Abstract
The growing demand for sustainable alternatives to animal and synthetic leathers has accelerated interest in mycelium-based materials as an eco-friendly solution for the fashion industry. This study explores the potential of mushroom mycelium to create leather-like materials that align with circular fashion principles. Five species of edible and medicinal mushrooms were cultivated on sawdust substrates and evaluated for their growth performance, physical properties, and suitability as leather substitutes. Growth analysis revealed distinct species-specific behaviors: Cubamyces flavidus and Lentinus squarrosulus exhibited rapid colonization, achieving full substrate coverage within five days and forming dense mycelial networks at 14 days. In contrast, despite growing more slowly, Sanghuangporus vaninii and Ganoderma gibbosum formed thicker, more compact mats that might be suitable for strong leather-like materials. Visual and structural assessments showed diverse textures, colors, and hyphal architectures resembling natural leather. Physical characterization revealed shrinkage ranging from 13.17% to 24.09%, higher than for cow tanned leather (>5%) and PU microfiber (0.1–1.2%), suggesting a need for stabilization treatments. Apparent densities ranged from 0.13 g/cm3 to 0.30 g/cm3, lower than those of cow leather (0.49 g/cm3) and PU leather (0.38 g/cm3), highlighting species-specific hyphal structures that influence flexibility, porosity, and strength. SEM imaging confirmed the presence of interwoven hyphal mats resembling the fibrous architecture of natural leather, with S. vaninii showing the most uniform and continuous structure. Water absorption was significantly higher in mycelium sheets, consistent with their microporous nature, though S. vaninii showed the lowest uptake, reflecting possible natural water absorption. Thermogravimetric analysis revealed three-stage degradation profiles, with S. vaninii and G. gibbosum retaining >35% mass at 400 °C, indicating strong thermal stability for processing techniques such as hot pressing and finishing. Overall, the results demonstrate mycelium-based leathers as a biodegradable, low-impact alternative that can replicate the visual and functional characteristics of traditional leather, with opportunities for further improvement in substrate optimization, eco-tanning, surface coating, and scalable production toward a sustainable fashion future.
1. Introduction
The growing need to address environmental degradation and reduce the ecological footprint of industries has driven innovation in sustainable material development [1]. Among these, the fashion industry, which has long been criticized for its environmental effect and resource-intensive methods, is aggressively looking at substitutes for conventional textiles, especially leather made from animals [2]. Globally, the leather industry is estimated to produce over 130 million tons of carbon dioxide emissions annually and generate approximately 25% of all chromium-containing waste released into the environment [3,4]. Traditional leather production not only relies heavily on livestock farming, which contributes greenhouse gas emissions, deforestation for raising cattle, high energy and water consumption, and waste management issues, but also involves tanning processes that utilize toxic chemicals such as chromium, posing serious risks to ecosystems and human health [5]. In response to these challenges, researchers and designers are turning toward bio-based, biodegradable, and cruelty-free materials that align with eco-friendly values and support the global movement toward circular economy models [6]. One such possible alternative is mycelium-like leather, a material derived from the vegetative root-like structure of fungi, which offers a sustainable and adaptable substitute for synthetic and animal-based leather [7].
Mycelium, the filamentous network of fungal hyphae, is well-known for its ability to grow rapidly on various lignocellulosic agricultural wastes, forming dense, fibrous mats that can be engineered into a range of shapes and textures. These unique properties make mycelium an attractive choice for use in the creation of leather-like materials that replicate the texture, flexibility, and appearance of animal leather [8]. The production process of mycelium-like leather (often referred to as “myco-leather”) involves cultivating various fungal strains under controlled conditions, followed by processing techniques such as drying and cross-linking treatment [9,10,11]. Mycelium-like leather is a feasible option for eco-conscious and sustainable fashion manufacturers together because it is renewable, biodegradable (over 90% degradation in natural environments within a few months), and requires less resources to produce than synthetic or animal-based alternatives [12,13].
This study explores the potential of mushroom mycelium from various fungal species to be developed into leather-like materials suitable for sustainable fashion applications, with the objective of obtaining viable alternatives to conventional leather. The research focuses on cultivating selected fungal species on rubber sawdust, an agro-industrial byproduct, to generate dense mycelial mats suitable for material testing. These mats were then tested through a number of primary physical and structural assessments. These properties provide information about the potential of mycelium-like leather to achieve the technical demands of fashion materials, including durability, ease, and appearance. The findings of this research not only demonstrate the viability of using mushroom mycelium as an alternative leather source but also additionally uncover important characteristics that influence its performance in various applications.
While the results are promising, there remain several challenges and opportunities for future development. Ensuring consistent quality, scalability of production, resistance to wear and tear, and consumer acceptance are critical steps before mycelium-like leather can compete with traditional materials in mainstream markets [7]. Additionally, surface coating, tanning, and combination layering techniques may be needed to enhance specific properties such as tensile strength and waterproofing [8,14,15,16]. Nonetheless, with growing consumer demand for ethical and sustainable alternatives, the integration of mycelium-like leather into the fashion industry presents an interesting pathway toward more responsible consumption and production patterns [17].
Thus, this study aims to link the knowledge divide between material science, biological innovation, and trends by providing a comprehensive examination of the structural and physical characteristics of leather-like materials made from mycelium in comparison to commonly used leather materials that include cow tanned leather and polyurethane microfiber leather. It also presents practical methods and ideas for simple, accessible production that link recently developed techniques for inducing mycelium-like leather with conventional methods reported in previous studies. Together, these investigations and methodological outcomes provide significant information on the properties of specific fungal species and the improvement of production processes. Furthermore, the study looks at future prospects, potential technological advancements, and key challenges that must be addressed to support the transition from experimental prototypes to sustainable, commercially viable products.
2. Materials and Methods
2.1. Source of Mushroom Mycelium and Inoculum Preparation
Five species of edible and medicinal mushroom mycelia were selected for this study based on their rapid growth and high potential for consistency in density, structure, and surface texture, which are key characteristics for producing efficient leather-like materials. These species were obtained from the culture collections of the Saisamorn Lumyoung Laboratory Culture Collection (SLLC), Faculty of Science, Chiang Mai University. The selected species included C. flavidus (CMU-AM011), G. gibbosum (CMU-WE017), L. squarrosulus (CMU-WE001), P. similis (CMU-AM006), and S. vaninii (CMU-WE058). Prior to use, each species was sub-cultured onto potato dextrose agar (PDA) plates and incubated at 30 °C for seven days in darkness to preserve active growth [18].
For inoculum preparation, sorghum grains were used as a nutrient-rich substrate. The grains were thoroughly washed and boiled for 20 min. After boiling, remaining water was carefully drained to prevent excess moisture during sterilization. A total of 150 g of the prepared grains were then put into glass bottles, sealed with cotton plugs, and sterilized by autoclaving at 121 °C for 20 min. Once cooled to room temperature, each bottle was inoculated with five 5 mm diameter mycelial plugs taken from the PDA cultures of the respective mushroom species. The inoculated bottles were then incubated at 30 °C for two weeks, allowing the mycelium to fully colonize the sorghum substrate [19].
