Abstract
Background: Erectile dysfunction (ED) affects approximately 20% of men worldwide, significantly affecting their quality of life. While phosphodiesterase type 5 inhibitors (PDE5-Is) are the standard first-line treatment, a substantial number of patients are non-responders. Second-line treatments, such as intracavernosal alprostadil, are effective but often limited by their invasive nature and the need for frequent injections. Intracavernosal onabotulinumtoxinA (BoNT-A) offers a promising new option. By inhibiting acetylcholine release and norepinephrine, as well as other neurotransmitters involved in detumescence, it facilitates cavernosal smooth muscle relaxation and enhances penile blood flow. Its effects may persist for up to six months following a single injection, potentially reducing treatment burden and improving adherence among men with refractory ED. Methods: A systematic review was performed in accordance with the PRISMA guidelines. Literature searches were conducted in PubMed, Embase, Cochrane Library, Scopus, and Clinicaltrials.gov from inception until August 2025 using a combination of keywords and MeSH terms related to ‘erectile dysfunction’ and ‘botulinum toxin’. After screening, 51 studies met the inclusion criteria. Due to significant heterogeneity in interventions (e.g., BoNT-A dosage, co-therapies), patient populations, and reported outcomes, the data were not suitable for meta-analysis. Consequently, a narrative synthesis was performed to summarize the findings. Results: Among the included studies, intracavernosal BoNT-A was associated with improvements in validated erectile function scores. Reported response rates, variably defined across studies, ranged from 40% to 77.5%. Several studies suggested that efficacy was higher in patients with mild-to-moderate ED and with repeated administration of 100 U doses. The treatment exhibited a favorable safety profile. The most common adverse event was mild, transient penile pain (reported incidence 1.5–6%). No studies reported serious systemic adverse events. The overall strength of the evidence was limited by significant heterogeneity among the included studies and their generally small sample sizes. Conclusions: Based on this systematic review, intracavernosal onabotulinumtoxinA (BoNT-A) may be a beneficial therapeutic option for patients with refractory ED, offering potential improvements in sexual function while reducing the need for invasive therapies. Future large-scale, placebo-controlled studies are essential to confirm these benefits and standardize their clinical application.
1. Introduction
Erectile dysfunction (ED) is a prevalent global health issue, affecting approximately 20% of men worldwide, with its incidence rising progressively with age. The condition markedly impairs quality of life, self-esteem, and intimate relationships. For over two decades, phosphodiesterase type 5 inhibitors (PDE5-Is) have served as the primary first-line therapy, offering a non-invasive and effective treatment for many patients [,]. Nevertheless, a significant clinical challenge persists, as a substantial subset of men, particularly those with severe vasculogenic, neurogenic, or diabetic ED, demonstrate inadequate responses to these agents [,].
This therapeutic gap necessitates consideration of second and third-line treatments []. Intracavernosal injections of vasoactive agents, such as alprostadil, are an effective second-line alternative []. However, their adoption is often limited by patient apprehension regarding needle use, the technical challenges of self-administration, risks including priapism, and the requirement for frequent, on-demand dosing, which may reduce spontaneity [,]. Consequently, many patients with refractory erectile dysfunction face a difficult choice between foregoing treatment or proceeding directly to penile prosthesis implantation, a definitive but invasive and irreversible option associated with its own risks and costs [].
This unmet clinical need has driven the exploration of novel, longer-lasting therapeutic modalities [,]. Among these, low-intensity shockwave therapy (Li-SWT) and platelet-rich plasma (PRP) injections are currently under active investigation [,,,]. Concurrently, intracavernosal administration of onabotulinumtoxinA (BoNT-A) has emerged as a particularly promising approach [,,,,,]. Three commercial formulations of BoNT-A are available: onabotulinumtoxinA, incobotulinumtoxinA, and abobotulinumtoxinA, and they are widely employed across various medical disciplines. BoNT-A, a potent neurotoxin, mediates its effect by inhibiting the presynaptic release of acetylcholine and other neurotransmitters at adrenergic nerve terminals within the corpus cavernosum. This results in prolonged chemical denervation, facilitating relaxation of cavernosal smooth muscle, reduction in sympathetic tone, and enhanced arterial inflow, thereby promoting erection [,]. A notable advantage of BoNT-A is its sustained duration of action, with clinical benefits reported to persist for several months following a single injection, potentially reducing treatment frequency and improving patient adherence [].
