Abstract
Neuropathic pain is a chronic and debilitating disorder of the somatosensory system that affects a significant proportion of the population and is characterized by abnormal responses such as hyperalgesia and allodynia. Voltage-gated ion channels, including sodium (NaV), calcium (CaV), and potassium (KV) channels, play a pivotal role in modulating neuronal excitability and pain signal transmission following nerve injury. This review intends to provide a comprehensive analysis of the molecular and cellular mechanisms by which dysregulation in the expression, localization, and function of specific NaV channel subtypes (mainly NaV1.7 and NaV1.8) and their auxiliary subunits contributes to aberrant neuronal activation, the generation of ectopic discharges, and sensitization in neuropathic pain. Likewise, special emphasis is placed on the crucial role of CaV channels, particularly CaV2.2 and the auxiliary subunit CaVα2δ, whose overexpression increases calcium influx, neurotransmitter release, and neuronal hyperexcitability, thus maintaining persistent pain states. Furthermore, KV channels (particularly KV7 channels) function as brakes on neuronal excitability, and their dysregulation facilitates the development and maintenance of neuropathic pain. Therefore, targeting specific KV channel subtypes to restore their function is also a promising therapeutic strategy for alleviating neuropathic pain symptoms. On the other hand, recent advances in the development of small molecules as selective modulators or inhibitors targeting voltage-gated ion channels are also discussed. These agents have improved efficacy and safety profiles in preclinical and clinical studies by attenuating pathophysiological channel activity and restoring neuronal function. This review seeks to contribute to guiding future research and drug development toward more effective mechanism-based treatments by discussing the molecular mechanisms underlying neuropathic pain and highlighting translational therapeutic opportunities.
1. Introduction
The somatosensory system enables us to perceive and interact with our environment and body through highly specialized peripheral sensory neurons in the skin, muscles, joints, and internal organs that detect touch, pressure, pain, and temperature. These neurons detect environmental stimuli, convert them into action potentials (APs), and transmit them to the brain via the spinal cord (SC). These same neurons transmit pain signals through the peripheral nerves to the SC, where second-order neurons transfer them to the thalamus. The thalamus receives these signals and projects them to the primary somatosensory cortex, where the information is integrated [1] (Figure 1).
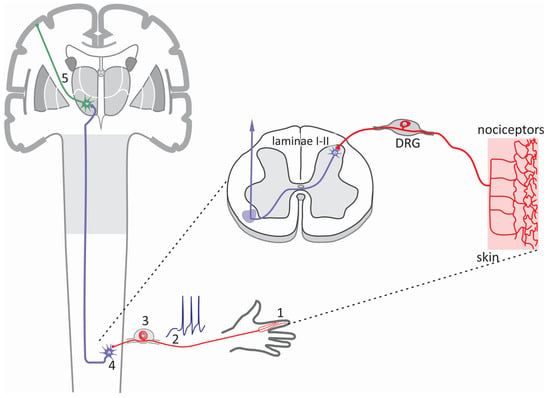
Figure 1.
The sensory pathway. The somatosensory system comprises an intricate network of sensory receptors distributed throughout the skin, muscles, joints, and internal organs. These receptors include nociceptors activated in response to noxious stimuli and generate pain signals. The information generated in the periphery (1) is transmitted as action potentials (2), primarily via primary afferent fibers of the Aδ and C type that have a peripheral axon innervating the distal regions, to the DRG (3), where the soma of the sensory neurons are located. The pain signals then travel to the second-order neurons in the laminae I-II of the spinal cord (4). Finally, these signals are transmitted to third-order neurons in the thalamus (5) and then to the primary somatosensory cortex to be integrated.
Neuropathic pain is a disorder of the somatosensory system that affects ~10% of the general population. It is more frequent in women and in individuals over 50 years of age and most frequently affects the lower back, upper and lower limbs, and the neck [2,3]. It is characterized by abnormal responses to stimuli, including hyperalgesia, an increased painful response to painful stimuli, and allodynia, the presence of pain associated with innocuous stimuli. Different conditions may cause neuropathic pain, with diabetes mellitus being one of the most significant [3]. However, neuropathic pain may also occur as a result of herpes virus infections, acquired immunodeficiency syndrome, or have a traumatic origin. Likewise, autoimmune disorders such as Guillain–Barré syndrome and multiple sclerosis, as well as some oncological treatments, may also cause peripheral neuropathy [1,3].
It is well-known that the changes responsible for neuropathic pain mechanisms lie in altered gene transcription or protein functional expression/localization in sensory neurons. Interestingly, after damage to peripheral sensory fibers, alterations in the different subunits that compose voltage-gated ion channels may contribute to the changes in pain transmission observed in allodynia and hyperalgesia, as we shall discuss next [1,4].
2. Voltage-Gated Sodium (NaV) Channels and Neuropathic Pain
2.1. Structure and Function of NaV Channels
NaV channels play a relevant role in the development and maintenance of neuropathic pain. These proteins are essential components of the excitability machinery in excitable cells, including neurons, and alterations in their expression or function after nerve injury may cause greater pain sensitivity. When the receptor potential is sufficient to reach the activation threshold of NaV channels, it will trigger the generation and propagation of regenerative action potentials (APs) in nociceptive neurons and the transmission of pain signals to the SC [3,4].
NaV channels are multimeric complexes formed by a main subunit (NaVα) that forms the ion-conducting pore (~250 kD), together with one or two auxiliary NaVβ subunits (30–40 kD) (Figure 2A). NaVα subunits are encoded by ten genes (SCN) that give rise to nine different proteins named NaV1.1–NaV1.9 and a novel subfamily known as NaVx [5,6,7] (Figure 2A). These proteins comprise twenty-four transmembrane segments organized into four homologous repeat domains, each containing six transmembrane segments. Likewise, the fourth of these segments, S4, is positively charged and considered as the region that serves as a sensor for transmembrane voltage changes. The loop that connects the S5 and S6 transmembrane segments forms the channel’s pore [6,7] (Figure 2A).
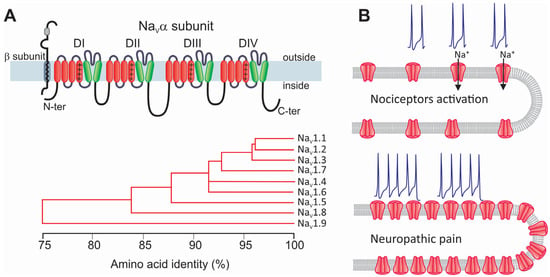
Figure 2.
Structure and classification of NaV channels and their participation in neuropathic pain. (A) The α subunit forms the ion-conducting region of NaV channels. This protein comprises four repeated homologous domains (DI–DIV), formed by six transmembrane segments connected by intracellular loops. Segment S4 (+) acts as the channel voltage sensor. Although the NaVα subunit alone can form a functional channel, it is generally associated with auxiliary subunits β (blue; NaVβ1–NaVβ4) that regulate its biophysical properties, its trafficking to the membrane, and its interaction with proteins extrinsic to the channel (upper panel). Nine α subunits have been identified (NaV1.1α–NaV1.9α) that share a similar membrane topology, each encoded by a different gene with different properties. The phylogenetic tree illustrates the amino acid sequence similarity of the mammal NaVα subunits encoding the nine identified NaV channels (lower panel). (B) In neuropathic pain, significant alterations occur in both the functional expression and the activity of NaV channels. These changes affect neuronal excitability, which translates as increased sensitivity to pain and produces hyperalgesia and allodynia.
On the other hand, the auxiliary subunits of NaV channels (NaVβ1 to NaVβ4) are glycoproteins containing one single transmembrane segment with a small intracellular domain and an extracellular region similar to that of cell adhesion molecules [8,9]. NaVα subunits alone are sufficient to reconstitute the sodium channel function. In contrast, NaVβ subunits modulate the voltage dependence and kinetics of the currents that flow through the pore-forming subunit [6,8,9,10].
2.2. The Role of NaV Channels in the Pathogenesis of Neuropathic Pain
Over the past few years, some mutations in NaV channel proteins and changes in their functional expression and post-translational processing have been associated with neuropathic pain [11,12,13,14,15]. Injuries or conditions affecting the peripheral nerves produce axonopathy and demyelination. This is because neuropathy alters the patterns of AP firing due to the remodeling of the neuron’s intrinsic electrical properties [16,17] (Figure 2B). Animal models of neuropathic pain have shown that after peripheral nerve injury, NaV channels are relocated, which is accompanied by changes in neuronal excitability that produce the ectopic activation of APs, ultimately leading to allodynia and hyperalgesia. After nerve injury, the percentage of neurons that can generate APs persistently and significantly increases. In parallel, this change causes subthreshold membrane potential oscillations and the emergence of pacemaker activity [17,18,19,20]. Augmented neuronal excitability and consequent increased discharge are the primary signals of neuropathic pain and trigger central sensitization [1,21].
NaV channels are concentrated in the proximal segment and nodes of Ranvier of healthy sensory neurons; however, after injury, it has been noted that NaV1.7 and NaV1.8 channels tend to concentrate at the axonal ends of neuromas generated at the site of injury, altering the excitability of injured cells [22,23]. This occurs due to a local buildup of vesicles transporting NaV channels. Some of these channels reach the cell membrane at the injury site, increasing their number and altering local electrogenic properties (Figure 2B).
However, in addition to changes in the localization of NaV channels, gene transcription is also regulated in dorsal root ganglion (DRG) sensory neurons during neuropathic pain [24,25]. In this regard, it is known that NaV1.1-, NaV1.2-, and NaV1.3-type channels are expressed in DRG neurons only in the embryonic stage, while adult cells predominantly express NaV1.7, NaV1.8, and NaV1.9, and to a lesser extent, NaV1.1 and NaV1.6 [26]. During neuropathic pain, there is a change in the expression profile of NaV channels that significantly impacts sensory neurons’ excitability by producing a hyperpolarization of the resting membrane potential [27]. This, in turn, allows NaV channels to transition from the inactivated refractory state to the closed state, increasing the fraction of channels available to be activated.
Sensitive to tetrodotoxin (TTX), NaV1.1 channels are crucial in the central nervous system (CNS) because they are key to generating and propagating APs. Likewise, given that their activity contributes to determining neurons’ activation threshold, the proper functioning of these proteins prevents the neuronal hyperexcitability that accompanies diverse CNS disorders, including neuropathic pain. Indeed, pharmacological inhibition of these channels has been shown to reduce mechanical pain in a peripheral nerve injury model [28]. In addition, from a molecular perspective, experimental evidence suggests an increased expression of NaV1.1 channels in DRG neurons shortly after nerve injury, both at the RNA and protein level, pointing to their possible role in the abnormal neuronal activity associated with neuropathic pain [29].
As mentioned earlier, NaV1.3 channels mediate a rapidly activating and inactivating current sensitive to TTX associated with neuropathic pain in axotomized DRG neurons [30]. These channels are expressed at low levels in adult primary sensory afferents, but are rapidly upregulated in DRG neurons after peripheral or spinal nerve injury or ligation [31,32,33,34].
Interestingly, it has been reported that the expression pattern of NaV channels undergoes an important change in the neuropathic pain model resulting from nerve injury. Indeed, this expression pattern changes from an increased expression of tetrodotoxin-resistant channels (TTX-R; NaV1.8 and NaV1.9) to an increased expression of toxin-sensitive channels (TTX-S; NaV1.3) in injured neurons [26,31,33,35]. In this context, it has been proposed that the augmentation in excitability observed in injured neurons is the result, at least in part, of this increase in the expression of NaV1.3 channels [36,37].
This idea is grounded in the fact that the biophysical properties of these channels are suitable for promoting spontaneous ectopic discharge, which, as mentioned above, is a hallmark of injured nerves. Although it is generally accepted that NaV1.3 channels may participate in initiating and maintaining neuropathic pain [36,37], unexpectedly, studies in NaV1.3 knockout animals have reported that such animals can develop neuropathic pain after peripheral nerve injury [38,39]. The origin of this difference is unknown, though it may lie in the changes observed in the expression patterns of other NaV channels, as we shall discuss next.
NaV1.7 channels are primarily expressed in nociceptive neurons and are essential in generating APs in response to noxious stimuli [23]. Regarding its role in the development of neuropathic pain, it has been speculated that nerve injury may lead to the upregulation of NaV1.7 channels, which would contribute to an increase in neuronal excitability and ectopic discharges, which are characteristic features of neuropathic pain [40]. However, the exact mechanisms by which NaV1.7 channels influence neuropathic pain remain poorly understood, and some studies suggest that compensatory mechanisms might mask their role in specific cellular contexts.
It has been hypothesized that nerve growth factor (NGF) may play an important role in the increased expression of NaV1.7 channels observed after nerve injury. Elevated levels of NGF lead to an increased expression of these channels through signaling pathways involving various transcription factors. These molecular events may promote the expression of NaV1.7 channels and eventually contribute to the general sensitization process and the maintenance of neuropathic pain [23,40].
Like other voltage-gated sodium channels, NaV1.8 plays an essential role in developing and maintaining neuropathic pain through its effects on neuronal excitability and the generation of abnormal electrical activity in sensory neurons [41]. After nerve injury, the expression of these channels is often significantly affected. Initially, NaV1.8 expression may be downregulated in injured neurons, though neighboring uninjured neurons often show increased expression [23]. In addition, the biophysical properties of NaV1.8 channels may also be affected after nerve injury. Changes in the properties of NaV channels can shift the activation curve toward hyperpolarized values, lowering the threshold for firing APs. This makes neurons more likely to activate repeatedly [42] (Figure 3). These changes may significantly contribute to the spontaneous discharges seen in hyperalgesia associated with neuropathic pain. Notably, changes in the expression of NaV1.7 and NaV1.8 channels may exacerbate neuronal excitability by converging via complex cell mechanisms to determine the persistence of neuropathic pain [43].
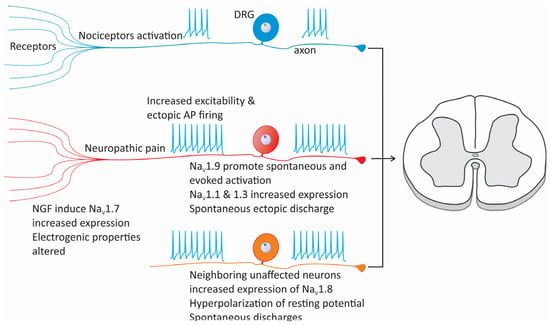
Figure 3.
Changes in NaV channel expression and cellular excitability in neuropathic pain. Alterations in the expression of various subtypes of sodium channels, such as NaV1.1, NaV1.3, NaV1.7, NaV1.8, and NaV1.9, can increase cellular excitability, reducing the activation threshold of nociceptors. Similarly, after nerve injury, neurons adjacent to the damaged area may experience changes in the expression of NaV channels, particularly NaV1.3 and NaV1.8, which causes the development of ectopic foci of neuronal activity. On the other hand, the expression of NaV1.8 channels in neurons neighboring an injured nerve can also be compromised, contributing to the maintenance of neuropathic pain.
Following nerve injury, cross-talk can occur between intact and injured neurons, where changes in NaV1.8 channel expression can influence the activity of nearby neurons expressing NaV1.7 channels (Figure 3). As mentioned above, the initially low expression of NaV1.8 channels in injured cells is increased in neighboring uninjured neurons, which ultimately results in increased NaV1.8 expression, generating ectopic activity. On the other hand, the upregulation of NaV1.7 channel expression in these cells increases the neuronal excitability and ectopic AP firing caused by changes in NaV1.8 expression [43]. Together, these events amplify pain signaling by causing a state of persistent neuronal hyperexcitability.
Finally, it has been proposed that NaV channel mRNA may be transported peripherally from DRGs to the sciatic nerve and translated locally. In particular, the local upregulation of NaV1.8 channel mRNA has been observed after nerve injury, which may contribute, at least in part, to the increase in channel protein levels observed at the peripheral level and in neuronal excitability. This, in turn, may play a relevant role in the aberrant nociception that characterizes neuropathic pain [44].
NaV1.9 channels are expressed primarily in nociceptive neurons, where they play a relevant role in pain signaling, especially in neuropathic pain conditions [24,27]. In addition to those reviewed in the preceding sections, these channels generate APs and amplify pain signals, especially after nerve injury. While NaV1.9 channels mediate TTX-R sodium currents that are crucial for maintaining neuronal excitability under physiological conditions [41,45], under pathological conditions, sodium currents through these channels can become dysregulated and contribute to chronic pain states. Numerous studies suggest that NaV1.9 channels favor spontaneous and evoked activation in DRG neurons, which, in abnormal conditions, leads to exacerbated pain perception [24,27].
2.3. The Role of the NaV Channel Auxiliary Subunits in Neuropathic Pain
Furthermore, as already mentioned, NaVβ auxiliary subunits regulate the kinetic properties and voltage dependence of the ion-conducting subunits of NaV channels [9,46,47]. Therefore, alterations in the functional expression of these proteins may influence the development of neuropathic pain due to their central role in the excitability of sensory neurons. On the other hand, it has also been observed that the expression of the auxiliary subunits NaVβ2 and NaVβ3 can be augmented after peripheral nerve injury, both in injured sensory neurons and neighboring uninjured nerve cells. This could be associated with neuronal hyperexcitability and the development of ectopic activity [48].
Previous work on NaVβ2 subunit expression has revealed that this protein is upregulated following peripheral nerve injury, which may affect neuronal excitability [48]. This idea is supported by results obtained in NaVβ2 null mice, showing decreased NaV TTX-S channel expression in DRG neurons [10,49]. Remarkably, mechanical allodynia associated with peripheral nerve injury was attenuated in the knockout animals, consistent with the role of this protein in neuropathic pain [10,49].
The auxiliary subunit NaVβ3 may also play a role in neuropathic pain. As discussed above, there is evidence that peripheral nerve injury induces an increase in currents passing through TTX-S NaV channels related to an increase in the expression of NaV1.3 in DRG neurons. This occurs in parallel with increased NaVβ3 mRNA and protein levels [50,51]. It is worth noting that the co-expression of the NaVβ3 subunit in heterologous expression systems produces changes in the activation and inactivation of NaV1.3 channels, faster recovery from inactivation, and slower kinetics of the current [10,52]. Likewise, the overexpression of Scn3b mRNA has been observed in multiple pain models, specifically depending on the type of fiber and the model used. In a chronic injury model, it was increased in C fibers, while in a diabetic neuropathy model, it was increased in medium Aδ fibers and the lumbar SC [50,51].
Therefore, it has been speculated that the overexpression of NaV1.3 channels and NaVβ3 subunits represents an attempt by sensory neurons to compensate for the decrease in the expression of NaV1.8 and NaV1.9 channels induced by nerve injury [25,32]. This causes alterations in the properties of the current, changing from slow activation through NaV1.8 channels to a faster one that flows through NaV1.3/NaVβ3 channels, which would reduce the activation threshold of APs and promote high-frequency firing, thus contributing to the hyperexcitability observed in injured sensory neurons [10,52]. However, NaV1.3 channels have been reported to be preferentially upregulated in medium- and large-sized DRG neurons after nerve injury and may not fully compensate for the loss in the functional expression of NaV1.8 and NaV1.9 channels in small-diameter sensory neurons [33,53].
The NaVβ1 subunit presents a complex scenario. Although its expression increases current density through NaV channels [54], its role in neuropathic pain remains unclear. NaVβ1 knockout mice die prematurely, hampering behavioral studies [55], but their DRG neurons are hyperexcitable, suggesting possible allodynia [56]. Furthermore, increased NaVβ1 mRNA levels in sympathetic nerve injury models complicate determining its precise role in neuropathic pain [46].
2.4. NaV Channels as Therapeutic Targets for Neuropathic Pain
Nerve conduction through peripheral nerves has long been blocked by inhibiting the activity of NaV channels to combat pain. Studies in animals and humans have validated sodium channels, mainly NaV1.7, NaV1.8, and NaV1.9, as viable targets for pain treatment [57]. This is because, as already mentioned, NaV channels play a crucial role in the hyperexcitability of nociceptors and, therefore, in the underlying mechanism of nerve signal conduction in neuropathic pain. However, the development of effective treatments targeting NaV channels has yet to advance decisively. Most studies emphasize the importance of using selective blockers for different NaV channel subtypes, which could offer pain relief and minimize side effects.
Creating specific blockers for NaV1.7 channels is a promising approach to treating neuropathic pain, as it may help to avoid the side effects linked to non-selective sodium channel blockers [58]. Initially, a series of drugs based on benzodiazepines developed to inhibit NaV1.7 channels specifically showed the inhibition of spontaneous neuronal activation in animal models. They reversed tactile allodynia in spinal nerve ligation (SNL) models. Subsequently, a series of imidazopyridine-based blockers with improved pharmacokinetics and a significantly greater efficacy in SNL models were also developed [59,60,61]. Other compounds selective for the inhibition of NaV1.7 channels include biphenylthiazolcarboxamides and biphenylpyrazoles, as well as ProTx-II, a peptide isolated from tarantula venom that selectively inhibits NaV1.7 with an approximately 100-fold selectivity over other isoforms [62,63]. However, the clinical use of these compounds has been limited by their affinity for other NaV channel isoforms and their ineffectiveness in reducing pain in the short term after administration [58].
Animal models in which NaV1.7 has been knocked out have revealed its contribution to neuropathic pain, and several works suggest that NaV1.7 activity regulates endogenous opioid release, such that the combination of a NaV1.7 channel inhibitor with an opioid may provide synergistic analgesia with fewer side effects [64,65]. Specifically, it has been shown that a complete blockade of the sodium currents in cultured wild-type DRG neurons with TTX increased the expression of opioid peptides and that the absence of NaV1.7 channels in the knockout mice was associated with the upregulation of Penk, the precursor of met-enkephalin, found at high levels in the sensory neuron terminals of NaV1.7-null mice [64]. Therefore, the combination of NaV1.7 channel antagonists with enkephalinase inhibitors or low-dose opioids has shown significant analgesic effects by reducing opioid-related side effects.
