Abstract
Porphyrias are a group of congenital and acquired diseases caused by an enzymatic impairment in the biosynthesis of heme. Depending on the specific enzyme involved, different types of porphyrias (i.e., chronic vs. acute, cutaneous vs. neurovisceral, hepatic vs. erythropoietic) are described, with different clinical presentations. Acute hepatic porphyrias (AHPs) are characterized by life-threatening acute neuro-visceral crises (acute porphyric attacks, APAs), featuring a wide range of neuropathic (central, peripheral, autonomic) manifestations. APAs are usually unleashed by external “porphyrinogenic” triggers, which are thought to cause an increased metabolic demand for heme. During APAs, the heme precursors δ-aminolevulinic acid (ALA) and porphobilinogen (PBG) accumulate in the bloodstream and urine. Even though several hypotheses have been developed to explain the protean clinical picture of APAs, the exact mechanism of neuronal damage in AHPs is still a matter of debate. In recent decades, a role has been proposed for oxidative damage caused by ALA, mitochondrial and synaptic ALA toxicity, dysfunction induced by relative heme deficiency on cytochromes and other hemeproteins (i.e., nitric oxide synthases), pyridoxal phosphate functional deficiency, derangements in the metabolic pathways of tryptophan, and other factors. Since the pathway leading to the biosynthesis of heme is inscribed into a complex network of interactions, which also includes some fundamental processes of basal metabolism, a disruption in any of the steps of this pathway is likely to have multiple pathogenic effects. Here, we aim to provide a comprehensive review of the current evidence regarding the mechanisms of neuronal damage in AHPs.
1. Introduction
Porphyrias are a group of congenital and acquired diseases characterized by an impairment of the heme biosynthetic pathway [1]. Depending on the distinct enzyme deficiency and the tissue-specific isoform involved, different kinds of porphyria are described, the main distinction being made between cutaneous (or non-acute) porphyrias (congenital erythropoietic porphyria, porphyria cutanea tarda, hepato-erythropoietic porphyria, and X-linked/erythropoietic protoporphyria), mainly featuring a clinical picture of cutaneous phototoxicity, and acute hepatic porphyrias (AHPs). The latter comprise ALA dehydratase deficiency porphyria (AlaD-P), acute intermittent porphyria (AIP), hereditary coproporphyria (HCP), and variegate porphyria (VP). Both AlaD-P and AIP present only with acute neurovisceral manifestations, whereas HCP and PV display neurovisceral as well as subacute photosensitivity symptoms [2].
From a clinical perspective, neurovisceral acute porphyric attacks (APAs) are the most dramatic manifestations of AHPs [2,3,4,5,6]; they are usually unleashed by external triggers, such as fasting, alcoholic intake, the luteal phase of menstrual cycles, or porphyrinogenic drugs [7] (antiepileptics, barbiturates, griseofulvin, hormonal replacement therapy, etc.) and display a wide range of neuropathic manifestations [2,3,4,5,8,9].
The involvement of the autonomic nervous system in the splanchnic district occurs in the form of abdominal pain, constipation, diarrhea, nausea, and/or vomiting. Tachycardia (or other arrhythmias), hypertension, postural hypotension, diaphoresis, and sphincter disturbances are also commonly reported. Peripheral neuropathy is usually present as an acute axonal motor neuropathy, characteristically starting at the upper limbs and proximal muscles (as compared to Guillain-Barré syndrome), often accompanied by sensory involvement. Somewhat similar to Guillain-Barré syndrome, respiratory paralysis (due to phrenic nerve involvement) or bulbar paralysis can be dreaded and potentially life-threatening complications. Central nervous system (CNS) disturbances may develop throughout the entire course of APAs, the most dramatic being encephalopathy of varying severity, seizures (complex partial, tonic-clonic, myoclonic, and absence), and hyponatremia due to syndrome of inappropriate secretion of antidiuretic hormone (SIADH); psychiatric symptoms may complete the picture, ranging from depression, irritability, hallucination, to overt psychosis or catatonia. In patients with symptomatic AHP, a wide spectrum of histopathologic (and, more recently, radiological) changes have been observed in almost every part of the central and peripheral nervous systems [4,10,11]. Between crises, AIP patients may present with hyperintense (unspecific) small, rounded white matter lesions in T2-weighted magnetic resonance images [12].
It is important to note that phenotype penetration in congenital AHPs is probably very low; a French study of the general population found a mutation rate of 1/1675 for the gene responsible for AIP (HMBS coding for hydroxymethylbilane synthase, the third enzyme of the heme biosynthetic pathway) [13], while the yearly incidence of acute porphyric attacks in Europe is estimated to be 0.5–1 over 100.000 [14]. Similarly, based on genomic/exomic database interrogations, a clinical penetrance of 1% was estimated for HMBS heterozygous mutations [15]. Early studies have attempted to link some mutations in genes that are causative of AHP with the presence of psychiatric illnesses in the absence of any other overt clinical manifestations [16,17,18]. Although interesting, these findings are debatable and should be reconfirmed by contemporary investigations that comply with today’s standards of diagnostic methodology.
During porphyric crises, increased levels of the heme precursors δ-amino levulinic acid (ALA) and porphobilinogen (PBG) are measured in urine, indicating an enhanced activity of liver ALA synthase (ALAS1), the first enzyme in the heme biosynthetic pathway [3,6]. ALAS1 is probably overexpressed as a result of greater demands for heme under “porphyrinogenic” stress conditions.
Some diseases, either congenital (i.e., hereditary tyrosinemia) or acquired (i.e., lead intoxication) present classically as AHP-like syndromes [19,20]. Type I hereditary tyrosinemia is a rare and inheritable metabolic disturbance of the phenylalanine metabolism, where, due to an enzymatic dysfunction (fumaryl-acetoacetate hydrolase deficiency), a build-up of tyrosine and succinylacetone (SA) occurs [19]. SA is a by-product of tyrosine breakdown, which inhibits δ-aminolevulinic acid dehydratase (ALAD), the second enzyme of the heme biosynthetic pathway [21], thus leading to the accumulation of ALA. Lead displaces a zinc ion from a zinc-cysteine coordination complex of ALAD, suppressing its activity by either steric hindrance or by catalytic impairment [22].
While the pathogenesis of cutaneous manifestations is chiefly attributed to the light-dependent release of cytotoxic reactive oxygen species (ROS) exerted by the type I/II photosensitized reactions induced by porphyrins (i.e., cyclic tetrapyrrolic heme precursors) [23,24], the exact mechanism underlying the neurologic impairment in AHP is still a matter of debate [4,5,25]. Despite some early evidence of direct porphobilinogen neurotoxicity [26], in recent decades, the majority of authors have focused on the occurrences of direct ALA neurotoxicity, neurologic damage following heme depletion, and a few others. The present work aims to provide a comprehensive review of the current evidence regarding the principal hypotheses for neuronal damage in AHPs.
Experimental Models for Studying AHPs
Several experimental models have helped to elucidate the pathophysiology of neurologic damage in AHPs.
As previously mentioned, succinyl acetone (SA) inhibits ALAD in mammalian cell cultures and rodents. Furthermore, a marked decrease in total heme content is observed in brains of SA-treated rats [27]. Otherwise, 2-allyl-2-isopropylacetamide (AIA) and 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) induce a series of enzymatic disruptions which are considered to resemble variegate porphyria [28]. Fasting induces ALAS1 expression via the peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) [29]. Griseofulvin is a well-known inducer of APAs and heme depletion [30,31]. Direct ALA administration has also been extensively utilized as a resource to study AHP in vitro and in vivo [32,33,34,35].
Some animal models for studying the three most common AHPs (AIP, HCP, and VP) are currently available [36], in particular, T1/T2 mice have residual 30% HMBS activity and are supposed to partially resemble the pathology of human AIP [37,38]. Quite recently, homozygous Hmbs knock-in mice have been produced that display 5% of normal HMBS activity and mimic the exceedingly rare homozygous dominant form of Acute Intermittent Porphyria [39].
2. δ-Aminolevulinic Acid Toxicity
2.1. Mechanisms of Transport and Uptake of δ-ALA in the CNS
When considering the hypothesis of a direct damaging effect of ALA to the brain, some issues arise regarding the possibility of ALA to effectively reach neurotoxic concentrations in the CNS under physiologic conditions.
In vivo studies have shown that the influx-rate constant for ALA movement from bloodstream to the brain is, in fact, quite low and decreases with age. Moreover, it seems to be a result of passive diffusion, except at the CSF-blood and choroid plexus-blood barriers, where the active transport of ALA has been described [40]. This is likely due to the presence of the di- and tripeptide transporter PEPT2 [40,41]. In the choroid plexus, ALA concentrations are considerably high compared to the rest of the brain. Additionally, PEPT2 is expressed at the apical (CNS-facing) side of choroid cells [42], where ALA uptake is seven-fold greater than at the basolateral (blood-facing) side [40]; thus, under physiologic conditions, the choroid plexus is likely to effectively drain whatever ALA might diffuse to the CNS. Both PEPT2 mRNA and protein expression have been localized in subependymal cells, ependymal cells, the choroid plexus [43,44], and, notably, in rat dorsal root ganglia satellite cells (but not in neurons) [44], where also ALA accumulation was described in earlier reports [45].
When incubated with high ALA concentrations (4.0 mM), rat cerebral cortex particles accumulate ALA intracellularly and ALAD activity is enhanced, while HMBS acts as a secondary control step, leading to a build-up of PBG [32,33]. More recently, in mice receiving ALA intraperitoneally, ALA accumulation in the encephalon was demonstrated, together with a plethora of effects on brain metabolism (a reduction of brain ALAS mRNA levels, an increase in cerebellar and hippocampal heme oxygenase activity, an increase in acetylcholinesterase activity, and alterations in factors involved in the management of oxidative stress) [34].
In neonatal rat astrocytes, PEPT2 is likely to be the main transporter of ALA; dipeptides, α-amino-containing cephalosporins, and a less acidic pH all negatively affect its transport rate [46]. Moreover, PEPT2 expression in astrocytes appears to decrease with age [47].
