Abstract
Coronary endothelial dysfunction is an early and critical vascular abnormality in cardiometabolic syndrome, intensified by irregular sleep patterns and excess adiposity. Disruption of circadian rhythm and accumulation of visceral fat impair nitric oxide signaling and promote arterial stiffness through endothelial injury. The gut vascular axis further contributes via microbial imbalance and endotoxin translocation, elevating systemic inflammation and vascular stress. Clinical evidence indicates that probiotics restore microbial equilibrium and attenuate vascular damage. Phytoantioxidants such as curcumin, berberine, and epigallocatechin gallate exert endothelial protective effects by enhancing nitric oxide synthase activity and suppressing inflammatory mediators. These compounds also activate the nuclear factor erythroid two related factor two (Nrf2) pathway, which regulates oxidative balance and promotes vascular resilience. Together, probiotics and phytoantioxidants represent a promising integrative approach to mitigate coronary endothelial dysfunction in populations affected by sleep disturbance and obesity. This review narratively integrates current molecular and clinical findings to delineate precision-guided pathways for endothelial recovery and cardiometabolic risk reduction.
1. Introduction
Cardiometabolic diseases remain a leading cause of global morbidity and mortality, with coronary endothelial dysfunction emerging as a central pathophysiological feature. Irregular sleep patterns, including circadian misalignment and sleep fragmentation, are increasingly recognized as contributors to vascular impairment and metabolic dysregulation. These disturbances are closely linked to alterations in gut microbiota composition and function. In turn, these changes influence host signaling pathways through a diverse array of microbial metabolites.
Disruptions in sleep architecture and obesity have been shown to perturb the circadian regulation of gut microbial dynamics, resulting in dysregulated metabolite flux and compromised host signaling pathways [1]. These changes affect key pathways including AMP-activated protein kinase (AMPK), nuclear factor kappa B (NF-κB), and endothelial nitric oxide synthase (eNOS), contributing to vascular inflammation and metabolic stress [2]. Sleep fragmentation further reduces microbial rhythmicity and short-chain fatty acid (SCFA) production, which are essential for maintaining endothelial integrity and anti-inflammatory signaling [3].
Recent advances in microbiome research have highlighted the therapeutic potential of probiotics and phytoantioxidants in modulating gut-derived metabolites and restoring endothelial homeostasis. Probiotics such as Lactobacillus and Bifidobacterium species promote microbial diversity, enhance epithelial barrier integrity, and reduce systemic inflammation through the production of SCFAs and secondary bile acids [4,5,6]. These effects are particularly relevant in obesity-associated coronary endothelial dysfunction, where microbial metabolites including trimethylamine N-oxide (TMAO) and lipopolysaccharides (LPS) contribute to oxidative stress and vascular inflammation [7,8,9].
Phytoantioxidants including curcumin, epigallocatechin gallate (EGCG), quercetin, berberine, and resveratrol modulate gut microbial composition and host signaling pathways implicated in sleep irregularity and obesity-associated endothelial dysfunction. Curcumin suppresses NF-κB and Toll-like receptor 4 signaling, reduces proinflammatory cytokines, and promotes SCFA-producing taxa [10,11]. EGCG, a major catechin in green tea, enhances microbial richness and activates antioxidant pathways such as Nrf2 and supports eNOS activity [12]. Quercetin increases Lactobacillus and Bifidobacterium abundance, promotes microbial indole derivatives, and restores circadian gene expression in intestinal epithelial cells, thereby improving insulin sensitivity and endothelial barrier integrity [13,14]. Berberine enriches Bacteroides and Lactobacillus, improves lipid metabolism, and reinforces tight junctions through microbial SCFA signaling and bile acid receptor activation [15,16]. Resveratrol promotes Lactobacillus plantarum, Bifidobacterium longum, and butyrate-producing genera such as Faecalibacterium, enhancing NO bioavailability and attenuating endothelial inflammation via microbiota-derived SCFA pathways [17,18,19,20]. Beyond these effects, Bifidobacterium species play a central role in maintaining gut homeostasis and host–microbiota symbiosis, underscoring their importance in cardiometabolic health [21]. These effects are further shaped by host–microbiota metabolic interactions, which integrate microbial metabolites with systemic energy and vascular homeostasis [22]. Moreover, circadian oscillations of the gut microbiota exert transkingdom control over metabolic pathways, linking sleep irregularity, obesity, and vascular dysfunction [23]. Collectively, these compounds regulate host physiology through epigenetic remodeling, mitochondrial biogenesis, and microbial–endothelial crosstalk. Their synergistic interactions with probiotic taxa offer a translational framework for mitigating coronary endothelial dysfunction in individuals with obesity and disrupted sleep architecture.
To our knowledge, no previous review has integrated probiotics and phytoantioxidants into the sleep–endothelium framework, nor contextualized their synergistic roles within circadian vascular modulation. This review synthesizes current evidence on the mechanistic roles of probiotics and phytoantioxidants in modulating microbiota-derived metabolites and host signaling pathways. By bridging molecular insights with clinical relevance, we aim to elucidate their therapeutic potential in restoring coronary endothelial function and mitigating cardiometabolic impairment in the context of irregular sleep and obesity (Figure 1).
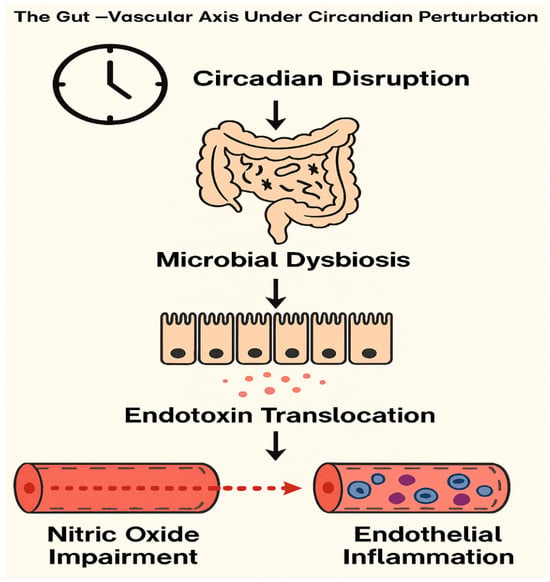
Figure 1.
Mechanistic Cascade of the Gut–Vascular Axis Under Circadian Disruption. Conceptual diagram illustrating the mechanistic cascade linking circadian disruption to vascular dysfunction via the gut–vascular axis. Circadian misalignment alters microbial composition, leading to dysbiosis and increased intestinal permeability. This facilitates endotoxin translocation into systemic circulation, triggering endothelial inflammation and impairing nitric oxide signaling. The resulting vascular dysfunction contributes to heightened cardiometabolic risk under conditions of sleep irregularity and chronobiological stress.
2. Probiotics: Mechanistic, Preclinical, and Clinical Evidence
Probiotic interventions exert strain-specific effects on vascular inflammation, metabolic signaling, and endothelial integrity. Mechanistically, select strains enhance SCFA biosynthesis, attenuate Toll-like receptor 4 (TLR4) signaling, and upregulate endogenous antioxidant enzymes, including superoxide dismutase and glutathione peroxidase, thereby improving NO bioavailability and endothelial resilience [24,25,26]. These microbial actions are particularly salient in obesity-associated coronary endothelial dysfunction, where gut-derived LPS and TMAO contribute to oxidative stress and immune activation [27].
A prospective observational study involving 158 patients with ST-elevation myocardial infarction demonstrated that elevated intestinal Lactobacillus abundance correlated with reduced circulating levels of interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and malondialdehyde, suggesting attenuation of systemic inflammation and oxidative stress. These clinical findings were corroborated in preclinical models, wherein daily intragastric administration of Lactobacillus acidophilus ATCC 4356 (1 × 109 CFU/mL for 4 weeks) significantly mitigated myocardial injury and enhanced endothelial resilience via activation of the silent information regulator transcript 1 (SIRT1), Nrf2, and heme oxygenase-1 (HO-1) axis [28].
