Development of a Novel Diagnostic Support Tool for Degenerative Cervical Myelopathy Combining 10-s Grip and Release Test and Grip Strength: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design
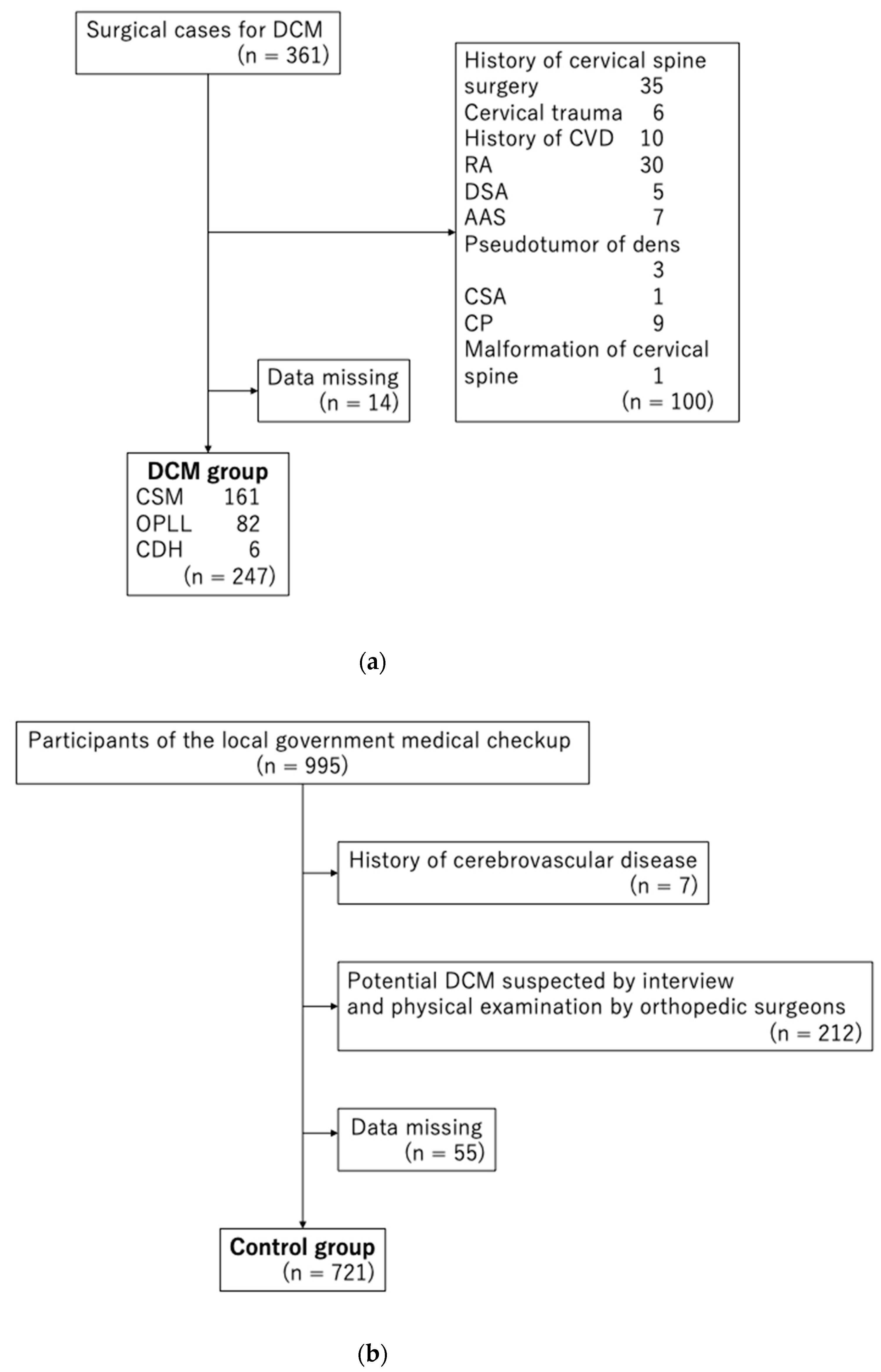
2.3. Study Participants
2.3.1. DCM Group
2.3.2. Control Group
2.4. Examination of GRT and Grip Strength
2.5. Statistical Analyses
3. Results
ROC Analyses
4. Discussion
4.1. Short Summary
4.2. Diagnosis of DCM
4.3. Advantages and Cautions in the Use of the DCM Diagnostic Support Tool
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nurick, S. The Pathogenesis of the Spinal Cord Disorder Associated with Cervical Spondylosis. Brain 1972, 95, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-C.; Ko, C.-C.; Yen, Y.-S.; Huang, W.-C.; Chen, Y.-C.; Liu, L.; Tu, T.-H.; Lo, S.-S.; Cheng, H. Epidemiology of Cervical Spondylotic Myelopathy and Its Risk of Causing Spinal Cord Injury: A National Cohort Study. Neurosurg. Focus 2013, 35, E10. [Google Scholar] [CrossRef] [PubMed]
- Lad, S.P.; Patil, C.G.; Berta, S.; Santarelli, J.G.; Ho, C.; Boakye, M. National Trends in Spinal Fusion for Cervical Spondylotic Myelopathy. Surg. Neurol. 2009, 71, 66–69; Discussion 69. [Google Scholar] [CrossRef]
- Boogaarts, H.D.; Bartels, R.H.M.A. Prevalence of Cervical Spondylotic Myelopathy. Eur. Spine J. 2015, 24 (Suppl. 2), 139–141. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Tadokoro, N.; Tetreault, L.; Arocho-Quinones, E.V.; Budde, M.D.; Kurpad, S.N.; Fehlings, M.G. Imaging Evaluation of Degenerative Cervical Myelopathy: Current State of the Art and Future Directions. Neurosurg. Clin. N. Am. 2018, 29, 33–45. [Google Scholar] [CrossRef]
- Aizawa, T.; Kokubun, S.; Ozawa, H.; Kusakabe, T.; Tanaka, Y.; Hoshikawa, T.; Hashimoto, K.; Kanno, H.; Morozumi, N.; Koizumi, Y.; et al. Increasing Incidence of Degenerative Spinal Diseases in Japan during 25 Years: The Registration System of Spinal Surgery in Tohoku University Spine Society. Tohoku J. Exp. Med. 2016, 238, 153–163. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Ahuja, C.S.; Akbar, M.A.; Witiw, C.D.; Nassiri, F.; Furlan, J.C.; Curt, A.; Wilson, J.R.; Fehlings, M.G. Degenerative Cervical Myelopathy—Update and Future Directions. Nat. Rev. Neurol. 2020, 16, 108–124. [Google Scholar] [CrossRef]
