Abstract
Cervical spinal cord injury causes severe functional impairment with limited spontaneous recovery, and while spinal cord stimulation has emerged as a promising neuromodulatory strategy, evidence for cervical applications remains fragmented. To address this gap, we conducted a systematic review synthesizing preclinical and clinical evidence on cervical spinal cord stimulation for functional rehabilitation following spinal cord injury. The review was registered on PROSPERO (CRD420251088804) and conducted in accordance with PRISMA guidelines, with PubMed, Embase, IEEE Xplore, and Web of Science searched from inception to July 2025 for animal and human studies of cervical spinal cord stimulation, including epidural, intraspinal, and transcutaneous approaches, reporting functional neurological outcomes. Risk of bias was assessed using the Cochrane RoB 2 and ROBINS-I tools, and due to substantial heterogeneity, results were synthesized narratively. Thirty-one studies comprising 119 animals and 156 human participants, met inclusion criteria. Across studies, outcome measures such as GRASSP, ISNCSCI, and dynamometry consistently demonstrated improvements in hand strength, dexterity, and voluntary motor activation. Several studies also reported gains in sensory and autonomic function, whereas respiratory outcomes were infrequently assessed. Adjunctive interventions, including cortical stimulation, brain–computer interface priming, and task-specific training frequently augmented recovery. Adverse events were generally mild, although overall risk of bias was predominantly serious. Overall, cervical spinal cord stimulation demonstrates preliminary assistive and therapeutic effects on motor recovery, with additional sensory, autonomic, and potential respiratory benefits.
1. Introduction
Spinal cord injury (SCI) affects primarily young and middle-aged adults, with traumatic cases occurring at a mean age of 35 years and individuals aged 21–40 accounting for over 40% of cases [1,2]. Cervical injuries frequently cause tetraparesis or tetraplegia, resulting in loss of independence in daily activities, caregiver reliance, and, in high cervical lesions, respiratory compromise requiring ventilatory support [3]. Chronic sequelae, including spasticity, neuropathic pain, cardiovascular dysregulation, and pressure ulcers, contribute further to long-term morbidity [4].
The intrinsic potential for recovery after high cervical SCI remains limited. Neurological improvement generally plateaus within the first year, and gains are modest even under intensive rehabilitation [5,6,7,8]. While incremental improvements can meaningfully impact independence, substantial restoration of function is rare in motor-complete or near-complete cervical SCI. Current treatment approaches focus on acute surgical decompression of the spinal cord and stabilization, prevention of secondary injury, pharmacological management, and long-term multidisciplinary rehabilitation. Although advances in rehabilitation techniques have led to incremental improvements, they remain insufficient for restoring meaningful function in patients with severe cervical SCI. Crucially, traditional therapies are limited by the absence of interventions that can directly modulate spinal circuitry and promote recovery beyond the modest natural course.
Spinal cord stimulation (SCS) has emerged as a promising neuromodulation strategy to address this gap. Originally introduced for analgesia in the 1960s based on the gate control theory of pain [9,10], subsequent work showed that activating spinal networks can also influence motor output. In individuals with chronic motor-complete paraplegia, lumbosacral epidural stimulation combined with intensive rehabilitation has enabled weight-bearing standing, volitional control of paralyzed muscles, and even overground walking [11,12,13]. These landmark studies established that residual spinal circuits below the lesion can generate functional motor patterns when appropriately facilitated [14].
Following successful demonstrations of SCS at the lumbosacral cord, attention shifted to the cervical spinal cord, given its critical role in upper limb and respiratory function. Several stimulation modalities have been explored, including intraspinal microstimulation (ISMS) via penetrating microelectrodes, epidural electrical stimulation (EES) using dorsal column arrays, and non-invasive transcutaneous stimulation (tSCS) over the dorsal cervical spine [15,16,17,18,19,20,21,22,23,24,25,26]. ISMS offers high anatomical and functional specificity in preclinical models but relies on penetrating electrodes that currently limit near-term clinical translation; EES enables more targeted segmental recruitment than tSCS but requires invasive surgical implantation; and tSCS provides a non-invasive and readily deployable approach at the expense of reduced spatial specificity. Preclinical studies demonstrate that cervical stimulation can selectively recruit forelimb and respiratory circuits and support recovery of skilled motor behaviors after injury [15,16,19,21,27,28,29,30,31,32,33,34,35,36,37,38]. These findings suggest that cervical neuromodulation may provide a means to restore upper limb and respiratory function beyond the modest natural course of recovery.
Against this background, the present systematic review synthesizes preclinical and clinical evidence on cervical SCS for functional rehabilitation after SCI, with a focus on motor, sensory, respiratory, and autonomic outcomes, and evaluates the methodological quality of the available studies.
2. Materials and Methods
2.1. Search Strategy
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. The review protocol was prospectively registered in PROSPERO (CRD420251088804). Reporting was conducted in accordance with the PRISMA guidelines [39]. The PRISMA 2020 flow diagram was generated using the PRISMA2020 R package and Shiny app version 1.1.1 [40]. A comprehensive search of the published literature was performed in MEDLINE/PubMed, Embase, Web of Science, and IEEE Xplore electronic databases in June–July 2025 by two independent reviewers (M.W. and P.A.), working independently and blinded to each other’s selections to ensure reliability. The systematic search combined both keywords and, where applicable, Medical Subject Headings (MeSH), using the following Boolean string: (“cervical spinal cord stimulation” OR “cervical spinal cord” OR “cervical stimulation”) AND (“spinal cord injury” OR “SCI”) AND (“stimulation” OR “neuromodulation”) AND (“recovery” OR “rehabilitation” OR “function” OR “movement” OR “sensory” OR “motor” OR “autonomic” OR “respiration”). Additionally, the reference lists of all included studies and relevant reviews were manually screened to identify any additional eligible reports not captured by the electronic search.
2.2. Study Selection Criteria
Study selection was guided by predefined PICOS (Population, Intervention, Comparison, Outcome, and Study design) criteria [41]. Studies were eligible for inclusion if they met the following criteria: (1) enrolled humans or animals with spinal cord injury; (2) applied electrical stimulation to the cervical spinal cord; (3) reported outcomes in at least one functional neurological domain (motor, sensory, autonomic, respiratory, or related function); and (4) employed an eligible study design, including case series, case reports, cohort studies, conference abstracts, preprints, or randomized controlled trials (RCTs). Studies were excluded if they (1) focused primarily on chronic pain, spasticity, or other non-functional outcomes; (2) targeted stimulation sites outside the cervical spinal cord and its associated roots; (3) involved participants with peripheral nerve injuries; (4) lacked sufficient extractable data, such as reviews, editorials, or protocol descriptions; or (5) were published in languages other than English.
2.3. Data Extraction
Two independent reviewers (M.W. and P.A.) screened abstracts, titles, and full texts of results yielded by the search strategy. Standardized data were extracted from eligible studies according to the following metrics: first author’s name and year of publication, type of animal in case of non-human studies, age and gender, clinical characteristics (level of injury, time since injury, ASIA Impairment Scale (AIS) grade), stimulator characteristics (type of device/manufacturer, number of leads, location of leads, and method of lead placement), stimulation parameters (frequency, pulse width, amplitude, stimulation time length, and optimization), functional neurological outcomes, and adverse effects.
2.4. Analysis of Neurological Functional Outcomes
Studies reporting functional neurological outcomes were reviewed in detail to identify unique cases and cohorts. Potentially overlapping participants were screened by cross-checking published subject identifiers or explicit references to previously reported cases. When multiple publications described the same cohort, only the most recent or comprehensive report was included. Functional outcomes were synthesized qualitatively across motor, sensory, respiratory, and autonomic domains. Where possible, standardized assessments such as Graded and Redefined Assessment of Strength, Sensibility, and Prehension (GRASSP), ISNCSCI motor scores, and dynamometry were compared descriptively to identify consistent patterns of improvement [42,43]. Due to heterogeneity in study designs, outcome measures, and reporting formats no quantitative pooling or effect size estimation was performed. Instead, findings were narratively summarized to highlight the range and frequency of reported improvements and the contexts in which stimulation was most effective. Functional neurological outcomes in preclinical studies were not systematically analyzed using standardized metrics, as animal experiments employed highly heterogeneous, task-specific outcome measures with incomplete and inconsistent reporting across studies.
To contextualize the magnitude of reported changes, frequently used clinical outcome scales are briefly summarized here. The ISNCSCI Upper Extremity Motor Score (UEMS) ranges from 0 to 50, with higher scores indicating better motor strength across ten key muscles in the upper limbs [43]. GRASSP is reported as separate subscores (not a single total): Strength, 0–50 per limb; Sensibility, 0–12 per limb; Prehension Ability, 0–30 per limb; and Prehension Performance, which is time-based with no maximum score [42]. Neurological severity was categorized using the ASIA Impairment Scale (AIS), where AIS A denotes motor and sensory complete injury and AIS E denotes normal function [44]. Functional independence was commonly measured using the Spinal Cord Independence Measure III (SCIM-III), which ranges from 0 to 100 and captures self-care, respiration, sphincter management, and mobility [45].
2.5. Bias Assessment
Two independent reviewers (M.W. and P.A.) assessed the risk of bias for all included human studies. Version 2 of the Cochrane risk-of-bias tool for randomized trials (Rob-2) was applied to randomized clinical trials and the ROBINS-I tool was used for non-randomized studies [46,47]. Risk of bias for preclinical animal studies was not formally tabulated but common limitations, including small sample sizes, heterogeneous injury models, and absence of blinding, are addressed in the Discussion.
