The Role of Lung Ultrasound in SARS-CoV-19 Pneumonia Management
Abstract
:1. Introduction
2. Materials and Method
2.1. Patients
2.2. Statement of Ethics
2.3. Laboratory Data
2.4. Oxygen Requirement
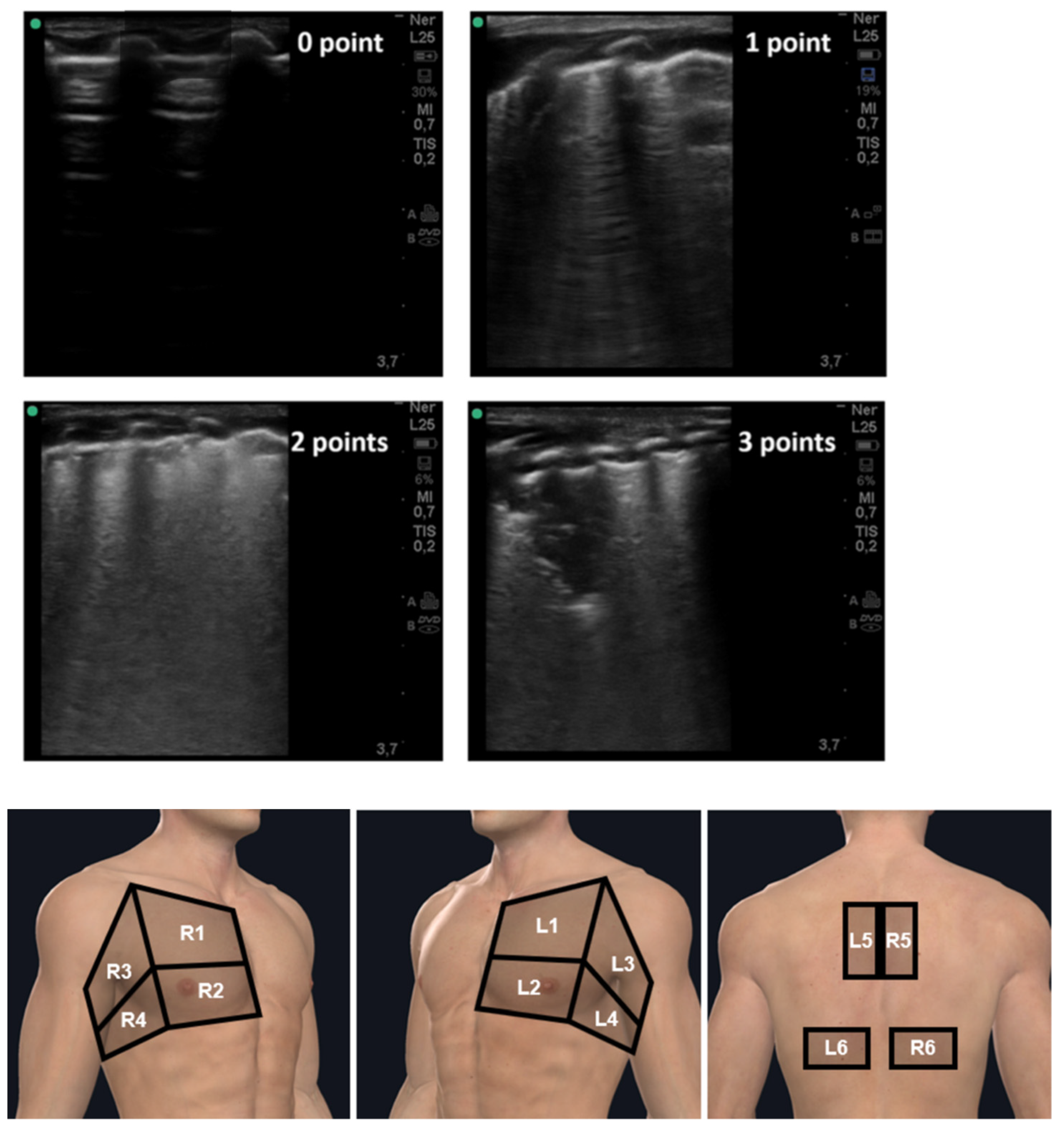