- Tetreault, L.; Kopjar, B.; Côté, P.; Arnold, P.; Fehlings, M.G. A Clinical Prediction Rule for Functional Outcomes in Patients Undergoing Surgery for Degenerative Cervical Myelopathy: Analysis of an International Prospective Multicenter Data Set of 757 Subjects. J. Bone Joint Surg. Am. 2015, 97, 2038–2046. [Google Scholar] [CrossRef]
- Behrbalk, E.; Salame, K.; Regev, G.J.; Keynan, O.; Boszczyk, B.; Lidar, Z. Delayed Diagnosis of Cervical Spondylotic Myelopathy by Primary Care Physicians. Neurosurg. Focus 2013, 35, E1. [Google Scholar] [CrossRef]
- Kobayashi, H.; Otani, K.; Nikaido, T.; Watanabe, K.; Kato, K.; Handa, J.; Yabuki, S.; Konno, S.-I. Grip Strength as a Screening Index for Severe Degenerative Cervical Myelopathy in Primary Care: Development of Cutoff Values Using Receiver Operating Curve Analysis. Int. J. Gen. Med. 2021, 14, 9863–9872. [Google Scholar] [CrossRef]
- Machino, M.; Ando, K.; Kobayashi, K.; Morozumi, M.; Tanaka, S.; Ito, K.; Kato, F.; Ishiguro, N.; Imagama, S. Cut off Value in Each Gender and Decade of 10-s Grip and Release and 10-s Step Test: A Comparative Study between 454 Patients with Cervical Spondylotic Myelopathy and 818 Healthy Subjects. Clin. Neurol. Neurosurg. 2019, 184, 105414. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Kikuchi, S.-I.; Yabuki, S.; Onda, A.; Nikaido, T.; Watanabe, K.; Konno, S.-I. Prospective One-Year Follow-up of Lumbar Spinal Stenosis in a Regional Community. J. Pain Res. 2018, 11, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R. The Cervical Syndrome; American Lecture Series; C. C. Thomas: Springfield, IL, USA, 1966. [Google Scholar]
- Spurling, R.G. Lateral Rupture of the Cervical Intervertebral Discs: A Common Cause of Shoulder and Arm Pain. Surg. Gynecol. Obstet. 1944, 78, 350–358. [Google Scholar]
- Ono, K.; Ebara, S.; Fuji, T.; Yonenobu, K.; Fujiwara, K.; Yamashita, K. Myelopathy Hand. New Clinical Signs of Cervical Cord Damage. J. Bone Joint Surg. Br. 1987, 69, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Fischer, J.E.; Bachmann, L.M.; Jaeschke, R. A Readers’ Guide to the Interpretation of Diagnostic Test Properties: Clinical Example of Sepsis. Intensive Care Med. 2003, 29, 1043–1051. [Google Scholar] [CrossRef]
- Arnold, J.G., Jr. The Clinical Manifestations of Spondylochondrosis (Spondylosis) of the Cervical Spine. Ann. Surg. 1955, 141, 872–889. [Google Scholar] [CrossRef]