3. Results
3.1. Study Selection
The database search identified 1847 records from PubMed/Medline, Web of Science, Embase, and IEEE Xplore. After removal of 623 duplicate records, 1224 records were screened by title and abstract. Of these, 1101 records were excluded during initial screening. A total of 123 reports were sought for retrieval, of which 40 reports were not retrieved. Eighty-three full-text reports were assessed for eligibility. Fifty-two reports were excluded for the following reasons: no functional outcomes reported (n = 27), prospective trial protocols (n = 10), stimulation not targeting the cervical spine (n = 8), absence of spinal cord injury (n = 4), or review articles (n = 3). In total, 31 studies met the inclusion criteria and were included in the qualitative synthesis. Figure 1 presents the PRISMA 2020 flow diagram summarizing the study selection process [39].
3.2. Study and Patient Characteristics
The included 31 studies were published between 2013 and 2025 and involved a total of 275 participants (156 humans, 119 animals) with cervical SCI. Study designs included five case reports, ten prospective case series, three RCTs, one conference abstract, one preprint, and eleven preclinical animal studies. Each study focused on only one type of interface: ISMS was investigated in two, tSCS in twenty-one, and EES in eight studies. Only one study investigated EES in humans with SCI. Injury chronicity varied from acute (e.g., same day) to 32 years post injury, and neurological severity ranged from AIS A–D, where applicable. Where follow-up assessments were carried out, duration ranged from 2 weeks to 9 months. Table 1 summarizes key study and patient characteristics.
3.3. Stimulation Location and Parameters
Stimulation parameters varied considerably across both animal and human studies (Table 2). Reported frequencies ranged from 20 to 300 Hz, with pulse widths typically between 200 and 1000 µs and amplitudes titrated to elicit visible or EMG-detected motor responses without discomfort or tissue injury. Electrode placement and optimization strategies differed substantially among stimulation modalities. ISMS was applied in preclinical rodent and primate studies using penetrating microelectrodes inserted directly into the ventral horn, most often from C6 to C7 or T1. These sites reliably recruited forelimb and hand muscles, with short pulse widths (200–300 µs) and variable frequencies and currents. Optimization involved systematic mapping of motor pools to identify electrode sites producing selective grasping or reaching movements. EES in animal models employed electrodes placed over the dorsal surface of the cervical enlargement, frequently lateralized to the dorsal root entry zones to maximize segmental recruitment. Frequencies typically ranged from 20 to 100 Hz with pulse widths between 200 and 500 µs. A respiratory-focused study in rats positioned electrodes at C3–C5 to engage phrenic motoneurons and elicit inspiratory activity. In human studies, stimulation optimization was guided by intraoperative neurophysiological monitoring or iterative adjustment to maximize functional responses. Transcutaneous spinal cord stimulation studies placed large surface electrodes over the posterior neck, typically spanning C3–C5, with reference electrodes over the iliac crests or shoulders. Later work often employed two adjacent cervical electrodes or three-patch configurations to broaden current spread. Frequencies ranged from 20 to 50 Hz, sometimes combined with carrier frequencies of 5 kHz to reduce discomfort and improve current penetration. Pulse widths varied from 500 to 1000 µs, and amplitudes reached 40–200 mA depending on electrode configuration and participant tolerance. Optimization strategies included titration to evoke surface motor evoked potentials (sMEPs), motor pool mapping, or real-time adjustments based on voluntary motor output during training tasks. Collectively, these findings indicate that ISMS enables selective recruitment of cervical motor pools but remains limited to preclinical applications. EES allows segmental control of forelimb and respiratory circuits yet requires surgical implantation, whereas tSCS provides a non-invasive alternative that produces broader, less specific activation patterns.
3.4. Outcomes Measured
Motor recovery was the most consistently assessed outcome domain. The synthesis below primarily reflects standardized outcome reporting in human studies, as functional outcomes in animal experiments were heterogeneous and often reported using non-uniform or task-specific measures. Improvements were captured by standardized tools including GRASSP, ISNCSCI upper extremity motor subscores, and dynamometry, supplemented by patient-reported outcomes and task-based assessments (Table 3).
GRASSP outcomes were most frequently reported for tSCS, assessed in nine studies that consistently demonstrated improvements in strength and prehension subscores. Inanici et al. documented a 52-point increase in total GRASSP score, with persistent benefits for more than three months after stimulation ceased; in their later study, similar improvements were maintained up to six months [48,49]. Zhang et al. reported 10–33-point increases in GRASSP scores, with multi-session stimulation plus training producing sustained gains at one to three months [50,51]. Benavides et al. showed measurable GRASSP improvements even after a single tSCS session, while Capozio et al. reported gains in 4 of 5 patients [52,53]. Additional single-patient or small series further reinforced consistent GRASSP improvements when tSCS was paired with adjunctive interventions such as brain–computer interface (BCI) priming or robotic exoskeleton training [54,55]. Similar trends were observed in the EES study by Lu et al., showing improved voluntary control and task performance in two patients after epidural stimulation [18].
ISNCSCI motor scores also demonstrated consistent improvements. Six tSCS studies reported increases of up to 23 points, often exceeding minimally important difference thresholds [48,49,53,54,56,57]. In a multicenter trial of 60 participants, Moritz et al. found that 72% of participants surpassed this threshold [56]. Some studies noted neurological level improvements in one to two segments, though sensory recovery did not consistently follow dermatome maps. Lu et al. documented sustained session-to-session increases in Upper Extremity Motor Scores in two patients treated with cervical EES [18].
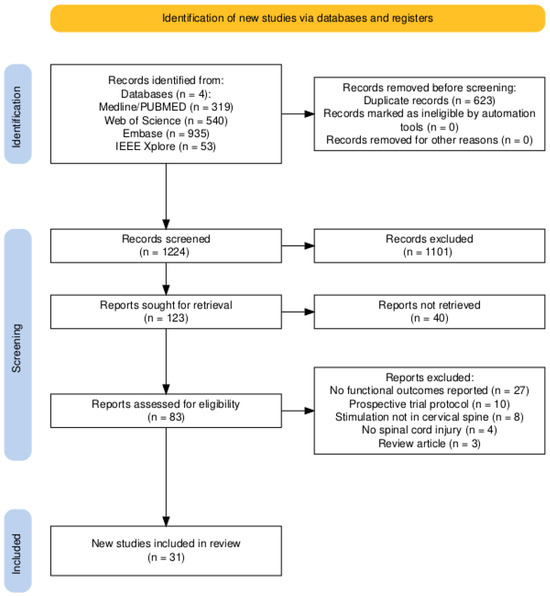
Figure 1.
PRISMA flow diagram describing the selection process.
Dynamometry outcomes were reported in thirteen tSCS studies, showing consistent gains in voluntary grip and pinch strength. Increases ranged from the first detectable grip force in previously paralyzed muscles to large magnitude gains such as a 325% increase in grip strength and >1000% increases in exerted force [51,58,59,60]. Huang et al. demonstrated that residual baseline grip was a strong predictor of response, with robust gains only in participants who had minimal pre-stimulation grip force [61]. Lu et al. similarly reported improved grip force in implanted participants [18].
Respiratory outcomes were less frequently evaluated. Gad et al. demonstrated in a chronic tetraplegic patient that cervical tSCS improved voluntary breathing and coughing, with effects persisting after stimulation was turned off [62]. In animal models, Bezdudnaya et al. showed that epidural stimulation at C4 maintained breathing with normal end-tidal and improved blood pressure [37].
Autonomic outcomes showed variable but promising findings. Inanici et al. observed improved bladder function, thermoregulation, and heart rate control following tSCS [48]. Singh et al. found cervical tSCS to be hemodynamically safe and feasible in a study of seven children with SCI [63]. Effects were inconsistent across studies, reflecting small sample sizes. Autonomic outcomes were not assessed in the single human EES study [18].
Patient-reported outcomes were included in five tSCS studies, emphasizing improved independence in hand use and quality of life. Moritz et al. reported significant improvements in SCIM-III scores and patient-reported outcome scores in parallel with objective motor gains [64]. EES patients similarly reported improvements in mobility and self-care alongside functional gains [18].
3.5. Combination Interventions
Several studies combined cervical spinal cord stimulation with additional interventions aimed at enhancing neuroplasticity and functional recovery. Four preclinical studies paired motor cortex stimulation with cervical SCS. These studies all demonstrated that concurrent cortical and spinal stimulation produced greater improvements in corticospinal excitability and forelimb motor outcomes than either intervention alone, suggesting synergistic effects on descending motor pathways and spinal circuitry [19,65,66,67].
Multimodal approaches were also explored in human studies. Samejima et al. conducted a human feasibility case study in which motor intention detected by a BCI was used to prime cervical tSCS. The intervention demonstrated safety and short-term improvements in voluntary hand function, supporting the concept that coupling stimulation with brain-driven signals may enhance recovery [68]. Capozio et al. combined cervical tSCS with motor imagery in eight participants. This single-session study showed acute improvements in manual dexterity and cortical excitability, suggesting that cognitive engagement of motor networks may potentiate stimulation effects [69]. García-Alén et al. performed a 15-patient clinical trial combining cervical tSCS with upper limb robotic-assisted rehabilitation. Additive improvements in strength and functional outcomes were seen in robotic exoskeleton training and tSCS compared to exoskeleton-assisted training alone [55].
Several additional studies integrated cervical tSCS with intensive task-specific training. These studies reported more durable functional improvements than stimulation alone, reinforcing the role of activity-dependent plasticity [50,70]. Collectively, these findings suggest that cervical SCS may be most effective not as a stand-alone therapy but when used with neuromodulatory adjuncts.