Interestingly, a specific regulatory sequence within the NaV1.7 channel structure involved in the molecular mechanism of chronic pain was identified and proposed as a new target for therapeutic intervention to alleviate neuropathic pain [66,67,68,69]. This sequence, called the collapsin response mediator protein 2 (CRMP2) regulatory sequence (CRS), seems to be crucial for the functional coupling between NaV1.7 channels and CRMP2, a cytosolic protein involved in regulating sodium channel activity [66,67]. A decoy peptide corresponding to the CRS reduced NaV1.7 currents and the presynaptic expression of the channels, decreasing the release of calcitonin gene-related peptide (CGRP) associated with pain signaling.
More importantly, the CRS peptide effectively reversed nerve-injury-induced mechanical allodynia in rodent models without causing motor impairment or altering normal physiological pain sensation [68]. Finally, an AAV-mediated gene therapy strategy introduced a plasmid encoding the NaV1.7–CRS gene into sensory neurons. This approach reduced the function of NaV1.7 channels in animal models, decreasing mechanical allodynia associated with nerve injury and chemotherapy-induced peripheral neuropathy [68]. These findings underscore the potential of the CRS domain as a therapeutic target for the management of neuropathic pain.
The selective targeting of NaV1.8 channels also represents a promising strategy for treating neuropathic pain. Several compounds have been developed to selectively inhibit these channels. In particular, A-803467 and A-887826 exhibit over a 100-fold selectivity for NaV1.8 (IC50 of 8 nM) compared to other sodium channel blockers and have shown efficacy in reducing neuropathic pain in rodent models [61,70,71,72]. This selectivity reduces the risk of unwanted systemic side effects associated with non-selective NaV channel blockers. Likewise, dexpramipexole, a benzothiazole-like compound, has shown a high selectivity for these channels, effectively blocking TTX-R conductance in DRG neurons with an IC50 of ~300 nM and analgesic effects in various pain models, including those induced by nerve injury and diabetes.
Lastly, VX-548 (suzetrigine) is a more recently discovered NaV1.8 channel selective inhibitor, effective in treating acute pain. By inhibiting these channels, VX-548 prevents sensory neurons from transmitting pain signals to the spinal cord and brain, significantly reducing painful sensations [73,74]. Therefore, VX-548 is a first-in-class non-opioid analgesic, approved recently by the Food and Drug Administration (FDA), for treating adult patients experiencing moderate to severe acute pain, such as pain following injury, illness, or surgery [73]. However, it is not yet approved for the management of neuropathic pain, though it is being evaluated for neuropathic pain conditions, including painful diabetic peripheral neuropathy (DPN) and painful lumbosacral radiculopathy (LSR). Data from Phase 2 and 3 trials for chronic pain (including neuropathic pain) have shown mixed results, with apparent efficacy for acute pain but unresolved questions regarding chronic pain conditions.
At the molecular level, VX-548 exhibits potent state-dependent inhibition of NaV1.8 channels, characterized by a “reverse use dependence” mechanism. This means that it binds tightly to the channels in their resting (closed) state, but this inhibition can only be rapidly relieved by extensive and prolonged depolarizations. Consequently, VX-548 maintains tonic inhibition of these channels under physiological conditions. This unique mechanism distinguishes VX-548 from other NaV channel inhibitors and supports its consistent and selective analgesic effect [75,76].
The clinical success of VX-548 validates NaV1.8 channels as a viable pharmacological target for treating acute pain, confirming their central role in peripheral nociceptive signaling. Furthermore, initial data from the evaluation of VX-548 in managing pain in DPN and LSR suggest that NaV1.8 channels also play a relevant signaling role during the development of neuropathic pain. However, further studies are required to validate this idea.
The treatment of neuropathic pain aimed at the selective inhibition of NaV1.8 channels offers several advantages. These compounds provide analgesia and may improve tolerability compared to other therapies. By targeting peripherally located NaV channels, these blockers may minimize the central side effects typically seen with more broad-spectrum NaV channel inhibitors. Preclinical and clinical research will contribute to a better understanding of their role and effectiveness in broader pain management contexts.
On the other hand, therapy targeting the molecular mechanisms associated with neuropathic pain involving NaV1.9 channels is still in development. Finding compounds that may alter NaV1.9 currents has proven difficult [61]. This is because the expression of these channels in heterologous systems is complex and tends to run down quickly in sensory neurons [77]. An innovative strategy was developed in which individual voltage-sensor paddles from NaV1.9 were transplanted into chimeric constructs of voltage-gated (KV) channels to identify toxins that may interact with native NaV1.9 channels [78]. Although this study showed that NaV1.9 channels have a distinctive pharmacological profile and that the voltage-sensor paddles could be promising targets, it was unclear to what extent the chimeric channels reproduced the pharmacological properties of native channels.
3. Voltage-Gated Calcium (CaV) Channels in Neuropathic Pain
3.1. Structure and Function of CaV Channels
CaV channels are the preferential route for the entry of calcium ions into excitable cells. These channels are activated in response to the depolarization of the plasma membrane and, thus, allow for the selective entry of calcium. In this way, CaV channels contribute to determining cell excitability. Additionally, calcium entering cells acts as a second chemical messenger that initiates and regulates multiple physiological processes, including gene expression and neurotransmitter release, among many others. Therefore, CaV channels play a dual role by linking electrical signals at the cell surface with biochemical responses within the cell [79,80,81,82].
Based on their biophysical and pharmacological properties, voltage-gated calcium (CaV) channels have been classified into T, L, N, P, Q, and R subtypes. However, the most used classification is based on the voltage range at which they apparently activate, separating them into the following two categories: low- and high-threshold channels, LVA and HVA, respectively. The T-type channel is the only low-threshold channel described, while the L-, N-, P-, Q-, and R-type channels are considered to be high-voltage-activated channels [79,80,81,82,83,84] (Figure 4A).
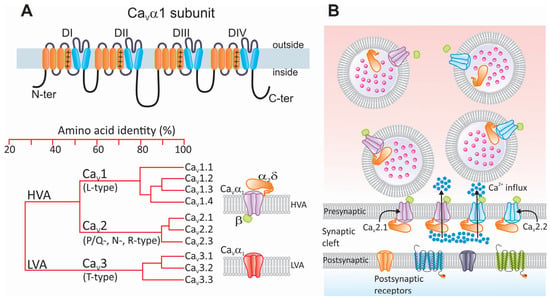
Figure 4.
Structure and classification of CaV channels and their participation in neurotransmission. (A) Schematic representation of the CaVα1 subunit illustrating its membrane topology. Like NaV channels and some members of the KV channel family, the main CaVα1 subunit is a protein composed of four relatively conserved homologous repeat domains (DI–DIV) containing six α helices each (upper panel). The fourth α helix of each repeat domain contains a sequence of regularly spaced positively charged (+) basic residues that sense changes in transmembrane voltage. The loops connecting the repeat domains, as well as the amino and carboxyl termini, are intracellular. The lower left panel shows a phylogenetic tree illustrating the evolutionary relationship among members of the CaV channel family. The structural homology comparison is based on the alignment of the human channels. LVA and HVA stand for high- and low-voltage-activated, respectively. HVA channels (CaV1 and CaV2) are oligomeric complexes whose composition, in addition to the pore-forming CaVα1 subunit, includes two auxiliary subunits called CaVβ and CaVα2δ (shown in green and orange, respectively). On the other hand, LVA (CaV3) channels function as monomers of the main CaVα1 subunit (lower right panel). (B) In response to membrane depolarization caused by the arrival of an AP, presynaptic CaV channels allow for the entry of calcium ions (blue dots) from the extracellular space to the synaptic terminals. The most relevant channel subtypes involved in this event are CaV2.1 and CaV2.2 (shown in purple and blue, respectively). Increased intracellular calcium concentration at active sites promotes fusion of neurotransmitter-containing vesicles through the SNARE complex. Neurotransmitters (pink dots) released into the synaptic cleft diffuse until they bind to their receptors located on the postsynaptic membrane.
From a molecular perspective, LVA (CaV3) channels are monomers formed solely by the main CaVα1 subunit. HVA channels (CaV1 and CaV2) are more complex oligomers formed by the CaVα1 subunit together with auxiliary subunits such as CaVβ and CaVα2δ. The structure of CaVα1 is similar to the NaVα subunit, consisting of four domains, each with six transmembrane segments [82,85] (Figure 4A).
CaV channels comprise a CaVα1 ion-conducting subunit and may have associated auxiliary subunits depending on the channel subtype. The CaVα1 subunit, in turn, consists of four homologous repeat domains, each with six transmembrane segments called S1 to S6. The S4 segment, having positively charged amino acids, can detect changes in transmembrane potential and functions as the channel’s voltage sensor. Between the S5 and S6 segments, the P segment is located, which contains the amino acids that form the ion selectivity filter. Furthermore, four isoforms of the CaVβ auxiliary subunit (CaVβ1 to CaVβ4) have been identified [86]. These proteins have an intracellular localization. They contribute to regulating the voltage dependence of the channels and the kinetic properties of the currents and allow the channel complex to interact with intracellular signaling molecules that modulate its activity [86,87].
Likewise, it is known that the CaVα2δ auxiliary subunits favor the membrane expression of CaV channels (Figure 4A). Four subtypes of these proteins have been described (CaVα2δ-1 to CaVα2δ-4), encoded by independent genes (CACNA2D). These genes are initially translated into precursor proteins that are proteolytically processed, giving rise to two peptides, CaVδ and CaVα2, with the first anchored to the plasma membrane through a GPI motif and the second being completely extracellular, which remain linked by a disulfide bond [88]. The CaVα2 peptide is highly glycosylated and contains diverse functional regions, including von Willebrand factor A (vWFA) motifs, a metal-ion-dependent adhesion site (MIDAS), and four cache regions. Similarly to the CaVβ subunit, the CaVα2δ subunits promote and stabilize the expression of CaV channels on the cell surface [79,85,89].
Finally, eight CaVγ subunits have been identified, which, according to phylogenic analyses, belong to a protein subfamily originating from a single gene. Biochemical and electrophysiological studies have shown the physical and functional interactions of these subunits with the CaV channel complex [90,91,92,93]. On the other hand, it is known that the CaVγ2 subunit can also bind to proteins containing the PDZ domain and that it participates in the intracellular trafficking of the AMPA receptor [94].
In mammals, ten different CaVα1 subunits encoded by independent genes (CACNA1) are expressed, which, from a molecular point of view, group CaV channels into three subfamilies [81,82]. The first (CaV1) includes L-type channels with four members (CaV1.1 to CaV1.4). The CaV2 subfamily has three members (CaV2.1 to CaV2.3), which give rise to currents through P/Q-type, N-type, and R-type neuronal channels, respectively. P- and Q-type channels result from the alternative splicing of the CACNA1A gene encoding the CaV2.1α1 subunit [95,96].
Lastly, the CaV3 subfamily groups low-activation-threshold channels and consists of three members, CaV3.1 to CaV3.3 (Figure 4A). These channels allow for a basal calcium influx called a window current, which helps to maintain the resting membrane potential. Likewise, since they are activated at more negative potentials than the other CaV channels, they can significantly influence cell excitability, contributing to the generation of APs and rhythmic electrical activity [79,80,81,82].
It is widely accepted that CaV channels may play a key role in the fundamental mechanisms of neuropathic pain. The contribution of these proteins to cell excitability and neurotransmission, as well as their potential role in the treatment of the condition, stresses the need to understand neuropathic pain at the cell and molecular levels [82,85,97]. The association of CaV channels with the pathogenesis of the disease occurs predominantly through the HVA channels of the P/Q- (CaV2.1) and N-types (CaV2.2) and the ancillary CaVα2δ subunit. However, it has also been reported that the LVA channels of the CaV3.2 class may also contribute significantly to the pathophysiology of the condition [98,99,100].
As mentioned above, the entry of calcium ions in response to the activation of CaV2 channels determines the release of neurotransmitters. The calcium that enters the nerve terminal promotes the assembly of a subset of scaffolding proteins essential for anchoring synaptic vesicles containing neurotransmitters to the cell membrane and their eventual fusion [87,101,102] (Figure 4B). Therefore, alterations in the functional expression of CaV2 channels will alter synaptic transmission and consequently may affect pain signaling.
3.2. Role of Different CaV2 Channel Subunits in Nociceptive Pathways and Neuropathic Pain
CaV channels regulate neuronal excitability, synaptic transmission, and pain signaling. As we will see below, there are three subtypes of CaV2 channels. Among them, CaV2.1 (P/Q-type) and CaV2.2 (N-type) are particularly important in neurotransmission between primary afferent fibers and neurons of the SC’s dorsal horn.
CaV2 channels contribute to the onset and maintenance of neuropathic pain through different cellular and molecular processes. Firstly, these channels are decisive in synaptic transmission, since their activation gives rise to transient increases in the concentration of intracellular calcium in the nerve terminals, which favors the release of neurotransmitters. In pathological conditions, however, synaptic transmission in sensory neurons may be altered, increasing the release of chemical pain mediators such as glutamate and substance P and inducing central sensitization, one of the main features of neuropathic pain. Second, the activity of CaV2 channels can be regulated by phosphorylation and other post-translational modifications, which may promote their functional expression during neuropathic pain. Finally, transient increases in intracellular calcium can cause, in pathological conditions, changes in gene expression patterns that promote the activation of transcription factors that target genes associated with chronic pain [103] (Figure 5A).
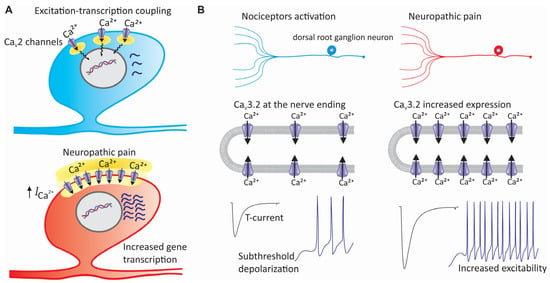
Figure 5.
Contribution of CaV channels to the pathogenesis of neuropathic pain. (A) In addition to its effects on the release of neurotransmitters, alteration in the expression of CaV2 channels participates in the pathophysiology of neuropathic pain by affecting the excitation–transcription coupling. This fundamental cell process links electrical activity in excitable cells to gene transcription. This implies that the calcium, once inside the cells, can activate transcription factors, either directly or through protein kinases and second messengers that control the activity of these factors. (B) Overexpression of CaV3 channels in sensory neurons during neuropathic pain increases their excitability, decreases the firing threshold of afferent fibers, and favors repetitive firing.
The contribution of CaV2.1 (P/Q-type) channels to pain perception is being studied in detail and is beginning to be revealed. These channels are crucial for neurotransmitter release in the CNS, and pharmacological studies suggest their involvement in the pathophysiology of pain. While initial studies showed that intrathecal injections of specific blockers, such as AgaIVA, did not have an apparent effect on CaV2.1 channels in neuropathic pain [104,105,106], subsequent research has indicated that they may influence descending pathways that modulate pain transmission in some areas of the brain [107,108]. Likewise, studies conducted in CaV2.1α1 subunit knockout mice have revealed alterations in their response to nociceptive stimuli [109]. However, eliminating the pore-forming subunit of CaV2.1 channels does not significantly modify the response to thermal stimuli [109].
CaV2.2 channels are predominantly expressed in the presynaptic nerve terminals of central and peripheral neurons [110]. They are crucial for releasing neurotransmitters relevant to generating pain signals, such as glutamate and GABA. In addition, the activity of these channels is modulated by the activation of various G protein-coupled receptors (GPCRs) involved in nociception, including opioid, cannabinoid, neuropeptide Y, and substance P receptors [111,112]. Research has shown a significant relationship between the knockout of CaV2.2 channels and neuropathic pain, consistent with their contribution to the molecular pathophysiology of this condition. Multiple studies suggest that the genetic ablation of these channels can result in reduced pain responses across various models of neuropathic pain [113,114]. The absence of CaV2.2 channels affects the release of substance P and CGRP, and this reduction in neurotransmitter release contributes to a decrease in overall pain signaling.
CaV2.2 channels are concentrated in nerve terminals located in laminae I and II of the dorsal horn of the spinal cord, where they transmit pain signals arriving on C and Aδ fibers. Interestingly, these channels in primary afferent fibers contribute to developing allodynia and hyperalgesia after nerve injury [115], as discussed below.
Several studies have shown that mutually exclusive splicing patterns in the gene encoding the CaV2.2α1 subunit modulate the function of N-type channels in sensory neurons and can influence pain transmission. In particular, an exon 37a-containing isoform whose expression is restricted to DRGs correlates closely with significantly larger N-type currents in nociceptive neurons [116,117]. The preferential inclusion of exon 37a in sensory neurons generates a module in the C-terminus of the CaV2.2α1 subunit that mediates channel inhibition in a voltage-independent manner, which requires tyrosine kinase activation [118,119]. Furthermore, exon 37a enhances the μ-opioid-receptor-mediated inhibition of N-type channels [120], contributing to defining the molecular nature of the voltage-independent inhibition of N-type channels in the pain pathway.
Likewise, research in animal pain models has shown that the expression of the CaV2.2α1 subunit is significantly increased [100]. Mice subjected to partial sciatic nerve ligation showed an increased current amplitude through N-type channels and increased mRNA levels for the gene encoding the CaV2.2α1 protein in DRG neurons [121,122]. Likewise, in a chronic constrictive nerve injury model, the CaV2.2α1 protein was upregulated in lamina II of the SC dorsal horn [123]. Furthermore, in nerve ligation models, a significant increase in the expression of CaV2.2 channels with a subsequent increase in the amplitude of the current was reported, which further facilitated the excitatory synaptic transmission of Aδ and C fibers in the SC dorsal horn [122].
Research on the cellular and molecular bases of neuropathic pain has shown that ubiquitination may contribute to its establishment and maintenance by regulating the turnover of synaptic proteins. Specifically, it has been described that the active zone protein RIM1α participates in the development of the condition by binding and positively regulating the expression of CaV2.2 channels. It is also known that RIM1α-associated spinal allodynia is mediated by Fbxo3, a protein that reduces the Fbxl2-dependent ubiquitination of RIM1α. When deubiquitinated, RIM1α can bind directly to these channels, increasing its expression in the nerve terminals of the dorsal horn of the SC [124].
The activation of nociceptin opioid peptide (NOP) receptors, also known as opioid-like receptor 1 (ORL-1), results in the G protein-dependent regulation of CaV2.2 channels [125,126,127]. This results in a decreased current amplitude, with consequent alterations in presynaptic calcium levels and impairment in neurotransmission [128]. Due to the widespread expression of both NOP and CaV2.2 channels in the brain, dorsal horn of the SC, and DRG, alterations in this system result in different neurological conditions, including neuropathic pain. As mentioned, CaV2.2 channels are crucial for pain processing by controlling the synaptic strength on C and Aδ afferent fibers. Thus, reducing calcium influx by activating NOP receptors may decrease the release of CGRP and substance P, neurotransmitters involved in pain signaling. This is beneficial in neuropathic pain, where the expression of CaV2.2 channels is generally upregulated [126,129].
3.3. The Role of the CaVα2δ-1 Auxiliary Subunit in Neuropathic Pain
The α2δ subunits of CaV2 channels have been shown to play crucial roles in nociceptive signaling [130,131,132,133]. As mentioned earlier, these proteins are essential in the function and regulation of these channels by contributing to the intracellular trafficking, voltage dependence, and kinetics of the currents [134,135,136,137,138]. In particular, the CaVα2δ-1 subunit, which is expressed in excitable cells, including neurons, is essential for presynaptic functions such as synapse formation, the regulation of synaptic plasticity, and the control of the calcium concentration in the synaptic cleft [138,139,140]. The protein contains several functional regions that allow for interactions with the channel complex and other synaptic molecules. Research on the structure of CaVα2δ has identified a von Willebrand factor A (VWA) region along with four cache domains. The VWA region is critical for interaction with the CaVα1 subunit [138].
It has been reported that CaVα2δ expression may increase at both the mRNA and protein levels in sensory neurons after spinal nerve ligation and in animal models of diabetic neuropathy [130,141,142,143,144,145]. This change is accompanied by AP discharges in the injured neurons due to an increase in the functional expression of CaV2.2 channels mediated by the exacerbated expression of the CaVα2δ subunit. Consistent with this, genetic ablation of the CaVα2δ-1 subunit significantly decreased the expression of CaV2.2 channels on the cell membrane of DRG neurons and in the dorsal horn of the SC [146]. This alteration in neuronal excitability can affect the release of neurotransmitters associated with neuropathic pain pathways [122]. Interestingly, mice overexpressing CaVα2δ-1 exhibit neuropathic pain symptoms without nerve damage, whereas CaVα2δ-1-deficient mice show deficits in sensitivity after nerve injury [147,148,149].
Several studies support an important role of the interaction between the CaVα2δ-1 subunit and thrombospondin-4 (TSP4), a glycoprotein found in the extracellular matrix, in nerve-injury-induced neuropathic pain, mediated through aberrant excitatory synapse formation and presynaptic neurotransmission in the SC [150,151,152]. Peripheral nerve injury induces the upregulation of both proteins in the SC that precedes the onset and correlates with the duration of neuropathic pain [130,141,142,143,153,154,155]. The inhibition of this regulation or the genetic ablation of CaVα2δ-1 or TSP4 prevent the onset and development of the disease [152,155,156]. The mechanism by which an exaggerated expression of TSP4 alters the function of CaV channels remains to be established. In this regard, it has been reported that TSP4 can differently affect the distinct types of channels in sensory neurons, decreasing the currents passing through HVA channels and increasing those flowing through LVA channels [154], which is paradoxical given that, unlike what occurs with HVA channels, a clear role for auxiliary subunits, including CaVα2δ-1, in the functional expression of LVA channels has not yet been established. Further research is required to better understand the origin of this discrepancy.