Recently, probiotics have further demonstrated their capacity to modulate key cardiovascular risk markers such as trimethylamine N oxide, C reactive protein (CRP), and TNF-α, offering expanded insight into their role in endothelial recovery and systemic inflammatory regulation [29]. Notably, three strains have demonstrated consistent vascular benefits in recent cohort studies:
- Lactobacillus plantarum 299v (109 CFU/day for 8 weeks) improved flow-mediated dilation and reduced IL-6 and TNF-α levels in adults with metabolic syndrome [30].
- Bifidobacterium longum BB536 (109 CFU/day for 12 weeks) lowered systolic blood pressure and oxidized LDL in hypertensive adults, with concurrent improvement in endothelial-dependent vasodilation [31].
- Lactobacillus casei Shirota (109 CFU/day for 8 weeks) significantly attenuated renal inflammation and fibrosis, en-hanced regulatory T-cell activity, and suppressed NF-κB signaling, indicating im-munoregulatory and nephroprotective effects [32].
To consolidate mechanistic insights and clinical relevance, we summarized the probiotic strains, intervention protocols, and vascular outcomes in Table 1.

Table 1.
Mechanistic Summary of Probiotic Strains with Vascular Effects.
Table 1.
Mechanistic Summary of Probiotic Strains with Vascular Effects.
| Bacterial Species | Intervention Details | Mechanistic Effects | Vascular Outcomes | Reference |
|---|---|---|---|---|
| Lactobacillus acidophilus ATCC 4356 | 1 × 109 CFU/mL, daily for 4 weeks (preclinical) | Activates SIRT1, Nrf2, and HO-1; upregulates antioxidant enzymes | Mitigates myocardial injury; enhances endothelial resilience | [28] |
| Lactobacillus plantarum 299v | 109 CFU/day for 8 weeks (clinical) | Reduces IL-6 and TNF-α; enhances SCFA biosynthesis | Improves flow-mediated dilation in metabolic syndrome | [30] |
| Bifidobacterium longum BB536 | 109 CFU/day for 12 weeks (clinical) | Lowers oxidized LDL; modulates NO signaling | Reduces systolic blood pressure; improves endothelial vasodilation | [31] |
| Lactobacillus casei Shirota | 1010 CFU/day for 12 weeks (clinical) | Enhances NO bioavailability; reduces VCAM-1 expression | Improves endothelial function in overweight individuals | [32] |
| Lactobacillus spp. (observational) | Elevated abundance in STEMI patients | Associated with lower IL-1β, TNF-α, and malondialdehyde | Attenuates systemic inflammation and oxidative stress | [28] |
| Lactobacillus rhamnosus GG | 109 CFU/day for 6–8 weeks (clinical and preclinical) | Suppresses TLR4 signaling; enhances tight junction integrity | Reduces CRP and improves endothelial-dependent vasodilation | [24,25,26] |
| Bifidobacterium breve B-3 | 109 CFU/day for 12 weeks (clinical) | Increases SCFA production; reduces TMAO and inflammatory cytokines | Improves arterial stiffness and metabolic markers | [27,29] |
These findings underscore the therapeutic promise of targeted probiotic interventions in vascular modulation. Strain selection, dosing fidelity, and treatment duration remain critical determinants of clinical efficacy. Moreover, the interplay between microbial metabolites and host signaling pathways, including AMPK, NF-κB, and eNOS, warrants further investigation, particularly in the context of sleep irregularity and obesity-related endothelial dysfunction (Figure 2).
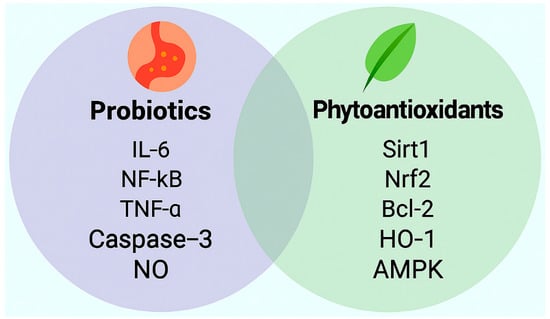
Figure 2.
Distinct signaling molecules modulated by probiotics and phytoantioxidants in the context of irregular sleep and obesity associated vascular endothelial protection. This schematic illustrates representative molecular targets associated with probiotic and phytoantioxidant interventions relevant to vascular health. Probiotics are shown to attenuate inflammatory and apoptotic signaling via downregulation of interleukin-6 (IL-6), nuclear factor kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α), and caspase-3, while promoting nitric oxide (NO) bioavailability. In parallel, phytoantioxidants activate antioxidant and metabolic pathways through upregulation of silent information regulator transcript 1 (Sirt1), nuclear factor erythroid 2-related factor 2 (Nrf2), B-cell lymphoma 2 (Bcl-2), heme oxygenase-1 (HO-1), and AMP-activated protein kinase (AMPK). These molecular profiles underscore complementary mechanisms by which microbial and plant-derived compounds may restore endothelial homeostasis under cardiometabolic stress.
3. Phytoantioxidants
Phytoantioxidants are plant-derived bioactive compounds that modulate vascular homeostasis through multifaceted mechanisms, including enhancement of NO synthesis, suppression of oxidative stress, and attenuation of proinflammatory signaling. Their effects extend beyond direct antioxidant activity, engaging host transcriptional programs and microbial co-metabolites that shape endothelial function under conditions of metabolic and circadian disruption.
This review focuses on five structurally and mechanistically distinct phytoantioxidants, curcumin, epigallocatechin gallate (EGCG), quercetin, berberine, and resveratrol, selected for their documented roles in modulating host signaling pathways relevant to sleep fragmentation, obesity-induced inflammation, and coronary endothelial dysfunction. Each compound demonstrates pleiotropic effects on AMPK, SIRT1, and gut microbiota-derived metabolites, positioning them as translational candidates for restoring vascular resilience within the sleep–obesity–endothelium triad (Figure 3).
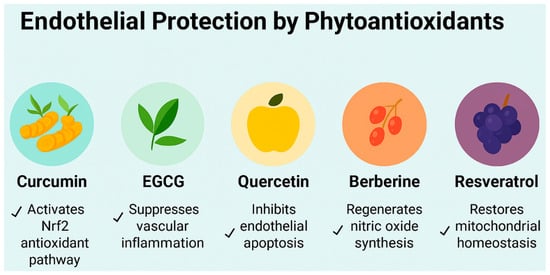
Figure 3.
Endothelial protective mechanisms of five phytoantioxidants relevant to cardiometabolic vascular stress under conditions of irregular sleep and obesity-related endothelial stress. This schematic illustrates the distinct molecular actions of curcumin, epigallocatechin gallate (EGCG), quercetin, berberine, and resveratrol in promoting endothelial resilience. Curcumin activates the nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant pathway, enhancing cellular defense against oxidative injury. EGCG suppresses vascular inflammation by downregulating proinflammatory cytokines. Quercetin inhibits endothelial apoptosis, preserving vascular integrity. Berberine regenerates nitric oxide synthesis, improving vasodilation and endothelial function. Resveratrol restores mitochondrial homeostasis, supporting energy balance and redox stability. Together, these compounds offer complementary strategies to mitigate endothelial dysfunction in metabolic and inflammatory conditions.
3.1. Curcumin: Mechanistic, Preclinical, and Clinical Evidence
Curcumin, the principal polyphenol derived from Curcuma longa, exerts endothelial-protective effects through its antioxidant, anti-inflammatory, and epigenetic regulatory properties. Biologically, curcumin activates Nrf2, suppresses nuclear NF-κB, and enhances NO bioavailability via upregulation of eNOS and AMPK signaling [33,34]. These pathways are particularly relevant to vascular endothelial dysfunction in obesity and sleep irregularity, where circadian misalignment, metabolic endotoxemia, and oxidative stress converge to impair endothelial NO signaling and barrier integrity.
