In Silico Optimization of Inhibitors of the 3-Chymotrypsin-like Protease of SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Training and Validation Set of Inhibitors
2.2. Model Building
2.3. Molecular Mechanics
2.4. Conformational Search
2.5. Solvation Gibbs Free Energies
2.6. Calculation of the Entropic Term
2.7. Calculation of Binding Affinity and QSAR Model
2.8. Interaction Energy
2.9. Generation of Pharmacophore
2.10. ADME Properties
2.11. Virtual Combinatorial Library Generation
2.12. Inhibitory Potency Prediction
2.13. Molecular Dynamics Simulations
3. Results
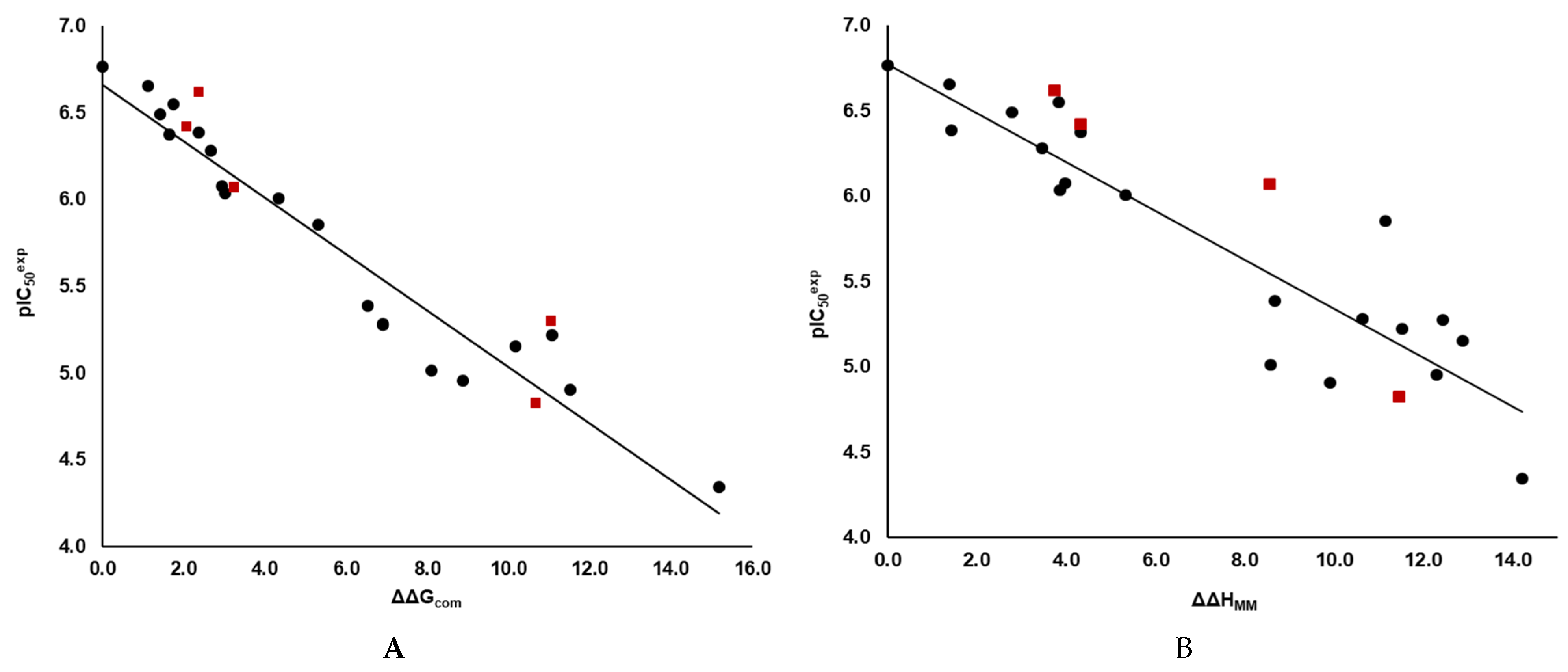
3.1. QSAR MODEL of 3CLpro Inhibition
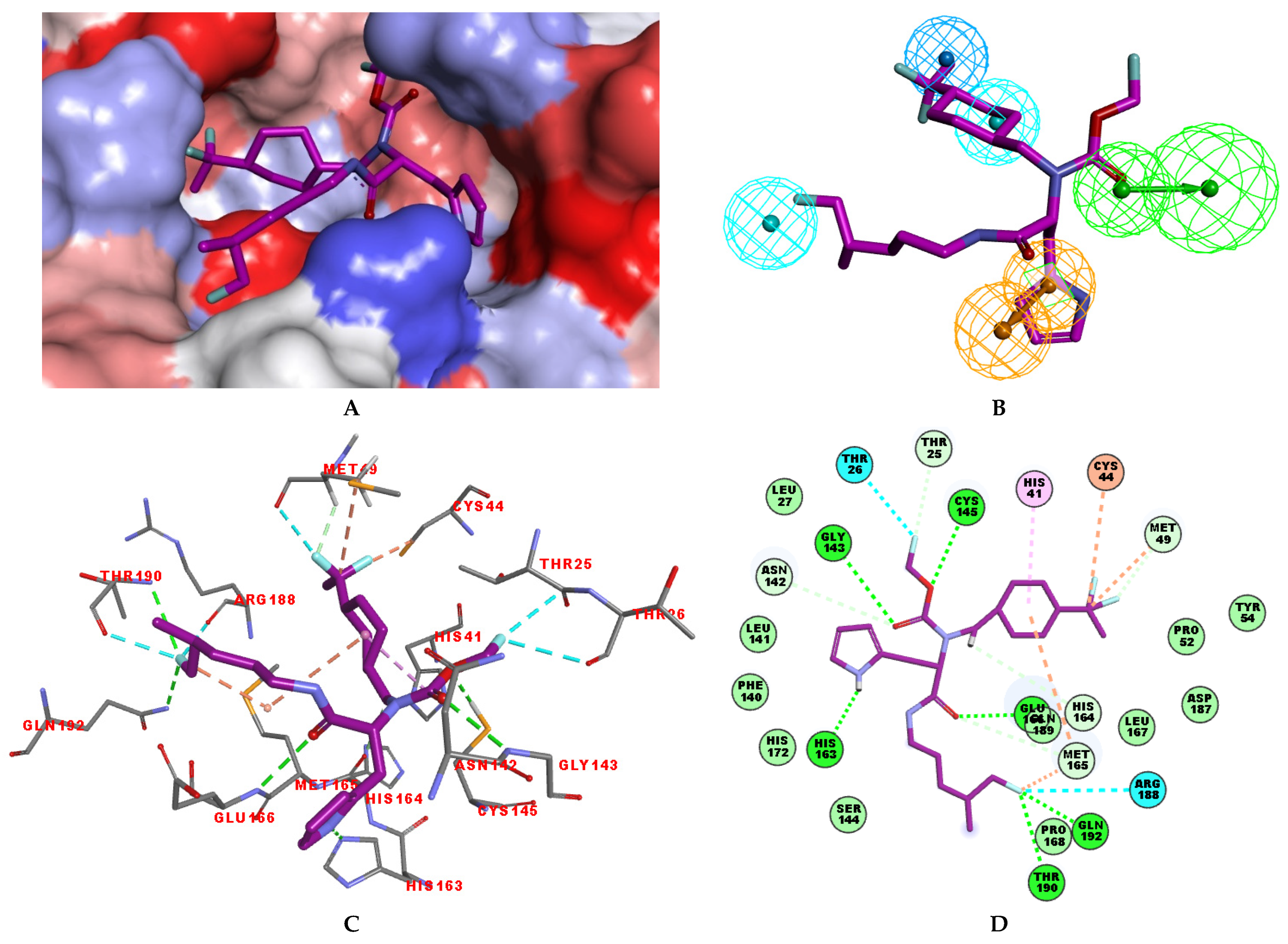
3.2. Binding Mode of IPCLs
3.3. Interaction Energy
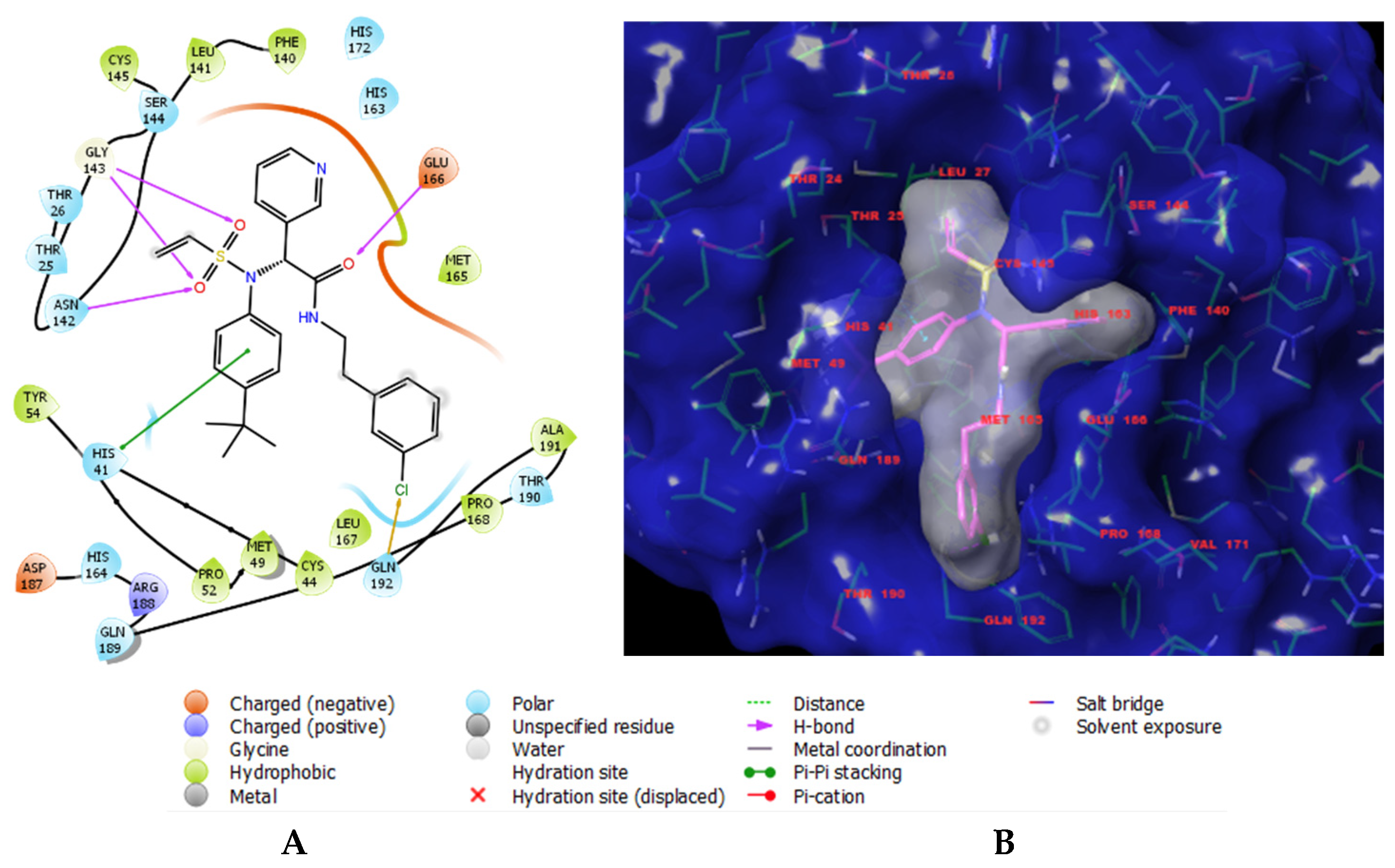
3.4. Pharmacophore Model
3.5. Virtual Combinatorial Library of IPCLs and In Silico Screening
3.6. New IPCL Analogues

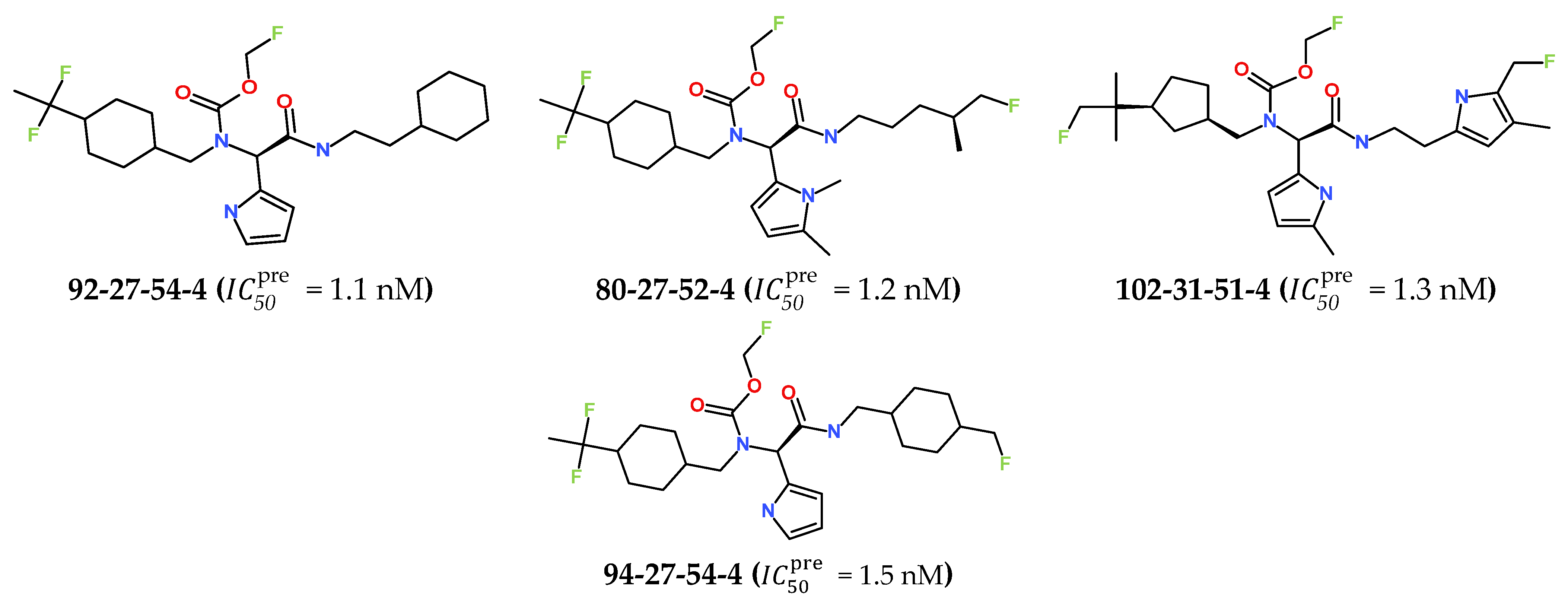
3.7. Predicted Pharmacokinetic Profile of New IPCL Analogues
3.8. Molecular Dynamics Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3CLpro | 3-Chymotrypsin-like Protease |
| 3D | Three-dimensional |
| ACE2 | Angiotensin Converting Enzyme II |
| ADME | Absorption, distribution, metabolism, and excretion |
| Ar | Ring aromatic |
| CAMD | Computer-aided molecular design |
| CFFII | Class II consistent force field |
| COVID-19 | Coronavirus disease 2019 |
| ΔEint | Interaction energy |
| GFE | Gibbs free energy |
| ΔΔGcom | Relative GFE of formation of enzyme–inhibitor complex E:I* |
| ΔΔGsol | Solvation component of the relative GFE |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| ΔΔHMM | Enthalpy component of the relative GFE |
| HOA | Human oral absorption |
| HYD | Hydrophobic |
| HYD-Al | Hydrophobic aliphatic |
| Observed half-maximal inhibitory concentration | |
| Predicted half-maximal inhibitory concentration | |
| IPCLx | Inhibitors included in TS and in VS |
| MD | Molecular dynamics |
| MERS | Middle East respiratory syndrome |
| MM | Molecular mechanics |
| MM-PBSA | Molecular mechanics–Poisson–Boltzmann surface area |
| PDB | Protein Data Bank |
| PH4 | Pharmacophore model |
| QSAR | Quantitative structure–activity relationship |
| RMSD | Root mean square deviation |
| RNA | Ribonucleic acid |
| SARS | Severe acute respiratory syndrome |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TS | Training set |
| ∆∆TSvib | Vibrational entropy contribution of the relative GFE |
| VS | Validation set |
References
- Lv, Z.; Cano, K.E.; Jia, L.; Drag, M.; Huang, T.T.; Olsen, S.K. Targeting SARS-CoV-2 Proteases for COVID-19 Antiviral Development. Front. Chem. 2022, 9, 819165. [Google Scholar] [CrossRef]
- World Health Organization (WHO). SARS. How a Global Epidemic Was Stopped; WHO: Geneva, Switzerland, 2006; Available online: https://iris.who.int/bitstream/handle/10665/207501/9290612134_eng.pdf (accessed on 7 December 2025).
- World Health Organization (WHO). The World Health Report: 2003: Shaping the Future; WHO: Geneva, Switzerland, 2003; Available online: https://iris.who.int/handle/10665/42789 (accessed on 7 December 2025).