3.6. Methodological Quality
Risk of bias was high across most human studies (Figure 2). Eleven studies were judged to be at serious risk of bias, primarily due to very small sample sizes, heterogeneous injury cohorts, lack of control groups, and unblinded outcome assessments. An additional seven studies were rated as serious–critical, reflecting the limitations of single-patient case reports, exploratory pilot designs, and sparse reporting (including one conference abstract and one preprint), which further constrain generalizability. These two studies were included to reflect the dynamic and rapidly evolving nature of the field; however, as they have not undergone full peer review, their findings should be interpreted with caution. Only two studies achieved lower overall ratings: Huang et al., a sham-controlled crossover trial in 10 participants, was judged as having moderate risk of bias, and the multicenter trial by Moritz et al. (n = 60) was rated moderate due to its larger sample size, standardized protocol, and comprehensive reporting, though it still lacked a sham-control condition [56,61]. Collectively, the evidence base remains dominated by small, uncontrolled studies. Similarly, while preclinical studies provide compelling mechanistic support for cervical spinal cord stimulation, they are constrained by small sample sizes, heterogeneous injury models, and frequent absence of blinding. Together, these limitations underscore that although findings across both animal and human studies are encouraging, the certainty of evidence remains low and highlights the need for larger, rigorously controlled clinical trials. Full results of the human study assessments are summarized in Appendix A, Table A1.
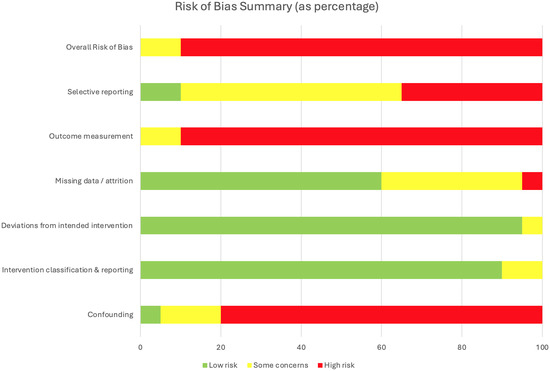
Figure 2.
Risk of bias summary for 20 included human studies assessed with the RoB 2 and ROBINS-I tools.
3.7. Adverse Effects
Adverse events were infrequently reported. Across 31 studies, no serious complications (e.g., permanent neurological worsening, infection requiring device removal) were documented. Mild or transient effects included skin irritation from transcutaneous electrodes, discomfort or muscle spasms during stimulation, and lead migration in epidural implants. Reporting of safety outcomes was inconsistent, and most studies did not include systematic adverse event monitoring.

Table 1.
Study characteristics of included animal and human studies.
Table 1.
Study characteristics of included animal and human studies.
| Authors | Country | Population | Sex | N | Age Range | Injury Level | Injury Duration | Injury Type/Severity | Modality |
|---|---|---|---|---|---|---|---|---|---|
| Animal studies | |||||||||
| Kasten et al. (2013) [15] | USA | Rats | F | 11 | Adult | C4–C5 | 4 weeks | Contusion injury | ISMS |
| McPherson et al. (2015) [17] | USA | Rats | F | 9 | Adult | C4–C5 | >8 weeks | Contusion injury | ISMS |
| Alam et al. (2015) [29] | USA | Rats | F | 12 | Adult | C4 | 1–2 weeks | Dorsal funiculi crush | EES |
| Alam et al. (2017) [30] | USA | Rats | F | 5 | Adult | C4 | 1 week | Dorsal funiculi crush | EES |
| Bezdudnaya et al. (2018) [37] | USA | Rats | M | 20 | Adult | C1 | Acute | Complete transection | EES |
| Yang et al. (2019) [66] | USA | Rats | F | 27 | 10–12 weeks | C3 | 11 days | Contusion injury | EES |
| Samejima et al. (2021) [68] | USA | Rats | F | 5 | Adult | C4 | 2 weeks | Contusion injury | EES |
| Barra et al. (2022) [19] | Switzerland | Macaca fascicularis | F | 3 | 3–9 years | C5–C6 | 1 week | Unilateral CST transection | EES |
| Pal et al. (2022) [71] | USA | Rats | F | 8 | Adult | C4 | 10 days | AAV-based CST inactivation | EES |
| Song et al. (2016) [67] | USA | Rats | F | 6 | Adult | C1 | 1 week | Transection at rostral medualla | tSCS |
| Zareen et al. (2017) [25] | USA | Rats | F | 13 | 10–12 weeks | C4 | 2 weeks | Contusion injury | tSCS |
| Human studies | |||||||||
| Lu et al. (2016) [18] | USA | Human | N.R. | 2 | N.R. | C5–C6 | >18 months | AIS B | EES |
| Murray et al. (2017) [26] | USA | Human | M | 1 | 27 years | C6–C7 | 9 years | AIS C | tSCS |
| Inanici et al. (2018) [49] | USA | Human | M | 1 | 62 years | C3 | 2 years | AIS D | tSCS |
| Gad et al. (2018) [59] | USA | Human | 1F 5M | 6 | 20–62 years | C4–C8 | 1–21 years | AIS A (n = 0), B (n = 2), C (n = 4), D (n = 0) | tSCS |
| Benavides et al. (2020) [52] | USA | Human | 4F 6M | 10 | 22–64 years | C4–C6 | >1 year | AIS A (n = 4), B (n = 3), C (n = 2), D (n = 1) | tSCS |
| Zhang et al. (2020) [51] | USA | Human | M | 1 | 38 years | C5 | 15 years | AIS A | tSCS |
| Gad et al. (2020) [62] | USA | Human | M | 1 | 39 years | C5 | 9 years | AIS A | tSCS |
| Tefertiller et al. (2021) [57] | USA | Human | 2F 5M | 7 | 18–55 years | C4–C6 | 15–38 months | AIS A (n = 0), B (n = 4), C (n = 2), D (n = 1) | tSCS |
| Inanici et al. (2021) [48] | USA | Human | 2F 4M | 6 | 28–62 years | C3–C5 | 1.5–12 years | AIS A (n = 0), B (n = 2), C (n = 2), D (n = 2) | tSCS |
| Huang et al. (2022) [61] | USA | Human | N.R. | 10 | 22–63 years | C3–C7 | 2–14 years | AIS A–B | tSCS |
| McGeady et al. (2022) [54] | UK/Hong Kong | Human | M | 1 | 48 years | C4 | 12 years | AIS A | tSCS |
| Zhang et al. (2023) [50] | USA | Human | 4M | 4 | 25–78 years | C1–C4 | 4–9 years | AIS A (n = 1), B (n = 1), C (n = 0), D (n = 2) | tSCS |
| Oh et al. (2023) [70] | USA | Human | 4M | 4 | 23–29 years | C4–C6 | 2–14 years | AIS A–B | tSCS |
| García-Alén et al. (2023) [55] | Spain | Human | 1F 14M | 15 | 18–70 years | C4–C7 | 3–10 months | AIS A–D | tSCS |
| Human studies | |||||||||
| Chandrasekaran et al. (2023) [60] | USA | Human | 2M | 2 | 20–39 years | C5 | 4–7 years | AIS A–B | tSCS |
| Moritz et al. (2024) [56] | International | Human | 10F 50M | 60 | years | C2–C7 | year | AIS B–D | tSCS |
| Singh et al. (2024) [63] | USA | Human (Children) | 1F 6M | 7 | 6–17 years | C1–C7 | >2 years | AIS A (n = 1), B (n = 4), C (n = 1), D (n = 1) | tSCS |
| Verma et al. (2025) [58] | USA | Human | 1F 4M | 5 | 19–67 years | C4–C7 | 3–18 years | AIS A (n = 3), B (n = 1), C (n = 1), D (n = 0) | tSCS |
| Capozio et al. (2025) [53] | UK | Human | 5F | 5 | 31–65 years | C3–C8 | 2–30 years | AIS A (n = 0), B (n = 0), C (n = 3), D (n = 2) | tSCS |
| Capozio et al. (2025) [69] | UK | Human | 4F 4M | 8 | 22–72 years | C2–C7 | 3–32 years | AIS A (n = 0), B (n = 1), C (n = 4), D (n = 3) | tSCS |
Abbreviations: AIS = ASIA Impairment Scale; CST = Cortico-Spinal Tract; AAV = Adeno-Associated Virus; N.R. = Not Reported.

Table 2.
Summary of stimulation parameters and adjunct interventions in animal and human studies.
Table 2.
Summary of stimulation parameters and adjunct interventions in animal and human studies.
