External Validation of a Risk Stratification Score for B3 Breast Lesions Detected at Ultrasound Core Needle Biopsy
Abstract
1. Introduction
2. Methods and Materials
2.1. Patient Population
2.2. Biopsy Technique
2.3. Data Collection and Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients and Lesions
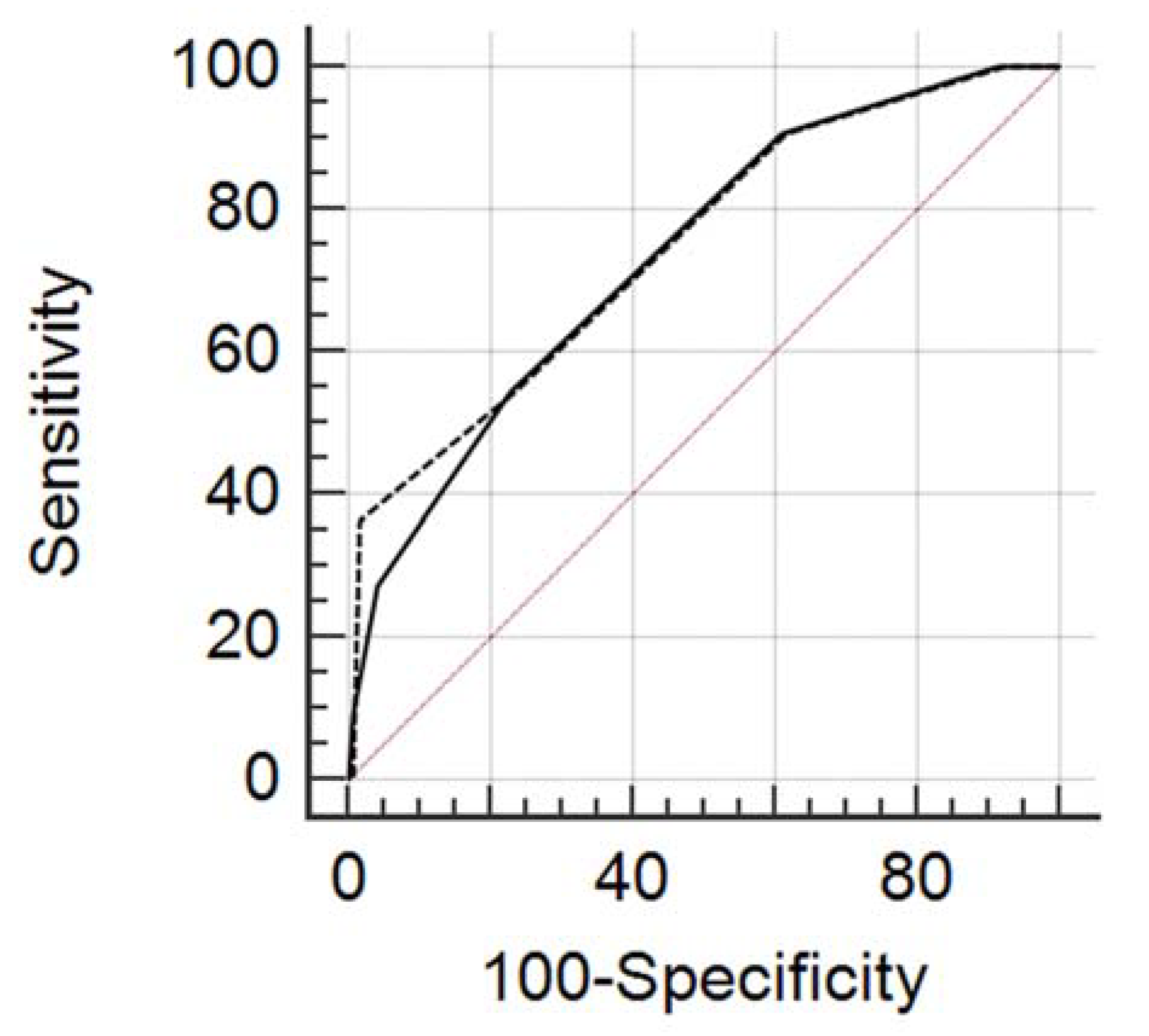
3.2. ROC Curve Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| AIDEP | atypical intraductal epithelial proliferation |
| ADH | atypical ductal hyperplasia |
| FEA | flat epithelial atypia |
| LN | lobular neoplasia |
| ALH | atypical lobular hyperplasia |
| LCIS | lobular carcinoma in situ |
| RS/CSL | radial scar/complex sclerosing lesions |
| PL | papillary lesions |
| FE | fibro-epithelial lesions with cellular stroma |
| VABB | vacuum assisted breast biopsy |
| US-CNB | ultrasound guided core needle biopsy |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| NPV | negative predictive value |
| PPV | positive predictive value |
| DCIS | ductal carcinoma in situ |
References
- Rakha, E.A.; Ho, B.C.; Naik, V.; Sen, S.; Hamilton, L.J.; Hodi, Z.; Ellis, I.O.; Lee, A.H. Outcome of breast lesions diagnosed as lesion of uncertain malignant potential (B3) or suspicious of malignancy (B4) on needle core biopsy, including detailed review of epithelial atypia. Histopathology 2011, 58, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.S.; Denley, H.E.; Pinder, S.E.; Ellis, I.O.; Elston, C.W.; Vujovic, P.; Macmillan, R.D.; Evans, A.J.; Nottingham Breast Team. Excision biopsy findings of patients with breast needle core biopsies reported as suspicious of malignancy (B4) or lesion of uncertain malignant potential (B3). Histopathology 2003, 42, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Caini, S.; Renne, G.; Cassano, E.; Ambrogetti, D.; Cattani, M.G.; Saguatti, G.; Chiaramondia, M.; Bellotti, E.; Bottiglieri, R.; et al. Positive predictive value for malignancy on surgical excision of breast lesions of uncertain malignant potential (B3) diagnosed by stereotactic vacuum-assisted needle core biopsy (VANCB): A large