Following ALA administration, PEPT2-deficient mice showed substantially lower ALA concentrations in the choroid plexus, cerebral cortex, kidney, eye, blood, and plasma (suggestive of a greater renal clearance), but a five-fold greater concentration in CSF, reaching an eight-fold greater CSF/blood concentration ratio. PEPT2 null mice also displayed a much higher level of susceptibility to ALA toxicity and developed neuromuscular dysfunction following ALA chronic administration; notably, in this study, ALA plasma levels, calculated as the area under the curve, were comparable to those observed in porphyric patients during APAs [48].
In a population of AIP patients, homozygous carriers of a PEPT2 variant with a higher affinity for ALA (PEPT2*1*1) were independently associated with worse renal function and a more severe annual decrease in eGFR, compared to heterozygous or homozygous carriers of a variant with lower affinity (PEPT2*1*2 and PEPT2*2*2) [49]. Conversely, the presence of the PEPT2*2*2 variant was associated with poorer motor dexterity and working memory in children, in the context of low-level lead exposure [50]. Following these pieces of evidence, it has been conjectured that PEPT2 polymorphisms as well as (reversible) functional impairment may act as a modifying factor in defining the penetrance of the AHP phenotype, or even the timing of porphyric attacks [48].
It should be noted that ALA is a substrate for uptake by members of the neurotransmitter sodium and chloride dependent transporter family, whose substrate specificity is, normally, suited to GABA or GABA-like substances such as taurine and β-alanine [51]. In vitro evidence of ALA uptake was found for the transporters SLC6A6, SLC6A13 (whose affinity for ALA was suggested also through homology modeling [52]), possibly SLC6A8 [53], SLC15A1, and SLC36A1 [54]. The latter, in particular, is present in most parts of the human gastroenteric tract, peaking in expression in the small bowel; its mRNA is also detected in the blood-nerve barrier transcriptome [55,56]. These findings, perhaps, should be kept in mind when one thinks of the heightened levels of ALA detected in the peripheral, compared to the central, nervous system [5,44], or of the spasmodic effect exerted by ALA on rat small-bowel preparations [57].
Two final findings are worth mentioning. First, it is interesting the case report of an 82-year-old man who developed a clinical and biochemical picture suggestive of variegate porphyria, immediately following an oral loading of ALA, administered as a prodrug in the context of a photodynamic therapy for Barrett’s esophagitis is interesting [58]. In this case, even allowing for a genetic predisposition of the patient, which may have precipitated a full porphyric syndrome, an -at least initial- direct neurotoxic effect of ALA is too compelling a hypothesis to be easily discarded. Second, it has been shown that Trypanosoma cruzi’s epimastigotes produce and excrete substantial amounts of ALA in their culture medium [59]. Trypanosoma cruzi is a parasite that constitutively lacks some cytosolic enzymes of the heme biosynthetic pathway [60] and relies on host heme to survive; it is the causative agent of Chagas disease, whose manifestations include, intriguingly, neuropathy and autonomic dysfunction [61,62,63].
2.2. Endogenous Production of δ-ALA in the CNS
In principle, in the presence of an enzyme dysfunction in heme biosynthesis, rather than being obtained from the bloodstream, ALA could also be endogenously overproduced by the induction of neuronal (or glial) ALA synthase. Objections against this argument have been posited due to the evidence that liver transplantation (LT) is curative in AHPs [64], with most patients who undergo LT also showing a significant improvement in chronic neurological symptoms. Conversely, it has been reported that patients who received liver grafts from AHP donors within “domino” procedures began to suffer from APAs [65]. Nonetheless, several studies have been conducted to ascertain the possibility of an endogenous brain production of ALA.
In mice, brain mitochondrial ALAS activity is very low at birth, it reaches a peak at about 15 days and then declines steadily during the first 12 months of age [66,67]. In vivo, it seems to be unaffected by fasting, ethanol, AIA, DDC, or barbiturates [66,68,69], whereas it decreases after the administration of cycloheximide or large doses of ALA, or its methyl ester [67]. In fact, brain ALAS mRNA levels in mice were shown to diminish following a chronic or acute intraperitoneal administration of ALA [34]. Notably, injected hematin and CoCl2 are not taken up by the brain in vivo and do not affect brain ALAS activity, contrary to the liver isoform [67]. Brain ALAS requires a much lower glycine concentration to reach maximum activity compared to liver, adrenal mitochondrial or heart mitochondrial isoforms [66], but its activity was found to be only 20% compared to the liver isoform. This should still suffice to support the brain’s own requirement for synthesis and turnover of its hemoproteins [67].
Quite interestingly, it has been shown that cerebellar ALAS does undergo upregulation when heme synthesis is disrupted by the intraventricular injection of SA in rats [68], although no changes were observed when SA was administered intraperitoneally [70].
More recently, SA-induced Alas1 upregulation, inhibited by heme administration, was also observed in vitro in primary cortical neuron cultures [71]. Furthermore, isoflurane and sevoflurane administration increased brain ALAS activity (60% and 163%, respectively) in T1/T2 female mice; sevoflurane was effective also on T1 female mice (550% ALAS activity induction) [69]. In this study, although brain ALAS activity was enhanced, no ALA accumulation, nor variations of ALAS protein expression were detected.
Intriguingly, homozygous Hmbs knock-in mice show markedly elevated concentrations of ALA and PBG in the whole CNS (i.e., cerebrum, cerebellum, upper brain stem, and lower brain stem), including the spinal cord, and in the CSF [39]. Differently from T1/T2 mice, Hmbs knock-in mice do not show an immediate porphyric biochemical response to porphyrinogenic stimuli. However, they develop a severe neuropathy which closely resembles homozygous dominant acute intermittent porphyria, thus reinforcing the hypothesis of direct neurotoxicity exerted by locally produced ALA (and possibly PBG) [39].
2.3. Oxidative Damage, Mitochondrial Alterations and Effects on Iron Homeostasis
From a biochemical perspective, ALA undergoes a phosphate-catalyzed auto-enolization and becomes an oxidizing agent, reacting with iron and O2 to produce superoxide anion (O2·), HO· radical, and ALA radical (ALA·); the latter, in turn, reduces iron and yields the oxidant species dioxovaleric acid (DOVA), by reacting with oxygen [72,73].
Oxidative damage is the main mechanism by which ALA is deemed to cause mitochondrial dysfunction. In vitro, ALA induces mitochondrial swelling and the loss of transmembrane potential [74], possibly due to ROS-driven thiol cross-linking, which may lead to the aggregation of giant pore-like proteins [75]. Interestingly, calcium chelators and (only in the initial phases) catalase and dithiothreitol were able to restore transmembrane potential [74,75]. In addition, in ALA-treated HepG2 cells, an increased expression of mitochondrial biogenesis-related factors and mitochondrial network disruption was observed [76]. Likewise, ALA treatment was shown to alter mitochondrial polarity in rat Schwann cells [35]. ALA-induced lipid peroxidation has also been observed in vitro in rat astrocytes [77], rat Schwann cells [35] and, notably, in cardiolipin-rich liposomes, with a significant increase in their permeability. More specifically, phosphatidylcholine and cardiolipin (a major component of inner mitochondrial membranes) seem to be particularly susceptible to ALA-driven oxidative damage, even when ALA concentrations are in the micromolar range [73]; this has been proposed as an alternative slow-acting (i.e., with a time scale of hours) mechanism contributing to mitochondrial damage [73].
The high susceptibility of myelin-producing cells to oxidation could also play a pathogenic role. It has been shown that rat Schwann cells cultures, when incubated with ALA, suffer a dose-dependent reduction of proteins involved both in the initial stages of myelin formation, as well as in myelin sheath maintenance; decreased levels of sphingomyelins, phosphatidylcholines, and lysophosphatidylcholines were measured accordingly. At the same time, increased levels of carbonylated proteins and peroxidated lipids were detected, suggesting the activation of oxidative events [35].
ALA-driven oxidation is also supposed to exert a disruptive effect on iron homeostasis. Incubation with ALA alters the secondary and tertiary structure of apoferritin (possibly due to selective oxidative damage in tryptophan and cysteine moieties) and impairs its iron uptake ability (which is dependent on L subunits), while keeping its ferroxidase activity intact (dependent on H subunits) [72]. Additionally, ALA-induced iron release from ferritin has been observed in vitro [78]. In previously fasted succinyl acetone methyl ester (SAME)-treated rats, significant ALA-driven increases of total non-heme iron, lipid peroxidation, and of the antioxidant copper zinc superoxide dismutase (CuZnSOD) activity were detected in the brain [79].
Similar results were obtained by intraperitoneal injections of ALA, which caused an increase in total non-heme iron and ferritin in the cortex, in ferritin content in the striatum, in CuZnSOD activity in brain homogenates, lipid peroxidation and protein carbonylation in synaptic membrane preparations of total brain tissue, and in calcium uptake by cortical synaptosomes [80].
The iron regulatory protein 1 (IRP1) is a post-transcriptional regulator that binds to specific mRNA stem-loop structures known as iron-responsive elements (IRE); IREs are present in the mRNA untranslated regions (UTRs) of several proteins pivotal to iron homeostasis. It is therefore worth noting that incubation with ALA or SAME leads to an increased activity of IRP1, which is preventable by the addition of the cell-permeable antioxidant N-acetylcysteine (but not extracellular-acting catalase or superoxide dismutase) [81]. The authors of this study conclude that intracellular ALA should be numbered amongst the co-sensors in the regulation of iron homeostasis.
2.4. Neurotransmitter Balance Disruption
The chemical structure of ALA, a five-carbon-chain ω-aminoacid, shares some similarities with those of neurotransmitters such as GABA or glutamate. Seminal studies showed that ALA acts as an agonist at presynaptic GABAA autoreceptors (GABAAR), displacing GABA and tampering the depolarization-induced GABA release from preloaded nerve endings [82,83]. Prolonged intraperitoneal treatment with ALA in rodents resulted in a decreased binding of 3H-muscimol (a potent, selective GABAAR agonist) in total brain membrane preparations, a result that has been confirmed in vitro in synaptic membranes [80]. Moreover, in vitro and in vivo evidence was provided that the density of GABAAR decreases in the presence of ALA and DOVA, possibly due to selective oxidative damage (i.e., unlike broad peroxidation) [84]; in this setting, physiologic neurodevelopment could be impaired, since morphologic changes (such as a reduction in the average length of cytoplasmic processes) have been described in P19 cells [84], a cell line which represents a well-established model for studying neuronal differentiation. ALA could also have some GABA mimetic effect on pinealocytes, decreasing melatonin production (see Section 3.3.1) [85].