| Authors | Population | Electrode Manufacturer | Electrode Placement | Pulse Width (s) | Frequency (Hz) (Carrier, kHz) | Amplitude | Adjunctive Intervention | Stimulation Optimization |
|---|---|---|---|---|---|---|---|---|
| Intraspinal microstimulation | ||||||||
| Kasten et al. (2013) [15] | Rats | Custom | C6–T1 ventral horn | 300 | N.R. | – | Set at threshold just to evoke forelimb movement when delivered via a single electrode | |
| McPherson et al. (2015) [17] | Rats | Custom | C6–C7 ventral horn | 200 | 50–100 | 30– | EMG-synchronized | 90% of motor threshold; synchronized ISMS below the injury with volitional motor commands |
| Epidural stimulation | ||||||||
| Alam et al. (2015) [29] | Rats | Custom | C6 and C8 | 200 | 40 | 400– | – | 70% of motor threshold for mono- and bipolar electrode pairings |
| Lu et al. (2016) [18] | Human | Boston Scientific | C5–T1 | 210 | 2–40 | 0.1–10 mA | – | Individually adjusted for motor evoked response |
| Alam et al. (2017) [30] | Rats | Custom | C6 and C8 | 200 | 20, 40, 60 | N.R. | – | 60–70% of sMEP threshold |
| Bezdudnaya et al. (2018) [37] | Rats | Custom | C3–C5 | 200–500 | 100–300 | 100– | – | Titrated to elicit phrenic motor responses and entrain breathing |
| Yang et al. (2019) [66] | Rats | LGMedSupply | C4–T2 | 200 | 330 | Up to 1.5 mA | Cortical stimulation | 75% of motor threshold for evoked response |
| Samejima et al. (2021) [68] | Rats | Custom | C6 | 400 | 50–100 | 300– | BCI | Individually set to invoke elbow extension |
| Barra et al. (2022) [19] | Macaca fascicularis | Custom | C6–T1 | 200–400 | 20–120 | 600– | Cortical stimulation | Stimulation triggered in phase with voluntary movement; individualized optimization |
| Pal et al. (2022) [71] | Rats | Custom | C5–C6 | 200 | 5 | N.R. | Cortical stimulation | M1 stimulation at 110% of the threshold for evoking a cortical MEP followed 10 ms later by spinal stimulation at 90% of the threshold for generating a spinal MEP |
| Transcutaneous stimulation | ||||||||
| Song et al. (2016) [67] | Rats | StimTentCom | C4–T2; M1 region | 200 | 100; 0.2 | 100–400/ 50– | Cortical stimulation | Paired M1 (0.2 Hz) and spinal stimulation (100 Hz, 5 pulses/burst) with 10 ms interstimulus interval |
| Murray et al. (2017) [26] | Human | UniPatch | C5–T2 | 1000 | 0.2 | Up to 150 mA | TMS | Intensities increased from sub-threshold to suprathreshold |
| Zareen et al. (2017) [25] | Rats | StimTentCom | C4–T2; M1 region | 200 | 330; 250 | Up to 1.5 mA | Cortical stimulation | 10 biphasic pulses at 250 Hz every 2 s; amplitude set at 75% of motor threshold |
| Inanici et al. (2018) [49] | Human | Axelgaard | C3–C4, C6–C7 | 1000 | 30 (10) | 80–120 mA | – | Individually titrated to comfort |
| Gad et al. (2018) [59] | Human | Axelgaard | C3–C4, C6–C7 | 1000 | 30 (10) | 10–250 mA | – | Adjusted to enable maximal grip strength without causing discomfort |
| Benavides et al. (2020) [52] | Human | Axelgaard | C5–C6 | 200 | 30 (5) | mA | TMS | Intensity set to evoke biceps root-evoked potential V in 5/10 trials |
| Zhang et al. (2020) [51] | Human | STIMEX | C3–C4, C7–T1 | 1000 | 30 (10) | Up to 80 mA | – | Adjusted to enable maximal grip strength without causing discomfort |
| Gad et al. (2020) [62] | Human | TESCoN | C5–C6 | 1000 | 30 (10) | 20 mA | – | Adjusted for dose–response curves of inspiratory capacities and forced expiratory volume |
| Tefertiller et al. (2021) [57] | Human | Axelgaard | C3–C4, C6–C7, T11–T12 | 1000 | 30 (10) | 45–150 mA | – | Adjusted until optimal functional movement of the targeted area was achieved in a joint below the level of injury |
| Inanici et al. (2021) [48] | Human | Axelgaard | C3–C4, C6–C7 | 1000 | 30 (10) | 80–120 mA | – | Individually titrated to comfort |
| Huang et al. (2022) [61] | Human | Axelgaard | C4–C5 | 1000 | 30 | 40–180 mA | – | Individually titrated to elicit maximal handgrip; stimulation timed concurrently to voluntary handgrip attempt |
| McGeady et al. (2022) [54] | Human | Axelgaard | C4–C6 | 1000 | 30 | 40–55 mA | Motor imagery | Individually titrated to comfort |
| Zhang et al. (2023) [50] | Human | STIMEX | C3–C5 | 1000 | 30 | Up to 120 mA | – | Individually adjusted for subthreshold upper extremity muscle activation |
| Oh et al. (2023) [70] | Human | Axelgaard | C6–T1 | 500 | 30 | 20–70 mA | – | Adjusted for maximum hand grip |
| García-Alén et al. (2023) [55] | Human | Axion | C3–C4, C6–C7 | 1000 | 30 (10) | 45–86 mA | Robotic exoskeleton | 90% of RMT induced by single-pulse tSCS at the abductor pollicis brevis |
| Chandrasekaran et al. (2023) [60] | Human | Custom | C4–T1 | 500 | 50 | 140–160 mA | – | Adjusted for maximal tolerable intensity during ABT |
| Moritz et al. (2024) [56] | Human | LIFT (ARCex) | C3–C7 | 1000 | 30 (10) | 10–180 mA | – | Individually titrated to comfort; electrode placement individualized based on desired effect |
| Singh et al. (2024) [63] | Human (Children) | Syrtenty | C3–C4, C6–C7, T10–T11 | 1000 | 30 (10) | 20–70 mA | – | Adjusted to enable maximal hand-grip force at subthreshold intensity |
| Verma et al. (2025) [58] | Human | Anuevo | C3–T1 | 500 | 30 | mA | – | Maximum tolerable intensity just below motor threshold |
| Capozio et al. (2025) [53] | Human | Axelgaard | Above/below lesion | 1000 | 30 | 40–70 mA | TMS | Individually titrated to 80–90% of RMT |
| Capozio et al. (2025) [69] | Human | Axelgaard | C5–C6 midline | 1000 | 30 (5) | 30–60 mA | TMS, motor imagery | Individually titrated to 80–90% of RMT |
Abbreviations: RMT = Resting Motor Threshold; BCI = brain–computer interface; TMS = Transcranial Magnetic Stimulation; ABT = Activity-Based Therapy; N.R. = Not Reported.

Table 3.
Summary of functional outcomes in human studies.
Table 3.
Summary of functional outcomes in human studies.
| Authors | GRASSP | ISNCSCI | Dyn. | Main Finding |
|---|---|---|---|---|
| Epidural stimulation | ||||
| Lu et al. (2016) [18] | – | ↑ | ↑ | Immediate and session-to-session motor gains in 2/2 participants; three-fold increase in hand strength with stimulation; upper-extremity motor score increased by 23 and 16 points; improved self-care and mobility. |
| Transcutaneous stimulation | ||||
| Murray et al. (2017) [26] | – | – | – | Reduced spasticity and spasms, reversal of anhidrosis. |
| Inanici et al. (2018) [49] | ↑ | ↑ | ↑ | Improvements of 52 points on GRASSP, 14 points on UEMS, 2- to 7-fold increase in pinch strength in left and right hand, respectively; overall sensation and neurological level of injury improved from C3 to C4. Functional gains persisted for over 3 months after stimulation. |
| Gad et al. (2018) [59] | – | – | ↑ | Grip strength increased by 325% with stimulation and 225% off stimulation after 8 sessions. |
| Benavides et al. (2020) [52] | ↑ | – | – | Immediate GRASSP improvements observed in a single session. |
| Zhang et al. (2020) [51] | ↑ | – | ↑ | GRASSP improved by 24 points and handgrip strength increased (left: 283%, right: 30%); gains sustained for 3 months. |
| Gad et al. (2020) [62] | – | – | – | Improved breathing and cough effectiveness; benefits persisted beyond the stimulation period. |
| Tefertiller et al. (2021) [57] | – | ↑ | – | AIS grade improved in 2/7 participants (B to C; C to D); sensation increased in 5/7; all 4/4 AIS A participants were able to activate lower extremities with stimulation. |
| Inanici et al. (2021) [48] | ↑ | ↑ | ↑ | GRASSP scores increased; UEMS improved by up to 8 points. AIS grade improved in 1/6 participants (AIS C to D); all 6/6 maintained gains for at least 3–6 months beyond stimulation. Additional improvements in bladder function, thermoregulation, heart-rate stability, and quality of life. |
| Huang et al. (2022) [61] | – | – | ↑ | Grip force increased in 5/10 participants with measurable baseline force. |
| McGeady et al. (2022) [54] | ↑ | ↑ | ↑ | GRASSP increased by 35 points with associated strength gains and improved neurological level of injury; brain–computer interface priming enhanced gains. |
| Zhang et al. (2023) [50] | ↑ | – | ↑ | Five-fold improvement in GRASSP in AIS A; GRASSP increased by 10–33 points; up to three-fold increase in upper-extremity strength; gains maintained for 1 month in all 4/4 participants. |
| Oh et al. (2023) [70] | – | – | ↑ | Grip strength increased only with combined stimulation and training. |
| García-Alén et al. (2023) [55] | ↑ | ↑ | ↑ | GRASSP strength and pinch improved; also increases in SCIM scores and quality of life. |
| Chandrasekaran et al. (2023) [60] | – | ↑ | ↑ | Up to 1136% increase in force; tactile sensation improved. |
| Moritz et al. (2024) [56] | ↑ | ↑ | ↑ | Seventy-two percent of participants exceeded MCID; pinch, grip, UEMS, and SCIM scores increased; autonomic function (bladder and blood-pressure regulation) improved in some; significant improvements in patient-reported quality of life. |
| Singh et al. (2024) [63] | – | – | ↑ | Handgrip increased in 6/7 participants; more than 20% force increase in 3 participants; safety and tolerability in children confirmed. |
| Verma et al. (2025) [58] | – | – | ↑ | Grip strength increased; minimal residual force at baseline predicted response. |
| Capozio et al. (2025) [53] | ↑ | – | – | GRASSP improved in 4/5 participants; sensation improved in 3/5. |
| Capozio et al. (2025) [69] | – | – | – | Dexterity increased after a single session, with or without stimulation. |
Abbreviations: GRASSP = Graded and Redefined Assessment of Strength, Sensibility, and Prehension; ISNCSCI = International Standards for Neurological Classification of Spinal Cord Injury; Dyn. = dynamometry; MCID = Minimal Clinically Important Difference; UEMS = Upper Extremity Motor Score; SCIM = Spinal Cord Independence Measure; AIS = ASIA Impairment Scale.