Initial clinical studies, including several randomized controlled trials and meta-analyses, have reported encouraging improvements in erectile function scores (e.g., IIEF, SHIM, EHS) [,,,,,,,]. These studies consistently report a favorable safety profile, with adverse events predominantly limited to mild and transient local pain [,]. However, the current literature is marked by considerable heterogeneity in terms of study design, patient populations, BoNT-A formulations, dosing regimens, and outcome measures. This variability, compounded by the lack of large, definitive trials, leads to ongoing uncertainty regarding the precise efficacy, optimal clinical application, and positioning of BoNT-A within existing erectile dysfunction treatment algorithms [,,].
Therefore, this systematic review aims to critically synthesize and evaluate the current evidence on the efficacy and safety of intracavernosal BoNT-A for the treatment of ED. By consolidating findings across studies, we seek to provide a clear summary of its therapeutic potential, identify factors predicting treatment success, and highlight key knowledge gaps to guide future clinical research [,].
2. Materials and Methods
2.1. Search Strategy and Study Registration
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines []. The review protocol was prospectively registered in the PROSPERO international prospective register of systematic reviews (Registration Number: CRD420251087894).
A comprehensive literature search was performed across multiple electronic databases, including PubMed, Embase, the Cochrane Library, Scopus, and ClinicalTrials.gov, from their inception until August 2025. To ensure search comprehensiveness, the AI-powered research platforms Consensus and Elicit were also utilized to screen for any additional relevant studies that may not have been captured by the database searches.
The search strategy was designed to incorporate a combination of relevant keywords and Medical Subject Headings (MeSH) terms. The core concepts included “Erectile Dysfunction,” “Botulinum Toxins,” and “Injections.” The following search string, adapted for each database, exemplifies the approach: (erectile dysfunction) AND (botulinum toxin OR Botox OR BoNT-A OR Xeomin OR Dysport) AND (intracavernosal injection OR penile injection).
2.2. Study Selection and Eligibility Criteria
Two independent reviewers screened the retrieved records by title and abstract, followed by a full-text assessment of potentially eligible studies. Any discrepancies between reviewers were resolved through discussion or by consultation with a third reviewer.
Studies were included if they investigated the use of intracavernosal botulinum toxin type A (BoNT-A) for the treatment of erectile dysfunction in human subjects. Randomized controlled trials, prospective and retrospective cohort studies, and case series with ≥10 patients were considered. Reviews, editorials, animal studies, and case reports with fewer than 10 patients were excluded.
2.3. Study Selection Process
The study selection process is detailed in the PRISMA flow diagram (Figure 1). A total of six relevant clinical trials were identified on ClinicalTrials.gov. Of these, results were publicly posted for three, and only one was associated with a peer-reviewed publication. Our initial database and register searches yielded 1052 records. After removing duplicates, 557 unique records were screened by title and abstract. Subsequently, 305 full-text articles were assessed for eligibility, resulting in 51 studies that met the inclusion criteria and were included in the final synthesis. To ensure a comprehensive capture of the literature, the reference lists of all eligible studies were manually screened.
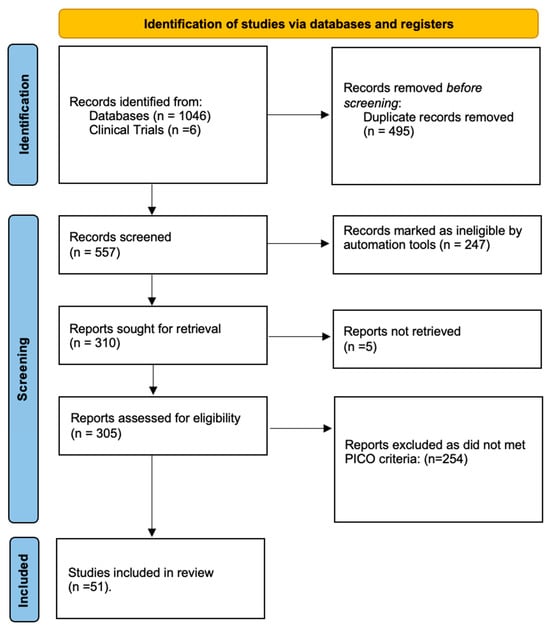
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews, which included searches of databases and registers.