It is important to acknowledge that, in clinical practice, gabapentinoids are generally viewed as safe, non-porphyrinogenic drugs for the treatment of seizures and control of neuropathic pain in AIP patients [86].
Intracerebral injections of substantial amounts of ALA are known to produce body asymmetry and convulsions in rats, preventable by glutamate receptor antagonists [87]. ALA inhibits glutamate uptake in a dose-dependent, non-competitive, and irreversible manner in rat astrocyte cultures, seemingly because of a disruption of the GLT1 subtype of the glutamate transporter (possibly related, again, to selective oxidation damage) [77]; it also non-competitively impairs glutamate uptake in synaptosomes [82].
With regard to cholinergic neurotransmission, mice receiving intraperitoneal injections of ALA showed an increase in cortex acetylcholinesterase activity after chronic treatment, whereas a reduction in cortical and an increase in hippocampal butyrylcholinesterase activity was observed after an acute treatment [34].
2.5. Other Effects of δ-Aminolevulinic Acid
Classical studies on rabbit brain microsomes and chick embryo neuronal cell cultures have shown that ALA exerts an inhibitory effect on Na+/K+ ATPase [88,89]. In rat cerebellar membranes, ALA impaired signal transduction by lowering the production of the intracellular second messenger cAMP, by a mechanism possibly involving direct oxidative damage to adenylate cyclase [90].
With regard to ALA and sugar metabolism, it is interesting to note that rat cerebral cortex particles displayed an increased glucose uptake (about 145%) when incubated with 2.4 mM ALA in the first hour, subsequently decreasing to around half the control values after 5 h [32]. Other studies on cerebellum particles observed a reduced glucose uptake following ALA administration (87% during 1 h incubation) [33].
3. Heme Deficiency-Induced Dysfunction
3.1. Alterations in Heme-Dependent Signal Transduction
Growing evidence is emerging in support of heme functioning as a key regulatory and signaling molecule, playing essential roles in the viability of neurons. SA-induced heme deficiency leads to impairments in nerve growth factor (NGF)-induced neuronal differentiation via the early disruption of gene expression [91,92]. In NGF-induced PC12 mature cells (rat pheochromocytoma clonal cells, resembling cells of neural crest origin and a standard model for studying neural development in vitro) SA causes caspase-dependent apoptosis, the activation of the pro-apoptotic c-Jun N-terminal kinase (JNK) and the inhibition of the pro-survival Ras-ERK 1/2 signaling pathways, with downstream inhibitory effects on the gene expression of some regulators (including p53, c-myc, PI3K, Ras, MAPK, JAK1, and MEKK1), and structural proteins (such as SVOP, NCAM, and NPY, and survival motor neuron protein), as well as the upregulation of several stress-induced genes (such as Hsp70, Hsp27, GLUT1, and transferrin receptor) [93]. Notably, mice that lack NPY expression were found to be much more susceptible to seizures [94]. The inactivation of ERK1/2 is likely linked to hypophosphorylation and the reversibly reduced expression of the N-Methyl-d-aspartate receptor (NMDAR) caused by heme depletion, with morphologic changes and neurite loss as a final result [71,95].
3.2. Cytochrome Dysfunction
In addition to oxidative damage, mitochondrial failure in AHP could also be driven by cytochrome dysfunction due to heme depletion. Of note, heme is yielded in mitochondria: following an initial series of reactions in the cytosol, coproporphyrin III is imported in mitochondria by ABCB6, a homodimeric porphyrin transporter located in the outer mitochondrial membrane, to undergo the final steps of heme biosynthesis [96]. Several alterations in brain oxidative phosphorylation have been reported in T1/T2 mice, with an increase of Complex II activity in the basal state, and a significant reduction of all four complexes after treatment with phenobarbital, compared to wild-type controls [97]. Recently, a pilot study found an altered mitochondrial bioenergetic profile in AHP patients with moderate-to-severe symptoms, who had a significantly lower oxygen consumption rate at the basal and maximal state, compared to controls and AHP patients with mild or no symptoms [98].
Somewhat unrelated to neuronal damage, it has been observed that a liver isoenzyme (CYP2A5) of the P450 cytochrome family suffers a heme-reversible inhibition in activity and mRNA levels in T1/T2 mice challenged with phenobarbital [99].
3.3. Effects on Tryptophan and Glucose Metabolism
Tryptophan 2,3-dioxygenase (TDO, formerly known as tryptophan pyrrolase) is a cytosolic hemoprotein that plays a rate-limiting role in tryptophan degradation [100]. When inhibited in the liver, plasma tryptophan build-up occurs, with augmented tryptophan brain uptake [101] and, reasonably, enhanced serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) synthesis. Early studies showed that heme-depleted rats present a dramatic reduction of hepatic TDO activity and an associated increased concentration of brain tryptophan, 5-HT, and 5-HIAA, which was almost completely reversed by parenteral administration of heme [102]. Interestingly, some authors have identified a resemblance between the neurovisceral manifestations of AHP and serotonin syndrome [102].
On the other hand, however, a combined treatment of AIA and DDC was shown to significantly impact the tryptophan metabolic pathway in rat liver in another direction: while serotonin levels decreased and tryptophan concentration rose, an unexpected dose-dependent boost of TDO activity and a reduction of saturation (holoenzyme/apoenzyme ratio) were noted, together with a dose-dependent inhibition of phosphoenolpyruvate carboxykinase (PEPCK) activity [103]. Thus, it has been speculated that the depletion of heme (and possibly pyridoxal phosphate -see below) by these porphyrinogenic drugs may lead to an enhanced substrate-mediated activity of TDO and a switch from the serotonin to the kynurenine pathway, with an increased conversion of tryptophan to kynurenine (whose formation is increased in porphyric animals [103]) and quinolinate, which is an inhibitor of PEPCK [103]. While urinary metabolome studies in AIP patients did not confirm any differences from controls in the serotoninergic route of tryptophan metabolism, the kynurenine pathway was significantly altered with higher urinary concentrations of kynurenine and its metabolites, thus, indirectly confirming an activation of TDO [104]. Notably, PEPCK is a key enzyme in the gluconeogenetic pathway, and the occurrence of its inhibition in a porphyric setting could help to elucidate the role played by glucose in the pathogenesis of AHP. Gluconeogenesis impairment, in the form of PEPCK [28,105] or glycogen phosphorylase (GP) [28] inhibition, has been demonstrated in rats variously treated with porphyrinogenic drugs (AIA, DDC, phenobarbital, or others).
Regarding glucose utilization, 18F-FDG PET scans of fasted T1/T2 mice showed a reduced glucose cerebral uptake in the primary somatosensorial and neocortex areas, which reverted to the control values after Hmbs-liver gene delivery. Additionally, increased levels of liver and serum ketone bodies, increased hepatic glycogen storage, and reduced pyruvate, lactate, and alanine were recorded, suggestive of a different metabolic response to fasting compared to control or Hmbs-liver gene supplied T1/T2 mice [106].
Finally, it is worth mentioning that xanthurenic acid, a product of kynurenine metabolism, has shown potent inhibitory effects on sepiapterin reductase, an enzyme involved in tetrahydrobiopterin (BH4) biosynthesis [107]. Tetrahydrobiopterin acts as a cofactor in the hydroxylation of tyrosine, phenylalanine, and tryptophan. An increase of its levels, as a consequence of impaired xanthurenic acid production, has been linked to hyperalgesia [108]. On the other hand, the increased activity of TDO, such as that observed in the aforementioned studies, or a higher production of xanthurenic acid ([109,110], see Section 4.2), could have an impact on tetrahydrobiopterin availability, with some effect, for instance, on nitric oxide biosynthesis ([111], see Section 3.4).
3.3.1. Effect on Melatonin and Circadian Cycles
Interestingly, it has been evidenced that melatonin, a neurohormone with antioxidizing properties and a derivative of serotonin, is able to revert some of the porphyrinogenic toxicity induced by ALA and DDC [112]. AIP patients are known to have decreased levels of plasma melatonin [113], a finding which was confirmed in ALA-treated animal models and in pinealocytes cultures [85]. The decrease in melatonin production has been attributed by some to GABA-like activity exerted by ALA on pinealocytes (whose vascular supply stands outside the BBB) [85].
In mammals, some of the most important factors involved in circadian oscillations depend on heme for their functions: Clock is a hemeprotein with gas-sensor properties, which forms complexes with NO and CO [114]; human Period-2 (hPer2) stability is regulated by heme, which acts as a regulatory ligand [115]; the nuclear hormone receptors (NHRs), REV-ERBα and REV-ERBb bind heme to regulate their negative action on transcription of their target genes [116,117,118]. Thus, it has been conjectured that heme depletion could be a factor that contributes to circadian disturbances in porphyria: in fact, mice fed with griseofulvin, a well-known inducer of porphyria and a heme depleter [30,31], have a shorter circadian period (as measured on the rhythm of core body temperature levels) phase advances in diurnal rhythm, which were all reversible upon heme supplementation [119].
