Abstract
Infection with high-risk (HR) Human Papillomavirus (HPV) is associated with the development of precancerous lesions or invasive carcinoma of the uterine cervix. Thus, the high viral load (VL) of HR-HPV DNA currently serves as a representative quantitative marker for cervical cancer. However, the clinical significance of low HPV DNA VL remains undetermined. This study aimed to evaluate the clinical association between the low HPV DNA VL and cytology/histologic diagnosis of cervical samples. We searched the electronic medical databases for the resultant analyses of HPV genotyping among patients who underwent treatment for any cervical lesion or who had undergone gynecological examinations with any positive HPV results according to the national cancer screening service between 2015 and 2016. HPV testing with genotyping and semi-quantitative VL measurement was conducted using an AnyplexTM II H28 Detection assay (H28 assay, Seegene, Seoul, Republic of Korea). The H28 assay is a multiplex semi-quantitative real-time PCR test using the tagging of oligonucleotide cleavage and extension (TOCE) technology. The VL was semi-quantified as high (3+; positive signal before 31 PCR cycles), intermediate (2+; positive between 31 and 39 PCR cycles), or low (1+; positive after 40 PCR cycles). Out of 5940 HPV VL analyses, 356 assays (5.99%) were reported as low VL (1+) of HPV DNA. Matched cytology diagnoses were mostly negative findings (n = 347, 97.5%), except for seven cases of atypical squamous cells of undetermined significance (1.9%) and two cases of atypical glandular cells (0.6%). During the follow-up periods, abnormal cytologic diagnoses were identified, including one case of high-grade squamous intraepithelial lesion (HSIL) and two low-grade squamous intraepithelial lesions (LSILs). The matched, confirmative histologic diagnosis of HSIL cytology was compatible with chronic inflammation, wherein the two LSILs had regular check-ups. None revealed clinically concerned outcomes associated with HPV-related squamous lesions. The cytology was most likely negative for malignancy when the VL of HPV DNA was low (1+). Additional strategic monitoring and management may thus be unnecessary.
1. Introduction
Worldwide, cervical cancer is the fourth most common cancer and the fourth leading cause of cancer death in women, with an estimated 604,000 new cases and 342,000 deaths in 2020 [1]. Cervical cancer is by far the most common Human Papillomavirus (HPV)-related disease; nearly all cases of cervical cancer (more than 95%) can be attributed to HPV infection.
More than 200 different HPV types have been listed by the International HPV Reference Center (www.hpvcenter.se; accessed on 20 May 2022), and this number continues to expand [2]. HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59, referred to as high-risk (HR) HPV types, have been classified as carcinogenic (IARC Group 1), and other HPV types, HPV26, 53, 66, 67, 68, 70, 73, and 82, have been classified as probably or possibly carcinogenic (IARC Groups 2A and 2B) [3,4]. HR-HPV types are the etiological agents of several cancers, such as those of the cervix, vagina, vulva, anus, penis, and oropharyngeal cancer [5]. HPV6 and 11, represented as LR-HPV types, induce benign genital warts or condyloma acuminata [6].
Notably, most cervical cancer (more than 90%) is due to HR-HPV [1]. Integration of HPV into the host genome is reported in a majority of cervical cancers (83%) [7]. HPV16 can be present as an episomal or integrated form, or both [8], whereas HPV18 has been found integrated into almost all cervical cancers [9]. HR-HPV types, i.e., HPV16 and 18, showed that both oncoproteins E6 and E7 play a key role in cervical cancer by altering pathways involved in the host immune response to establish persistent infection and promote cellular transformation [9].
As an effective screening test for cervical cancer, cervical cytology has so far played a key role in reducing the incidence of cervical cancer in countries with organized screening [10,11,12,13,14]; however, it has limitations in terms of test sensitivity, specificity, encouraging participation in the screening program, etc. In particular, the HPV DNA test has further contributed to a reduction in the incidence of cervical cancer [4,15], which highlights its importance for primary screening and management of cervical cancer [16,17].
To date, the viral load (VL) of HPV has been proposed as a potent discriminator of significance from insignificant HPV infections [18,19]. Several research studies have shown high VL of HPV to be an alternate indicator for persistent HPV infection and a significant predictor of the risk of squamous intraepithelial lesions (SILs) [20,21]; however, debates remain on this issue. Other studies using quantitative real-time polymerase chain reaction (qRT-PCR) targeting HPV16 (E6 and L1 genes) have shown mixed results with respect to the association between mean viral burden and grade of SIL [22,23]. The opposite results have also been reported, which show no relevance between the incidence of cervical lesions and HPV VL [24,25].