2.5. LUS Protocol
Lung Ultrasound Scoring System
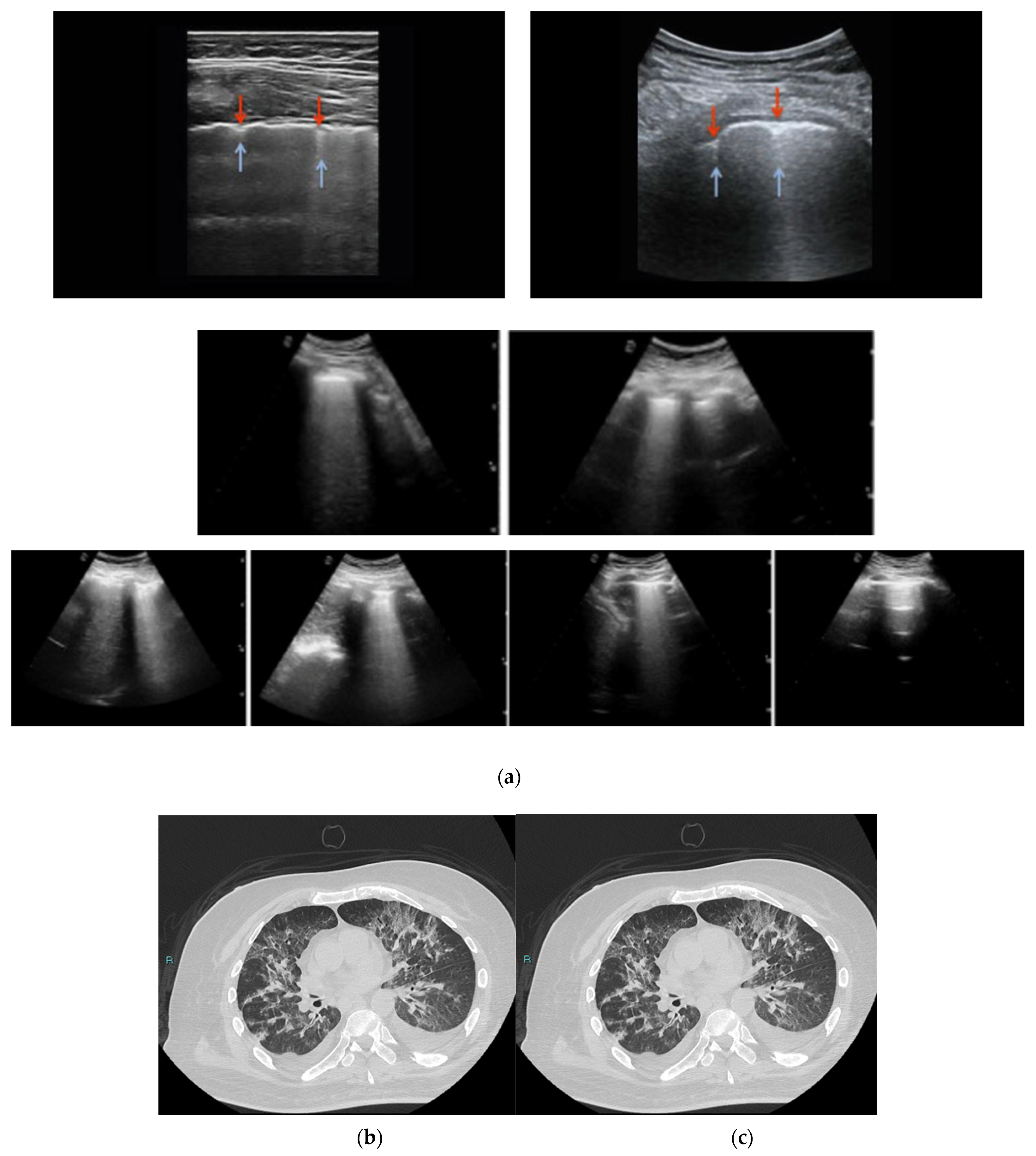
2.6. High Resolution CT (HRCT)
2.7. End Points of the Study
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 16 February 2022).