2.2. Substrate Use and Preparation
Rubber tree sawdust was selected as the primary substrate for this study. The sawdust was sourced from a sawmill in northern Thailand. Prior to use, it was sieved to obtain particles between ≤2 mm in size and then oven-dried at 60 °C until completely moisture-free, as investigated by visual inspection and stable weight measurements [20].
2.3. Measurement of Mycelial Development on Sawdust
Sawdust was combined with the nutritional supplement for mushroom growing (5% rice bran, 1% calcium carbonate, 2% calcium sulfate, and 0.2% sodium sulfate). The mixtures were then adjusted to 60% relative humidity by adding water. A 10 g portion of each substrate mixture was placed onto a glass plate (90 mm in diameter) and sterilized at 121 °C for 60 min. After cooling, a 10 mm mycelial disk was placed at the center, and plates were incubated at 30 °C for 14 days [18]. Mycelial growth was measured daily by recording the colony diameter along two perpendicular axes, and the radial growth rate (mm/day) was calculated based on the increase in average colony diameter over time. Growth density was evaluated on day 7 and at the end of incubation, classified as very thin (+), thin (++), thick (+++), or very thick (++++) following the criteria described by Raman et al. [12]. Each treatment was conducted in five replicates.
2.4. Mushroom Mycelium Cultivation on Substrate
The dried substrate was mixed with the nutritional supplement (as described in Section 2.3) based on dry weight, and adjusted to a final moisture content of approximately 60% relative humidity. A total of 600 g of the prepared substrate was packed into polypropylene bags (3.5 × 12.5 in). Each bag was sealed using cotton-plugged polyvinyl chloride pipe rings and covered with paper. The bags were sterilized in an autoclave at 121 °C for 60 min. After sterilization, the bags were allowed to cool to room temperature over 24 h. Each bag was then inoculated with six grams of mycelial culture, achieving a substrate-to-inoculum ratio of 100:1 (w/w). The inoculated bags were incubated in darkness at 30 °C for 30 days, or until the mushroom mycelium fully colonized the substrate [21].
2.5. Induction of Mycelium-like Leather
Fully colonized substrate bags for each mushroom species were used to induce mycelium-like leather following modified methods from previous studies (Figure 1) [12,22,23]. The colonized spawn substrate was carefully removed from the bags and placed in polypropylene plastic boxes (38.8 × 28.3 × 14.2 mm3), then incubated in the dark at 28–30 °C (room temperature) with 80–90% humidity for 30 days to allow for further mycelial growth around the outer surfaces. After 30 days, dense mycelial mats were carefully separated from the substrate and cut into sheets measuring 10 × 20 cm2. Any residual substrate adhering to the sheets was carefully scraped off using a scalpel and cleaned with a soft brush. The fresh volume of each sheet was recorded to calculate average shrinkage. Sheets were then air-dried for 24 h, followed by oven-drying at a controlled temperature (70 °C) for 24–48 h until a constant weight was achieved [12]. The dried mycelial sheets were then stored in desiccators for further experiments.

Figure 1.
An overview of the primary processes involved in inducing mycelium-like leather in this study: (A) colonized spawn substrate, (B) incubation of the spawn, (C) development of dense mycelial mats, and (D) separation of the compact mats from the substrate.
2.6. Investigating the Physical Properties and Characteristics of Mycelium-like Leather
2.6.1. Assessing Visual Characteristics
A preliminary visual assessment was carried out to examine the morphological features of the mycelium-like leather sheets after drying, following a modified protocol adapted from Crawford [23]. Comparative observations were conducted to evaluate overall appearance, with particular attention given to color and texture. Color characterization was recorded using the Methuen Handbook of Colour as a reference standard [24], focusing on the parts with the most surface area to achieve similarity. In addition to surface appearance, each sheet was systematically analyzed according to mushroom species, decay type, and hyphal system, based on the morphological characteristics of their fruiting bodies. The classification followed the guidelines provided in manuals of common edible and medicinal mushrooms, complemented by relevant academic literature [25,26,27,28]. These parameters provided a comprehensive dataset crucial for identifying the most suitable fungal species for high-quality mycelium-like leather production.
2.6.2. Shrinkage and Density Measurements
Shrinkage of the mycelium-like leather sheets was measured by comparing their wet and dry volumes, which were calculated from measurements of length, width, and thickness, following the modified method described by Pahlawan & Griyanitasari [29]. The shrinkage percentage was calculated using the formula: Shrinkage (%) = [(V1 − V2)/V1] × 100, where V1 represents the wet volume and V2 the dry volume of the sample. After shrinkage measurements, the dried mycelium-like leather sheets were cut into 5 × 5 cm2 squares. Density was determined using the dry mass and volume, following the method described by Raman et al. [12] and adapted to the International Organization for Standardization (ISO) 2420 [30]. Each sample was measured five times for certainty. The resulting differences in physical characteristics among the mycelium-like leather sheets are illustrated in Figure 2 for comparative analysis.
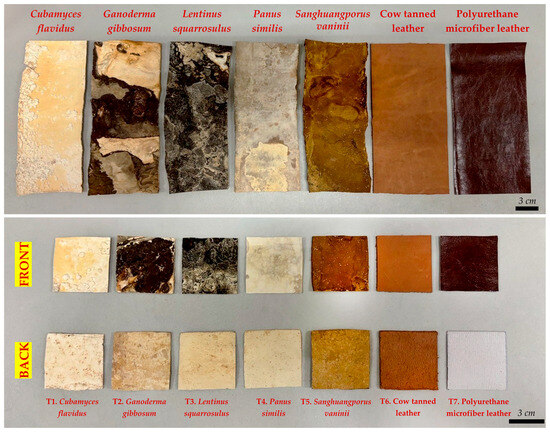
Figure 2.
Comparative specifications of mycelium-like leather sheets versus conventional synthetic and animal-derived leather.
2.6.3. Scanning Electron Microscope Observation
The surface structures of the mycelium-like leather sheets were examined using scanning electron microscopy (SEM JSM-IT300, JEOL, Tokyo, Japan). Dehydrated sheets were cut into small squares approximately 5 × 5 mm2 with a scalpel. These pieces were then mounted on 10 mm2 stub adapters using 2 mm2 double-sided carbon tape. The samples were coated with gold for two minutes under high vacuum conditions to enhance conductivity. SEM imaging was performed at a high voltage of 15 kV at the Science and Technology Service Center, Faculty of Science, Chiang Mai University, Thailand. Surface structures from each treatment group were analyzed by comparing the captured SEM images to identify differences [21].
2.6.4. Water Absorption Testing
The surface hydrophobicity of the mycelium-like leather sheets was assessed by measuring water absorption, following a modified method from Zhang et al. [31]. The samples were cut into small pieces (approximately 5 × 5 cm) and weighed to obtain the initial mass (W0). Each piece was then submerged in a beaker containing an appropriate volume of water and removed at intervals of 30, 60, 90, 150, 210, 270, and 330 min. After removal, the surface water was gently blotted using filter paper, and the wet mass (Wt) was recorded. The following formula was used to determine water absorption: Water absorption (%) = (Wt − W0)/W0 × 100. Each sample was tested five times, and the average value was reported.