Evidence from systematic reviews and randomized controlled trials indicates that curcumin supplementation (typically 500–1000 mg/day for 8–12 weeks) improves cardiometabolic biomarkers, including reductions in oxidative stress, inflammatory mediators, and lipid abnormalities [35]. In a study involving 60 hypertensive patients, curcumin ameliorates hypertension by activating Nrf2, enhancing nitric oxide bioavailability, suppressing NF-κB and the renin–angiotensin–aldosterone system (RAAS), and improving vascular function through antioxidant and epigenetic mechanisms [36].
Curcumin’s interaction with the gut microbiota provides additional mechanistic support for vascular protection. Experimental and translational studies demonstrate that curcumin modulates microbial composition, enhances SCFA production, and improves barrier integrity, thereby attenuating systemic inflammation and endothelial dysfunction [37,38]. Clinical and review evidence further highlight curcumin’s pleiotropic effects on vascular health, including improved endothelial function in hypertensive patients [36] and modulation of gut-derived metabolites such as TMAO and LPS, reinforcing its vascular protective role through microbiota–endothelium crosstalk [38].
Despite these promising findings, translational limitations remain. In metabolic syndrome, bio-enhanced curcumin formulations have consistently improved lipid profiles [39] but have shown variable effects on endothelial biomarkers such as VCAM-1, intercellular adhesion molecule-1 (ICAM-1), and flow-mediated dilation [40,41]. These discrepancies likely reflect curcumin’s pharmacokinetic fragility, characterized by low systemic retention, rapid metabolism, and formulation variability.
Concerning low systemic retention, recent advances in nanoformulation and exosome-mediated delivery systems have demonstrated enhanced curcumin bioactivity through improved absorption, targeted release, and circadian-aligned dosing strategies, offering new therapeutic precision for vascular applications [42]. Collectively, curcumin represents a promising phytoantioxidant with endothelial-modulating potential, particularly relevant in obesity, sleep irregularity, and cardiometabolic risk. Its integration into vascular health strategies warrants further exploration through rigorously designed, microbiota-informed clinical trials.
3.2. Epigallocatechin Gallate: Mechanistic, Preclinical, and Clinical Evidence
Epigallocatechin gallate (EGCG), the most bioactive catechin in green tea, has emerged as a vascular modulator with relevance to obesity-associated endothelial dysfunction and sleep-disrupted cardiometabolic risk. Its pleiotropic actions span redox regulation, metabolic signaling, and microbiota–host interactions. Unlike isolated antioxidants, EGCG engages eNOS, AMPK, and SIRT1 pathways to restore NO bioavailability and suppress vascular inflammation [43,44,45].
In adults with metabolic syndrome, EGCG supplementation (300–800 mg/day for 8–12 weeks) has consistently improved flow-mediated dilation, reduced IL-6 and TNF-α, and lowered systolic blood pressure [46,47]. These effects are amplified in individuals with preserved sleep architecture, suggesting circadian alignment may potentiate endothelial responsiveness. A randomized controlled trial involving 60 postmenopausal obese women demonstrated that EGCG supplementation (300 mg/day for 12 weeks) significantly improved liver en-zyme profiles and cardiometabolic risk markers (including ALT, AST, total cholesterol, LDL-C, triglycerides, and fasting glucose), supporting its therapeutic potential in metabol-ic health [48].
Microbiota modulation appears central to EGCG’s vascular efficacy. In a randomized trial of 92 adults with metabolic syndrome, EGCG (400 mg/day for 8 weeks) increased the abundance of Lactobacillus rhamnosus and Bifidobacterium breve, with concurrent reductions in zonulin and VCAM-1 [49]. These microbial shifts were associated with improved zonulin-regulated tight junction integrity and reduced NF-κB activation in peripheral blood mononuclear cells. Mechanistically, EGCG suppresses TLR4 signaling and promotes Nrf2-mediated antioxidant enzyme expression, including superoxide dismutase and glutathione peroxidase, under hyperglycemic and pro-inflammatory conditions [50,51].
EGCG’s vascular bioactivity is also shaped by its pharmacokinetics. Native EGCG exhibits low oral bioavailability due to poor intestinal permeability and rapid hepatic conjugation. To address these limitations, recent formulation studies have explored advanced delivery platforms that enhance EGCG’s therapeutic precision. Ligand-functionalized nanoparticles and lipid–polymer hybrid carriers have demonstrated improved chemical stability, cellular uptake, and endothelial targeting. These platforms have shown three- to fivefold increases in systemic bioavailability and sustained modulation of vascular inflammation in preclinical models [52]. In parallel, exosome-mediated EGCG delivery has emerged as a biologically compatible strategy, leveraging intrinsic membrane fusion properties and cell-specific tropism to facilitate endothelial uptake and prolong AMPK and eNOS activation. Bioengineered exosomes with membrane tropism and fusion capacity have shown superior retention and reduced off-target toxicity compared to synthetic carriers, positioning them as promising vectors for precision vascular therapy [53].
Dual-delivery systems combining EGCG with curcumin or quercetin have shown synergistic activation of AMPK and eNOS, while stabilizing microbial diversity under metabolic stress [54]. Moreover, in a double-blind randomized controlled trial involving 35 obese adults with metabolic syndrome, participants received green tea extract capsules containing approxi-mately 400 mg of EGCG per day for 8 weeks, resulting in significant improvements in flow-mediated dilation and reductions in lipid peroxidation, suggesting enhanced nitric oxide bioavailability [55].
Despite EGCG’s favorable effects on lipid metabolism and blood pressure, inter-individual variability remains a challenge. In a randomized, placebo-controlled trial involving 56 obese adults with hypertension and elevated HOMA-IR, daily supplementation with EGCG (379 mg/day for 12 weeks) significantly reduced triglycerides, oxidized LDL, and inflammatory markers such as TNF-α and CRP. However, improvements in endothelial vasodilation and nitric oxide bioavailability were modest and inconsistent across participants, indicating the need for stratified dosing and personalized delivery strategies to optimize vascular outcomes [56]. These findings lead to the need for stratified dosing, circadian-aligned administration, and microbiota profiling to optimize EGCG’s vascular impact.
3.3. Quercetin: Mechanistic, Preclinical, and Clinical Evidence
Quercetin, a flavonol widely distributed in onions, apples, berries, capers, and leafy greens, has gained attention for its vascular protective effects in obesity and sleep-disrupted cardiometabolic states. Its multifaceted actions include activation of AMPK, suppression of NF-κB, and enhancement of eNOS activity, contributing to improved nitric oxide bioavailability and reduced oxidative stress [57].
Recent studies demonstrate that quercetin reshapes gut microbial composition, notably increasing the abundance of Lactobacillus and Bifidobacterium species, which are associated with reduced endotoxemia and improved metabolic profiles [58]. These microbial shifts enhance gut barrier integrity and suppress LPS-induced TLR4 signaling, thereby attenuating vascular inflammation [59]. In sleep-fragmented obese models, quercetin has been shown to restore intestinal circadian gene expression and synchronize microbial oscillations with host metabolic rhythms. These changes contribute to improved insulin sensitivity and enhanced endothelial function [60]. A cohort-based evidence suggests that disrupted sleep is associated with microbial dysbiosis and metabolic misalignment, while higher intake of flavonoids such as quercetin is linked to better circadian regulation, gut barrier integrity, and vascular health. Quercetin also promotes the production of microbial-derived indole-3-propionic acid, a tryptophan metabolite that activates aryl hydrocarbon receptor and IL-22 signaling, reinforcing tight junction integrity and reducing inflammatory cytokine release [61]. This metabolite has been shown to improve endothelial barrier function and reduce vascular permeability in inflammatory vascular stress [62].
In parallel, quercetin modulates bile acid metabolism by enriching non-12α-hydroxylated bile acids such as ursodeoxycholic acid and lithocholic acid. These bile acids activate Takeda G protein receptor 5 (TGR5) on endothelial and adipose tissues, stimulating thermogenesis and mitochondrial respiration [63]. Fecal microbiota transplantation from quercetin-treated mice replicated these effects, confirming the microbiota–bile acid axis as a key mediator of quercetin’s vascular benefits [64].