Similarly, research has demonstrated that the CaVα2δ-1 subunit interacts with N-methyl-D-aspartate receptors (NMDARs) to create a complex that increases their activity by promoting their trafficking to synapses [157,158]. NMDARs are preferentially expressed in postsynaptic neurons, but are also present in presynaptic neurons, influencing neurotransmitter release and synaptic plasticity [159]. The activation of presynaptic NMDARs leads to increased calcium and the exocytosis of secretory vesicles, resulting in greater glutamate release. Under normal conditions, NMDARs are inactive; however, these receptors become tonically active in neuropathic pain [160]. Notably, models of neuropathic pain have shown an elevated expression of CaVα2δ-1/NMDAR complexes, indicating their potential involvement in pain mechanisms [149].
3.4. Contribution of the CaV3.2 Channels to Neuropathic Pain
On the other hand, presynaptic T-type calcium channels play a crucial role in nociceptive signaling, and their dysregulation can lead to the development of neuropathic pain [97,98,99]. Notably, the CaV3.2 isoform, expressed in primary afferent neurons, spinal dorsal horn neurons, and supraspinal brain regions, is particularly important in processing pain signals.
It is known that CaV3.2 channels participate in the regulation of neuronal excitability. Their activation lowers the threshold for APs, which can alter pain signaling under pathological conditions [97,161,162]. An increased expression of these channels in DRG neurons has been linked to heightened neuronal firing and chronic pain (Figure 5B). In contrast, silencing CaV3.2 channels in DRG neurons using antisense oligonucleotides or siRNA significantly reduces mechanical nociception and tactile allodynia [163,164]. In addition, experimental evidence indicates that antagonists targeting these channels may reduce neuronal excitability and provide analgesia in models of neuropathic pain [99]. However, beyond peripheral and spinal mechanisms, CaV3.2 channels may also play an important role in specific brain areas, contributing to pain perception and modulation [161,162]. Interestingly, the inhibition of these channels in the brain has also been found to have analgesic effects.
Likewise, experimental evidence shows that although the total expression of CaV3.2 in DRG neurons may increase in neuropathic pain models, the membrane expression of these channels is significantly augmented without changes in total expression [99,165]. Additionally, the accumulation of CaV3.2 in uninjured nerves may contribute to neuropathic pain due to interactions with injured axons, mediated by increased levels of NGF and tumor necrosis factor-α (TNF-α), which regulate T-type calcium channels [166]. In the early stages of chronic pain, an increase in CaV3.2 expression is favored by the transcription factor Egr-1 [167].
Phosphorylation, ubiquitination, and other post-translational modifications are known to contribute to the development of neuropathic pain after nerve injury [97,99,168]. In this regard, it has also been documented that, after spinal nerve injury, there is an upregulation in the expression of CaV3.2 channels and the Cdk5 kinase in DRG and SC dorsal horn neurons, which is associated with mechanical allodynia. Cdk5 directly phosphorylates CaV3.2 channels, increasing their functional expression and enhancing neuronal excitability, contributing to neuropathic pain. In contrast, the inhibition of Cdk5 decreases the firing of compound APs in spinal nerves and modifies the paw withdrawal threshold in animals with allodynia induced by spinal nerve ligation (SLN) [169]. Interestingly, the study of functional and cellular localization changes in CaV3.2, as well as CaV2.2 channels and Cdk5 within intact L3-4 afferent fibers adjacent to the injured peripheral nerve at L5-6, show that both the channels and the kinase are altered in intact neurons after injury, evidencing an additional molecular mechanism underlying neuropathic pain. Furthermore, nerve injury at L5-6 has been shown to modify the slow and fast components of compound APs recorded in the L4 dorsal root, and these changes may be mediated by the effects of Cdk5 on CaV channel function and localization [169].
Finally, it is worth mentioning that the ubiquitin–proteasome system also regulates CaV3.2 channels, influencing their expression and functional activity in neuropathic pain [99]. Research shows that USP5 is a deubiquitinating enzyme that decreases the ubiquitination of CaV3.2 channels during neuropathic pain [167,170]. This action prevents the channels from being internalized and increases their presence on the cell membrane, leading to higher T-type calcium currents and pain sensitivity. In the early stages of neuropathic pain, the transcription factor Egr-1 controls the expression of CaV3.2 channels, while in later stages, USP5 is responsible for further increasing CaV3.2 expression. Studies have shown that knocking down USP5 results in an increased ubiquitination of CaV3.2, decreased protein levels of the channel, and reduced whole-cell currents [170,171,172]. Conversely, increasing USP5 activity leads to greater activity of CaV3.2 channels in models of neuropathic pain.
3.5. CaV Channels as Potential Therapeutic Targets for Neuropathic Pain
Gaining insight into the function and potential of CaV channels as therapeutic targets has offered valuable information for managing chronic pain conditions. For instance, CaV2.2 channels are key in pain signaling because they help release neurotransmitters from sensory neurons. Blocking these channels can prevent the release of neuropeptides that transmit pain, making them a promising target for treating neuropathic pain.
Several studies stress significant advancements in studying CaV2.2 channels for treating neuropathic pain. Ziconotide, a synthetic version of the marine peptide ω-conotoxin MVIIA, has been established as an effective blocker of these channels for treating severe chronic pain [111,173]. It is administered intrathecally and has shown efficacy in several neuropathic pain models. The mechanism of ziconotide involves blocking calcium entry, which is crucial for releasing the neuropeptides substance P and CGRP in sensory neurons [174].
Furthermore, in animal models, treatment with ziconotide can prevent hyperalgesia and allodynia, confirming the role of CaV2.2 channels in establishing neuropathic pain. Ziconotide is about ten times more potent than intrathecally administered morphine [175]. However, its clinical use is limited by side effects [111]. Leconotide is a newer blocker of CaV2.2 channels, which has emerged as an alternative to ziconotide. This compound has been shown to have antihyperalgesic effects and offers a better side effect profile [176]. On the other hand, an alternative for pain relief has focused on developing small molecules that function as inhibitors of CaV2.2 channel activity. These molecules aim to provide similar benefits to ziconotide and leconotide without the disadvantages associated with peptide administration. Some of these peptides have been designed to disrupt the coupling of the main subunit CaVα1 with other intrinsic or extrinsic proteins in the channel complex. In this context, various studies suggest that collapsin response mediator protein 2 (CRMP-2) is an important molecular interactor of CaV2.2, regulating its function and, consequently, the release of neurotransmitters in sensory neurons. The overexpression of CRMP-2 increases the current density through CaV2.2 channels and enhances the release of CGRP, which participates in pain transmission.
It has also been reported that disrupting the CRMP-2/CaV2.2 complex with specific peptides, such as TAT-CBD3, Ct-dis, and R9-CBD3-A6K, reduces the excessive neurotransmitter release associated with chronic pain, showing antinociceptive effects in neuropathic pain models [177,178,179,180]. Moreover, the potential of quinazolines and benzoylpyrazolines as agents that disrupt the coupling between CaVα1 and CaVβ subunits has been investigated. These compounds have shown the ability to decrease currents through CaV2.2 channels, alter their presynaptic localization, and inhibit the release of CGRP, exhibiting antinociceptive properties in various pain models, including neuropathic pain [181].
Likewise, Khanna and his colleagues also showed that the inhibition of the interaction between CaVα1 and CaVβ subunits reduces the excitability of DRG neurons, leading to a decrease in acute and neuropathic pain in several animal models [182]. Specifically, the authors developed a molecule identified as IPPQ that selectively binds to CaVβ, inhibiting its coupling with the CaVα1 subunit of CaV2.2 channels. This leads to the delocalization of presynaptic channels, a decrease in the amplitude of calcium currents in sensory neurons, and a reduction in the release of the nociceptive neurotransmitter CGRP in the SC. This same research group recently designed a small peptidomimetic molecule derived from the CRMP2 peptide called CBD3063. This compound selectively inhibits the interaction between CaV2.2 channels and CRMP2, reducing calcium entry and neurotransmitters’ release linked to pain signaling. Additionally, CBD3063 showed efficacy in animal models by reversing neuropathic pain without affecting sensory or cognitive functions, suggesting a favorable side effect profile [183].
Likewise, C2230 is a novel use- and state-dependent blocker of CaV2.2 channels, recently reported for its potential as an analgesic across various pain models [184]. It effectively reverses pain behaviors associated with neuropathic pain without negatively impacting motor or cardiovascular functions. The compound stabilizes CaV2.2 channels in the inactivated state, resulting in use-dependent inhibition during high-frequency stimulation. This reduces calcium influx in sensory neurons, significantly decreasing excitatory postsynaptic currents and neurotransmitter release in the SC. Additionally, C2230 lowers calcium responses in the parabrachial nucleus, a critical area for pain processing, and alleviates adverse reactions to mechanical stimuli following neuropathic injury [184].
Recent research has highlighted the role of CaVα2δ-1 auxiliary subunit ligands as targets for treating neuropathic pain. Gabapentin (GBP) and pregabalin, first- and second-generation CaVα2δ-1 ligands, have been shown to effectively alleviate the symptoms of this condition [185,186,187]. These drugs work by preferentially binding to the CaVα2δ-1 and CaVα2δ-2 subunits of CaV channels [188], modulating calcium influx, and reducing the release of excitatory neurotransmitters, which contributes to their analgesic effects [189,190].
GBP has been shown to inhibit calcium entry through CaV channels by acting directly on the CaVα2δ subunit [188]. Findings suggest that GBP’s ability to downregulate CaV2.2 channels contributes to its analgesic effects, particularly in the management of neuropathic pain, by reducing the release of neurotransmitters at nerve terminals. GBP’s mechanism of action was initially proposed by our research group and is based on the premise that the drug enters cells through an L amino acid transport system and interacts with the subunit of the channels inside the cell, disrupting (or preventing) the association of CaVα2δ-1 with the channel complex. This results in inadequate trafficking of the channels to the plasma membrane [191].
Subsequent studies provided experimental support for this mechanism, demonstrating that the VWA domain of CaVα2δ subunits plays a critical role in the intracellular trafficking of CaV channels. Furthermore, these studies revealed that interaction with GBP can disrupt the normal functioning of this domain [192]. Additionally, it has been found that pregabalin can affect the trafficking of CaVα2δ-1 to the presynaptic terminals of DRG neurons, likely using a mechanism similar to that of GBP, thereby reducing calcium entry and neurotransmitter release in the SC [144]. Finally, it has also been shown that some derivatives of γ-aminobutyric acid (GABA), which exhibit anticonvulsant and antinociceptive properties, relate to their ability to bind to the CaVα2δ subunit of CaV2.2 channels, similar to the mechanism observed with GBP and pregabalin [193,194].
The novel CaVα2δ ligand mirogabalin represents a third-generation option aimed explicitly at peripheral neuropathic pain and shows promising clinical applications [195,196]. NVA1309, another next-generation ligand, may offer a greater efficacy with fewer side effects than existing treatments [195,197]. NVA1309 is a gabapentinoid that binds to the CaVα2δ-1 subunit at a specific site (R243), which is also utilized by other gabapentinoids like pregabalin. NVA1309 and mirogabalin inhibit CaV2.2 currents in vitro and reduce CaV2.2 expression in the plasma membrane more effectively than pregabalin [195].
Therefore, auxiliary subunits of calcium channels, particularly CaVα2δ, represent promising targets for treating neuropathic pain due to their significant role in regulating calcium channel activity and neuronal excitability. By targeting the auxiliary subunits instead of the main CaVα1 channel subunit, therapies with fewer side effects than traditional channel blockers can be created. This approach may lead to treatment options that are better tolerated by patients with chronic pain.
CaV3 channel blockers, such as ethosuximide, have shown the ability to improve symptoms of neuropathic pain in animals with nerve injury. However, clinical studies have been inconclusive due to their low patient effectiveness. Similar observations have been made with small molecules that block CaV3.2 channels, such as ABT-639, TTA-P2, TTA-A2, and Z944, which have not produced relevant results despite their promising start in preclinical studies [99,198,199,200,201,202]. Additionally, cannabinoids have shown effectiveness in alleviating neuropathic pain by inhibiting CaV3 channels and increasing potassium currents through BK channels. An intrathecal injection of the CB1/CB2 receptor agonist NMP-7 has been shown to inhibit neuropathic pain induced by nerve injury in animal models through mechanisms involving CB2 receptors and CaV3.2 channels [201,203].
Interestingly, some natural compounds have shown the ability to alleviate inflammatory and neuropathic pain through their dual inhibitory action on CaV channels. Specifically, recent studies have documented that Icariside II, a prenyl-flavonol derived from the traditional Chinese herb epimedium, may have a beneficial effect on neuropathic pain by inhibiting CaV3 channels and disrupting the interaction between USP5 and CaV3.2 channels [204]. Furthermore, Icariside II reduces the excitability of sensory neurons and, in models of neuropathic pain, significantly decreases mechanical allodynia and thermal hyperalgesia, indicating its analgesic potential [204].
Likewise, a novel therapeutic strategy for neuropathic pain involves the synthetic peptide RD2, which selectively inhibits CaV2.2 channels when administered orally. RD2 competes with ziconotide for binding to these channels at nanomolar concentrations, demonstrating a high selectivity compared to other CaV channel subtypes. In preclinical studies, RD2 significantly alleviated mechanical allodynia in rats with sciatic nerve inflammatory neuritis [205]. Unlike ziconotide, which requires intrathecal delivery and is associated with logistical challenges and side effects, RD2 offers the advantage of oral administration. However, despite its promising selectivity in vitro, RD2’s long-term effects on other systems remain uncertain, as CaV2.2 channels are implicated in essential physiological processes like neurotransmission. Furthermore, efficacy data are currently limited to rodent models, with no evidence yet from primate or human studies. These factors highlight ongoing challenges in ensuring the safety and specificity of RD2 for clinical applications.
Recently, Colecraft’s research group developed an ingenious maneuver that could increase the arsenal of molecular tools available for treating neuropathic pain. Specifically, these authors showed that the targeted ubiquitination of CaV channels in sensory neurons may reduce neuropathic pain [206,207]. The system selectively ubiquitinated CaV channels in sensory neurons, reducing their number at the cell membrane and decreasing current density. The CaV-aβlator system consists of a molecule designed to post-translationally reduce the number of HVA-type CaV channels in the membrane by specifically targeting CaVβ accessory subunits. It comprises a nanobody that indiscriminately binds to all four CaVβ isoforms and is fused to the HECT catalytic domain of the E3 ubiquitin ligase Nedd4L. This fusion promotes the ubiquitination of both CaVα1 and CaVβ subunits, removing the channel complex from the cell membrane, thus resulting in the potent inhibition of calcium currents in various cell types [206,207].
This targeted reduction in the functional expression of CaV channels decreases neuropathic-pain-associated behaviors in animal models, showing a potential new avenue for pain therapy [207,208]. This study provides evidence that manipulating the post-translational modification, such as ubiquitination, of voltage-gated ion channels may be an effective strategy for controlling neuropathic pain, providing an alternative to current pharmacological approaches, which often have significant side effects or a limited efficacy.
The CaV-aβlator strategy acts as a potent and selective inhibitor that reduces the number of CaV channels on the cell surface, especially CaV2.2 (N-type), which are key for neurotransmitter release in pain pathways and an essential target for treating neuropathic pain. This is achieved through the targeted ubiquitination of CaVβ subunits, resulting in channel internalization and degradation. Thus, CaV-aβlator enables the precise control of these calcium channel functions and holds significant therapeutic potential for cardiovascular and neurological diseases, where these channels play a pivotal role.
4. Voltage-Gated Potassium (KV) Channels in Neuropathic Pain
4.1. Structure and Function of KV Channels
KV channels are classified into three major structural families (Figure 6A). Members of the first family correspond to the inward rectifier (Kir) channels that follow the structural pattern of the KcsA channel. This primitive channel consists of a tetramer formed by four identical subunits containing two transmembrane domains connected by a pore region, where the ion selectivity filter resides [209,210].
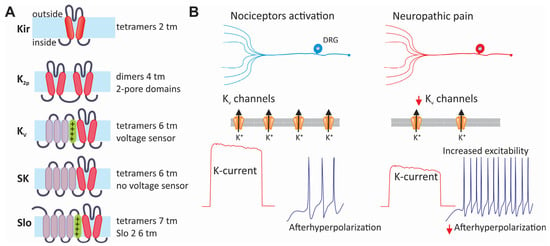
Figure 6.
Classification and contribution of KV channels to neuropathic pain. (A) The family of potassium channels arranged according to the structure of their main α subunits. The members of this family can be grouped into those formed by tetramers of two (Kir) or dimers of four (two pores) transmembrane segments. Likewise, channels formed by six transmembrane segments, the predominant voltage-sensitive potassium channels, assemble into a tetramer to form a functional channel. The same is also valid for the small-conductance calcium-activated potassium (SK) channels and the large-conductance channels activated by both changes in intracellular calcium and membrane voltage. (B) The expression of KV channels is often decreased in neuropathic pain. This decreases the outward current, which usually helps to stabilize the membrane potential and opposes excitatory signals. The decreased activity of KV channels during neuropathic pain may cause an increase in neuronal excitability, affecting the frequency and duration of APs.
In mammals, Kir channels are encoded by 15 genes grouped into seven subfamilies [211]. On the other hand, members of the second family of potassium channels are formed by two pores (K2p) and four transmembrane segments; unlike the other families, their subunits assemble as dimers. Fifteen genes of this family have been found in mammals [211,212,213]. The third family of potassium channels comprises six transmembrane segments and a single pore-forming domain for ion conduction (α-subunit), including the subfamily of voltage-gated channels (KV1 to KV4). The KV1 subfamily is the largest, with eight different genes. Like NaV and CaV channels, these channels contain a voltage sensor domain, where the fourth segment (S4) contains an array of positively charged amino acids that function as voltage-sensing elements. This also includes the KV7 (KCNQ), KV10 (ether-a-go-go), KV11 (erg), and KV12.2 (elk) subfamilies [211] (Figure 6A).
Interestingly, the KV5, KV6, KV8, and KV9 channels do not form functional channels even though they share the same general structure as the other members of the KV family. For this reason, these proteins have been called silent subunits (KVS). However, by forming heterotetrameric channels with the KV2α and KV3α subunits, they can modulate their biophysical properties and inhibit their functional expression [211,214]. This family of six transmembrane segments includes the small-conductance calcium-activated potassium channels (SKCa) and the Slo channel subfamily [215]. Though the structure of Slo channels is similar to that of KV channels, the α subunits of Slo1 and Slo3 have seven transmembrane domains instead of six. Furthermore, the α subunits of Slo channels have a large C-terminal domain [216].
KV channels participate in multiple functions and are expressed in all eukaryotic cells. These channels determine the resting membrane potential in most cells and are fundamental components of the electrical activity of the cell membrane in virtually all tissues. Additionally, they help to determine the shape, duration, and frequency of APs in excitable cells. The function of voltage-gated potassium channels (KV) in excitable cells can often be inferred from their subunit composition, which determines their biophysical properties and interactions with second messengers, as well as their spatial and temporal expression and regulation in pathophysiological processes [211,217].
4.2. The Role of KV Channels in Neuropathic Pain
Research on mutant mice lacking specific subunits of KV channels has highlighted their direct role in nociceptive circuits, enhancing our understanding of KV channel subunits in healthy and diseased conditions [217,218]. KV channels typically counteract membrane depolarization that activates NaV and CaV channels, thereby reducing the excitability of sensory neurons. However, a decrease in KV channel activity is associated with hyperexcitability in various pain syndromes, including traumatic injuries and painful diabetic neuropathy (Figure 6B). The KV1.1/KV1.2 subunits influence the AP threshold and firing frequency in primary afferent fibers and can be affected by nerve injuries [217]. In contrast, the KV2.1/KV2.2 subunits are recruited slower, primarily impacting repolarization and AP firing frequency. High-threshold KV3 channels limit AP duration and neurotransmitter release at central terminals. Moreover, the downregulation of KV4.3 channels after peripheral axotomy contributes to mechanical hypersensitivity. At the same time, inflammatory mediators in chronic pain states can modify the functional expression of KV7.2/KV7.3 channels, increasing the excitability of DRG neurons [217,218].
Interestingly, the KV9.1 subunit has been postulated as a predictor of neuropathic pain. Studies showing the downregulation of its expression after nerve injury have confirmed its relevance [217,218,219]. KV9.1 belongs to the KVS subfamily, which only conducts currents with other subunits like KV2.1, a high-threshold channel distinguished by its slow activation and inactivation kinetics [220]. These characteristics suggest that its effect becomes more significant in the later phases of the AP and is enhanced by prolonged stimulation.
Spinal nerve ligation reduces the expression of KV1.2 channels in DRG neurons, presumably by affecting the expression of the enzyme Tet methylcytosine dioxygenase 1 (TET1). In line with this, the overexpression of the enzyme rescues the deficient expression of the channels by reducing methylation in the promoter of the KCNA2 gene [221]. Furthermore, histone deacetylase 2 (HDAC2) controlled the expression of KV1.2 in sensory neurons in an animal model of chronic nerve constriction [222]. Finally, the microRNA miR-137 is known to regulate the function of KV1.2 channels, while its silencing rescues the expression and function of these channels, reducing allodynia in neuropathic animals [223].
Although spinal nerve ligation reduces mRNA levels for KV1.2 [224,225], changes in the functional expression of these channels could reflect post-translational processes such as phosphorylation or intracellular trafficking, independent of changes in gene expression [224]. This idea is supported by the observation that currents passing through delayed rectifier potassium channels are reduced in DRG neurons without being accompanied by changes in mRNA levels for KV1 and KV2 channels [226].
A proposed mechanism for the involvement of KV2.1 channels in neuropathic pain is decreased functional expression. Indeed, research has found reductions in neuronal KV2.1 expression following nerve damage [218,227,228,229]. On the other hand, electrophysiological recordings in DRG neurons have shown that the inhibition of KV2.1 channels affects posthyperpolarization following the firing of APs, modifying the refractory period between spikes. Therefore, it has been postulated that, in neuropathic pain, alterations in the expression of these channels may affect AP propagation along the axon during repeated firing [218,219,227,229]. Indeed, the excitability of sensory neurons increases after a blockade of KV2.1 channels, allowing for a higher firing rate. This suggests that KV2.1 channels may function as a brake on neuronal excitability, which is significantly altered under neuropathic pain conditions.
