Hybrid Strategies for CTO PCI: A Systematic Review and Meta-Analysis of Antegrade and Retrograde Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction and Management
- Study characteristics: year, country, design, sample size;
- Patient population: age, sex, comorbidities (when reported);
- Procedural strategy: ADR, RWE, RDR, hybrid;
- Devices used: IVUS, CrossBoss, Stingray, microcatheters;
- Primary outcomes:
- –
- Technical success (successful CTO crossing with restoration of TIMI flow and <30% residual stenosis);
- –
- Procedural success (technical success without in-hospital MACE);
- –
- In-hospital MACE: death, MI, stroke, or target vessel revascularization.
- Secondary outcomes: revascularization, restenosis, perforation, tamponade, mortality.
2.5. Quality Assessment
2.6. Data Synthesis and Quantitative Analysis
3. Results
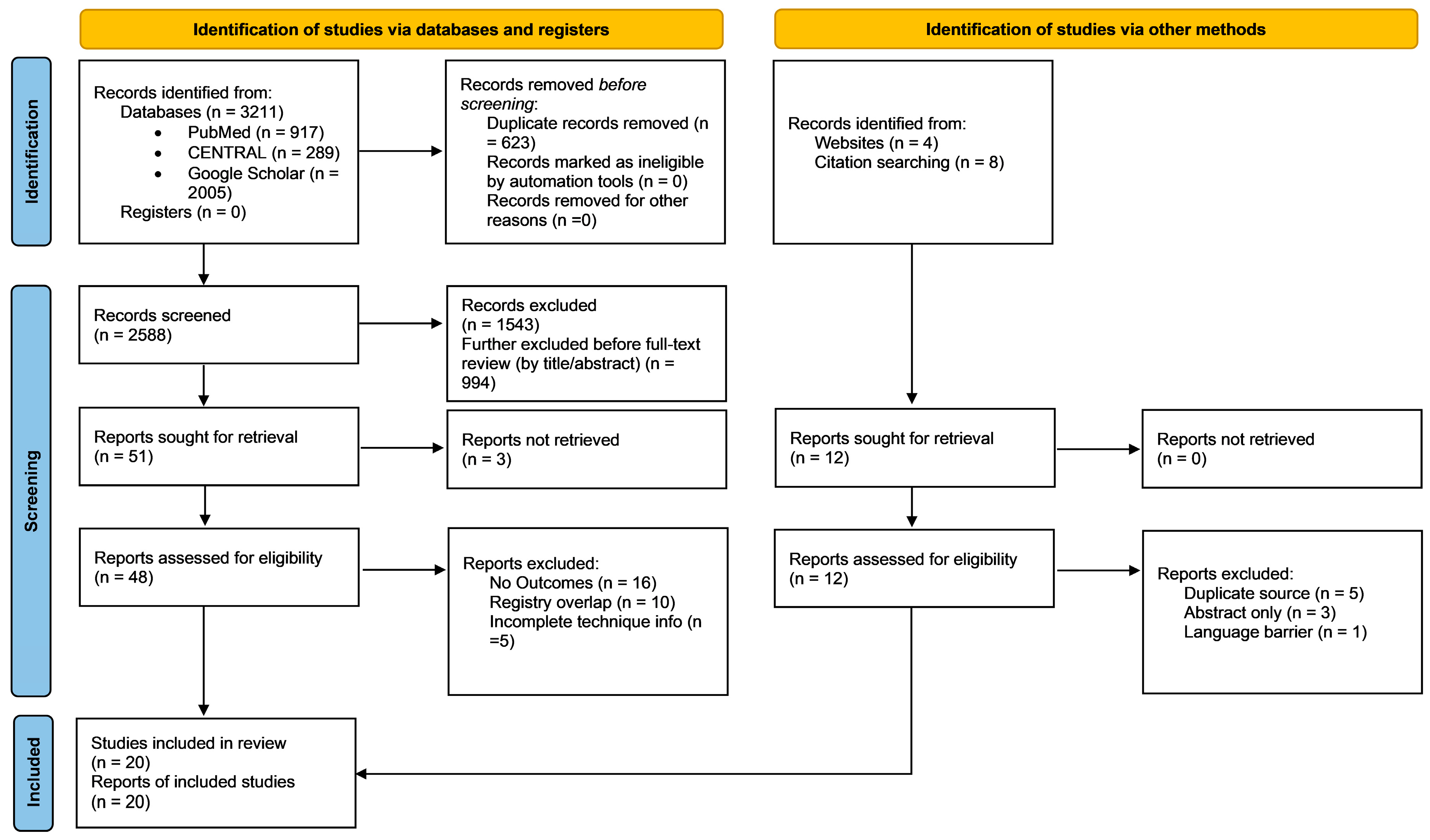
3.1. Search Results
3.2. Study Characteristics
3.3. Technical Approaches
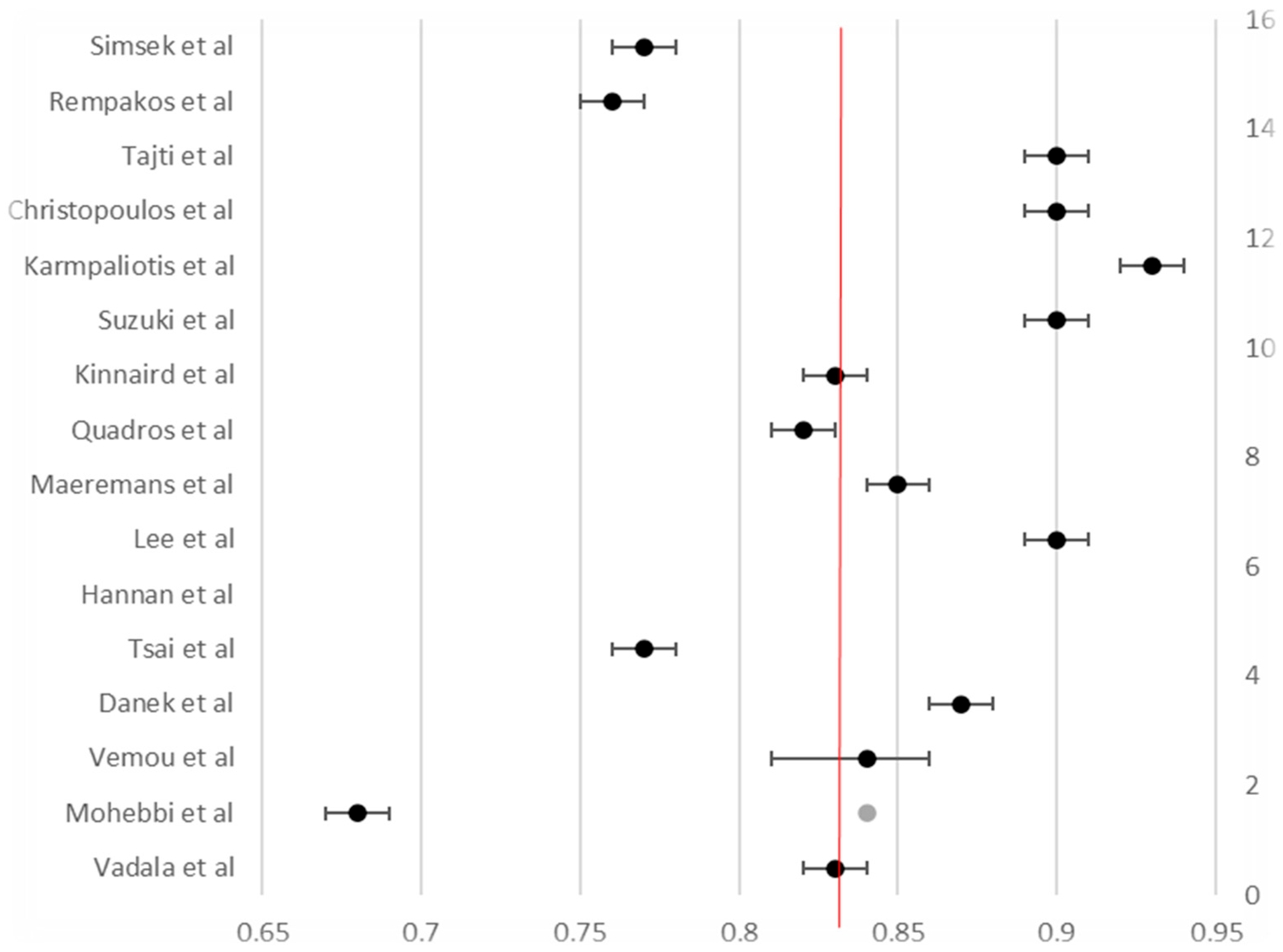
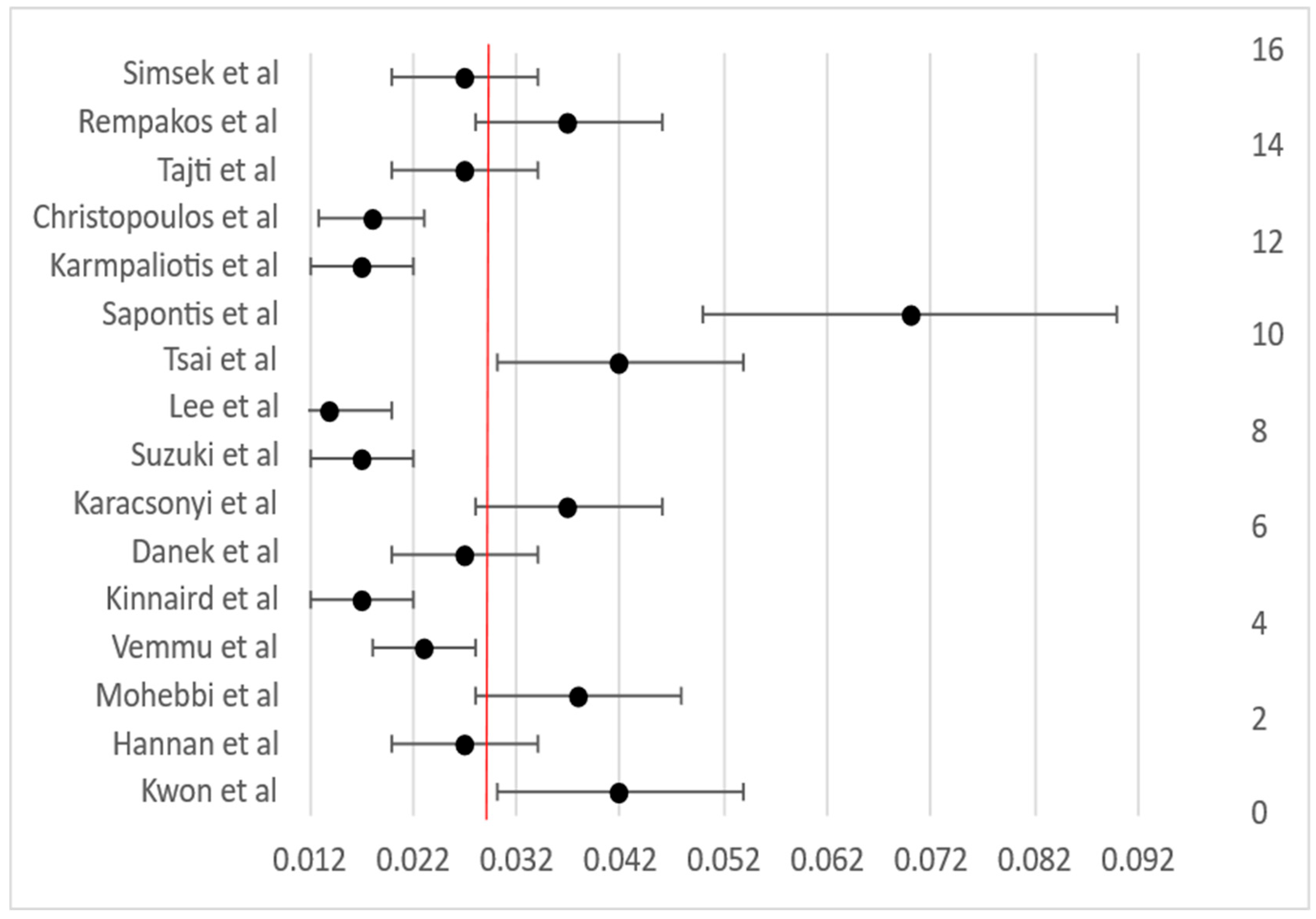
3.4. Procedural Outcomes
3.5. Long-Term Clinical Outcomes
3.6. Quality Assessment Results
4. Discussion
4.1. Review Limitations
4.2. Implications for Clinical Practice and Future Research
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | Antegrade Dissection and Reentry |
| AFR | Antegrade Fenestration and Reentry |
| AWE | Antegrade Wire Escalation |
| BASE | Balloon-Assisted Subintimal Entry |
| CABG | Coronary Artery Bypass Grafting |
| CAD | Coronary Artery Disease |
| CART | Controlled Antegrade and Retrograde Tracking |
| CTO | Chronic Total Occlusion |
| HDR | Helical Dissection Re-entry |
| ISR | In-Stent Restenosis |
| IVUS | Intravascular Ultrasound |
| MACE | Major Adverse Cardiac Events |
| PAA | Primary Antegrade Approach |
| PCI | Percutaneous Coronary Intervention |
| PRA | Primary Retrograde Approach |
| RDR | Retrograde Dissection and Re-entry |
| Reverse CART | Reverse Controlled Antegrade and Retrograde Tracking |