2.6.5. Thermogravimetric Analysis (TGA)
The thermal degradation behavior of mycelium-like leather sheets was analyzed using a thermogravimetric analyzer (Rigaku: Thermo Plus EVO2, Nihon Rigaku Co., Tokyo, Japan). Approximately 2.5–3.0 mg of each sample was placed in an alumina crucible and heated from 25 °C to 600 °C at a constant rate of 10 °C/min under a nitrogen atmosphere [12].
2.7. Statistical Analysis
Statistical analysis of the experiments was performed using one-way analysis of variance (ANOVA) with SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test was applied to determine significant differences between mean values at a significance level of p ≤ 0.05.
3. Results and Discussion
3.1. Mycelial Growth Characteristics
The mycelial growth characteristics of the five edible and medicinal mushroom species cultivated on sawdust substrates revealed notable differences in growth rates, density, and expansion patterns (Figure 3), providing considerable guidelines for developing leather-like materials. Cubamyces flavidus and L. squarrosulus exhibited the highest growth rates, 16.77 mm/day and 16.39 mm/day, respectively (Figure 3B), exhibiting full plate colonization around day 5 (Figure 3A), demonstrating rapid substrate colonization and high potential for producing dense mycelial mats (Figure 3C). Their mycelial density was also high, particularly for C. flavidus, which achieved a rating of +++ at day 7 and ++++ at day 14 (Figure 4), indicating an excellent and tightly interwoven hyphal network suitable for forming leather-like textures. Panus similis displayed moderate growth (13.70 mm/day), achieving complete expansion by day 6–7, while G. gibbosum and S. vaninii showed slower growth rates (12.17 mm/day and 11.00 mm/day, respectively), requiring until day 8 for full plate coverage. Despite slower rates, these species still formed dense mycelial networks over time, suggesting potential for applications requiring thicker or more resilient material structures.
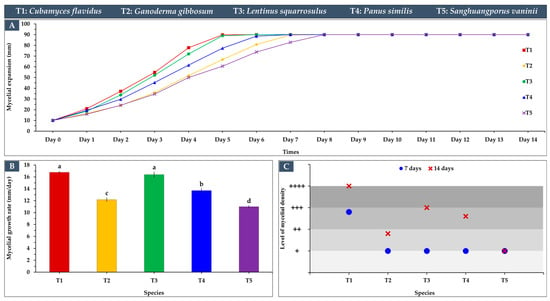
Figure 3.
Mycelial growth characteristics of five edible and medicinal mushroom species cultivated on sawdust substrates: (A) mycelial expansion patterns, (B) growth rate across the incubation period (means ± SD), with statistical differences indicated by letters (a–d) using Duncan’s multiple range test (p ≤ 0.05), and (C) mycelial density levels.
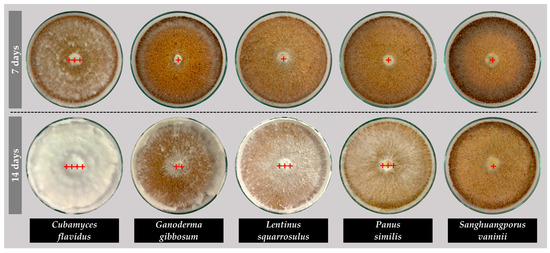
Figure 4.
Mycelial density of five edible and medicinal mushroom species grown on sawdust-based substrates. Density level was defined as very thin (+), thin (++), thick (+++), and very thick (++++) and is indicated by red plus marks.
The expansion patterns demonstrated that rapid early growth, as seen in C. flavidus and L. squarrosulus, contributed to uniform coverage and denser mycelial mats, which are critical factors in achieving leather-like texture, durability, and reducing the risk of contamination [12]. Conversely, slower-growing species such as S. vaninii and G. gibbosum may require longer cultivation periods or optimization of substrate composition to reach comparable density and uniformity. These differences highlight the fact that mycelial growth characteristics vary widely among species, influenced by critical factors such as culture type, substrate composition, and ambient environmental conditions [32].
These results suggest that species selection and management of mycelial growth parameters are key to producing sustainable, eco-friendly leather-like materials, aligning with current trends in green fashion by utilizing renewable fungal biomass. Overall, the study demonstrates that edible and medicinal mushrooms possess diverse growth characteristics that can be strategically harnessed to develop biodegradable and high-performance fungal materials for long-term uses.
3.2. Physical Properties and Characteristics
3.2.1. Visual Characteristics
The visual assessment of mushroom mycelium skin revealed distinct differences in color, texture, and surface uniformity among the species (Table 1 and Figure 2), which are critical for evaluating their potential as mycelium-based material products for sustainable design applications [33]. Cubamyces flavidus exhibited a range of whitish to yellowish tones, from pale yellow to light orange, with a predominantly smooth surface interrupted by scattered primordia-like bumps. This combination of light coloration and subtle texture variation suggests potential for applications where a delicate, natural appearance is required. On the other hand, G. gibbosum exhibited a broader color spectrum, ranging from whitish and orange-white to reddish golden, brown, and deep brown shades. Its surface was mostly rough with noticeably coarse areas, indicating a more rugged and textured appearance that could mimic certain vintage leather styles but may require post-processing for uniformity. Lentinus squarrosulus produced sheets with a consistent rough and hard texture, spanning whitish and pale orange to dark brown and brownish gray. The uniform roughness and dense appearance suggest strong structural integrity but a less pliable feel compared to smoother mycelium sheets, which might make it as suitable for applications where durability is prioritized over softness. Panus similis showed a smooth, largely uniform surface across a gradient from whitish and orange-white to rich brown shades, with only minor rough areas. Its balance of smoothness and visual depth makes it an achievable possibility for high-quality mycelium-like leather with both esthetic appeal and functional flexibility. Interestingly, S. vaninii demonstrated the smooth and most consistent surface among the mushroom mycelium sheets, with only minor variations in texture. Its uniform appearance and refined tactile quality closely resemble conventional leather, suggesting its possibilities for exclusive, decorative uses.

Table 1.
Initial characteristics and classification of mushroom mycelia to determine the best possible species for high-quality mycelium-like leather.
For comparison, cow tanned leather displayed a warm brownish orange palette with a consistently smooth surface, which reflected the beautiful look of actual leather products [40]. PU microfiber leather exhibited deep chocolate brown tones with an exceptionally smooth and uniform surface, typical of synthetic leathers designed for a signature look [41].
These observations suggest that mycelium-like leather sheets, particularly those from P. similis and S. vaninii, can achieve primary visual and textural qualities similar to traditional leather. Variations in color and roughness across species may offer opportunities of personalizing feeling and esthetic characteristics for various design uses. The ability to balance smoothness, texture, and natural coloration supports the feasibility of using mushroom mycelium as a sustainable, eco-friendly alternative to conventional leather, aligning with contemporary trends in environmentally conscious design.
