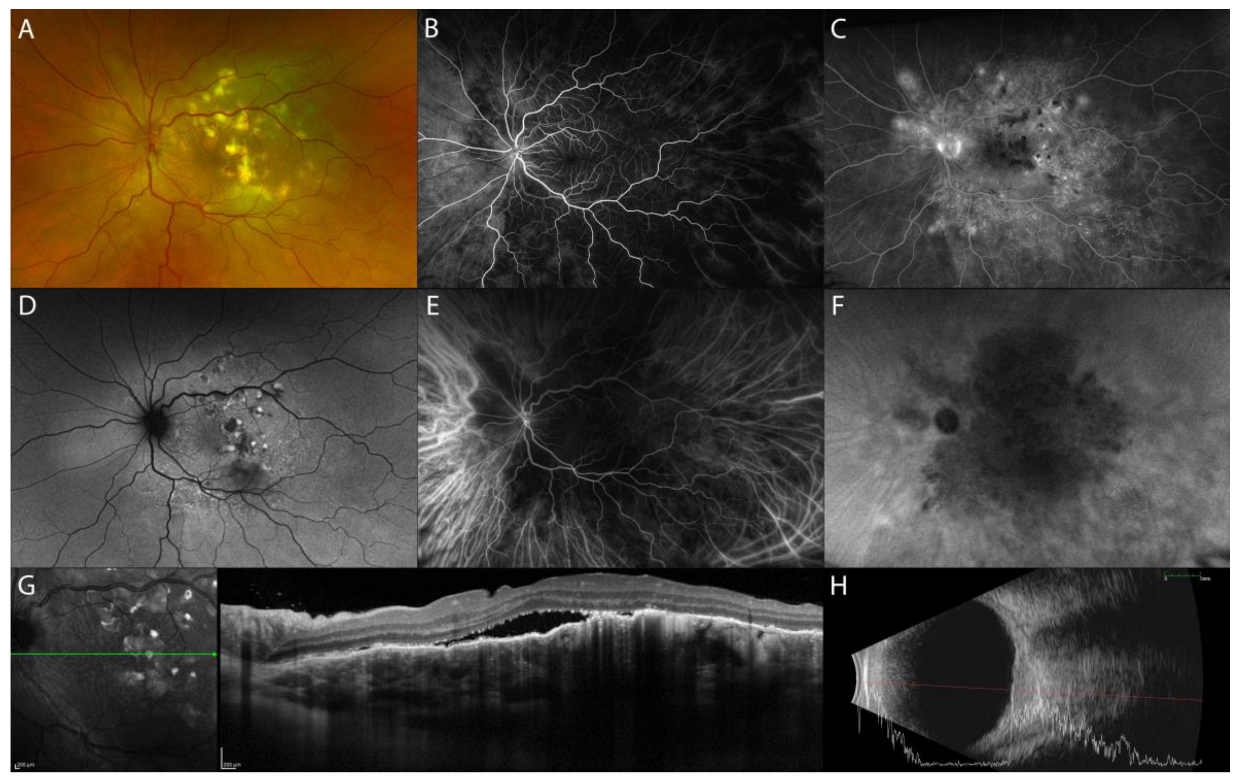
Nodular Posterior Scleritis
3. Neoplastic Lesions
3.1. Choroidal Melanoma
3.2. Choroidal Metastasis
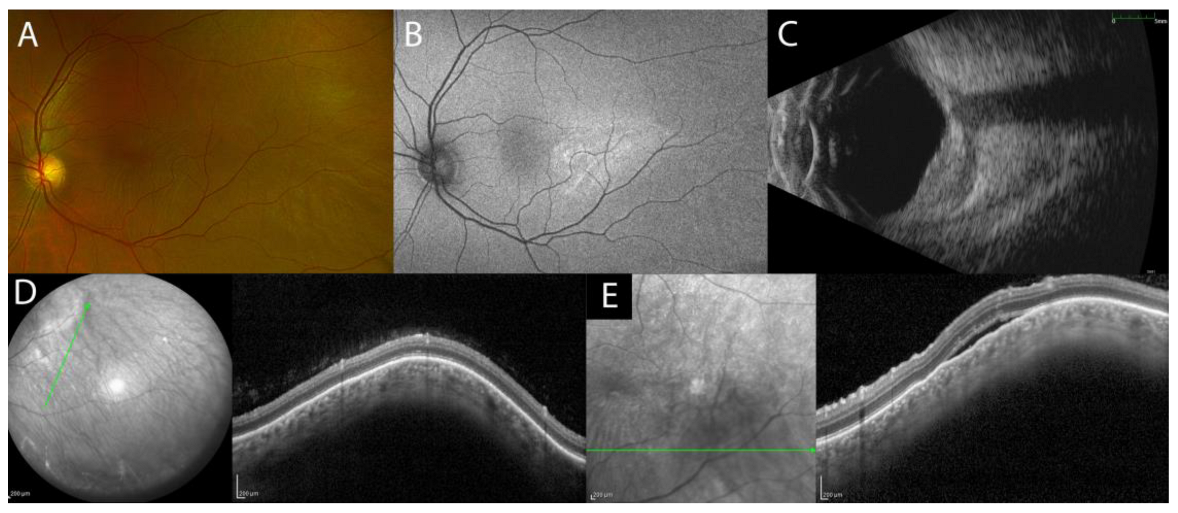
3.3. Choroidal Hemangioma
3.4. Choroidal Lymphoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steiner, M.; Esteban-Ortega, M.d.M.; Muñoz-Fernández, S. Choroidal and retinal thickness in systemic autoimmune and inflammatory diseases: A review. Surv. Ophthalmol. 2019, 64, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Vupparaboina, K.K.; Goud, A.; Dansingani, K.K.; Chhablani, J. Choroidal imaging biomarkers. Surv. Ophthalmol. 2019, 64, 312–333. [Google Scholar] [CrossRef]
- Cimino, L.; Aldigeri, R.; Marchi, S.; Mastrofilippo, V.; Viscogliosi, F.; Coassin, M.; Soldani, A.; Savoldi, L.; De Fanti, A.; Belloni, L.; et al. Changes in patterns of uveitis at a tertiary referral center in Northern Italy: Analysis of 990 consecutive cases. Int. Ophthalmol. 2018, 38, 133–142. [Google Scholar] [CrossRef]
- Urzua, C.A.; Herbort, C.; Valenzuela, R.A.; Abu El-Asrar, A.M.; Arellanes-Garcia, L.; Schlaen, A.; Yamamoto, J.; Pavesio, C. Initial-onset acute and chronic recurrent stages are two distinctive courses of Vogt-Koyanagi-Harada disease. J. Ophthalmic Inflamm. Infect. 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Abouammoh, M.A.; Gupta, V.; Hemachandran, S.; Herbort, C.P.; Abu El-Asrar, A.M. Indocyanine green angiographic findings in initial-onset acute Vogt–Koyanagi–Harada disease. Acta Ophthalmol. 2016, 94, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Forster, D.J.; Cano, M.R.; Green, R.L.; Rao, N.A. Echographic Features of the Vogt-Koyanagi-Harada Syndrome. Arch. Ophthalmol. 1990, 108, 1421–1426. [Google Scholar] [CrossRef]
- Hosoda, Y.; Uji, A.; Hangai, M.; Morooka, S.; Nishijima, K.; Yoshimura, N. Relationship between retinal lesions and inward choroidal bulging in vogt-koyanagi-harada disease. Am. J. Ophthalmol. 2014, 157, 1056–1063.e1. [Google Scholar] [CrossRef]
- Chee, S.P.; Afrin, M.; Tumulak, M.J.G.; Siak, J. Role of Optical Coherence Tomography in the Prognosis of Vogt–Koyanagi–Harada Disease. Ocul. Immunol. Inflamm. 2019, 29, 118–123. [Google Scholar] [CrossRef]
- Nakayama, M.; Keino, H.; Okada, A.A.; Watanabe, T.; Taki, W.; Inoue, M.; Hirakata, A. Enhanced depth imaging optical coherence tomography of the choroid in Vogt-Koyanagi-Harada disease. Retina 2012, 32, 2061–2069. [Google Scholar] [CrossRef]
