Abstract
Ventricular arrhythmias (VA) are a major cause of sudden cardiac death (SCD). Echocardiography is the first widely available imaging tool which guides VA management strategies. Along with other invasive and noninvasive imaging techniques, it provides essential information for identification of VA substrate such as differentiation between ischemic and non-ischemic etiology and identification of structural heart disease. Both classic as well as novel echocardiographic techniques such as left ventricular strain measurement and mechanical dispersion assessment provide prognostic information and assist in risk stratification. Furthermore, intracardiac echocardiography may have an adjunctive role for the VA ablation by providing real-time visualization of cardiac structures, continuous monitoring of catheter location and early recognition of procedural complications. This review gathers all relevant information that echocardiography may offer prior to VA ablation procedures.
1. Introduction
Ventricular arrhythmias (VA) consist of a large spectrum of ventricular rhythm disturbances ranging from premature ventricular complexes to ventricular tachycardia and fibrillation. VA are a major cause of sudden cardiac death (SCD) and a significant burden on the patient’s quality of life. In the majority of cases, VA are associated with structural heart disease.
Cardiac imaging plays an essential role in revealing the underlying etiology of VA and for SCD risk stratification. Echocardiography is the first readily available, inexpensive and accurate imaging tool used to identify the structural changes associated with VA. It is usually followed by multimodality imaging techniques such as cardiac magnetic resonance (CMR), cardiac computed tomography (CT) and nuclear imaging which bring additional information about the VA substrate. Basic echocardiography measurements such as left ventricle ejection fraction (LVEF) have long been used to assess the risk of SCD and current guidelines still rely on it for the indication of a implantable cardiac defibrillator (ICD) [1].
Substrate ablation is an efficient interventional treatment for VA. For a safe and successful ablation there is a need to understand anatomical VA substrate along with the electric substrate defined by the electroanatomic mapping (EAM). This review will focus on the necessary information provided by the echocardiography prior to the VA ablation procedure.
2. Role of Echocardiography in Identification of VA Substrate
2.1. VA in Structural Heart Disease
Echocardiography is useful in the diagnosis of structural heart disease [2]. Transthoracic echocardiography (TTE) is a widely available imaging technique which offers valuable information about VA substrate. The main objectives of TTE evaluation are assessment of left ventricle (LV) systolic function with estimation of LVEF, differential diagnosis between ischemic versus nonischemic etiology, assessment of valve function [3], identification of congenital heart disease [3] and different cardiomyopathies [3].
For the evaluation of LV systolic function, LVEF is the main parameter used in VA management. The ESC guidelines recommend performing echocardiography in all patients with suspected or known VA for assessment of LV function and identification of structural substrate [1]. In patients with structural heart disease, a LVEF < 35% estimated by echocardiography is associated with increased risk of VA and SCD [1]. Furthermore, indications for ICDs in primary prevention of SCD rely on LVEF [1]. The LVEF may be estimated using 2D or 3D LV volumes [1]. Furthermore, TTE can identify segmental LV contraction abnormalities and describe myocardial scars which may represent VA substrate (Figure 1).
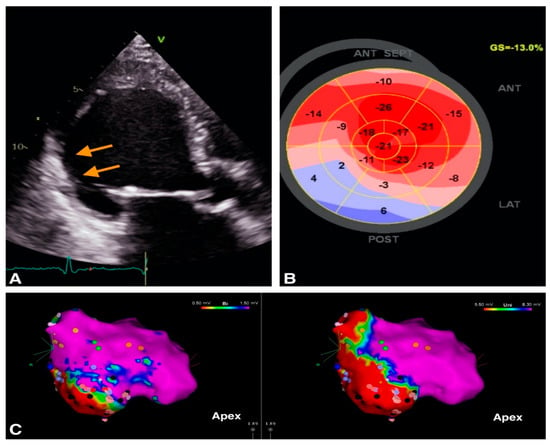
Figure 1.