- World Health Organization (WHO). COVID-19 Epidemiological Update, 177th ed.; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 7 December 2025).
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; El-Ella, D.M.A.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mohsen, M.O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2021, 54, 524–540. [Google Scholar] [CrossRef]
- Islam, M.A. A review of SARS-CoV-2 variants and vaccines: Viral properties, mutations, vaccine efficacy, and safety. Infect. Med. 2023, 2, 247–261. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Albayat, H.; Alwarthan, S.; Alhajri, M.; Najim, M.A.; AlShehail, B.M.; Al-Adsani, W.; Alghadeer, A.; Abduljabbar, W.A.; et al. Variants of SARS-CoV-2: Influences on the Vaccines’ Effectiveness and Possible Strategies to Overcome Their Consequences. Medicina 2023, 59, 507. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921. [Google Scholar] [CrossRef] [PubMed]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Cortese, M.; Winter, S.L.; Wachsmuth-Melm, M.; Neufeldt, C.J.; Cerikan, B.; Stanifer, M.L.; Boulant, S.; Bartenschlager, R.; Chlanda, P. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun. 2020, 11, 5885. [Google Scholar] [CrossRef]
- Anand, K.; Ziebuhr, J.; Wadhwani, P.; Mesters, J.R.; Hilgenfeld, R. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science 2003, 300, 1763–1767. [Google Scholar] [CrossRef]
- Cantrelle, F.; Boll, E.; Brier, L.; Moschidi, D.; Belouzard, S.; Landry, V.; Leroux, F.; Dewitte, F.; Landrieu, I.; Dubuisson, J.; et al. NMR spectroscopy of the main protease of SARS-CoV-2 and fragment-based screening identify three protein hotspots and an antiviral fragment. Angew. Chem. Int. Ed. 2021, 60, 25428–25435. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved a-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Pacte Mondial Réseau France. Les 17 Objectifs de Développement Durable et Leurs 169 Cibles. 2024. Available online: www.pactemondial.org (accessed on 4 March 2025).
- Stille, J.K.; Tjutrins, J.; Wang, G.; Venegas, F.A.; Hennecker, C.; Rueda, A.M.; Sharon, I.; Blaine, N.; Miron, C.E.; Pinus, S.; et al. Design, synthesis and in vitro evaluation of novel SARS-CoV-2 3CLpro covalent inhibitors. Eur. J. Med. Chem. 2022, 229, 114046. [Google Scholar] [CrossRef] [PubMed]
- Insight-II and Discover Molecular Modeling and Simulation Package, Version 2005; Accelrys, Inc.: San Diego, CA, USA, 2005.
- Keita, M.; Kumar, A.; Dali, B.; Megnassan, E.; Siddiqi, M.I.; Frecer, V.; Miertus, S. Quantitative structure-activity relationships and design of thymine-like inhibitors of thymidine monophosphate kinase of Mycobacterium tuberculosis with favourable pharmacokinetic profiles. RSC Adv. 2014, 4, 55853–55866. [Google Scholar] [CrossRef]
- Kouassi, A.F.; Kone, M.; Keita, M.; Esmel, A.; Megnassan, E.; N’guessan, Y.T.; Frecer, V.; Miertus, S. Computer-aided design of orally bioavailable pyrrolidine carboxamide inhibitors of enoyl-acyl carrier protein reductase of Mycobacterium tuberculosis with favorable pharmacokinetic profiles. Int. J. Mol. Sci. 2015, 16, 29744–29771. [Google Scholar] [CrossRef]