An alternative possibility to reduce the activity of KV2.1 channels that occurs in neuropathic pain is through the deregulation of KVS subunits, such as KV9.1, which participates in the formation of the functional tetrameric channel [227,230]; KV9.1 is the only KVS subunit implicated in the pathophysiology of neuropathic pain. In animal models, it shows a significant and rapid downregulation in DRGs after nerve injury, which correlates with the presence of the pain phenotype [218,227]. Likewise, the inhibition of KV9.1 with intrathecally applied siRNAs recapitulates mechanical allodynia phenotypes. KV9.1 silencing results in lower firing thresholds and shortening after hyperpolarization in DRG neurons, a phenotype reminiscent of the inhibition of KV2.1 channels [218]. These data suggest that the alteration in KV2.1 channel activity during neuropathic pain signaling is due, at least in part, to a loss of its functional interaction with KV9.1.
KV4 channels are also expressed in sensory neurons of the DRG [231]. Electrophysiological and molecular studies using antisense probes implicate the KV4.1 and KV4.3 channels as the molecular correlates of A-type potassium currents in DRG neurons [232]. The function and expression of these channels are regulated by various signaling pathways, accessory subunits such as KV4-channel-interacting proteins (KChIPs), and transcription factors such as restrictive neuron silencing factor (REST) [233,234], which, interestingly, suppresses the transcription of the KCND3 gene encoding KV4.3 channels after nerve injury [235]. The downregulation of mRNA for KV4.3 channels and their membrane expression occurs in DRG neurons in several nerve injury models, implicating the dysfunction of these channels in neuropathic pain [217].
Furthermore, the regulatory subunits KChIP1, KChIP2, and DPP10 form a complex with KV4.3 channels in DRG neurons, and spinal nerve ligation is known to downregulate the expression of components of this molecular complex. Conversely, the overexpression of KV4.3, KChIP1, and DPP10 is accompanied by the attenuation of nerve-ligation-induced mechanical hypersensitivity and the partial recovery of membrane levels of the complex members in injured DRGs. These data show that potassium channel modulatory subunits participate in developing KV4.3-mediated neuropathic pain [236].
Likewise, a current called M that flows through KV channels also contributes to the regulation of pain pathways. This low-threshold non-inactivating current is regulated by the activation of muscarinic receptors. The M current, like other potassium currents, regulates membrane potential and, consequently, neuronal excitability [218,237,238]. This current arises from the activity of homomeric KV7.2 channels, also known as KCNQ2, or heteromeric channels formed by the combination of KV7.2 and KV7.3, also called KCNQ3 [239,240,241]. It is also known that sensory neurons in the DRG express both types of channels, and that they also display M currents upon electrophysiological examination [242].
Furthermore, it has been reported that the expression of the KV7.2 and KV7.3 channels decreases in neuropathic pain models due to nerve constriction, and that, in consequence, the M current is reduced in sensory neurons of the DRG [243,244]. Molecular studies on the mechanisms associated with the downregulation of these channels in response to nerve injury show that the genes encoding KV7 channels have binding sites for repressor element 1 (NRSE) and that the effects of nerve ligation are linked to the transcription factor REST, whose expression increases in sensory neurons in response to nerve injury [244,245,246].
4.3. KV Channels in Neuropathic Pain Therapy
Although KV1.2 channels have been identified as molecular actors in the pathophysiology of neuropathic pain as regulators of neuronal excitability, they have not yet been used as a therapeutic target. However, there is experimental evidence that restoring normal levels of KV1.2 in DRGs and dorsal horn of the SC decreases pain in neuropathic animals, indicating its potential as a therapeutic target [223]. Similarly, KV4 channels have not yet been targeted for clinical treatments for neuropathic pain. Still, they are considered as promising candidates for the future development of analgesic drugs because, in experimental studies, rescuing the negative regulation of these channels has shown potential to relieve pain in animal models.
Since there are no KV4 channel activators, targeting auxiliary subunits is presented as a promising therapeutic alternative. These proteins not only regulate the activity of KV4 channels, but also intervene in their assembly and transport to the cell membrane. Interestingly, in this context, the compound NS5806 has been shown to potentiate currents through KV4 channels in a ChiP2-dependent manner, in addition to attenuating allodynia in a neuropathic pain model [247,248].
On the other hand, some studies have documented the residual expression of KV2.1 after the pain phenotype has been established, which could be therapeutically exploited by activators of these channels to increase their conductance. Indeed, retigabine, a positive allosteric modulator of KV2–KV5 channels, increases these currents and reduces nerve transmission through Aδ and C fibers to the dorsal horn of the SC [242], in addition to producing the hyperpolarization of primary afferent fibers [249]. As a consequence of these effects, it has been suggested that retigabine could exert an analgesic action in some models of neuropathic pain [238,242,250].
It should be noted that retigabine can also increase M currents besides acting on KV2 channels. In this case, the compound causes a shift in the KV7 channel conductance–voltage curve in the hyperpolarizing direction, which is associated with an increase in the channel’s opening time and a significant decrease in its deactivation kinetics [251,252,253]. However, due to its side effects, clinical use of retigabine has been discontinued. A therapeutic alternative has emerged with flupirtine, a structural analogue of retigabine. Flupirtine also shares this mechanism of action, acting on KV7 channels, although it has also been shown to enhance analgesia mediated by GABAA receptors [254,255,256].
5. Voltage-Gated Chloride and Proton Channels
5.1. Cl− Channels and Nociception
The excitability of primary afferent neurons has been traditionally associated with cation fluxes across the plasma membrane, key determinants in the generation and propagation of action potentials, as well as in their ability to respond to tissue-damaging stimuli. However, recent research has highlighted the role of chloride (Cl−) homeostasis and its fluxes across the cell membrane in nociception. In particular, these processes are gaining relevance due to their involvement in the development and maintenance of neuropathic pain. Recent studies suggest the implication of alterations in chloride channel activity and Cl− homeostasis in primary afferent nociceptors [257].
It is worth mentioning that the intracellular concentration of Cl− ions is regulated by transmembrane transporters, such as the Na+-K+-2Cl− cotransporter 1 (NKCC1) and K+-Cl− cotransporter 2 (KCC2). The deregulation of these transporters under pathological conditions can lead to an increase in intracellular chloride levels, which can facilitate the depolarization of nociceptive neurons and increase pain signaling [257]. The changes caused in chloride homeostasis and the equilibrium potential of the ion by the deregulation of membrane transporters can be so drastic that they can cause the neurotransmitter GABA acting on its extrasynaptic receptors to have a depolarizing and, therefore, pronociceptive effect [258].
Therefore, most studies focus on the role of chloride homeostasis and other chloride channels, such as calcium-activated or ligand-gated channels and GABAA receptors, respectively, and the NKCC1 and KCC2 transporters, in modulating nociceptor excitability and pain perception. However, this exciting topic falls outside the scope of this review, as it is centered on voltage-gated channels.
Likewise, the ClC-2 channel, a specific type of voltage-gated chloride channel, has been associated with nociception due to its altered expression and activity in certain pain states beyond neuropathic pain. This channel belongs to a large family of proteins encoded by nine different genes in mammals. Based on sequence homology, these channels can be grouped into three branches. The first branch comprises five channels expressed in the cell membrane and corresponds to the ClC-0, ClC-1, ClC-2, ClC-Ka, and ClC-Kb channels. In contrast, the proteins encoded by the other two branches reside predominantly in intracellular membranes [259].
ClC-2 channels exhibit a symmetrical homodimeric structure, with each subunit containing an independent pathway for ion permeation. The transmembrane domain generates two chloride-conducting pores with an independent gating mechanism. The activation of ClC-2 channels occurs through electrostatic and steric repulsion when intracellular chloride ions occupy the pore, inducing conformational changes in the residues responsible for gating. Unlike most ClC family homologs, ClC-2 is activated by hyperpolarization rather than depolarization, making it unique in its physiological roles [259]. However, the chloride equilibrium potential may be altered under neuropathic pain conditions [257]. As a result, the activation of these channels could depolarize the cell membrane and increase its excitability, a mechanism similar to that observed with extrasynaptic GABAA receptors [258].
Although the direct role of the ClC-2 channel in neuropathic pain is yet to be determined, it could play a key role in regulating synaptic inhibition by controlling chloride gradients at the spinal level in the nervous system [260,261]. These gradients directly affect GABAergic neurotransmission and, therefore, contribute to the pathophysiology of neuropathic pain.
5.2. Proton Channels and Neuropathic Pain
While voltage-gated H⁺ channels (particularly the Hv1 subtype) have been implicated in the pathogenesis of neuropathic pain through microglial regulation and inflammatory signaling, their mechanistic roles remain poorly characterized. Emerging evidence indicates that Hv1 mediates nociceptive signaling by amplifying microglial reactive oxygen species production and potentiating the release of proinflammatory cytokines in the central nervous system.
Voltage-gated H⁺ channels allow for the flow of protons across the cell membrane in response to changes in electrical potential and play important roles in various cellular functions. When activated during membrane depolarization, they allow proton efflux, thus helping to maintain the acid–base balance within cells. Furthermore, Hv1 proton channels may directly influence neuronal excitability by conducting ions that depolarize the neuronal membrane. As mentioned above, they may also indirectly modulate excitability through their effects on ROS production, pH regulation, injury, and microglial activity.
The molecular structure of Hv1 channels differs from other voltage-gated ion channels due to their unique composition and function [262]. These channels consist solely of a voltage-sensing domain (VSD) composed of four transmembrane helices (S1–S4) and an additional amphipathic helix (S0) at the N-terminus. They are composed of two subunits, each with its own proton permeability pathway within the VSD [262,263]. The S4 helix in the VSD is crucial for sensing changes in transmembrane voltage. It undergoes outward displacement in response to depolarization, which changes the internal salt bridge network and reconfigures the proton permeability pathway [262]. The two subunits of Hv1 channels interact during the channel opening process, showing a positive cooperativity that modulates the voltage response of the two permeation pathways [264]. Furthermore, Hv1 channels exhibit unidirectional conductance, allowing protons to exit the cell but not enter it, which is essential for maintaining intracellular pH homeostasis.
The involvement of ROS in the development of neuropathic pain is reinforced by the fact that, following spinal cord injury, a large proportion of patients develop neuropathic pain [265]. Furthermore, NOX2-derived ROS in microglia have been associated with neuropathic pain induced by nerve injury [266]. It is also known that, in response to peripheral nerve injury, macrophages are recruited by DRGs and increase ROS production through a NOX2-dependent mechanism. Interestingly, NOX2−/− mice display a reduced neuropathic pain phenotype [267]. Given the established link between Hv1–NOX and NOX2 and the development of neuropathic pain, it was initially suggested that microglial Hv1 channels could initiate and maintain neuropathic pain after spinal cord injury [268].
Indeed, Hv1 channels are functionally expressed in spinal cord microglia and show significant upregulation following peripheral nerve injury [269]. Furthermore, the activation of these channels contributes to the onset of neuropathic pain by favoring the production of reactive oxygen species (ROS). This production of ROS is associated with astrocyte activation, which worsens pain sensitivity [269]. Likewise, Hv1-null mice display decreased pain sensitivity after nerve injury compared to wild-type mice. Together these data suggest that Hv1 channels are relevant in mediating pain responses through microglial and astrocytic interactions.
6. Voltage-Gated Ion Channel Dysregulation in Supraspinal Pathways
Pain transmission involves complex neural circuits that run from the periphery to the brain. While spinal mechanisms are crucial for the initial processing of nociceptive signals, supraspinal pathways, predominantly cortical and brainstem circuits, are essential for the integration, modulation, and conscious perception of pain.
The cortex plays a central role in pain perception, integrating sensory, emotional, and cognitive aspects. Key regions include the somatosensory cortices (S1 and S2), which are responsible for the sensory-discriminative aspects of pain, such as location and intensity. S1 receives nociceptive inputs from the thalamus, while S2 integrates information from multiple body regions. The insula and anterior cingulate cortex (ACC) provide pain’s affective and emotional dimensions. The insula contributes to the subjective experience, while the ACC is related to emotional and motivational responses. The prefrontal cortex participates in cognitive pain evaluation, including attention and modulation. Its dysfunction is associated with chronic pain.
The brainstem bridges cortical centers and spinal circuits, modulating pain through several structures, including the periaqueductal gray matter that integrates descending pain control signals, producing analgesia by inhibiting nociceptive neurons. The rostral ventromedial nucleus of the medulla oblongata contains neurons that facilitate and impede pain, exerting bidirectional control. Finally, the parabrachial area involves pain’s affective and motivational aspects and contributes to the transition from acute to chronic pain.
While alterations in peripheral voltage-gated ion channels predominate in initiating neuropathic pain, supraspinal mechanisms may maintain pain through central sensitization and altered descending control. However, direct evidence for the involvement of voltage-gated ion channels in supraspinal pathways is scarce and contrasts with their well-documented contribution to peripheral and spinal mechanisms. In this context, it is worth noting that neuropathic pain may involve important changes in neuronal excitability and synaptic transmission along the peripheral and supraspinal pathways. Indeed, a key event in the development of neuropathic pain is central sensitization, which involves alterations in neuronal excitability and synaptic transmission. Though the involvement of voltage-gated ion channels has not been directly documented in the supraspinal context, their contribution to neuronal excitability suggests that they may be indirectly involved in neuropathic pain, as discussed below.
It has been documented that NaV1.3 channels, especially in the thalamus, can generate and maintain neuropathic pain due to their role in central hyperexcitability [14,201]. Particularly, after a spinal cord injury, NaV1.3 channel expression increases significantly in thalamic neurons, which is associated with increased spontaneous neuronal activity contributing to pain generation. Furthermore, it has been observed that spinal cord injuries can trigger supraspinal changes in NaV channel expression in thalamic neurons [14,37,270].
Specifically, four weeks after injury, immunostaining for NaV1.3 channels was significantly increased in neurons of the ventral posterolateral nucleus of the thalamus. Electrophysiological recordings from neurons in this region in SCI animals showed a high rate of spontaneous activity, independent of ascending afferent input. Interestingly, antisense oligonucleotides targeting NaV1.3 channel messengers reduced their expression in the thalamus and reversed the increase in spontaneous activity. Furthermore, the exacerbated spontaneous activity persisted even after complete spinal cord transection, indicating that afferent input is not essential for maintaining thalamic hyperexcitability, suggesting that this region may function as an intrinsic pain generator [37,270] and supporting the finding that NaV1.3 overexpression in the post-spinal cord injury thalamus contributes to spontaneous neuronal activity and neuropathic pain. Although further research is needed to fully understand NaV channels’ contributions to neuropathic pain, their involvement in central sensitization and hyperexcitability suggests a potential role in the molecular mechanisms of the condition.
On the other hand, T-type calcium channels, especially those of the CaV3.2 class, are not only involved in neuropathic pain at the peripheral level, but also appear to play a role at the supraspinal level. Specifically, it has been found that CaV3.2 channels are expressed in GABAergic neurons and contribute to high-frequency firing activity in the reticular thalamic nucleus and anterior pretectum (APT), a region involved in pain perception [162,271]. This activity is increased in animal models of neuropathic pain, while the specific elimination of CaV3.2 channels in APT neurons reduces mechanical allodynia.
Likewise, the upregulation of T-type channels in the anterior cingulate cortex (ACC) has been shown to alleviate neuropathic pain [272]. After chronic nerve constriction injury, the upregulation of CaV3.2 channels has been observed in the ACC of rats, suggesting that these channels may be involved in the development of neuropathic pain. These findings are associated with a significant increase in T-type calcium currents, which results in increased neuronal activity in the ACC. Furthermore, the T-type channel inhibitor NNC 55-0396 reduced the frequency of postsynaptic excitatory synaptic currents (mEPSCs) and reduced neuronal firing frequency in the ACC [272]. Finally, a microinjection of the T-channel inhibitor into the ACC partially alleviated mechanical and thermal allodynia in rats with neuropathic pain. Taken together, these data suggest that T channels, particularly those of the CaV3.2 class, could contribute to neuronal hyperexcitability in this region and, thus, participate in the development of neuropathic pain.
Finally, like NaV and CaV channels, KV channels in supraspinal pathways are less well-characterized than their spinal and peripheral counterparts. While further research is needed to clarify their role in specific brain regions, some evidence supports their possible involvement in neuropathic pain through cellular mechanisms such as thalamocortical hyperexcitability and microglial activation.
KV7 channels regulate neuronal excitability in the thalamus and the ACC, among other brain regions [273,274]. Furthermore, alterations in the functional expression of these proteins at the supraspinal level are recognized as contributing to central sensitization, a central feature of neuropathic pain. As mentioned earlier, retigabine and flupirtine, KV7 channel activators, effectively prevent neuropathic pain in animal models by reducing neuronal excitability [229,255,256]. Thus, by activating KV7 channels, these drugs reduce the heightened neuronal activity associated with the condition, suggesting a possible role for these channels in pain processing at the supraspinal level.
Likewise, KV1.3 channels in spinal cord microglia are implicated in neuroinflammation linked to neuropathic pain [275]. Studies in animal models show that neuropathic pain is associated with an increased expression of M1 (proinflammatory) and M2 (anti-inflammatory) microglial phenotypes in the spinal cord, along with elevated levels of NLRP3 inflammasome components (NLRP3, caspase-1, and IL-1β), markers of neuroinflammation. The inhibition of KV1.3 with PAP-1 reduced hyperalgesia, M1 polarization, and the expression of NLRP3, caspase-1, and IL-1β, suggesting that KV1.3 channels may play a key role in neuropathic pain by promoting M1 microglial polarization and activating the NLRP3 inflammasome [275].
In this context, the scorpion-venom-derived peptide αKtx12 has been investigated for its neuroprotective potential by acting as a selective KV1.3 channel blocker [276]. Studies show that αKtx12 increases the viability of SH-SY5Y neuroblastoma cells and promotes hippocampal pyramidal cell proliferation in animal models. Furthermore, the peptide reduces microglial activation and the production of proinflammatory cytokines, generating a neuroprotective environment. Although this mechanism is spinal, similar KV1.3-mediated activation could occur in supraspinal regions such as the periaqueductal gray, even though direct corroborating evidence is still lacking. These findings suggest that KV1.3 inhibition by αKtx12 might be considered an alternative for treating neuropathic pain associated with nervous system injuries.
7. Genetic Defects in Voltage-Gated Ion Channels Associated with Neuropathic Pain
Although neuropathic pain is defined as an event caused by nerve disease or injury, a few channelopathies could fit this framework, because they represent a form of nerve dysfunction at the molecular level. Therefore, some mutations in voltage-gated ion channels, specifically NaV channels, which alter the normal electrical properties of nerves, can cause neuronal hyperexcitability and generate pain signals, even without apparent structural nerve damage. Therefore, in these cases, the underlying “lesion” is genetic and functional rather than anatomical.
As mentioned earlier, genetic alterations in NaV channels may cause neuropathic pain. These mutations disrupt channel function, leading to the hyperexcitability of nociceptive neurons and contributing to various painful conditions. For example, gain-of-function mutations exist in SCN9A and SCN10A, which encode the NaV1.7 and NaV1.8 channels, respectively [277,278,279,280]. These mutations are associated with inherited erythromelalgia (IEM) and paroxysmal extreme pain disorder (PEPD), both characterized by severe spontaneous pain due to the hyperexcitability of nociceptive neurons [277,279,281,282,283].
NaV1.7 mutations associated with IME cause a hyperpolarizing shift in activation and slower channel deactivation. These changes lower the threshold for generating action potentials in sensory neurons, increasing their excitability [279,284,285]. Furthermore, the mutations enhance the response of NaV1.7 to slow depolarizations, amplifying small-input signals close to the resting potential. Because NaV1.7 is primarily expressed in small sensory and nociceptive neurons, these alterations contribute to the chronic pain experienced by IME patients [279].
Generally speaking, these mutations alter the biophysical properties of NaV channels, resulting in a reduction in the activation threshold, with a consequent increase in neuronal excitability and the spontaneous firing of nociceptive neurons. These changes give rise to characteristic symptoms of neuropathic pain, such as spontaneous pain, hyperalgesia, and allodynia. Understanding these genetic defects provides insight into the molecular basis of neuropathic pain and guides the development of targeted therapies, such as selective Nav channel inhibitors.
On the other hand, genetic defects in CaV and KV channels linked explicitly to neuropathic pain, without association with different types of pain, are not well defined. Although these channels play a fundamental role in neurotransmitter release and neuronal excitability and alterations in their function are implicated in chronic and neuropathic pain, most evidence points to their involvement in a wide spectrum of painful conditions, and not exclusively in neuropathic pain. Thus, although CaV and KV channel dysfunction is mechanistically crucial in neuropathic pain, it has not been established that any specific genetic alteration in these channels causes neuropathic pain independently of other pain or neurological conditions.
8. Neuropathic Pain Trials: Testing Ion-Channel-Targeting Drugs
The landscape of clinical trials for voltage-gated ion channel antagonists or blockers for the treatment of neuropathic pain, although rapidly expanding, is currently still limited. The clinical trial pipeline is growing for NaV channels, with several pharmaceutical companies developing candidates targeting NaV1.7 and NaV1.8 subtypes, which are critical in pain transmission. In particular, NaV1.8 channel blockers have shown potential, as NaV1.8 is primarily expressed in peripheral sensory neurons involved in pain transmission and is less likely to cause side effects in the central nervous system [286]. A partial blockade of NaV1.8 has been shown to reduce neuronal hyperexcitability significantly in models of chronic neuropathic pain. As previously mentioned, one notable compound is VX-548, a selective NaV1.8 blocker approved by the FDA for moderate to severe acute pain, being studied for its potential in neuropathic pain [73,74]. It offers an alternative to opioids, with a favorable side effect profile and no addictive potential.