- Valdenassi, L.; Franzini, M.; Ricevuti, G.; Rinaldi, L.; Galoforo, A.C.; Tirelli, U. Letter to the Editor: Potential mechanisms by which the oxygen-ozone (O2–O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4059–4061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Kudlinski, B.; Zgoła, D.; Stolińska, M.; Murkos, M.; Kania, J.; Nowak, P.; Noga, A.; Wojciech, M.; Zaborniak, G.; Zembron-Lacny, A. Systemic Inflammatory Predictors of In-Hospital Mortality in COVID-19 Patients: A Retrospective Study. Diagnostics 2022, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Léonard-Lorant, I.; Delabranche, X.; Séverac, F.; Helms, J.; Pauzet, C.; Collange, O.; Ohana, M. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology 2020, 296, E189–E191. [Google Scholar] [CrossRef] [Green Version]
- Poyiadji, N.; Cormier, P.; Patel, P.Y.; Hadied, M.O.; Bhargava, P.; Khanna, K.; Nadig, J.; Keimig, T.; Spizarny, D.; Reeser, N.; et al. Acute Pulmonary Embolism and COVID-19. Radiology 2020, 297, E335–E338. [Google Scholar] [CrossRef] [PubMed]
- Grillet, F.; Behr, J.; Calame, P.; Aubry, S.; Delabrousse, E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology 2020, 296, E186–E188. [Google Scholar] [CrossRef] [Green Version]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The Role of Biomarkers in Diagnosis of COVID-19—A Systematic Review. Life Sci. 2020, 254, 117788. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295, 200463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumagalii, C.; Rozzini, R.; Vannini, M.; Coccia, F.; Cesaroni, G.; Mazzeo, F.; Cola, M.; Bartoloni, A.; Fontanari, P.; Lavorini, F.; et al. Clinical risk score to predict in-hospital mortality in COVID-19 patients: A retrospective cohort study. BMJ Open 2020, 10, e040629. [Google Scholar] [CrossRef]
- Leonardi, A.; Scipione, R.; Alfieri, G.; Petrillo, R.; Dolciami, M.; Ciccarelli, F.; Perotti, S.; Cartocci, G.; Scala, A.; Imperiale, C.; et al. Role of computed tomography in predicting critical disease in patients with COVID-19 pneumonia: A retrospective study using a semiautomatic quantitative method. Eur. J. Radiol. 2020, 130, 109202. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, S.; Zhang, Y.; Jiang, Y.; Jiang, Y.; Wang, Y.; Li, H.; Huang, C.; Zhang, S.; Li, X.; et al. Chest CT Features of 182 Patients with Mild Coronavirus Disease 2019 (COVID-19) Pneumonia: A Longitudinal, Retrospective and Descriptive Study. Infect. Dis. Ther. 2020, 9, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Li, H.; Yu, H.; Liu, X.; Zeng, X.; Peng, D.; Zhang, W. 2019 novel coronavirus (COVID-19) pneumonia: CT manifestations and pattern of evolution in 110 patients in Jiangxi, China. Eur. Radiol. 2021, 31, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients with COVID-19: A Simple, Quantitative, Reproducible Method. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Mayo, P.H.; Copetti, R.; Feller-Kopman, D.; Mathis, G.; Maury, E.; Mongodi, S.; Mojoli, F.; Volpicelli, G.; Zanobetti, M. Thoracic ultrasonography: A narrative review. Intensive Care Med. 2019, 45, 1200–1211. [Google Scholar] [CrossRef]