- Allangba, K.N.P.G.; Keita, M.; N’guessan, R.K.; Megnassan, E.; Frecer, V.; Miertus, S. Virtual design of novel Plasmodium falciparum cysteine protease falcipain-2 hybrid lactone–chalcone and isatin–chalcone inhibitors probing the S2 active site pocket. J. Enzym. Inhib. Med. Chem. 2019, 34, 547–561. [Google Scholar] [CrossRef]
- Frecer, V.; Burello, E.; Miertus, S. Combinatorial design of nonsymmetrical cyclic urea inhibitors of aspartic protease of HIV-1. Bioorg. Med. Chem. 2005, 13, 5492–5501. [Google Scholar] [CrossRef]
- Dali, B.; Keita, M.; Megnassan, E.; Frecer, V.; Miertus, S. Insight into selectivity of peptidomimetic inhibitors with modified statine core for plasmepsin II of Plasmodium falciparum over human cathepsin D. Chem. Biol. Drug Des. 2012, 79, 411–430. [Google Scholar] [CrossRef]
- Megnassan, E.; Keita, M.; Bieri, C.; Esmel, A.; Frecer, V.; Miertus, S. Design of novel dihydroxynaphthoic acid inhibitors of Plasmodium falciparum lactate dehydrogenase. Med. Chem. 2012, 8, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Owono Owono, L.C.; Keita, M.; Megnassan, E.; Frecer, V.; Miertus, S. Design of thymidine analogs targeting thymidilate kinase of Mycobacterium tuberculosis. Tuberc. Res. Treat. 2013, 2013, 670836. [Google Scholar] [CrossRef]
- Frecer, V.; Berti, F.; Benedetti, F.; Miertus, S. Design of peptidomimetic inhibitors of aspartic protease of HIV-1 containing -Phe Psi Pro- core and displaying favourable ADME-related properties. J. Mol. Graph. Model. 2008, 27, 376–387. [Google Scholar] [CrossRef]
- Frecer, V.; Seneci, P.; Miertus, S. Computer-assisted combinatorial design of bicyclic thymidine analogs as inhibitors of Mycobacterium tuberculosis thymidine monophosphate kinase. J. Comput.-Aided Mol. Des. 2011, 25, 31–49. [Google Scholar] [CrossRef]
- Megnassan, E.; Fofana, I.; Dali, B.; Koblavi, F.M.; Frecer, V.; Miertus, S.; Eugene, M.; Mansilla-Koblavi, F.; Desk, S. Structure-Based Design of Tetrahydroisoquinoline-Based Hydroxamic Acid Derivatives Inhibiting Human Histone Deacetylase 8. J. Comput. Chem. Mol. Model. 2021, 5, 504–538. [Google Scholar] [CrossRef]
- Frecer, V.; Miertus, S.; Tossi, A.; Romeo, D. Rational design of inhibitors for drug-resistant HIV-1 aspartic protease mutants. Drug Des. Discov. 1998, 15, 211–231. [Google Scholar] [PubMed]
- OECD. Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(Q)SAR] Models; OECD Series on Testing and Assessment, No. 69; OECD Publishing: Paris, France, 2014. [Google Scholar] [CrossRef]
- Sharon, I.; Stille, J.; Tjutrins, J.; Wang, G.; Venegas, F.A.; Hennecker, C.; Rueda, A.M.; Miron, C.E.; Pinus, S.; Labarre, A. Crystal Structure of SARS-CoV-2 Main Protease (3CLpro/Mpro) Co-valently Bound to Compound C63. RCSB Protein Data Bank 2021. [Google Scholar] [CrossRef]
- Discovery Studio. Molecular Modeling and Simulation Software, Release 2.5; Accelrys Inc.: San Diego, CA, USA, 2009.