The VL of HPV can be measured by various methods, including semi-quantitative (e.g., Hybrid Capture 2) or quantitative polymerase chain reaction (PCR) [26,27]. Most recently, a droplet digital PCR (ddPCR) method has been developed to detect and quantify HPV DNA from various HPV types simultaneously. Rotondo et al. showed that the ddPCR method had high sensitivity, accuracy, specificity, and reproducibility in quantifying HPV DNA sequences [28].
In recent practice, a qRT-PCR method is commonly used to estimate the amount of HPV DNA. The AnyplexTM II HPV28 Detection assay (H28 assay, Seegene, Seoul, Republic of Korea) is a multiplex qRT-PCR test using the tagging of oligonucleotide cleavage and extension (TOCE) technology for simultaneous detection and genotyping of 19 high-risk (HR) HPV types (HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 69, 73, and 82), and 9 low-risk (LR) HPV types (HPV6, 11, 40, 42, 43, 44, 54, 61, and 70) in a single reaction (http://www.seegene.com/assays; accessed on 20 May 2022). The H28 assay also estimates the VL through a repeated melting temperature analysis during the TOCE reaction and reports a semi-quantitative system as low (1+; positive for 40 PCR cycles), intermediate (2+; positive within 31–39 cycles), or high (3+; positive before 31 cycles) [29].
Meanwhile, unlike the high VL of HPV, there has been little research on the clinicopathologic significance of low (+) VL of HPV based on H28 assay in cervical lesions. It is not surprising that the clinical association between low HPV VL and cytology/histologic diagnosis of cervical samples remains undetermined, mainly because of the over-sensitivity and inconsistent reproducibility of the test. Such a little-known characteristic may impede gynecologic physicians’ and patients’ best shared decision making for therapeutic plans and consequently bring about unexpected problems, such as excessive levels of patient-perceived anxiety or unnecessary waste of time and money. Therefore, a clear association between low HPV VL and cytology/histologic diagnosis of cervical samples using H28 assay needs to be investigated thoroughly.
In this study, we aimed to evaluate the clinicopathologic significance of an HPV infection with a low (1+) viral DNA load on cervical/vaginal cytology samples obtained from patients with a clinical follow-up due to previous cervical lesions, such as SIL or invasive cervical carcinoma, and from patients who have shown a positive result for HPV in an earlier HPV examination.
2. Materials and Methods
2.1. Case Selection
We searched the electronic medical database of the Department of Pathology of Kyungpook National University Chilgok Hospital in the Republic of Korea for HPV genotyping analyses among patients with any previous cervical lesions or who had undergone gynecological examinations according to the National Cancer Screening Services, between January 2015 and December 2016 at Kyungpook National University Chilgok Hospital.
A total of 5940 HPV analysis results were obtained. Each of the matched clinical characteristics included age and HPV status at the initial cytology/histopathologic diagnosis plus the follow-up information for the subsequent cytology/histopathologic diagnosis and HPV status. A flowchart shows the sample selection and analysis process in this study (Figure 1).
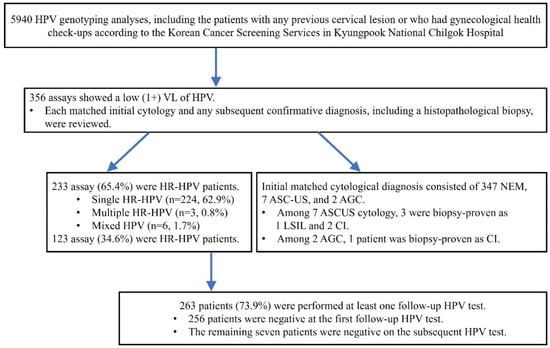
Figure 1.
Flowchart of the sample selection and analysis process in this study.
2.2. AnyplexTM II HPV H28 Detection Assay
Briefly, liquid-based cytology (LBC) samples were collected using broom-like devices with detachable heads (BD SurePathTM collection vial, Becton, Dickinson and Company, BD Life Sciences-Integrated Diagnostic Solutions, Sparks, MD, USA) according to the manufacturer’s instructions. The cervical broom was placed into the cervical canal and was rotated 360 degrees around the entire cervical canal. The detachable head was placed in a vial with preservative fluid. The LBC sample collection and transient storage temperature followed the manufacturer’s instructions.
Subsequently, the HPV test with Anyplex HPV28 assay using LBC specimens was performed according to the manufacturers’ instructions. DNA from each LBC sample was extracted using STARMag 96 X4 Universal Cartridge Kit (Seegene, Seoul, Korea). HPV genotyping was conducted using a multiplex polymerase chain reaction (PCR) using a CFX96 PCR Thermal Cycler (Bio-Rad, Hercules, CA, USA), according to manufacturers’ guidelines, using 5 μL of template DNA in a total volume of 20 μL for Anyplex™ II HPV28.