- Rinaldi, L.; Milione, S.; Fascione, M.C.; Pafundi, P.C.; Altruda, C.; Di Caterino, M.; Monaco, L.; Reginelli, A.; Perrotta, F.; Porta, G.; et al. Relevance of lung ultrasound in the diagnostic algorithm of respiratory diseases in a real-life setting: A multicentre prospective study. Respirology 2020, 25, 535–542. [Google Scholar] [CrossRef]
- Jalil, B.A.; Khan, A.; Kugasia, I.R.; Ijaz, M. Lung ultrasound in early SARS-CoV-2 pneumonia and the LUS-CoV criteria. In Baylor University Medical Center Proceedings; Taylor & Francis: Oxfordshire, UK, 2020; Volume 34, pp. 1–4. [Google Scholar] [CrossRef]
- Lugara, M.; Oliva, G.; Pafundi, P.C.; Tamburrini, S.; Nevola, R.; Gjeloshi, K.; Rinaldi, L. Clinical application of lung ultrasound score on COVID-19 setting: A regional experience in Southern Italy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3623–3631. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, E.; Goffi, A.; Tizzani, M.; Locatelli, S.M.; Porrino, G.; Losano, I.; Leone, D.; Calzolari, G.; Vesan., M.; Steri, F.; et al. Lung Ultrasonography for the Diagnosis of SARS-CoV-2 Pneumonia in the Emergency Department. Ann. Emerg. Med. 2021, 77, 385–394. [Google Scholar] [CrossRef]
- Buonsenso, D.; Piano, A.; Raffaelli, F.; Bonadia, N.; de Gaetano Donati, K.; Franceschi, F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: A case report and potential applications during COVID-19 outbreak. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2776–2780. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M.; Smargiassi, A.; Inchingolo, R.; Demi, L. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert. Rev. Respir. Med. 2019, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Delle Sedie, A.; Carli, L.; Cioffi, E.; Bombardieri, S.; Riente, L. The promising role of lung ultrasound in systemic sclerosis. Clin. Rheumatol. 2012, 31, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Salaffi, F.; Carotti, M.; Tardella, M.; Pineda, C.; Bertolazzi, C.; Bichisecchi, E.; Filippucci, E.; Grassi, W. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders--preliminary results. Arthritis Res. Ther. 2011, 13, R134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruaro, B.; Baratella, E.; Confalonieri, P.; Confalonieri, M.; Vassallo, F.G.; Wade, B.; Geri, P.; Pozzan, R.; Caforio, G.; Marrocchio, C.; et al. High-Resolution Computed Tomography and Lung Ultrasound in Patients with Systemic Sclerosis: Which One to Choose? Diagnostics 2021, 11, 2293. [Google Scholar] [CrossRef]
- Lokuge, A.; Alexander, J.; Land, B.; McAllister, A. Lung ultrasound in a respiratory pandemic. Emerg. Med. Australas. 2020, 32, 883–889. [Google Scholar] [CrossRef]