3.4. Effects on Nitric Oxide Synthase
From a clinical and radiological perspective, some CNS manifestations of porphyria have been likened to a picture of posterior reversible encephalopathy syndrome (PRES) [10,11]. The physiopathologic mechanism underlying PRES is generally considered to involve, together with endothelial dysfunction, a reduction of vasodilatory nitric oxide [10,120]. Nitric oxide synthases are hemoproteins, for which the prosthetic heme group is –at least in the neuronal form (nNOS)- a requisite for the dimerization of the enzyme subunits and the correct binding of substrates [121]; interestingly, they also require tetrahydro-biopteroate as a cofactor, and relative BH4 deficiency has been linked to uncoupled reactions and the excessive production of the highly reactive species peroxinitrite [111]. NOS is also found throughout the neuronal populations of the myenteric plexus [122], where its dysfunction causes a number of dysautonomic gastrointestinal symptoms, as has been described for patients with nocturnal paroxysmal hemoglobinuria and sickle cell anemia [123,124]. Thus, an impairment in NOS activity following acute or acute-on-chronic heme depletion has been proposed as an explanation for the neurovisceral manifestation of AHP [125]. While nNOS, together with brain soluble guanylate cyclase and heme oxygenase 2, was found to maintain normal levels of activity in the brain as a whole in T1/T2 mice [99], subsequent studies in wild-type mice found a decrease of mitochondrial NOS and an increase of (inducible) iNOS glial expression following an acute ALA intraperitoneal injection [34,126]. Rats treated with SA intraperitoneal injections displayed significantly reduced nitrite/nitrate urinary output, soluble guanylate cyclase activity, kidney homogenate NOS activity, and diminished vascular sensitivity to acetylcholine and MAHMA-NONOate, a NO donor, even in the absence of overt cardiovascular dysregulation [127,128].
4. Other Proposed Mechanisms of Toxicity
4.1. Cataplerosis Induced by Succinyl-CoA Deficiency
Massive consumption of succinyl-CoA, caused by the overactivation of ALAS, has been proposed as a possible mechanism of mitochondrial damage leading to bioenergetic failure. Indeed, succinyl-CoA is needed as a substrate for the tricarboxylic acid cycle, which in turn provides the reduced species NADPH and FADH2 to the mitochondrial respiratory chain. Substrate depletion, or cataplerosis, has been observed in hepatocytes, since T1/T1 mice exposed to phenobarbital displayed selectively reduced liver activities of the TCA cycle enzymes citrate synthase, α-ketoglutarate dehydrogenase, and succinate dehydrogenase (the last two are directly involved in the synthesis or utilization of succinyl-CoA) [129]. The hypothesis of a reprogramming of the glucose metabolism in hepatic porphyrias is also supported by studies on urinary metabolome: asymptomatic AIP patients, compared to patients with porphyria cutanea tarda, display higher urinary concentrations in glycolytic intermediates such as acetate, citrate and pyruvate [130], thus hinting at a possible specific dysregulation in the balance among glycolysis, TCA cycle, and phosphorylative oxidation in patients with HMBS mutations.
4.2. Pyridoxal Phosphate Consumption
An interesting hypothesis has been proposed regarding vitamin B6 (functional) deficiency in patients with AHP. ALA synthase requires pyridoxal phosphate to exert its enzymatic activity, so that ALAS over-induction might, theoretically, lead to depletion of vitamin B6.
An early report described a case of an AIP patient whose ALA and PBG urinary excretion decreased following induction of vitamin B6 deficiency, and rose following pyridoxine supplementation [131], corroborating the hypothesis of a pivotal role played by pyridoxal phosphate in the heme biosynthetic pathway. A few years later, a seminal study found significantly lower concentrations of plasma pyridoxal phosphate and significantly higher xanthurenic acid excretion in a group of 21 patients with AIP, compared to an age-matched healthy control group [109]. In a more recent unindexed study, a population of 50 patients with HMBS mutations were found to have normal plasma pyridoxal phosphate levels, but a significantly higher urinary 3-hydroxykynurenine/xanthurenic acid ratio, homocysteine and methionine levels compared to controls, suggestive of a functional vitamin B6 deficiency in the AIP group [110]. Of further interest, a poorer vitamin status was detected in a population of AIP and VP patients that concerned vitamin B6, vitamin B12, and red blood cell folates. In this study, symptomatic patients displayed lower vitamin B group levels and a significant association was identified between lower plasma pyridoxal phosphate and a history of recurrent attacks [132]. Previously mentioned urinary metabolome studies also found an impairment in the vitamin B6-mediated conversion of kynurenine to kynurenic acid in AIP patients [104].
Inborn enzymatic dysfunctions, leading to pyridoxal phosphate deficiency, are at the origin of some forms of vitamin B-responsive epilepsies (pyridoxine dependent epilepsy and pyridoxal phosphate dependent epilepsy) [133,134,135]. Moreover, toxicity due to isoniazid, an antitubercular drug that impairs vitamin B6 metabolism, appears to cause nerve lesions and a clinical picture comparable to those found in AIP [136,137]. Thus, it might be speculated that pyridoxal phosphate may also play a role in defining the neurologic clinical picture of patients with AHP.
5. Conclusions
The pathway leading to the biosynthesis of heme is inscribed into a complex network of interactions that also includes some of the most fundamental processes of basal metabolism. Therefore, a disruption in any of the steps of this pathway is likely to interfere in a pleiotropic fashion with the viability of cells and tissues, thus giving rise to multiple possible mechanisms of pathogenesis (Figure 1). Here, we have endeavored to outline the (sometimes contradictory) evidence that has been gathered to date, regarding the mechanisms of neurotoxicity in acute hepatic porphyrias.
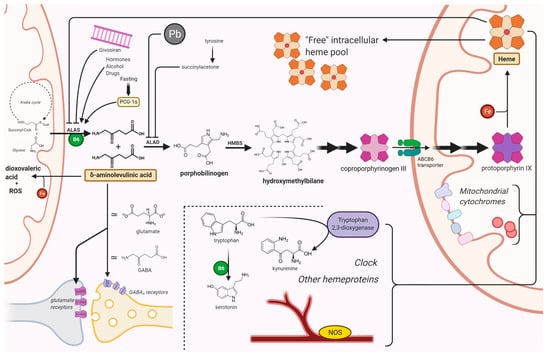
Figure 1.
Mechanisms of neuronal damage in acute hepatic porphyrias. ALAS condenses glycine and succinyl-CoA into ALA, in the first step of the biosynthesis of heme. After an initial series of reactions in the cytosol, coproporphyrin III is imported in mitochondria by ABCB6, a homodimeric porphyrin transporter located in the outer mitochondrial membrane. ALAS is induced by porphyrinogenic stimuli (e.g., fasting, alcohol, or certain drugs) which supposedly induce an increased metabolic demand for heme. In particular, fasting induces ALAS1 expression via the peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α). Lead and succinyl acetone cause a porphyria-like picture since they inhibit ALAD. Acute intermittent porphyria, the most common AHP, is an autosomal dominant disease caused by an abnormal function of HMBS. ALA and (in most cases) PBG accumulate in patients with acute porphyrias during neurovisceral attacks. Givosiran, a siRNA-based drug for the treatment of AHPs, acts by impairing ALAS mRNA translation in the liver. Among other toxic effects, ALA undergoes auto-enolization to yield the highly reactive dioxovaleric acid (DOVA) and other oxidant species; it also interferes with GABA and glutamate receptors. Lack of heme has pleiotropic effects on cytochromes, nitric oxide synthases, tryptophan 2,3-dioxygenase, and several other hemeprotein; it may also affect the regulatory functions of the intracellular “free” heme pool. Pyridoxal phosphate figures among the factors involved in this highly connected network of reactions. Other possible mechanisms of neuronal damage are described in the text. ABCB6, ATP-binding cassette transporter B6; ALA, δ-aminolevulinic acid; ALAD, ALA dehydratase; ALAS, ALA synthase; B6, pyridoxal phosphate; Fe, iron; GABA, γ-aminobutyric acid; HMBS, hydroxymethylbilane synthase; NOS, nitric oxide synthase; Pb, lead. Created with BioRender.com (last accessed date: 22 November 2021).
Porphyric patients suffer a considerable burden of disease [138], with both debilitating chronic symptoms and the always lurking menace of life-threatening porphyric attacks. Successfully recognizing an acute hepatic porphyria represents a major diagnostic challenge for the clinician [139,140]. Until recently, therapeutic options consisted of the treatment of acute attacks with parenteral heme arginate and glucose infusions, avoidance of triggering factors, and—in extreme cases—liver transplantation [141,142].
A promising new drug, givosiran, has provided potential avenues as a new effective weapon in the armamentarium of the clinician [143]. Givosiran is a small interfering RNA (siRNA) that targets ALAS mRNA in hepatocytes, exploiting the RNA-induced silencing complex (RISC) to impair its translation. Even though some adverse events, possibly related to induction of relative heme deficiency [144,145,146] and partly reversible [144,147], have been recorded, givosiran has proved to be very effective in reducing the biochemical markers of disease, lowering the mean annualized rate of porphyric attacks, and improving the quality of life of patients [143].
In our opinion, this does not abate the need to clarify the mechanisms of pathogenesis in AHPs. On the contrary, the encouraging perspective offered by these therapeutic advancements raises the stakes for answering the unsolved questions posed by such a fascinating and challenging field of research.
6. Acronyms
5-HIAA 5-hydroxyindoleacetic acid. 5-HT 5-hydroxytryptamine (or serotonin). ABCB6 ATP-binding cassette transporter B6. ADP ALA dehydratase deficiency porphyria. AHP acute hepatic porphyrias. AIA 2-allyl-2-isopropylacetamide. AIP acute intermittent porphyria. ALA aminolevulinic acid. ALAD aminolevulinic acid-dehydratase. ALAS aminolevulinic acid synthase. BH4 tetrahydrobiopteroate. CNS Central Nervous System. CSF cerebro-spinal fluid. CuZnSOD copper-zinc superoxide dismutase. DDC 3:5-diethoxycarbonyl-1,4-dihydrocollidine. DOVA dioxovaleric acid. HCP hereditary coproporphyria. HMBS hydroxymethylbilane-synthase or porphobilinogen-deaminase (PBGD). IRE iron-responsive element. IRP1 iron regulatory protein 1. JNK(s) c-Jun N-terminal kinase(s). LT liver transplantation. MAHMA-NONOate methylamine hexamethylene methylamine NONOate. NGF nerve growth factor. nNOS neuronal nitric oxyde synthase. PBG porphobilinogen. PC12 (cells) rat pheochromocytoma clonal cells. PEPCK phosphoenolpyruvate carboxykinase. PRES Posterior reversible encephalopathy syndrome. SA succinylacetone. SAME succinylacetone methyl esther. TCA tricarboxylic acid. TDO tryptophan 2:3-dioxygenase. UTR untranslated region. VP variegate porphyria.