At the initial examination and at least once during a follow-up visit, HPV testing with genotyping and semi-quantitative VL measurement were conducted according to the manufacturer’s instructions using the AnyplexTM II H28 Detection kit (Cat No. HP7S00X, Seegene, Seoul, Korea).
2.3. Statistical Analyses
We performed a double-blind test for the cytology/histopathology diagnosis and the HPV status using the AnyplexTM II H28 assay. Descriptive statistical analysis using SPSS Statistics software for Windows, version 21.0 (IBM Corporation, Armonk, NY, USA) was only conducted for the demographic data, including age and follow-up.
3. Results
At a baseline examination for the cytology and HPV typing, the mean age was 48.1 (±11.5, standard deviation) years old. The mean follow-up period was 58.3 ± 12.2 months.
Out of 5940 HPV analyses, 356 assays (5.99%) were reported as a low (1+) VL of HPV. Of 356 cases with low (1+) VL of HPV, 150 patients had a previous confirmative diagnosis of HPV-related epithelial lesion. The results are summarized in Table 1.

Table 1.
Previous HPV-related lesions of the cases with low (1+) VL of HPV.
From the HPV test at the starting time (baseline) of this study, 233 (65.4%) out of the 356 cases were HR-HPV genotypes (Table 2). However, the baseline cytology showed mostly negative findings (n = 347, 97.5%), seven atypical squamous cells of undetermined significance (ASC-US) (1.9%), and two atypical glandular cells (AGC) (0.6%) (Table 3). At that time, three ASC-US cases were determined by a punch biopsy: one case of low-grade squamous epithelial lesion (LSIL) and the other two cases of chronic inflammation. One of the AGC cases was also diagnosed as chronic inflammation on the punch biopsy. Detailed clinical characteristics of abnormal baseline cytology are shown in Table 4.

Table 2.
Baseline HPV types in patients with low (1+) VL of HPV.

Table 3.
Baseline cytological diagnosis in patients with low (1+) VL of HPV.

Table 4.
Clinical characteristics of abnormal baseline cytology.
During the follow-up periods, 263 (73.9%) of 356 patients with a low VL of HPV DNA underwent at least one repeated HPV test. Almost all (97.3%) showed a negative HPV result at the initial repeated test, while the remaining patients (n = 7) were finally negative for HPV at the subsequent tests (Figure 2). Furthermore, three patients were identified to have abnormal cytology: one high-grade squamous epithelial lesion (HSIL) and two LSILs. The patient with HSIL cytology was confirmed as chronic inflammation, not evident for HSIL, with no immunoreactivity for P16 immunohistochemistry on the punch biopsy. Two patients who reported LSIL cytology were regularly followed. Each of the baseline HPV types was HPV53 (HR) for HSIL cytology, and HPV59 (HR) and HPV54 (LR) for LSIL cytology. None revealed medically concerned outcomes associated with HPV-related epithelial lesions during the follow-up periods.
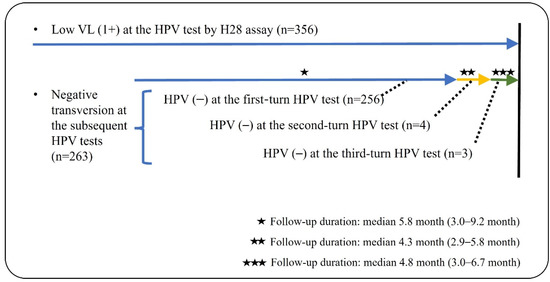
Figure 2.
Illustration for clinical follow-ups of low VL (1+) HPV test in this study.
4. Discussion
HR-HPV genomic integrations into the host genome are associated with a persistent infection [30]. Either acquired genetic or epigenetic alteration based on the viral genome integration could lead to carcinogenesis in the uterine cervix [30,31,32,33]. Therefore, HPV-related premalignant or malignant lesions contain integrated HPV DNA [34,35], extrachromosomal viral DNA [36], or both [37]; for instance, a study analysis from The Cancer Genome Atlas showed that HPV integration occurred in >80% of HPV-related cervical cancers [38].