Example of image integration from a patient with VT and old myocardial infarction; (A). 2D apical 3 chambers view showing the thin, hyperechogenic inferolateral wall (orange arrows). (B). Bull’s eye representation of peak longitudinal strain showing positive values in inferior wall providing a description of the myocardial scar (C) Electroanatomic bipolar (left) and unipolar (right) voltage maps in septal view, with an inferior area of low voltage corresponding to the scar identified by echocardiography.
Transthoracic and transesophageal echocardiography are the routine tools for the diagnosis and severity assessment of underlying valvular heart disease which may contribute to VA occurrence.
Echocardiography plays a significant role in the diagnosis and in management of patients with congenital heart disease and VA. Several studies have shown that right ventricle (RV) and LV dysfunction are risk factors for VA in congenital heart disease patients [2]. It is mandatory to establish the current status of the congenital heart disease and the need of correcting residual complications in order to limit the risk of VA recurrence. A frequent clinical scenario is the operated patient with tetralogy of Fallot with severe residual pulmonary regurgitation and severe dilatation of RV who develops ventricular tachycardia (VT). The treatment of pulmonary stenosis/regurgitation is discussed to promote RV reverse remodeling and maybe decrease the risk of malignant polymorphic VA [4].
A multicenter study performed by Koyak et al. showed that moderate to severe systemic or subpulmonary ventricular dysfunction were associated with a high risk of SCD, especially in adults with transposition of great arteries, Eisenmenger physiology and surgically repaired tetralogy of Fallot [5].
Finally, echocardiography may diagnose cardiomyopathy as the substrate for VA (Figure 2).
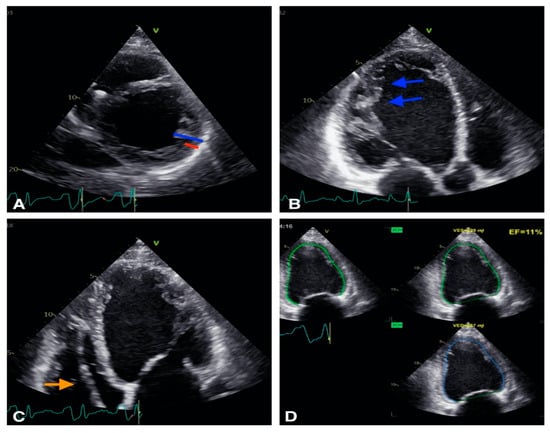
Figure 2.
Example of a patient with non-compaction cardiomiopathy. (A): 2D short axis view, the non compaction myocardium (blue) is twice thicker than the compacted myocardium (red). (B): 2D apical 3 chambers view, trabeculations are visible (blue arrows). (C): 2D apical 4 chambers view: defibrillator lead is visibile in right ventricle (orange arrow). (D): Biplane EF is calculated at 11% showing severe LV systolic dysfunction.
The established echocardiographic criteria for diagnosis of the different cardiomyopathies are summarized in Table 1. However, CMR provides more precise information of VA substrate, as it is able to distinguish between ischemic and non-ischemic etiology and detect subtle structural abnormalities such as myocarditis, sarcoid or amyloid cardiomyopathies [6]. CMR also brings incremental information for the diagnosis of HCM and ARVD [6].

Table 1.
Echocardiographic criteria for the diagnosis of cardiomyopathy.
2.2. VA in Structurally Normal Hearts
Almost 50% of SCD events occur in patients without structural heart disease [1]. Table 2 summarizes the possible etiology of VA in patients without any obvious structural changes. There is data that “normal” structural hearts associated with VA and channelopathies actually show structural changes. A group by Scheirlynck et al. studied 175 patients with Brugada Syndrome (BrS) compared to 82 controls and found that BrS patients had lower longitudinal strain and more heterogeneous contractions than healthy controls [13]. Furthermore, BrS patients with a history of life-threatening VA had more heterogeneous LV contractions [13]. Therefore, LV mechanical dispersion may be a risk marker in BrS and its evaluation in prospective studies is needed [9]. Studies on RV function also seem to be relevant in BrS patients, in whom subtle contractile RV mechanical abnormalities were demonstrated, including impaired RV longitudinal strain and a greater contraction delay between the lateral and the septal aspect of the RV [14].