- Konate, S.; Allangba, K.N.P.G.; Fofana, I.; N’guessan, R.K.; Megnassan, E.; Miertus, S.; Frecer, V. Improved Inhibitors Targeting the Thymidylate Kinase of Multidrug-Resistant Mycobacterium tuberculosis with Favorable Pharmacokinetics. Life 2025, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Bieri, C.; Esmel, A.; Keita, M.; Owono, L.C.O.; Dali, B.; Megnassan, E.; Miertus, S.; Frecer, V. Structure-Based Design and Pharmacophore-Based Virtual Screening of Combinatorial Library of Triclosan Analogs Active against Enoyl-Acyl Carrier Protein Reductase of Plasmodium falciparum with Favourable ADME Profiles. Int. J. Mol. Sci. 2023, 24, 6916. [Google Scholar] [CrossRef]
- Nanan, L.F.; Esmel, A.E.; Dali, B.L.; Keita, M.; Koblavi-Mansilla, F.; Megnassan, E. Computer-Aided Design and Pharmacophore-Based Screening of a Diverse Combinatorial Library of Phytoselective Aryloxyacetic Acid Derivatives as HPPD Inhibitors. J. Agric. Food Chem. 2025, 73, 8129–8147. [Google Scholar] [CrossRef]
- Miertus, S.; Frecer, V.; Chiellini, E.; Chiellini, F.; Solaro, R.; Tomasi, J. Molecular interactions and inclusion phenomena in substituted β-cyclodextrins:: Simple inclusion probes: H2O, C, CH4, C6H6, NH4+, HCOO−. J. Incl. Phenom. 1998, 32, 23–46. [Google Scholar] [CrossRef]
- Frecer, V.; Májeková, M.; Miertuš, S. Approximate methods for solvent effect calculations on biomolecules. J. Mol. Struct. THEOCHEM 1989, 183, 403–419. [Google Scholar] [CrossRef]
- Maple, J.R.; Hwang, M.; Stockfisch, T.P.; Dinur, U.; Waldman, M.; Ewig, C.S.; Hagler, A.T. Derivation of class II force fields. I. Methodology and quantum force field for the alkyl functional group and alkane molecules. J. Comput. Chem. 1994, 15, 162–182. [Google Scholar] [CrossRef]
- Rocchia, W.; Sridharan, S.; Nicholls, A.; Alexov, E.; Chiabrera, A.; Honig, B. Rapid grid-based construction of the molecular surface and the use of induced surface charge to calculate reaction field energies: Applications to the molecular systems and geometric objects. J. Comput. Chem. 2002, 23, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Gilson, M.K.; Honig, B. The inclusion of electrostatic hydration energies in molecular mechanics calculations. J. Comput.-Aided Mol. Des. 1991, 5, 5–20. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson−Boltzmann Surface Area Method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Fischer, S.; Smith, J.C.; Verma, C.S. Dissecting the vibrational entropy change on protein/ligand binding: Burial of a water molecule in bovine pancreatic trypsin inhibitor. J. Phys. Chem. B 2001, 105, 8050–8055. [Google Scholar] [CrossRef]
- Schwarzl, S.M.; Tschopp, T.B.; Smith, J.C.; Fischer, S. Can the calculation of ligand binding free energies be improved with continuum solvent electrostatics and an ideal-gas entropy correction? J. Comput. Chem. 2002, 23, 1143–1149. [Google Scholar] [CrossRef]
- Wienen-Schmidt, B.; Jonker, H.R.A.; Wulsdorf, T.; Gerber, H.-D.; Saxena, K.; Kudlinzki, D.; Sreeramulu, S.; Parigi, G.; Luchinat, C.; Heine, A.; et al. Paradoxically, Most Flexible Ligand Binds Most Entropy-Favored: Intriguing Impact of Ligand Flexibility and Solvation on Drug-Kinase Binding. J. Med. Chem. 2018, 61, 5922–5933. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics; Wiley-VCH: Weinheim, Germany, 2009; Volume I–II. [Google Scholar] [CrossRef]
- Frecer, V.; Miertus, S. Interactions of ligands with macromolecules: Rational design of specific inhibitors of aspartic protease of HIV-1. Macromol. Chem. Phys. 2002, 203, 1650–1657. [Google Scholar] [CrossRef]
- Seidel, T.; Wieder, O.; Garon, A.; Langer, T. Applications of the Pharmacophore Concept in Natural Product inspired Drug Design. Mol. Inform. 2020, 39, 2000059. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y. Pharmacophore modeling and applications in drug discovery: Challenges and recent advances. Drug Discov. Today 2010, 15, 444–450. [Google Scholar] [CrossRef]
- Giordano, D.; Biancaniello, C.; Argenio, M.A.; Facchiano, A. Drug Design by Pharmacophore and Virtual Screening Approach. Pharmaceuticals 2022, 15, 646. [Google Scholar] [CrossRef]
- Schueler, F.W. Chemobiodynamics and Drug Design; McGraw-Hill: New York, NY, USA, 1960. [Google Scholar]
- Güner, O.F. History and evolution of the pharmacophore concept in computer-aided drug design. Curr. Top. Med. Chem. 2002, 2, 1321–1332. [Google Scholar] [CrossRef]
- Güner, O.F.; Bowen, J.P. Setting the record straight: The origin of the pharmacophore concept. J. Chem. Inf. Model. 2014, 54, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.K. Pharmacophore Perception, Development and Use in Drug Design. Edited by Osman F. Güner. Molecules 2000, 5, 987–989. [Google Scholar] [CrossRef]
- QikProp 6.5, Release 139; Schrödinger LLC.: New York, NY, USA, 2019.