4. Discussion
The aim of this study was to obtain a validated overview of the current evidence of cervical SCS for functional rehabilitation after SCI, with a focus on motor, sensory, respiratory, and autonomic outcomes, and to evaluate the methodological quality of the available studies. To this end, we conducted a systematic literature review, including human and animal studies, with accompanying risk-of-bias assessment for human studies. Across the included human studies, cervical SCS was generally well tolerated within the limitations of small, largely uncontrolled studies with no major stimulation-related adverse events recorded. Minor side effects, such as transient discomfort, were infrequent and consistent with the established safety profile of SCS in other neurological contexts [10,72].
Preclinical studies offer important mechanistic insights. They demonstrate that cervical stimulation can activate forelimb motor pools, strengthen spared corticospinal connections, and facilitate long-term improvements in skilled motor function [17,27,30,71,73]. Notably, some models demonstrate that stimulation can induce lasting plastic changes rather than only transient facilitation. McPherson et al. showed that activity-dependent stimulation produced durable recovery of reaching ability accompanied by strengthening of spared corticospinal projections, while Zareen et al. demonstrated axonal outgrowth of corticospinal fibers when spinal stimulation was paired with cortical neuromodulation [17,25]. These data suggest that cervical SCS may act not just as an assistive technology but as a therapeutic intervention promoting structural reorganization. Additional work highlights its ability to engage respiratory-related circuits, reinforcing relevance for patients with high cervical injuries [37,62,74,75]. The emerging body of human research reinforces and extends the preclinical evidence base. Across 20 included clinical studies, cervical SCS has been shown to have preliminary assistive and therapeutic effects on upper limb motor function, with occasional reports of enhanced respiratory capacity. However, most investigations were uncontrolled pilot trials or case reports, leaving the certainty of evidence low. Only Huang et al., a sham-controlled crossover trial in 10 participants, and Moritz et al., a multicenter prospective study with 60 participants, were judged to be at a moderate risk of bias [56,61]. These provide early but important steps toward rigorous evaluation. Importantly, aside from the trial by Lu et al., epidural cervical SCS studies in SCI remain limited to animal studies, with no larger clinical series demonstrating sustained functional outcomes [18]. By contrast, epidural approaches at the cervical level have been explored more recently in poststroke hemiparesis, though these still required laminectomy and open surgical implantation, whereas SCI research has largely pivoted toward non-invasive transcutaneous stimulation [20,22]. This shift likely reflects the technical and safety challenges associated with cervical epidural lead implantation in the setting of SCI, including altered anatomy, scarring, and concerns regarding surgical risk in a neurologically vulnerable region. In addition to stimulation alone, several studies integrated adjunctive interventions such as motor cortex stimulation, brain–computer interface priming, motor imagery, robotic exoskeleton training, and task-specific activity-based practice. Although heterogeneous in design, these approaches consistently reported greater or more durable functional improvements than stimulation alone. This supports the hypothesis that cervical SCS works best not as a stand-alone restorative therapy but rather as a facilitator of neuroplasticity, amplifying the effects of motor practice and supraspinal input. These findings corroborate reports on lumbosacral SCS, where task-specific training has been effective in translating the acute effects of stimulation into lasting functional gains [11,12,76]. Future clinical trials should therefore prioritize protocols that systematically pair stimulation with rehabilitation or other neuromodulation methods, as this combined approach is most likely to yield meaningful and durable recovery. A recurring clinical question concerns the degree of residual function required to benefit from cervical SCS. The studies included in this review encompassed both motor-complete (AIS A–B) and motor-incomplete (AIS C–D) injuries. Improvements in hand strength and dexterity were reported even in some participants classified as AIS A; however, these were often single cases and typically involved at least minimal residual motor output detectable on EMG or dynamometry. Larger series and controlled studies suggest that individuals with preserved voluntary activation, even if weak (AIS B–D), are more likely to experience robust gains. For example, Huang et al. and Verma et al. showed that minimal baseline handgrip force was a prerequisite for substantial strength improvements [58,61]. Current evidence therefore indicates that cervical SCS can modulate function across a spectrum of injury severities, but that the predictability and magnitude of response are greatest when some descending drive is preserved. More systematic, stratified trials are needed to define clear thresholds of residual function and to determine whether truly motor-complete injuries without any measurable output can benefit to a similar extent.
Important limitations remain. The current literature is dominated by small sample sizes, heterogeneous injury profiles, variable stimulation protocols, and unblinded outcome assessments. These factors limit generalizability and make it difficult to identify which patients are most likely to benefit. Although motor outcomes in the human studies were assessed using validated measures, substantial variability in the choice of assessments and the frequent use of adjunctive interventions limited comparability across studies and precluded a formal meta-analysis. Few studies incorporated patient-reported outcomes or long-term follow-up, leaving gaps in understanding durability and real-world impact [77].
5. Conclusions
In summary, this systematic review addresses its stated objectives by synthesizing available preclinical and clinical evidence on cervical spinal cord stimulation for functional rehabilitation following SCI, identifying preliminary assistive and therapeutic effects, and evaluating the methodological limitations that currently constrain the strength of this evidence. Based on the reviewed evidence, several priorities for future research emerge. First, larger sham-controlled and blinded clinical trials are essential to establish efficacy and to separate stimulation-specific effects from placebo or training-related gains. To overcome this challenge, future studies may consider alternative electrode placements that preserve cutaneous sensation without engaging spinal circuits or the use of active comparator conditions rather than inert shams. Second, optimization, personalization, and safety of stimulation parameters should be pursued through systematic exploration of electrode placement, optimal stimulation configurations, and activity-dependent paradigms. Third, integration with adjunctive rehabilitation strategies appears critical, as stimulation likely acts as a facilitator of neuroplasticity rather than a stand-alone intervention. Finally, identifying which patient subgroups are most likely to benefit, including potential age- and sex-related differences in functional responsiveness, will be critical for guiding clinical decision-making and resource allocation. Given the profound impact that even modest improvements in hand or respiratory function can have for people with tetraplegia, the continued systematic development and rigorous evaluation of cervical SCS remains a clear research priority.
Author Contributions
Conceptualization, M.C.W. and P.H.; Methodology, M.C.W., P.A., and P.H.; Literature Search, M.C.W. and P.A.; Data Curation, M.C.W. and P.A.; Formal Analysis, M.C.W. and P.A.; Visualization, M.C.W.; Writing—Original Draft, M.C.W.; Writing—Review and Editing, M.C.W., V.V.-V., P.A., and P.H.; Supervision, P.H. and V.V.-V.; Project Administration, P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This article is a systematic review and does not contain any studies with human participants or animals performed by any of the authors. Ethical approval was therefore not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AIS | ASIA Impairment Scale |
| ASIA | American Spinal Injury Association |
| BCI | Brain–Computer Interface |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| EES | Epidural Electrical Stimulation |
| EMG | Electromyography |
| GRASSP | Graded and Redefined Assessment of Strength, Sensibility, and Prehension |
| ISMS | Intraspinal Microstimulation |
| ISNCSCI | International Standards for Neurological Classification of Spinal Cord Injury |
| MCID | Minimal Clinically Important Difference |
| MEP | Motor Evoked Potential |
| MRI | Magnetic Resonance Imaging |
| N.A. | Not Applicable |
| N.R. | Not Reported |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRO | Patient-Reported Outcome |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized Controlled Trial |
| RMT | Resting Motor Threshold |
| SCAP | Spinal Cord Associative Plasticity |
| SCI | Spinal Cord Injury |
| SCIM | Spinal Cord Independence Measure |
| SCIM-III | Spinal Cord Independence Measure, Version III |
| SCS | Spinal Cord Stimulation |
| sMEP | Surface Motor Evoked Potential |
| tSCS | Transcutaneous Spinal Cord Stimulation |
| TMS | Transcranial Magnetic Stimulation |
| UEMS | Upper Extremity Motor Score |
| QoL | Quality of Life |
Appendix A

Table A1.
Risk of bias assessment for all included human studies.
Table A1.
Risk of bias assessment for all included human studies.