- Chee, S.P.; Chan, S.W.N.; Jap, A. Comparison of Enhanced Depth Imaging and Swept Source Optical Coherence Tomography in Assessment of Choroidal Thickness in Vogt–Koyanagi–Harada Disease. Ocul. Immunol. Inflamm. 2017, 25, 528–532. [Google Scholar] [CrossRef]
- Bacsal, K.; Wen, D.S.H.; Chee, S.P. Concomitant Choroidal Inflammation during Anterior Segment Recurrence in Vogt-Koyanagi-Harada Disease. Am. J. Ophthalmol. 2008, 145, 480–486. [Google Scholar] [CrossRef]
- Tagawa, Y.; Namba, K.; Mizuuchi, K.; Takemoto, Y.; Iwata, D.; Uno, T.; Fukuhara, T.; Hirooka, K.; Kitaichi, N.; Ohno, S.; et al. Choroidal thickening prior to anterior recurrence in patients with Vogt-Koyanagi-Harada disease. Br. J. Ophthalmol. 2016, 100, 473–477. [Google Scholar] [CrossRef]
- Liu, S.; Du, L.; Zhou, Q.; Zhang, Q.; Hu, K.; Qi, J.; Liang, L.; Zhou, C.; Kijlstra, A.; Yang, P. The Choroidal Vascularity Index Decreases and Choroidal Thickness Increases in Vogt–Koyanagi–Harada Disease Patients During a Recurrent Anterior Uveitis Attack. Ocul. Immunol. Inflamm. 2018, 26, 1237–1243. [Google Scholar] [CrossRef]
- Sakata, V.M.; Da Silva, F.T.; Hirata, C.E.; Takahashi, W.Y.; Costa, R.A.; Yamamoto, J.H. Choroidal bulging in patients with Vogt-Koyanagi-Harada disease in the non-acute uveitic stage. J. Ophthalmic Inflamm. Infect. 2014, 4, 6. [Google Scholar] [CrossRef]
- Da Silva, F.T.; Sakata, V.M.; Nakashima, A.; Hirata, C.E.; Olivalves, E.; Takahashi, W.Y.; Costa, R.A.; Yamamoto, J.H. Enhanced depth imaging optical coherence tomography in long-standing Vogt-Koyanagi-Harada disease. Br. J. Ophthalmol. 2013, 97, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Kee, A.R.; Ang, L.; Tun Hang, Y.; Gupta, V.; Kon, O.M.; Mitchell, D.; Zierhut, M.; Pavesio, C. Tuberculosis or sarcoidosis: Opposite ends of the same disease spectrum? Tuberculosis 2016, 98, 21–26. [Google Scholar] [CrossRef] [PubMed]
- De Simone, L.; Bonacini, M.; Aldigeri, R.; Alessandrello, F.; Mastrofilippo, V.; Gozzi, F.; Bolletta, E.; Adani, C.; Zerbini, A.; Cavallini, G.M.; et al. Could different aqueous humor and plasma cytokine profiles help differentiate between ocular sarcoidosis and ocular tuberculosis? Inflamm. Res. 2022, 71, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Cutrufello, N.J.; Karakousis, P.C.; Fishler, J.; Albini, T.A. Intraocular tuberculosis. Ocul. Immunol. Inflamm. 2010, 18, 281–291. [Google Scholar] [CrossRef]
- Helm, C.J.; Holland, G.N. Ocular tuberculosis. Surv. Ophthalmol. 1993, 38, 229–256. [Google Scholar] [CrossRef]