- Burkert, J.; Jarman, R.; Deol, P. Evolution of Lung Abnormalities on Lung Ultrasound in Recovery from COVID-19 Disease—A Prospective, Longitudinal Observational Cohort Study. J. Ultrasound. Med. 2022. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J. Ultrasound. Med. 2020, 39, 1459–1462. [Google Scholar] [CrossRef] [Green Version]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Sher, S.; Nenna, R.; Valente, S.; Inchingolo, C.D.; Corbo, G.M. Lung Ultrasonography may Provide an Indirect Estimation of Lung Porosity and Airspace Geometry. Respiration 2014, 88, 458–468. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Demi, L.; Inchingolo, R. Artifactual lung ultrasonography: It is a matter of traps, order, and disorder. Appl. Sci. 2020, 10, 1570. [Google Scholar] [CrossRef] [Green Version]
- Shafqat, A.; Shafqat, S.; Salameh, S.A.; Kashir, J.; Alkattan, K.; Yaqinuddin, A. Mechanistic insights into the immune pathophysiology of COVID-19; an in-depth review. Front. Immunol. 2022, 13, 835104. [Google Scholar] [CrossRef]
- Gattinoni, L.; Gattarello, S.; Steinberg, I.; Busana, M.; Palermo, P.; Lazzari, S.; Romitti, F.; Quintel, M.; Meissner, K.; Marini, J.J.; et al. COVID-19 pneumonia: Pathophysiology and management. Eur. Respir. Rev. 2021, 30, 210138. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M. What Is COVID 19 Teaching Us about Pulmonary Ultrasound? Diagnostics 2022, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Mongodi, S.; De Luca, D.; Colombo, A.; Stella, A.; Santangelo, E.; Corradi, F.; Gargani, L.; Rovida, S.; Volpicelli, G. Quantitative Lung Ultrasound: Technical aspects and clinical applications. Anesthesiology 2021, 134, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Gaujard, G.; Guinot, P.G.; Bouhemad, B.; L.U. S. Study Group. Using the lung ultrasound score to monitor disease progression for COVID-19-associated ARDS. Intensive Care Med. 2021, 47, 1329–1331. [Google Scholar] [CrossRef] [PubMed]
- Perrone, T.; Soldati, G.; Padovini, L.; Fiengo, A.; Lettieri, G.; Sabatini, U.; Gori, G.; Lepore, F.; Garolfi, M.; Palumbo, I.; et al. A New Lung Ultrasound Protocol Able to Predict Worsening in Patients Affected by Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. J. Ultrasound Med. 2020; in press. [Google Scholar] [CrossRef] [PubMed]
- Demi, M.; Prediletto, R.; Soldati, G.; Demi, L. Physical Mechanisms Providing Clinical Information from Ultrasound Lung Images: Hypotheses and Early Confirmations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 612–623. [Google Scholar] [CrossRef]
- Kumar, A.; Weng, I.; Graglia, S.; Lew, T.; Gandhi, K.; Lalani, F.; Chia, D.; Duanmu, Y.; Jensen, T.; Lobo, V.; et al. Point-of-Care Ultrasound predicts clinical outcomes in patients with COVID-19. J. Ultrasound Med. 2022, 67, 1367–1375. [Google Scholar] [CrossRef]
- Stecher, S.S.; Anton, S.; Fraccaroli, A.; Götschke, G. Lung ultrasound predicts clinical course but not outcome in COVID-19 ICU patients: A retrospective single-center analysis. BMC Anesthesiol. 2021, 21, 178. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bulla, P.; Jödicke, L.; Klein, C.; Bott, S.M.; Keller, R.; Malek, N.; Fröhlich, E.; Göpel, S.; Blumenstock, G.; et al. Can follow up lung ultrasound in Coronavirus Disease-19 patients indicate clinical outcome? PLoS ONE 2021, 16, e0256359. [Google Scholar] [CrossRef]