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from Monte Carlo simulations. Bioorg. Med. Chem. Lett. 2000, 10, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef]
- Duffy, E.M.; Jorgensen, W. Prediction of Properties from Simulations: Free Energies of Solvation in Hexadecane, Octanol, and Water. J. Am. Chem. Soc. 2000, 122, 2878–2888. [Google Scholar] [CrossRef]
- Jorgensen, W.L. Efficient Drug Lead Discovery and Optimization. Acc. Chem. Res. 2009, 42, 724–733. [Google Scholar] [CrossRef]
- Jorgensen, W.L. Computational Methods for the Study of Drug-Likeness. Drug Discov. Today 2004, 9, 37–44. [Google Scholar] [CrossRef]
- Desmond Molecular Dynamics System, Release 2021–2; Schrödinger LLC: New York, NY, USA, 2021.
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the SC’06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; p. 43. [Google Scholar] [CrossRef]
- Frecer, V.; Miertus, S. Antiviral agents against COVID-19: Structure-based design of specific peptidomimetic inhibitors of SARS-CoV-2 main protease. RSC Adv. 2020, 10, 40244–40263. [Google Scholar] [CrossRef]
- Frecer, V.; Ho, B.; Ding, J.L. Molecular dynamics study on lipid A from Escherichia coli: Insights into its mechanism of biological action. Biochim. Et Biophys. Acta 2000, 1466, 87–104. [Google Scholar] [CrossRef]
- Garbett, N.C.; Chaires, J.B. Thermodynamic studies for drug design and screening. Expert Opin. Drug Discov. 2012, 7, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Klebe, G.; Böhm, H.-J. Energetic and entropic factors determining binding affinity in protein-ligand complexes. J. Recept. Signal Transduct. Res. 1997, 17, 459–473. [Google Scholar] [CrossRef]
- Freire, E. Do enthalpy and entropy distinguish first in class from best in class? Drug Discov. Today 2008, 13, 869–874. [Google Scholar] [CrossRef]
- Sapse, A.; Schweitzer, B.S.; Dicker, A.P.; Bertino, J.R.; Frecer, V. Ab initio studies of aromatic-aromatic and aromatic-polar interactions in the binding of substrate and inhibitor to dihydrofolate reductase. Int. J. Pept. Protein Res. 1992, 39, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.; Lombardo, F.; Dominy, B.; Feeney, P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Aly, O. Molecular Docking Reveals the Potential of Aliskiren, Dipyridamole, Mopidamol, Rosuvastatin, Rolitetracycline and Metamizole to Inhibit COVID-19 Virus Main Protease. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, B.; Jiang, X.-M.; Su, H.; Li, J.; Zhao, Y.; Xie, X.; Jin, Z.; Peng, J.; Liu, F.; et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, 368, 1331–1335. [Google Scholar] [CrossRef]
- Klemm, T.; Ebert, G.; Calleja, D.J.; Allison, C.C.; Richardson, L.W.; Bernardini, J.P.; Lu, B.G.; Kuchel, N.W.; Grohmann, C.; Shibata, Y.; et al. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020, 39, e106275. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.C.; Pokhrel, S.; Labbe, A.D.; Mathews, I.I.; Cooper, C.J.; Davidson, R.B.; Phillips, G.; Weiss, K.L.; Zhang, Q.; O’nEill, H.; et al. Potent and Selective Covalent Inhibition of the Papain-like Protease from SARS-CoV-2. Res. Sq. 2022, 14, 1733. [Google Scholar] [CrossRef]
- Nemčovičová, I.; Lopušná, K.; Štibrániová, I.; Benedetti, F.; Berti, F.; Felluga, F.; Drioli, S.; Vidali, M.; Katrlík, J.; Pažitná, L.; et al. Identification and evaluation of antiviral activity of novel compounds targeting SARS-CoV-2 virus by enzymatic and antiviral assays, and computational analysis. J. Enzym. Inhib. Med. Chem. 2024, 39, 2301772. [Google Scholar] [CrossRef]
- Kerti, L.; Frecer, V. Design of inhibitors of SARS-CoV-2 papain-like protease deriving from GRL0617: Structure-activity relationships. Bioorg. Med. Chem. 2024, 113, 117909. [Google Scholar] [CrossRef]
- Vuong, W.; Fischer, C.; Khan, M.B.; van Belkum, M.J.; Lamer, T.; Willoughby, K.D.; Lu, J.; Arutyunova, E.; Joyce, M.A.; Saffran, H.A.; et al. Improved SARS-CoV-2 Mpro inhibitors based on feline antiviral drug GC376: Structural enhancements, increased solubility, and micellar studies. Eur. J. Med. Chem. 2021, 222, 113584. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V.; Hayashi, Y.; Jung, S.H. An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy. J. Med. Chem. 2016, 59, 6595–6628. [Google Scholar] [CrossRef]
- Yang, H.; Xie, W.; Xue, X.; Yang, K.; Ma, J.; Liang, W.; Zhao, Q.; Zhou, Z.; Pei, D.; Ziebuhr, J.; et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. Biol. PLoS 2005, 3, e324. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M.; Shahabi, D.; Chapman, M.E.; Mesecar, A.D. Drug development and medicinal chemistry efforts toward SARS-coronavirus and COVID-19 therapeutics. ChemMedChem 2020, 15, 907–932. [Google Scholar] [CrossRef] [PubMed]
- Sencanski, M.; Perovic, V.; Pajovic, S.B.; Adzic, M.; Paessler, S.; Glisic, S. Drug Repurposing for Candidate SARS-CoV-2 Main Protease Inhibitors by a Novel In Silico Method. Molecules 2020, 25, 3830. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Iketani, S.; Zask, A.; Khanizeman, N.; Bednarova, E.; Forouhar, F.; Fowler, B.; Hong, S.J.; Mohri, H.; Nair, M.S.; et al. Development of optimized drug-like small molecule inhibitors of the SARS-CoV-2 3CL protease for treatment of COVID-19. Nat. Commun. 2022, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Li, G.D.; Hilgenfeld, R.; Whitley, R.; de Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]