Author Contributions
Conceptualization, A.R. and P.V.; writing—original draft preparation, A.R.; writing—review and editing, E.D.P., M.M. and P.V.; supervision, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bissell, D.M.; Anderson, K.E.; Bonkovsky, H.L. Porphyria. N. Eng. J. Med. 2017, 377, 862–872. [Google Scholar] [CrossRef]
- Simon, N.G.; Herkes, G.K. The neurologic manifestations of the acute porphyrias. J. Clin. Neurosci. 2011, 18, 1147–1153. [Google Scholar] [CrossRef]
- Bissell, D.M.; Wang, B. Acute Hepatic Porphyria. J. Clin. Transl. Hepatol. 2015, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bonkowsky, H.L.; Schady, W. Neurologic manifestations of acute porphyria. Semin. Liver Dis. 1982, 2, 108–124. [Google Scholar] [CrossRef]
- Meyer, U.A.; Schuurmans, M.M.; Lindberg, R.L. Acute porphyrias: Pathogenesis of neurological manifestations. Semin. Liver Dis. 1998, 18, 43–52. [Google Scholar] [CrossRef]
- Ventura, P.; Cuoghi, C.; Marcacci, M. The acute porphyric attack: A difficult diagnosis for a potential lethal event in emergency medicine. J. Emerg. Med. Trauma Surg. Care 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Roveri, G.; Nascimbeni, F.; Rocchi, E.; Ventura, P. Drugs and acute porphyrias: Reasons for a hazardous relationship. Postgrad. Med. 2014, 126, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Pischik, E.; Kauppinen, R. Neurological Manifestations of Acute Intermittent Porphyria. Cell. Mol. Biol. 2015, 55, 72–83. [Google Scholar]
- Souza, P.V.S.; Badia, B.M.L.; Farias, I.B.; Gonçalves, E.A.; Pinto, W.B.V.R.; Oliveira, A.S.B. Acute hepatic porphyrias for the neurologist: Current concepts and perspectives. Arq. Neuropsiquiatr. 2021, 79, 68–80. [Google Scholar] [CrossRef]
- Jaramillo-Calle, D.A.; Solano, J.M.; Rabinstein, A.A.; Bonkovsky, H.L. Porphyria-induced posterior reversible encephalopathy syndrome and central nervous system dysfunction. Mol. Genet. Metab. 2019, 128, 242–253. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, X.; Wang, Y.; Zhao, R.; Qu, L.; Pei, H.; Tuo, M.; Zhang, Y.; Song, Y.; Ji, X.; et al. Acute intermittent porphyria presenting with seizures and posterior reversible encephalopathy syndrome: Two case reports and a literature review. Medicine 2018, 97, e11665. [Google Scholar] [CrossRef]
- Bylesjö, I.; Brekke, O.L.; Prytz, J.; Skjeflo, T.; Salvesen, R. Brain magnetic resonance imaging white-matter lesions and cerebrospinal fluid findings in patients with acute intermittent porphyria. Eur. Neurol. 2004, 51, 1–5. [Google Scholar] [CrossRef]
- Nordmann, Y.; Puy, H.; Da Silva, V.; Simonin, S.; Robreau, A.M.; Bonaiti, C.; Phung, L.N.; Deybach, J.C. Acute intermittent porphyria: Prevalence of mutations in the porphobilinogen deaminase gene in blood donors in france. J. Intern. Med. 1997, 242, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.; Harper, P.; Badminton, M.; Sandberg, S.; Deybach, J.C. The incidence of inherited porphyrias in europe. J. Inherit. Metab. Dis. 2013, 36, 849–857. [Google Scholar] [CrossRef]
- Chen, B.; Solis-Villa, C.; Hakenberg, J.; Qiao, W.; Srinivasan, R.R.; Yasuda, M.; Balwani, M.; Doheny, D.; Peter, I.; Chen, R.; et al. Acute intermittent porphyria: Predicted pathogenicity of HMBS variants indicates extremely low penetrance of the autosomal dominant disease. Hum. Mut. 2016, 37, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- McEwin, R.; Lawn, J.; Jonas, C.T. A survey of porphyria among psychiatric patients. Med. J. Aust. 1972, 2, 303–306. [Google Scholar] [CrossRef]
- Golechha, G.R.; Chatterjee, S.B.; Sethi, B.B.; Agarwal, S.S. Acute porphyria amongst psychiatric patients. Indian J. Psychiatry 1981, 23, 365–369. [Google Scholar]
- Tishler, P.V.; Woodward, B.; O’Connor, J.; Holbrook, D.A.; Seidman, L.J.; Hallett, M.; Knighton, D.J. High prevalence of intermittent acute porphyria in a psychiatric patient population. Am. J. Psychiatry 1985, 142, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Larochelle, J.; Lambert, M.; de Weerd, A.W.; Gianella-Borradori, A.; Michaud, J.; Grenier, A.; Ogier, H.; Gauthier, M.; Lacroix, J.; et al. Neurologic Crises in Hereditary Tyrosinemia. N. Engl. J. Med. 1990, 322, 432–437. [Google Scholar] [CrossRef]
- Bissell, D.M.; Lai, J.C.; Meister, R.K.; Blanc, P.D. Role of delta-aminolevulinic acid in the symptoms of acute porphyria. Am. J. Med. 2015, 128, 313–317. [Google Scholar] [CrossRef]
- Sassa, S.; Kappas, A. Hereditary tyrosinemia and the heme biosynthetic pathway. profound inhibition of δ-aminolevulinic acid de- hydratase activity by succinylacetone. J. Clin. Investig. 1983, 71, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.J.; Cooper, J.B.; Wood, S.P.; Shoolingin-Jordan, P.M. Lead poisoning, haem synthesis and 5-aminolaevulinic acid dehydratase. Trends Biochem. Sci. 1998, 23, 217–221. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Brun, A.; Sandberg, S. Mechanisms of photosensitivity in porphyric patients with special emphasis on erythropoietic protoporphyria. J. Photochem. Photobiol. B 1991, 10, 285–302. [Google Scholar] [CrossRef]
- Souza, P.V.S.; Badia, B.M.L.; Farias, I.B.; Pinto, W.B.V.R.; Oliveira, A.S.B. Acute hepatic porphyria: Pathophysiological basis of neuromuscular manifestations. Front. Neurosci. 2021, 15, 715523. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.S.; Levere, R.D.; Lieberman, J.S.; Cardinal, R.A.; Watson, C.J. Presynaptic neuromuscular inhibition by porphobilinogen and porphobilin. Proc. Natl. Acad. Sci. USA 1971, 68, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Wyss, P.A.; Boynton, S.; Chu, J.; Roth, A.S. Tissue distribution of succinylacetone in the rat in vivo: A possible basis for neurotoxicity in hereditary infantile tyrosinemia. Biochim. Biophys. Acta Mol. Basis Dis. 1993, 1182, 323–328. [Google Scholar] [CrossRef]
- Lelli, S.M.; de Viale, L.C.S.M.; Mazzetti, M.B. Response of glucose metabolism enzymes in an acute porphyria model: Role of reactive oxygen species. Toxicology 2005, 216, 49–58. [Google Scholar] [CrossRef]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.K.; Chin, S.; Wu, P.H.; Meyer, U.A.; Spiegelman, B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1α. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef]
- De Matteis, F.; Rimington, C. Disturbance of porphyrin metabolism caused by griseofulvin in mice. Br. J. Dermatol. 1963, 75, 91–104. [Google Scholar] [CrossRef]
- Inafuku, K.; Takamiyagi, A.; Oshiro, M.; Kinjo, T.; Nakashima, Y.; Nonaka, S. Alteration of mRNA levels of δ-aminolevulinic acid synthase, ferrochelatase and heme oxygenase-1 in griseofulvin induced protoporphyria mice. J. Dermatol. Sci. 1999, 19, 189–198. [Google Scholar] [CrossRef]
- Juknat, A.A.; Kotler, M.L.; del Carmen Batlle, A.M. High δ-aminolevulinic acid uptake in rat cerebral cortex: Effect on porphyrin biosynthesis. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1995, 111, 143–150. [Google Scholar] [CrossRef]
- Princ, F.G.; Juknat, A.A.; Batlle, A.M.D.C. Porphyrinogenesis in rat cerebellum. Effect of high δ-aminolevulinic acid concentration. Gen. Pharmacol. Vasc. Syst. 1994, 25, 761–766. [Google Scholar] [CrossRef]
- Lavandera, J.; Rodr’ıguez, J.; Ruspini, S.; Meiss, R.; Zuccoli, J.R.; Mart’ınez, M.D.C.; Gerez, E.; Batlle, A.; Buzaleh, A.M. Pleiotropic effects of 5-aminolevulinic acid in mouse brain. Biochem. Cell Biol. 2016, 94, 297–305. [Google Scholar] [CrossRef]
- Felitsyn, N.; McLeod, C.; Shroads, A.L.; Stacpoole, P.W.; Notterpek, L. The heme precursor delta-aminolevulinate blocks peripheral myelin formation. J. Neurochem. 2008, 106, 2068–2079. [Google Scholar] [CrossRef]
- Yasuda, M.; Desnick, R.J. Murine models of the human porphyrias: Contributions toward understanding disease pathogenesis and the development of new therapies. Mol. Genet. Metab. 2019, 128, 332–341. [Google Scholar] [CrossRef]
- Lindberg, R.L.; Porcher, C.; Grandchamp, B.; Ledermann, B.; Bu¨rki, K.; Brandner, S.; Aguzzi, A.; Meyer, U.A. Porphobilinogen deaminase deficiency in mice causes a neuropathy resembling that of human hepatic porphyria. Nat. Genet. 1996, 12, 195–199. [Google Scholar] [CrossRef]
- Lindberg, R.L.; Martini, R.; Baumgartner, M.; Erne, B.; Borg, J.; Zielasek, J.; Ricker, K.; Steck, A.; Toyka, K.V.; Meyer, U.A. Motor neuropathy in porphobilinogen deaminase-deficient mice imitates the peripheral neuropathy of human acute porphyria. J. Clin. Investig. 1999, 103, 1127–1134. [Google Scholar] [CrossRef]