3.2.2. Shrinkage and Density
The physical evaluation of mycelium-like leathers revealed notable differences in shrinkage and density among the species studied (Table 2), as well as in comparison to traditional cow tanned and PU microfiber leathers. Shrinkage values varied significantly, with C. flavidus and P. similis exhibiting the highest shrinkage rates at 24.09% and 22.68%, respectively, while G. gibbosum displayed the lowest among the mycelium species at 13.17%. Sanghuangporus vaninii and L. squarrosulus showed average shrinkage values of 18.96% and 21.44%, respectively. Compared to cow tanned leather with shrinkage greater than 5% and PU microfiber leather with minimal shrinkage (0.1–1.2%), the mycelium-based materials generally exhibited a lack of dimensional stability [14,42], suggesting that post-processing stabilization may be needed to improve shape retention.
Apparent density is a key parameter for evaluating materials, as it serves as a reliable predictor of other important properties such as porosity, water absorption, mechanical strength, and thermal properties, making it a valuable indicator of overall mechanical performance [14,43]. Cow tanned leather had the highest density at 0.49 g/cm3, reflecting its tightly packed collagen matrix, followed by PU microfiber leather at 0.38 g/cm3. Among the mycelium-based leathers, G. gibbosum (0.30 g/cm3) and S. vaninii (0.21 g/cm3) were relatively denser, whereas C. flavidus (0.13 g/cm3) and P. similis (0.14 g/cm3) exhibited lower densities, indicating more porous, loose hyphal networks. For L. squarrosulus, the density was average at 0.17 g/cm3. These differences in density correlate with the microstructural observations, where looser hyphal arrangements create lighter, while compact and continuous mycelial networks may improve strength, flexibility, and shape retention [44].

Table 2.
Overall physical properties of mycelium-like leathers produced from the mycelia of various edible and medicinal mushroom species in comparison to traditional synthetic and animal leathers.
Table 2.
Overall physical properties of mycelium-like leathers produced from the mycelia of various edible and medicinal mushroom species in comparison to traditional synthetic and animal leathers.
| Species/Types | Shrinkage (%) | Thickness (mm) | Weight (g) | Density (g/cm3) |
|---|---|---|---|---|
| T1. Cubamyces flavidus | 24.09 ± 1.37 a | 1.29 ± 0.20 c | 0.42 ± 0.08 d | 0.13 ± 0.01 f |
| T2. Ganoderma gibbosum | 13.17 ± 3.39 c | 1.87 ± 0.26 b | 1.40 ± 0.18 b | 0.30 ± 0.02 c |
| T3. Lentinus squarrosulus | 21.44 ± 1.38 ab | 1.06 ± 0.05 c | 0.44 ± 0.04 d | 0.17 ± 0.02 e |
| T4. Panus similis | 22.68 ± 1.02 a | 0.50 ± 0.04 d | 0.17 ± 0.02 e | 0.14 ± 0.01 f |
| T5. Sanghuangporus vaninii | 18.96 ± 1.61 b | 1.76 ± 0.41 b | 0.92 ± 0.23 c | 0.21 ± 0.03 d |
| T6. Cow tanned leather | >5 [45] | 2.39 ± 0.05 a | 2.91 ± 0.12 a | 0.49 ± 0.02 a |
| T7. PU microfiber leather | 0.1–1.2 [46] | 1.06 ± 0.03 c | 1.02 ± 0.02 c | 0.38 ± 0.01 b |
Note: Values are presented as the mean ± standard deviation. Within each species or type of leather, values followed by different letters in the same column are significantly different, as determined by Duncan’s multiple range test (p ≤ 0.05).
The combination of shrinkage and density data suggests that the mechanical and dimensional properties of mycelium-like leathers can be tuned by selecting specific fungal species and optimizing growth conditions. Lower-density species such as C. flavidus and P. similis may offer enhanced softness and breathability, whereas denser species like G. gibbosum and S. vaninii could provide greater durability and structural integrity. Compared with traditional leathers, mycelium-based materials present a unique balance between lightweight, flexibility, and eco-friendly biodegradability, supporting their potential use in green fashion applications. Post-processing treatments, including controlled drying, pressing, and substrate refinement, could further improve shrinkage control and density uniformity, producing mycelium leathers that meet industry standards for performance and esthetics.
3.3. Surface Structures of Mycelium-like Leather Sheets
SEM offered significant information into the microstructural properties of the mycelium-like leather sheets compared with traditional cow leather and PU microfiber leather (Figure 5). The overall surface textures of the mycelium-derived materials revealed unique natural patterns that closely simulate the textured appearance of animal leather, with some species such as L. squarrosulus, P. similis, and S. vaninii displaying more consistent and compact surface morphology. The front surfaces of the mycelium sheets exhibited a dense network of interwoven hyphae, forming a continuous and cohesive mat. This interconnected hyphal arrangement is essential for imparting mechanical strength, flexibility, and the leather-like feel that is desirable for fashion applications [12,44].
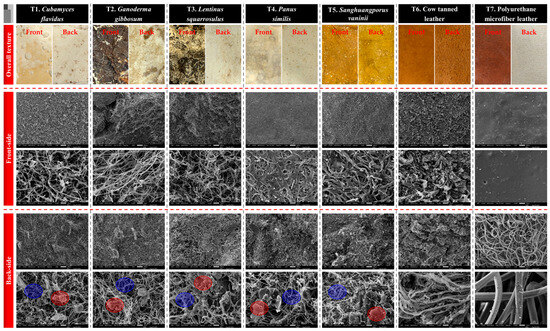
Figure 5.
Scanning electron microscope images showing the surface structures of mycelium-like leather sheets derived from the mycelia of five edible and medicinal mushroom species within study, as versus traditional synthetic and animal leathers. The front and back surfaces are presented, with blue highlights indicating areas of pure mycelium, while red highlights mark regions on the backside where sawdust particle residues remain.
On the backside, SEM images highlighted notable differences among species. Blue-highlighted regions represented areas of pure, well-colonized mycelium, whereas red-highlighted areas indicated residual sawdust particles partially contained within the mycelial network. These residues were more apparent in mycelium sheets than in cow leather or PU leather, suggesting that optimizing substrate composition or improving post-harvest cleaning could enhance surface purity and uniformity.
When compared to cow tanned leather, which displayed a fibrous collagen matrix [47,48], the mycelium sheets appeared simpler and more porous, providing enhanced breathability, and lightweight characteristics [11,49]. PU microfiber leather, on the other hand, exhibited a highly uniform and synthetic surface lacking the natural fibrous microstructure, pores, and smell found in both cow leather or mycelium sheets [50]. This structural difference highlights the inherent advantage of mycelium-based materials in offering a natural, biodegradable alternative with a microstructure that supports both physical comfort and environmental compatibility.
All things considered, the SEM results demonstrate that mycelium-like leathers can potentially approach the hierarchical structure of conventional leathers while providing unique opportunities to modify texture, porosity, and physical characteristics by carefully selecting the appropriate substrate and growth conditions. The presence of sawdust residues on the back surface may be further minimized through substrate refinement or surface finishing treatments, leading to a more consistent product that aligns with the expectations of eco-conscious fashion industries.