On the other hand, NaV1.7 channel inhibitors have faced challenges regarding selectivity and efficacy, though research continues on these compounds as promising non-opioid treatments for neuropathic pain. Some trials have shown promising results; however, most candidates have failed in advanced phases due to efficacy issues and study design discrepancies [287]. One such trial specifically focused on vixotrigine (BIIB074) for treating trigeminal neuralgia [286,288]. However, its Phase II trial for neuropathic pain was discontinued [286]. Therefore, many research teams and companies are prioritizing NaV1.8 inhibitors, following the stalling of NaV1.7 trials.
There are also ongoing clinical trials of CaV channel blockers or antagonists for treating neuropathic pain, specifically targeting CaV2.2 (N-type) channels. Thus, the novel compound C2230, which potently and selectively blocks these channels [184], has been documented to have analgesic effects in multiple pain models, including neuropathic pain, through systemic and intranasal administration without generating tolerance or adverse behavioral effects. This compound is considered to be a promising candidate for further clinical development [289]. Other CaV2.2 channel blockers, such as RD2, have shown efficacy in animal models of neuropathic pain and a good tolerability in healthy human volunteers, reinforcing the potential for targeting these channels [205,289,290]. In addition to CaV2.2 channels, CaV3 channel blockers, such as ABT-639 [291], have also been studied in clinical trials for diabetic peripheral neuropathic pain. However, more recent reviews and data indicate that ABT-639 and other similar T-type channel blockers have not advanced further in clinical development for neuropathic pain due to their lack of efficacy [292].
Furthermore, KV channel activators, particularly targeting KV7 subtypes (KCNQ), are under active preclinical and clinical investigation for neuropathic pain. Novel compounds such as SCR2682 and advances in chemical optimization highlight the potential of this approach to deliver novel non-opioid therapies for chronic neuropathic pain. This compound potentiates M currents in dorsal root ganglion neurons and reduces nerve-injury-induced pain in animal models, showing potential as a therapeutic tool [293,294]. Research also focuses on overcoming toxicity and improving selectivity by modifying compounds like retigabine. The modulation of KV channels, especially KV7, remains a promising strategy for treating neuropathic pain, with ongoing efforts to develop safer and more effective activators. Other KV7 activators, such as retigabine, approved for epilepsy but limited by side effects, and newer compounds, such as ASP0819, have been explored in preclinical studies and early clinical trials to improve selectivity and reduce toxicity in pain management [201].
Likewise, BHV-7000 is a late-stage drug candidate that selectively activates KV7.2/7.3 potassium channels, primarily developed for epilepsy and mood disorders, with ongoing exploration for pain treatment [295]. Its clinical development highlights its potential to regulate neuronal hyperexcitability with improved safety and tolerability profiles. Interestingly, gabapentin, widely used for neuropathic pain due to its binding to the CaVα2δ auxiliary subunit of HVA-type CaV channels, has also been found to activate KV7 channels [296], suggesting that part of its analgesic effect may involve KV channel modulation.
9. Challenges and Future Directions
9.1. Key Hurdles in Ion Channel Research for Neuropathic Pain
There are still significant challenges in investigating voltage-gated ion channels in neuropathic pain. The first of these is related to the complexity of the disease. Neuropathic pain is a multifactorial and heterogeneous condition with diverse etiologies and multiple cellular and molecular mechanisms. Although NaV channels are the most studied, their dysfunction is detected in <20% of patients, suggesting that other ion channels and mechanisms are also involved. This complexity hampers the identification of universal molecular targets and the development of effective therapies.
Second, there is a great diversity of ion channels. As detailed throughout this manuscript, multiple types of voltage-gated ion channels, each with distinct expression patterns and functions, are implicated in the pathophysiology of neuropathic pain. The redundancy and overlap among these channels complicate efforts to identify the most relevant candidates and design selective modulators without off-target effects.
There is also a significant translational gap between animal models and humans. Many of the findings on the involvement of voltage-gated ion channels in neuropathic pain come from animal models, which may not fully replicate the human disease. Differences in ion channel expression and regulation may limit the translation of preclinical findings into effective clinical treatments.
On the other hand, nonselective ion channel blockers can be effective in animal models, but they often have a limited clinical efficacy and cause adverse effects. Therefore, developing more selective compounds that target peripheral channels without central or cardiac impacts remains a significant challenge. Likewise, following nerve injury, the functional expression of voltage-gated ion channels can change dynamically, influenced by inflammatory mediators and neuronal activity. Understanding these temporal and spatial changes is crucial for producing effective therapeutic responses.
Lastly, the proteins that form voltage-gated ion channel complexes are targets of post-translational modifications such as phosphorylation, ubiquitination, SUMOylation, and glycosylation, which affect their activity, intracellular trafficking, and half-life. Studying how these processes are affected during the development of neuropathic pain would be a topic of interest for future studies, especially considering its therapeutic potential (see below). Furthermore, it would be of great interest to expand our knowledge of how the release of cytokines and chemokines during neuroinflammation can affect the function and expression of ion channels and how these processes can contribute to the molecular pathophysiology of neuropathic pain. Likewise, studying how DNA methylation or histone acetylation and other epigenetic modifications can affect the expression patterns of ion channels is another topic of great relevance, since these modifications can affect pain perception and promote the development of neuropathic pain.
9.2. Small Molecules Versus Biologics in the Future of Voltage-Gated Ion Channel Therapies in Neuropathic Pain
Small molecules remain the primary and most promising approach to targeting voltage-gated ion channels in neuropathic pain. Recent advances include highly selective inhibitors for NaV and CaV channels, with successful clinical trials generating significant momentum for this approach. Small molecules offer advantages such as oral bioavailability, ease of synthesis, and the ability to modulate channel activity or trafficking in a reversible and tunable manner.
Innovative small-molecule strategies are emerging, such as targeted protein degradation (PROTACs), which can selectively degrade specific ion channel subtypes and potentially offer longer-lasting effects [206,207,208,297]. Furthermore, small molecules are being developed to disrupt protein–protein interactions critical to channel function and trafficking, expanding the scope of therapeutic mechanisms. PROTACs in the ion channel field represent a novel strategy to modulate ion channel function by targeting the channel proteins rather than through traditional inhibition, potentially offering increased specificity and overcoming drug resistance mechanisms. They are small, heterobifunctional molecules designed to selectively degrade target proteins by exploiting the cell’s ubiquitin–proteasome system (UPS). They are composed of two ligands connected by a linker—one ligand binds to the protein of interest, and the other recruits an E3 ubiquitin ligase. This proximity induces the ubiquitination of the target protein, marking it for degradation by the 26S proteasome, effectively reducing the cellular levels of the protein rather than simply inhibiting its function [206,207,208].
NaV1.7 and NaV1.8 are also susceptible to PROTAC-mediated targeted protein degradation. Engineered PROTACs have been used to induce the rapid and near-complete degradation of these channels in vitro, revealing that NaV channels can be targeted intracellularly by PROTACs for degradation via the proteasome rather than the lysosomal pathway.
Indeed, developing new therapeutic strategies for neuropathic pain has also found a promising target in the NaV1.7 and NaV1.8 channels. Recent research has shown the feasibility of selectively degrading these channels using small molecules to induce targeted protein degradation. To achieve this, degron-labeled systems, such as dTAG, fused to NaV1.7 and NaV1.8 channels have been used, allowing for the evaluation of the efficacy of different PROTACs that recruit E3 ligases to label the protein targeted for degradation [298]. Chimeras have been designed with degron tags at both the C- and N-termini of the channels, observing that the C-terminal location favors more efficient degradation. The recruitment of E3 ligases such as CRBN and VHL effectively mediates degradation, showing vigorous activity for NaV1.8. Furthermore, degradation is proteasome-dependent, as it is blocked by specific inhibitors such as MG132, but not by lysosomal inhibitors [298].
These findings represent the first experimental evidence for the small-molecule-directed degradation of ion channels, providing a significant framework for the pharmacology of NaV channels related to neuropathic pain. The therapeutic potential of these findings is considerable, as the precise control of NaV channel abundance in preclinical models using these techniques complements existing genetic tools and will pave the way for the development of novel non-opioid analgesics.
On the other hand, biologics, including peptide toxins, have demonstrated efficacy in preclinical models and some clinical trials, especially for calcium channels. These agents can offer specificity but often face administration route, stability, and production challenges. Although they represent innovative tools and can inspire new drug designs, their widespread adoption is limited compared to small molecules.
Given the complexity of neuropathic pain, future advances could also come from rational combination therapies, using small molecules, biologics, or both to target multiple pathways simultaneously. Specifically, integrating molecular engineering techniques, mechanistic analysis, and pharmacology opens new avenues for the selective modulation of key proteins involved in pain molecular pathophysiology, with a potentially transformative impact on the medical management of neuropathic pain.
Author Contributions
Conceptualization, R.F., A.C.-L. and A.S.; resources, R.F. and A.S.; writing—original draft preparation, R.F., A.C.-L. and A.S.; writing—review and editing, R.F., A.C.-L. and A.S.; funding acquisition, R.F. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Secretaria de Educación, Ciencia y Tecnología de la Ciudad de México, grant number SECTEI/146/2024” awarded to R.F., and “Programa de Apoyo a Proyectos de Investigación e innivación Tecnológica PAPIIT of the National Autonomous University of México (UNAM), grant number IN211524” awarded to A.S. A.C.L. was supported by UNAM Posdoctoral Program (POSDOC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef]
- St John Smith, E. Advances in understanding nociception and neuropathic pain. J. Neurol. 2018, 265, 231–238. [Google Scholar] [CrossRef]
- Bagal, S.K.; Marron, B.E.; Owen, R.M.; Storer, R.I.; Swain, N.A. Voltage-gated sodium channels as drug discovery targets. Channels 2015, 9, 360–366. [Google Scholar] [CrossRef]
- Catterall, W.A.; Lenaeus, M.J.; Gamal El-Din, T.M. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 133–154. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated sodium and calcium channels: Discovery, structure, function, and pharmacology. Channels 2023, 17, 2281714. [Google Scholar] [CrossRef]
- Namadurai, S.; Yereddi, N.R.; Cusdin, F.S.; Huang, C.L.; Chirgadze, D.Y.; Jackson, A.P. A new look at sodium channel β subunits. Open Biol. 2015, 5, 140192. [Google Scholar] [CrossRef]
- O’Malley, H.A.; Isom, L.L. Sodium channel β subunits: Emerging targets in channelopathies. Annu. Rev. Physiol. 2015, 77, 481–504. [Google Scholar] [CrossRef]
- Chahine, M.; O’Leary, M.E. Regulatory role of voltage-gated Na channel β subunits in sensory neurons. Front. Pharmacol. 2011, 2, 70. [Google Scholar] [CrossRef]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Lampert, A.; O’Reilly, A.O.; Reeh, P.; Leffler, A. Sodium channelopathies and pain. Pflügers Arch. 2010, 460, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.; McMahon, S.B. The physiological function of different voltage-gated sodium channels in pain. Nat. Rev. Neurosci. 2021, 22, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.L.H.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The role of voltage-gated sodium channels in pain signaling. Physiol. Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef]
- Comini, M.; Themistocleous, A.C.; Bennett, D.L.H. Human pain channelopathies. Handb. Clin. Neurol. 2024, 203, 89–109. [Google Scholar]
- Zhang, J.M.; Donnelly, D.F.; Song, X.J.; Lamotte, R.H. Axotomy increases the excitability of dorsal root ganglion cells with unmyelinated axons. J. Neurophysiol. 1997, 78, 2790–2794. [Google Scholar] [CrossRef]
- Devor, M. Sodium channels and mechanisms of neuropathic pain. J. Pain 2006, 7 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef]
- Liu, C.N.; Michaelis, M.; Amir, R.; Devor, M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: Relation to neuropathic pain. J. Neurophysiol. 2000, 84, 205–215. [Google Scholar] [CrossRef]
- Amir, R.; Michaelis, M.; Devor, M. Burst discharge in primary sensory neurons: Triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J. Neurosci. 2002, 22, 1187–1198. [Google Scholar] [CrossRef]
- Gold, M.S.; Weinreich, D.; Kim, C.S.; Wang, R.; Treanor, J.; Porreca, F.; Lai, J. Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. J. Neurosci. 2003, 23, 158–166. [Google Scholar] [CrossRef]
- Gracely, R.H.; Lynch, S.A.; Bennett, G.J. Painful neuropathy: Altered central processing maintained dynamically by peripheral input. Pain 1992, 51, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Bao, L. Trafficking regulates the subcellular distribution of voltage-gated sodium channels in primary sensory neurons. Mol. Pain 2015, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S. NaV1.7 and NaV.8: Role in the pathophysiology of pain. Mol. Pain 2019, 15, 1744806919858801. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Black, J.A.; Waxman, S.G. Voltage-gated sodium channels: Therapeutic targets for pain. Pain Med. 2009, 10, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, A.A.; Cummins, T.R.; Dib-Hajj, S.D.; Hormuzdiar, W.; Tyrrell, L.; Waxman, S.G.; Black, J.A. Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root neurons after sciatic nerve injury but not rhizotomy. J. Neurosci. 2000, 20, 7279–7289. [Google Scholar] [CrossRef]
- Yin, R.; Liu, D.; Chhoa, M.; Li, C.M.; Luo, Y.; Zhang, M.; Lehto, S.G.; Immke, D.C.; Moyer, B.D. Voltage-gated sodium channel function and expression in injured and uninjured rat dorsal root ganglia neurons. Int. J. Neurosci. 2016, 126, 182–192. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Black, J.A.; Waxman, S.G. NaV1.9: A sodium channel linked to human pain. Nat. Rev. Neurosci. 2015, 16, 511–519. [Google Scholar] [CrossRef]
- Pineda-Farias, J.B.; Loeza-Alcocer, E.; Nagarajan, V.; Gold, M.S.; Sekula, R.F., Jr. Mechanisms underlying the selective therapeutic efficacy of carbamazepine for attenuation of trigeminal nerve injury pain. J. Neurosci. 2021, 41, 8991–9007. [Google Scholar] [CrossRef]
- Wang, W.; Atianjoh, F.; Gauda, E.B.; Yaster, M.; Li, Y.; Tao, Y.X. Increased expression of sodium channel subunit NaV1.1 in the injured dorsal root ganglion after peripheral nerve injury. Anat. Rec. 2011, 294, 1406–1411. [Google Scholar] [CrossRef]
- Cummins, T.R.; Waxman, S.G. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J. Neurosci. 1997, 17, 3503–3514. [Google Scholar] [CrossRef]
- Waxman, S.G.; Kocsis, J.D.; Black, J.A. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons and is reexpressed following axotomy. J. Neurophysiol. 1994, 72, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Fjell, J.; Cummins, T.R.; Zheng, Z.; Fried, K.; LaMotte, R.; Black, J.A.; Waxman, S.G. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain 1999, 83, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Oh, Y.; Chung, J.M.; Chung, K. The changes in expression of three subtypes of TTX sensitive sodium channels in sensory neurons after spinal nerve ligation. Brain Res. Mol. Brain Res. 2001, 95, 153–161. [Google Scholar] [CrossRef]
- Black, J.A.; Cummins, T.R.; Plumpton, C.; Chen, Y.H.; Hormuzdiar, W.; Clare, J.J.; Waxman, S.G. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J. Neurophysiol. 1999, 82, 2776–2785. [Google Scholar] [CrossRef]
- Casals-Díaz, L.; Casas, C.; Navarro, X. Changes of voltage-gated sodium channels in sensory nerve regeneration and neuropathic pain models. Restor. Neurol. Neurosci. 2015, 33, 321–334. [Google Scholar] [CrossRef]
- Liao, S.; Liu, T.; Yang, R.; Tan, W.; Gu, J.; Deng, M. Structure and function of sodium channel NaV1.3 in neurological disorders. Cell. Mol. Neurobiol. 2023, 43, 575–584. [Google Scholar] [CrossRef]
- Hains, B.C.; Klein, J.P.; Saab, C.Y.; Craner, M.J.; Black, J.A.; Waxman, S.G. Upregulation of sodium channel NaV1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 2003, 23, 8881–8892. [Google Scholar] [CrossRef]
- Nassar, M.A.; Baker, M.D.; Levato, A.; Ingram, R.; Mallucci, G.; McMahon, S.B.; Wood, J.N. Nerve injury induces robust allodynia and ectopic discharges in NaV1.3 null mutant mice. Mol. Pain 2006, 22, 33. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wickenden, A.D.; Chaplan, S.R. Sodium channel blockers for the treatment of neuropathic pain. Neurotherapeutics 2009, 66, 663–678. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, L.; Zhu, F.; Xia, W.; Wen, T.; Xia, R.; Yu, X.; Xu, D.; Peng, C. Ectopic expression of NaV1.7 in spinal dorsal horn neurons induced by NGF contributes to neuropathic pain in a mouse spinal cord injury model. Front. Mol. Neurosci. 2023, 16, 1091096. [Google Scholar] [CrossRef]
- Alsaloum, M.; Labau, J.I.R.; Liu, S.; Estacion, M.; Zhao, P.; Dib-Hajj, F.; Waxman, S.G. Contributions of NaV1.8 and NaV1.9 to excitability in human induced pluripotent stem-cell derived somatosensory neurons. Sci. Rep. 2021, 11, 24283. [Google Scholar] [CrossRef] [PubMed]
- Kan, P.; Zhu, Y.F.; Ma, J.; Singh, G. Computational modeling to study the impact of changes in NaV1.8 sodium channel on neuropathic pain. Front. Comput. Neurosci. 2024, 18, 1327986. [Google Scholar] [CrossRef] [PubMed]
- Vasylyev, D.V.; Zhao, P.; Schulman, B.R.; Waxman, S.G. Interplay of NaV1.8 and NaV1.7 channels drives neuronal hyperexcitability in neuropathic pain. J. Gen. Physiol. 2024, 156, e202413596. [Google Scholar] [CrossRef] [PubMed]
- Thakor, D.K.; Lin, A.; Matsuka, Y.; Meyer, E.M.; Ruangsri, S.; Nishimura, I.; Spigelman, I. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol. Pain 2009, 5, 14. [Google Scholar] [CrossRef]