- Yasuda, M.; Gan, L.; Chen, B.; Yu, C.; Zhang, J.; Gama-Sosa, M.A.; Pollak, D.D.; Berger, S.; Phillips, J.D.; Edelmann, W.; et al. Homozygous hydroxymethylbilane synthase knock-in mice provide pathogenic insights into the severe neurological impairments present in human homozygous dominant acute intermittent porphyria. Hum. Mol. Genet. 2019, 28, 1755–1767. [Google Scholar] [CrossRef]
- Ennis, S.R.; Novotny, A.; Xiang, J.; Shakui, P.; Masada, T.; Stummer, W.; Smith, D.E.; Keep, R.F. Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 2003, 959, 226–234. [Google Scholar] [CrossRef]
- Novotny, A.; Xiang, J.; Stummer, W.; Teuscher, N.S.; Smith, D.E.; Keep, R.F. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J. Neurochem. 2000, 75, 321–328. [Google Scholar] [CrossRef]
- Shu, C.; Shen, H.; Teuscher, N.S.; Lorenzi, P.J.; Keep, R.F.; Smith, D.E. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: Studies in rat choroid plexus epithelial cells in primary culture. J. Pharmacol. Exp. Ther. 2002, 301, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Berger, U.V.; Hediger, M.A. Distribution of peptide transporter PEPT2 mRNA in the rat nervous system. Anat. Embryol. 1999, 199, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Groneberg, D.A.; D¨oring, F.; Nickolaus, M.; Daniel, H.; Fischer, A. Expression of PEPT2 peptide transporter mRNA and protein in glial cells of rat dorsal root ganglia. Neurosci. Lett. 2001, 304, 181–184. [Google Scholar] [CrossRef]
- Whetsell, W.O. Porphyrin-heme biosynthesis in organotypic cultures of mouse dorsal root ganglia. Effects of heme and lead on porphyrin synthesis and peripheral myelin. J. Clin. Investig. 1984, 74, 600–607. [Google Scholar] [CrossRef]
- Xiang, J.; Hu, Y.; Smith, D.E.; Keep, R.F. PEPT2-mediated transport of 5-aminolevulinic acid and carnosine in astrocytes. Brain Res. 2006, 1122, 18–23. [Google Scholar] [CrossRef]
- Shen, H.; Smith, D.E.; Keep, R.F.; Brosius, F.C. Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol. Pharm. 2004, 1, 248–256. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, H.; Keep, R.F.; Smith, D.E. Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J. Neurochem. 2007, 103, 2058–2065. [Google Scholar] [CrossRef]
- Tchernitchko, D.; Tavernier, Q.; Lamoril, J.; Schmitt, C.; Talbi, N.; Lyoumi, S.; Robreau, A.M.; Karim, Z.; Gouya, L.; Thervet, E.; et al. A Variant of Peptide Transporter 2 Predicts the Severity of Porphyria-Associated Kidney Disease. J. Am. Soc. Nephrol. 2017, 28, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Sobin, C.; Flores-Montoya, M.G.; Gutierrez, M.; Parisi, N.; Schaub, T. δ-Aminolevulinic acid dehydratase single nucleotide polymorphism 2 (ALAD2) and peptide transporter 2*2 haplotype (hPEPT2*2) differently influence neurobehavior in low-level lead exposed children. Neurotoxicol. Teratol. 2015, 47, 137–145. [Google Scholar] [CrossRef]
- Chen, N.H.; Reith, M.E.; Quick, M.W. Synaptic uptake and beyond: The sodium-and chloride-dependent neurotransmitter transporter family SLC6. Pflüger’s Gers Arch. 2004, 447, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Baglo, Y.; Gabrielsen, M.; Sylte, I.; Gederaas, O.A. Homology Modeling of Human γ-Butyric Acid Transporters and the Binding of Pro-Drugs 5-Aminolevulinic Acid and Methyl Aminolevulinic Acid Used in Photodynamic Therapy. PLoS ONE 2013, 8, e65200. [Google Scholar] [CrossRef]
- Tran, T.T.; Mu, A.; Adachi, Y.; Adachi, Y.; Taketani, S. Neurotransmitter transporter family including SLC6A6 and SLC6A13 contributes to the 5-aminolevulinic acid (ALA)-induced accumulation of protoporphyrin IX and photodamage, through uptake of ALA by cancerous cells. Photochem. Photobiol. 2014, 90, 1136–1143. [Google Scholar] [CrossRef]
- Frølund, S.; Marquez, O.C.; Larsen, M.; Brodin, B.; Nielsen, C.U. δ-aminolevulinic acid is a substrate for the amino acid transporter SLC36A1 (HPAT1). Br. J. Pharmacol. 2010, 159, 1339–1353. [Google Scholar] [CrossRef]
- Palladino, S.P.; Helton, E.S.; Jain, P.; Dong, C.; Crowley, M.R.; Crossman, D.K.; Ubogu, E.E. The human blood-nerve barrier transcriptome. Sci. Rep. 2017, 7, 17477. [Google Scholar] [CrossRef] [PubMed]
- Kazamel, M.; Desnick, R.J.; Quigley, J.G. Porphyric neuropathy: Pathophysiology, diagnosis, and updated management. Curr. Neurol. Neurosci. Rep. 2020, 20, 56. [Google Scholar] [CrossRef]
- Cutler, M.G.; Turner, J.M.; Moore, M.R. A comparative study of the effects of δ-aminolaevulinic acid and the GABAA agonist, muscimol, in rat jejunal preparations. Pharmacol. Toxicol. 1991, 69, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Sylantiev, C.; Schoenfeld, N.; Mamet, R.; Groozman, G.B.; Drory, V.E. Acute neuropathy mimicking porphyria induced by aminolevulinic acid during photodynamic therapy. Muscle Nerve 2005, 31, 390–393. [Google Scholar] [CrossRef]
- Lombardo, M.E.; Araujo, L.S.; Batlle, A. 5-Aminolevulinic acid synthesis in epimastigotes of Trypanosoma cruzi. Int. J. Biochem. Cell Biol. 2003, 35, 1263–1271. [Google Scholar] [CrossRef]
- Alves, J.M.; Voegtly, L.; Matveyev, A.V.; Lara, A.M.; da Silva, F.M.; Serrano, M.G.; Buck, G.A.; Teixeira, M.M.; Camargo, E.P. Identification and phylogenetic analysis of heme synthesis genes in trypanosomatids and their bacterial endosymbionts. PLoS ONE 2011, 6, e23518. [Google Scholar] [CrossRef][Green Version]
- Sica, R.; Gonzalez, S.C.; Sanz, O.; Mirkin, G. Peripheral nervous system involvement in human and experimental chronic american trypanosomiasis. Bull Soc. Pathol. Exot. 1995, 88, 156–163. [Google Scholar] [PubMed]
- Lopes, E.R.; Tafuri, W.L. Involvement of the autonomic nervous system in Chagas heart disease. Rev. Soc. Bras. Med. Trop. 1983, 16, 206–212. [Google Scholar] [CrossRef][Green Version]
- P´erez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Lissing, M.; Nowak, G.; Adam, R.; Karam, V.; Boyd, A.; Gouya, L.; Meersseman, W.; Melum, E.; O-ldakowska-Jedynak, U.; Reiter, F.P.; et al. Liver transplantation for acute intermittent porphyria. Liver Transplant. 2021, 27, 491–501. [Google Scholar] [CrossRef]
- Dowman, J.K.; Gunson, B.K.; Bramhall, S.; Newsome, P.N.; Badminton, M.N. Liver transplantation from donors with acute intermittent porphyria. Ann. Intern. Med. 2011, 154, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, J.R.; Simone, J.J.; Beattie, D.S. Detection and regulation of δ-aminolevulinic acid synthetase activity in the rat brain. Arch. Biochem. Biophys. 1978, 189, 86–91. [Google Scholar] [CrossRef]
- De Matteis, F.; Zetterlund, P.; Wetterberg, L. Brain 5-aminolaevulinate synthase. Developmental aspects and evidence for regulatory role. Biochem. J. 1981, 196, 811–817. [Google Scholar] [CrossRef]
- De Matteis, F.; Ray, D.E. Studies on Cerebellar Haem Metabolism in the Rat In Vivo. J. Neurochem. 1982, 39, 551–556. [Google Scholar] [CrossRef]
- Ruspini, S.F.; Zuccoli, J.R.; Lavandera, J.V.; Mart´ınez, M.D.C.; Oliveri, L.M.; Gerez, E.N.; Batlle, A.M.D.C.; Buzaleh, A.M. Effects of volatile anaesthetics on heme metabolism in a murine genetic model of Acute Intermittent Porphyria. A comparative study with other por-phyrinogenic drugs. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1296–1305. [Google Scholar] [CrossRef]
- Kang, K.; Anderson-Burham, L.A.; Bloomer, J.R. Effect of succinylacetone administration on brain heme metabolism and behavior in mice. Biochem. Pharmacol. 1987, 36, 3084–3086. [Google Scholar] [CrossRef]
- Chernova, T.; Steinert, J.R.; Guerin, C.J.; Nicotera, P.; Forsythe, I.D.; Smith, A.G. Neurite degeneration induced by heme deficiency mediated via inhibition of NMDA receptor-dependent extracellular signal- regulated kinase 1/2 activation. J. Neurosci. 2007, 27, 8475–8485. [Google Scholar] [CrossRef]
- Rocha, M.E.M.; Dutra, F.; Bandy, B.; Baldini, R.L.; Gomes, S.L.; Faljoni-Al´ario, A.; Liria, C.W.; Miranda, M.T.M.; Bechara, E.J.H. Oxidative damage to ferritin by 5-aminolevulinic acid. Arch. Biochem. Biophys. 2003, 409, 349–356. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Bechara, E.J. 5-Aminolevulinic acid induces lipid peroxidation in cardiolipin-rich liposomes. Arch. Biochem. Biophys. 1993, 305, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Castilho, R.F.; Valle, V.G.; Bechara, E.J.; Vercesi, A.E. Calcium-dependent mitochondrial oxidative damage pro moted by 5-aminolevulinic acid. Biochim. Biophys. Acta Mol. Basis Dis. 1992, 1180, 201–206. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Castilho, R.F.; Meinicke, A.R.; Valle, V.G.; Hermes-Lima, M.; Bechara, E.J. Oxidative damage of mitochondria induced by 5-aminolevulinic acid: Role of Ca2+ and membrane protein thiols. Biochim. Biophys. Acta (BBA)—Bioenerg. 1994, 1188, 86–92. [Google Scholar] [CrossRef]