2.4. Data Extraction
Data were systematically extracted from the included studies using a standardized data collection form. The extracted information included the following:
- -
- Study characteristics: First author, publication year, country, and study design.
- -
- Patient demographics: Sample size, mean age, and ED etiology and severity.
- -
- Intervention details: BoNT-A formulation (e.g., onabotulinumtoxinA), total dose, number and frequency of injections, injection technique, and any concomitant ED therapies.
- -
- Outcome data: As outlined below.
2.5. Outcomes
The primary outcome was the change in erectile function from baseline, measured by validated instruments, including the International Index of Erectile Function (IIEF), the Sexual Health Inventory for Men (SHIM), and the Erection Hardness Score (EHS). Where available, mean differences with standard deviations (SD) or 95% confidence intervals (CIs) were extracted.
Secondary outcomes included the following:
- -
- The safety and adverse event profile, categorized by nature and frequency.
- -
- The duration of the therapeutic effect.
- -
- Changes in hemodynamic parameters as assessed by penile duplex ultrasonography.
2.6. Data Synthesis and Risk of Bias Assessment
Due to substantial clinical and methodological heterogeneity across the included studies in terms of design, patient populations, and outcome reporting, a quantitative meta-analysis was deemed inappropriate. Therefore, the findings were synthesized narratively.
The risk of bias for included randomized controlled trials was assessed using the Cochrane Risk of Bias tool (RoB 2, 2019 version), and the overall judgments were visualized graphically using the robvis (Risk-of-Bias Visualization, 2019 version) tool.
3. Results
3.1. Characteristics of Included Studies
The 51 studies included in this synthesis encompassed a range of designs, including randomized controlled trials (RCTs), systematic reviews, meta-analyses, retrospective case series, and pilot studies. The sample sizes of the primary clinical studies varied substantially, from small pilot cohorts (n = 15–70) to larger multicenter trials (n = 176). The systematic reviews and meta-analyses aggregated data from multiple primary studies [,,,,,], key papers are shown in Table 1. Most of the clinical studies focused on men with ED refractory to PDE5-Is, while a minority included broader ED populations. As pre-specified in the protocol, articles were excluded if they were not published in English, the full text was not accessible, or they did not involve human subjects.

Table 1.
Comparison of key studies on BoNT-A penile injection for ED.
3.2. Efficacy Outcomes
Intracavernosal BoNT-A injection was associated with significant improvements in erectile function across multiple studies. These improvements were consistently demonstrated using validated patient-reported outcome measures, including IIEF, SHIM, and EHS, as well as objective parameters from penile Doppler ultrasound [,,,,,]. The calculated response rates, defined as achieving a minimal clinically important difference in the IIEF-EF domain, ranged from 40% to 77.5%. Subgroup analyses indicated that higher efficacy was observed in patients with less severe ED and in those who received repeated injection cycles [,,,,]. These findings are supported by meta-analyses, which confirm statistically significant improvements in erectile function scores compared to placebo, with a more pronounced effect noted particularly at the 100 U dose [,,].
3.3. Safety and Adverse Events
The intracavernosal administration of BoNT-A was found to be generally well-tolerated. The most frequently reported adverse event was mild and transient penile pain or discomfort at the injection site, with an incidence ranging from 1.5% to 6% across studies [,,,,]. Serious adverse events were rare, with only isolated case reports of priapism or localized tissue reactions [,,,,,,]. Positively, no systemic side effects related to the toxin were reported in any of the included studies.
3.4. Predictors of Response and Durability
Analysis of the included studies identified several factors associated with improved and sustained treatment outcomes. The administered dose was a significant moderator of effect durability; higher doses of 100 U consistently demonstrated a longer duration of efficacy, maintaining therapeutic benefit for up to six months in certain cohorts, compared to lower 50 U regimens [,]. Furthermore, observational data indicate that repeated BoNT-A injection cycles may enhance and prolong the therapeutic response compared to a single administration [,]. Emerging evidence also suggests that objective biomarkers, such as penile shear wave elastography, may help identify patients with favorable tissue compliance who are most likely to benefit from the treatment, although this requires further validation [].