multi-institutional study in Italy. Breast 2011, 20, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Rageth, C.J.; O’Flynn, E.A.; Pinker, K.; Kubik-Huch, R.A.; Mundinger, A.; Decker, T.; Tausch, C.; Dammann, F.; Baltzer, P.A.; Fallenberg, E.M.; et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res. Treat. 2018, 174, 279–296. [Google Scholar] [CrossRef]
- Pinder, S.E.; Shaaban, A.; Deb, R.; Desai, A.; Gandhi, A.; Lee, A.H.S.; Pain, S.; Wilkinson, L.; Sharma, N. NHS Breast Screening multidisciplinary working group guidelines for the diagnosis and management of breast lesions of uncertain malignant potential on core biopsy (B3 lesions). Clin. Radiol. 2018, 73, 682–692. [Google Scholar] [CrossRef]
- Ellis, I.O.; Humphreys, S.; Michell, M.; Pinder, S.E.; Wells, C.A.; Zakhour, H.D. Guidelines for breast needle core biopsy handling and reporting in breast screening assessment. J. Clin. Pathol. 2004, 57, 897–902. [Google Scholar] [CrossRef]
- Rakha, E.A.; Ellis, I.O. An overview of assessment of prognostic and predictive factors in breast cancer needle core biopsy specimens. J. Clin. Pathol. 2007, 60, 1300–1306. [Google Scholar] [CrossRef]
- Golub, R.M.; Bennett, C.L.; Stinson, T.; Venta, L.; Morrow, M. Cost minimization study of image-guided core biopsy versus surgical excisional biopsy for women with abnormal mammograms. J. Clin. Oncol. 2004, 22, 2430–2437. [Google Scholar] [CrossRef]
- Tonegutti, M.; Girardi, V.; Ciatto, S.; Manfrin, E.; Bonetti, F. B3 breast lesions determined by vacuum-assisted biopsy: How to reduce the frequency of benign excision biopsies. Radiol. Med. 2010, 115, 1246–1257. [Google Scholar] [CrossRef]
- Bianchi, S.; Bendinelli, B.; Saladino, V.; Vezzosi, V.; Brancato, B.; Nori, J.; Palli, D. Non-Malignant Breast Papillary Lesions—B3 Diagnosed on Ultrasound—Guided 14-Gauge Needle Core Biopsy: Analysis of 114 Cases from a Single Institution and Review of the Literature. Pathol. Oncol. Res. 2015, 21, 535–546. [Google Scholar] [CrossRef]