 | |||||||
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| R-Group |  | 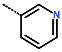 | 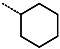 | 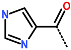 |  |  |  |
| No. | 8 | 9 | 10 | 11 | 12 | 13 | |
| R-Group |  |  | 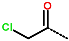 | 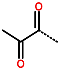 |  |  | |
| No. | 14 | 15 | 16 | 17 | 18 | 19 | |
| R-Group |  | 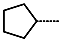 |  |  |  | 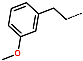 | |
| No. | 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| R-Group |  |  | 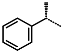 | 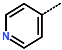 |  |  |  |
| Training set | IPCL1 | IPCL2 | IPCL3 | IPCL4 | IPCL5 | IPCL6 | IPCL7 |
| R3–R2–R1–R1′ | 18-1-2-9 | 21-1-2-9 | 17-1-2-9 | 22-1-2-9 | 3-1-2-10 | 3-1-2-9 | 20-1-2-9 |
| (µM) | 0.17 | 0.22 | 0.28 | 0.32 | 0.41 | 0.42 | 0.52 |
| Training set | IPCL8 | IPCL9 | IPCL10 | IPCL11 | IPCL12 | IPCL13 | IPCL14 |
| R3–R2–R1–R1′ | 3-1-23-10 | 16-1-2-10 | 3-1-24-10 | 6-1-2-5 | 3-1-2-4 | 3-1-2-11 | 3-1-2-8 |
| [µM] | 0.84 | 0.92 | 0.98 | 1.40 | 4.1 | 5.2 | 5.3 |
| Training set | IPCL15 * | IPCL16 | IPCL17 | IPCL18 | IPCL19 | IPCL20 | |
| R3–R2–R1–R1′ | 22-1-2-9 | 3-1-2-12 | 15-1-2-8 | 3-1-2-7 | 3-1-2-13 | 3-26-2-8 | |
| [µM] | 6.0 | 7.0 | 9.7 | 11.1 | 12.4 | 45.1 | |
| Validation set | IPCL21 | IPCL22 | IPCL23 | IPCL24 | IPCL25 | ||
| R3–R2–R1–R1′ | 19-1-2-9 | 15-1-2-10 | 3-1-2-14 | 3-1-25-8 | 6-1-2-8 | ||
| [µM] | 0.24 | 0.38 | 0.85 | 5.0 | 15.0 | ||
| Training Set a | Mw b | ∆∆HMM c | ∆∆Gsol d | ΔΔTSvib e | ∆∆Gcom f | g |
| [g·mol−1] | [kcal·mol−1] | [kcal·mol−1] | [kcal·mol−1] | [kcal·mol−1] | [µM] | |
| IPCL1 * | 512 | 0.0 | 0.0 | 0.0 | 0.0 | 0.17 |
| IPCL2 | 547 | 1.4 | 1.5 | 1.7 | 1.1 | 0.22 |
| IPCL3 | 508 | 3.8 | 1.3 | 3.4 | 1.8 | 0.28 |
| IPCL4 | 478 | 2.8 | 1.0 | 2.4 | 1.4 | 0.32 |
| IPCL5 | 442 | 1.4 | 2.7 | 1.7 | 2.4 | 0.41 |
| IPCL6 | 456 | 4.3 | 0.7 | 3.4 | 1.7 | 0.42 |
| IPCL7 | 492 | 3.5 | 3.1 | 3.9 | 2.7 | 0.52 |
| IPCL8 | 437 | 4.0 | −0.9 | 0.2 | 3.0 | 0.84 |
| IPCL9 | 450 | 3.9 | −0.9 | 0.0 | 3.0 | 0.92 |
| IPCL10 | 452 | 5.3 | −1.6 | −0.6 | 4.3 | 0.98 |
| IPCL11 | 434 | 11.1 | −6.5 | −0.7 | 5.3 | 1.4 |
| IPCL12 | 460 | 8.7 | −0.2 | 1.9 | 6.5 | 4.1 |
| IPCL13 | 436 | 10.6 | 0.8 | 4.5 | 6.9 | 5.2 |
| IPCL14 | 432 | 12.4 | −2.1 | 3.4 | 6.9 | 5.3 |
| IPCL15 | 479 | 11.5 | −1.9 | −1.4 | 11.1 | 6 |
| IPCL16 | 433 | 12.9 | 0.7 | 3.5 | 10.2 | 7 |
| IPCL17 | 418 | 8.6 | 2.0 | 2.4 | 8.1 | 9.7 |
| IPCL18 | 420 | 12.3 | −0.3 | 3.1 | 8.9 | 11.1 |
| IPCL19 | 490 | 9.9 | 0.3 | −1.3 | 11.5 | 12.4 |
| IPCL20 | 441 | 14.2 | −0.8 | −1.8 | 15.2 | 45.1 |
| Validation Set | Mw b | ∆∆HMM c | ∆∆Gsol d | ΔΔTSvib e | ∆∆Gcom f | h |
| [g·mol−1] | [kcal·mol−1] | [kcal·mol−1] | [kcal·mol−1] | [kcal·mol−1] | ||
| IPCL21 | 508 | 3.7 | 2.6 | 4.0 | 2.4 | 0.95 |
| IPCL22 | 428 | 4.3 | −0.2 | 2.0 | 2.1 | 0.99 |
| IPCL23 | 448 | 8.6 | −1.2 | 4.1 | 3.3 | 1.01 |
| IPCL24 | 482 | 15.3 | 0.0 | 4.3 | 11.1 | 0.91 |
| IPCL25 | 406 | 11.5 | 2.1 | 2.8 | 10.7 | 1.02 |
| Statistical Data of Linear Regression | (A) | (B) |
|---|---|---|
| (A) | ||
| (B) | ||
| Number of compounds n | 20 | 20 |
| Squared correlation coefficient of regression | 0.84 | 0.93 |
| Leave-one-out cross-validated squared predictive correlation coefficient | 0.81 | 0.91 |
| Standard error of regression σ | 0.290 | 0.193 |
| Statistical significance of regression, Fisher F-test | 94.021 | 235.338 |
| Level of statistical significance α | >95% | >95% |
| Range of half-maximal inhibitory concentrations [µM] | 0.17–45.1 | |
| Squared correlation coefficient of regression of validation set | 0.75 | 0.90 |
| Predictive squared correlation coefficient of regression of validation set | 0.53 | 0.85 |
| Root mean square error of validation set | 0.47 | 0.26 |
| Mean absolute error of validation set | 0.44 | 0.21 |
| Aggregated metrics of QSAR model (B) for iterative reshuffling of the TS and vs. (Y-Randomization) | ||
| Squared correlation coefficient of regression | 0.92 ± 0.02 | |
| Leave-one-out cross-validated squared predictive correlation coefficient | 0.90 ± 0.02 | |
| Predictive squared correlation coefficient of regression of validation set | 0.80 ± 0.33 | |
| Root mean square error of validation set | 0.21 ± 0.06 | |
| Mean absolute error of validation set | 0.18 ± 0.05 | |
| Hypothesis | RMSD a | R2 b | Total Costs c | Costs Difference d | Closest Random e | Features f |
|---|---|---|---|---|---|---|