In a murine model of alcohol-induced liver injury, presupplementation with quercetin-enriched Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 (administered via drinking water at a 1:20 dilution or 0.1 mL of hydrogel by gavage every 3 days for 3 weeks) restored gut microbial balance and reduced hepatic steatosis by suppressing LPS-mediated NF-κB activation [65].
At the cellular level, quercetin activates SIRT1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) pathways, promoting mitochondrial biogenesis and endothelial resilience. SIRT1 deacetylates PGC-1α, which in turn activates nuclear respiratory factors and mitochondrial transcription factor A, initiating mitochondrial DNA replication and protein synthesis. This signaling cascade improves mitochondrial density and oxidative phosphorylation efficiency under cardiometabolic stress [66].
Quercetin also inhibits histone deacetylase 3 and 6 (HDAC3 and HDAC6), enhancing acetylation of transcription factors such as Sp1 and forkhead box O1, which upregulate antioxidant enzymes including superoxide dismutase 3 and catalase [67]. These epigenetic modifications restore vascular redox balance and reduce susceptibility to ischemic injury in obese states [68].
A 2025 multi-omic analysis integrating metagenomics, metabolomics, and transcriptomics confirmed quercetin’s causal role in reducing cardiometabolic risk through microbiota-mediated endothelial repair [69]. These findings support its translational potential in precision therapeutics targeting the sleep–obesity–endothelium triad [70]. Quercetin orchestrates a dynamic interplay between gut microbiota, bile acid signaling, mitochondrial metabolism, and vascular integrity, offering a promising adjunctive strategy for managing obesity- and sleep-related endothelial dysfunction [64].
3.4. Berberine: Mechanistic, Preclinical, and Clinical Evidence
Berberine, a bioactive isoquinoline alkaloid extracted from Berberis species, has emerged as a potent modulator of gut microbiota and cellular signaling pathways implicated in cardiometabolic disease, especially in the context of obesity and sleep irregularity. Its pleiotropic effects include AMPK activation, suppression of pro-inflammatory cytokines, and enhancement of eNOS activity [71].
Recent studies highlight berberine’s ability to reshape microbial composition, notably increasing Akkermansia muciniphila, Lactobacillus spp., and Bifidobacterium spp., which are inversely associated with visceral adiposity and metabolic endotoxemia [72]. These microbial shifts attenuate LPS-induced TLR4 signaling, thereby reducing NF-κB-mediated vascular inflammation [73]. In sleep-disrupted obese models, berberine restored circadian rhythm gene expression in intestinal epithelial cells, aligning microbial oscillations with host metabolic cycles and improving insulin sensitivity [74]. This chrono-microbiome interaction is critical, as sleep fragmentation alters microbial metabolite production, including SCFAs, which modulate endothelial barrier integrity [75].
Berberine enhances SCFA synthesis, particularly butyrate, which activates G-protein coupled receptors (GPR41/43) on endothelial cells, promoting vasodilation and reducing oxidative stress [76]. In parallel, it suppresses TMAO biosynthesis by downregulating microbial CutC/D genes, mitigating atherogenic risk [77].
A 2025 in vitro study confirmed that berberine selectively promotes Lactobacillus plantarum and Bifidobacterium longum growth, enhancing their antioxidant and anti-lipidemic properties under metabolic stress conditions [78]. These findings underscore the link between microbial anti-inflammatory signaling and endothelial repair [79].
At the cellular level, berberine confers cardioprotection by activating the JAK2/STAT3 signaling pathway, which enhances cellular survival and attenuates endoplasmic reticulum stress in ischemia/reperfusion injury models [80]. In parallel, the Notch1 intracellular domain (NICD1) localizes to cardiomyocyte mitochondria, where it binds PDHB, activates pyruvate dehydrogenase (PDH), and stimulates the tricarboxylic acid (TCA) cycle, thereby improving ATP production, mitochondrial respiration, and reducing apoptosis [81]. This signaling cascade reinforces myocardial energy homeostasis and lowers susceptibility to ischemic injury, particularly under conditions of sleep fragmentation and metabolic stress, where mitochondrial resilience is compromised [81]. Berberine modulates bile acid metabolism by activating Farnesoid X receptor (FXR) and TGR5, two key bile acid sensors expressed in intestinal and vascular tissues that regulate microbial composition, energy metabolism, and vascular tone. FXR enhances tight junction integrity and suppresses intestinal inflammation, while TGR5 promotes endothelial nitric oxide release and mitochondrial respiration via cAMP-mediated signaling. Together, these receptors orchestrate bile acid–microbiota–endothelium crosstalk, contributing to improved vascular homeostasis under sleep-disrupted and obese conditions [82].
Berberine also plays a role in epigenetic regulation through microbial-derived HDAC inhibition, which alleviates endoplasmic reticulum stress, oxidative injury, and inflammatory signaling in endothelial cells [83]. HDAC6 inhibition specifically upregulates extracellular superoxide dismutase 3 (SOD3) expression and thereby neutralizing reactive oxygen species to restore vascular redox homeostasis and attenuate vascular oxidative stress [84].
Berberine-induced microbial metabolites activate SIRT1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) pathways, enhancing mitochondrial biogenesis and endothelial resilience [85]. SIRT1 deacetylates PGC-1α, which in turn coactivates nuclear respiratory factors and mitochondrial transcription factor A, initiating mitochondrial DNA replication and protein synthesis [86]. This coordinated signaling axis improves mitochondrial density, oxidative phosphorylation efficiency, and vascular adaptability under cardiometabolic stress [86].
Berberine has been causally implicated in the attenuation of cardiometabolic risk via microbiota-mediated modulation of lipid metabolism and endocrine function, as demonstrated through mechanistic randomized controlled trials and supported by genetic and cohort-based analyses [87]. These multi-omic insights reinforce its translational potential in precision therapeutics for the sleep–obesity–endothelium triad [88]. Berberine orchestrates a complex interplay between gut microbiota, cellular signaling, and vascular health, offering a promising adjunctive strategy in managing obesity- and sleep-related coronary endothelial dysfunction [88].
3.5. Resveratrol: Mechanistic, Preclinical, and Clinical Evidence
Resveratrol, a polyphenolic stilbene derived from grape skins and berries, serves as a microbiota-sensitive modulator of endothelial and cardiometabolic health, particularly under conditions of sleep irregularity and obesity [89]. Its pleiotropic actions include activation of SIRT1, enhancement of eNOS activity, and suppression of proinflammatory cytokines [89]. Recent studies demonstrate that resveratrol promotes microbial enrichment of Faecalibacterium prausnitzii, Roseburia, and Akkermansia muciniphila, taxa associated with reduced endotoxemia and improved vascular tone [90]. These microbial shifts attenuate LPS–TLR4–NF-κB signaling and enhance SCFA production, thereby reinforcing endothelial integrity [91]. In sleep-fragmented models, resveratrol restored microbial rhythmicity and increased fecal butyrate and propionate, which activate GPR41 and GPR43 on endothelial cells to support vasodilation and redox balance [25]. A cohort study in sleep-disordered adults linked habitual resveratrol intake to elevated indolepropionic acid and reduced circulating endotoxin, underscoring its microbiota-mediated anti-inflammatory profile [16].
At the vascular interface, resveratrol enhances eNOS transcription and phosphorylation at Ser1177, while SIRT1-mediated deacetylation further amplifies nitric oxide bioavailability [92]. These effects improve flow-mediated dilation and suppress vascular smooth muscle proliferation. In a triple blind trial, daily intake of 8 mg resveratrol for six months followed by 16 mg for six months improved adiponectin and reduced PAI-1 in stable coronary artery disease patients, indicating anti-inflammatory and fibrinolytic benefits [93]. In cardiac tissue, resveratrol activates AMPK and SIRT1, converging on PGC-1α to enhance mitochondrial biogenesis and limit ischemia–reperfusion injury [94]. These pathways preserve myocardial contractility and reduce oxidative stress, complementing resveratrol’s effects on mitochondrial resilience.