- Taylor, A.R. Mechanism and Treatment of Spinal-Cord Disorders Associated with Cervical Spondylosis. Lancet 1953, 261, 717–720. [Google Scholar] [CrossRef]
- Penning, L. Some Aspects of Plain Radiography of the Cervical Spine in Chronic Myelopathy. Neurology 1962, 12, 513–519. [Google Scholar] [CrossRef]
- Ono, K.; Ota, H.; Tada, K.; Yamamoto, T. Cervical Myelopathy Secondary to Multiple Spondylotic Protrusions: A Clinicopathologic Study. Spine 1977, 2, 109. [Google Scholar] [CrossRef]
- Glaser, J.A.; Curé, J.K.; Bailey, K.L.; Morrow, D.L. Cervical Spinal Cord Compression and the Hoffmann Sign. Iowa Orthop. J. 2001, 21, 49–52. [Google Scholar] [PubMed]
- Chaiyamongkol, W.; Laohawiriyakamol, T.; Tangtrakulwanich, B.; Tanutit, P.; Bintachitt, P.; Siribumrungwong, K. The Significance of the Trömner Sign in Cervical Spondylotic Myelopathy Patient. Clin. Spine Surg. 2017, 30, E1315–E1320. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Yoshimura, N.; Muraki, S.; Hashizume, H.; Ishimoto, Y.; Yamada, H.; Takiguchi, N.; Nakagawa, Y.; Oka, H.; Kawaguchi, H.; et al. Prevalence of Cervical Cord Compression and Its Association with Physical Performance in a Population-Based Cohort in Japan: The Wakayama Spine Study. Spine 2012, 37, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Otani, K.; Sekiguchi, M.; Kikuchi, S.-I.; Konno, S.-I. Epidemiological Study of Cervical Cord Compression and Its Clinical Symptoms in Community-Dwelling Residents. PLoS ONE 2021, 16, e0256732. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, L.; Casey, A.; Laing, R.; Statham, P.; Fehlings, M.G. A Summary of Assessment Tools for Patients Suffering from Cervical Spondylotic Myelopathy: A Systematic Review on Validity, Reliability and Responsiveness. Eur. Spine J. 2015, 24 (Suppl. 2), 209–228. [Google Scholar] [CrossRef]
- Nouri, A.; Martin, A.R.; Mikulis, D.; Fehlings, M.G. Magnetic Resonance Imaging Assessment of Degenerative Cervical Myelopathy: A Review of Structural Changes and Measurement Techniques. Neurosurg. Focus 2016, 40, E5. [Google Scholar] [CrossRef]
- Martin, A.R.; De Leener, B.; Cohen-Adad, J.; Cadotte, D.W.; Nouri, A.; Wilson, J.R.; Tetreault, L.; Crawley, A.P.; Mikulis, D.J.; Ginsberg, H.; et al. Can Microstructural MRI Detect Subclinical Tissue Injury in Subjects with Asymptomatic Cervical Spinal Cord Compression? A Prospective Cohort Study. BMJ Open 2018, 8, e019809. [Google Scholar] [CrossRef]