| Study (Year) | Study Design | Confounding (Patient Selection, Injury Severity) | Intervention Classification & Reporting | Deviations from Intended Intervention | Missing Data/Attrition | Outcome Measurement (Blinding/Objectivity) | Selective Reporting | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Lu et al. (2016) [18] | Case series (cervical epidural stimulation in chronic SCI) | Serious—very small sample size (n = 2), no control group, variability in chronicity | Low–intervention (epidural electrode placement, stimulation parameters) clearly described | Low–stimulation applied as intended, intraoperative testing documented | Moderate—limited reporting on attrition; unclear if any patients excluded post-implantation | Serious—outcomes (hand strength, EMG, volitional control) assessed without blinding; risk of expectation bias | Moderate—selective emphasis on functional improvements, limited discussion of null or adverse findings | Serious |
| Murray et al. (2017) [26] | Case study (cervicothoracic tSCS in SCI, n = 1) | Critical—single participant, exploratory design, no control group | Low—stimulation protocol (site, frequency, sessions) described in detail | Low—intervention delivered as intended | Low—no attrition (single case) | Serious—outcomes (EEG, MEPs, motor activity) assessed without blinding; subjective interpretation risk | Moderate—selective focus on neurophysiological improvements, limited discussion of null or negative findings | Serious–Critical |
| Inanici et al. (2018) [49] | Case study (cervical tSCS in chronic tetraplegia, n = 1) | Critical—single participant, no control group | Low—stimulation parameters (site, intensity, frequency) well described | Low—intervention delivered as intended | Low—no attrition (single case) | Serious—motor and functional outcomes assessed without blinding; potential observer bias | Moderate—focus on positive motor improvements, limited reporting of variability or negative results | Serious–Critical |
| Gad et al. (2018) [59] | Case series (cervical tSCS in SCI, n = 6) | Serious—small heterogeneous cohort, varied chronicity and injury severity, no control group | Low—stimulation protocol (site, electrode configuration, parameters) clearly described | Low—intervention delivered as intended across sessions | Moderate—some incomplete follow-up and variability in session numbers | Serious—outcomes (hand strength, EMG, functional tasks) assessed without blinding; observer bias possible | Moderate—emphasis on positive motor outcomes, limited discussion of non-responders | Serious |
| Benavides et al. (2020) [52] | Case series (cervical tSCS in chronic tetraparesis, n = 10) | Serious—small cohort, varied injury severity and chronicity, no control group within SCI sample | Low—stimulation protocol (electrode placement, frequency, parameters) clearly described | Low—intervention delivered as intended | Moderate—follow-up limited to short-term sessions; attrition not clearly detailed | Serious—outcomes (TMS, MEPs, motor responses) assessed without blinding; potential interpretive bias | Moderate—selective emphasis on corticospinal excitability changes, limited reporting on individual variability | Serious |
| Zhang et al. (2020) [51] | Case study (cervical tSCS in complete tetraplegia, n = 1) | Critical—single participant, no comparator, no ability to control for confounding | Low—intervention (electrode placement, frequency, intensity) well described | Low—stimulation delivered as intended | Low—no attrition (single case) | Serious—outcomes (upper extremity strength, hand function tests) assessed without blinding; observer and reporting bias likely | Moderate—emphasis on positive motor outcomes, limited acknowledgment of limitations of single case | Serious–Critical |
| Gad et al. (2020) [62] | Case study (cervical tSCS for respiratory control in chronic tetraplegia, n = 1) | Critical—single participant, no control group, findings not generalizable | Low—intervention (electrode placement, stimulation parameters) well described | Low—intervention delivered as intended | Low—no attrition (single case) | Serious—respiratory outcomes (ventilation, EMG) assessed without blinding; observer bias possible | Moderate—emphasis on positive physiological responses, limited reporting of variability or potential null effects | Serious–Critical |
| Tefertiller et al. (2021) [57] | Case series (tSCS in chronic SCI, n = 7) | Serious—small heterogeneous cohort with varied injury levels and severities, no control group | Low—stimulation protocol (electrode placement, parameters, training) well described | Low—intervention delivered as intended | Moderate—limited follow-up, incomplete reporting of attrition | Serious—outcomes (motor function, grip, task performance) assessed without blinding; potential observer bias | Moderate—selective emphasis on functional improvements, limited reporting of negative or null results | Serious |
| Inanici et al. (2021) [48] | Prospective cohort study (cervical tSCS in chronic SCI, n = 6) | Serious—small cohort, varied AIS grades and chronicity, no control group | Low—intervention (tSCS protocol, electrode placement, parameters) clearly described | Low—intervention delivered as intended | Low—all participants completed planned sessions, minimal attrition | Serious—motor and functional outcomes assessed without blinding; possible observer and participant bias | Low to Moderate—outcomes comprehensively reported, but limited negative data presented | Serious |
| Huang et al. (2022) [61] | Randomized, sham-controlled crossover trial (cervical tSCS in severe SCI, n = 10) | Serious—small cohort, heterogeneous injury severity, limited generalizability despite crossover design | Low—stimulation parameters (site, intensity, frequency) clearly reported | Low—intervention delivered as intended | Low—all participants completed protocol, no attrition | Moderate—motor outcomes assessed without full blinding of assessors; participants blinded to sham vs. active | Low—outcomes comprehensively reported, including individual data | Moderate |
| McGeady et al. (2022) [54] | Case study (BCI priming + cervical tSCS in chronic SCI, n = 1) | Critical—single participant, exploratory design, no control or comparator | Low—intervention (BCI priming protocol and tSCS parameters) described in detail | Low—intervention delivered as intended | Low—no attrition (single case) | Serious—motor and functional outcomes assessed without blinding; high risk of expectation and observer bias | Moderate—focus on feasibility and positive effects, limited discussion of null findings or generalizability | Serious–Critical |
| Zhang et al. (2023) [50] | Conference abstract (tSCS + activity-based training in cervical SCI, n = 4) | Critical—very small cohort, conference abstract format, limited methodological detail, no control group | Serious—intervention parameters only briefly described; limited reproducibility | Moderate—intervention likely delivered as intended but insufficient detail to confirm | Serious—attrition and follow-up not reported | Serious—outcomes reported in summary only; no blinding; high reporting bias risk | Critical—selective emphasis on feasibility and positive findings; lack of detailed data | Serious–Critical |
| Oh et al. (2023) [70] | Case series (cervical tSCS + task-specific training in chronic SCI, n = 4) | Serious—very small sample, heterogeneous injuries, no control group | Low—stimulation parameters (electrode placement, frequency, intensity) and training protocol described in detail | Low—intervention delivered as intended | Moderate—follow-up limited, attrition reporting incomplete | Serious—motor outcomes (hand strength, task performance) assessed without blinding; observer and participant bias possible | Moderate—focus on positive findings, limited reporting of non-responders or null results | Serious |
| García-Alén et al. (2023) [55] | Clinical trial (cervical tSCS + robotic exoskeleton rehabilitation, n = 15) | Serious—modest sample size, heterogeneous chronic cervical injuries, limited randomization details, no blinded control | Low—intervention protocols (tSCS parameters, exoskeleton training) described clearly | Low—interventions delivered as intended | Moderate—short-term intervention; attrition not fully described | Serious—outcomes (motor scores, functional tasks) assessed without blinding; potential observer bias | Moderate—results emphasize improvements; limited discussion of non-responders or null effects | Serious |
| Chandrasekaran et al. (2023) [78] | Pilot study (targeted cervical tSCS in chronic SCI, n = 7) | Serious—small cohort, mixed injury severities, no control group | Low—stimulation protocol (targeted electrode placement, parameters) described in detail | Low—intervention delivered as intended | Low—all participants completed intervention, minimal attrition | Serious—outcomes (strength, tactile sensation, functional tasks) assessed without blinding; observer and participant bias possible | Moderate—selective emphasis on improvements, limited detail on negative results or variability | Serious |
| Moritz et al. (2024) [56] | Multicenter prospective clinical trial (cervical tSCS in chronic tetraparesis, n = 60+) | Moderate—relatively large sample, but no sham-control group; heterogeneous injury levels and chronicity | Low—intervention (tSCS protocol, electrode placement, stimulation parameters, training regimen) described in detail | Low—intervention delivered as intended across sites with standardized procedures | Low—minimal attrition reported; safety and adherence tracked | Moderate—outcomes (grip strength, hand function, SCIM, PROs) not blinded; potential performance and observer bias | Low—outcomes comprehensively reported per protocol, including adverse events and non-responders | Moderate |