- De Beça, F.F.; Rasteiro, C.; Correia, A.; Costa, S.; Amendoeira, I. Improved malignancy prediction by B3 breast lesions subclassification. Ann. Diagn. Pathol. 2013, 17, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Bednarova, I.; Londero, V.; Linda, A.; Girometti, R.; Lorenzon, M.; Bednarova, S.; Zuiani, C. Do clinical and radiologic features help predict malignancy of B3 breast lesions without epithelial atypia (B3a)? Radiol. Med. 2018, 123, 809–817. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.; Sickles, E.; Mendelson, E.; Morris, E. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Giuliani, M.; Rinaldi, P.; Rella, R.; D’Angelo, A.; Carlino, G.; Infante, A.; Romani, M.; Bufi, E.; Belli, P.; Manfredi, R. A new risk stratification score for the management of ultrasound-detected B3 breast lesions. Breast J. 2018, 24, 965–970. [Google Scholar] [CrossRef]
- Altman, D.G.; Vergouwe, Y.; Royston, P.; Moons, K.G.M. Prognosis and prognostic research: Validating a prognostic model. BMJ 2009, 338, b605. [Google Scholar] [CrossRef]
- Wöckel, A.; Kreienberg, R. First revision of the German S3 guideline “diagnosis, therapy, and follow-up of breast cancer”. Breast Care 2008, 3, 82–86. [Google Scholar] [PubMed]
- Kirshenbaum, K.; Keppke, A.; Hou, K.; Dickerson, M.; Gajjar, M.; Kirshenbaum, G. Reassessing Specimen Number and Diagnostic Yield of Ultrasound Guided Breast Core Biopsy. Breast J. 2012, 18, 464–469. [Google Scholar] [CrossRef]
- Uematsu, T. How to choose needles and probes for ultrasonographically guided percutaneous breast biopsy: A systematic approach. Breast Cancer 2012, 19, 238–241. [Google Scholar] [CrossRef]
- Kil, W.-H.; Cho, E.Y.; Kim, J.H.; Nam, S.-J.; Yang, J.-H. Is surgical excision necessary in benign papillary lesions initially diagnosed at core biopsy? Breast 2008, 17, 258–262. [Google Scholar] [CrossRef]
- Chang, J.M.; Moon, W.K.; Cho, N.; Han, W.; Noh, D.Y.; Park, I.A.; Jung, E.J. Risk of carcinoma after subsequent excision of benign papilloma initially diagnosed with an ultrasound (US)-guided 14-gauge core needle biopsy: A prospective observational study. Eur. Radiol. 2010, 20, 1093–1100. [Google Scholar] [CrossRef]