The recurrence risk of SIL or invasive carcinoma remains higher among women who undergo treatment for primary HPV-related epithelial lesions. For that reason, such patients need to be put under close surveillance and follow-up examination [39]. Given that between 5% and 20% of cases develop a recurrence within three years and have a more than fivefold increased risk of invasive carcinoma than that of the general population [40,41,42,43], it is essential for those patients to have a continuous cervical/vaginal cytology and HPV testing (with genotyping) in the follow-up periods [44]. However, it is worthwhile to note that most of the previous research for the VL of HPV has focused on the significance of a high load of HPV. As a result, the evidence that a high load of HPV is associated with the persistence of HPV DNA [45] and that DNA persistence is a potent indicator of disease clearance or recurrence [46] seems to have more than enough support. Notably, HPV DNA load decreased with time during the follow-up periods, and HR-HPV DNA was significantly reduced from 90% to 20% at six months after treatment [40,47]. All of these suggest that a quantitative analysis for HPV VL could be worthwhile to monitor for treatment effectiveness and disease recurrence.
The AnyplexTM II HPV28 assay is a multiplex semi-quantitative real-time PCR assay using TOCE technology. The viral load is semi-quantified as high (3+; positive signal before 31 PCR cycles), intermediate (2+; positive between 31 and 39 PCR cycles), or low (1+; positive after 40 PCR cycles) [48]. TOCE technology consistently produces the predicted melting temperature profile in singleplex and multiplex assays and distinguishes multiple targets in a single channel in a homogeneous real-time PCR reaction [49]. The sensitivity of TOCE is reported to be comparable to a standard hydrolysis probe reaction, with a limit of detection of one copy/reaction [50]. The assay also detects the internal-control target gene (human β-globin gene) to ensure the quality of prepared nucleic acids [50].
The H28 assay has been validated in recent HPV-related studies. Cornall et al. showed that both AnyplexTM II HPV28 and AnyplexTM II HPV HR assays had superior performances with higher sensitivity than HC2 (p < 0.0001) and were concordant with other commercial assays for HR-HPV detection despite presented semi-quantitative results [51]. Furthermore, although the study design was not identical, Lee et al. evaluated performances of the AnyplexTM II HR assay, sharing the same TOCE technology as the AnyplexTM II HPV28 assay, compared to that of Cobas 4800 HPV and HC2. They assessed the precision of the assay by comparing the positive control results from 15 replicates using clinical specimens with low (quantitation cycle, Cq < 37), intermediate (Cq < 32.2), and high (Cq < 26.7) concentrations of HPV DNA in triplicate per run a day on five different days [50]. The precision tests showed 100% detection of three different levels (low, medium, and high concentration) of pooled clinical specimens using the AnyplexTM II HR assay and the Cobas 4800 [50]. Therefore, we could assume that AnyplexTM II tests, including the H28 assay, would be helpful in follow-up testing and patient management by providing genotyping information beyond HPV 16 and HPV 18 positivity [50].
However, few studies have been conducted on the significance of low VL of HPV. Its value has been virtually ignored due to the difficulty in accurate quantification of low VL and (in some cases) the possibility of contamination by sexual partners or another viral status, which may lead to a false-positive reaction of viral DNA for a short term [45]. Such a dearth of reasonable evidence has made it difficult for either physicians or patients to decide or discuss the best practices and strategies for patient-centered management of cervical lesions when confronted with a medical report indicating low VL of HPV. A partial understanding of VL consequences may consequently lead to wasteful use of limited healthcare resources and accordingly impede patient-centered, value-based care delivery [52].
We initiated this study to contribute to medical science by addressing the clinical relevance between the low VL of HPV and the risk of cervical lesions. In this study, none of the patients who reported as low (1+) VL of HPV revealed clinically concerned outcomes associated with HPV-related epithelial lesions during follow-up. Therefore, in accordance with what the study found, we carefully propose that clinicians and patients would have a better shared decision making with simply the monitoring and management of HPV VL, instead of unnecessary additional tests or procedures, when the low (1+) VL of HPV status is detected in an H28 assay.
In particular, there are two further significant implications obtained from this study. Although cervical cancer can develop as long as HPV infection persists, a quantitative contribution to cervical carcinogenesis remains unclear regarding the duration and viral load of HPV infection. Fortunately, our results could provide a practical reference for the clinical treatment of patients. Those with a low VL of HPV should be managed more conservatively and safely or in a step-by-step manner considering familial and social environmental factors, such as pregnancy, rather than immediate management.
The other is that, even though the LR-HPVs are not subject to screening and follow-up for cervical cancer, it is impossible to know whether the viral subtype is from an LR- or HR-HPV group before performing the HPV subtyping test. Moreover, one should be aware of the possibility of cross-reaction or multiple HPV infections. This study demonstrated the clinical significance of the low VL of HPV in practical management. In other words, the cases with a lower VL of LR-HPV, as expected, did not have any precancerous or cancerous lesions during the follow-up; furthermore, in cases of HR-HPV groups, they did show identical findings. Therefore, we proposed that the low VL, regardless of LR- and HR-HPV subtyping, can emphasize the significance of conservative strategies for screening or follow-up of cervical cancer.