| RFR | Reentry with Focal Reentry |
| RWE | Retrograde Wire Escalation |
| Side-BASE | Side-Branch Assisted Subintimal Entry |
| STAR | Subintimal Tracking and Reentry |
| STEMI | ST-Elevation Myocardial Infarction |
| TIMI | Thrombolysis in Myocardial Infarction |
| TVF | Target Vessel Failure |
| TVR | Target Vessel Revascularization |
References
- Arnold, R.; Gervasoni, R.; Leclercq, F. Chronic total occlusions: Current approaches, evidence and outcomes. J. Clin. Med. 2025, 14, 4695. [Google Scholar] [CrossRef] [PubMed]
- Sianos, G. CTO PCI, the evolution of the revolution: Time for consensus on definitions. EuroIntervention 2018, 14, 31–33. [Google Scholar] [CrossRef]
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current perspectives on coronary chronic total occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef]
- Claessen, B.E.; Dangas, G.D.; Weisz, G.; Witzenbichler, B.; Guagliumi, G.; Möckel, M.; Brener, S.J.; Xu, K.; Henriques, J.P.; Mehran, R.; et al. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur. Heart J. 2012, 33, 768–775. [Google Scholar] [CrossRef]
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Morino, Y.; Abe, M.; Morimoto, T.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: The J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc. Interv. 2011, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Sliman, H.; Sliman, R.K.; Knaapen, P.; Nap, A.; Sobieszek, G.; Opolski, M.P. The role of intravascular imaging in coronary chronic total occlusion PCI: Enhancing procedural success through real-time visualization. J. Pers. Med. 2025, 15, 318. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, P.H.; Ahn, J.M.; Park, D.W.; Yun, S.C.; Han, S.; Kang, H.; Kang, S.J.; Kim, Y.H.; Lee, C.W.; et al. Randomized Trial Evaluating Percutaneous Coronary Intervention for the Treatment of Chronic Total Occlusion. Circulation 2019, 139, 1674–1683. [Google Scholar] [CrossRef]