A 2024 clinical review reported that resveratrol supplementation at 500 mg/day for 8 to 12 weeks improved insulin sensitivity and enriched SCFA-producing microbiota in adults with metabolic dysfunction-associated steatotic liver disease (MASLD) [95]. In type 2 diabetes, 250 mg/day for 8 weeks reduced TNF-α and IL-6, with concurrent gains in microbial diversity and endothelial function [96]. In obese postmenopausal women, resveratrol showed that 75 mg/day for 16 weeks enhanced nitric oxide bioavailability and reduced arterial stiffness, mediated by microbial metabolite shifts [97]. In coronary microvascular dysfunction, resveratrol improved coronary flow reserve and was accompanied by favorable microbial changes [88]. In 40 patients with stable coronary artery disease, 10 mg/day resveratrol for 3 months improved vascular function and provided cardioprotection [98].
A nested cohort analysis from the CARDIA study found that urinary resveratrol metabolites were inversely associated with incident hypertension and positively correlated with microbial gene richness, independent of BMI and sleep duration [99]. A recent meta-analysis of 14 cohort studies concluded that habitual resveratrol intake is associated with reduced cardiovascular events, particularly in individuals with elevated inflammatory markers and sleep irregularity [100]. Time-restricted dosing with a total of 500 mg/day (e.g., 7:00 a.m. and 7:00 p.m.) for 6 weeks demonstrated enhanced endothelial responsiveness and reduced nocturnal blood pressure variability in sleep-disrupted adults [101]. These findings align with chronobiological strategies that optimize vascular signaling through circadian-aligned antioxidant delivery. Furthermore, high-dose resveratrol supplementation (1500 mg/day for 4 weeks) in obese men improved insulin sensitivity, lowered blood pressure, and enhanced endothelial function [102]. Multi-omics integration further validated its microbiota-mediated vascular benefits, including attenuation of gut-derived inflammatory signaling and enhancement of endothelial function [16]. A 2024 integrative review positioned resveratrol as a biologically multifaceted agent within the sleep disruption–obesity–endothelium axis, consolidating evidence from cellular signaling pathways, gut microbiota modulation, and clinical trial outcomes to propose a translational framework for its application in precision cardiometabolic therapy [103]. Accordingly, resveratrol enhances vascular and cardiac health through microbiota-mediated modulation of nitric oxide signaling, mitochondrial resilience, and inflammatory suppression, emphasizing its unique role as a bridge compound linking circadian biology, microbiota modulation, and endothelial repair and reinforcing its potential as a clinically relevant adjunct in managing obesity- and sleep-related coronary endothelial dysfunction. Furthermore, by enriching SCFA-producing taxa and reducing pro-atherogenic metabolites such as TMAO and endotoxin, it restores endothelial barrier integrity and vascular tone. Clinical trials across diverse cardiometabolic phenotypes, using doses between 75 and 1500 mg/day, demonstrate duration-dependent improvements in microbial composition and endothelial function, supporting its role as a microbiota-sensitive adjunct in sleep- and obesity-related coronary endothelial dysfunction. We conclude a comparative overview of clinical efficacy across curcumin, EGCG, quercetin, berberine, and resveratrol in Table 2.

Table 2.
Classifications, Mechanisms, and Clinical Parameters of Phytoantioxidants.
Table 2.
Classifications, Mechanisms, and Clinical Parameters of Phytoantioxidants.
| Class | Mechanisms | Dosage & Duration | Reference | |
|---|---|---|---|---|
| Curcumin | Non-flavonoid polyphenol | NF-κB inhibition, AMPK activation, gut barrier modulation | 500–1000 mg/day, 8-12 weeks | [33,34,35,36,37,38,39,40,41,42] |
| EGCG | Flavonoid | Antioxidant, anti-inflammatory, microbiota–gut–brain axis modulation | 300–800 mg/day, 8–12 weeks | [43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
| Quercetin | Flavonoid | Nrf2 activation, endothelial protection, metabolic regulation | 300–800 mg/day, 8–12 weeks | [57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| Berberine | Alkaloid | FXR/TGR5 modulation, insulin sensitization, bile acid signaling | 1000 mg twice daily, 12 weeks | [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] |
| Resveratrol | Non-flavonoid polyphenol | SIRT1 activation, mitochondrial biogenesis, anti-inflammatory and vascular effects | 150–500 mg/day, 8–12 weeks | [16,25,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] |
AMPK: AMP-activated protein kinase; CRP: C-reactive protein; EGCG: Epigallocatechin gallate; eNOS: Endothelial nitric oxide synthase; FMD: Flow-mediated dilation; FPG: Fasting plasma glucose; HbA1c: Hemoglobin A1c; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; LDL-C: Low-density lipoprotein cholesterol; NO: Nitric oxide; SCFA: Short-chain fatty acid; SBP: Systolic blood pressure; TG: Triglycerides.
4. Translational Outlook
Despite compelling mechanistic and clinical evidence supporting microbiota-targeted and antioxidant-based interventions, several translational barriers hinder their integration into routine cardiovascular care. Regulatory heterogeneity across countries complicates the standardization of probiotic strains and plant-derived antioxidant formulations, limiting reproducibility and scalability. To advance clinical applicability, trial designs must incorporate validated endothelial function metrics such as flow-mediated dilation, pulse wave velocity, and circulating adhesion molecules alongside microbial and inflammatory biomarkers, particularly in populations affected by sleep disruption and cardiometabolic stress.
Recent experimental studies have demonstrated that specific probiotic strains, such as Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis, can significantly reduce infarct size and modulate inflammatory and oxidative stress responses in rat models of cardiac ischemia–reperfusion injury [104]. These findings are further supported by evidence showing improved hemodynamic stability and endothelial protection following probiotic therapy in dysbiotic and inflamed rat models [105], underscoring the translational relevance of microbiota-targeted interventions in cardiovascular contexts.
Integrating these vascular markers with microbial metabolites and systemic inflammatory indices will strengthen mechanistic interpretation and therapeutic precision. Longitudinal cohort studies employing multi-omics profiling will be critical to elucidate causal pathways and refine intervention targets. Interdisciplinary collaboration, bridging cardiology, sleep medicine, microbiology, and nutritional science, will be essential for adaptive trial frameworks and real-world implementation strategies.
Furthermore, precision nutrition strategies increasingly emphasize individualized pairings of probiotics and polyphenols, tailored to the host’s microbiome composition and metabolic profile. This concept is supported by enterotype-specific microbial functions that influence polyphenol metabolism and systemic outcomes [106]. Individuals with Prevotella-dominant enterotypes, which are characterized by enhanced carbohydrate fermentation and elevated production of SCFAs, may experience amplified glycemic and lipid responses to berberine. Berberine interacts with microbial metabolites to modulate bile acid pathways and activate AMP-activated protein kinase signaling [107,108]. In contrast, epigallocatechin gallate shows improved neurovascular efficacy when administered alongside specific Lactobacillus strains, particularly Lactobacillus rhamnosus and Lactobacillus plantarum. These strains enhance catechin bioavailability and stimulate eNOS through gut–brain axis signaling [12,109]. Such strain specific interactions may influence blood–brain barrier integrity, vascular tone, and cognitive resilience under conditions of circadian disruption [110].
In summary, coronary endothelial dysfunction represents a mechanistic nexus linking sleep irregularity, cardiometabolic stress, and gut–vascular signaling. Probiotics and phytoantioxidants offer biologically grounded, complementary strategies to restore endothelial integrity. This review highlights their therapeutic promise within a personalized framework. Realizing their full clinical potential will require rigorous validation, stratified intervention models, and collaborative translational research to transform mechanistic insights into effective cardiovascular prevention.