3.4. Water Absorption
The water absorption profiles of the mycelium-like leather sheets and conventional leathers over a 330 min period are presented in Figure 6. Distinct patterns were observed between the mycelium-based samples and the conventional controls (cow tanned leather and PU microfiber leather). Most of the mycelium-based samples exhibited rapid water uptake within the first 90 min, followed by a gradual stabilization phase. Among the tested species, P. similis demonstrated the highest water absorption capacity, reaching 560.67% at 330 min, whereas S. vaninii absorbed considerably less, with a final value of 177.26%. Notably, L. squarrosulus showed a balanced absorption profile, achieving 375.18% at 330 min, suggesting a dense yet moderately porous mycelial network. C. flavidus and G. gibbosum followed similar patterns, stabilizing at 319.77% and 283.43%, respectively. In contrast, the conventional leather controls exhibited minimal water uptake. PU microfiber leather absorbed only 34.22%, while cow tanned leather showed the lowest overall absorption (33.74%), indicating strong resistance to water penetration compared with the mycelium-like leathers.

Figure 6.
Water absorption behaviors of mycelium-like leather sheets compared with conventional leathers. Data are presented as the mean values, with error bars representing ± standard deviation. Asterisks (*) indicate statistically significant differences at each point, as determined by Duncan’s multiple range test (p ≤ 0.05).
The differences in water absorption among fungal species can be attributed to variations in overall density, microporous structure, and the characteristics of the outer hydrophobic layer. Mycelium with higher density and more compact hyphal networks tends to exhibit lower water absorption, whereas more porous structures allow greater moisture uptake [51,52]. Overall, the results clearly demonstrate that mycelium-based leather sheets exhibit markedly higher water absorption than conventional animal-derived and synthetic leathers. This tendency arises from the characteristic porosity and water-attracting nature of fungal mycelia, which form capillary-like networks that easily get and retain moisture [53,54]. For example, P. similis, L. squarrosulus, and C. flavidus likely develop looser overall mycelial networks (both front and backside) with larger capillary spaces, leading to greater water uptake, while S. vaninii forms denser, more compact structures that provide better resistance to moisture. These findings align with previous studies, which highlight that the porous structure of mycelial skin and its biochemical composition play key roles in influencing mechanical strength, structural characteristics, as well as water barrier performance [14,54,55]. Notably, water absorption in mycelium-based leathers and pure mycelium materials generally falls within the range of 53.08–496% [47,56,57], highlighting both their potential and the need for further material optimization.
From a sustainability perspective, while the high-water absorption of mycelium leathers poses a challenge for practical use in fashion applications, it also provides opportunities for targeted material engineering. Strategies such as surface modification, blending with hydrophobic biopolymers, or incorporating natural waxes could significantly reduce water uptake while maintaining eco-friendly attributes. Importantly, the data suggest that selective species choice, favoring naturally lower-absorption fungi like S. vaninii, may already offer a viable starting point for developing water-resistant mycelium leathers without extensive modification. All things considered, mycelium-like leathers offer beneficial structural and sustainable advantages, though controlling water absorption is essential to following conventional leather standards in eco-friendly fashion.
3.5. Thermogravimetric Analysis
TGA revealed distinct thermal degradation profiles for mycelium-like leather materials compared with conventional leathers (Figure 7), offering critical details into their thermal stability and potential application in sustainable fashion. All mycelium-derived samples (C. flavidus, G. gibbosum, L. squarrosulus, P. similis, and S. vaninii) exhibited a three-stage weight loss pattern: an initial minor mass loss below 150 °C associated with moisture evaporation, followed by a major decomposition phase between 200 and 400 °C, and a final slow degradation phase above 450 °C linked to the breakdown of chitin, carbohydrates, amino acids, and other cell-wall components [58]. Among the fungal species tested, S. vaninii and G. gibbosum demonstrated slightly higher thermal stability, retaining more than 35% of their mass at 400 °C, suggesting a denser, more thermally resistant network structure.

Figure 7.
Thermogravimetric analysis of mycelium-like leathers derived from the mycelia of five edible and medicinal mushroom species investigated in this study, compared with conventional synthetic and animal leathers.
When compared with cow tanned leather and PU microfiber leather, mycelium-like leather sheets exhibited comparable or even superior thermal degradation behavior in the mid-temperature range (250–400 °C), with some species such as L. squarrosulus closely replicating the thermal profile of animal leather. Notably, PU microfiber leather showed a delayed onset of weight loss but a more gradual and extended degradation curve, reflecting its synthetic polymer composition. This property might provide better dimensional stability at moderate temperatures but raises concerns regarding recyclability and end-of-life environmental impact due to persistent polymer residues.
These results collectively indicate that mycelium-like leathers offer a combination between biodegradability and thermal stability that may be appropriate for use in designs. Their thermal performance is robust enough to withstand conventional product manufacturing processes such as hot pressing, stamping, and heavy steaming, while still aligning with the sustainability goals of biodegradability and lower carbon footprint. Optimizing substrate formulations and growth conditions could further enhance the thermal resistance of these biomaterials, bringing them even closer to, or surpassing, the performance of traditional leather products while offering a more circular and eco-friendlier alternative.
3.6. Further Activities and Future Perspectives
To advance the development of mushroom mycelium as a viable leather-like material aligned with eco-friendly and sustainable fashion trends, several future directions are proposed. A promising approach involves reinforcing mycelium with natural fibers such as cheesecloth, woven fabrics, and other biodegradable materials to improve structural strength, flexibility, and surface texture [12,59], offering a composite solution that remains biodegradable and sustainable.
In addition, the adoption of eco-friendly tanning methods presents a valuable opportunity to improve material performance while avoiding harmful chemicals used in conventional leather processing. Techniques such as vegetable tanning and enzymatic treatments (e.g., microbial proteases) can increase the leather’s softness, flexibility, and uniformity, while preserving its natural feel, without the need for substances like chrome, glycerol, ethylene glycol, or synthetic tanning oils [12,23,60].
Surface coating also plays a vital role in enhancing the performance, functionality, and esthetic appeal of mycelium leather. Biodegradable coating agents, like natural dyes, resins, oils, bio-paraffins, polylactic acid, and bio-based polymers, can be applied using techniques like air spraying, curtain coating, or dip coating. These coatings improve water resistance, color stability, and surface finish while staying true to the principles of sustainability [7,15,57]. Future research should also explore how mycelium leather responds to washing, including its resistance to wear, retention of color, and stability of texture, as well as its interactions with different detergents. Understanding these factors is essential for evaluating the durability and care requirements of fashion products made from this material. The determination of cleaning agents is generally equally important. For instance, strong or alcohol-based cleansers should be avoided since they may dry up or fade the material. Therefore, comfortable, pH-balanced solutions with natural ingredients should be used [61]. However, one key limitation that remains underexplored is the long-term stabilization and aging behavior of mycelium-like leather under various environmental conditions. Over time, environmental factors such as humidity and sunlight exposure could influence the material’s flexibility, color, and structural integrity. Investigating these aging mechanisms and developing stabilization strategies, such as natural cross-linking or eco-friendly maintaining treatments, will be essential to maximize the material’s long-term use and market acceptance.