- Maeremans, J.; Walsh, S.; Knaapen, P.; Spratt, J.C.; Avran, A.; Hanratty, C.G.; Faurie, B.; Agostoni, P.; Bressollette, E.; Kayaert, P.; et al. The hybrid algorithm for treating chronic total occlusions in Europe: The RECHARGE registry. J. Am. Coll. Cardiol. 2016, 68, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tsuchikane, E.; Katoh, O.; Muramatsu, T.; Muto, M.; Kishi, K.; Hamazaki, Y.; Oikawa, Y.; Kawasaki, T.; Okamura, A. Outcomes of percutaneous coronary interventions for chronic total occlusion performed by highly experienced Japanese specialists: The first report from the Japanese CTO-PCI Expert Registry. JACC Cardiovasc. Interv. 2017, 10, 2144–2154. [Google Scholar] [CrossRef]
- Sapontis, J.; Salisbury, A.C.; Yeh, R.W.; Cohen, D.J.; Hirai, T.; Lombardi, W.; McCabe, J.M.; Karmpaliotis, D.; Moses, J.; Nicholson, W.J.; et al. Early procedural and health status outcomes after chronic total occlusion angioplasty: A report from the OPEN-CTO registry. JACC Cardiovasc. Interv. 2017, 14, 1523–1534. [Google Scholar] [CrossRef]
- Kinnaird, T.; Gallagher, S.; Cockburn, J.; Sirker, A.; Ludman, P.; de Belder, M.; Smith, M.; Anderson, R.; Strange, J.; Mamas, M.; et al. Procedural success and outcomes with increasing use of enabling strategies for chronic total occlusion intervention. Circ. Cardiovasc. Interv. 2018, 11, e006436. [Google Scholar] [CrossRef]
- Tsai, T.T.; Stanislawski, M.A.; Shunk, K.A.; Armstrong, E.J.; Grunwald, G.K.; Schob, A.H.; Maddox, T.M.; Bradley, S.M.; Rao, S.V.; Messenger, J.C.; et al. Contemporary incidence, management, and long-term outcomes of percutaneous coronary interventions for chronic coronary artery total occlusions: Insights from the VA CART program. J. Am. Coll. Cardiol. Interv. 2017, 10, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Hannan, E.L.; Zhong, Y.; Jacobs, A.K.; Stamato, N.J.; Berger, P.B.; Walford, G.; Sharma, S.; Venditti, F.J.; King, S.B. Patients with chronic total occlusions undergoing percutaneous coronary interventions: Characteristics, success, and outcomes. Circ. Cardiovasc. Interv. 2016, 9, e003586. [Google Scholar] [CrossRef]