Author Contributions
Original Draft Preparation, C.-N.T.; Conceptualization, Organization, Writing—Review and Editing, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This review was supported by Chang Gung Memorial Hospital, Linkou Branch, Taiwan, R.O.C. (CMRPG3F1831, CMRPG3F1832, CMRPG3H0991, CMRPG3H0992, CMRPG3H1801, CMRPG3K0051, CMRPG3K0052, CMRPG3K0221, CMRPG3K0222, CMRPG3K1901, CMRPG3M0121) and from National Science and Technology Council, Taiwan, R.O.C. (NMRPG3G0091, NMRPG3J0511, NMRPG3M0421).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sejbuk, M.; Siebieszuk, A.; Witkowska, A.M. The role of gut microbiome in sleep quality and health: Dietary strategies for microbiota support. Nutrients 2024, 16, 2259. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut microbiota and cardiovascular disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Circadian rhythms: A regulator of gastrointestinal health and dysfunction. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Chassaing, B.; Langella, P. Exploring the interaction and impact of probiotic and commensal bacteria on vitamins, minerals and short chain fatty acids metabolism. Microb. Cell Fact. 2024, 23, 172. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A.; Atkin, S.L.; Johnston, T.P.; Jamialahmadi, T.; et al. Curcumin and endothelial function: A systematic review. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef]
- Alam, M.; Gulzar, M.; Akhtar, M.S.; Rashid, S.; Zulfareen; Tanuja; Shamsi, A.; Hassan, M.I. Epigallocatechin-3-gallate therapeutic potential in human diseases: Molecular mechanisms and clinical studies. Mol. Biomed. 2024, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Wang, Y.; Tu, S. Potential implications of quercetin in autoimmune diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Phytother. Res. 2020, 155, 104722. [Google Scholar] [CrossRef]
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a promising nutraceutical: Implications in gut microbiota modulation, inflammatory disorders, and colorectal cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef]
- Cheang, W.S.; Wong, W.T.; Wang, L.; Cheng, C.K.; Lau, C.W.; Ma, R.C.W.; Xu, A.; Wang, N.; Huang, Y.; Tian, X.Y. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor δ. Pharmacol. Res. 2019, 139, 384–394. [Google Scholar] [CrossRef]
- Sung, M.M.; Dyck, J.R. Therapeutic potential of resveratrol in heart failure. Ann. N. Y. Acad. Sci. 2015, 1348, 123–131. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Ruiz-Ros, J.A.; María, T.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory biomarkers and improves cardiovascular health. Am. J. Cardiol. 2012, 110, 356–363. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, Z.; Zhang, D.X.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host–gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Nutr. Rev. 2014, 72, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Zhao, X.; Shang, C.; Xiang, M.; Li, L.; Cui, X. Microbiota-derived short-chain fatty acids: Implications for cardiovascular and metabolic disease. Front. Cardiovasc. Med. 2022, 9, 900381. [Google Scholar] [CrossRef]
- Kavyani, B.; Ahmadi, S.; Nabizadeh, E.; Abdi, M. Anti-oxidative activity of probiotics; focused on cardiovascular disease, cancer, aging, and obesity. Microb. Pathog. 2024, 196, 107001. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Wang, Q.; Zhou, H. Gut-derived endotoxemia and TMAO in coronary endothelial dysfunction: Role of microbial metabolites. J. Clin. Med. 2022, 11, 2987. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, L.; Zhang, X.; Chen, Y.; Wang, H.; Liu, Q.; Lu, P.; Wang, J.; Liu, Y.; Gao, J.; et al. Lactobacillus ameliorates myocardial ischemia–reperfusion injury by attenuating apoptosis, inflammation, oxidative stress, and ferroptosis. BMC Med. 2025, 23, 377. [Google Scholar] [CrossRef]
- Lopes, M.R.; Direito, R.; Guiguer, E.L.; Strozze Catharin, V.C.; Zutin, T.L.M.; Rubira, C.J.; Sloan, K.P.; Sloan, L.A.; Yanaguizawa Junior, J.L.; Laurindo, L.F.; et al. Bridging the gut microbiota and the brain, kidney, and cardiovascular health: The role of probiotics. Probiotics Antimicrob. Proteins 2025, 17, e10680. [Google Scholar] [CrossRef]
- Andersson, K.; Nilsson, A.; Johansson, M.; Bergström, J. Lactobacillus plantarum 299v improves endothelial function in metabolic syndrome: A cohort study. Microorganisms 2023, 11, 456. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Chan, C.W.; Chen, Y.T.; Lin, B.F. Renal protective and immunoregulatory effects of Lactobacillus casei strain Shirota in nephropathy-prone mice. Front. Nutr. 2024, 11, 1438327. [Google Scholar] [CrossRef]
- Alam, M.S.; Anwar, M.J.; Maity, M.K. Curcumin modulates eNOS and NF-κB signaling in vascular inflammation. Pharmaceuticals 2024, 17, 1674. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Phytother. Res. 2014, 28, 514–518. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.C.; Ursoniu, S.; Banach, M. Effect of curcuminoids on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Joshi, P.; Joshi, S.; Semwal, D.K.; Rawat, S.; Bhatt, V.; Nautiyal, V.; Verma, K.; Dwivedi, J.; Sharma, S.; Semwal, R.B.; et al. Role of curcumin in ameliorating hypertension and associated conditions: A mechanistic insight. Mol. Cell. Biochem. 2022, 488, 45–62. [Google Scholar] [CrossRef]
- Feng, J. Role of curcumin in altering gut microbiota for anti-obesity and anti-hyperlipidemic effects. Front. Microbiol. 2025, 16, 1625098. [Google Scholar] [CrossRef]
- Zhu, J.; He, L. The modulatory effects of curcumin on the gut microbiota: A potential strategy for disease treatment and health promotion. Microorganisms 2024, 12, 642. [Google Scholar] [CrossRef]
- Unhapipatpong, C.; Julanon, N.; Chattranukulchai Shantavasinkul, P.; Polruang, N.; Numthavaj, P.; Thakkinstian, A. Umbrella Review of Systematic Reviews and Meta-Analyses of Randomized Controlled Trials Investigating the Effect of Curcumin Supplementation on Lipid Profiles. Nutr. Rev. 2025, 83, 1520–1536. [Google Scholar] [CrossRef]
- Ferreira, M.; Tan, J.; Alavi, S.; Chen, Y.; Kumar, R.; Singh, A.; Patel, V.; Gupta, N.; Wang, L.; Zhang, Y.; et al. Curcumin–phospholipid complex improves lipid profile but not endothelial biomarkers in adults with metabolic syndrome: A longitudinal cohort analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1121–1130. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R. Enhancing the bioavailability and bioactivity of curcumin for disease prevention and treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.; Chen, Y.; Shen, H.; Chen, L.; Ding, L.; Tang, Q.; Yang, Z.; Chen, W.; Shen, Z. Precision cardiac targeting: Empowering curcumin therapy through smart exosome-mediated drug delivery. Regen. Biomater. 2024, 11, rbad108. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A.V. Innovative delivery systems for curcumin: Exploring nanosized and conventional formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ellis, K.; Scipione, C.A.; Fish, J.E.; Howe, K.L. Epigallocatechin gallate (EGCG) modulates senescent endothelial cell–monocyte communication in age-related vascular inflammation. Front. Cardiovasc. Med. 2025, 11, 1506360. [Google Scholar] [CrossRef]