| Singh et al. (2024) [63] | Prospective feasibility study (cervical and thoracic tSCS in pediatric chronic SCI, n = 6) | Serious—very small sample, pediatric population with variable injury levels and chronicity, no control group | Low—intervention (cervical/thoracic electrode placement, stimulation parameters, safety monitoring) clearly described | Low—intervention delivered as intended | Low—no attrition reported during short-term intervention | Serious—motor function outcomes assessed without blinding; potential observer bias | Moderate—emphasis on feasibility and safety; limited discussion of negative or null results | Serious |
| Verma et al. (2025) [58] | Prospective case series (cervical tSCS in chronic SCI, n = 5) | Serious—very small cohort, varied injury severities and chronicity, no control group within SCI | Low—stimulation parameters (cervical electrode placement, frequency, intensity) well described | Low—intervention delivered as intended | Low—all participants completed protocol, no attrition | Serious—outcomes (motoneuron firing via EMG, hand motor function) assessed without blinding; risk of observer bias | Moderate—focus on facilitation and motor improvements, limited discussion of non-responders or null findings | Serious |
| Capozio et al. (2025a) [53] | Preprint (bimanual task practice + cervical tSCS in chronic SCI, n = 5) | Critical—preprint (not peer-reviewed), very small sample, no control group, heterogeneous injuries | Serious—intervention parameters described but some methodological details limited in preprint format | Low—intervention delivered as intended | Moderate—short-term follow-up; attrition reporting incomplete | Serious—outcomes (hand motor function, spinal plasticity measures) assessed without blinding; risk of observer bias | Serious—emphasis on positive findings, limited reporting of null or negative outcomes; preprint format limits transparency | Serious–Critical |
| Capozio et al. (2025b) [69] | Single-session experimental study (motor imagery + cervical tSCS in chronic SCI, n = 8) | Serious—very small sample, heterogeneous injuries, no control/sham condition | Low—stimulation protocol and motor imagery task clearly described | Low—intervention delivered as intended in all participants | Low—no attrition (single-session study) | Serious—outcomes (manual dexterity, cortical excitability via TMS) assessed without blinding; potential measurement bias | Moderate—focus on positive acute effects, limited reporting of variability or null findings | Serious |
References
- Khadour, F.A.; Khadour, Y.A.; Meng, L.; Cui, X.; Xu, T. Epidemiological features of traumatic and non-traumatic spinal cord injury in Wuhan, China. Sci. Rep. 2024, 14, 1640. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shang, Z.; Zhang, W.; Pang, M.; Hu, X.; Dai, Y.; Shen, R.; Wu, Y.; Liu, C.; Luo, T.; et al. Global incidence and characteristics of spinal cord injury since 2000–2021: A systematic review and meta-analysis. BMC Med. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Hagen, E.M. Acute complications of spinal cord injuries. World J. Orthop. 2015, 6, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sezer, N.; Akkus, S.; Ugurlu, F.G. Chronic complications of spinal cord injury. World J. Orthop. 2015, 6, 24–33. [Google Scholar] [CrossRef]
- Kirshblum, S.; Snider, B.; Eren, F.; Guest, J. Characterizing natural recovery after traumatic spinal cord injury. J. Neurotrauma 2021, 38, 1267–1284. [Google Scholar] [CrossRef]
- Steeves, J.D.; Kramer, J.K.; Fawcett, J.W.; Cragg, J.; Lammertse, D.P.; Blight, A.R.; Marino, R.J.; Ditunno, J.F.; Coleman, W.P.; Geisler, F.H.; et al. Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord 2011, 49, 257–265. [Google Scholar] [CrossRef]
- Ter Wengel, P.V.; De Haan, Y.; Feller, R.E.; Oner, F.C.; Vandertop, W.P. Complete Traumatic Spinal Cord Injury: Current Insights Regarding Timing of Surgery and Level of Injury. Glob. Spine J. 2020, 10, 324–331. [Google Scholar] [CrossRef]
- Dengler, J.; Steeves, J.D.; Curt, A.; Mehra, M.; Novak, C.B.; Fox, I.K. Spontaneous motor recovery after cervical spinal cord injury: Issues for nerve transfer surgery decision making. Spinal Cord 2022, 60, 922–927. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth. Analg. 1967, 46, 489–491. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.B.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rowald, A.; Komi, S.; Demesmaeker, R.; Baaklini, E.; Hernandez-Charpak, S.D.; Paoles, E.; Montanaro, H.; Cassara, A.; Becce, F.; Lloyd, B.; et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat. Med. 2022, 28, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Chalif, J.I.; Chavarro, V.S.; Mensah, E.; Johnston, B.; Fields, D.P.; Chalif, E.J.; Chiang, M.; Sutton, O.; Yong, R.; Trumbower, R.; et al. Epidural spinal cord stimulation for spinal cord injury in humans: A systematic review. J. Clin. Med. 2024, 13, 1090. [Google Scholar] [CrossRef]
- Kasten, M.R.; Sunshine, M.D.; Secrist, E.S.; Horner, P.J.; Moritz, C.T. Therapeutic intraspinal microstimulation improves forelimb function after cervical contusion injury. J. Neural Eng. 2013, 10, 044001. [Google Scholar] [CrossRef][Green Version]
- Sunshine, M.D.; Cho, F.S.; Lockwood, D.R.; Fechko, A.S.; Kasten, M.R.; Moritz, C.T. Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J. Neural Eng. 2013, 10, 036001. [Google Scholar] [CrossRef]
- McPherson, J.G.; Miller, R.R.; Perlmutter, S.I.; Poo, M.M. Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proc. Natl. Acad. Sci. USA 2015, 112, 12193–12198. [Google Scholar] [CrossRef]
- Lu, D.C.; Edgerton, V.R.; Modaber, M.; AuYong, N.; Morikawa, E.; Zdunowski, S.; Sarino, M.E.; Sarrafzadeh, M.; Nuwer, M.R.; Roy, R.R.; et al. Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Neurorehabilit. Neural Repair 2016, 30, 951–962. [Google Scholar] [CrossRef]
- Barra, B.; Conti, S.; Perich, M.G.; Zhuang, K.; Schiavone, G.; Fallegger, F.; Galan, K.; James, N.D.; Barraud, Q.; Delacombaz, M.; et al. Epidural electrical stimulation of the cervical dorsal roots restores voluntary upper limb control in paralyzed monkeys. Nat. Neurosci. 2022, 25, 924–934. [Google Scholar] [CrossRef]
- de Freitas, R.M.; Bhatia, S.; Sorensen, E.; Verma, N.; Carranza, E.; Ensel, S.; Borda, L.; Boos, A.; Goldsmith, J.; Fisher, L.E.; et al. Spinal cord stimulation improves motor function and spasticity in chronic post-stroke upper limb hemiparesis. medRxiv 2025. [Google Scholar] [CrossRef]
- Greiner, N.; Barra, B.; Schiavone, G.; Lorach, H.; James, N.; Conti, S.; Kaeser, M.; Fallegger, F.; Borgognon, S.; Lacour, S.; et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat. Commun. 2021, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.P.; Verma, N.; Sorensen, E.; Carranza, E.; Boos, A.; Fields, D.P.; Roy, S.; Ensel, S.; Barra, B.; Balzer, J.; et al. Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis. Nat. Med. 2023, 29, 689–699. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Edgerton, V.R. Transcutaneous electrical spinal cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef]
- Song, W.G.; Martin, J.H. Spinal cord direct current stimulation differentially modulates neuronal activity in the dorsal and ventral spinal cord. J. Neurophysiol. 2017, 117, 1143–1155. [Google Scholar] [CrossRef]
- Zareen, N.; Shinozaki, M.; Ryan, D.; Alexander, H.; Amer, A.; Truong, D.Q.; Khadka, N.; Sarkar, A.; Naeem, S.; Bikson, M.; et al. Motor cortex and spinal cord neuromodulation promote corticospinal tract axonal outgrowth and motor recovery after cervical contusion spinal cord injury. Exp. Neurol. 2017, 297, 179–189. [Google Scholar] [CrossRef]
- Murray, L.M.; Knikou, M. Remodeling brain activity by repetitive cervicothoracic transspinal stimulation after human spinal cord injury. Front. Neurol. 2017, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.T.; Lucas, T.H.; Perlmutter, S.I.; Fetz, E.E. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J. Neurophysiol. 2007, 97, 110–120. [Google Scholar] [CrossRef]
- Zimmermann, J.B.; Seki, K.; Jackson, A. Reanimating the arm and hand with intraspinal microstimulation. J. Neural Eng. 2011, 8, 054001. [Google Scholar] [CrossRef]
- Alam, M.; Garcia-Alias, G.; Shah, P.K.; Gerasimenko, Y.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Evaluation of optimal electrode configurations for epidural spinal cord stimulation in cervical spinal cord injured rats. J. Neurosci. Methods 2015, 247, 50–57. [Google Scholar] [CrossRef][Green Version]
- Alam, M.; Garcia-Alias, G.; Jin, B.; Keyes, J.; Zhong, H.; Roy, R.R.; Gerasimenko, Y.; Lu, D.C.; Edgerton, V.R. Electrical neuromodulation of the cervical spinal cord facilitates forelimb skilled function recovery in spinal cord injured rats. Exp. Neurol. 2017, 291, 141–150. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.; Murray, L.; Joiner, E.; Goldberg, J.; Thuet, E.; Modik, O.; Shelkov, E.; Lombardi, J.; Sardar, Z.; Lehman, R.; et al. Invasive and non-invasive synergies between motor cortex and cervical spinal stimulation in humans. Neuromodulation 2024, 27, S185. [Google Scholar] [CrossRef]
- DiMarco, A.F.; Kowalski, K.E. High-frequency spinal cord stimulation of inspiratory muscles in dogs: A new method of inspiratory muscle pacing. J. Appl. Physiol. 2009, 107, 662–669. [Google Scholar] [CrossRef]
- DiMarco, A.F.; Kowalski, K.E.; Geertman, R.T.; Hromyak, D.R. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: Results of a National Institutes of Health-sponsored clinical trial. Part I: Methodology and effectiveness of expiratory muscle activation. Arch. Phys. Med. Rehabil. 2009, 90, 717–725. [Google Scholar] [CrossRef]
- Routal, R.V.; Pal, G.P. Location of the phrenic nucleus in the human spinal cord. J. Anat. 1999, 195, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Verin, E.; Marie, J.P.; Similowski, T. Cartography of human diaphragmatic innervation: Preliminary data. Respir. Physiol. Neurobiol. 2011, 176, 68–71. [Google Scholar] [CrossRef]