- Agrawal, R.; Agarwal, A.; Jabs, D.A.; Kee, A.; Testi, I.; Mahajan, S.; McCluskey, P.J.; Gupta, A.; Palestine, A.; Denniston, A.; et al. Standardization of Nomenclature for Ocular Tuberculosis—Results of Collaborative Ocular Tuberculosis Study (COTS) Workshop. Ocul. Immunol. Inflamm. 2020, 28, 74–84. [Google Scholar] [CrossRef]
- Bolletta, E.; Mastrofilippo, V.; Invernizzi, A.; Aldigeri, R.; Spaggiari, L.; Besutti, G.; Borrelli, R.; Lococo, F.; Ricchetti, T.; Rapicetta, C.; et al. Clinical Relevance of Subcentimetric Lymph Node Biopsy in the Diagnosis of Ocular Sarcoidosis. Ocul. Immunol. Inflamm. 2022, 30, 717–720. [Google Scholar] [CrossRef]
- Obenauf, C.D.; Shaw, H.E.; Sydnor, C.F.; Klintworth, G.K. Sarcoidosis and its ophthalmic manifestations. Am. J. Ophthalmol. 1978, 86, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Demirci, H.; Shields, C.L.; Shields, J.A.; Eagle, R.C.J. Ocular tuberculosis masquerading as ocular tumors. Surv. Ophthalmol. 2004, 49, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.G.; Wahab, C.H.; Kheir, W.J. Choroidal sarcoid granuloma: A case report and review of the literature. J. Ophthalmic Inflamm. Infect. 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Invernizzi, A.; Markan, A.; Testi, I.; Keane, P.A.; Agrawal, R.; Nguyen, Q.D.; Pavesio, C.; Gupta, V. Imaging in Tubercular Choroiditis: Current Concepts. Ocul. Immunol. Inflamm. 2020, 28, 1223–1238. [Google Scholar] [CrossRef]
- Modi, Y.S.; Epstein, A.; Bhaleeya, S.; Harbour, J.W.; Albini, T. Multimodal imaging of sarcoid choroidal granulomas. J. Ophthalmic Inflamm. Infect. 2013, 3, 58. [Google Scholar] [CrossRef]
- Invernizzi, A.; Mapelli, C.; Viola, F.; Cigada, M.; Cimino, L.; Ratiglia, R.; Staurenghi, G.; Gupta, A. Choroidal granulomas visualized by enhanced depth imaging optical coherence tomography. Retina 2015, 35, 525–531. [Google Scholar] [CrossRef]
- Salman, A.; Parmar, P.; Rajamohan, M.; Vanila, C.G.; Thomas, P.A.; Jesudasan, C.A.N. Optical Coherence Tomography in Choroidal Tuberculosis. Am. J. Ophthalmol. 2006, 142, 170–172. [Google Scholar] [CrossRef]
- Mehta, N.; Chong, J.; Tsui, E.; Duncan, J.L.; Curcio, C.A.; Freund, K.B.; Modi, Y. Presumed Foveal Bacillary Layer Detachment in a Patient With Toxoplasmosis Chorioretinitis and Pachychoroid Disease. Retin. Cases Brief Rep. 2021, 15, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Rostaqui, O.; Querques, G.; Haymann, P.; Fardeau, C.; Coscas, G.; Souied, E.H. Visualization of sarcoid choroidal granuloma by enhanced depth imaging optical coherence tomography. Ocul. Immunol. Inflamm. 2014, 22, 239–241. [Google Scholar] [CrossRef]