- Das, M.; Yagnik, U.; Raninga, I. EGCG improves endothelial biomarkers in metabolic syndrome. Front. Endocrinol. 2025, 16, 1655875. [Google Scholar] [CrossRef]
- Rezaei, M.; Akhavan, N.; Fathi, F.; Alavi, S.M.; Fadaii, M.J.; Askarpour, M. Effect of green tea supplementation on blood pressure in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Blood Press. 2025, 34, e2517122. [Google Scholar] [CrossRef]
- Yan, R.; Cao, Y. EGCG improves short-chain fatty acid levels and endothelial function in adults with poor sleep quality. Biomedicines 2025, 13, 206. [Google Scholar] [CrossRef]
- Margareto, J. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Sun, Y. EGCG suppresses TLR4 signaling and enhances Nrf2 antioxidant response. Mol. Nutr. Food Res. 2025, 69, e2300123. [Google Scholar] [CrossRef]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef]
- Wu, S.; Dong, R.; Xie, Y.; Chen, W.; Liu, W.; Weng, Y. CO-loaded hemoglobin/EGCG nanoparticles functional coatings for inflammation modulation of vascular implants. Regen. Biomater. 2025, 12, rbae148. [Google Scholar] [CrossRef]
- Patel, P.; Garala, K.; Singh, S.; Prajapati, B.G.; Chittasupho, C. Lipid-based nanoparticles in delivering bioactive compounds for improving therapeutic efficacy. Pharmaceuticals 2025, 17, 329. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Alharbi, H.O.; Alshebremi, M.; Babiker, A.Y.; Rahmani, A.H. Quercetin as a modulator of endothelial nitric oxide and oxidative stress pathways. Biomolecules 2025, 15, 151. [Google Scholar] [CrossRef]
- Mi, W.; Hu, Z.; Xu, L.; Bian, X.; Lian, W.; Yin, S.; Zhao, S.; Gao, W.; Guo, C.; Shi, T. Quercetin positively affects gene expression profiles and metabolic pathways of antibiotic-treated mouse gut microbiota. Front. Microbiol. 2022, 13, 983358. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, K.; Huang, Y.; Zhang, X.; Yang, T.; Zhan, K.; Zhao, G. Quercetin alleviates lipopolyssacharide-induced oxidative stress and inflammatory responses via regulation of the TLR4–NF-κB signaling pathway in bovine rumen epithelial cells. Toxins 2023, 15, 512. [Google Scholar] [CrossRef]
- Seong, H.J.; Baek, Y.; Lee, S.; Jin, H.J. Gut microbiome and metabolic pathways linked to sleep quality. Front. Microbiol. 2024, 15, 1418773. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Y.; Zhang, Y.; Xie, J.; Guo, Q.; Yang, H.; Yang, Y.; Chen, J.; Su, L. Quercetin ameliorates obesity and inflammation via microbial metabolite indole-3-propionic acid in high-fat diet-induced obese mice. Front. Nutr. 2025, 12, 1574792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lv, H.; Ainiwan, M.; Yesitayi, G.; Abudesimu, A.; Siti, D.; Aizitiaili, A.; Ma, X. Untargeted metabolomics identifies indole-3-propionic acid to relieve Ang II-induced endothelial dysfunction in aortic dissection. Mol. Cell. Biochem. 2024, 479, 1767–1786. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, W.; Zhang, P.; Qu, Y.; Zhong, H.; Zhou, L.; Zhou, W.; Yang, W.; Xu, H.; Zhao, X.; et al. The gut microbiota–bile acid–TGR5 axis orchestrates platelet activation and atherothrombosis. Nat. Cardiovasc. Res. 2025, 4, 584–601. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Kerby, R.L.; Aquino-Martinez, R.; Evered, A.H.; Cross, T.-W.L.; Everhart, J.; Ulland, T.K.; Kay, C.D.; Bolling, B.W.; Bäckhed, F.; et al. Gut microbes modulate the effects of the flavonoid quercetin on atherosclerosis. NPJ Biofilms Microbiomes 2025, 11, 12. [Google Scholar] [CrossRef]
- Kim, W.G.; Kim, H.I.; Kwon, E.K.; Han, M.J.; Kim, D.H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food Funct. 2018, 9, 4255–4265. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin reprograms immunometabolism of macrophages via the SIRT1/PGC-1α signaling pathway to ameliorate lipopolysaccharide-induced oxidative damage. Int. J. Mol. Sci. 2023, 24, 5542. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Su, L.; Zeng, Y.; Li, G.; Chen, J.; Chen, X. Quercetin improves high-fat diet-induced obesity by modulating gut microbiota and metabolites in C57BL/6J mice. Phytother Res. 2022, 36, 4558. [Google Scholar] [CrossRef]
- Shi, M.; Sun, L.; Wei, J.; Shen, Y.; Wang, J.; Zhang, P.; Yang, X.; Ding, Y.; Yin, W.; Lu, X.; et al. Quercetin alleviates endothelial dysfunction in preeclampsia by inhibiting ferroptosis and inflammation through EGFR binding. Commun. Biol. 2025, 8, 547. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Menni, C.; Berry, S.E.; Valdes, A.M.; Spector, T.D.; Segata, N. Cardiometabolic health, diet and the gut microbiome: A meta-omics perspective. Nat. Med. 2023, 29, 1174–1182. [Google Scholar] [CrossRef]
- Zieniuk, B.; Pawełkowicz, M. Berberine as a Bioactive Alkaloid: Multi-Omics Perspectives on Its Role in Obesity Management. Metabolites 2025, 15, 467. [Google Scholar] [CrossRef]
- Chang, J.; Sun, C.; Wang, M.; Li, W.; Jia, Y.; Zhang, J.; Qiu, F. Berberine inhibits phagocytosis through the TLR4-PI3K-CDC42 pathway. Acta Mater. Medica 2025, 4, 280. [Google Scholar] [CrossRef]
- Dong, C.; Yu, J.; Yang, Y.; Zhang, F.; Su, W.; Fan, Q.; Wu, C.; Wu, S. Berberine, a potential prebiotic to indirectly promote Akkermansia growth through stimulating gut mucin secretion. Biomed. Pharmacother. 2021, 139, 111595. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ji, L.; Wang, Y.; Zhang, Y.; Wang, H.; Wang, J.; Zhu, Q.; Xie, M.; Ou, W.; Liu, J.; et al. Acetate enables metabolic fitness and cognitive performance during sleep disruption. Cell Metab. 2024, 36, 1998–2014.e15. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Hu, J.; Deng, Y.; Zou, J.; Ding, W.; Peng, Q.; Duan, R.; Sun, J.; Zhu, J. Berberine mediates the production of butyrate to ameliorate cerebral ischemia via the gut microbiota in mice. Nutrients 2023, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, B.; Dong, X.; Sun, J.; Miao, Z.; Pan, L. Gut microbiota-derived butyrate prevents aortic dissection via GPR41. Acta Pharmacol. Sin. 2025, 46, 1123–1135. [Google Scholar] [CrossRef]
- Ma, S.R.; Tong, Q.; Lin, Y.; Pan, L.B.; Fu, J.; Peng, R.; Zhang, X.-F.; Zhao, Z.X.; Li, Y.; Yu, J.B.; et al. Berberine treats atherosclerosis via a vitamin-like effect down-regulating choline–TMA–TMAO production pathway in gut microbiota. Signal Transduct. Target Ther. 2022, 7, 207. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y.-J.; Park, W. Berberine modulates hyper-inflammation in mouse macrophages stimulated with polyinosinic-polycytidylic acid via calcium–CHOP/STAT pathway. Sci. Rep. 2021, 11, 11234. [Google Scholar] [CrossRef]