- Galer, E.L.; Huang, R.; Madhavan, M.; Wang, E.; Zhou, Y.; Leiter, J.C.; Lu, D.C. Cervical epidural electrical stimulation increases respiratory activity through somatostatin-expressing neurons in the dorsal cervical spinal cord in rats. J. Neurosci. 2023, 43, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Bezdudnaya, T.; Lane, M.A.; Marchenko, V. Paced breathing and phrenic nerve responses evoked by epidural stimulation following complete high cervical spinal cord injury in rats. J. Appl. Physiol. 2018, 125, 687–696. [Google Scholar] [CrossRef]
- Mickle, A.R.; Peñaloza-Aponte, J.D.; Coffey, R.; Hall, N.A.; Baekey, D.; Dale, E.A. Closed-loop cervical epidural stimulation partially restores ipsilesional diaphragm EMG after acute C2 hemisection. Respir. Physiol. Neurobiol. 2024, 320, 104182. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haddaway, N.R.; McGuinness, L.A.; Pritchard, C.C. PRISMA2020: An R package and Shiny app for producing PRISMA 2020 flow diagrams. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Kalsi-Ryan, S.; Beaton, D.; Curt, A.; Duff, S.; Popovic, M.R.; Rudhe, C.; Fehlings, M.G. Development of the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP): Reliability and validity. J. Neurotrauma 2012, 29, 905–914. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sørensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Maynard, F.M.; Bracken, M.B.; Creasey, G.; Ditunno, J.F.; Donovan, W.H.; Ducker, T.B.; Garber, S.L.; Marino, R.J.; Stover, S.L.; Tator, C.H.; et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord 1997, 35, 266–274. [Google Scholar] [CrossRef]
- Catz, A.; Itzkovich, M.; Agranov, E.; Ring, H.; Tamir, A. The Spinal Cord Independence Measure (SCIM) version III: Reliability and validity in a multi-center international study. Disabil. Rehabil. 2007, 29, 1926–1933. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef]
- Inanici, F.; Samejima, S.; Gad, P.; Edgerton, V.R.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1272–1278. [Google Scholar] [CrossRef]
- Zhang, F.; Carnahan, J.; Ravi, M.; Bheemreddy, A.; Kirshblum, S.; Forrest, G.F. Combining Spinal Cord Transcutaneous Stimulation with Activity-Based Training to Improve Upper Extremity Function Following Cervical Spinal Cord Injury. In Proceedings of the 45th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Zhang, F.; Momeni, K.; Ramanujam, A.; Ravi, M.; Carnahan, J.; Kirshblum, S.; Forrest, G.F. Cervical Spinal Cord Transcutaneous Stimulation Improves Upper Extremity and Hand Function in People with Complete Tetraplegia: A Case Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 3167–3174. [Google Scholar] [CrossRef]
- Benavides, F.D.; Jo, H.J.; Lundell, H.; Edgerton, V.R.; Gerasimenko, Y.; Perez, M.A. Cortical and subcortical effects of transcutaneous spinal cord stimulation in humans with tetraplegia. J. Neurosci. 2020, 40, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Capozio, A.; Chowdhury, S.T.; Chakrabarty, S.; Delis, I.; Horne, M.; Sivan, M.; Gad, P.; Holt, R.; Ichiyama, R.; Astill, S.L. Bimanual upper limb task practice and transcutaneous electrical stimulation enhance spinal plasticity and hand function after chronic cervical spinal cord injury. medRxiv 2025. [Google Scholar] [CrossRef]
- McGeady, C.; Vuckovic, A.; Tharu, N.S.; Zheng, Y.P.; Alam, M. Brain–computer interface priming for cervical transcutaneous spinal cord stimulation therapy: An exploratory case study. Front. Rehabil. Sci. 2022, 3, 896766. [Google Scholar] [CrossRef]
- García-Alén, L.; Kumru, H.; Castillo-Escario, Y.; Benito-Penalva, J.; Medina-Casanovas, J.; Gerasimenko, Y.P.; Edgerton, V.R.; García-Alías, G.; Vidal, J. Transcutaneous cervical spinal cord stimulation combined with robotic exoskeleton rehabilitation for the upper limbs in subjects with cervical spinal cord injury: Clinical trial. Biomedicines 2023, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.T.; Field-Fote, E.C.; Tefertiller, C.; van Nes, I.J.W.; Trumbower, R.D.; Kalsi-Ryan, S.; Purcell, M.; Janssen, T.W.J.; Krassioukov, A.; Morse, L.R.; et al. Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: A safety and efficacy trial. Nat. Med. 2024, 30, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Tefertiller, C.; Rozwod, M.; VandeGriend, E.; Bartelt, P.; Sevigny, M.; Smith, A.C. Transcutaneous Electrical Spinal Cord Stimulation to Promote Recovery in Chronic Spinal Cord Injury. Front. Rehabil. Sci. 2021, 2, 740307. [Google Scholar] [CrossRef]
- Verma, N.; Oh, J.; Bedoy, E.; Chetty, N.; Steele, A.G.; Park, S.J.; Guerrero, J.R.; Faraji, A.H.; Weber, D.; Sayenko, D.G. Transcutaneous stimulation of the cervical spinal cord facilitates motoneuron firing and improves hand-motor function after spinal cord injury. J. Neurophysiol. 2025, 134, 128–143. [Google Scholar] [CrossRef]
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.; Edgerton, V.R. Non-invasive activation of cervical spinal networks after severe paralysis. J. Neurotrauma 2018, 35, 2145–2158. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Bhagat, N.A.; Ramdeo, R.; Ebrahimi, S.; Sharma, P.D.; Griffin, D.G.; Stein, A.; Harkema, S.J.; Bouton, C.E. Targeted transcutaneous spinal cord stimulation promotes persistent recovery of upper limb strength and tactile sensation in spinal cord injury: A pilot study. Front. Neurosci. 2023, 17, 1210328. [Google Scholar] [CrossRef]
- Huang, R.; Nikooyan, A.A.; Moore, L.D.; Zdunowski, S.; Morikawa, E.; Sierro, T.; Sayenko, D.; Gad, P.; Homsey, T.; Le, T.; et al. Minimal handgrip force is needed for transcutaneous electrical stimulation to improve hand functions of patients with severe spinal cord injury. Sci. Rep. 2022, 12, 7733. [Google Scholar] [CrossRef]
- Gad, P.; Kreydin, E.; Zhong, H.; Edgerton, V.R. Enabling respiratory control after severe chronic tetraplegia: An exploratory case study. J. Neurophysiol. 2020, 124, 774–780. [Google Scholar] [CrossRef]
- Singh, G.; Keller, A.; Lucas, K.; Borders, C.; Stout, D.; King, M.; Parikh, P.; Stepp, N.; Ugiliweneza, B.; D’Amico, J.M.; et al. Safety and Feasibility of Cervical and Thoracic Transcutaneous Spinal Cord Stimulation to Improve Hand Motor Function in Children With Chronic Spinal Cord Injury. Neuromodulation 2024, 27, 661–671. [Google Scholar] [CrossRef]
- Moritz, C.T.; Field-Fote, E.C.; van Nes, I.J.W.; Trumbower, R.D.; Kalsi-Ryan, S.; Purcell, M.; Janssen, T.; Krassioukov, A.; Morse, L.; Zhao, K.; et al. Transcutaneous Spinal Cord Stimulation Parameters and Programming Strategies for Effective Upper Extremity Rehabilitation Following Tetraplegia. Neuromodulation 2024, 27, S263. [Google Scholar] [CrossRef]
- Pal, A.; Garcia-Sandoval, A.; Ratnadurai-Giridharan, S.; Yang, Q.; Bethea, T.; Ramamurthy, A.; Wen, T.C.; Voit, W.; Carmel, J. Paired brain and spinal cord stimulation strengthens spared spinal circuits after injury. Neurorehabilit. Neural Repair 2018, 32, 1071–1072. [Google Scholar] [CrossRef]
- Yang, Q.; Ramamurthy, A.; Lall, S.; Santos, J.; Ratnadurai-Giridharan, S.; Zareen, N.; Alexander, H.; Ryan, D.; Martin, J.H.; Carmel, J.B. Independent replication of motor cortex and cervical spinal cord electrical stimulation to promote forelimb motor function after spinal cord injury in rats. Exp. Neurol. 2019, 320, 112962. [Google Scholar] [CrossRef]
- Song, W.G.; Amer, A.; Ryan, D.; Martin, J.H. Combined motor cortex and spinal cord neuromodulation promotes corticospinal system functional and structural plasticity and motor function after injury. Exp. Neurol. 2016, 277, 46–57. [Google Scholar] [CrossRef]
- Samejima, S.; Khorasani, A.; Ranganathan, V.; Nakahara, J.; Tolley, N.M.; Boissenin, A.; Shalchyan, V.; Daliri, M.R.; Smith, J.R.; Moritz, C.T. Brain-Computer-Spinal Interface Restores Upper Limb Function After Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1233–1242. [Google Scholar] [CrossRef]
- Capozio, A.; Graham, M.; Ichiyama, R.; Astill, S.L. A single session of motor imagery paired with spinal stimulation improves manual dexterity and increases cortical excitability after spinal cord injury. Clin. Neurophysiol. 2025, 174, 160–168. [Google Scholar] [CrossRef]
- Oh, J.; Scheffler, M.S.; Mahan, E.E.; King, S.T.; Martin, C.A.; Dinh, J.; Steele, A.G.; O’Malley, M.K.; Sayenko, D.G. Combinatorial Effects of Transcutaneous Spinal Stimulation and Task-Specific Training to Enhance Hand Motor Output after Paralysis. Top. Spinal Cord Inj. Rehabil. 2023, 29, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Park, H.; Ramamurthy, A.; Asan, A.S.; Bethea, T.; Johnkutty, M.; Carmel, J.B. Spinal cord associative plasticity improves forelimb sensorimotor function after cervical injury. Brain 2022, 145, 4531–4544. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, N.A.; Mathews, M.; Nageeb, F.; Guirguis, M.; Mekhail, M.N.; Cheng, J. Retrospective review of 707 cases of spinal cord stimulation: Indications and complications. Pain Pract. 2011, 11, 148–153. [Google Scholar] [CrossRef]
- Yang, Q.; Ramamurthy, A.; Lall, S.; Zareen, N.; Ryan, H.; Martin, J.; Carmel, J. Motor Cortex and Cervical Spinal Cord Electrical Stimulation Promotes Forelimb Function after Spinal Cord Injury in Rats. Ann. Neurol. 2018, 84, S229–S230. [Google Scholar] [CrossRef] [PubMed]
- Malone, I.; Kelly, M.; Nosacka, R.; Nash, M.; Mitchell, G.; Otto, K.; Dale, E. Chronic, closed-loop, cervical epidural stimulation elicits diaphragm motor plasticity after spinal cord injury. FASEB J. 2021, 35, 04848. [Google Scholar] [CrossRef]
- Malone, I.G.; Kelly, M.N.; Mitchell, G.S.; Otto, K.J.; Dale, E.A. Long-term closed-loop cervical epidural stimulation elicits plasticity in diaphragm motor output. FASEB J. 2020, 34, 07185. [Google Scholar] [CrossRef]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Biering-Sørensen, F.; Noonan, V.K. Standardization of Data for Clinical Use and Research in Spinal Cord Injury. Brain Sci. 2016, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Bhagat, N.; Ramdeo, R.; Ebrahimi, S.; Sharma, P.; Stein, A.; Griffin, D.; Harkema, S.; Bouton, C. Targeted transcutaneous cervical spinal cord stimulation promotes lasting upper limb recovery in spinal cord and peripheral nerve injury. Brain Stimul. 2023, 16, 373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.