Table 2.
Etiology of VA.
Other studies show an overlap between different VA etiology as for BrS and arrhythmogenic cardiomyopathy [15]. The presence of arrhythmogenic cardiomyopathy diagnostic criteria in BrS patients was associated with a trend towards higher arrhythmic risk [15].
Prior to VA ablation, echocardiographic data can be completed with cardiac magnetic resonance (CMR). CMR may be later combined with EAM information. Several studies have confirmed that low endocardial voltage corresponds to the presence of scar tissue as defined by the late gadolinium enhanced CMR [16]. Pre-procedural CMRs have been extremely helpful in localizing scar tissue in the ventricles and have helped to guide and target the arrhythmogenic tissue with catheter ablation [17].
3. Echocardiography for Risk Stratification in Patients with VA
Echocardiography plays an important role in SCD risk stratification. Reduced LVEF is associated with a high risk of VA and mortality, but the ability to predict VA is limited [2]. Some of the patients with SCD after myocardial infarction have LVEF > 35% which proves the poor sensitivity of LVEF for risk stratification [2].
Other echocardiographic markers, which are additive to LVEF, are associated with high risk of VA: low global and/or regional strain, low relative wall thickness, worsening mechanical dyssynchrony, high peak strain dispersion (Table 3) [18].

Table 3.
Echocardiographic arrhythmogenic risk stratification in structural heart disease.
Global wall motion score index (GWMSI) is a prognostic echocardiographic parameter that evaluate wall motion abnormalities and helps to differentiate between ischemic and nonischemic etiology [2]. Mahenthiran et al. showed a predictive value for the composite endpoint of appropriate ICD therapy and all-cause mortality [23]. Restrictive mitral filling pattern and E/E’ ratio are diastolic functional parameters assessed easily by echocardiography, and Bruch et al. suggested that in patients with systolic dysfunction and ICD these parameters were independent predictors of ICD discharge and cardiac death [2].
The evaluation of exercise- or dobutamine-induced wall motion abnormalities could provide additional value to LVEF for risk stratification for VA in patients with coronary artery disease. Also, Elhendy et al. showed that ischemia during stress echocardiography is an independent predictor of death and ICD therapy in patients with coronary heart disease at high risk of arrhythmic death [24].
Relative wall thickness (RWT) is defined as twice the posterior wall thickness divided by the LV diastolic diameter. A study which included patients enrolled in the MADIT-CRT trial demonstrated a powerful predictive value of low RWT for estimating the risk of VA and VA risk or death in either ischemic or nonischemic cardiomyopathy, heart failure and left bundle branch block (LBBB) [2,11].
The reduction of LV global longitudinal strain (GLS) and regional longitudinal myocardial deformation, especially low inferior strain, was associated with the occurrence of VA, irrespective of etiology of cardiomyopathy [18,25,26]. Abnormalities of myocardial deformation or strain can indicate the presence of scar [27]. Furthermore, Trivedi et al. demonstrated that strain abnormalities can quantify the extent of low-voltage scar detected by EAM in ischemic [27] and non-ischemic cardiomyopathy [28]. The results of these studies indicate that performing noninvasive speckle tracking echocardiography may provide valuable information on scar burden and location prior to invasive EAM [28].
Mechanical dispersion is a measure of myocardial deformation heterogeneity and it is regarded as a novel risk parameter of VA, independently of LVEF [2]. It is defined as the standard deviation of time to peak negative strain in all LV myocardial segments [2]. A prospective study by Haugaa et al. conducted upon 569 patients at 40 days after myocardial infarction (MI) show that a combination of mechanical dispersion and global strain may improve the selection of patients after MI for implantable cardioverter defibrillator therapy, particularly in patients with LVEFs > 35% who did not fulfill current ICD indications [29].
In patients with ischemic cardiomyopathy, Mahenthiran et al. showed that GWMSI and right coronary region infero-posterior akinesia identified those at increased risk of events [23]. Ersboll et al. revealed that novel echocardiographic parameters such as GLS and mechanical dispersion were significantly and independently related to SCD or VA after acute MI [25].