| Hypo1 | 1.855 | 0.97 | 82.10 | 457.86 | 170.21 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo2 | 2.845 | 0.92 | 130.92 | 409.04 | 193.30 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo3 | 3.550 | 0.87 | 176.24 | 363.73 | 199.38 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo4 | 3.580 | 0.86 | 178.80 | 361.16 | 256.42 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo5 | 3.635 | 0.86 | 181.48 | 358.48 | 265.72 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo6 | 3.784 | 0.85 | 192.02 | 347.95 | 286.74 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo7 | 3.768 | 0.85 | 193.96 | 346.01 | 294.29 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo8 | 4.065 | 0.82 | 215.69 | 324.28 | 300.22 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo9 | 4.146 | 0.81 | 222.35 | 317.62 | 321.99 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Hypo10 | 4.156 | 0.81 | 224.63 | 315.34 | 323.16 | HBA-HYD_Al-HYD-HYD-RING_Ar |
| Fixed Cost | 0 | 1 | 45.55 | 494.42 | ||
| Null Cost | 6.991 | 0 | 539.97 | 0 |
 | ||||||
| R-Group a,b | ||||||
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
| R-Group | 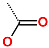 |  | 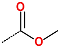 | 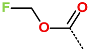 |  |  |
| No. | 7 | 8 | 9 | 10 | 11 | 12 |
| R-Group |  | 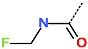 |  | 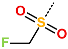 | 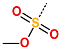 | 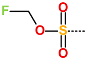 |
| No. | 13 | 14 | 15 | 16 | 17 | 18 |
| R-Group |  |  |  | 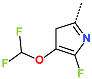 | 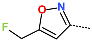 |  |
| No. | 19 | 20 | 21 | 22 | 23 | 24 |
| R-Group | 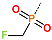 |  |  | 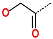 |  |  |
| No. | 25 | 26 | 27 | 28 | 29 | 30 |
| R-Group |  |  | 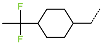 |  | 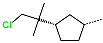 |  |
| No. | 31 | 32 | 33 | 34 | 35 | 36 |
| R-Group | 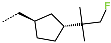 |  |  |  |  | 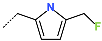 |
| No. | 37 | 38 | 39 | 40 | 41 | 42 |
| R-Group | 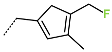 | 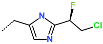 | 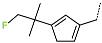 | 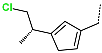 |  | 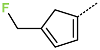 |
| No. | 43 | 44 | 45 | 46 | 47 | 48 |
| R-Group | 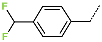 |  | 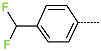 |  | 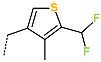 |  |
| No. | 49 | 50 | 51 | 52 | 53 | 54 |
| R-Group |  |  |  |  |  |  |
| No. | 55 | 56 | 57 | 58 | 59 | 60 |
| R-Group |  |  | 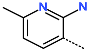 | 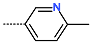 | 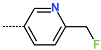 |  |
| No. | 61 | 62 | 63 | 64 | 65 | 66 |
| R-Group |  |  | 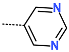 | 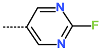 | 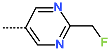 | 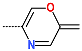 |
| No. | 67 | 68 | 69 | 70 | 71 | 72 |
| R-Group | 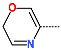 |  | 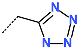 | 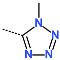 |  | 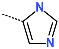 |
| No. | 73 | 74 | 75 | 76 | 77 | 78 |
| R-Group |  | 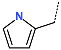 | 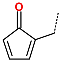 |  |  |  |
| No. | 79 | 80 | 81 | 82 | 83 | 84 |
| R-Group |  | 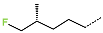 |  |  |  |  |
| No. | 85 | 86 | 87 | 88 | 89 | 90 |
| R-Group |  | 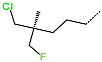 |  |  | 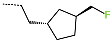 |  |
| No. | 91 | 92 | 93 | 94 | 95 | 96 |
| R-Group |  | 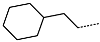 | 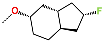 |  | 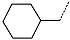 |  |
| No. | 97 | 98 | 99 | 100 | 101 | 102 |
| R-Group | 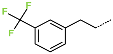 |  |  | 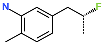 |  | 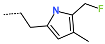 |
| No. | 103 | 104 | 105 | 106 | 107 | 108 |
| R-Group | 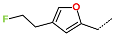 | 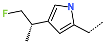 |  |  | 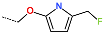 |  |
| No. | 109 | 110 | 111 | |||
| R-Group |  |  |  | |||
| New IPCL Analogues | R3-R2-R1-R1′ R-Groups | Mw a | ∆∆HMM b | ∆∆Gsol c | ∆∆TS d | ∆∆Gcom e | f |
|---|---|---|---|---|---|---|---|
| [g·mol−1] | [kcal·mol−1] | [kcal·mol−1] | [kcal·mol−1] | [kcal·mol−1] | [nM] | ||
| Ref. | IPCL1 | 512 | 0 | 0 | 0 | 0 | 170 g |
| 1 | 79-32-57-22 | 493 | −0.6 | 4.3 | 9.9 | −6.3 | 20.3 |
| 2 | 85-32-57-22 | 529 | −4.2 | 3.6 | 6.7 | −7.3 | 13.5 |
| 3 | 85-30-57-22 | 563 | −5.9 | 5.0 | 4.9 | −5.8 | 23.7 |
| 4 | 87-32-57-22 | 511 | −3.5 | 4.6 | 7.8 | −6.7 | 17.1 |
| 5 | 79-31-57-22 | 511 | −4.5 | 5.0 | 7.6 | −7.2 | 14.4 |
| 6 | 86-32-57-22 | 527 | −2.3 | 2.9 | 8.0 | −7.4 | 13.0 |
| 7 | 86-31-57-22 | 545 | 2.2 | 2.1 | 6.2 | −2.0 | 103.6 |
| 8 | 109-31-57-22 | 563 | 1.2 | 2.7 | 4.5 | −0.5 | 177.4 |
| 9 | 88-50-57-22 | 499 | 3.2 | 1.0 | 10.1 | −5.9 | 23.3 |
| 10 | 79-32-57-4 | 511 | −0.2 | 0.7 | 9.5 | −8.9 | 7.5 |
| 11 | 79-31-57-4 | 529 | −2.7 | 1.1 | 6.8 | −8.4 | 9.0 |
| 12 | 79-48-52-4 | 489 | −6.5 | −0.5 | 0.7 | −7.7 | 11.9 |
| 13 | 111-47-73-22 | 500 | −1.7 | −4.4 | −0.7 | −5.4 | 28.3 |
| 14 | 80-27-53-22 | 491 | −2.6 | 4.1 | 7.2 | −5.7 | 25.0 |
| 15 | 80-27-53-4 | 509 | −4.6 | 2.6 | 6.7 | −8.7 | 8.0 |