- Konstantinidis, N.V.; Werner, G.S.; Deftereos, S.; Di Mario, C.; Galassi, A.R.; Buettner, J.H.; Avran, A.; Reifart, N.; Goktekin, O.; Garbo, R.; et al. Temporal trends in chronic total occlusion interventions in Europe. Circ. Cardiovasc. Interv. 2018, 11, e006229. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Mashayekhi, K.; Boukhris, M.; Behnes, M.; Pyxaras, S.; Christiansen, E.H.; Gutiérrez-Chico, J.L.; Maniscalco, L.; Stojkovic, S.; Bozinovic, N.Z.; et al. Reclassification of CTO crossing strategies in the ERCTO Registry according to the CTO-ARC consensus recommendations. JACC Cardiovasc. Interv. 2024, 17, 2425–2437. [Google Scholar] [CrossRef]
- Christopoulos, G.; Karmpaliotis, D.; Alaswad, K.; Yeh, R.W.; Jaffer, F.A.; Wyman, R.M.; Lombardi, W.L.; Menon, R.V.; Grantham, J.A.; Kandzari, D.E.; et al. Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry. Int. J. Cardiol. 2015, 198, 222–228. [Google Scholar] [CrossRef]
- Tajti, P.; Karmpaliotis, D.; Alaswad, K.; Jaffer, F.A.; Yeh, R.W.; Patel, M.; Mahmud, E.; Choi, J.W.; Burke, M.N.; Doing, A.H.; et al. The hybrid approach to chronic total occlusion percutaneous coronary intervention: Update from the PROGRESS CTO registry. JACC Cardiovasc. Interv. 2018, 11, 1325–1335. [Google Scholar] [CrossRef]
- Danek, B.A.; Karatasakis, A.; Karmpaliotis, D.; Alaswad, K.; Yeh, R.W.; Jaffer, F.A.; Patel, M.; Bahadorani, J.; Lombardi, W.L.; Wyman, M.R.; et al. Use of antegrade dissection re-entry in coronary chronic total occlusion percutaneous coronary intervention in a contemporary multicenter registry. Int. J. Cardiol. 2016, 214, 428–437. [Google Scholar] [CrossRef]
- Rempakos, A.; Alexandrou, M.; Simsek, B.; Kostantinis, S.; Karacsonyi, J.; Mutlu, D.; Ybarra, L.F.; Bagur, R.; Choi, J.W.; Poommipanit, P.; et al. Trends and outcomes of antegrade dissection and re-entry in chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc. Interv. 2023, 16, 2736–2747. [Google Scholar] [CrossRef]