- Wang, M.; Luo, X.; Wang, L.; Estill, J.; Lv, M.; Zhu, Y.; Wang, Q.; Xiao, X.; Song, Y.; Lee, M.S.; et al. A comparison of lung Ultrasound and Computed Tomography in the diagnosis of patients with COVID-19: A systematic Review and Meta-Analysis. Diagnostics 2021, 11, 1351. [Google Scholar] [CrossRef]
- Buda, N.; Cylwik, J.; Mróz, K.; Rudzińska, R.; Dubik, P.; Malczewska, A.; Oraczewska, A.; Skoczyński, S.; Suska, A.; Górecki, T.; et al. Lung Ultrasound examination in patients with SARS-CoV-2 Infection: Multicenter study. J. Clin. Med. 2021, 10, 3255. [Google Scholar] [CrossRef] [PubMed]
- Lichter, Y.; Topilsky, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Oz, A.G.; Vine, J.; Goren, O.; Cohen, B.; et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020, 46, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yu, K.; Zhao, Q.; Tian, R.; Xie, H.; Xie, L.; Deng, P.; Xie, G.; Bao, A.; Du, J. Lung Ultrasound Score in Evaluating the Severity of Coronavirus Disease 2019 (COVID-19) Pneumonia. Ultrasound Med. Biol. 2020, 46, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Falster, C.; Jacobsen, N.; Wulff Madsen, L.; Dahlerup Rasmussen, L.; Davidsen, J.R.; Christie Knudtzen, F.; Nielsen, S.L.; Johansen, I.S.; Laursen, C.B. Lung ultrasound may be a valuable aid in decision making for patients admitted with COVID-19 disease. Eur. Clin. Respir. J. 2021, 8, 1909521. [Google Scholar] [CrossRef]
- Nishiura, H.; Kobayash, T.; Miyama, T.; Suzuki, A.; Jung, S.M.; Hayashi, K.; Kinoshita, R.; Yang, Y.; Yuan, B.; Akhmetzhanov, A.R.; et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int. J. Infect. Dis. 2020, 94, 154–155. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 2020, 25, 2000180. [Google Scholar] [CrossRef] [Green Version]
- Puylaert, C.A.J.; Scheijmans, J.C.G.; Borgstein, A.B.J.; Andeweg, C.S.; Bartels-Rutten, A.; Beets, G.L.; van Berge Henegouwen, M.I.; Braak, S.J.; Couvreur, R.; Daams, F.; et al. Yield of Screening for COVID-19 in Asymptomatic patients before Elective or Emergency Surgery Using Chest CT and RT-PCR (SCOUT). Ann. Surg. 2020, 272, 919–924. [Google Scholar] [CrossRef]
- Pelosi, P.; Tonelli, R.; Torregiani, C.; Baratella, E.; Confalonieri, M.; Battaglini, D.; Marchioni, A.; Confalonieri, P.; Clini, E.; Salton, F.; et al. Different Methods to Improve the Monitoring of Noninvasive Respiratory Support of Patients with Severe Pneumonia/ARDS Due to COVID-19: An Update. J. Clin. Med. 2022, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Bouhemad, B.; Liu, Z.H.; Arbelot, C.; Zhang, M.; Ferarri, F.; Le-Guen, M.; Girard, M.; Lu, Q.; Rouby, J.-J. Ultrasound assessment of anti- biotic-induced pulmonary reaeration in ventilator-associated pneumo-nia. Crit. Care Med. 2010, 38, 84–92. [Google Scholar]
- Baldi, G.; Gargani, L.; Abramo, A.; D’Errico, L.; Caramella, D.; Picano, E.; Giunta, F.; Forfori, F. Lung water assessment by lung ultrasonography in intensive care: A pilot study. Intensive Care Med. 2013, 39, 74–84. [Google Scholar] [CrossRef]