- McGrath, R.; Tomkinson, G.R.; Clark, B.C.; Cawthon, P.M.; Cesari, M.; Al Snih, S.; Jurivich, D.A.; Hackney, K.J. Assessing Additional Characteristics of Muscle Function with Digital Handgrip Dynamometry and Accelerometry: Framework for a Novel Handgrip Strength Protocol. J. Am. Med. Dir. Assoc. 2021, 22, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Mowforth, O.D.; Davies, B.M.; Kotter, M.R. The Use of Smart Technology in an Online Community of Patients with Degenerative Cervical Myelopathy. JMIR Form. Res. 2019, 3, e11364. [Google Scholar] [CrossRef]
- Zhan, A.; Mohan, S.; Tarolli, C.; Schneider, R.B.; Adams, J.L.; Sharma, S.; Elson, M.J.; Spear, K.L.; Glidden, A.M.; Little, M.A.; et al. Using Smartphones and Machine Learning to Quantify Parkinson Disease Severity: The Mobile Parkinson Disease Score. JAMA Neurol. 2018, 75, 876–880. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Azer, S.A.; Algrain, H.A.; AlKhelaif, R.A.; AlEshaiwi, S.M. Evaluation of the Educational Value of YouTube Videos about Physical Examination of the Cardiovascular and Respiratory Systems. J. Med. Internet Res. 2013, 15, e241. [Google Scholar] [CrossRef] [PubMed]
- Bobos, P.; Nazari, G.; Lu, Z.; MacDermid, J.C. Measurement Properties of the Hand Grip Strength Assessment: A Systematic Review with Meta-Analysis. Arch. Phys. Med. Rehabil. 2020, 101, 553–565. [Google Scholar] [CrossRef]
- Nagata, K.; Yoshimura, N.; Hashizume, H.; Yamada, H.; Ishimoto, Y.; Muraki, S.; Nakagawa, Y.; Minamide, A.; Oka, H.; Kawaguchi, H.; et al. Physical Performance Decreases in the Early Stage of Cervical Myelopathy before the Myelopathic Signs Appear: The Wakayama Spine Study. Eur. Spine J. 2019, 28, 1217–1224. [Google Scholar] [CrossRef]
- Lees, F.; Turner, J.W. Natural history and prognosis of cervical spondylosis. Br. Med. J. 1963, 2, 1607–1610. [Google Scholar] [CrossRef]
- Fujimori, T.; Le, H.; Hu, S.S.; Chin, C.; Pekmezci, M.; Schairer, W.; Tay, B.K.; Hamasaki, T.; Yoshikawa, H.; Iwasaki, M. Ossification of the Posterior Longitudinal Ligament of the Cervical Spine in 3161 Patients: A CT-Based Study. Spine 2015, 40, E394–E403. [Google Scholar] [CrossRef]
- Murone, I. The Importance of the Sagittal Diameters of the Cervical Spinal Canal in Relation to Spondylosis and Myelopathy. J. Bone Joint Surg. Br. 1974, 56, 30–36. [Google Scholar]