- Kwon, O.; Lee, P.H.; Lee, S.W.; Lee, J.Y.; Kang, D.Y.; Ahn, J.M.; Park, D.W.; Kang, S.J.; Kim, Y.H.; Lee, C.W.; et al. Retrograde approach for the percutaneous recanalisation of coronary chronic total occlusions: Contribution to clinical practice and long-term outcomes. EuroIntervention 2019, 15, e354–e361. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, B.; Sadeghipour, P.; Zolfaghari, R.; Vadalà, G.; Khalilipour, E.; Zahedmehr, A.; Diana, D.; Maadani, M.; Shakerian, F.; Kiani, R.; et al. Outcomes of chronic total occlusion percutaneous coronary intervention from the RAIAN (RAjaie-Iran) registry. Indian Heart J. 2023, 75, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Karacsonyi, J.; Tajti, P.; Rangan, B.V.; Halligan, S.C.; Allen, R.H.; Nicholson, W.J.; Harvey, J.E.; Spaedy, A.J.; Jaffer, F.A.; Grantham, J.A.; et al. Randomized comparison of a CrossBoss first versus standard wire escalation strategy for crossing coronary chronic total occlusions: The CrossBoss First trial. JACC Cardiovasc. Interv. 2018, 11, 225–233. [Google Scholar] [CrossRef]
- Karmpaliotis, D.; Karatasakis, A.; Alaswad, K.; Jaffer, F.A.; Yeh, R.W.; Wyman, R.M.; Lombardi, W.L.; Grantham, J.A.; Kandzari, D.E.; Lembo, N.J.; et al. Outcomes with the use of the retrograde approach for coronary chronic total occlusion interventions in a contemporary multicenter US registry. Circ. Cardiovasc. Interv. 2016, 9, e003434. [Google Scholar] [CrossRef]
- Simsek, B.; Kostantinis, S.; Karacsonyi, J.; Alaswad, K.; Jaffer, F.A.; Doshi, D.; Gorgulu, S.; Goktekin, O.; Kerrigan, J.; Haddad, E.; et al. Antegrade dissection and re-entry versus parallel wiring in chronic total occlusion percutaneous coronary intervention: Insights from the PROGRESS-CTO registry. Catheter. Cardiovasc. Interv. 2022, 100, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Vemmou, E.; Quadros, A.S.; Dens, J.A.; Abi Rafeh, N.; Agostoni, P.; Alaswad, K.; Avran, A.; Belli, K.C.; Carlino, M.; Choi, J.W.; et al. In-stent CTO percutaneous coronary intervention: Individual patient data pooled analysis of 4 multicenter registries. JACC Cardiovasc. Interv. 2021, 14, 1308–1319. [Google Scholar] [CrossRef]