- Biswas, J.; Badrinath, S.S. Ocular morbidity in patients with active systemic tuberculosis. Int. Ophthalmol. 1995, 19, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Vermeirsch, S.; Testi, I.; Pavesio, C. Choroidal involvement in non-infectious posterior scleritis. J. Ophthalmic Inflamm. Infect. 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Alsarhani, W.K.; Abu El-Asrar, A.M. Multimodal Imaging of Nodular Posterior Scleritis: Case Report and Review of the Literature. Middle East Afr. J. Ophthalmol. 2020, 27, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Lavric, A.; Restori, M.; Pavesio, C.; Sagoo, M.S. Nodular posterior scleritis: Clinico-sonographic characteristics and proposed diagnostic criteria. Retina 2016, 36, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Demirci, H.; Shields, C.L.; Honavar, S.G.; Shields, J.A.; Bardenstein, D.S. Long-term follow-up of giant nodular posterior scleritis simulating choroidal melanoma. Arch. Ophthalmol. 2000, 118, 1290–1292. [Google Scholar] [CrossRef]
- Pérez-Campagne, E.; Guex-Crosier, Y.; Schalenbourg, A.; Uffer, S.; Zografos, L. Giant nodular posterior scleritis compatible with ocular sarcoidosis simulating choroidal melanoma. Arch. Soc. Esp. Oftalmol. 2007, 82, 563–566. [Google Scholar] [CrossRef]
- Hage, R.; Jean-Charles, A.; Guyomarch, J.; Rahimian, O.; Donnio, A.; Merle, H. Nodular posterior scleritis mimicking choroidal metastasis: A report of two cases. Clin. Ophthalmol. 2011, 5, 877–880. [Google Scholar] [CrossRef][Green Version]
- Liu, A.T.; Luk, F.O.; Chan, C.K. A case of giant nodular posterior scleritis mimicking choroidal malignancy. Indian J. Ophthalmol. 2015, 63, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.J.; Newman, J.H.; Honig, S.E.; Mayro, E.L.; McGarrey, M.; Graf, A.E.; Selzer, E.B.; Acaba-Berrocal, L.A.; Considine, S.P.; Malik, K.; et al. Choroidal amelanotic tumours: Clinical differentiation of benign from malignant lesions in 5586 cases. Br. J. Ophthalmol. 2020, 104, 194–201. [Google Scholar] [CrossRef]
- McCluskey, P.J.; Watson, P.G.; Lightman, S.; Haybittle, J.; Restori, M.; Branley, M. Posterior scleritis: Clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology 1999, 106, 2380–2386. [Google Scholar] [CrossRef]
- Cunningham, E.T.; McCluskey, P.; Pavesio, C.; Wakefield, D.; Zierhut, M. Scleritis. Ocul. Immunol. Inflamm. 2016, 24, 2–5. [Google Scholar] [CrossRef]