| 16 | 80-27-52-22 | 488 | −0.6 | 3.9 | 11.0 | −7.6 | 12.0 |
| 17 | 80-27-52-4 | 506 | −6.7 | −0.1 | 6.9 | −13.7 | 1.2 |
| 18 | 80-27-75-4 | 505 | −6.1 | −0.3 | 7.7 | −14.0 | 1.1 |
| 19 | 80-27-74-4 | 492 | −13.1 | 1.6 | 3.1 | −14.6 | 0.8 |
| 20 | 77-50-76-4 | 490 | 9.9 | 4.2 | 7.0 | 7.1 | 3250.6 |
| 21 | 80-32-52-6 | 498 | −1.0 | −0.4 | 10.8 | −12.1 | 2.2 |
| 22 | 78-26-58-11 | 494 | 4.5 | −0.9 | 4.0 | −0.3 | 192.2 |
| 23 | 102-31-52-6 | 553 | −7.4 | −0.3 | 3.1 | −10.9 | 3.5 |
| 24 | 102-31-51-6 | 539 | −18.3 | 10.1 | 3.0 | −11.2 | 3.1 |
| 25 | 102-31-51-4 | 525 | −17.7 | 9.4 | 5.2 | −13.4 | 1.3 |
| 26 | 102-31-51-1 | 493 | −3.7 | 5.1 | 9.1 | −7.8 | 11.5 |
| 27 | 88-29-58-1 | 504 | −1.1 | −0.6 | 6.3 | −8.0 | 10.4 |
| 28 | 103-29-58-4 | 526 | 2.7 | 0.9 | 3.9 | −0.3 | 191.1 |
| 29 | 103-29-58-1 | 494 | 4.0 | 0.9 | 1.4 | 3.5 | 833.4 |
| 30 | 108-43-58-1 | 496 | −1.9 | −1.2 | −7.1 | 3.9 | 967.9 |
| 31 | 98-45-63-1 | 526 | 4.5 | −2.3 | −6.5 | 8.8 | 6024.8 |
| 32 | 97-45-63-1 | 494 | 4.9 | −2.8 | −4.6 | 6.6 | 2653.6 |
| 33 | 80-27-54-4 | 478 | −9.6 | 2.1 | 6.6 | −14.2 | 1.0 |
| 34 | 91-27-54-4 | 474 | -8.2 | 1.2 | 4.8 | −11.8 | 2.4 |
| 35 | 92-27-54-4 | 486 | −5.4 | −0.5 | 8.1 | −14.0 | 1.1 |
| 36 | 94-27-54-4 | 504 | −8.9 | 2.3 | 6.6 | −13.2 | 1.5 |
| 37 | 95-27-54-4 | 472 | −4.0 | 0.9 | 6.6 | −9.7 | 5.5 |
| 38 | 101-27-54-4 | 512 | −1.2 | 1.6 | 2.4 | −1.9 | 104.0 |
| 39 | 107-27-54-4 | 503 | −7.0 | −0.3 | −2.7 | −4.6 | 38.0 |
| IPCLx a | #stars b | Mwc [g·mol−1] | Smold [Å2] | Smol,hfoe [Å2] | Vmolf [Å3] | RotB g | HBdon h | HBacc i | logPo/w j | logSwat k | logKHSA l | logB/B m | BIPcaco n [nm·s−1] | #meta o | [nM] p | HOA q | %HOA r |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPCL1 | 1 | 512.1 | 848.6 | 98.3 | 1568.7 | 10 | 1 | 8.5 | 4.9 | −6.7 | 0.4 | −1.0 | 899.3 | 3 | 170 * | 1 | 96 |
| 80-27-52-4 | 2 | 505.6 | 849.4 | 51.0 | 1596.7 | 10 | 1 | 5 | 6.9 | −8.5 | 1.2 | −0.2 | 2548.8 | 2 | 1.2 | 1 | 100 |
| 80-27-75-4 | 1 | 504.6 | 832.3 | 83.7 | 1579.2 | 11 | 1 | 7 | 5.8 | −7.3 | 0.7 | −0.6 | 1316.5 | 2 | 1.1 | 1 | 91 |
| 80-27-74-4 | 1 | 491.6 | 812.9 | 68.4 | 1544.0 | 11 | 2 | 5 | 6.3 | −7.6 | 1.0 | −0.5 | 1809.6 | 2 | 0.8 | 1 | 100 |
| 80-32-52-6 | 2 | 497.7 | 872.5 | 44.6 | 1667.0 | 11 | 1 | 5 | 7.2 | −8.5 | 1.4 | −0.4 | 2997.6 | 2 | 2.2 | 1 | 100 |
| 102-31-52-6 | 4 | 552.7 | 974.8 | 82.6 | 1802.6 | 10 | 2 | 5 | 7.7 | −10.5 | 1.7 | −0.8 | 1278.1 | 5 | 3.5 | 1 | 100 |
| 102-31-51-6 | 3 | 538.7 | 896.4 | 73.1 | 1719.6 | 10 | 2 | 4 | 7.7 | −9.4 | 1.7 | −0.6 | 1529.1 | 5 | 3.1 | 1 | 100 |
| 102-31-51-4 | 3 | 524.6 | 885.8 | 79.7 | 1678.8 | 9 | 2 | 4 | 7.4 | −9.3 | 1.6 | −0.6 | 1316.1 | 5 | 1.3 | 1 | 100 |
| 80-27-54-4 | 2 | 477.5 | 807.4 | 69.1 | 1500.8 | 10 | 1 | 4 | 6.7 | −8.1 | 1.2 | −0.4 | 1689.2 | 1 | 1.0 | 1 | 100 |
| 91-27-54-4 | 1 | 473.5 | 773.4 | 70.3 | 1458.6 | 9 | 1 | 5.7 | 5.5 | −6.9 | 0.8 | −0.4 | 1750.9 | 1 | 2.4 | 1 | 100 |
| 92-27-54-4 | 2 | 485.6 | 806.4 | 72.4 | 1532.1 | 9 | 1 | 4 | 6.7 | −8.0 | 1.3 | −0.5 | 1520.6 | 1 | 1.1 | 1 | 100 |
| 94-27-54-4 | 2 | 503.6 | 788.3 | 60.2 | 1536.9 | 8 | 1 | 4 | 6.9 | −8.1 | 1.4 | −0.2 | 2167.3 | 1 | 1.5 | 1 | 100 |
| 95-27-54-4 | 1 | 471.6 | 751.9 | 66.5 | 1453.0 | 8 | 1 | 4 | 6.2 | −7.2 | 1.2 | −0.3 | 1792.3 | 1 | 5.5 | 1 | 100 |
| Veklury | 5 | 600.6 | 894.9 | 260.9 | 1721.0 | 16 * | 4 | 17.9 | 1.0 | −4.7 | −0.9 | −3.2 * | 33.3 | 6 | 1 | 34 | |
| Lagevrio | 0 | 329.3 | 579.5 | 253.6 | 998.7 | 7 | 4 | 13.3 | −1.5 | −2.1 | −1.1 | −2.3 | 39.0 | 4 | 2 | 47 | |
| Nirmatrelvir | 0 | 499.5 | 713.4 | 183.4 | 1423.3 | 8 | 2.3 | 11.3 | 0.1 | −2.6 | −1.3 | −1.3 | 36.4 | 4 | 2 | 56 | |
| Ritonavir | 11 | 720.9 | 1110.9 * | 134.3 | 2177.9 * | 18 * | 3.3 | 11.0 | 6.6 * | −8.4 * | 0.8 | −2.1 | 309.3 | 9 * | 1 | 71 | |
| Dexamethasone | 0 | 392.5 | 605.6 | 177.8 | 1141.4 | 5 | 3 | 8.2 | 1.9 | −3.6 | 0.0 | −1.3 | 204.1 | 4 | 3 | 80 | |
| Baricitinib | 0 | 371.4 | 617.9 | 191.1 | 1103.5 | 4 | 1 | 8.5 | 1.6 | −4.8 | −0.2 | −1.5 | 152.7 | 2 | 3 | 75 | |
| Lopinavir | 5 | 628.8 | 1018.2 * | 121.2 | 1992.2 | 16 * | 4 | 9.5 | 5.8 | −6.9 * | 0.6 | −1.8 | 339.0 | 8 | 1 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Fofana, I.; Dali, B.; Koné, M.; Sujova, K.; Megnassan, E.; Miertus, S.; Frecer, V. In Silico Optimization of Inhibitors of the 3-Chymotrypsin-like Protease of SARS-CoV-2. Life 2026, 16, 6. https://doi.org/10.3390/life16010006
Fofana I, Dali B, Koné M, Sujova K, Megnassan E, Miertus S, Frecer V. In Silico Optimization of Inhibitors of the 3-Chymotrypsin-like Protease of SARS-CoV-2. Life. 2026; 16(1):6. https://doi.org/10.3390/life16010006
Chicago/Turabian StyleFofana, Issouf, Brice Dali, Mawa Koné, Katarina Sujova, Eugene Megnassan, Stanislav Miertus, and Vladimir Frecer. 2026. "In Silico Optimization of Inhibitors of the 3-Chymotrypsin-like Protease of SARS-CoV-2" Life 16, no. 1: 6. https://doi.org/10.3390/life16010006
APA StyleFofana, I., Dali, B., Koné, M., Sujova, K., Megnassan, E., Miertus, S., & Frecer, V. (2026). In Silico Optimization of Inhibitors of the 3-Chymotrypsin-like Protease of SARS-CoV-2. Life, 16(1), 6. https://doi.org/10.3390/life16010006