- Demi, M.; Buda, N.; Soldati, G. Vertical Artifacts in Lung Ultrasonography: Some Common Clinician Questions and the Related Engineer Answers. Diagnostics 2022, 12, 215. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Values |
|---|---|
| Age (years), median [IQR] | 71 [58–78] |
| Sex, n (%) M F | 68 (68.7) 31 (31.3) |
| Signs and Symptoms | |
| Presence of fever, n (%) | 90 (90.9) |
| Presence of cough, n (%) | 90 (90.9) |
| Pharyngeal hyperemia, n (%) | 63 (63.6) |
| Asthenia, n (%) | 76 (76.8) |
| Vomiting, n (%) | 5 (5.1) |
| Diarrhea, n (%) | 11 (11.1) |
| Dyspnea, n (%) | 89 (89.9) |
| Tachycardia, n (%) | 67 (67.7) |
| Phenotype, n (%) type 1 type 2 type 3 type 4 | 7 (7.1) 21 (21.2) 60 (60.6) 11 (11.1) |
| Pre-existing Comorbidities | Values |
| Hypertension, n (%) | 85 (85.9) |
| Diabetes, n (%) | 34 (34.3) |
| Atrial fibrillation, n (%) | 13 (13.1) |
| Ischemic heart disease, n (%) | 33 (33.3) |
| Ictus, n (%) | 14 (14.1) |
| Dementia, n (%) | 31 (31.3) |
| Chronic obstructive pulmonary disease (COPD), n (%) | 56 (56.6) |
| Active cancer in the last five years, n (%) | 14 (14.1) |
| Hyperinflammatory syndrome % | 84 (84.8) |
| Smoke, n (%) | 66 (66.7) |
| Obesity, n (%) No Grade I Grade II Grade III | 58 (59.6) 30 (30.3) 8 (8.1) 2 (2) |
| Chronic liver disease, n (%) | 7 (7.1) |
| Chronic kidney disease, n (%) | 31 (31.3) |
| Mortality, n (%) | 21 (21.2) |
| Days of hospitalization, median [IQR] | 30 [20–40] |
| Ventilation, median [IQR] | 0.50 [0.28–0.60] |
| Oxygen interface, n (%) | |
| None Nasal cannula Venturi Mask CPAP/Bi-level Orotracheal Intubation | 14 (14.1) 18 (18.2) 40 (40.4) 25 (25.3) 2 (2) |
| Therapy | |
| Anticoagulants, n (%) | 72 (72.7) |
| Antiplatelets, n (%) | 35 (35.4) |
| ACE Inhibitors, n (%) | 32 (32.3) |
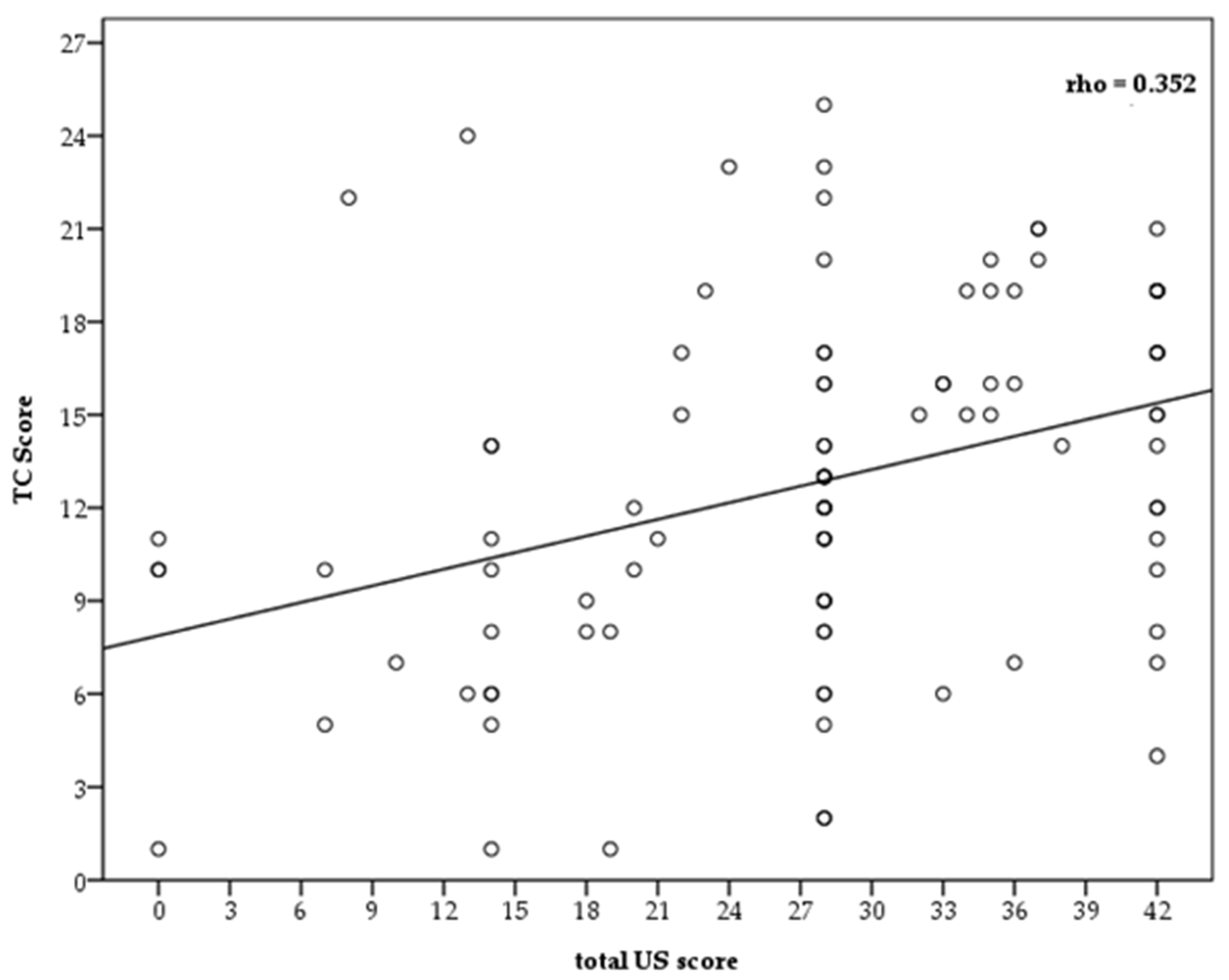
| Ultrasound thoracic, median [IQR] | 28 [20–36] |
| Ultrasound score, n (%) | |
| Score 0 | 2 (2) |
| Score 1 | 18 (18.2) |
| Score 2 | 47 (47.6) |
| Score 3 | 32 (32.2) |
| CT score, mean (SD) | 12.63 (5.72) |
| CT score, n (%) Score 1 Score 2 Score 3 | 20 (20.2) 59 (59.6) 20 (20.2) |
| Laboratory | Values |
|---|---|
| Hb (mg/dL), mean (SD) | 12.2 (2.2) |
| White blood cells (×103), mean (SD) | 9.73 (4.38) |
| Lymphocytes (a.v.), median [IQR] | 0.8 [0.6–1.3] |
| Neutrophils (a.v.), mean (SD) | 7.56 (3.15) |
| Platelets, mean (SD) | 282,098 (141,397) |
| Azotemia (mg/dL), mean (SD) | 54.7 (34.85) |
| Creatinine (mg/dL), median [IQR] | 0.9 [0.8–1.2] |
| Sodium (mmol/L), mean (SD) | 138.8 (4.2) |
| Potassium (mmol/L), mean (SD) | 4.8 (3.9) |
| AST (U/L), mean (SD) | 42.6 (124.6) |
| ALT (U/L), mean (SD) | 48.8 (136.8) |
| Glycemia (mg/dL), median [IQR] | 110 [88.3–175] |
| CRP (mg/dL), median [IQR] | 5 [2.6–12] |
| INR, median [IQR] | 1.12 [1.10–1.20] |
| aPTT (s), mean (SD) | 31 (6.8) |
| Fibrinogen (mg/dL), mean (SD) | 483.3 (141.4) |
| Nt-pro-bnp (pg/mL), median [IQR] | 1578 [600–3500] |
| D-Dimer (pg/mL), median [IQR] | 2300 [782.5–4210] |
| LDH (mU/mL), mean (SD) | 361.5 (138.4) |
| Troponin (ng/mL), median [IQR] | 0.032 [0.014–0.090] |
| Procalcitonin (ng/mL), median [IQR] | 0.2 [0.03–0.90] |