3.5. Risk of Bias Assessment
Methodological quality was appraised using the Cochrane Risk of Bias tool for randomized trials (RoB 2). A summary of the judgments for each domain across all studies is visualized in Figure 2. While most studies demonstrated low risk of bias in key areas such as random sequence generation and outcome measurement, a common area of concern was the lack of blinding of participants and personnel due to the interventional nature of the treatment.
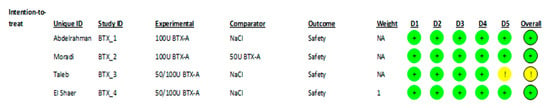
Figure 2.
Risk of bias assessment for each parallel clinical trial included (RoB2). Green circles indicate “low risk of bias”; yellow circles indicate “some concerns” according to the RoB 2 assessment criteria. [,,,].
4. Discussion
ED remains a highly prevalent condition, and the treatment of cases refractory to first-line therapies continues to pose a considerable clinical challenge. This persistent unmet need has driven the exploration of novel therapeutic strategies, among which intracavernosal injection of BoNT-A has emerged as a particularly promising intervention.
The basis for BoNT-A use lies in the underlying mechanisms of ED, which often include endothelial dysfunction, disrupted nitric oxide (NO) signaling, and excessive sympathetic activity, causing increased cavernosal smooth muscle contraction. BoNT-A counteracts this by inhibiting the presynaptic release of acetylcholine and, crucially, norepinephrine from adrenergic nerves within the corpus cavernosum [,,]. This reduces sympathetic-driven vasoconstriction, promoting smooth muscle relaxation. The resulting improvement in penile blood flow and erection rigidity is especially relevant for patients with vasculogenic or neurogenic ED who have failed to respond to PDE5-Is or intracavernosal prostaglandins [,,].
Moreover, the sustained efficacy observed with repeated injections suggests a prolonged neuromodulatory effect, reinforcing BoNT-A’s potential as a mechanism-based therapy that directly targets an underlying pathological component of refractory ED [].
While botulinum toxin has a well-established role in urology for conditions such as overactive bladder, its application in ED represents a novel and promising frontier in sexual medicine []. This review synthesizes evidence from several key randomized controlled trials (RCTs), including four double-blind studies conducted by El-Shaer et al. [], Moradi et al. [], Taleb et al. [], and Abdelrahman et al. [], which form a critical part of the evidence base.
These four RCTs exclusively enrolled men with vasculogenic ED refractory to conventional medical therapy. The interventions varied in formulation and dose: three trials utilized onabotulinumtoxinA at doses of 100 U (Abdelrahman et al., n = 70) or a range of 50–100 U (Taleb et al., n = 45; El-Shaer et al., n = 176) [,,]. In contrast, the study by Moradi et al. (n = 40) employed abobotulinumtoxinA []. A summary of these trials is provided in Table 2. Notably, none of these studies reported any systemic adverse effects, underscoring the localized action of the treatment [,,,].

Table 2.
Main RCTs about intracavernosal injection of BoNT-A.
Collectively, the current literature supports intracavernosal BoNT-A as a safe and moderately effective treatment for ED, particularly for patients who have not responded to standard pharmacological therapies (Table 3) [,,,,,,,,,]. The improvements in ED function scores are clinically meaningful for a substantial proportion of patients, and the safety profile is highly favorable, dominated by minor and transient local adverse events [,,,,,,,,]. The evidence is particularly compelling for its use as an adjunct to PDE5-Is or prostaglandin E1 (PGE1) injections, potentially enhancing their efficacy [,,].

Table 3.
Key claims and supporting evidence identified in this paper.
Furthermore, real-world observational data from Giuliano et al. suggest that repeated BoNT-A injections may augment and prolong the therapeutic effect, indicating a potential benefit from multiple treatment sessions [,]. This is consistent with the drug’s proposed mechanism, whereby the induced relaxation of cavernosal smooth muscle directly improves penile hemodynamics and rigidity, an effect demonstrated across multiple studies in men with vasculogenic ED [,,,,,,].