- Lai, J.; Porreca, F.; Hunter, J.C.; Gold, M.S. Voltage-gated sodium channels and hyperalgesia. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 371–397. [Google Scholar] [CrossRef]
- Blackburn-Munro, G.; Fleetwood-Walker, S.M. The sodium channel auxiliary subunits beta1 and β2 are differentially expressed in the spinal cord of neuropathic rats. Neuroscience 1999, 90, 153–164. [Google Scholar] [CrossRef]
- Calhoun, J.D.; Isom, L.L. The role of non-pore-forming β subunits in physiology and pathophysiology of voltage-gated sodium channels. Handb. Exp. Pharmacol. 2014, 221, 51–89. [Google Scholar]
- Pertin, M.; Ji, R.R.; Berta, T.; Powell, A.J.; Karchewski, L.; Tate, S.N.; Isom, L.L.; Woolf, C.J.; Gilliard, N.; Spahn, D.R.; et al. Upregulation of the voltage-gated sodium channel β2 subunit in neuropathic pain models: Characterization of expression in injured and non-injured primary sensory neurons. J. Neurosci. 2005, 25, 10970–10980. [Google Scholar] [CrossRef]
- Lopez-Santiago, L.F.; Pertin, M.; Morisod, X.; Chen, C.; Hong, S.; Wiley, J.; Decosterd, I.; Isom, L.L. Sodium channel β2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J. Neurosci. 2006, 26, 7984–7994. [Google Scholar] [CrossRef]
- Shah, B.S.; Stevens, E.B.; Gonzalez, M.I.; Bramwell, S.; Pinnock, R.D.; Lee, K.; Dixon, A.K. β3, a novel auxiliary subunit for the voltage-gated sodium channel, is expressed preferentially in sensory neurons and is upregulated in the chronic constriction injury model of neuropathic pain. Eur. J. Neurosci. 2000, 12, 3985–3990. [Google Scholar] [CrossRef]
- Takahashi, N.; Kikuchi, S.; Dai, Y.; Kobayashi, K.; Fukuoka, T.; Noguchi, K. Expression of auxiliary β subunits of sodium channels in primary afferent neurons and the effect of nerve injury. Neuroscience 2003, 121, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.R.; Aglieco, F.; Renganathan, M.; Herzog, R.I.; Dib-Hajj, S.D.; Waxman, S.G. NaV1.3 sodium channels: Rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J. Neurosci. 2001, 21, 5952–5961. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Kobayashi, K.; Yamanaka, H.; Obata, K.; Dai, Y.; Noguchi, K. Comparative study of the distribution of the α-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J. Comp. Neurol. 2008, 510, 188–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; O’Leary, M.E.; Chahine, M. Regulation of NaV1.6 and NaV1.8 peripheral nerve Na+ channels by auxiliary β-subunits. J. Neurophysiol. 2011, 106, 608–619. [Google Scholar] [CrossRef][Green Version]
- Chen, C.; Westenbroek, R.E.; Xu, X.; Edwards, C.A.; Sorenson, D.R.; Chen, Y.; McEwen, D.P.; O’Malley, H.A.; Bharucha, V.; Meadows, L.S.; et al. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J. Neurosci. 2004, 24, 4030–4042. [Google Scholar] [CrossRef]
- Lopez-Santiago, L.F.; Brackenbury, W.J.; Chen, C.; Isom, L.L. Na+ channel Scn1b gene regulates dorsal root ganglion nociceptor excitability in vivo. J. Biol. Chem. 2011, 286, 22913–22923. [Google Scholar] [CrossRef]
- Ma, R.S.Y.; Kayani, K.; Whyte-Oshodi, D.; Whyte-Oshodi, A.; Nachiappan, N.; Gnanarajah, S.; Mohammed, R. Voltage gated sodium channels as therapeutic targets for chronic pain. J. Pain Res. 2019, 12, 2709–2722. [Google Scholar] [CrossRef]
- Eagles, D.A.; Chow, C.Y.; King, G.F. Fifteen years of NaV1.7 channels as an analgesic target: Why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy? Br. J. Pharmacol. 2022, 179, 3592–3611. [Google Scholar] [CrossRef]
- London, C.; Hoyt, S.B.; Parsons, W.H.; Williams, B.S.; Warren, V.A.; Tschirret-Guth, R.; Smith, M.M.; Priest, B.T.; McGowan, E.; Martin, W.J.; et al. Imidazopyridines: A novel class of hNaV1.7 channel blockers. Bioorg. Med. Chem. Lett. 2008, 18, 1696–1701. [Google Scholar] [CrossRef]
- Hoyt, S.B.; London, C.; Ok, H.; Gonzalez, E.; Duffy, J.L.; Abbadie, C.; Dean, B.; Felix, J.P.; Garcia, M.L.; Jochnowitz, N.; et al. Benzazepinone NaV1.7 blockers: Potential treatments for neuropathic pain. Bioorg. Med. Chem. Lett. 2007, 17, 6172–6177. [Google Scholar] [CrossRef]
- Theile, J.W.; Cummins, T.R. Recent developments regarding voltage-gated sodium channel blockers for the treatment of inherited and acquired neuropathic pain syndromes. Front. Pharmacol. 2011, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Schmalhofer, W.A.; Calhoun, J.; Burrows, R.; Bailey, T.; Kohler, M.G.; Weinglass, A.B.; Kaczorowski, G.J.; Garcia, M.L.; Koltzenburg, M.; Priest, B.T. ProTx-II: A selective inhibitor of NaV1.7 sodium channels blocks action potential propagation in nociceptors. Mol. Pharmacol. 2008, 74, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Tyagarajan, S.; Chakravarty, P.K.; Zhou, B.; Taylor, B.; Fisher, M.H.; Wyvratt, M.J.; Lyons, K.; Klatt, T.; Li, X.; Kumar, S.; et al. Substituted biaryl pyrazoles as sodium channel blockers. Bioorg. Med. Chem. Lett. 2010, 20, 5480–5483. [Google Scholar] [CrossRef]
- Minett, M.S.; Pereira, V.; Sikandar, S. Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel NaV1.7. Nat. Commun. 2015, 6, 8967. [Google Scholar] [CrossRef]
- Emery, E.C.; Luiz, A.P.; Wood, J.N. NaV1.7 and other voltage-gated sodium channels as drug targets for pain relief. Expert Opin. Ther. Targets 2016, 20, 975–983. [Google Scholar] [CrossRef]
- Dustrude, E.T.; Perez-Miller, S.; François-Moutal, L.; Moutal, A.; Khanna, M.; Khanna, R. A single structurally conserved SUMOylation site in CRMP2 controls NaV1.7 function. Channels 2017, 11, 316–328. [Google Scholar] [CrossRef]
- François-Moutal, L.; Dustrude, E.T.; Wang, Y.; Brustovetsky, T.; Dorame, A.; Ju, W.; Moutal, A.; Perez-Miller, S.; Brustovetsky, N.; Gokhale, V.; et al. Inhibition of the Ubc9 E2 SUMO-conjugating enzyme-CRMP2 interaction decreases NaV1.7 currents and reverses experimental neuropathic pain. Pain 2018, 159, 2115–2127. [Google Scholar] [CrossRef]
- Gomez, K.; Stratton, H.J.; Duran, P.; Loya, S.; Tang, C.; Calderon-Rivera, A.; François-Moutal, L.; Khanna, M.; Madura, C.L.; Luo, S.; et al. Identification and targeting of a unique NaV1.7 domain driving chronic pain. Proc. Natl. Acad. Sci. USA 2023, 120, e2217800120. [Google Scholar] [CrossRef]
- Hestehave, S.; Allen, H.N.; Gomez, K.; Duran, P.; Calderon-Rivera, A.; Loya-López, S.; Rodríguez-Palma, E.J.; Khanna, R. Small molecule targeting NaV1.7 via inhibition of CRMP2-Ubc9 interaction reduces pain-related outcomes in a rodent osteoarthritic model. Pain 2025, 166, 99–111. [Google Scholar] [CrossRef]
- Jarvis, M.F.; Honore, P.; Shieh, C.C.; Chapman, M.; Joshi, S.; Zhang, X.F.; Kort, M.; Carroll, W.; Marron, B.; Atkinson, R.; et al. A-803467: A potent and selective NaV1.8 sodium channel blocker attenuates neuropathic and inflammatory pain in the rat. Proc. Natl. Acad. Sci. USA 2007, 104, 8520–8525. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shieh, C.C.; Chapman, M.L.; Matulenko, M.A.; Hakeem, A.H.; Atkinson, R.N.; Kort, M.E.; Marron, B.E.; Joshi, S.; Honore, P.; et al. A-887826 is a structurally novel, potent and voltage-dependent NaV1.8 sodium channel blocker that attenuates neuropathic tactile allodynia in rats. Neuropharmacology 2010, 59, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Zhang, H.B.; Bean, B.P. Use-dependent relief of inhibition of NaV1.8 channels by A-887826. Mol. Pharmacol. 2023, 103, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lyu, D.; Gao, J. Suzetrigine: The first Nav1.8 inhibitor approved for the treatment of moderate to severe acute pain. Drug Discov. Ther. 2025, 19, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Osteen, J.D.; Immani, S.; Tapley, T.L.; Indersmitten, T.; Hurst, N.W.; Healey, T.; Aertgeerts, K.; Negulescu, P.A.; Lechner, S.M. Pharmacology and Mechanism of Action of Suzetrigine, a Potent and Selective NaV1.8 Pain Signal Inhibitor for the Treatment of Moderate to Severe Pain. Pain Ther. 2025, 14, 655–674. [Google Scholar] [CrossRef]
- Vaelli, P.; Fujita, A.; Jo, S.; Zhang, H.B.; Osorno, T.; Ma, X.; Bean, B.P. State-Dependent Inhibition of Nav1.8 Sodium Channels by VX-150 and VX-548. Mol. Pharmacol. 2024, 106, 298–308. [Google Scholar] [CrossRef]
- Jo, S.; Fujita, A.; Osorno, T.; Stewart, R.G.; Vaelli, P.M.; Bean, B.P. Differential state-dependent Nav1.8 inhibition by suzetrigine, LTGO-33, and A-887826. J. Gen. Physiol. 2025, 157, e202413719. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, Z.; Xu, Y.; Zhang, Y.; Tang, D.; Wu, X.; Tang, C.; Chen, M.; Shi, X.; Chen, P.; et al. Electrophysiological and pharmacological analyses of Nav1.9 voltage-gated sodium channel by establishing a heterologous expression system. Front. Pharmacol. 2017, 8, 852. [Google Scholar] [CrossRef]
- Bosmans, F.; Puopolo, M.; Martin-Eauclaire, M.F.; Bean, B.P.; Swartz, K.J. Functional properties and toxin pharmacology of a dorsal root ganglion sodium channel viewed through its voltage sensors. J. Gen. Physiol. 2011, 138, 59–72. [Google Scholar] [CrossRef]
- Felix, R. Molecular regulation of voltage-gated Ca2+ channels. J. Recept. Signal Transduct. Res. 2005, 25, 57–71. [Google Scholar] [CrossRef]
- Lacinová, L. Voltage-dependent calcium channels. Gen. Physiol. Biophys. 2005, 24, 1–78. [Google Scholar]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.W. Calcium channels in excitable cell membranes. Annu. Rev. Physiol. 1983, 45, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.W.; Fox, A.P.; Hess, P.; McCleskey, E.W.; Nilius, B.; Nowycky, M.C.; Rosenberg, R.L. Multiple types of calcium channel in excitable cells. Soc. Gen. Physiol. Ser. 1987, 41, 167–187. [Google Scholar]
- Dolphin, A.C. Voltage-gated calcium channels and their auxiliary subunits: Physiology, pathophysiology, and pharmacology. J. Physiol. 2016, 594, 5369–5390. [Google Scholar] [CrossRef]
- Buraei, Z.; Yang, J. Structure and function of the β subunit of voltage-gated Ca2+ channels. Biochim. Biophys. Acta 2013, 1828, 1530–1540. [Google Scholar] [CrossRef]
- Gandini, M.A.; Felix, R. Molecular and functional interplay of voltage-gated Ca2+ channels with the cytoskeleton. Curr. Mol. Pharmacol. 2015, 8, 69–80. [Google Scholar] [CrossRef]
- Calderón-Rivera, A.; Andrade, A.; Hernández-Hernández, O.; González-Ramírez, R.; Sandoval, A.; Rivera, M.; Gomora, J.C.; Felix, R. Identification of a disulfide bridge essential for structure and function of the voltage-gated Ca2+ channel α2δ-1 auxiliary subunit. Cell Calcium 2012, 51, 22–30. [Google Scholar] [CrossRef][Green Version]
- Obermair, G.J.; Tuluc, P.; Flucher, B.E. Auxiliary Ca2+ channel subunits: Lessons learned from muscle. Curr. Opin. Pharmacol. 2008, 8, 311–318. [Google Scholar] [CrossRef]
- Kang, M.G.; Chen, C.C.; Felix, R.; Letts, V.A.; Frankel, W.N.; Mori, Y.; Campbell, K.P. Biochemical and biophysical evidence for γ2 subunit association with neuronal voltage-activated Ca2+ channels. J. Biol. Chem. 2001, 276, 32917–32924. [Google Scholar] [CrossRef]
- Kang, M.G.; Campbell, K.P. Gamma subunit of voltage-activated calcium channels. J. Biol. Chem. 2003, 278, 21315–21318. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, A.; Andrade, A.; Beedle, A.M.; Campbell, K.P.; Felix, R. Inhibition of recombinant N-type CaV channels by the γ2 subunit involves unfolded protein response (UPR)-dependent and UPR-independent mechanisms. J. Neurosci. 2007, 27, 3317–3327. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Herrera, D.; Calderón-Rivera, A.; Zarco, N.; Corzo-Lopez, A.; Leyva-Leyva, M.; Monjaraz, E.; Sandoval, A.; Oviedo, N.; González-Ramírez, R.; Felix, R. Molecular cloning of the gene promoter encoding the human CaVγ2/Stargazin divergent transcript (CACNG2-DT): Characterization and regulation by the cAMP-PKA/CREB signaling pathway. Front. Physiol. 2023, 14, 1286808. [Google Scholar] [CrossRef]
- Payne, H.L. The role of transmembrane AMPA receptor regulatory proteins (TARPs) in neurotransmission and receptor trafficking (Review). Mol. Membr. Biol. 2008, 25, 353–362. [Google Scholar] [CrossRef]
- Bourinet, E.; Soong, T.W.; Sutton, K.; Slaymaker, S.; Mathews, E.; Monteil, A.; Zamponi, G.W.; Nargeot, J.; Snutch, T.P. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat. Neurosci. 1999, 2, 407–415. [Google Scholar] [CrossRef]
- Richards, K.S.; Swensen, A.M.; Lipscombe, D.; Bommert, K. Novel CaV2.1 clone replicates many properties of Purkinje cell CaV2.1 current. Eur. J. Neurosci. 2007, 26, 2950–2961. [Google Scholar] [CrossRef]
- Bourinet, E.; Francois, A.; Laffray, S. T-type calcium channels in neuropathic pain. Pain 2016, 157 (Suppl. S1), S15–S22. [Google Scholar] [CrossRef]
- Todorovic, S.M.; Jevtovic-Todorovic, V. Neuropathic pain: Role for presynaptic T-type channels in nociceptive signaling. Pflugers Arch. 2013, 465, 921–927. [Google Scholar] [CrossRef]
- Cai, S.; Gomez, K.; Moutal, A.; Khanna, R. Targeting T-type/CaV3.2 channels for chronic pain. Transl. Res. 2021, 234, 20–30. [Google Scholar] [CrossRef]
- Hoppanova, L.; Lacinova, L. Voltage-dependent CaV3.2 and CaV2.2 channels in nociceptive pathways. Pflugers Arch. 2022, 474, 421–434. [Google Scholar] [CrossRef]
- Gandini, M.A.; Zamponi, G.W. Voltage-gated calcium channel nanodomains: Molecular composition and function. FEBS J. 2022, 289, 614–633. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S. Presynaptic calcium channels. Int. J. Mol. Sci. 2019, 20, 2217. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.; Sandoval, A.; Barragán-Iglesias, P.; Granados-Soto, V.; Delgado-Lezama, R.; Felix, R.; González-Ramírez, R. Transcription factor Sp1 regulates the expression of calcium channel α2δ-1 subunit in neuropathic pain. Neuroscience 2019, 412, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Pogrel, J.W.; Yaksh, T.L. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J. Pharmacol. Exp. Ther. 1994, 269, 1117–1123. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sakashita, Y. Differential effects of intrathecally administered N- and P-type voltage-sensitive calcium channel blockers upon two models of experimental mononeuropathy in the rat. Brain Res. 1998, 794, 329–332. [Google Scholar] [CrossRef]
- White, D.M.; Cousins, M.J. Effect of subcutaneous administration of calcium channel blockers on nerve injury-induced hyperalgesia. Brain Res. 1998, 801, 50–58. [Google Scholar] [CrossRef]
- Knight, Y.E.; Bartsch, T.; Kaube, H.; Goadsby, P.J. P/Q-type calcium-channel blockade in the periaqueductal gray facilitates trigeminal nociception: A functional genetic link for migraine? J. Neurosci. 2002, 22, RC213. [Google Scholar] [CrossRef]
- Urban, M.O.; Ren, K.; Sablad, M.; Park, K.T. Medullary N-type and P/Q-type calcium channels contribute to neuropathy-induced allodynia. Neuroreport 2005, 16, 563–566. [Google Scholar] [CrossRef]
- Luvisetto, S.; Marinelli, S.; Panasiti, M.S.; D’Amato, F.R.; Fletcher, C.F.; Pavone, F.; Pietrobon, D. Pain sensitivity in mice lacking the CaV2.1 α1 subunit of P/Q-type Ca2+ channels. Neuroscience 2006, 142, 823–832. [Google Scholar] [CrossRef]
- Park, J.; Luo, Z.D. Calcium channel functions in pain processing. Channels 2010, 4, 510–517. [Google Scholar] [CrossRef]
- Snutch, T.P. Targeting chronic and neuropathic pain: The N-type calcium channel comes of age. NeuroRx 2005, 2, 662–670. [Google Scholar] [CrossRef]
- Tedford, H.W.; Zamponi, G.W. Direct G protein modulation of CaV2 calcium channels. Pharmacol. Rev. 2006, 58, 837–862. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, H.; Kurihara, T.; Zong, S.; Kazuno, A.; Matsuda, Y.; Nonaka, T.; Han, W.; Toriyama, H.; Tanabe, T. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001, 20, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jun, K.; Lee, T.; Kim, S.S.; McEnery, M.W.; Chin, H.; Kim, H.L.; Park, J.M.; Kim, D.K.; Jung, S.J.; et al. Altered nociceptive response in mice deficient in the β1B subunit of the voltage-dependent calcium channel. Mol. Cell. Neurosci. 2001, 18, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xie, M.X.; Hu, L.; Wang, X.F.; Mai, J.Z.; Li, Y.Y.; Wu, N.; Zhang, C.; Li, J.; Pang, R.P.; et al. Upregulation of N-type calcium channels in the soma of uninjured dorsal root ganglion neurons contributes to neuropathic pain by increasing neuronal excitability following peripheral nerve injury. Brain Behav. Immun. 2018, 71, 52–65. [Google Scholar] [CrossRef]
- Bell, T.J.; Thaler, C.; Castiglioni, A.J.; Helton, T.D.; Lipscombe, D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron 2004, 41, 127–138. [Google Scholar] [CrossRef]
- Castiglioni, A.J.; Raingo, J.; Lipscombe, D. Alternative splicing in the C-terminus of CaV2.2 controls expression and gating of N-type calcium channels. J. Physiol. 2006, 576, 119–134. [Google Scholar] [CrossRef]
- Altier, C.; Dale, C.S.; Kisilevsky, A.E.; Chapman, K.; Castiglioni, A.J.; Matthews, E.A.; Evans, R.M.; Dickenson, A.H.; Lipscombe, D.; Vergnolle, N.; et al. Differential role of N-type calcium channel splice isoforms in pain. J. Neurosci. 2007, 27, 6363–6373. [Google Scholar] [CrossRef]
- Raingo, J.; Castiglioni, A.J.; Lipscombe, D. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat. Neurosci. 2007, 10, 285–292. [Google Scholar] [CrossRef]
- Andrade, A.; Denome, S.; Jiang, Y.Q.; Marangoudakis, S.; Lipscombe, D. Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat. Neurosci. 2010, 13, 1249–1256. [Google Scholar] [CrossRef]
- Murali, S.S.; Napier, I.A.; Mohammadi, S.A.; Alewood, P.F.; Lewis, R.J.; Christie, M.J. High-voltage-activated calcium current subtypes in mouse DRG neurons adapt in a subpopulation-specific manner after nerve injury. J. Neurophysiol. 2015, 113, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Ogawa, K.; Minami, K.; Shinohara, S.; Kato, A. Injury-specific functional alteration of N-type voltage-gated calcium channels in synaptic transmission of primary afferent C-fibers in the rat spinal superficial dorsal horn. Eur. J. Pharmacol. 2016, 772, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Marsala, J.; Lukacova, N.; Marsala, M.; Jergova, S.; Orendacova, J.; Yaksh, T.L. Localization of N-type Ca2+ channels in the rat spinal cord following chronic constrictive nerve injury. Exp. Brain. Res. 2002, 147, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Ho, Y.C.; Hsieh, M.C.; Wang, H.H.; Cheng, J.K.; Chau, Y.P.; Peng, H.Y. Spinal Fbxo3-dependent Fbxl2 ubiquitination of active zone protein RIM1α mediates neuropathic allodynia through CaV2.2 activation. J. Neurosci. 2016, 36, 9722–9738. [Google Scholar] [CrossRef]
- Borgland, S.L.; Connor, M.; Christie, M.J. Nociceptin inhibits calcium channel currents in a subpopulation of small nociceptive trigeminal ganglion neurons in mouse. J. Physiol. 2001, 536 Pt 1, 35–47. [Google Scholar] [CrossRef]
- Beedle, A.M.; McRory, J.E.; Poirot, O.; Doering, C.J.; Altier, C.; Barrere, C.; Hamid, J.; Nargeot, J.; Bourinet, E.; Zamponi, G.W. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat. Neurosci. 2004, 7, 118–125. [Google Scholar] [CrossRef]
- Seseña, E.; Soto, E.; Bueno, J.; Vega, R. Nociceptin/orphanin FQ peptide receptor mediates inhibition of N-type calcium currents in vestibular afferent neurons of the rat. J. Neurophysiol. 2020, 124, 1605–1614. [Google Scholar] [CrossRef]
- Caminski, E.S.; Antunes, F.T.T.; Souza, I.A.; Dallegrave, E.; Zamponi, G.W. Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders. Mol. Brain 2022, 15, 95. [Google Scholar] [CrossRef]
- Altier, C.; Khosravani, H.; Evans, R.M.; Hameed, S.; Peloquin, J.B.; Vartian, B.A.; Chen, L.; Beedle, A.M.; Ferguson, S.S.; Mezghrani, A.; et al. ORL1 receptor-mediated internalization of N-type calcium channels. Nat. Neurosci. 2006, 9, 31–40. [Google Scholar] [CrossRef]