- Shields, C.; Kaliki, D.; Rojanaporn, S.R.; Ferenczy, J.A.S. Enhanced Depth Imaging Optical Coherence Tomography of Small Choroidal Melanoma: Comparison With Choroidal Nevus. Arch. Ophthalmol. 2012, 130, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, W.-J.; Chen, R.-J.; Qian, J.; Luo, C.-Q.; Zhang, Y.-J.; Shen, Y.; Ye, X.-F.; Gao, Q.-Y. Fundus fluorescein angiography in metastatic choroidal carcinomas and differentiating metastatic choroidal carcinomas from primary choroidal melanomas. Zhonghua. Yan Ke Za Zhi 2011, 47, 27–34. [Google Scholar] [PubMed]
- Krause, L.; Bechrakis, N.E.; Kreusel, K.M.; Servetopoulou, F.; Heinrich, S.; Foerster, M.H. Indocyanine green angiography in choroid metastases. Ophthalmologe 2002, 99, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Demirci, H.; Cullen, A.; Sundstrom, J.M. Enhanced depth imaging optical coherence tomography of choroidal metastasis. Retina 2014, 34, 1354–1359. [Google Scholar] [CrossRef]
- Witkin, A.J.; Fischer, D.H.; Shields, C.L.; Reichstein, D.; Shields, J.A. Enhanced depth imaging spectral-domain optical coherence tomography of a subtle choroidal metastasis. Eye 2012, 26, 1598–1599. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Arepalli, S.; Pellegrini, M.; Mashayekhi, A.; Shields, J.A. Choroidal lymphoma shows calm, rippled, or undulating topography on enhanced depth imaging optical coherence tomography in 14 eyes. Retina 2014, 34, 1347–1353. [Google Scholar] [CrossRef]
- Al-Dahmash, S.A.; Shields, C.L.; Kaliki, S.; Johnson, T.; Shields, J.A. Enhanced depth imaging optical coherence tomography of choroidal metastasis in 14 eyes. Retina 2014, 34, 1588–1593. [Google Scholar] [CrossRef]
- Karimi, S.; Nourinia, R.; Mashayekhi, A. Circumscribed Choroidal Hemangioma. J. Ophthalmic Vis. Res. 2015, 10, 320–328. [Google Scholar] [CrossRef]
- Stroszczynski, C.; Hosten, N.; Bornfeld, N.; Wiegel, T.; Schueler, A.; Foerster, P.; Lemke, A.J.; Hoffmann, K.T.; Felix, R. Choroidal hemangioma: MR findings and differentiation from uveal melanoma. Am. J. Neuroradiol. 1998, 19, 1441–1447. [Google Scholar]
- Day, S.; Mruthyunjaya, P. Chapter 153—Circumscribed Choroidal Hemangioma. In Retina, 5th ed.; Ryan, S.J., Sadda, S.R., Hinton, D.R., Schachat, A.P., Sadda, S.R., Wilkinson, C.P., Wiedemann, P., Schachat, A.P.B.T.-R., Eds.; W.B. Saunders: London, UK, 2013; pp. 2340–2350. ISBN 978-1-4557-0737-9. [Google Scholar]
- Rojanaporn, D.; Kaliki, S.; Ferenczy, S.R.; Shields, C.L. Enhanced depth imaging optical coherence tomography of circumscribed choroidal hemangioma in 10 consecutive cases. Middle East Afr. J. Ophthalmol. 2015, 22, 192–197. [Google Scholar] [CrossRef]