4.1. Limitations
Despite these encouraging findings (Table 3), this systematic review highlights several important limitations within the extant literature. The current evidence is constrained by the preponderance of studies with small sample sizes, significant heterogeneity in trial design and outcome measures, and short follow-up durations [,,,]. Furthermore, the predominance of positive outcomes raises concerns regarding potential publication bias, wherein negative or null results may be underrepresented.
These limitations necessitate a cautious interpretation of the results and underscore a clear need for larger, multicenter, randomized controlled trials employing standardized protocols. Such studies are essential to confirm efficacy, establish long-term safety, and define optimal dosing strategies [,,,]. Future research should also prioritize the identification of reliable predictors of treatment response, including the validation of imaging biomarkers such as shear-wave elastography []. Additionally, critical gaps remain in understanding the synergistic potential of BoNT-A when combined with PDE5 inhibitors and its comparative effectiveness against other second-line therapies, such as low-intensity shockwave therapy or platelet-rich plasma injections. The absence of direct head-to-head comparisons prevents a definitive assessment of BoNT-A relative position within the ED treatment algorithm [,,,,,,,].
4.2. Unresolved Questions
While the current evidence positions intracavernosal BoNT-A as a promising therapy for refractory ED, its pathway to becoming a standardized treatment is contingent upon resolving three fundamental areas of uncertainty, as outlined in Table 4. First, the long-term therapeutic landscape remains largely uncharted. There is a critical need for prospective studies with extended follow-up periods to definitively establish the durability of effect and the cumulative safety profile of repeated BoNT-A injections. This data is indispensable for therapy risk-benefit ratio over time and for shaping sustainable clinical guidelines. Second, to move beyond a one-size-fits-all approach, a concerted effort is required to identify and validate predictive biomarkers—whether derived from clinical characteristics, imaging modalities like shear-wave elastography, or molecular profiles. The ability to pre-emptively identify optimal responders would revolutionize patient selection, maximizing therapeutic success rates and avoiding unnecessary procedures. Finally, the current heterogeneity in dosing and technique underscores the necessity for rigorous, controlled trials to establish an evidence-based optimal dosing regimen and injection protocol. Standardizing these parameters is a crucial prerequisite for ensuring consistent, reproducible clinical outcomes and facilitating the widespread, reliable adoption of this treatment across diverse healthcare settings and patient populations.

Table 4.
Key open research questions for future studies.
5. Conclusions
Intracavernosal botulinum toxin (BoNT-A) injection represents a promising and well-tolerated therapeutic strategy for men with ED refractory to standard pharmacological treatments. Current evidence indicates it is a moderately effective intervention capable of producing clinically meaningful improvements in erectile function. These benefits likely extend beyond questionnaire scores, potentially enhancing sexual satisfaction, confidence, and overall quality of life. By offering a durable effect from a minimally invasive procedure, BoNT-A may also reduce the need for more invasive options like penile prosthesis implantation in selected patients.
However, the translation of this promise into routine clinical practice is constrained by significant evidence gaps. The existing literature is largely composed of small-scale, short-term, and methodologically heterogeneous studies, limiting the strength of conclusions and generalizability of findings. To advance this treatment paradigm, a critical need exists for larger, long-term, multicenter randomized controlled trials. These studies must prioritize establishing robust patient selection criteria, defining the durability of effect, refining optimal dosing and injection protocols, and validating predictive biomarkers. Furthermore, investigating the synergistic potential of BoNT-A in combination with PDE5-Is could yield strategies for optimizing outcomes. Until such high-quality data is available, BoNT-A should be considered an investigational therapy within the broader management algorithm for difficult-to-treat ED.