- Laafi, J.; Homedan, C.; Jacques, C.; Gueguen, N.; Schmitt, C.; Puy, H.; Reynier, P.; Carmen Martinez, M.; Malthi‘ery, Y. Pro-oxidant effect of ALA is implicated in mitochondrial dysfunction of HepG2 cells. Biochimie 2014, 106, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Emanuelli, T.; Pagel, F.W.; Porciu´ncula, L.O.; Souza, D.O. Effects of 5-aminolevulinic acid on the glutamatergic neurotransmission. Neurochem. Int. 2003, 42, 115–121. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Kleinman, C.G.; Demasi, M.; Bechara, E.J. 5-aminolevulinic acid induces iron release from ferritin. Arch. Biochem. Biophys. 1995, 316, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.E.; Bandy, B.; Costa, C.A.; de Barros, M.P.; Pinto, A.M.; Bechara, E.J. Iron mobilization by succinylacetone methyl ester in rats. A model study for hereditary tyrosinemia and porphyrias charac- terized by 5-aminolevulinic acid overload. Free Radic. Res. 2000, 32, 343–353. [Google Scholar] [CrossRef]
- Demasi, M.; Penatti, C.A.; DeLucia, R.; Bechara, E.J. The prooxidant effect of 5-aminolevulinic acid in the brain tissue of rats: Impli- cations in neuropsychiatric manifestations in porphyrias. Free Radic. Biol. Med. 1996, 20, 291–299. [Google Scholar] [CrossRef]
- Carvalho, H.; Bechara, E.J.; Meneghini, R.; Demasi, M. Haem precursor delta-aminolaevulinic acid induces activation of the cytosolic iron regulatory protein 1. Biochem. J. 1997, 328 Pt 3, 827–832. [Google Scholar] [CrossRef]
- Brennan, M.J.; Cantrill, R.C. δ-Aminolaevulinic acid is a potent agonist for GABA autoreceptors. Nature 1979, 280, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.J.; Cantrill, R.C.; Kramer, S. Effect of delta- aminolaevulinic acid on GABA receptor binding in synaptic plasma membranes. Int. J. Biochem. 1980, 12, 833–835. [Google Scholar] [CrossRef]
- Adhikari, A.; Penatti, C.A.A.; Resende, R.R.; Ulrich, H.; Britto, L.R.G.; Bechara, E.J.H. 5-Aminolevulinate and 4, 5-dioxovalerate ions decrease GABA(A) receptor density in neuronal cells, synaptosomes and rat brain. Brain Res. 2006, 1093, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Puy, H.; Deybach, J.C.; Bogdan, A.; Callebert, J.; Baumgartner, M.; Voisin, P.; Nordmann, Y.; Touitou, Y. Increased δ aminolevulinic acid and decreased pineal melatonin production: A common event in acute porphyria studies in the rat. J. Clin. Investig. 1996, 97, 104–110. [Google Scholar] [CrossRef]
- Lin, T.C.; Lai, S.L.; Hsu, S.P.; Ro, L.S. Treatment of neuropathic pain in acute intermittent porphyria with gabapentin. J. Formos Med. Assoc. 2013, 112, 578–579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Emanuelli, T.; Prauchner, C.A.; Dacanal, J.; Zeni, A.; Reis, E.C.; De Mello, C.F.; De Souza, D.O. Intrastriatal administration of 5-aminolevulinic acid induces convulsions and body asymmetry through glutamatergic mechanisms. Brain Res. 2000, 868, 88–94. [Google Scholar] [CrossRef]
- Becker, D.; Viljoen, D.; Kramer, S. The inhibition of red cell and brain atpase by δ-aminolaevulinic acid. Biochim. Biophys. Acta Biomembr. 1971, 225, 26–34. [Google Scholar] [CrossRef]
- Russell, V.A.; Lamm, M.C.; Taljaard, J.J. Inhibition of Na+, K+-ATPase activity by delta-aminolevulinic acid. Neurochem. Res. 1983, 8, 1407–1415. [Google Scholar] [CrossRef]
- Emanuelli, T.; Pagel, F.W.; Alves, L.B.; Regner, A.; Souza, D.O. Inhibition of adenylate cyclase activity by 5-aminolevulinic acid in rat and human brain. Neurochem. Int. 2001, 38, 213–218. [Google Scholar] [CrossRef]
- Zhu, Y.; Hon, T.; Ye, W.; Zhang, L. Heme deficiency interferes with the Ras-mitogen-activated protein kinase signaling pathway and expression of a subset of neuronal genes. Cell Growth Differ. 2002, 13, 431–439. [Google Scholar] [PubMed]
- Zhu, Y.; Lee, H.C.; Zhang, L. An Examination of Heme Action in Gene Expression: Heme and Heme Deficiency Affect the Expression of Diverse Genes in Erythroid K562 and Neuronal PC12 Cells. DNA Cell Biol. 2002, 21, 333–346. [Google Scholar] [CrossRef]
- Sengupta, A.; Hon, T.; Zhang, L. Heme deficiency suppresses the expression of key neuronal genes and causes neuronal cell death. Brain Res. Mol. Brain Res. 2005, 137, 23–30. [Google Scholar] [CrossRef]
- Weinshenker, D.; Szot, P.; Miller, N.S.; Rust, N.C.; Hohmann, J.G.; Pyati, U.; White, S.S.; Palmiter, R.D. Genetic comparison of seizure control by norepinephrine and neuropeptide Y. J. Neurosci. 2001, 21, 7764–7769. [Google Scholar] [CrossRef]
- Chernova, T.; Nicotera, P.; Smith, A.G. Heme deficiency is associated with senescence and causes suppression of N-methyl-D-aspartate receptor subunits expression in primary cortical neurons. Mol. Phar. Macol. 2006, 69, 697–705. [Google Scholar] [CrossRef]
- Song, G.; Zhang, S.; Tian, M.; Zhang, L.; Guo, R.; Zhuo, W.; Yang, M. Molecular insights into the human ABCB6 transporter. Cell Discovery 2021, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Homedan, C.; Schmitt, C.; Laafi, J.; Gueguen, N.; Desquiret-Dumas, V.; Lenglet, H.; Karim, Z.; Gouya, L.; Deybach, J.C.; Simard, G.; et al. Mitochondrial energetic defects in muscle and brain of a Hmbs-/- mouse model of acute intermittent porphyria. Hum. Mol. Genet. 2015, 24, 5015–5023. [Google Scholar] [CrossRef]
- Dixon, N.; Li, T.; Marion, B.; Faust, D.; Dozier, S.; Molina, A.; Rud- Nick, S.; Bonkovsky, H.L. Pilot study of mitochondrial bioenergetics in subjects with acute porphyrias. Mol. Genet. Metab. 2019, 128, 228–235. [Google Scholar] [CrossRef]
- Jover, R.; Hoffmann, F.; Scheffler-Koch, V.; Lindberg, R.L. Limited heme synthesis in porphobilinogen deaminase-deficient mice impairs transcriptional activation of specific cytochrome P450 genes by phenobarbital. Eur. J. Biochem. 2000, 267, 7128–7137. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B. The functions and regulation of tryptophan pyrrolase. Life Sci. 1977, 21, 755–767. [Google Scholar] [CrossRef]
- Salter, M.; Hazelwood, R.; Pogson, C.I.; Iyer, R.; Madge, D.J. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem. Pharmacol. 1995, 49, 1435–1442. [Google Scholar] [CrossRef]
- Litman, D.A.; Correia, M.A. L-tryptophan: A common denominator of biochemical and neurological events of acute hepatic porphyria? Science 1983, 222, 1031–1033. [Google Scholar] [CrossRef]
- Lelli, S.M.; Mazzetti, M.B.; San Mart´ın de Viale, L.C. Hepatic alteration of tryptophan metabolism in an acute porphyria model Its relation with gluconeogenic blockage. Biochem. Pharmacol. 2008, 75, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, A.; Marcos, J.; Aguilera, P.; To-Figueras, J.; Pozo, O.J. Comprehensive analysis of the tryptophan metabolome in urine of patients with acute intermittent porphyria. J. Chromatogr. B 2017, 1060, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Lunetta, J. Acute hepatic heme depletion: Impaired gluconeogenesis in rats. Semin. Hematol. 1989, 26, 120–127. [Google Scholar] [PubMed]
- Collantes, M.; Serrano-Mendioroz, I.; Benito, M.; Molinet-Dronda, F.; Delgado, M.; Vinaixa, M.; Sampedro, A.; Enr´ıquez de Salamanca, R.; Prieto, E.; Pozo, M.A.; et al. Glucose metabolism during fasting is altered in experimental porphobilinogen deaminase deficiency. Hum. Mol. Genet. 2016, 25, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Haruki, H.; Hovius, R.; Pedersen, M.G.; Johnsson, K. Tetrahydro- biopterin biosynthesis as a potential target of the kynurenine pathway metabolite xanthurenic acid. J. Biol. Chem. 2016, 291, 652–657. [Google Scholar] [CrossRef]
- Staats Pires, A.; Tan, V.X.; Heng, B.; Guillemin, G.J.; Latini, A. Kynurenine and tetrahydrobiopterin pathways crosstalk in pain hypersensitivity. Front. Neurosci. 2020, 14, 620. [Google Scholar] [CrossRef]
- Hamfelt, A.; Wetterberg, L. Pyridoxal phosphate in acute intermittent porphyria. Ann. N. Y. Acad. Sci. 1969, 166, 361–364. [Google Scholar] [CrossRef]
- Haugen, V.E.; Storjord, E.; Bjørke Monsen, A.; Brekke, O.; Sandberg, S.; Dahl, J.; Landsem, A.; Mollnes, T.; Waage Nielsen, E.; Ueland, P.; et al. Impaired vitamin b6 status in patients with acute intermittent porphyria. In Proceedings of the International Congress on Porphyrins and Porphyrias, Bordeaux, France, 3 July 2017. [Google Scholar]