In hypertrophic cardiomyopathy (HCM), the most frequent fatal arrhythmia is spontaneous fast monomorphic ventricular tachycardia (VT), whereas non-sustained ventricular tachycardia (NSVT) is often found on ECG monitoring [9]. HCM risk SCD variables include age, family history of SCD, unexplained syncope, NSVT, LV outflow gradient, maximum LV wall thickness, LA diameter [2]. The last three can be easily assessed by echocardiography, but strain parameters may improve risk stratification of VA [2]. Extreme LV hypertrophy defined as maximal wall thickness > 30 mm is associated with higher risk for VA, especially in young patients [30]. Haland et al. showed that worse GLS, more pronounced mechanical dispersion and higher percent of late gadolinium enhancement (%LGE) on CMR were identified in patients with VA, and mechanical dispersion correlated with extent of fibrosis and was an independent risk factor for VA [31].
Echocardiography plays a central role in diagnosis of arrhythmogenic right ventricular cardiomyopathy (ARVC), showing regional RV akinesia, dyskinesia or aneurysm [2]. In patients with early ARVC, larger RV diameter, increased RV mechanical dispersion and reduced LV and RV function are echocardiographic parameters which improve risk stratification of arrhythmic events [32]. Moreover, Mast et al. demonstrated that a prolonged subtricuspid electromechanical interval was an echocardiographic parameter significantly associated with arrhythmogenesis in asymptomatic mutation carriers [33].
In non-compaction cardiomyopathy (NCM) echocardiography is the first-line imaging modality but cannot provide tissue characterization or accurate evaluation of RV function [34]. CMR provides a 3D approach allowing imaging of the entire heart, including both left and right ventricle, with low operator variability or limitations due to patient’s body structure [34] proving the pivotal role of multimodality cardiovascular imaging in the identification and risk stratification of non-compaction cardiomyopathy patients. Left ventricular non compaction (LVNC) is also prevalent in patient with congenital heart disease and might contribute to myocardial dysfunction or arrhythmias [20].
Patients with dilated cardiomyopathy (DCM) are at high risk of SCD and the annual event rate of sustained VA is 4.5% [22]. LVEF < 30–35% was investigated in several studies as a predictor of arrhythmic events, and a 5% or 10% decrease of LVEF is associated with high arrhythmic risk [22].
Furthermore, Perry et al. demonstrated that in patients with moderate and severe decreased LVEF, mechanical dispersion ≥ 75 ms is related to VA, with a 9-fold increase in risk of VA events [35]. In a prospective study, patients with DCM and arrhythmic events had reduced LVEF and GLS, while mechanical dispersion predicted VA independently of LVEF [36].
4. Ecocardiography as an Adjunctive Tool for VA Ablation
Echocardiography may also offer specific relevant information prior to the ablation procedure. It is important to diagnose pericardial fluid prior to or during catheter ablation procedure. The presence of a cardiac thrombus should also be diagnosed before VA ablation. Traditionally, TTE was the main imaging tool to diagnose thrombosis, however recently CMR and cardiac CT are frequently used [6]. The guidelines suggest that although LV endocardial ablation can be performed in patients with a laminated thrombus, the presence of a mobile thrombus represents a contraindication [37]. In selected patients with mobile thrombi, ablation with epicardial access only has been demonstrated to be a good alternative [38].
Echocardiography has little contribuition in deciding the access route before catheter ablation. When femoral access is required, ultrasound-guided femoral arterial and venous access has been widely implemented in electrophysiological procedures in an effort to reduce vascular complications [39]. Sharma et al. reported the rate of major vascular access complications in patients undergoing VA ablation, which was 8.9% in the conventional group and 0% in the ultrasound-guided group [40]. Epicardial access is associated with higher complication rates and is not usually performed as the first line approach [6]. The decision depends on the underlying etiology and VT ECG characteristics. Late gadolinium enhancement CMR defines the location of epicardial or intramural scar and thus is more useful to establish the access route [6]. Imaging with cardiac CT or CMR can be used to assess the anatomic relationship between the pericardial space and the surrounding structures and this may limit the damage related to epicardial access [6].