Looking ahead, scaling up production through an adjusted process, particularly by increasing the size and length of mushroom spawns, will available the generation of larger mycelium-like leather sheets. These innovations will be integrated into the design and production of stylish, fully biodegradable prototypes, such as shoes, wallets, jackets, handbags, hats, handmade watch straps, belts, notebook covers, leather box sets, keychains, sheets, and other fashion accessories and garments (Figure 8). These next steps are essential to validating the material’s real-world performance, design versatility, and consumer appeal. Ultimately, this approach supports the change toward a circular fashion economy, reducing the environmental footprint of synthetic leathers and decreasing reliance on traditional animal-based products.

Figure 8.
Future directions and opportunities for incorporating mycelium-like leather innovations into the creation of stylish and completely biodegradable prototypes.
4. Conclusions
This study demonstrates the promising potential of mushroom mycelium and provides a comparative analysis of five species cultivated on sawdust substrates as a sustainable alternative for developing leather-like materials aligned with eco-friendly fashion trends. Among the five species evaluated, C. flavidus and L. squarrosulus exhibited the fastest mycelial growth rates, achieving full substrate colonization by day 5 and forming dense, tightly interwoven hyphal networks. Such rapid growth is critical for producing consistent, strong mats suitable for leather-like applications. Species with slower growth, including G. gibbosum and S. vaninii, required longer cultivation periods but still developed compact structures, suggesting their suitability for products requiring thicker or more durable materials. Visual and microstructural analyses indicated that P. similis and S. vaninii produced the smoothest and most uniform surfaces, closely resembling conventional cow leather, whereas C. flavidus, G. gibbosum, and L. squarrosulus offered unique textural features that might expand the range of possibilities. Shrinkage and density measurements demonstrated the adaptable physical properties of mycelium-like leathers: C. flavidus and P. similis exhibited higher shrinkage with lower densities, favoring softness and airflow, whereas denser species such as G. gibbosum and S. vaninii offered greater structural integrity. TGA revealed robust stability, with S. vaninii and G. gibbosum retaining over 35% mass at 400 °C, indicating their capacity to withstand conventional manufacturing processes while remaining biodegradable. Water absorption studies further illustrated species-specific behavior, with P. similis exhibiting the highest uptake and S. vaninii the lowest, demonstrating the significance of structural density in moisture resistance. Collectively, these findings highlight that mycelium-like leathers can combine the desirable mechanical strength, flexibility, and esthetic appeal with environmentally sustainable attributes. Future optimization through substrate refinement, eco-friendly tanning, surface coatings, and fiber reinforcement can enhance performance, water resistance, and uniformity. With these advancements, mycelium holds strong potential as a renewable and biodegradable alternative to animal and synthetic leather, offering opportunities to support the circular economy, minimize environmental impact, and drive innovation in sustainable fashion.
Author Contributions
Conceptualization, W.A. and S.L.; methodology, W.A., T.P., Y.K., A.R., P.P., P.S. and O.X.; investigation, W.A., T.P., Y.K., A.R., P.P. and S.L.; software, W.A. and P.J.; validation, W.A., T.P., Y.K., A.R., P.P., P.J. and S.L.; formal analysis, W.A., T.P., Y.K., A.R., P.P., P.J., O.X., K.J. and S.L.; data curation, W.A., P.J., O.X., K.J. and S.L.; writing—original draft preparation—W.A., P.J., P.S., O.X., K.J. and S.L.; writing—review and editing—W.A., T.P., Y.K., A.R., P.P., P.J., P.S., O.X., K.J. and S.L.; supervision, S.L.; project administration, W.A.; funding acquisition, W.A. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the CMU Proactive Researcher program, Chiang Mai University (Grant No. 784/2567), Chiang Mai, Thailand. Additional funding was provided by the National Research Council of Thailand (NRCT) under the 2024 fiscal year grant for the New Researcher Career Path (Grant No. N42A670707), Bangkok, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research work was partially supported by Chiang Mai University, Chiang Mai, Thailand. Saisamorn Lumyong also acknowledges partial support from the Academy of Science, The Royal Society of Thailand.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gu, W. Research on strategy optimization of sustainable development towards green consumption of eco-friendly materials. J. King Saud. Univ. Sci. 2024, 36, 103190. [Google Scholar] [CrossRef]
- Kellen, C.; Zhafirah, L.; Kasih, T.P. Transforming fast fashion: Exploring bio-leather as a sustainable alternative. In Proceedings of the 8th International Conference on Eco Engineering Development, Semarang, Indonesia, 6–7 November 2025; IOP Conference Series: Earth and Environmental Science. p. 012039. [Google Scholar]
- Yorgancioglu, A.; Başaran, B.; Sancakli, A. Value Addition to Leather Industry Wastes and By-Products: Hydrolyzed Collagen and Collagen Peptides. In Waste in Textile and Leather Sectors; IntechOpen: London, UK, 2020; pp. 1–26. [Google Scholar]
- Sointukangas, K.; Pulkkis, N. Meet the Scientists of the Future Bioeconomy Part 5: Engineering Fungal Mycelium to Produce Leather Without Cows. Available online: https://www.bioeconomy.fi/meet-the-scientists-of-the-future-bioeconomy-part-5-geza-szilvay-is-engineering-fungal-mycelium-to-produce-leather-without-cows/ (accessed on 20 September 2025).
- Speranskaya, O. The Environmental Impact of Leather Production on Climate Change. Available online: https://sustainfashion.info/the-environmental-impact-of-leather-production-on-climate-change/ (accessed on 25 July 2025).
- Dhanda, V.; Arsalan, S.; Kaushal, S. Revolutionizing material: The rise of bio leather as eco-friendly and sustainable approach. Int. J. Agron. 2024, 7, 121–128. [Google Scholar] [CrossRef]
- Birlie, A.A. Transforming the leather industry: A comprehensive review on leather alternatives. J. Renew. Mater. 2025, 13, 1783–1802. [Google Scholar] [CrossRef]
- Shin, H.J.; Ro, H.S.; Kawauchi, M.; Honda, Y. Review on mushroom mycelium-based products and their production process: From upstream to downstream. Bioresour. Bioprocess. 2025, 12, 3. [Google Scholar] [CrossRef]
- Blundell, R. Innovative Mycelium Composites: Pioneering Sustainable Solutions. Available online: https://www.independent.com.mt/articles/2024-06-30/health/Innovative-mycelium-composites-Pioneering-sustainable-solutions-6736262323 (accessed on 25 July 2025).