- Luo, Z.D.; Chaplan, S.R.; Higuera, E.S.; Sorkin, L.S.; Stauderman, K.A.; Williams, M.E.; Yaksh, T.L. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J. Neurosci. 2001, 21, 1868–1875. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, X.L.; Matthews, E.A.; Li, K.W.; Kurwa, A.; Boroujerdi, A.; Gross, J.; Gold, M.S. Calcium channel α2δ1 subunit mediates spinal hyperexcitability in pain modulation. Pain 2006, 125, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Li, K.W.; Yu, Y.P.; Zhou, C.; Kim, D.S.; Lin, B.; Sharp, K.; Steward, O.; Luo, Z.D. Calcium channel α2δ1 proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J. Biol. Chem. 2014, 289, 7025–7037. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Park, J.; Luo, Z.D. Injury-induced maladaptation and dysregulation of calcium channel α2δ subunit proteins and its contribution to neuropathic pain development. Br. J. Pharmacol. 2018, 175, 2231–2243. [Google Scholar] [CrossRef]
- Gurnett, C.A.; Felix, R.; Campbell, K.P. Extracellular interaction of the voltage-dependent Ca2+ channel α2δ and α1 subunits. J. Biol. Chem. 1997, 272, 18508–18512. [Google Scholar] [CrossRef]
- Felix, R.; Gurnett, C.A.; De Waard, M.; Campbell, K.P. Dissection of functional domains of the voltage-dependent Ca2+ channel α2δ subunit. J. Neurosci. 1997, 17, 6884–6891. [Google Scholar] [CrossRef]
- Andrade, A.; Sandoval, A.; Oviedo, N.; De Waard, M.; Elias, D.; Felix, R. Proteolytic cleavage of the voltage-gated Ca2+ channel α2δ subunit: Structural and functional features. Eur. J. Neurosci. 2007, 25, 1705–1710. [Google Scholar] [CrossRef]
- Andrade, A.; Sandoval, A.; González-Ramírez, R.; Lipscombe, D.; Campbell, K.P.; Felix, R. The α2δ subunit augments functional expression and modifies the pharmacology of CaV1.3 L-type channels. Cell Calcium 2009, 46, 282–292. [Google Scholar] [CrossRef]
- Dolphin, A.C.; Obermair, G.J. Regulation of calcium channels and synaptic function by auxiliary α2δ subunits. In Voltage-Gated Calcium Channels; Zamponi, G.W., Weiss, N., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Geisler, S.; Schöpf, C.L.; Obermair, G.J. Emerging evidence for specific neuronal functions of auxiliary calcium channel α2δ subunits. Gen. Physiol. Biophys. 2015, 34, 105–118. [Google Scholar] [CrossRef]
- Bikbaev, A.; Ciuraszkiewicz-Wojciech, A.; Heck, J.; Klatt, O.; Freund, R.; Mitlöhner, J.; Enrile Lacalle, S.; Sun, M.; Repetto, D.; Frischknecht, R.; et al. Auxiliary α2δ1 and α2δ3 subunits of calcium channels drive excitatory and inhibitory neuronal network development. J. Neurosci. 2020, 40, 4824–4841. [Google Scholar] [CrossRef]
- Li, C.Y.; Song, Y.H.; Higuera, E.S.; Luo, Z.D. Spinal dorsal horn calcium channel α2δ-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J. Neurosci. 2004, 24, 8494–8499. [Google Scholar] [CrossRef]
- Luo, Z.D.; Calcutt, N.A.; Higuera, E.S.; Valder, C.R.; Song, Y.H.; Svensson, C.I.; Myers, R.R. Injury type-specific calcium channel α2δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharmacol. Exp. Ther. 2002, 303, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.A.; Bingham, S.; Case, P.C.; Sanger, G.J.; Lawson, S.N. Dorsal root ganglion neurons show increased expression of the calcium channel α2δ-1 subunit following partial sciatic nerve injury. Brain Res. Mol. Brain Res. 2001, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.S.; Nieto-Rostro, M.; Rahman, W.; Tran-Van-Minh, A.; Ferron, L.; Douglas, L.; Kadurin, I.; Sri Ranjan, Y. The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by α2δ ligand pregabalin. J. Neurosci. 2009, 29, 4076–4088. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Rostro, M.; Patel, R.; Dickenson, A.H.; Dolphin, A.C. Nerve injury increases native CaV2.2 trafficking in dorsal root ganglion mechanoreceptors. Pain 2023, 164, 1264–1279. [Google Scholar] [CrossRef]
- Nieto-Rostro, M.; Ramgoolam, K.; Pratt, W.S.; Kulik, A.; Dolphin, A.C. Ablation of α2δ-1 inhibits cell-surface trafficking of endogenous N-type calcium channels in the pain pathway in vivo. Proc. Natl. Acad. Sci. USA 2018, 115, E12043–E12052. [Google Scholar] [CrossRef]
- Patel, R.; Bauer, C.S.; Nieto-Rostro, M.; Margas, W.; Ferron, L.; Chaggar, K.; Crews, K.; Ramirez, J.D.; Bennett, D.L.H.; Schwartz, A.; et al. The α2δ-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. J. Neurosci. 2013, 33, 16412–16426. [Google Scholar] [CrossRef]
- Margas, W.; Ferron, L.; Nieto-Rostro, M.; Schwartz, A.; Dolphin, A.C. Effect of knockout of α2δ-1 on action potentials in mouse sensory neurons. Philos. Trans. R. Soc. Lond B Biol. Sci. 2016, 371, 20150430. [Google Scholar] [CrossRef]
- Cui, W.; Wu, H.; Yu, X.; Song, T.; Xu, X.; Xu, F. The calcium channel α2δ1 subunit: Interactional targets in primary sensory neurons and role in neuropathic pain. Front. Cell. Neurosci. 2021, 15, 699731. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, Z.D. Electrophysiological characterization of spinal neuron sensitization by elevated calcium channel α2δ-1 subunit protein. Eur. J. Pain 2014, 18, 649–658. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, Z.D. Nerve injury-induced calcium channel α2δ-1 protein dysregulation leads to increased pre-synaptic excitatory input into deep dorsal horn neurons and neuropathic allodynia. Eur. J. Pain 2015, 19, 1267–1276. [Google Scholar] [CrossRef]
- Park, J.; Yu, Y.P.; Zhou, C.Y.; Li, K.W.; Wang, D.; Chang, E.; Kim, D.S. Central mechanisms mediating thrombospondin-4-induced pain states. J. Biol. Chem. 2016, 291, 13335–13348. [Google Scholar] [CrossRef] [PubMed]
- Li, K.W.; Kim, D.S.; Zaucke, F.; Luo, Z.D. Trigeminal nerve injury-induced thrombospondin-4 up-regulation contributes to orofacial neuropathic pain states in a rat model. Eur. J. Pain 2014, 18, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Guo, Y.; Wu, H.E.; Park, J.; Trinh, V.N.; Luo, Z.D.; Hogan, Q.H. Thrombospondin-4 divergently regulates voltage-gated Ca2+ channel subtypes in sensory neurons after nerve injury. Pain 2016, 157, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Li, K.W.; Boroujerdi, A.; Peter Yu, Y.; Zhou, C.Y.; Deng, P.; Park, J.; Zhang, X.; Lee, J.; Corpe, M.; et al. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J. Neurosci. 2012, 32, 8977–8987. [Google Scholar] [CrossRef]
- Boroujerdi, A.; Zeng, J.; Sharp, K.; Kim, D.; Steward, O.; Luo, D.Z. Calcium channel α2δ-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain 2011, 152, 649–655. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Chen, S.R.; Chen, H.; Xie, J.D.; Sirrieh, R.E.; MacLean, D.M.; Zhang, Y.; Zhou, M.H.; Jayaraman, V.; et al. The α2δ-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018, 22, 2307–2321. [Google Scholar] [CrossRef]
- Wu, T.; Chen, S.R.; Pan, H.L.; Luo, Y. The α2δ-1-NMDA receptor complex and its potential as a therapeutic target for ischemic stroke. Front. Neurol. 2023, 14, 1148697. [Google Scholar] [CrossRef]
- Deng, M.; Chen, S.R.; Pan, H.L. Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain. Cell. Mol. Life. Sci. 2019, 76, 1889–1899. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, E.; Gao, M.; Weng, H.R. Endogenous activation of presynaptic NMDA receptors enhances glutamate release from the primary afferents in the spinal dorsal horn in a rat model of neuropathic pain. J. Physiol. 2013, 591, 2001–2019. [Google Scholar] [CrossRef]
- Candelas, M.; Reynders, A.; Arango-Lievano, M.; Neumayer, C.; Fruquière, A.; Demes, E.; Hamid, J.; Lemmers, C.; Bernat, C.; Monteil, A.; et al. CaV3.2 T-type calcium channels shape electrical firing in mouse Lamina II neurons. Sci. Rep. 2019, 9, 3112. [Google Scholar] [CrossRef]
- Fayad, S.L.; Ourties, G.; Le Gac, B.; Jouffre, B.; Lamoine, S.; Fruquière, A.; Laffray, S.; Gasmi, L.; Cauli, B.; Mallet, C.; et al. Centrally expressed CaV3.2 T-type calcium channel is critical for the initiation and maintenance of neuropathic pain. eLife 2022, 11, e79018. [Google Scholar] [CrossRef] [PubMed]
- Bourinet, E.; Alloui, A.; Monteil, A.; Barrère, C.; Couette, B.; Poirot, O.; Pages, A.; McRory, J.; Snutch, T.P.; Eschalier, A.; et al. Silencing of the CaV3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005, 24, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Messinger, R.B.; Naik, A.K.; Jagodic, M.M.; Nelson, M.T.; Lee, W.Y.; Choe, W.J.; Orestes, P.; Latham, J.R.; Todorovic, S.M.; Jevtovic-Todorovic, V. In vivo silencing of the Ca(V)3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain 2009, 145, 184–195. [Google Scholar] [CrossRef]
- Gomez, K.; Calderón-Rivera, A.; Sandoval, A.; González-Ramírez, R.; Vargas-Parada, A.; Ojeda-Alonso, J.; Granados-Soto, V.; Delgado-Lezama, R.; Felix, R. Cdk5-dependent phosphorylation of CaV3.2 T-Type channels: Possible role in nerve ligation-induced neuropathic allodynia and the compound action potential in primary afferent C fibers. J. Neurosci. 2020, 40, 283–296. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Chen, W.; Cui, S.; Liao, F.F.; Yi, M.; Liu, F.Y.; Wan, Y. Upregulation of CaV3.2 T-type calcium channels in adjacent intact L4 dorsal root ganglion neurons in neuropathic pain rats with L5 spinal nerve ligation. Neurosci. Res. 2019, 142, 30–37. [Google Scholar] [CrossRef]
- Tomita, S.; Sekiguchi, F.; Kasanami, Y.; Naoe, K.; Tsubota, M.; Wake, H.; Nishibori, M.; Kawabata, A. CaV3.2 overexpression in L4 dorsal root ganglion neurons after cdk spinal nerve cutting involves Egr-1 USP5 and HMGB1 in rats: An emerging signaling pathway for neuropathic pain. Eur. J. Pharmacol. 2020, 888, 173587. [Google Scholar] [CrossRef]
- Gomez, K.; Vallecillo, T.G.M.; Moutal, A.; Perez-Miller, S.; Delgado-Lezama, R.; Felix, R.; Khanna, R. The role of cyclin-dependent kinase 5 in neuropathic pain. Pain 2020, 161, 2674–2689. [Google Scholar] [CrossRef]
- Gomez, K.; Vargas-Parada, A.; Duran, P.; Sandoval, A.; Delgado-Lezama, R.; Khanna, R.; Felix, R. L5-6 spinal nerve ligation-induced neuropathy changes the location and function of Ca2+ channels and Cdk5 and affects the compound action potential in adjacent intact L4 afferent fibers. Neuroscience 2021, 471, 20–31. [Google Scholar] [CrossRef]
- García-Caballero, A.; Gadotti, V.M.; Stemkowski, P.; Weiss, N.; Souza, I.A.; Hodgkinson, V.; Bladen, C.; Chen, L.; Hamid, J.; Pizzoccaro, A.; et al. The deubiquitinating enzyme USP5 modulates neuropathic and inflammatory pain by enhancing CaV3.2 channel activity. Neuron 2014, 83, 1144–1158. [Google Scholar] [CrossRef]
- Garcia-Caballero, A.; Gadotti, V.M.; Chen, L.; Zamponi, G.W. A cell-permeant peptide corresponding to the cUBP domain of USP5 reverses inflammatory and neuropathic pain. Mol. Pain 2016, 12, 1744806916642444. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Caballero, A.G.; Berger, N.D.; Gladding, C.M.; Chen, L.; Pfeifer, T.A.; Zamponi, G.W. Small organic molecule disruptors of CaV3.2-USP5 interactions reverse inflammatory and neuropathic pain. Mol. Pain 2015, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, H.; Schaible, H.-G. Effects of antagonists to high-threshold Ca channels upon spinal mechanisms of pain, hyperalgesia and allodynia. Pain 2000, 85, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.R.; Nicol, G.D.; Vasko, M.R. Differential regulation of evoked peptide release by voltage-sensitive calcium channels in rat sensory neurons. Brain Res. 1996, 712, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Gao, D.; Pettus, M.; Phillips, C.; Bowersox, S.S. Interactions of intrathecally administered ziconotide, a selective blocker of neuronal N-type voltage-sensitive Ca channels, with morphine on nociception in rats. Pain 2000, 84, 271–281. [Google Scholar] [CrossRef]
- Kolosov, A.; Aurini, L.; Williams, E.D.; Cooke, I.; Goodchild, C.S. Intravenous injection of leconotide, an omega conotoxin: Synergistic antihyperalgesic effects with morphine in a rat model of bone cancer pain. Pain Med. 2011, 12, 923–941. [Google Scholar] [CrossRef]
- Wilson, S.M.; Brittain, J.M.; Piekarz, A.D.; Ballard, C.J.; Ripsch, M.S.; Cummins, T.R.; Hurley, J.H.; Khanna, M.; Hammes, N.M.; Samuels, B.C.; et al. Further insights into the antinociceptive potential of a peptide disrupting the N-type calcium channel-CRMP-2 signaling complex. Channels 2011, 5, 449–456. [Google Scholar] [CrossRef]
- Wilson, S.M.; Schmutzler, B.S.; Brittain, J.M.; Dustrude, E.T.; Ripsch, M.S.; Pellman, J.J.; Yeum, T.S.; Hurley, J.H.; Hingtgen, C.M.; White, F.A.; et al. Inhibition of transmitter release and attenuation of anti-retroviral-associated and tibial nerve injury-related painful peripheral neuropathy by novel synthetic Ca2+ channel peptides. J. Biol. Chem. 2012, 287, 35065–35077. [Google Scholar] [CrossRef]
- Xie, J.Y.; Chea, L.A.; Yang, X.; Wang, Y.; Qu, C.; Wang, Y.; Federici, L.M.; Fitz, S.D.; Ripsch, M.S.; Que, M.R.; et al. Sustained relief of ongoing experimental neuropathic pain by a CRMP2 peptide aptamer with low abuse potential. Pain 2016, 157, 2124–2140. [Google Scholar] [CrossRef]
- Perez-Miller, S.; Gomez, K.; Khanna, R. Peptide and peptidomimetic inhibitors targeting the interaction of collapsin response mediator protein 2 with the N-type calcium channel for pain relief. ACS Pharmacol. Transl. Sci. 2024, 7, 1916–1936. [Google Scholar] [CrossRef]
- Ran, D.; Gomez, K.; Moutal, A.; Patek, M.; Perez-Miller, S.; Khanna, R. Comparison of quinazoline and benzoylpyrazoline chemotypes targeting the CaVα-β interaction as antagonists of the N-type CaV2.2 channel. Channels 2021, 15, 128–135. [Google Scholar] [CrossRef]
- Khanna, R.; Yu, J.; Yang, X.; Moutal, A.; Chefdeville, A.; Gokhale, V.; Shuja, Z.; Chew, L.A.; Bellampalli, S.S.; Luo, S.; et al. Targeting the CaVα- CaVβ interaction yields an antagonist of the N-type CaV2.2 channel with broad antinociceptive efficacy. Pain 2019, 160, 1644–1661. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.; Santiago, U.; Nelson, T.S.; Allen, H.N.; Calderon-Rivera, A.; Hestehave, S.; Rodríguez Palma, E.J.; Zhou, Y.; Duran, P.; Loya-Lopez, S. A peptidomimetic modulator of the CaV2.2 N-type calcium channel for chronic pain. Proc. Natl. Acad. Sci. USA 2023, 120, e2305215120. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Gomez, K.; Chen, Y.; Allen, H.N.; Hestehave, S.; Rodríguez-Palma, E.J.; Loya-Lopez, S.; Calderon-Rivera, A.; Duran, P.; Nelson, T.S.; et al. C2230, a preferential use- and state-dependent CaV2.2 channel blocker, mitigates pain behaviors across multiple pain models. J. Clin. Investig. 2024, 135, e177429. [Google Scholar] [CrossRef]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. α2δ ligands, gabapentin, pregabalin and mirogabalin: A review of their clinical pharmacology and therapeutic use. Expert Rev. Neurother. 2016, 16, 1263–1277. [Google Scholar] [CrossRef]
- Davari, M.; Amani, B.; Khanijahani, A.; Akbarzadeh, A.; Shabestan, R. Pregabalin and gabapentin in neuropathic pain management after spinal cord injury: A systematic review and meta-analysis. Korean J. Pain 2020, 33, 3–12. [Google Scholar] [CrossRef]
- Ablinger, C.; Eibl, C.; Roznovcova, M.; Cottrell, G.S.; Stephens, G.J.; Obermair, G.J. The presynaptic α2δ protein family and their therapeutic potential. In Ion Channels as Targets in Drug Discovery; Stephens, G., Stevens, E., Eds.; Springer International Publishing AG: Cham, Switzerland, 2024; pp. 57–89. [Google Scholar]
- Ferron, L.; Gandini, M.A.; Zamponi, G.W. Fighting pain: The structure of gabapentin and its binding site in the Cavα2δ subunit. Nat. Struct. Mol. Biol. 2023, 30, 717–719. [Google Scholar] [CrossRef]
- Sills, G.J. The mechanisms of action of gabapentin and pregabalin. Curr. Opin. Pharmacol. 2006, 6, 108–113. [Google Scholar] [CrossRef]
- Uchitel, O.D.; Di Guilmi, M.N.; Urbano, F.J.; Gonzalez-Inchauspe, C. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels 2010, 4, 490–496. [Google Scholar] [CrossRef]
- Vega-Hernández, A.; Felix, R. Down-regulation of N-type voltage-activated Ca2+ channels by gabapentin. Cell. Mol. Neurobiol. 2002, 22, 185–190. [Google Scholar] [CrossRef]
- Hendrich, J.; Van Minh, A.T.; Heblich, F.; Nieto-Rostro, M.; Watschinger, K.; Striessnig, J.; Wratten, J.; Davies, A.; Dolphin, A.C. Pharmacological disruption of calcium channel trafficking by the α2δ ligand gabapentin. Proc. Natl. Acad. Sci. USA 2008, 105, 3628–3633. [Google Scholar] [CrossRef]
- Zoidis, G.; Papanastasiou, I.; Dotsikas, I.; Sandoval, A.; Dos Santos, R.G.; Papadopoulou-Daifoti, Z.; Vamvakides, A.; Kolocouris, N.; Felix, R. The novel GABA adamantane derivative (AdGABA): Design, synthesis, and activity relationship with gabapentin. Bioorg. Med. Chem. 2005, 13, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, E.; Sandoval, A.; González-Ramírez, R.; Zoidis, G.; Felix, R. Inhibition of recombinant N-type and native high voltage-gated neuronal Ca2+ channels by AdGABA: Mechanism of action studies. Toxicol. Appl. Pharmacol. 2011, 250, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.A.; Gandini, M.A.; Ali, M.Y.; Kricek, F.; Skouteris, G.; Zamponi, G.W. Determinants of interactions of a novel next-generation gabapentinoid NVA1309 and mirogabalin with the CaVα2δ-1 subunit. Mol. Brain 2024, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Y.; Zhang, M.; Yu, S. Mirogabalin as a novel calcium channel α2δ ligand for the treatment of neuropathic pain: A review of clinical update. Front. Pharmacol. 2024, 15, 1491570. [Google Scholar] [CrossRef]
- Kricek, F.; Ruf, C.; Meghani, P.; Souza, I.A.; Gandini, M.A.; Zamponi, G.W.; Skouteris, G. A next generation peripherally restricted CaVα2δ-1 ligand with inhibitory action on CaV2.2 channels and utility in neuropathic pain. Biomed. Pharmacother. 2024, 174, 116472. [Google Scholar] [CrossRef]
- Dogrul, A.; Gardell, L.R.; Ossipov, M.H.; Tulunay, F.C.; Lai, J.; Porreca, F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain 2003, 105, 159–168. [Google Scholar] [CrossRef]
- Choe, W.; Messinger, R.B.; Leach, E.; Eckle, V.S.; Obradovic, A.; Salajegheh, R.; Jevtovic-Todorovic, V.; Todorovic, S.M. TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent. Mol. Pharmacol. 2011, 80, 900–910. [Google Scholar] [CrossRef]
- Jarvis, M.F.; Scott, V.E.; McGaraughty, S.; Chu, K.L.; Xu, J.; Niforatos, W.; Milicic, I.; Joshi, S.; Zhang, Q.; Xia, Z. A peripherally acting, selective T-type calcium channel blocker ABT-639 effectively reduces nociceptive and neuropathic pain in rats. Biochem. Pharmacol. 2014, 89, 536–544. [Google Scholar] [CrossRef]
- Alles, S.R.A.; Smith, P.A. Peripheral voltage-gated cation channels in neuropathic pain and their potential as therapeutic targets. Front. Pain Res. 2021, 2, 750583. [Google Scholar] [CrossRef]
- Harding, E.K.; Dedek, A.; Bonin, R.P.; Salter, M.W.; Snutch, T.P.; Hildebrand, M.E. The T-type calcium channel antagonist Z944 reduces spinal excitability and pain hypersensitivity. Br. J. Pharmacol. 2021, 178, 3517–3532. [Google Scholar] [CrossRef]
- Berger, N.D.; Gadotti, V.M.; Petrov, R.R.; Chapman, K.; Diaz, P.; Zamponi, G.W. NMP-7 inhibits chronic inflammatory and neuropathic pain via block of CaV3.2 T-type calcium channels and activation of CB2 receptors. Mol. Pain 2014, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Gadotti, V.M.; Huang, S.; Garcia-Caballero, A.; Antunes, F.T.T.; Jung, H.A.; Choi, J.S.; Zamponi, G.W. Icariside II: A prenyl-flavonol alleviates inflammatory and neuropathic pain by inhibiting T-type calcium channels and USP5-CaV3.2 interactions. ACS Chem. Neurosci. 2023, 14, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Kutzsche, J.; Guzman, G.A.; Willuweit, A.; Kletke, O.; Wollert, E.; Gering, I.; Jürgens, D.; Breitkreutz, J.; Stark, H.; Beck-Sickinger, A.G.; et al. An orally available CaV2.2 calcium channel inhibitor for the treatment of neuropathic pain. Br. J. Pharmacol. 2024, 181, 1734–1756. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, T.J.; Park, J.; Fan, Q.R.; Colecraft, H.M. A potent voltage-gated calcium channel inhibitor engineered from a nanobody targeted to auxiliary CaVβ subunits. eLife 2019, 8, e49253. [Google Scholar] [CrossRef]
- Sun, L.; Tong, C.K.; Morgenstern, T.J.; Zhou, H.; Yang, G.; Colecraft, H.M. Targeted ubiquitination of sensory neuron calcium channels reduces the development of neuropathic pain. Proc. Natl. Acad. Sci. USA 2022, 119, e2118129119. [Google Scholar] [CrossRef]