Intracardiac echocardiography (ICE) generates 3-dimensional ventricular reconstruction that can be integrated in real-time with electroanatomic mapping. ICE provides real-time imaging of ventricular anatomy, catheter contact, and facilitates catheter manipulation during ablation [41].
In patients with scar-related VT, ICE may identify the thinned, akinetic myocardium and therefore it may localize the scar. There are studies which found a good correlation between scar area identified by ICE and that defined by EAM [42]. Furthermore, based on scar echo density, scar core has been differentiated from scar border zone as defined by EAM. ICE scar description has been validated against LGE-CMR and MDCT, but the technique is less effective [42]. In nonischemic cardiomyopathy, ICE can also detect the presence of midmyocardial and epicardial scar, which correlates with unipolar endocardial voltage abnormalities and epicardial bipolar voltage abnormalities [43].
LV access for endocardial mapping and VA ablation can be achieved either by retrograde transaortic or by antegrade transseptal approach. ICE has an important role in gaining safe left atrial access during transseptal puncture since it enables real-time visualization of the sheath-needle assembly, the fossa tenting and the puncturing the interatrial septum [41]. The technique is particularly valuable for VAs arising from sites with complex anatomy, such as periaortic VT and papillary muscle VT + RV free wall. ICE appears to be the most suitable imaging method for assessment of the exact location of the focus on papillary muscles in association with activation mapping [44].
ICE also provides early recognition of procedural complications, such as pericardial effusion or thrombus formation on sheaths and catheters. The presence of clots in the LV apex, especially in the setting of anteroapical aneurysms, can be evaluated with ICE to avoid catheter manipulation and prevent embolism [43]. Additional benefits are excellent patient tolerance and lack of need for general anesthesia or a second operator. For these reasons, ICE has largely replaced transesophageal echocardiography as ideal imaging modality for guiding catheter ablation of VA [43].
Futhermore, ICE facilitates significant reduction of radiation exposure during catheter ablation [45]. Zero fluoroscopy VT ablation is feasible using a combination of electroanatomic systems and ICE and achieving high acute success and low recurrence rates [41].
5. Conclusions
Conventional echocardiography has an established role in the management of patients suffering from ventricular arrhythmias, while novel echocardiographic techniques such as LV strain measurement and mechanical dispersion assessment upgrade the diagnostic and prognostic role of the technique. Additionally, intracardiac echocardiography may have an integrative role in real-time assessment of LV anatomy during VA ablation and early recognition of procedural complications.
Author Contributions
Conceptualization, A.D. and S.D.; resources, I.P.; writing—original draft preparation, A.D. and S.D.; writing—review and editing, A.D. and G.M.; supervision, R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac deathThe Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar]
- Papadopoulos, C.H.; Oikonomidis, D.; Lazaris, E.; Nihoyannopoulos, P. Echocardiography and cardiac arrhythmias. Hell. J. Cardiol. 2018, 59, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Saric, M.; Armour, A.C.; Arnaout, M.S.; Chaudhry, F.A.; Grimm, R.A.; Kronzon, I.; Landeck, B.F.; Maganti, K.; Michelena, H.I.; Tolstrup, K. Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. J. Am. Soc. Echocardiogr. 2016, 29, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Therrien, J.; Siu, S.C.; Harris, L.; Dore, A.; Niwa, K.; Janousek, J.; Williams, W.G.; Webb, G.; Gatzoulis, M.A. Impact of Pulmonary Valve Replacement on Arrhythmia Propensity Late After Repair of Tetralogy of Fallot. Circulation 2001, 103, 2489–2494. [Google Scholar] [CrossRef]