- Liao, K.-H. Hand Grip Strength in Low, Medium, and High Body Mass Index Males and Females. Middle East J. Rehabil. Health 2016, 3, e53229. [Google Scholar] [CrossRef] [Green Version]
| (a) | ||||||
| Age (y) | n | GRT (Times) | p-Value | |||
| CTRL | DCM | CTRL | DCM | |||
| Male | 40–59 | 82 | 62 | 27.0 ± 5.6 | 18.5 ± 8.2 | <0.0001 |
| 60–69 | 62 | 48 | 23.5 ± 4.7 | 13.9 ± 6.0 | <0.0001 | |
| 70–79 | 97 | 43 | 20.2 ± 5.2 | 15.0 ± 5.4 | <0.0001 | |
| 80–89 | 34 | 12 | 20.1 ± 5.0 | 11.4 ± 4.7 | <0.0001 | |
| Female | 40–49 | 119 | 22 | 23.5 ± 4.8 | 16.6 ± 5.3 | <0.0001 |
| 60–69 | 132 | 18 | 20.7 ± 4.7 | 12.5 ± 4.8 | <0.0001 | |
| 70–79 | 154 | 27 | 19.2 ± 4.8 | 12.2 ± 3.9 | <0.0001 | |
| 80–89 | 41 | 15 | 17.8 ± 4.3 | 9.5 ± 3.8 | <0.0001 | |
| (b) | ||||||
| Age (y) | n | Grip Strength (kg) | p-Value | |||
| CTRL | DCM | CTRL | DCM | |||
| Male | 40–59 | 82 | 62 | 42.6 ± 7.7 | 23.3 ± 12.1 | <0.0001 |
| 60–69 | 62 | 48 | 35.5 ± 7.8 | 16.5 ± 9.1 | <0.0001 | |
| 70–79 | 97 | 43 | 30.1 ± 7.5 | 16.5 ± 8.0 | <0.0001 | |
| 80–89 | 34 | 12 | 26.0 ± 5.7 | 10.8 ± 6.6 | <0.0001 | |
| Female | 40–49 | 119 | 22 | 25.6 ± 6.8 | 14.5 ± 7.4 | <0.0001 |
| 60–69 | 132 | 18 | 20.3 ± 4.6 | 10.0 ± 6.2 | <0.0001 | |
| 70–79 | 154 | 27 | 17.6 ± 4.8 | 8.5 ± 4.9 | <0.0001 | |
| 80–89 | 41 | 15 | 15.2 ± 3.8 | 4.4 ± 4.0 | <0.0001 | |
| Sex | AUC | Cutoff by Youden Index (Times) | Sensitivity | Specificity | LR (+) | LR (−) | |
| Male | 40–59 | 0.82 | 21 | 0.68 | 0.85 | 4.53 | 0.38 |
| 60–69 | 0.91 | 17 | 0.79 | 0.90 | 7.90 | 0.23 | |
| 70–79 | 0.75 | 15 | 0.58 | 0.85 | 3.87 | 0.49 | |
| 80–89 | 0.90 | 11 | 0.67 | 1.00 | #DIV/0! † | 0.33 | |
| Female | 40–49 | 0.84 | 18 | 0.73 | 0.89 | 6.64 | 0.30 |
| 60–69 | 0.91 | 17 | 0.94 | 0.64 | 2.61 | 0.09 | |
| 70–79 | 0.87 | 15 | 0.78 | 0.75 | 3.12 | 0.29 | |
| 80–89 | 0.92 | 12 | 0.87 | 0.93 | 12.43 | 0.14 | |
| Total | 0.73 | 0.83 | 4.31 | 0.33 | |||
| (a) | |||||||
| Sex | AUC | Cutoff by Youden Index (kg) | Sensitivity | Specificity | LR (+) | LR (−) | |
| Male | 40–59 | 0.92 | 32 | 0.68 | 0.85 | 11.57 | 0.20 |
| 60–69 | 0.94 | 29 | 0.96 | 0.81 | 5.05 | 0.05 | |
| 70–79 | 0.89 | 21 | 0.70 | 0.91 | 7.78 | 0.33 | |
| 80–89 | 0.97 | 19 | 0.83 | 0.94 | 13.83 | 0.18 | |
| Female | 40–49 | 0.87 | 20 | 0.73 | 0.82 | 4.06 | 0.33 |