It is also obvious that quality control of the laboratory performance is very important for either the primary screening test or a strategic assessment of the post-treatment testing utility for HPV infection. The laboratory performance should adhere to a well-established protocol of intra-laboratory quality assurance and be kept under close review, thereby ensuring a reliable data processing system within the institution. Once an individual laboratory is accredited for HPV molecular testing, intra-institutional strategic guidelines may also be proposed, which ultimately contribute to a standardized decision on “what to do” for the best follow-up care.
This study had several inherent limitations regarding small sample size, sample type, and analytic methods. In detail, first, by selecting patients with HPV infection from one tertiary medical institute in the Republic of Korea, we might have biased our cohorts towards patients with HPV DNA persistence, thus undermining generalization. Second, we only assessed the HPV DNA load using a semi-quantitative measurement by a repeated melting temperature analysis of the HPV28 assay, which may damage validity to some extent. Further studies should propose a clinical cut-off for the viral load for the reliability of HPV testing. Third, the method of HPV28 detection may present inflated positive results due to its excessive sensitivity for the primary HPV screening test. By extension, there remain the possibilities of transient HPV infection or contamination, requiring further efforts to refine the sensitivity of the measurement and increase reliability in future studies. These limitations support a need to verify the study finding through large data obtained from rigorous replication of research in multi-institute settings and the general population. However, we believe that our results outweigh the limitations in that it is the first study to provide at least a minimum of medical evidence on the possibility of strategic utilization of low VL of HPV for monitoring cervical lesions.
5. Conclusions
This study showed that none of the patients with a low VL of HPV DNA revealed clinically concerned outcomes associated with HPV-related squamous lesions, regardless of HR- or LR-HPV genotypes. Therefore, we could provide a practical guideline for the patient with a low VL of HPV DNA. When referring to the strategic monitoring and management of patients who undergo treatment for previous HPV-related epithelial lesions and patients who had an HR-HPV detection earlier, those with a low VL of HPV using the AnyplexTM II H28 assay should be managed in a conservative and safe or step-by-step manner considering familial and social environmental factors, such as pregnancy, rather than immediate management. In these particular situations, the accuracy and confidence of laboratory HPV testing need to be consistently guaranteed.
Author Contributions
N.J.-Y.P.: data curation, formal analysis, investigation, methodology, project administration, resources, writing (original draft); M.K.: investigation, methodology, validation; J.Y.J.: investigation, methodology, validation; S.H.Y.: writing (review and editing); G.O.C.: data curation, formal analysis, supervision; D.G.H.: data curation, formal analysis, supervision; C.S.-Y.P.: validation, writing (review and editing); J.Y.P.: conceptualization, funding acquisition, supervision, writing (review and editing). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Biomedical Research Institute grant, Kyungpook National University Hospital (Young investigator-15-25).
Institutional Review Board Statement
The study protocol was approved by the Institutional Review Board of the Kyungpook National University Chilgok Hospital with a reference number of KNUCH 2019–04-002-001. All methods were carried out in accordance with the rules of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical issue.
Acknowledgments
The authors sincerely thank Ji Eun Kim for the robust molecular work.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
AGC: Atypical glandular cells; ASC-US, Atypical squamous cells of undetermined significance; Bx, Biopsy; CI, Chronic inflammation; CIN, Cervical intraepithelial lesion; HPV, Human Papillomavirus; HR, High-risk; HSIL, High-grade squamous intraepithelial lesion; LR, Low-risk; LSIL, Low-grade squamous intraepithelial lesions; PCR, Polymerase chain reaction; qRT-PCR, Quantitative real-time polymerase chain reaction; TOCE, Tagging of oligonucleotide cleavage and extension; VAIN, vaginal intraepithelial lesion; VL, viral load.