| Ferritin (ng/mL), median [IQR] | 450 [280–700] |
| Blood Gas Analysis | |
| pH, median [IQR] | 7.45 [7.40–7.47] |
| pO2 (mmHg), median [IQR] | 68 [58.3–84.8] |
| pCO2 (mmHg), median [IQR] | 35 [33–42] |
| HCO3- (mmol/L), median [IQR] | 25 [23–26] |
| spO2 (%), median [IQR] | 93.1 [90–96] |
| Lactates (mmol/L), median [IQR] | 2.25 (1.02) |
| P/F, median [IQR] | 231 [136.3–295.3] |
| FiO2 admission, median [IQR] | 0.30 [0.21–0.50] |
| Parameters | Correlation Coefficient | p |
|---|---|---|
| Age (years) | 0.289 | 0.034 |
| Dyspnoea | 0.319 | 0.051 |
| Tachycardia | 0.457 | 0.002 |
| COVID-19 phenotype | 0.589 | <0.001 |
| Dementia | 0.197 | 0.256 |
| Platelets | −0.118 | 0.286 |
| Prothrombin time | 0.057 | 0.917 |
| NT-proBNP | 0.174 | 0.419 |
| D-dimer | 0.218 | 0.047 |
| pH | −0.469 | 0.008 |
| pO2 | −0.486 | 0.003 |
| spO2 | −0.226 | 0.467 |
| P/F | −0.689 | <0.001 |
| Death of patients | 0.492 | 0.008 |
| Ventilation | 0.562 | <0.001 |
| Lactates | 0.479 | 0.001 |
| Parameters | Correlation Coefficient | p |
|---|---|---|
| Age (years) | 0.369 | 0.029 |
| Dyspnoea | 0.488 | <0.001 |
| Tachycardia | 0.321 | 0.007 |
| COVID-19 phenotype | 0.639 | <0.001 |
| Dementia | 0.124 | 0.298 |
| Platelets | −0.189 | 0.321 |
| Prothrombin time | 0.025 | 0.874 |
| NT-proBNP | 0.098 | 0.513 |
| D-dimer | 0.289 | 0.041 |
| pH | −0.396 | 0.019 |
| pO2 | −0.470 | <0.001 |
| spO2 | −0.199 | 0.148 |
| P/F | −0.762 | <0.001 |
| Death of patients | 0.466 | 0.001 |
| Ventilation | 0.503 | <0.001 |
| Lactates | 0.442 | 0.001 |
| AUC of LUS Score | AUC (95% CI) |
|---|---|
| Dyspnea | 0.77 (0.61–0.92) |
| Tachycardia | 0.79 (0.68–0.879 |
| Dementia | 0.57 (0.45–0.69) |
| Mortality | 0.70 (0.59–0.82) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugarà, M.; Tamburrini, S.; Coppola, M.G.; Oliva, G.; Fiorini, V.; Catalano, M.; Carbone, R.; Saturnino, P.P.; Rosano, N.; Pesce, A.; et al. The Role of Lung Ultrasound in SARS-CoV-19 Pneumonia Management. Diagnostics 2022, 12, 1856. https://doi.org/10.3390/diagnostics12081856
Lugarà M, Tamburrini S, Coppola MG, Oliva G, Fiorini V, Catalano M, Carbone R, Saturnino PP, Rosano N, Pesce A, et al. The Role of Lung Ultrasound in SARS-CoV-19 Pneumonia Management. Diagnostics. 2022; 12(8):1856. https://doi.org/10.3390/diagnostics12081856
Chicago/Turabian StyleLugarà, Marina, Stefania Tamburrini, Maria Gabriella Coppola, Gabriella Oliva, Valeria Fiorini, Marco Catalano, Roberto Carbone, Pietro Paolo Saturnino, Nicola Rosano, Antonella Pesce, and et al. 2022. "The Role of Lung Ultrasound in SARS-CoV-19 Pneumonia Management" Diagnostics 12, no. 8: 1856. https://doi.org/10.3390/diagnostics12081856
APA StyleLugarà, M., Tamburrini, S., Coppola, M. G., Oliva, G., Fiorini, V., Catalano, M., Carbone, R., Saturnino, P. P., Rosano, N., Pesce, A., Galiero, R., Ferrara, R., Iannuzzi, M., Vincenzo, D., Negro, A., Somma, F., Fasano, F., Perrella, A., Vitiello, G., ... Rinaldi, L. (2022). The Role of Lung Ultrasound in SARS-CoV-19 Pneumonia Management. Diagnostics, 12(8), 1856. https://doi.org/10.3390/diagnostics12081856