| 60–69 | 0.91 | 13 | 0.78 | 0.96 | 19.50 | 0.23 | |
| 70–79 | 0.91 | 15 | 0.96 | 0.71 | 3.31 | 0.06 | |
| 80–89 | 0.97 | 10 | 0.93 | 0.90 | 9.30 | 0.08 | |
| Total | 0.83 | 0.86 | 5.78 | 0.19 | |||
| (b) | |||||||
| Cutoff | |||
|---|---|---|---|
| Sex | GRT (Times) | Grip Strength (kg) | |
| Male | 40–59 | 21 | 32 |
| 60–69 | 17 | 29 | |
| 70–79 | 15 | 21 | |
| 80–89 | 11 | 19 | |
| Female | 40–49 | 18 | 20 |
| 60–69 | 17 | 13 | |
| 70–79 | 15 | 15 | |
| 80–89 | 12 | 10 | |
| Sex | Age Group (y) | Sensitivity | Specificity | LR (+) | LR (−) |
|---|---|---|---|---|---|
| Male | 40–59 | 0.90 | 0.82 | 4.94 | 0.12 |
| 60–69 | 0.96 | 0.74 | 3.71 | 0.06 | |
| 70–79 | 0.79 | 0.78 | 3.65 | 0.27 | |
| 80–89 | 0.92 | 0.94 | 15.58 | 0.09 | |
| Female | 40–59 | 0.91 | 0.77 | 4.01 | 0.12 |
| 60–69 | 0.94 | 0.70 | 3.20 | 0.08 | |
| 70–79 | 1.00 | 0.56 | 2.26 | 0.00 | |
| 80–89 | 0.93 | 0.83 | 5.47 | 0.08 | |
| Total | 0.91 | 0.73 | 3.37 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, H.; Otani, K.; Nikaido, T.; Watanabe, K.; Kato, K.; Kobayashi, Y.; Yabuki, S.; Konno, S.-i. Development of a Novel Diagnostic Support Tool for Degenerative Cervical Myelopathy Combining 10-s Grip and Release Test and Grip Strength: A Pilot Study. Diagnostics 2022, 12, 2108. https://doi.org/10.3390/diagnostics12092108
Kobayashi H, Otani K, Nikaido T, Watanabe K, Kato K, Kobayashi Y, Yabuki S, Konno S-i. Development of a Novel Diagnostic Support Tool for Degenerative Cervical Myelopathy Combining 10-s Grip and Release Test and Grip Strength: A Pilot Study. Diagnostics. 2022; 12(9):2108. https://doi.org/10.3390/diagnostics12092108
Chicago/Turabian StyleKobayashi, Hiroshi, Koji Otani, Takuya Nikaido, Kazuyuki Watanabe, Kinshi Kato, Yoshihiro Kobayashi, Shoji Yabuki, and Shin-ichi Konno. 2022. "Development of a Novel Diagnostic Support Tool for Degenerative Cervical Myelopathy Combining 10-s Grip and Release Test and Grip Strength: A Pilot Study" Diagnostics 12, no. 9: 2108. https://doi.org/10.3390/diagnostics12092108
APA StyleKobayashi, H., Otani, K., Nikaido, T., Watanabe, K., Kato, K., Kobayashi, Y., Yabuki, S., & Konno, S.-i. (2022). Development of a Novel Diagnostic Support Tool for Degenerative Cervical Myelopathy Combining 10-s Grip and Release Test and Grip Strength: A Pilot Study. Diagnostics, 12(9), 2108. https://doi.org/10.3390/diagnostics12092108