- Quadros, A.S.; Belli, K.C.; de Paula, J.E.T.; de Magalhães Campos, C.A.H.; da Silva, A.C.; Santiago, R.; Ribeiro, M.C.; de Oliveira, P.D.; Lamelas, P.; Abelin, A.M.; et al. Chronic total occlusion percutaneous coronary intervention in Latin America. Catheter. Cardiovasc. Interv. 2020, 95, 1202–1210. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Rangan, B.V.; Kotsia, A.; Karmpaliotis, D.; Lembo, N.; Banerjee, S. The hybrid approach to intervention of chronic total occlusions. Curr. Cardiol. Rev. 2015, 11, 299–304. [Google Scholar] [CrossRef]
- Schumacher, S.P.; Stuijfzand, W.J.; Opolski, M.P.; van Rossum, A.C.; Nap, A.; Knaapen, P. Percutaneous coronary intervention of chronic total occlusions: When and how to treat. Cardiovasc. Revasc Med. 2019, 20, 513–522. [Google Scholar] [CrossRef]
- Dąbrowski, E.J.; Sorysz, D.; Mizia-Stec, K.; Kruk, M. Ten-Year Trends in Chronic Total Occlusion Interventions in Poland in the Context of the European CTO Club Consensus. J. Clin. Med. 2023, 12, 3762. [Google Scholar] [CrossRef] [PubMed]
- Macherey-Meyer, S.; Salem, K.; Heyne, S.; Meertens, M.M.; Finke, K.; Mauri, V.; Baldus, S.; Adler, C.; Lee, S. Percutaneous Coronary Intervention versus Optimal Medical Therapy in Patients with Chronic Total Occlusion: A Meta-Analysis. J. Clin. Med. 2024, 13, 2919. [Google Scholar] [CrossRef] [PubMed]
- Aljabbary, T.; Katyukha, A.; Elbaz-Greener, G.; Gressmann, K.; Bagai, A.; Graham, J.J.; Vijayaraghavan, R.; Kalra, S.; Vo, M.; Wijeysundera, H.C. Overview of Contemporary Chronic Total Occlusion Percutaneous Coronary Intervention Techniques: A Narrative Systematic Review. CJC Open 2021, 3, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]