- Mashayekhi, A.; Shukla, S.Y.; Shields, J.A.; Shields, C.L. Choroidal lymphoma: Clinical features and association with systemic lymphoma. Ophthalmology 2014, 121, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Preziosa, C.; Yaghy, A.; Ruben, M.; Invernizzi, A.; Fung, A.T.; Staurenghi, G.; Shields, C.L. Choroidal Lymphoma: Diagnostic Value of Combined Indocyanine Green Angiography and Optical Coherence Tomography. Ocul. Immunol. Inflamm. 2023, 31, 263–270. [Google Scholar] [CrossRef]
- Invernizzi, A.; Pellegrini, M.; Cornish, E.; Teo, K.Y.C.; Cereda, M.; Chabblani, J. Imaging the choroid: From indocyanine green angiography to optical coherence tomography angiography. Asia-Pac. J. Ophthalmol. 2020, 9, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Manalac, J.; Das, C.; Saktanasate, J.; Shields, J.A. Review of spectral domain enhanced depth imaging optical coherence tomography of tumors of the choroid. Indian J. Ophthalmol. 2015, 63, 117–121. [Google Scholar] [CrossRef] [PubMed]
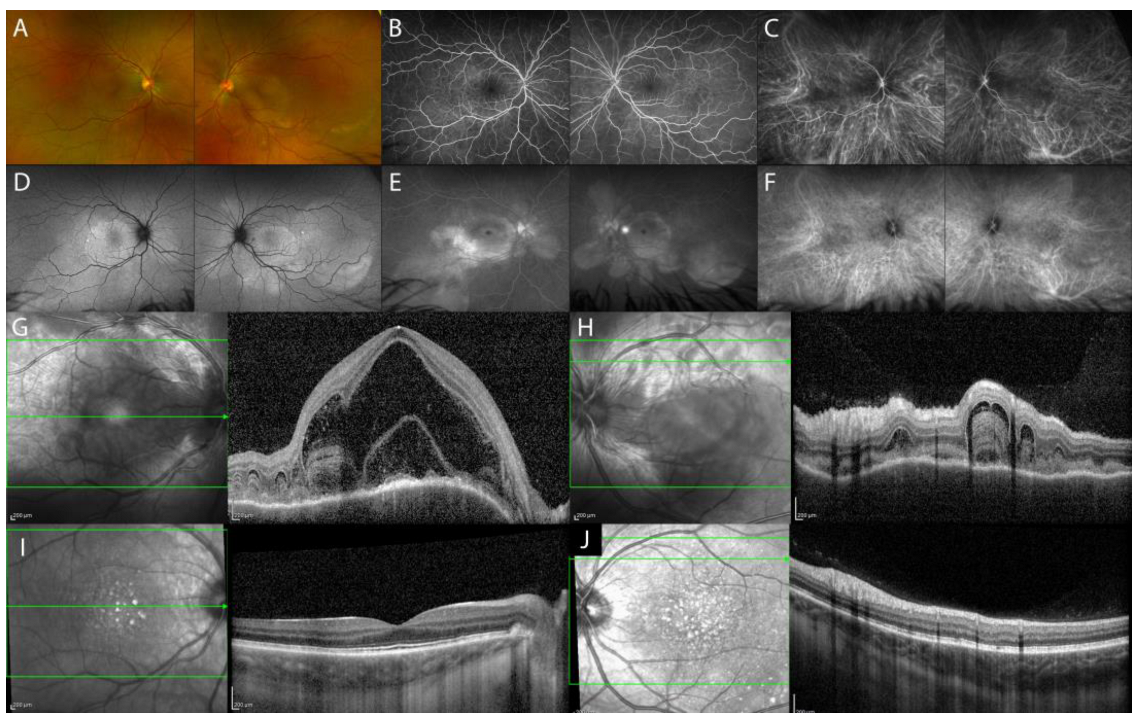

| Imaging Findings—Inflammatory Lesions | |||
|---|---|---|---|
| Lesion | OCT/EDI-OCT/SS-OCT | FA/ICGA | US |
| Acute uveitic phase of VKH disease | serous retinal detachment with typical fibrinous septa, diffuse choroidal thickening |
|
|
| Chronic recurrent uveitic phase of VKH disease | increased choroidal thickness, choroidal localized thickening with ‘choroidal bulging’ |
|
|
| Isolated sarcoid choroid granulomas | hyporeflective focal choroidal thickening with increased signal transmission, possible hyperreflectivity of outer retinal layers overlying the choroidal lesion (inflammatory infiltrate) |
|
|
| Tuberculoma | hyporeflective lesion in the choroidal stroma with increased signal transmission, possible “contact sign” |
|
|
| Nodular posterior scleritis | elevated retinal profile, possible: focal or multifocal ERD, retinal/choroidal folds, macular edema |
|
|
| Imaging Findings—Neoplastic Lesions | |||
|---|---|---|---|
| Lesion | OCT/EDI-OCT/SS-OCT | FA/ICGA | US/MRI |
| Choroidal melanoma | dome-shaped solid choroidal elevation with smooth and regular surface, choriocapillaris compression, possible: SRF, lipofuscin deposition, shaggy photoreceptors |
|
|
| Choroidal metastasis | irregular and lobulated contour, irregular and “lumpy bumpy” anterior surface |
|
|
| Choroidal hemangioma | smooth, gently sloping anterior contour, choroidal vessels expansion without choriocapillaris compression |
|
|
|
| ||