- Crawford, A.; Miller, S.R.; Branco, S.; Fletcher, J.; Stefanov, D. Growing mycelium leather: A paste substrate approach with post-treatments. Res. Dir. Biotechnol. Des. 2024, 2, e6. [Google Scholar] [CrossRef]
- d’Errico, A.; Schröpfer, M.; Mondschein, A.; Safeer, A.A.; Baldus, M.; Wösten, H.A. Cross-linking impacts the physical properties of mycelium leather alternatives by targeting hydroxyl groups of polysaccharides and amino groups of proteins. Heliyon 2024, 10, e36263. [Google Scholar] [CrossRef]
- Raman, J.; Kim, D.S.; Kim, H.S.; Oh, D.S.; Shin, H.J. Mycofabrication of mycelium-based leather from brown-rot fungi. J. Fungi 2022, 8, 317. [Google Scholar] [CrossRef]
- Jones, M.; Gandia, A.; John, S.; Bismarck, A. Leather-like material biofabrication using fungi. Nat. Sustain. 2021, 4, 9–16. [Google Scholar] [CrossRef]
- Amobonye, A.; Lalung, J.; Awasthi, M.K.; Pillai, S. Fungal mycelium as leather alternative: A sustainable biogenic material for the fashion industry. Sustain. Mater. Technol. 2023, 38, e00724. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Peeters, E. Recent technological innovations in mycelium materials as leather substitutes: A patent review. Front. Bioeng. Biotechnol. 2023, 11, 1204861. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Jahan, M.S.; Rahman, M.L.; Ruhane, T.A.; Ahmed, M.; Khan, M.A. Revolutionizing sustainable fashion: Jute–mycelium vegan leather reinforced with polyhydroxyalkanoate biopolymer crosslinking from novel bacteria. Adv. Polym. Technol. 2024, 1, 1304800. [Google Scholar] [CrossRef]
- Verified Market Research. Mycelium Leather Market Size and Forecast. Available online: https://www.verifiedmarketresearch.com/product/mycelium-leather-market/ (accessed on 25 July 2025).
- Aiduang, W.; Suwannarach, N.; Kumla, J.; Thamjaree, W.; Lumyong, S. Valorization of agricultural waste to produce myco-composite materials from mushroom mycelia and their physical properties. Agric. Nat. Resour. 2022, 56, 1083–1090. [Google Scholar] [CrossRef]
- Teeraphantuvat, T.; Jatuwong, K.; Jinanukul, P.; Thamjaree, W.; Lumyong, S.; Aiduang, W. Improving the physical and mechanical properties of mycelium-based green composites using paper waste. Polymers 2024, 16, 262. [Google Scholar] [CrossRef] [PubMed]
- Aiduang, W.; Jatuwong, K.; Jinanukul, P.; Suwannarach, N.; Kumla, J.; Thamjaree, W.; Teeraphantuvat, T.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S. Sustainable innovation: Fabrication and characterization of mycelium-based green composites for modern interior materials using agro-industrial wastes and different species of fungi. Polymers 2024, 16, 550. [Google Scholar] [CrossRef]
- Aiduang, W.; Kumla, J.; Srinuanpan, S.; Thamjaree, W.; Lumyong, S.; Suwannarach, N. Mechanical, physical, and chemical properties of mycelium-based composites produced from various lignocellulosic residues and fungal species. J. Fungi 2022, 8, 1125. [Google Scholar] [CrossRef]
- Reuters. Time for Fungus? Indonesian Watchmaker Turns to Mushroom Leather. Available online: https://www.reuters.com/article/lifestyle/time-for-fungus-indonesian-watchmaker-turns-to-mushroom-leather-idUSKBN1XA18U/ (accessed on 27 July 2025).
- Crawford, A. Species-specific mycelium growth pattern variations analysis for bio-design. In Proceedings of the International Conference on Research into Design, Bangalore, India, 9–11 January 2023; pp. 977–988. [Google Scholar]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Eyre Methuen: London, UK, 1978; pp. 1–252. [Google Scholar]
- Christensen, C.M. Common Edible Mushrooms; The University of Minnesota Press: Minneapolis, MN, USA, 1972. [Google Scholar]
- Sharma, A.; Bhardwaj, G.; Nayik, G.A. (Eds.) Edible and Medicinal Mushrooms of the Himalayas: Climate Change, Critically Endangered Species, and the Call for Sustainable Development; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Ghobad-Nejhad, M.; Zhou, L.W.; Tomšovský, M.; Angelini, P.; Cusumano, G.; Angeles Flores, G.; Venanzoni, R.; Wang, X.W.; Chaharmiri-Dokhaharani, S.; Moridi, F.M.; et al. Unlocking nature’s pharmacy: Diversity of medicinal properties and mycochemicals in the family Hymenochaetaceae (Agaricomycetes, Basidiomycota). Mycosphere 2024, 15, 6347–6438. [Google Scholar] [CrossRef]
- Aiduang, W.; Jatuwong, K.; Luangharn, T.; Jinanukul, P.; Thamjaree, W.; Teeraphantuvat, T.; Waroonkun, T.; Lumyong, S. A review delving into the factors influencing mycelium-based green composites (MBCs) production and their properties for long-term sustainability targets. Biomimetics 2024, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Pahlawan, I.F.; Griyanitasari, G. Physico-mechanical properties of cattle hide leather for working gloves with flame retardant addition. Livest. Anim. Res. 2020, 2, 151–159. [Google Scholar] [CrossRef]
- ISO 2420:2017; Leather—Physical and Mechanical Tests—Determination of Apparent Density and Mass per Unit Area. International Organization for Standardization: Geneva, Switzerland, 2017.
- Zhang, Z.; Liu, Y.; Wang, J.; Xie, T.; Sun, L.; Li, Z. A chrome-free combination tanning strategy: Based on silicic acid and plant tannin. J. Leather Sci. Eng. 2021, 3, 15. [Google Scholar] [CrossRef]
- Kashyap, A.; Rawat, R.; Chandurkar, P.; Tripathi, N.; Choudhary, A.; Gurjar, N. Comparative study on the influence of culture type, substrate composition, and environmental conditions on mycelial growth of selected fungi. J. Mol. Sci. 2025, 35, 140–145. [Google Scholar]
- Globa, A.; Soh, E.; Le Ferrand, H. Living textures and mycelium skin co-creation: Designing colour, pattern, and performance for bio-aesthetic expression in mycelium-bound composites. Biomimetics 2025, 10, 573. [Google Scholar] [CrossRef]
- Srinivasarao, B.; Nagadesi, P.K. New records of wood decay fungi from Eastern Ghats of Andhra Pradesh, India. Saudi J. Pathol. Microbiol. 2021, 6, 451–459. [Google Scholar]
- Luangharn, T.; Karunarathna, S.C.; Dutta, A.K.; Paloi, S.; Promputtha, I.; Hyde, K.D.; Xu, J.; Mortimer, P.E. Ganoderma (Ganodermataceae, Basidiomycota) species from the greater Mekong subregion. J. Fungi 2021, 7, 819. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Galappaththi, M.C.A.; Khan, M.B.; Ullah, S.; Fiaz, M.; Khalid, A.N. Lentinus squarrosulus an edible macro-fungus reported from Pakistan. Stud. Fungi 2022, 7, 6. [Google Scholar] [CrossRef]
- Yue, L.; Chen, J.; Tuo, Y.; Qi, Z.; Liu, Y.; He, X.L.; Zhang, B.; Hu, J.; Li, Y. Taxonomy and phylogeny of Panus (Polyporales, Panaceae) in China and its relationship with allies. MycoKeys 2024, 105, 267. [Google Scholar] [CrossRef]
- Shin, K.S. Identification of some Phellinus spp. Mycobiology 2001, 29, 190–193. [Google Scholar] [CrossRef]
- Balan, V.; Zhu, W.; Krishnamoorthy, H.; Benhaddou, D.; Mowrer, J.; Husain, H.; Eskandari, A. Challenges and opportunities in producing high-quality edible mushrooms from lignocellulosic biomass in a small scale. Appl. Microbiol. Biotechnol. 2022, 106, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- International Leather Club. Types of Leather: All Qualities, Grades, Finishes, & Cuts. Available online: https://www.internationalleatherclub.com/types-of-leather/?srsltid=AfmBOoqq8R-ObRu4zztf9HXqVZTxq3tSznaNnaDvkuozVxnpnP6o8kPF (accessed on 19 September 2025).