- Harding, E.K.; Zamponi, G.W. The calcium channel terminator: Hasta la vista pain. Trends Pharmacol. Sci. 2022, 43, 801–803. [Google Scholar] [CrossRef]
- Doyle, D.A.; Morais Cabral, J.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef]
- Armstrong, C.M. Voltage-gated K channels. Sci. STKE 2003, 2003, re10. [Google Scholar] [CrossRef][Green Version]
- González, C.; Baez-Nieto, D.; Valencia, I.; Oyarzún, I.; Rojas, P.; Naranjo, D.; Latorre, R. K+ channels: Function-structural overview. Compr. Physiol. 2012, 2, 2087–2149. [Google Scholar] [CrossRef]
- González, W.; Valdebenito, B.; Caballero, J.; Riadi, G.; Riedelsberger, J.; Martínez, G.; Ramírez, D.; Zúñiga, L.; Sepúlveda, F.V.; Dreyer, I.; et al. K2p channels in plants and animals. Pflugers Arch. 2015, 467, 1091–1104. [Google Scholar] [CrossRef]
- Renigunta, V.; Schlichthörl, G.; Daut, J. Much more than a leak: Structure and function of K2p-channels. Pflugers Arch. 2015, 467, 867–894. [Google Scholar] [CrossRef] [PubMed]
- Bocksteins, E. KV5, KV6, KV8, and KV9 subunits: No simple silent bystanders. J. Gen. Physiol. 2016, 147, 105–125. [Google Scholar] [CrossRef]
- Köhler, M.; Hirschberg, B.; Bond, C.T.; Kinzie, J.M.; Marrion, N.V.; Maylie, J.; Adelman, J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 1996, 273, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Salkoff, L.; Butler, A.; Ferreira, G.; Santi, C.; Wei, A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 2006, 7, 921–931. [Google Scholar] [CrossRef]
- Smith, P.A. K+ channels in primary afferents and their role in nerve injury-induced pain. Front. Cell. Neurosci. 2020, 14, 566418. [Google Scholar] [CrossRef] [PubMed]
- Busserolles, J.; Tsantoulas, C.; Eschalier, A.; López García, J.A. Potassium channels in neuropathic pain: Advances, challenges, and emerging ideas. Pain 2016, 157 (Suppl. S1), S7–S14. [Google Scholar] [CrossRef]
- Tsantoulas, C. Emerging potassium channel targets for the treatment of pain. Curr. Opin. Support. Palliat. Care 2015, 9, 147–154. [Google Scholar] [CrossRef]
- Misonou, H.; Mohapatra, D.P.; Trimmer, J.S. KV2.1: A voltage-gated K+ channel critical to dynamic control of neuronal excitability. Neurotoxicology 2005, 26, 743–752. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, G.; Ji, F.; Jia, S.; Wu, S.; Guo, X.; He, L.; Pan, Z.; Miao, X.; Mao, Q.; et al. TET1 overexpression mitigates neuropathic pain through rescuing the expression of µ-opioid receptor and KV1.2 in the primary sensory neurons. Neurotherapeutics 2019, 16, 491–504. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Ren, X.; Rong, L.; Huang, M.; Cao, J.; Zang, W. HDAC2, but not HDAC1, regulates KV1.2 expression to mediate neuropathic pain in cci rats. Neuroscience 2019, 408, 339–348. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, L.; Shao, J.; Zhang, Y.; Liu, Y.; Zhao, S.; Li, L.; Yu, W.; Zhang, M.; Ren, X.; et al. Epigenetic restoration of voltage-gated potassium channel KV1.2 alleviates nerve injury-induced neuropathic pain. J. Neurochem. 2021, 156, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Guan, X.; Wang, W.; Zhao, J.Y.; Zhang, H.; Tiwari, V.; Hoffman, P.N.; Li, M.; Tao, Y.X. Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit KV1.2 in primary afferent neurons. Mol. Pain 2014, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Vacher, H.; Park, K.S.; Clark, E.; Trimmer, J.S. Trafficking-dependent phosphorylation of KV1.2 regulates voltage-gated potassium channel cell surface expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20055–20060. [Google Scholar] [CrossRef]
- Cao, X.H.; Byun, H.S.; Chen, S.R.; Cai, Y.Q.; Pan, H.L. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: Role of brain-derived neurotrophic factor. J. Neurochem. 2010, 114, 1460–1475. [Google Scholar] [CrossRef]
- Tsantoulas, C.; Zhu, L.; Yip, P.; Grist, J.; Michael, G.J.; McMahon, S.B. KV2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input. Exp. Neurol. 2014, 251, 115–126. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tanaka, M.; Black, J.A.; Waxman, S.G. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve 1999, 22, 502–507. [Google Scholar] [CrossRef]
- Tsantoulas, C.; McMahon, S.B. Opening paths to novel analgesics: The role of potassium channels in chronic pain. Trends Neurosci. 2014, 37, 146–158. [Google Scholar] [CrossRef]
- Richardson, F.C.; Kaczmarek, L.K. Modification of delayed rectifier potassium currents by the KV9.1 potassium channel subunit. Hear Res. 2000, 147, 21–30. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, J.O.; Rim, H.D.; Cho, H.J. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res. Mol. Brain Res. 2002, 105, 146–152. [Google Scholar] [CrossRef]
- Phuket, T.R.; Covarrubias, M. KV4 channels underlie the subthreshold-operating a-type k-current in nociceptive dorsal root ganglion neurons. Front. Mol. Neurosci. 2009, 2, 3. [Google Scholar] [CrossRef]
- Grabauskas, G.; Heldsinger, A.; Wu, X.; Xu, D.; Zhou, S.; Owyang, C. Diabetic visceral hypersensitivity is associated with activation of mitogen-activated kinase in rat dorsal root ganglia. Diabetes 2011, 60, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Chavira-Suárez, E.; Sandoval, A.; Felix, R.; Lamas, M. Expression and high glucose-mediated regulation of K+ channel interacting protein 3 (KChIP3) and KV4 channels in retinal Müller Glial Cells. Biochem. Biophys. Res. Commun. 2011, 404, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Sasaki, K.; Ma, L.; Ueda, H. Neuron-Restrictive Silencer Factor Causes Epigenetic Silencing of KV4.3 Gene after Peripheral Nerve Injury. Neuroscience 2010, 166, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.L.; Cheng, J.K.; Hou, W.H.; Chang, Y.C.; Du, P.H.; Jian, J.J.; Rau, R.H.; Yang, J.H.; Lien, C.C.; Tsaur, M.L. K+ channel modulatory subunits KChIP and DPP participate in KV4-mediated mechanical pain control. J. Neurosci. 2017, 37, 4391–4404. [Google Scholar] [CrossRef]
- Selyanko, A.A.; Hadley, J.K.; Wood, I.C.; Abogadie, F.C.; Delmas, P.; Buckley, N.J.; London, B.; Brown, D.A. Two types of K+ channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell. J. Neurosci. 1999, 19, 7742–7756. [Google Scholar] [CrossRef]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (KV7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef]
- Wang, H.S.; Pan, Z.; Shi, W.; Brown, B.S.; Wymore, R.S.; Cohen, I.S.; Dixon, J.E.; McKinnon, D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science 1998, 282, 1890–1893. [Google Scholar] [CrossRef]
- Hadley, J.K.; Passmore, G.M.; Tatulian, L.; Al-Qatari, M.; Ye, F.; Wickenden, A.D.; Brown, D.A. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J. Neurosci. 2003, 23, 5012–5019. [Google Scholar] [CrossRef]
- Schwarz, J.R.; Glassmeier, G.; Cooper, E.C.; Kao, T.C.; Nodera, H.; Tabuena, D.; Kaji, R.; Bostock, H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J. Physiol. 2006, 573, 17–34. [Google Scholar] [CrossRef]
- Passmore, G.M.; Selyanko, A.A.; Mistry, M.; Al-Qatari, M.; Marsh, S.J.; Matthews, E.A.; Dickenson, A.H.; Brown, T.A.; Burbidge, S.A.; Main, M.; et al. KCNQ/M currents in sensory neurons: Significance for pain therapy. J. Neurosci. 2003, 23, 7227–7236. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, D.; Liu, M.; Cai, J.; Wan, Y.; Han, J.S.; Xing, G.G. Suppression of KCNQ/M (KV7) Potassium Channels in Dorsal Root Ganglion Neurons Contributes to the Development of Bone Cancer Pain in a Rat Model. Pain 2013, 154, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Ooi, L.; Dalle, C.; Robertson, B.; Wood, I.C.; Gamper, N. Transcriptional repression of the M channel subunit KV7.2 in chronic nerve injury. Pain 2011, 152, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M.; Ooi, L.; Linley, J.E.; Mordaka, P.; Dalle, C.; Robertson, B.; Gamper, N.; Wood, I.C. Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J. Neurosci. 2010, 30, 13235–13245. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Ma, L.; Ueda, H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J. Neurosci. 2010, 30, 4806–4814. [Google Scholar] [CrossRef]
- Lundby, A.; Jespersen, T.; Schmitt, N.; Grunnet, M.; Olesen, S.P.; Cordeiro, J.M.; Calloe, K. Effect of the Ito activator NS5806 on cloned KV4 channels depends on the accessory protein KChIP2. Br. J. Pharmacol. 2010, 160, 2028–2044. [Google Scholar] [CrossRef]
- Kanda, H.; Ling, J.; Chang, Y.T.; Erol, F.; Viatchenko-Karpinski, V.; Yamada, A.; Noguchi, K.; Gu, J.G. KV4.3 channel dysfunction contributes to trigeminal neuropathic pain manifested with orofacial cold hypersensitivity in rats. J. Neurosci. 2021, 41, 2091–2105. [Google Scholar] [CrossRef]
- Rivera-Arconada, I.; Lopez-Garcia, J.A. Retigabine-induced population primary afferent hyperpolarisation in vitro. Neuropharmacology 2006, 51, 756–763. [Google Scholar] [CrossRef]
- Blackburn-Munro, G.; Jensen, B.S. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur. J. Pharmacol. 2003, 460, 109–116. [Google Scholar] [CrossRef]
- Main, M.J.; Cryan, J.E.; Dupere, J.R.; Cox, B.; Clare, J.J.; Burbidge, S.A. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol. Pharmacol. 2000, 58, 253–262. [Google Scholar] [CrossRef]
- Wickenden, A.D.; Yu, W.; Zou, A.; Jegla, T.; Wagoner, P.K. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol. Pharmacol. 2000, 58, 591–600. [Google Scholar] [CrossRef]
- Tatulian, L.; Delmas, P.; Abogadie, F.C.; Brown, D.A. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J. Neurosci. 2001, 21, 5535–5545. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, L.; Brown, D.A. Effect of the KCNQ potassium channel opener retigabine on single KCNQ2/3 channels expressed in CHO cells. J. Physiol. 2003, 549, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Szelenyi, I. Flupirtine, a re-discovered drug, revisited. Inflamm. Res. 2013, 62, 251–258. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; Jackson, M.; Gu, S.L.; Fiala, K.; Gu, J. Neuropathic pain and KV7 voltage-gated potassium channels: The potential role of KV7 activators in the treatment of neuropathic pain. Mol. Pain 2019, 15, 1744806919864256. [Google Scholar] [CrossRef]
- Wilke, B.U.; Kummer, K.K.; Leitner, M.G.; Kress, M. Chloride—The underrated ion in nociceptors. Front. Neurosci. 2020, 14, 287. [Google Scholar] [CrossRef]
- Delgado-Lezama, R.; Bravo-Hernández, M.; Franco-Enzástiga, U.; De la Luz-Cuellar, Y.E.; Alvarado-Cervantes, N.S.; Raya-Tafolla, G.; Martínez-Zaldivar, L.A.; Vargas-Parada, A.; Rodríguez-Palma, E.J.; Vidal-Cantú, G.C.; et al. The role of spinal cord extrasynaptic α5 GABAA receptors in chronic pain. Physiol. Rep. 2021, 9, e14984. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Pusch, M. CLC chloride channels and transporters: Structure, function, physiology, and disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef]
- Rinke, I.; Artmann, J.; Stein, V. ClC-2 voltage-gated channels constitute part of the background conductance and assist chloride extrusion. J. Neurosci. 2010, 30, 4776–4786. [Google Scholar] [CrossRef]
- Ratté, S.; Prescott, S.A. ClC-2 channels regulate neuronal excitability, not intracellular chloride levels. J. Neurosci. 2011, 31, 15838–15843. [Google Scholar] [CrossRef]
- Tombola, F.; Ulbrich, M.H.; Isacoff, E.Y. Architecture and gating of Hv1 proton channels. J. Physiol. 2009, 587 Pt 22, 5325–5329. [Google Scholar] [CrossRef]
- Lee, S.Y.; Letts, J.A.; Mackinnon, R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl. Acad. Sci. USA 2008, 105, 7692–7695. [Google Scholar] [CrossRef] [PubMed]
- Tombola, F.; Ulbrich, M.H.; Kohout, S.C.; Isacoff, E.Y. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat. Struct. Mol. Biol. 2010, 17, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhao, X.; Hatch, M.; Chun, S.; Chang, E. Central Neuropathic Pain in Spinal Cord Injury. Crit. Rev. Phys. Rehabil. Med. 2013, 25, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; You, B.; Jo, E.K.; Han, S.K.; Simon, M.I.; Lee, S.J. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14851–14856. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Hohmann, S.W.; Syhr, K.M.; Schröder, K.; Sisignano, M.; Weigert, A.; Lorenz, J.E.; Lu, R.; Brüne, B.; Brandes, R.P.; et al. Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain 2014, 155, 2161–2170. [Google Scholar] [CrossRef]
- Zheng, J.; Murugan, M.; Wang, L.; Wu, L.J. Microglial voltage-gated proton channel Hv1 in spinal cord injury. Neural Regen. Res. 2022, 17, 1183–1189. [Google Scholar]
- Peng, J.; Yi, M.H.; Jeong, H.; McEwan, P.P.; Zheng, J.; Wu, G.; Ganatra, S.; Ren, Y.; Richardson, J.R.; Oh, S.B.; et al. The voltage-gated proton channel Hv1 promotes microglia-astrocyte communication and neuropathic pain after peripheral nerve injury. Mol. Brain. 2021, 14, 99. [Google Scholar] [CrossRef]
- Hains, B.C.; Saab, C.Y.; Waxman, S.G. Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain 2005, 128 Pt 10, 2359–2371. [Google Scholar] [CrossRef]
- Liao, Y.F.; Tsai, M.L.; Chen, C.C.; Yen, C.T. Involvement of the CaV3.2 T-type calcium channel in thalamic neuron discharge patterns. Mol. Pain. 2011, 7, 43. [Google Scholar] [CrossRef]
- Shen, F.Y.; Chen, Z.Y.; Zhong, W.; Ma, L.Q.; Chen, C.; Yang, Z.J.; Xie, W.L.; Wang, Y.W. Alleviation of neuropathic pain by regulating T-type calcium channels in rat anterior cingulate cortex. Mol. Pain. 2015, 11, 7. [Google Scholar] [CrossRef]
- Cerina, M.; Szkudlarek, H.J.; Coulon, P.; Meuth, P.; Kanyshkova, T.; Nguyen, X.V.; Göbel, K.; Seidenbecher, T.; Meuth, S.G.; Pape, H.C.; et al. Thalamic KV7 channels: Pharmacological properties and activity control during noxious signal processing. Br. J. Pharmacol. 2015, 172, 3126–3140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Li, Y. KCNQ potassium channels in sensory system and neural circuits. Acta Pharmacol. Sin. 2016, 37, 25–33. [Google Scholar] [CrossRef]
- Yuan, X.; Han, S.; Manyande, A.; Gao, F.; Wang, J.; Zhang, W.; Tian, X. Spinal voltage-gated potassium channel Kv1.3 contributes to neuropathic pain via the promotion of microglial M1 polarization and activation of the NLRP3 inflammasome. Eur. J. Pain. 2023, 27, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Beraldo-Neto, E.; Ferreira, V.F.; Vigerelli, H.; Fernandes, K.R.; Juliano, M.A.; Nencioni, A.L.A.; Pimenta, D.C. Unraveling neuroprotection with Kv1.3 potassium channel blockade by a scorpion venom peptide. Sci. Rep. 2024, 14, 27888. [Google Scholar] [CrossRef] [PubMed]
- Waxman, S.G. NaV1.7, its mutations, and the syndromes that they cause. Neurology 2007, 69, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Estacion, M.; Jarecki, B.W.; Tyrrell, L.; Fischer, T.Z.; Lawden, M.; Cummins, T.R.; Waxman, S.G. Paroxysmal extreme pain disorder M1627K mutation in human Nav1.7 renders DRG neurons hyperexcitable. Mol. Pain. 2008, 4, 37. [Google Scholar] [CrossRef]
- Fischer, T.Z.; Waxman, S.G. Familial pain syndromes from mutations of the NaV1.7 sodium channel. Ann. NY Acad. Sci. 2010, 1184, 196–207. [Google Scholar] [CrossRef]
- Faber, C.G.; Lauria, G.; Merkies, I.S.; Cheng, X.; Han, C.; Ahn, H.S.; Persson, A.K.; Hoeijmakers, J.G.; Gerrits, M.M.; Pierro, T.; et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc. Natl. Acad. Sci. USA 2012, 109, 19444–19449. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Rush, A.M.; Cummins, T.R.; Hisama, F.M.; Novella, S.; Tyrrell, L.; Marshall, L.; Waxman, S.G. Gain-of-function mutation in NaV1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain 2005, 128 Pt 8, 1847–1854. [Google Scholar] [CrossRef]
- Estacion, M.; Dib-Hajj, S.D.; Benke, P.J.; Te Morsche, R.H.; Eastman, E.M.; Macala, L.J.; Drenth, J.P.; Waxman, S.G. Nav1.7 gain-of-function mutations as a continuum: A1632E displays physiological changes associated with erythromelalgia and paroxysmal extreme pain disorder mutations and produces symptoms of both disorders. J. Neurosci. 2008, 28, 11079–11088. [Google Scholar] [CrossRef]
- Eberhardt, M.; Nakajima, J.; Klinger, A.B.; Neacsu, C.; Hühne, K.; O’Reilly, A.O.; Kist, A.M.; Lampe, A.K.; Fischer, K.; Gibson, J.; et al. Inherited pain: Sodium channel Nav1.7 A1632T mutation causes erythromelalgia due to a shift of fast inactivation. J. Biol. Chem. 2014, 289, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.R.; Dib-Hajj, S.D.; Waxman, S.G. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J. Neurosci. 2004, 24, 8232–8236. [Google Scholar] [CrossRef] [PubMed]
- Harty, T.P.; Dib-Hajj, S.D.; Tyrrell, L.; Blackman, R.; Hisama, F.M.; Rose, J.B.; Waxman, S.G. Nav1.7 mutant A863P in erythromelalgia: Effects of altered activation and steady-state inactivation on excitability of nociceptive dorsal root ganglion neurons. J. Neurosci. 2006, 26, 12566–12575. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liu, Y.; Qian, L.; Yi, M.; Yin, H.; Wang, S.; Xiang, B. Sodium channels as a new target for pain treatment. Front. Pharmacol. 2025, 16, 1573254. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Y.F.; Smith, R.; Ratté, S.; Prescott, S.A. Discordance between preclinical and clinical testing of Nav1.7-selective inhibitors for pain. Pain. 2025, 166, 481–501. [Google Scholar] [CrossRef]
- Dormer, A.; Narayanan, M.; Schentag, J.; Achinko, D.; Norman, E.; Kerrigan, J.; Jay, G.; Heydorn, W. A Review of the therapeutic targeting of SCN9A and Nav1.7 for pain relief in current human clinical trials. J. Pain. Res. 2023, 16, 1487–1498. [Google Scholar] [CrossRef]
- Gandini, M.A.; Zamponi, G.W. The N-type calcium channel rises from the ashes. J. Clin. Investig. 2025, 135, e189308. [Google Scholar] [CrossRef]
- Kutzsche, J.; Jürgens, D.; Willuweit, A.; Adermann, K.; Fuchs, C.; Simons, S.; Windisch, M.; Hümpel, M.; Rossberg, W.; Wolzt, M.; et al. Safety and pharmacokinetics of the orally available antiprionic compound PRI-002: A single and multiple ascending dose phase I study. Alzheimer’s Dement. 2020, 6, e12001. [Google Scholar] [CrossRef]
- Antunes, F.T.T.; Huang, S.; Chen, L.; Zamponi, G.W. Effect of ABT-639 on CaV3.2 channel activity and its analgesic actions in mouse models of inflammatory and neuropathic pain. Eur. J. Pharmacol. 2024, 967, 176416. [Google Scholar] [CrossRef]
- Ziegler, D.; Duan, W.R.; An, G.; Thomas, J.W.; Nothaft, W. A randomized double-blind, placebo-, and active-controlled study of T-type calcium channel blocker ABT-639 in patients with diabetic peripheral neuropathic pain. Pain 2015, 156, 2013–2020. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Tang, F.; Liang, B.; Chen, H.; Zhang, H.; Wang, K. Electrophysiological and pharmacological characterization of a novel and potent neuronal Kv7 channel opener SCR2682 for antiepilepsy. FASEB J. 2019, 33, 9154–9166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Hu, F.; Yang, J.; Guo, X.; Hou, X.; Ju, C.; Wang, K. Activation of neuronal voltage-gated potassium Kv7/KCNQ/M-current by a novel channel opener SCR2682 for alleviation of chronic pain. J. Pharmacol. Exp. Ther. 2021, 377, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Taglialatela, M. Targeting Kv7 Potassium channels for epilepsy. CNS Drugs 2025, 39, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Manville, R.W.; Abbott, G.W. Gabapentin is a potent activator of KCNQ3 and KCNQ5 potassium channels. Mol. Pharmacol. 2018, 94, 1155–1163. [Google Scholar] [CrossRef]
- He, M.; Cao, C.; Ni, Z.; Liu, Y.; Song, P.; Hao, S.; He, Y.; Sun, X.; Rao, Y. PROTACs: Great opportunities for academia and industry (an update from 2020 to 2021). Signal Transduct. Target. Ther. 2022, 7, 181. [Google Scholar] [CrossRef]
- Chamessian, A.; Payne, M.; Gordon, I.; Zhou, M.; Gereau, R. Small molecule-mediated targeted protein degradation of voltage-gated sodium channels involved in pain. bioRxiv 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).