| Study | Country/Registry | Design | n | Primary Techniques | Key Devices | Technical/Procedural Success (%) | In-Hospital MACE (%) |
|---|---|---|---|---|---|---|---|
| Simsek et al. [27] | Multinational | Registry | 1725 | ADR vs. PW | Stingray, CrossBoss | 78/75 (ADR/PW); 75.6/72.6 (Proc) | 3.7/1.9 |
| Rempakos et al. [22] | USA & Intl (PROGRESS-CTO) | Registry | 12,568 | ADR (primary–tertiary) | Stingray, CrossBoss, STAR | 77/75.5 | 3.7 (ADR); 1.6 (non-ADR) |
| Vadalà et al. [18] | Europe (ERCTO) | Prospective | 2395 | AWE, ADR, RWE, RDR | Standard CTO sets | 93.1 (AWE); 84.5 (ADR); 87.6 (RWE); 82.6 (RDR) | 3.4 (ADR); 6.6 (RDR) |
| Tajti et al. [20] | USA & Intl (PROGRESS-CTO) | Registry | 1313 | Hybrid (AWE, ADR, RWE, RDR) | NR | 90.7/85.2 | 2.7 |
| Maeremans et al. [11] | Europe (RECHARGE) | Prospective | 1177 | Hybrid (AWE, ADR, RWE, RDR) | CrossBoss (38%), Stingray (34%) | 86/NR | 3.6 |
| Christopoulos et al. [19] | USA (PROGRESS-CTO) | Registry | 1036 | Hybrid (AWE, ADR, Retrograde) | CrossBoss, Stingray, etc. | 91/90 | 1.7 |
| Karmpaliotis et al. [26] | USA (11 centres) | Registry | 1301 | Antegrade/Retrograde | NR | 94/85 (Tech); 93/82 (Proc) | 1.1/4.3 |
| Konstantinidis et al. [17] | Europe (ERCTO) | Prospective | 17,626 | Antegrade, Retrograde, ADR | NR | 79.7 → 89.3 (2008–15) | ≈5 |
| Sapontis et al. [13] | USA (OPEN-CTO) | Prospective | 1000 pts/1054 lesions | Hybrid | CrossBoss, Stingray | 86 (core), 90 (op.)/81–85 | 7.0 |
| Tsai et al. [15] | USA (VA CART) | National registry | 2394 pts/2516 lesions | Standard CTO PCI | DES 81%, BMS 19% | 79.8/79.7 | 4.3 |
| Lee et al. [10] | Asia (DECISION-CTO) | RCT | 834 | Antegrade ± Retrograde (25%) | DES | 90.6/≈90 | 0.9 |
| Suzuki et al. [12] | Japan (41 centres) | Prospective | 2846 | PAA, PRA, RRA | IVUS, microcatheters | 91/87/78 (Tech); 88.8 (Proc) | 1.7 |
| Karacsonyi et al. [25] | USA (1 centre) | RCT | 246 | CrossBoss ADR vs. AWE | CrossBoss, Stingray | 87.8/87.1 (Tech); 85.3/83.1 (Proc) | 3.3/4.0 |