- Koyak, Z.; Harris, L.; de Groot, J.R.; Silversides, C.K.; Oechslin, E.N.; Bouma, B.J.; Budts, W.; Zwinderman, A.H.; Van Gelder, I.C.; Mulder, B.J.M. Sudden Cardiac Death in Adult Congenital Heart Disease. Circulation 2012, 126, 1944–1954. [Google Scholar] [CrossRef]
- Mahida, S.; Sacher, F.; Dubois, R.; Sermesant, M.; Bogun, F.; Haïssaguerre, M.; Jaïs, P.; Cochet, H. Cardiac Imaging in Patients With Ventricular Tachycardia. Circulation 2017, 136, 2491–2507. [Google Scholar] [CrossRef]
- Williams, L.K.; Frenneaux, M.P.; Steeds, R.P. Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role in management. Eur. J. Echocardiogr. 2009, 10, iii9–iii14. [Google Scholar] [CrossRef] [PubMed]
- Hagège, A.A.; Dubourg, O.; Desnos, M.; Mirochnik, R.; Isnard, G.; Bonne, G.; Carrier, L.; Guicheney, P.; Bouhour, J.-B.; Schwartz, K.; et al. Familial hypertrophic cardiomyopathy. Cardiac ultrasonic abnormalities in genetically affected subjects without echocardiographic evidence of left ventricular hypertrophy. Eur. Heart J. 1998, 19, 490–499. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.P.J.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed Modification of the Task Force Criteria. Eur. Heart J. 2010, 31, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Chebrolu, L.H.; Mehta, A.M.; Nanda, N.C. Noncompaction cardiomyopathy: The role of advanced multimodality imaging techniques in diagnosis and assessment. Echocardiography 2017, 34, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.; Williams, L.; Navaratnam, G.; Rana, B.; Wheeler, R.; Collins, K.; Harkness, A.; Jones, R.; Knight, D.; O’Gallagher, K.; et al. Diagnosis and assessment of dilated cardiomyopathy: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2017, 4, G1–G13. [Google Scholar] [CrossRef] [PubMed]
- Scheirlynck, E.; Malderen, S.V.; Motoc, A.; Lie, Ø.H.; de Asmundis, C.; Sieira, J.; Chierchia, G.-B.; Brugada, P.; Cosyns, B.; Droogmans, S. Contraction alterations in Brugada syndrome; association with life-threatening ventricular arrhythmias. Int. J. Cardiol. 2020, 299, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mitroi, C.; García-Izquierdo, E.; García-Lunar, I.; Castro-Urda, V.; Toquero-Ramos, J.; Moñivas-Palomero, V.; Mingo-Santos, S.; Cavero, M.A.; Brugada, J.; Fernández-Lozano, I. Right ventricular function and dyssynchrony in Brugada syndrome: Highlighting the importance of the mechanical substrate in the right ventricular outflow tract. Int. J. Cardiol. 2021, 333, 233–238. [Google Scholar] [CrossRef]
- Scheirlynck, E.; Chivulescu, M.; Lie, Ø.H.; Motoc, A.; Koulalis, J.; de Asmundis, C.; Sieira, J.; Chierchia, G.-B.; Brugada, P.; Cosyns, B.; et al. Worse Prognosis in Brugada Syndrome Patients With Arrhythmogenic Cardiomyopathy Features. JACC Clin. Electrophysiol. 2020, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Silva, G.V.; Sarmento-Leite, R.; Sousa, A.L.S.; Howell, M.; Muthupillai, R.; Lambert, B.; Vaughn, W.K.; Flamm, S.D. Assessing Myocardial Viability and Infarct Transmurality With Left Ventricular Electromechanical Mapping in Patients With Stable Coronary Artery Disease. Circulation 2002, 106, 957–961. [Google Scholar] [CrossRef]
- Njeim, M.; Desjardins, B.; Bogun, F. Multimodality Imaging for Guiding EP Ablation Procedures. JACC Cardiovasc. Imaging 2016, 9, 873–886. [Google Scholar] [CrossRef]