- Youk, J.H.; Kim, E.-K.; Kwak, J.Y.; Son, E.J.; Park, B.-W.; Kim, S.I. Benign Papilloma without Atypia Diagnosed at US-guided 14-gauge Core-Needle Biopsy: Clinical and US Features Predictive of Upgrade to Malignancy. Radiology 2011, 258, 81–88. [Google Scholar] [CrossRef]
- Chou, W.Y.Y.; Veis, D.J.; Aft, R. Radial scar on image-guided breast biopsy: Is surgical excision necessary? Breast Cancer Res. Treat. 2018, 170, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, S.; Houssami, N.; Ambrogetti, D.; Bianchi, S.; Bonardi, R.; Brancato, B.; Catarzi, S.; Risso, G.G. Accuracy and Underestimation of Malignancy of Breast Core Needle Biopsy: The Florence Experience of Over 4000 Consecutive Biopsies. Breast Cancer Res. Treat. 2007, 101, 291–297. [Google Scholar] [CrossRef]
- Bennani-Baiti, B.; Baltzer, P.A. MR Imaging for Diagnosis of Malignancy in Mammographic Microcalcifications: A Systematic Review and Meta-Analysis. Radiology 2016, 283, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Bennani-Baiti, B.; Bennani-Baiti, N.; Baltzer, P.A. Diagnostic Performance of Breast Magnetic Resonance Imaging in Non-Calcified Equivocal Breast Findings: Results from a Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0160346. [Google Scholar] [CrossRef] [PubMed]
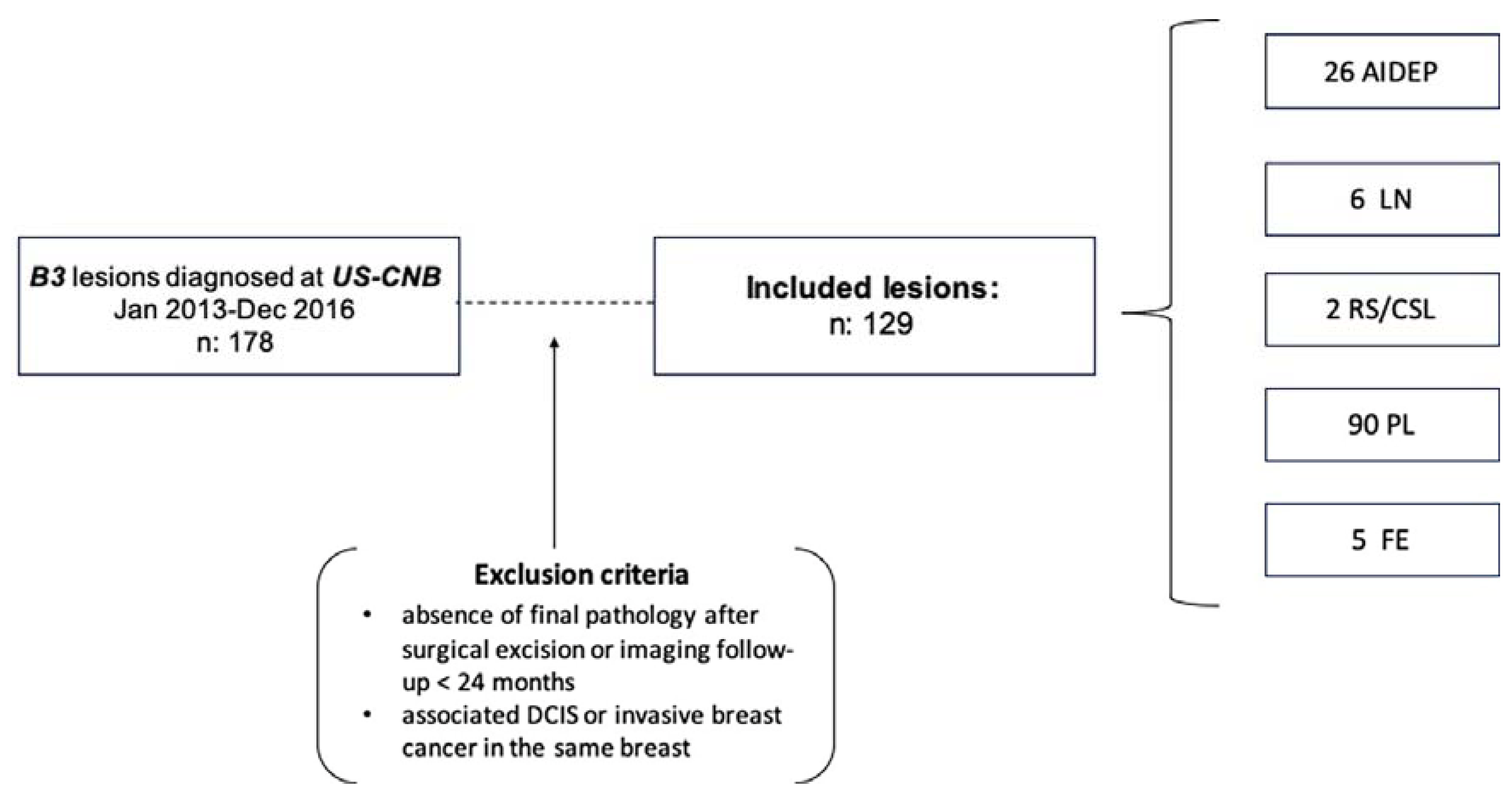

| US-CNB | N | % | FINAL DIAGNOSIS | PPV | |
|---|---|---|---|---|---|
| Non Malignant | Malignant | ||||
| AIDEP | 26 | 20.1 | 20 | 6 | 23%, 6/26 |
| LN | 6 | 4.6 | 5 | 1 | 16%, 1/6 |
| RS/CSL | 2 | 1.6 | 1 | 1 | 50%, 1/2 |
| PL | 90 | 69.8 | 87 | 3 | 3%, 3/90 |
| FE | 5 | 3.9 | 5 | 0 | 0% |
| Total | 129 | 100 | 118 | 11 | 8%, 11/129 |
| All Lesions | ||||||
|---|---|---|---|---|---|---|
| Cut-Off | Sens. | 95% CI | Spec. | 95% CI | +LR | −LR |
| ≥0 | 100%, 11/11 | 71.5–100.0 | 0%, 0/118 | 0.0–3.1 | 1.00 | |
| >0 | 100%, 11/11 | 71.5–100.0 | 8.47%, 10/118 | 4.1–15.0 | 1.09 | 0.00 |
| >1 | 90.91%, 10/11 | 58.7–99.8 | 38.98%, 46/118 | 30.1–48.4 | 1.49 | 0.23 |
| >2 | 54.55%, 6/11 | 23.4–83.3 | 77.12%, 91/118 | 68.5–84.3 | 2.38 | 0.59 |
| >3 | 27.27%, 3/11 | 6.0–61.0 | 95.76%, 113/118 | 90.4–98.6 | 6.44 | 0.76 |