- Winiw. Understanding PU Leather: What It Is and How It’s Created. 2025. Available online: https://www.microfiberleather.com/th/content/understanding-pu-leather-created/ (accessed on 19 September 2025).
- Benetti, B.; Conti, F.; Dimitriadis, P. Mycelium-based leather: A review on post-processing treatments and material enhancements. Environ. Clim. Technol. 2025, 29, 390–404. [Google Scholar] [CrossRef]
- Angelova, G.; Yemendzhiev, H.; Zaharieva, R.; Brazkova, M.; Koleva, R.; Stefanova, P.; Baldzhieva, R.; Vladev, V.; Krastanov, A. Mycelium-based composites derived from lignocellulosic residual by-products: An insight into their physico-mechanical properties and biodegradation profile. Appl. Sci. 2025, 15, 6333. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Lankinen, P.; Hietala, S.; Mikkonen, K.S. Dense and continuous networks of aerial hyphae improve flexibility and shape retention of mycelium composite in the wet state. Compos.-A Appl. Sci. Manuf. 2022, 152, 106688. [Google Scholar] [CrossRef]
- Zugno, L. Modern Cow Leather Processing. Available online: https://www.leathernaturally.org/wp-content/uploads/2023/02/LN-Guide_to_leather_making_PART_TWO.pdf (accessed on 15 August 2025).
- Krusetraining. Polymer Shrinkage. Available online: https://krusetraining.com/mandarin/wp-content/uploads/sites/3/2017/10/Polymer-Shrinkage.pdf (accessed on 15 August 2025).
- Kelly, S.J.R.; Weinkamer, R.; Bertinetti, L.; Edmonds, R.L.; Sizeland, K.H.; Wells, H.C.; Fratzl, P.; Haverkamp, R.G. Effect of collagen packing and moisture content on leather stiffness. J. Mech. Behav. Biomed. Mater. 2019, 90, 1–10. [Google Scholar] [CrossRef]
- Karunarathne, A.; Nabiyeva, G.; Rasmussen, C.J.; Alkhoury, K.; Assem, N.; Bauer, J.; Chester, S.A.; Khalizov, A.F.; Gor, G.Y. Effects of humidity on mycelium-based leather. ACS Appl. Bio Mater. 2024, 7, 6441–6450. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, H.; Tian, D.; Zhang, W.; Shi, B. Scalable production of robust, moisture-wicking, and breathable superfine mycelium fiber/waterborne polyurethane leather-like textile via direct casting and oven-drying. Ind. Crops Prod. 2025, 226, 120632. [Google Scholar] [CrossRef]
- Smith, J. What is Microfiber Leather? Available online: https://leatherskinshop.com/blogs/default-blog/what-is-microfiber-leather-1 (accessed on 17 September 2025).
- Parhizi, Z.; Dearnaley, J.; Kauter, K.; Mikkelsen, D.; Pal, P.; Shelley, T.; Burey, P.P. The fungus among us: Innovations and applications of mycelium-based composites. J. Fungi 2025, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Kohphaisansombat, C.; Jongpipitaporn, Y.; Laoratanakul, P.; Tantipaibulvut, S.; Euanorasetr, J.; Rungjindamai, N.; Chuaseeharonnachai, C.; Kwantong, P.; Somrithipol, S.; Boonyuen, N. Fabrication of mycelium (oyster mushroom)-based composites derived from spent coffee grounds with pineapple fibre reinforcement. Mycology 2024, 15, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Tajvidi, M.; Howell, C.; Hunt, C.G. Insight into mycelium-lignocellulosic bio-composites: Essential factors and properties. Compos.-A Appl. Sci. Manuf. 2022, 161, 107125. [Google Scholar] [CrossRef]
- Daâssi, D.; M Alhumairi, A.; Mellah, B.; Fdhil, N.; Baccar, N.; Chamkha, M. Physico-mechanical, thermal, and biodegradation performance of mycelium biocomposites derived from residual agrowastes. Polym. Bull. 2025, 82, 11295–11321. [Google Scholar] [CrossRef]
- Olivero, E.; Gawronska, E.; Manimuda, P.; Jivani, D.; Chaggan, F.Z.; Corey, Z.; de Almeida, T.S.; Kaplan-Bie, J.; McIntyre, G.; Nalam, P.C. Gradient porous structures of mycelium: A quantitative structure–mechanical property analysis. Sci. Rep. 2023, 13, 19285. [Google Scholar] [CrossRef]
- Appels, F.V.; van den Brandhof, J.G.; Dijksterhuis, J.; de Kort, G.W.; Wösten, H.A. Fungal mycelium classified in different material families based on glycerol treatment. Commun. Biol. 2020, 3, 334. [Google Scholar] [CrossRef]
- Elsacker, E.; Zhang, M.; Dade-Robertson, M. Fungal engineered living materials: The viability of pure mycelium materials with self-healing functionalities. Adv. Funct. Mater. 2023, 33, 2301875. [Google Scholar] [CrossRef]
- Chulikavit, N.; Huynh, T.; Dekiwadia, C.; Khatibi, A.; Mouritz, A.; Kandare, E. Influence of growth rates, microstructural properties and biochemical composition on the thermal stability of mycelia fungi. Sci. Rep. 2022, 12, 15105. [Google Scholar] [CrossRef] [PubMed]
- Kniep, J.; Graupner, N.; Reimer, J.J.; Müssig, J. Mycelium-based biomimetic composite structures as a sustainable leather alternative. Mater. Today Commun. 2024, 39, 109100. [Google Scholar] [CrossRef]
- Lasoń-Rydel, M.; Sieczyńska, K.; Gendaszewska, D.; Ławińska, K.; Olejnik, T.P. Use of enzymatic processes in the tanning of leather materials. AUTEX Res. J. 2024, 24, 20230012. [Google Scholar] [CrossRef]
- Laveri. Why Leather Bags Attract Dust—And How to Keep Them Clean Longer. Available online: https://mylaveri.com/blogs/news/why-leather-bags-attract-dust-and-how-to-keep-them-clean-longer?srsltid=AfmBOoqHiz-Rmp1qaNRkThe2-H6wbJzbGoygwp90PFfaHCJSYqODoxuB (accessed on 20 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).