References
- WHO Classification of Tumours Editorial Board. Female Genital Tumours. In WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2020; Volume 4. [Google Scholar]
- Brancaccio, R.N.; Robitaille, A.; Dutta, S.; Cuenin, C.; Santare, D.; Skenders, G.; Gheit, T. Generation of a novel next-generation sequencing-based method for the isolation of new human papillomavirus types. Virology 2018, 520, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Halec, G.; Schmitt, M.; Dondog, B.; Sharkhuu, E.; Wentzensen, N.; Gheit, T.; Pawlita, M. Biological activity of probable/possible high-risk human papillomavirus types in cervical cancer. Int. J. Cancer 2013, 132, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Cogliano, V. A review of human carcinogens–Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Haedicke, J.; Iftner, T. Human papillomaviruses and cancer. Radiother. Oncol. 2013, 108, 397–402. [Google Scholar] [CrossRef]
- Ball, S.L.; Winder, D.M.; Vaughan, K.; Hanna, N.; Levy, J.; Sterling, J.C.; Goon, P.K. Analyses of human papillomavirus genotypes and viral loads in anogenital warts. J. Med. Virol. 2011, 83, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Liu, J.; Hu, Y.; Lu, X.; Li, B.; Li, Y.; Cheng, X. Viral E6 is overexpressed via high viral load in invasive cervical cancer with episomal HPV16. BMC Cancer 2017, 17, 136. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Arbyn, M.; Benoy, I.; Simoens, C.; Bogers, J.; Beutels, P.; Depuydt, C. Prevaccination Distribution of Human Papillomavirus Types in Women Attending at Cervical Cancer Screening in Belgium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 321–330. [Google Scholar] [CrossRef]
- Arbyn, M.; Raifu, A.O.; Weiderpass, E.; Bray, F.; Anttila, A. Trends of Cervical Cancer Mortality in the Member States of the European Union. Eur. J. Cancer 2009, 45, 2640–2648. [Google Scholar] [CrossRef]
- Arbyn, M.; Sasieni, P.; Meijer, C.J.; Clavel, C.; Koliopoulos, G.; Dillner, J. Clinical Applications of Hpv Testing: A Summary of Meta-Analyses. Vaccine 2006, 24 (Suppl. 3), 78–79. [Google Scholar] [CrossRef] [PubMed]
- Badaracco, G.; Savarese, A.; Micheli, A.; Rizzo, C.; Paolini, F.; Carosi, M.; Venuti, A. Persistence of Hpv after Radio-Chemotherapy in Locally Advanced Cervical Cancer. Oncol. Rep. 2010, 23, 1093–1099. [Google Scholar] [PubMed]
- Berkhof, J.; Coupe, V.M.; Bogaards, J.A.; van Kemenade, F.J.; Helmerhorst, T.J.; Snijders, P.J.; Meijer, C.J. The Health and Economic Effects of Hpv DNA Screening in the Netherlands. Int. J. Cancer 2010, 127, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Sengupta, S. Cpg Methylation of Hpv 16 Lcr at E2 Binding Site Proximal to P97 Is Associated with Cervical Cancer in Presence of Intact E2. Virology 2006, 354, 280–285. [Google Scholar] [CrossRef]
- Bray, F.; Loos, A.H.; McCarron, P.; Weiderpass, E.; Arbyn, M.; Moller, H.M.; Parkin, D.M. Trends in Cervical Squamous Cell Carcinoma Incidence in 13 European Countries: Changing Risk and the Effects of Screening. Cancer Epidemiol. Biomark. Prev. 2005, 14, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Chaiwongkot, A.; Vinokurova, S.; Pientong, C.; Ekalaksananan, T.; Kongyingyoes, B.; Kleebkaow, P.; von Knebel Doeberitz, M. Differential Methylation of E2 Binding Sites in Episomal and Integrated Hpv 16 Genomes in Preinvasive and Invasive Cervical Lesions. Int. J. Cancer 2013, 132, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; De Simone, P.; Venturoli, S.; Cricca, M.; Zerbini, M.L.; Musiani, M.; Syrjänen, K. Factors Predicting Human Papillomavirus Clearance in Cervical Intraepithelial Neoplasia Lesions Treated by Conization. Gynecol. Oncol. 2003, 90, 358–365. [Google Scholar] [CrossRef]
- Costa, S.; Venturoli, S.; Origoni, M.; Preti, M.; Mariani, L.; Cristoforoni, P.; Sandri, M.T. Performance of Hpv DNA Testing in the Follow-up after Treatment of High-Grade Cervical Lesions, Adenocarcinoma in Situ (Ais) and Microinvasive Carcinoma. Ecancermedicalscience 2015, 9, 528. [Google Scholar]
- Cheung, J.L.; Lo, K.W.; Cheung, T.H.; Tang, J.W.; Chan, P.K. Viral Load, E2 Gene Disruption Status, and Lineage of Human Papillomavirus Type 16 Infection in Cervical Neoplasia. J. Infect. Dis. 2006, 194, 1706–1712. [Google Scholar] [CrossRef]