| >4 | 9.09%, 1/11 | 0.2–41.3 | 99.15%, 117/118 | 95.4–100.0 | 10.73 | 0.92 |
| >5 | 0%, 0/11 | 0.0–28.5 | 100.00%, 118/118 | 96.9–100.0 | 1.00 | |
| All Lesions | ||||||
|---|---|---|---|---|---|---|
| Cut-Off | Sens. | 95% CI | Spec. | 95% CI | +LR | −LR |
| ≥0 | 100%, 11/11 | 71.5–100.0 | 0%, 0/118 | 0.0–3.1 | 1.00 | |
| >0 | 100%, 11/11 | 71.5–100.0 | 7.63%, 9/118 | 3.5–14.0 | 1.08 | 0.00 |
| >1 | 90.91%, 1/11 | 58.7–99.8 | 3814%, 45/118 | 29.4–47.5 | 1.47 | 0.24 |
| >2 | 54.55%, 6/11 | 23.4–83.3 | 76.27%, 90/118 | 67.6–83.6 | 2.30 | 0.60 |
| >3 | 36.36%, 4/11 | 10.9–69.2 | 98.31%, 116/118 | 94.0–99.8 | 21.45 | 0.65 |
| >4 | 0%, 0/11 | 0.0–28.5 | 99.15%, 117/118 | 95.4–100.0 | 0.00 | 1.01 |
| >5 | 0%, 0/11 | 0.0–28.5 | 100%, 118/118 | 96.9–100.0 | 1.00 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grippo, C.; Jagmohan, P.; Clauser, P.; Kapetas, P.; Meier, A.; Stöger, A.M.; D’Angelo, A.; Baltzer, P.A.T. External Validation of a Risk Stratification Score for B3 Breast Lesions Detected at Ultrasound Core Needle Biopsy. Diagnostics 2020, 10, 181. https://doi.org/10.3390/diagnostics10040181
Grippo C, Jagmohan P, Clauser P, Kapetas P, Meier A, Stöger AM, D’Angelo A, Baltzer PAT. External Validation of a Risk Stratification Score for B3 Breast Lesions Detected at Ultrasound Core Needle Biopsy. Diagnostics. 2020; 10(4):181. https://doi.org/10.3390/diagnostics10040181
Chicago/Turabian StyleGrippo, Cristina, Pooja Jagmohan, Paola Clauser, Panagiotis Kapetas, Arthur Meier, Annabel M. Stöger, Anna D’Angelo, and Pascal A. T. Baltzer. 2020. "External Validation of a Risk Stratification Score for B3 Breast Lesions Detected at Ultrasound Core Needle Biopsy" Diagnostics 10, no. 4: 181. https://doi.org/10.3390/diagnostics10040181
APA StyleGrippo, C., Jagmohan, P., Clauser, P., Kapetas, P., Meier, A., Stöger, A. M., D’Angelo, A., & Baltzer, P. A. T. (2020). External Validation of a Risk Stratification Score for B3 Breast Lesions Detected at Ultrasound Core Needle Biopsy. Diagnostics, 10(4), 181. https://doi.org/10.3390/diagnostics10040181