Author Contributions
Conceptualization: V.T.C.; methodology: C.A.M.B. and L.L.H.; software: F.J.D.-C.A.; validation: M.T.R. and B.M.L.; formal analysis: F.G.-G.; investigation: V.T.C. and J.E.R.G.; resources: M.D.T.T.; data curation: F.G.-G.; writing—original draft preparation: V.T.C.; writing—review and editing: A.C.L. and J.E.R.G.; visualization: C.A.Y.R. and F.J.A.M.; supervision: J.E.R.G. and D.S.Z.; project administration: F.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ED | Erectile Dysfunction |
| PDE5-Is | Phosphodiesterase Type 5 Inhibitors |
| BoNT-A | Botulinum Toxin Type A |
| FDA | U.S. Food and Drug Administration |
| IIEF | International Index of Erectile Function |
| SHIM | Sexual Health Inventory for Men |
| EHS | Erection Hardness Score |
| CIs | Confidence Intervals |
| RCT | Randomized Controlled Trial |
| NO | Nitric Oxide |
References
- Pang, K.H. The effectiveness and safety of intracavernosal botulinum toxin injections in the management of erectile dysfunction: A systematic review and meta-analysis of clinical studies. Sex. Med. 2025, 13, qfaf034. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.; Denys, P.; Joussain, C. Safety and Effectiveness of Repeated Botulinum Toxin A Intracavernosal Injections in Men with Erectile Dysfunction Unresponsive to Approved Pharmacological Treatments: Real-World Observational Data. Toxins 2023, 15, 382. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.; Joussain, C.; Denys, P. Safety and Efficacy of Intracavernosal Injections of AbobotulinumtoxinA (Dysport®) as Add on Therapy to Phosphosdiesterase Type 5 Inhibitors or Prostaglandin E1 for Erectile Dysfunction—Case Studies. Toxins 2019, 11, 283. [Google Scholar] [CrossRef]
- Abdelrahman, I.F.S.; Raheem, A.A.; Elkhiat, Y.; Aburahma, A.A.; Abdel-Raheem, T.; Ghanem, H. Safety and efficacy of botulinum neurotoxin in the treatment of erectile dysfunction refractory to phosphodiesterase inhibitors: Results of a randomized controlled trial. Andrology 2022, 10, 254–261. [Google Scholar] [CrossRef]
- Chung, E. A review of current and emerging therapeutic options for erectile dysfunction. Med. Sci. 2019, 7, 91. [Google Scholar] [CrossRef]
- Duncan, C.; Omran, G.; Teh, J.; Davis, N.; Bolton, D.; Lawrentschuk, N. Erectile dysfunction: A global review of intracavernosal injectables. World J. Urol. 2019, 37, 1007–1014. [Google Scholar] [CrossRef]
- Moradi, S.; Khazaeli, D.; Dadfar, M.; Bakhtiari, N. Efficacy of Intracavernosal Injections of 50-Unit versus 100-Unit Doses of AbobotulinumtoxinA (Masport®) in Vasculogenic Erectile Dysfunction with Phosphodiesterase Type 5 Inhibitors Resistant. Nephrourol. Mon. 2022, 14, e119131. [Google Scholar] [CrossRef]
- Zahr, R.A.; Kheir, G.B.; Mjaess, G.; Jabbour, T.; Chalhoub, K.; Diamand, R.; Roumeguère, T. Intra-Cavernosal injection of botulinum toxin in the treatment of erectile dysfunction: A systematic review and meta-analysis. Urology 2022, 170, 5–13. [Google Scholar] [CrossRef]
- Ragab, M.W.; Ramzy, D.; Zidane, A.; El-Bohy, A.; Bendary, A.Z.; A Shawky, K.; El Shorbagy, G. (335) A Novel Ultrasound Technique Can Predict Efficacy of Botox Injection in Patients with Erectile Dysfunction. J. Sex. Med. 2024, 21, qdae041-012. [Google Scholar] [CrossRef]
- Langarizadeh, M.A.; Salary, A.; Tavakoli, M.R.; Nejad, B.G.; Fadaei, S.; Jahani, Z.; Forootanfar, H. An overview of the history, current strategies, and potential future treatment approaches in erectile dysfunction: A comprehensive review. Sex. Med. Rev. 2023, 11, 253–267. [Google Scholar] [CrossRef]
- Liu, M.; Chang, M.-L.; Wang, Y.-C.; Chen, W.-H.; Wu, C.; Yeh, S. Revisiting the regenerative therapeutic advances towards erectile dysfunction. Cells 2020, 9, 1250. [Google Scholar] [CrossRef]