- Cuzick, J.; Clavel, C.; Petry, K.U.; Meijer, C.J.; Hoyer, H.; Ratnam, S.; Iftner, T. Overview of the European and North American Studies on Hpv Testing in Primary Cervical Cancer Screening. Int. J. Cancer 2006, 119, 1095–1101. [Google Scholar] [CrossRef]
- Dong, X.P.; Stubenrauch, F.; Beyer-Finkler, E.; Pfister, H. Prevalence of Deletions of Yy1-Binding Sites in Episomal Hpv 16 DNA from Cervical Cancers. Int. J. Cancer 1994, 58, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Papenfuss, M.; Klimecki, W.T.; Giuliano, A.R. Cross-Sectional Analysis of Oncogenic Hpv Viral Load and Cervical Intraepithelial Neoplasia. Int. J. Cancer 2006, 118, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.; Gravitt, P.; Duh, L.M.; Lefevre, J.; Pourreaux, K.; Hankins, C.; Canadian Women’s HIV Study Group. High Level of Correlation of Human Papillomavirus-16 DNA Viral Load Estimates Generated by Three Real-Time Pcr Assays Applied on Genital Specimens. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2200–2207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gervais, F.; Dunton, K.; Jiang, Y.; Largeron, N. Systematic Review of Cost-Effectiveness Analyses for Combinations of Prevention Strategies against Human Papillomavirus (Hpv) Infection: A General Trend. BMC Public Health 2017, 17, 283. [Google Scholar] [CrossRef]
- Gravitt, P.E.; Kovacic, M.B.; Herrero, R.; Schiffman, M.; Bratti, C.; Hildesheim, A.; Burk, R.D. High Load for Most High Risk Human Papillomavirus Genotypes Is Associated with Prevalent Cervical Cancer Precursors but Only Hpv16 Load Predicts the Development of Incident Disease. Int. J. Cancer 2007, 121, 2787–2793. [Google Scholar] [CrossRef]
- Hesselink, A.T.; Berkhof, J.; Heideman, D.A.; Bulkmans, N.W.; van Tellingen, J.E.; Meijer, C.J.; Snijders, P.J. High-Risk Human Papillomavirus DNA Load in a Population-Based Cervical Screening Cohort in Relation to the Detection of High-Grade Cervical Intraepithelial Neoplasia and Cervical Cancer. Int. J. Cancer 2009, 124, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Oton-Gonzalez, L.; Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Tognon, M.; Martini, F. Simultaneous Detection and Viral DNA Load Quantification of Different Human Papillomavirus Types in Clinical Specimens by the High Analytical Droplet Digital PCR Method. Front. Microbiol. 2020, 11, 591452. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, A.T.; Sahli, R.; Berkhof, J.; Snijders, P.J.; van der Salm, M.L.; Agard, D.; Heideman, D.A.M. Clinical Validation of Anyplex Ii Hpv Hr Detection According to the Guidelines for Hpv Test Requirements for Cervical Cancer Screening. J. Clin. Virol. 2016, 76, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.T. Cyclic-Cmta: An Innovative Concept in Multiplex Quantification. Seegene Bull. 2012, 1, 11–15. [Google Scholar]
- Jordan, J.; Martin-Hirsch, P.; Arbyn, M.; Schenck, U.; Baldauf, J.J.; Da Silva, D.; Prendiville, W. European Guidelines for Clinical Management of Abnormal Cervical Cytology, Part 2. Cytopathology 2009, 20, 5–16. [Google Scholar] [CrossRef]
- Karim-Kos, H.E.; de Vries, E.; Soerjomataram, I.; Lemmens, V.; Siesling, S.; Coebergh, J.W. Recent Trends of Cancer in Europe: A Combined Approach of Incidence, Survival and Mortality for 17 Cancer Sites since the 1990s. Eur. J. Cancer 2008, 44, 1345–1389. [Google Scholar] [CrossRef] [PubMed]
- Kocken, M.; Helmerhorst, T.J.; Berkhof, J.; Louwers, J.A.; Nobbenhuis, M.A.; Bais, A.H.; Meijer, C.J. Risk of Recurrent High-Grade Cervical Intraepithelial Neoplasia after Successful Treatment: A Long-Term Multi-Cohort Study. Lancet Oncol. 2011, 12, 441–450. [Google Scholar] [CrossRef]
- Kristiansen, E.; Jenkins, A.; Holm, R. Coexistence of Episomal and Integrated Hpv16 DNA in Squamous Cell Carcinoma of the Cervix. J. Clin. Pathol. 1994, 47, 253–256. [Google Scholar] [CrossRef]
- Marongiu, L.; Godi, A.; Parry, J.V.; Beddows, S. Human Papillomavirus 16, 18, 31 and 45 Viral Load, Integration and Methylation Status Stratified by Cervical Disease Stage. BMC Cancer 2014, 30, 384. [Google Scholar] [CrossRef] [PubMed]