- Zhao, G.-L.; Yu, L.-M.; Gao, W.-L.; Duan, W.-X.; Jiang, B.; Liu, X.-D.; Zhang, B.; Liu, Z.-H.; Zhai, M.-E.; Jin, Z.-X.; et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 2016, 37, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, R.; Xu, S.; Zhou, X.-Y.; Cai, K.; Chen, Y.-L.; Zhou, Z.-Y.; Sun, X.; Shi, Y.; Wang, F.; et al. NOTCH1 mitochondria localization during heart development promotes mitochondrial metabolism and the endothelial-to-mesenchymal transition in mice. Nat. Commun. 2024, 15, 7074. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, C.; Jiang, Z.; Yang, Y.; Zhang, Y.; Yang, M.; Zhang, X.; Du, Y.; Zhang, J.; Wang, L.; et al. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, B.; Meng, X.; Yao, S.; Jin, L.; Yang, J.; Wang, J.; Zhang, H.; Zhang, Z.; Cai, D.; et al. Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci. Rep. 2016, 6, 20848. [Google Scholar] [CrossRef]
- He, R.; Liu, B.; Geng, B.; Li, N.; Geng, Q. The role of HDAC3 and its inhibitors in regulation of oxidative stress and chronic diseases. Cell Death Discov. 2023, 9, 1399. [Google Scholar] [CrossRef]
- Cole, L.K.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Berberine elevates cardiolipin in heart of offspring from mouse dams with high-fat diet-induced gestational diabetes mellitus. Sci. Rep. 2021, 11, 95353. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.Y.; Engelke, R.; Bhagwat, A.M.; Abdesselem, H.; Agha, M.V.; Vakayil, M.; Courjaret, R.; Goswami, N.; et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci Rep. 2021, 11, 87759. [Google Scholar] [CrossRef]
- Zhao, J.V.; Yeung, W.F.; Chan, Y.H.; Vackova, D.; Leung, J.Y.Y.; Ip, D.K.M.; Zhao, J.; Ho, W.K.; Tse, H.F.; Schooling, C.M. Effect of berberine on cardiovascular disease risk factors: A mechanistic randomized controlled trial. Nutrients 2021, 13, 2550. [Google Scholar] [CrossRef]
- Kong, Y.; Yang, H.; Nie, R.; Zhang, X.; Zhang, H.; Nian, X.; Liu, Y.; Chen, J.; Wang, Q.; Li, M. Berberine as a multi-target therapeutic agent for obesity: From pharmacological mechanisms to clinical evidence. Eur. J. Med. Res. 2025, 30, 477. [Google Scholar] [CrossRef]
- Godos, J.; Romano, G.L.; Gozzo, L.; Laudani, S.; Paladino, N.; Dominguez Azpíroz, I.; Martínez López, N.M.; Giampieri, F.; Quiles, J.L.; Battino, M.; et al. Resveratrol and vascular health: Evidence from clinical studies and mechanisms of actions related to its metabolites produced by gut microbiota. Front. Pharmacol. 2024, 15, 1368949. [Google Scholar] [CrossRef]
- Gostimirovic, M.; Rajkovic, J.; Bukarica, A.; Simanovic, J.; Gojkovic-Bukarica, L. Resveratrol and gut microbiota synergy: Preventive and therapeutic effects. Int. J. Mol. Sci. 2024, 24, 17573. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Resveratrol and the interaction between gut microbiota and arterial remodelling. Nutrients 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Förstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind placebo-controlled one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Wang, F.; Zhang, J. Resveratrol improves mitochondrial biogenesis by activating SIRT1/PGC-1α signaling in SAP. Sci. Rep. 2024, 14, 26216. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Niziński, P.; Kasprzak, P.; Kondracka, A.; Oniszczuk, T.; Rusinek, A.; Oniszczuk, A. Does resveratrol improve metabolic dysfunction-associated steatotic liver disease (MASLD)? Int. J. Mol. Sci. 2024, 25, 3746. [Google Scholar] [CrossRef]
- Fernandez-Quintela, A.; Macarulla, M.T.; Gómez-Zorita, S.; González, M.; Milton-Laskibar, I.; Portillo, M.P. Relationship between changes in microbiota induced by resveratrol and its anti-diabetic effect on type 2 diabetes. Front. Nutr. 2023, 9, 1084702. [Google Scholar] [CrossRef]
- Wu, W.; Meng, T.; Jin, F.; Li, J.; Huang, J.; Guo, Z.; Yu, M.; Zhou, Y. Effects of resveratrol on postmenopausal women: A systematic review and meta-analysis. Front. Pharmacol. 2025, 16, 1588284. [Google Scholar] [CrossRef]
- Damay, V.A.; Ivan, I. Resveratrol as an anti-inflammatory agent in coronary artery disease: A systematic review, meta-analysis and meta-regression. Chin. J. Integr. Med. 2024, 30, 927–937. [Google Scholar] [CrossRef]
- Bullón-Vela, V.; Abete, I.; Zulet, M.A.; Xu, Y.; Martínez-González, M.A.; Sayón-Orea, C.; Ruiz-Canela, M.; Toledo, E.; Martín Sánchez, V.; Estruch, R.; et al. Urinary resveratrol metabolites output: Differential associations with cardiometabolic markers and liver enzymes in house-dwelling subjects featuring metabolic syndrome. Molecules 2020, 25, 4340. [Google Scholar] [CrossRef]
- Molani-Gol, R.; Rafraf, M. Effects of resveratrol on anthropometric indices and inflammatory markers: An umbrella meta-analysis. Eur. J. Nutr. 2024, 63, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gong, A.; Zhang, B.; Cheng, H.; Huang, L.; Wu, X.; Zhang, D.; Dai, W.; Li, S.; Xu, H. The chronobiological and neuroprotective mechanisms of resveratrol in improving sleep. Mediators Inflamm. 2025, 2025, 4954030. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O.L. High-dose resveratrol supplementation (1500 mg/day for 4 weeks) in obese men: A randomized, placebo-controlled trial of insulin sensitivity, blood pressure, and endothelial function. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jia, Y.; Ren, F. Multidimensional biological activities of resveratrol and its prospects and challenges in the health field. Front. Nutr. 2024, 11, 1408651. [Google Scholar] [CrossRef]
- Borshchev, Y.Y.; Burovenko, I.Y.; Karaseva, A.B.; Minasian, S.M.; Protsak, E.S.; Borshchev, V.Y.; Semenova, N.Y.; Borshcheva, O.V.; Suvorov, A.N.; Galagudza, M.M. Probiotic therapy with Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis results in infarct size limitation in rats with obesity and chemically induced colitis. Microorganisms 2022, 10, 2293. [Google Scholar] [CrossRef]
- Borshchev, Y.Y.; Protsak, E.S.; Burovenko, I.Y.; Semenova, N.Y.; Zubkov, I.G.; Galagudza, M.M. Effect of probiotic therapy on hemodynamic response associated with systemic inflammatory reaction and antibiotic-induced dysbiosis in chronic experiments in rats. Bull. Exp. Biol. Med. 2022, 172, 676–680. [Google Scholar] [CrossRef]
- Hu, J.; Mesnage, R.; Tuohy, K.; Heiss, C.; Rodriguez-Mateos, A. (Poly)phenol-related gut metabotypes and human health: An update. Food Funct. 2024, 15, 2814–2835. [Google Scholar] [CrossRef]
- Bah, Y.R.; Baba, K.; Mustafa, D.N.A.B.; Watanabe, S.; Takeda, A.K.; Yamashita, T.; Kasahara, K. Bacteroides and Prevotella enriched gut microbial clusters associate with metabolic risks. Gut Pathog. 2025, 17, 55. [Google Scholar] [CrossRef]
- Ji, L.; Ma, J.; Ma, Y.; Cheng, Z.; Gan, S.; Yuan, G.; Liu, D.; Li, S.; Liu, Y.; Xue, X.; et al. Berberine ursodeoxycholate for the treatment of type 2 diabetes: A randomized clinical trial. JAMA Netw. Open 2025, 8, e2462185. [Google Scholar] [CrossRef]
- Li, X.; Zheng, P.; Cao, W.; Cao, Y.; She, X.; Yang, H.; Ma, K.; Wu, F.; Gao, X.; Fu, Y.; et al. Lactobacillus rhamnosus GG ameliorates noise induced cognitive deficits and systemic inflammation in rats by modulating the gut brain axis. Front. Cell Infect. Microbiol. 2023, 13, 1067367. [Google Scholar] [CrossRef]
- Srivastava, R.; Gupta, M.K. Gut bacteria derived metabolites and their implications in mental health and neurological diseases. World J. Microbiol. Biotechnol. 2025, 41, 423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).