- John Gorcsan, I.I.I.; Haugaa, K.H. Ventricular Arrhythmias and Reduced Echocardiographic Inferior Wall Strain. Circ. Cardiovasc. Imaging 2017, 10, e005900. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary. Circulation 2020, 142, e533–e557. [Google Scholar] [CrossRef]
- Ichida, F. Left ventricular noncompaction − Risk stratification and genetic consideration. J. Cardiol. 2020, 75, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Takasaki, A.; Ozawa, S.W.; Nakaoka, H.; Okabe, M.; Miyao, N.; Saito, K.; Ibuki, K.; Hirono, K.; Yoshimura, N.; et al. Long-Term Prognosis of Patients With Left Ventricular Noncompaction—Comparison Between Infantile and Juvenile Types. Circ. J. 2017, 81, 694–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammani, A.; Kayvanpour, E.; Bosman, L.P.; Sedaghat-Hamedani, F.; Proctor, T.; Gi, W.-T.; Broezel, A.; Jensen, K.; Katus, H.A.; te Riele, A.S.J.M.; et al. Predicting sustained ventricular arrhythmias in dilated cardiomyopathy: A meta-analysis and systematic review. ESC Heart Fail. 2020, 7, 1430–1441. [Google Scholar] [CrossRef]
- Mahenthiran, J.; Das, M.K.; Bhakta, D.; Ghumman, W.; Feigenbaum, H.; Sawada, S.G. Prognostic Importance of Wall Motion Abnormalities in Patients With Ischemic Cardiomyopathy and an Implantable Cardioverter-Defibrillator. Am. J. Cardiol. 2006, 98, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Elhendy, A.; Chapman, S.; Porter, T.R.; Windle, J. Association of Myocardial Ischemia With Mortality and Implantable Cardioverter-Defibrillator Therapy in Patients With Coronary Artery Disease at Risk of Arrhythmic Death. J. Am. Coll. Cardiol. 2005, 46, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Ersbøll, M.; Valeur, N.; Andersen, M.J.; Mogensen, U.M.; Vinther, M.; Svendsen, J.H.; Møller, J.E.; Kisslo, J.; Velazquez, E.J.; Hassager, C.; et al. Early Echocardiographic Deformation Analysis for the Prediction of Sudden Cardiac Death and Life-Threatening Arrhythmias After Myocardial Infarction. JACC Cardiovasc. Imaging 2013, 6, 851–860. [Google Scholar] [CrossRef]
- Biering-Sørensen, T.; Knappe, D.; Pouleur, A.-C.; Claggett, B.; Wang, P.J.; Moss, A.J.; Solomon, S.D.; Kutyifa, V. Regional Longitudinal Deformation Improves Prediction of Ventricular Tachyarrhythmias in Patients With Heart Failure With Reduced Ejection Fraction. Circ. Cardiovasc. Imaging 2017, 10, e005096. [Google Scholar] [CrossRef]
- Trivedi, S.J.; Campbell, T.; Stefani, L.D.; Thomas, L.; Kumar, S. Strain by speckle tracking echocardiography correlates with electroanatomic scar location and burden in ischaemic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 855–865. [Google Scholar] [CrossRef]
- Trivedi, S.J.; Campbell, T.; Davey, C.J.; Stefani, L.; Thomas, L.; Kumar, S. Longitudinal strain with speckle-tracking echocardiography predicts electroanatomic substrate for ventricular tachycardia in nonischemic cardiomyopathy patients. Heart Rhythm O2 2022, 3, 176–185. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain Echocardiography Improves Risk Prediction of Ventricular Arrhythmias After Myocardial Infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar] [CrossRef]
- Spirito, P.; Bellone, P.; Harris, K.M.; Bernabò, P.; Bruzzi, P.; Maron, B.J. Magnitude of Left Ventricular Hypertrophy and Risk of Sudden Death in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2000, 342, 1778–1785. [Google Scholar] [CrossRef]