- Giuliano, F.; Joussain, C.; Denys, P. Long Term Effectiveness and Safety of Intracavernosal Botulinum Toxin A as an Add-on Therapy to Phosphosdiesterase Type 5 Inhibitors or Prostaglandin E1 Injections for Erectile Dysfunction. J. Sex. Med. 2021, 19, 83–89. [Google Scholar] [CrossRef]
- Giuliano, F.; Denys, P.; Joussain, C. Effectiveness and Safety of Intracavernosal IncobotulinumtoxinA (Xeomin®) 100 U as an Add-on Therapy to Standard Pharmacological Treatment for Difficult-to-Treat Erectile Dysfunction: A Case Series. Toxins 2022, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Preto, M.; Keskin, H.; Vazquez, J.F.S.; Banthia, R.; Mahendran, T.; Deger, M.D.; Vinod, K.V.; Putra, R.; Sethupathy, T.; et al. Clinical effects and safety outcomes of platelet-rich plasma therapy in patients with vasculogenic erectile dysfunction: A systematic review and meta-analysis. World J. Men’s Health 2025, 43, e16. [Google Scholar] [CrossRef] [PubMed]
- Masterson, T.A.; Molina, M.; Ledesma, B.; Zucker, I.; Saltzman, R.; Ibrahim, E.; Han, S.; Reis, I.M.; Ramasamy, R. Platelet-rich plasma for the treatment of erectile dysfunction: A prospective, randomized, double-blind, placebo-controlled clinical trial. J. Urol. 2023, 210, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, H.; Raheem, A.; Abdelrahman, I.; Johnson, M.; Abdel-Raheem, T. Botulinum neurotoxin and its potential role in the treatment of erectile dysfunction. Sex. Med. Rev. 2018, 6, 135–142. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Cho, S.; Chung, H.; Rajasekaran, M. Novel emerging therapies for erectile dysfunction. World J. Men’s Health 2021, 39, 48–64. [Google Scholar] [CrossRef]
- Krivoborodov, G.G.; Kuzmin, I.V.; Slesarevskaya, M.N.; Efremov, N.S.; Gontar, A.A. Botulinum toxin therapy in urology: Historical aspect. Urol. Vedom. 2024, 14, 163–174. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Palea, S. Embracing the versatility of botulinum neurotoxins in conventional and new therapeutic applications. Toxins 2024, 16, 261. [Google Scholar] [CrossRef]
- Reddy, A.; Dick, B.; Natale, C.; Akula, K.; Yousif, A.; Hellstrom, W. Application of botulinum neurotoxin in male sexual dysfunction: Where are we now? Sex. Med. Rev. 2021, 9, 320–330. [Google Scholar] [CrossRef]
- Shaher, H.; Noah, K.; Abdelzaher, M.; Kandil, W.; Ahmed, I.S.; Nouh, I. Is bulbospongiosus muscle botox injection safe and effective in treating lifelong premature ejaculation? Randomised controlled study. World J. Urol. 2024, 42, 218. [Google Scholar] [CrossRef] [PubMed]
- Ragab, M.W.; Shawky, K.A.; Bendary, A.Z.E.F.; Albohy, A.-A.M. Penile shear-wave elastography predicts the outcome of botulinum neurotoxin (Botox) in the management of non-responders to phosphodiesterase-5-inhibitors: A pilot study. Arab. J. Urol. 2025, 23, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; EI-Tabey, M.; El-Shaer, W.; Ghanem, H.; Wahab, T. Comparative Study between Intracavernosal Injection of Botulinum Toxin type A [50 and 100 unit], Efficacy and Durability in the Treatment of Vascular Erectile Dysfunction. Benha J. Appl. Sci. 2019, 4, 31–35. [Google Scholar] [CrossRef]
- El-Shaer, W.; Ghanem, H.; Diab, T.; Abo-Taleb, A.; Kandeel, W. Intra-cavernous injection of BOTOX® (50 and 100 Units) for treatment of vasculogenic erectile dysfunction: Randomized controlled trial. Andrology 2021, 9, 1166–1175. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Raheem, A.; Abdelrahman, I.; Abdel-Raheem, T.; Tawfik, A.; Elkhayat, Y.; Ghanem, H. Botulinum neurotoxin in the treatment of erectile dysfunction. J. Sex. Med. 2022, 19, S9–S10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).