- Melnikow, J.; McGahan, C.; Sawaya, G.F.; Ehlen, T.; Coldman, A. Cervical Intraepithelial Neoplasia Outcomes after Treatment: Long-Term Follow-up from the British Columbia Cohort Study. J. Natl. Cancer Inst. 2009, 101, 721–728. [Google Scholar] [CrossRef]
- Min, K.J.; Lee, Y.J.; Suh, M.; Yoo, C.W.; Lim, M.C.; Choi, J.; Lee, J.K. The Korean Guideline for Cervical Cancer Screening. J. Gynecol. Oncol. 2015, 26, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Oyervides-Munoz, M.A.; Perez-Maya, A.A.; Rodriguez-Gutierrez, H.F.; Gomez-Macias, G.S.; Fajardo-Ramirez, O.R.; Trevino, V.; Barrera-Saldana, H.A.; Garza-Rodriguez, M.L. Understanding the Hpv Integration and Its Progression to Cervical Cancer. Infect. Genet. Evol. 2018, 61, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, M.; Pedamallu, C.S.; Gehlenborg, N.; Freeman, S.S.; Danilova, L.; Bristow, C.A.; Cancer Genome Atlas Network. Characterization of Hpv and Host Genome Interactions in Primary Head and Neck Cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 15544–15549. [Google Scholar] [CrossRef]
- Park, C.S.-Y. Thinking “Outside the Box”. J. Adv. Nurs. 2018, 74, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Stransky, N.; Couturier, J.; Hupe, P.; Barillot, E.; de Cremoux, P.; Sastre-Garau, X. Frequent Genomic Structural Alterations at Hpv Insertion Sites in Cervical Carcinoma. J. Pathol. 2010, 221, 320–330. [Google Scholar] [CrossRef]
- Sarian, L.O.; Derchain, S.F.; Pitta Dda, R.; Morais, S.S.; Rabelo-Santos, S.H. Factors Associated with Hpv Persistence after Treatment for High-Grade Cervical Intra-Epithelial Neoplasia with Large Loop Excision of the Transformation Zone (Lletz). J. Clin. Virol. 2004, 31, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, N.F.; Trevisan, A.; Duarte-Franco, E.; Rohan, T.E.; Ferenczy, A.; Villa, L.L.; Franco, E.L. Viral Load as a Predictor of the Risk of Cervical Intraepithelial Neoplasia. Int. J. Cancer 2003, 103, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, H.; Sohn, I.S.; Hwang, H.S.; Kwon, H.S.; Lee, S.J.; Chang, S. Nationwide Cervical Cancer Screening in Korea: Data from the National Health Insurance Service Cancer Screening Program and National Cancer Screening Program, 2009-2014. J. Gynecol. Oncol. 2017, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends--an Update. Cancer Epidemiol. Biomarkers. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Vinokurova, S.; von Knebel Doeberitz, M. Systematic Review of Genomic Integration Sites of Human Papillomavirus Genomes in Epithelial Dysplasia and Invasive Cancer of the Female Lower Genital Tract. Cancer Res. 2004, 64, 3878–3884. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Hughes, J.P.; Edelstein, Z.R.; Kiviat, N.B.; Koutsky, L.A.; Mao, C.; Schiffman, M. Human Papillomavirus (Hpv) Type 16 and Type 18 DNA Loads at Baseline and Persistence of Type-Specific Infection During a 2-Year Follow-Up. J. Infect. Dis. 2009, 200, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Baasland, I.; Romundstad, P.R.; Eide, M.L.; Jonassen, C.M. Clinical performance of Anyplex II HPV28 by human papillomavirus type and viral load in a referral population. PLoS ONE 2019, 14, e0210997. [Google Scholar] [CrossRef]
- Lee, D.-H. TOCE: Innovative Technology for High Multiplex Real-time PCR. Seegene Bull. 2012, 1, 5–10. [Google Scholar]
- Lee, D.H.; Hwang, N.R.; Lim, M.C.; Yoo, C.W.; Joo, J.; Kim, J.-Y.; Park, S.-Y.; Hwang, H.-S. Comparison of the performance of Anyplex II HPV HR, the Cobas 4800 human papillomavirus test and Hybrid Capture 2. Ann. Clin. Biochem. 2016, 53 Pt 5, 561–567. [Google Scholar] [CrossRef]
- Cornall, A.M.; Poljak, M.; Garland, S.M.; Phillips, S.; Tan, J.H.; Machalek, D.A.; Quinn, M.A.; Tabrizi, S.N. Anyplex II HPV28 detection and Anyplex II HPV HR detection assays are highly concordant with other commercial assays for detection of high-risk HPV genotypes in women with high grade cervical abnormalities. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 545–551. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses and Cancer: From Basic Studies to Clinical Application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).