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Leren, I.S.; Saberniak, J.; Haland, T.F.; Edvardsen, T.; Haugaa, K.H. Combination of ECG and Echocardiography for Identification of Arrhythmic Events in Early ARVC. JACC Cardiovasc. Imaging 2017, 10, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Mast, T.P.; Teske, A.J.; Te Riele, A.S.; Groeneweg, J.A.; Van Der Heijden, J.F.; Velthuis, B.K.; Loh, P.; Doevendans, P.A.; Van Veen, T.A.; Dooijes, D.; et al. Prolonged Electromechanical Interval Unmasks Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy in the Subclinical Stage. J. Cardiovasc. Electrophysiol. 2016, 27, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.I.; Markousis-Mavrogenis, G.; Vartela, V.; Manolopoulou, D.; Abate, E.; Hamadanchi, A.; Rigopoulos, A.G.; Kolovou, G.; Noutsias, M. The pivotal role of cardiovascular imaging in the identification and risk stratification of non-compaction cardiomyopathy patients. Heart Fail. Rev. 2020, 25, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.; Patil, S.; Marx, C.; Horsfall, M.; Chew, D.P.; Sree Raman, K.; Daril, N.D.M.; Tiver, K.; Joseph, M.X.; Ganesan, A.N.; et al. Advanced Echocardiographic Imaging for Prediction of SCD in Moderate and Severe LV Systolic Function. JACC Cardiovasc. Imaging 2020, 13, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Goebel, B.; Dahlslett, T.; Meyer, K.; Jung, C.; Lauten, A.; Figulla, H.R.; Poerner, T.C.; Edvardsen, T. Risk Assessment of Ventricular Arrhythmias in Patients with Nonischemic Dilated Cardiomyopathy by Strain Echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Aliot, E.M.; Stevenson, W.G.; Almendral-Garrote, J.M.; Bogun, F.; Calkins, C.H.; Delacretaz, E.; Bella, P.D.; Hindricks, G.; Jaïs, P.; Josephson, M.E.; et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: Developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). EP Eur. 2009, 11, 771–817. [Google Scholar]
- Berte, B.; Yamashita, S.; Sacher, F.; Cochet, H.; Hooks, D.; Aljefairi, N.; Amraoui, S.; Denis, A.; Derval, N.; Hocini, M.; et al. Epicardial only mapping and ablation of ventricular tachycardia: A case series. EP Eur. 2016, 18, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. EP Eur. 2019, 21, 1143–1144. [Google Scholar]
- Sharma, P.S.; Padala, S.K.; Gunda, S.; Koneru, J.N.; Ellenbogen, K.A. Vascular Complications During Catheter Ablation of Cardiac Arrhythmias: A Comparison Between Vascular Ultrasound Guided Access and Conventional Vascular Access. J. Cardiovasc. Electrophysiol. 2016, 27, 1160–1166. [Google Scholar] [CrossRef]
- Asvestas, D.; Xenos, T.; Tzeis, S. The contribution of intracardiac echocardiography in catheter ablation of ventricular arrhythmias. Rev. Cardiovasc. Med. 2022, 23, 25. [Google Scholar] [CrossRef]
- Bunch, T.J.; Weiss, J.P.; Crandall, B.G.; Day, J.D.; Dimarco, J.P.; Ferguson, J.D.; Mason, P.K.; McDANIEL, G.; Osborn, J.S.; Wiggins, D.; et al. Image Integration Using Intracardiac Ultrasound and 3D Reconstruction for Scar Mapping and Ablation of Ventricular Tachycardia. J. Cardiovasc. Electrophysiol. 2010, 21, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; Garcia, F. Use of Intracardiac Echocardiography in Interventional Cardiology. Circulation 2018, 137, 2278–2294. [Google Scholar] [CrossRef] [PubMed]
- Kautzner, J.; Peichl, P. Papillary Muscle Ventricular Tachycardia or Ectopy: Diagnostics, Catheter Ablation and the Role of Intracardiac Echocardiography. Arrhythm. Electrophysiol. Rev. 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Proietti, R.; Rivera, S.; Dussault, C.; Essebag, V.; Bernier, M.L.; Ayala-Paredes, F.; Badra-Verdu, M.; Roux, J.-F. Intracardiac echo-facilitated 3D electroanatomical mapping of ventricular arrhythmias from the papillary muscles: Assessing the ‘fourth dimension’ during ablation. EP Eur. 2017, 19, 21–28. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).