- Alkaitis, M.S.; Crabtree, M.J. Recoupling the cardiac nitric oxide synthases: Tetrahydrobiopterin synthesis and recycling. Curr. Heart Fail. Rep. 2012, 9, 200–210. [Google Scholar] [CrossRef]
- Lelli, S.M.; Mazzetti, M.B.; de Viale, L.C.S.M. Melatonin modulates drug-induced acute porphyria. Toxicol. Rep. 2016, 3, 141–147. [Google Scholar] [CrossRef]
- Puy, H.; Deybach, J.C.; Baudry, P.; Callebert, J.; Touitou, Y.; Nordmann, Y. Decreased nocturnal plasma melatonin levels in patients with recurrent acute intermittent porphyria attacks. Life Sci. 1993, 53, 621–627. [Google Scholar] [CrossRef]
- Lukat-Rodgers, G.S.; Correia, C.; Botuyan, M.V.; Mer, G.; Rodgers, K.R. Heme-based sensing by the mammalian circadian protein CLOCK. Inorg. Chem. 2010, 49, 6349–6365. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Kim, K.D.; Lucas, A.; Drahos, K.E.; Santos, C.S.; Mury, S.P.; Capelluto, D.G.S.; Finkielstein, C.V. A novel heme-regulatory motif mediates heme-dependent degradation of the circadian factor Period 2. Mol. Cell. Biol. 2008, 28, 4697–4711. [Google Scholar] [CrossRef]
- Burris, T.P. Nuclear Hormone Receptors for Heme: REV-ERBα and REV-ERBβ Are Ligand-Regulated Components of the Mammalian Clock. Mol. Endocrinol. 2008, 22, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef]
- Rogers, P.M.; Ying, L.; Burris, T.P. Relationship between circadian oscillations of REV-ERBα expression and intracellular levels of its ligand, heme. Biochem. Biophys. Res. Commun. 2008, 368, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Iwadate, R.; Satoh, Y.; Watanabe, Y.; Kawai, H.; Kudo, N.; Kawashima, Y.; Mashino, T.; Mitsumoto, A. Impairment of heme biosynthesis induces short circadian period in body temperature rhythms in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R8–R18. [Google Scholar] [CrossRef] [PubMed]
- Thachil, J. L-Asparaginase, nitric oxide and posterior reversible encephalopathy syndrome. Ann. Hematol. 2013, 92, 141–142. [Google Scholar] [CrossRef] [PubMed]
- Klatt, P.; Pfeiffer, S.; List, B.M.; Lehner, D.; Glatter, O.; B¨achinger, H.P.; Werner, E.R.; Schmidt, K.; Mayer, B. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J. Biol. Chem. 1996, 271, 7336–7342. [Google Scholar] [CrossRef]
- Bredt, D.S.; Hwang, P.M.; Snyder, S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 1990, 347, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.D.; Wang, X.; Tanus-Santos, J.E.; Hogg, N.; Cannon, R.O.; Schechter, A.N.; Gladwin, M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002, 8, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Wang, X.; Sapsford, R.J.; Russell, R.P.; Farrell, A.L.; Jessop, H.A.; McGawley, G.M.; Oxborough, D.L.; Pleasants, P.; Richards, S.J.; et al. Nitric oxide consumption and pulmonary hypertension in patients with paroxysmal nocturnal hemoglobinuria. Blood 2005, 106, 1046. [Google Scholar] [CrossRef]
- Thachil, J. Nitric oxide and the clinical manifestations of acute porphyria. Intern. Med. J. 2008, 38, 732–735. [Google Scholar] [CrossRef]
- Buzaleh, A.; Meiss, R.; Lavandera, J.; Vallecorsa, P.; Ruspini, S.; Batlle, A. Óxido nítrico sintasa y hemo oxigenasa en encéfalo de ratones tratados con anestésicos volatiles y otros agentes porfirinogénicos: Estudio inmunohistoquímico de la expresión proteica. Medicina 2012, 72, 121. [Google Scholar]
- Bourque, S.L.; Benjamin, C.D.; Adams, M.A.; Nakatsu, K. Lack of hemodynamic effects after extended heme synthesis inhibition by succinylacetone in rats. J. Pharmacol. Exp. Ther. 2010, 333, 290–296. [Google Scholar] [CrossRef]
- Soong, J.; Adams, M.A.; Nakatsu, K. Acute depletion of heme by succinylacetone alters vascular responses but does not induce hyperten-sion. Can. J. Physiol. Pharmacol. 2008, 86, 613–619. [Google Scholar] [CrossRef]
- Homedan, C.; Laafi, J.; Schmitt, C.; Gueguen, N.; Lefebvre, T.; Karim, Z.; Desquiret-Dumas, V.; Wetterwald, C.; Deybach, J.C.; Gouya, L.; et al. Acute intermittent porphyria causes hepatic mitochondrial energetic failure in a mouse model. Int. J. Biochem. Cell Biol. 2014, 51, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Luck, M.; Schmitt, C.; Talbi, N.; Gouya, L.; Caradeuc, C.; Puy, H.; Bertho, G.; Pallet, N. Urinary metabolic profiling of asymptomatic acute intermittent porphyria using a rule-mining-based algorithm. Metabolomics 2018, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Elder, T.; Mengel, C.E. Effect of pyridoxine deficiency on porphyrin precursor excretion in acute intermittent porphyria. Am. J. Med. 1966, 41, 369–374. [Google Scholar] [CrossRef]
- Ventura, P.; Marcacci, M.; Marchini, S.; Cuoghi, C.; Vaccari, D.; Pietrangelo, A. Is poor vitamin status a reliable target for treatment of symptomatic patients with hepatic acute porphyrias? Dig. Liver Dis. 2019, 51, e23–e24. [Google Scholar] [CrossRef]
- Mills, P.B.; Footitt, E.J.; Mills, K.A.; Tuschl, K.; Aylett, S.; Varadkar, S.; Hemingway, C.; Marlow, N.; Rennie, J.; Baxter, P.; et al. Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (aldh7a1 deficiency). Brain 2010, 133, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Bagci, S.; Zschocke, J.; Hoffmann, G.; Bast, T.; Klepper, J.; Mu¨ller, A.; Heep, A.; Bartmann, P.; Franz, A. Pyridoxal phosphate-dependent neonatal epileptic encephalopathy. BMJ Case Rep. 2009, 2009, bcr1120081247. [Google Scholar] [CrossRef]
- Plecko, B.; St¨ockler, S. Vitamin B6 dependent seizures. Can. J. Neurol. Sci. 2009, 36 (Suppl. 2), S73–S77. [Google Scholar]
- Romero, J.A.; Kuczler, F.J., Jr. Isoniazid overdose: Recognition and treatment. Am. Fam. Physician 1998, 57, 749. [Google Scholar] [PubMed]
- Cavanagh, J. On the pattern of change in peripheral nerves produced by isoniazid intoxication in rats. J. Neurol. Neurosurg. Psychiatry 1967, 30, 26. [Google Scholar] [CrossRef]
- Gouya, L.; Ventura, P.; Balwani, M.; Bissell, D.M.; Rees, D.C.; St¨olzel, U.; Phillips, J.D.; Kauppinen, R.; Langendonk, J.G.; Desnick, R.J.; et al. Explore: A prospective, multinational, natural history study of patients with acute hepatic porphyria with recurrent attacks. Hepatology 2020, 71, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Cappellini, M.D.; Rocchi, E. The acute porphyrias: A diagnostic and therapeutic challenge in internal and emergency medicine. Intern. Emerg. Med. 2009, 4, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Cappellini, M.D.; Biolcati, G.; Guida, C.C.; Rocchi, E.; Porfiria, G.I. A challenging diagnosis for potential fatal diseases: Recommendations for diagnosing acute porphyrias. Eur. J. Intern. Med. 2014, 25, 497–505. [Google Scholar] [CrossRef]
- Soonawalla, Z.F.; Orug, T.; Badminton, M.N.; Elder, G.H.; Rhodes, J.M.; Bramhall, S.R.; Elias, E. Liver transplantation as a cure for acute intermittent porphyria. Lancet 2004, 363, 705–706. [Google Scholar] [CrossRef]
- Singal, A.K.; Parker, C.; Bowden, C.; Thapar, M.; Liu, L.; McGuire, B.M. Liver transplantation in the management of porphyria. Hepatology 2014, 60, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Sardh, E.; Ventura, P.; Peir´o, P.A.; Rees, D.C.; St¨olzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N. Eng. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef]
- Lazareth, H.; Poli, A.; Bignon, Y.; Mirmiran, A.; Rabant, M.; Cohen, R.; Schmitt, C.; Puy, H.; Karras, A.; Gouya, L.; et al. Renal function decline under therapy with small interfering RNA silencing ALAS1 for acute intermittent porphyria. Kidney Int. Rep. 2021, 6, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Petrides, P.E.; Klein, M.; Schuhmann, E.; Torkler, H.; Molitor, B.; Loehr, C.; Obermeier, Z.; Beykirch, M.K. Severe homocysteinemia in two givosiran treated porphyria patients: Is free heme deficiency the culprit? Ann. Hematol. 2021, 100, 1685–1693. [Google Scholar] [CrossRef]
- To-Figueras, J.; Wijngaard, R.; García-Villoria, J.; Aarsand, A.K.; Aguilera, P.; Deulofeu, R.; Brunet, M.; Gómez-Gómez, À.; Pozo, O.J.; Sandberg, S. Dysregulation of homocysteine homeostasis in acute intermittent porphyria patients receiving heme arginate or Givosiran. J. Inherit. Metab. Dis. 2021, 44, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Marcacci, M.; Cuoghi, C.; Pietrangelo, A.; Ventura, P. Hyperhomocysteinemia in patients with acute porphyrias: A possible effect of ALAS1 modulation by siRNAm therapy and its control by vitamin supplementation. Eur. J. Intern. Med. 2021, 92, 121–123. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).