| Choroidal lymphoma | diffuse choroidal infiltration, choroidal surface depending on tumor thickness:
|
|
|
| OCT Imaging Findings—Inflammatory and Neoplastic Lesions | |||
|---|---|---|---|
| Inflammatory Lesion | OCT/EDI-OCT/SS-OCT | Neoplastic Lesion | OCT/EDI-OCT/SS-OCT |
| Acute VKH disease | diffuse choroidal thickening | Choroidal melanoma | smooth, regular solid choroidal elevation with choriocapillaris compression |
| Chronic recurrent VKH disease | choroidal localized thickening with ‘choroidal bulging’ | Choroidal metastasis | irregular and “lumpy bumpy” anterior surface |
| Isolated sarcoid choroid granulomas | hyporeflective focal choroidal thickening with increased signal transmission | Choroidal hemangioma | smooth choroidal elevation without choriocapillaris compression, choroidal vessels expansion |
| Tuberculoma | hyporeflective focal choroidal thickening with increased signal transmission and possible “contact sign” | Choroidal lymphoma | choriocapillaris compressed inward, choroidal surface varies depending on the tumor thickness: calm (thin infiltration), rippled (medium infiltration), undulating or “seasick” (thick infiltration) |
| Nodular posterior scleritis | elevated retinal profile with normal choroid tissue beneath | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolletta, E.; De Simone, L.; Pellegrini, M.; Preziosa, C.; Mastrofilippo, V.; Adani, C.; Gentile, P.; Gozzi, F.; Cimino, L. Optical Coherence Tomography in Inflammatory and Neoplastic Lesions Deforming the Choroidal Profile. Diagnostics 2023, 13, 1991. https://doi.org/10.3390/diagnostics13121991
Bolletta E, De Simone L, Pellegrini M, Preziosa C, Mastrofilippo V, Adani C, Gentile P, Gozzi F, Cimino L. Optical Coherence Tomography in Inflammatory and Neoplastic Lesions Deforming the Choroidal Profile. Diagnostics. 2023; 13(12):1991. https://doi.org/10.3390/diagnostics13121991
Chicago/Turabian StyleBolletta, Elena, Luca De Simone, Marco Pellegrini, Chiara Preziosa, Valentina Mastrofilippo, Chantal Adani, Pietro Gentile, Fabrizio Gozzi, and Luca Cimino. 2023. "Optical Coherence Tomography in Inflammatory and Neoplastic Lesions Deforming the Choroidal Profile" Diagnostics 13, no. 12: 1991. https://doi.org/10.3390/diagnostics13121991
APA StyleBolletta, E., De Simone, L., Pellegrini, M., Preziosa, C., Mastrofilippo, V., Adani, C., Gentile, P., Gozzi, F., & Cimino, L. (2023). Optical Coherence Tomography in Inflammatory and Neoplastic Lesions Deforming the Choroidal Profile. Diagnostics, 13(12), 1991. https://doi.org/10.3390/diagnostics13121991