| Danek et al. [21] | USA (11 centres) | Registry | 1313 | ADR vs. AWE/Retrograde | CrossBoss, Stingray > 50% | 86.9/91.8 (Tech); 85.0/90.7 (Proc) | 2.9/2.2 |
| Kinnaird et al. [14] | UK (BCIS) | National | 28,050 | CTO PCI with enabling strategies | IVUS, atherectomy, etc. | 56.8 → 83.8 | 0.7 → 2.0 |
| Vemmou et al. [28] | Multinational | Pooled analysis | 11,961 | ISR vs. De novo CTO PCI | IVUS/OCT frequent in ISR | 84.9/85.2 (Tech); 83.7/83.9 (Proc) | 1.9/2.5 |
| Quadros et al. [29] | Latin America (7 countries) | Registry | 1040 | AWE 81%, ADR 8%, Retro 11% | Guidewires, IVUS variable | 82.5 (range 65–100) | 3.1 |
| Mohebbi et al. [24] | Iran (RAIAN) | Single-centre | 790 | Hybrid (antegrade, ADR, retrograde) | IVUS, microcatheters | 70.3/70.3 | 3.5 |
| Hannan et al. [16] | USA (NY State) | Population registry | 4030 | CTO PCI (techniques NR) | NR | 63.6 (success) | ≈3 (MACE) |
| Kwon et al. [23] | Korea (Asan) | Single-centre | 1635 | Antegrade/Retrograde | Stenting routine | 79.5 → 87.1 (post-retrograde) | 4.3/4.1 |
| Trial | Study Question | Intervention(s) | Comparator/Control | Principal Findings and Interpretation |
|---|---|---|---|---|
| DECISION-CTO [10] | Does CTO PCI improve outcomes compared with optimal medical therapy? | CTO PCI using operator-selected antegrade or retrograde techniques (≈25% retrograde use) | Optimal medical therapy alone | PCI achieved excellent technical success (~90%) with very low periprocedural complication rates. While hard outcomes (death, MI, stroke, revascularization) were similar to medical therapy, PCI provided sustained improvements in angina and quality of life at 1–3 years. High crossover limited statistical power. |
| CrossBoss First Trial [25] | Does an up-front device-based ADR approach improve efficiency versus standard wire escalation? | CrossBoss/Stingray ADR system | Conventional antegrade wire escalation | Both techniques achieved nearly identical technical and procedural success with low complication rates. No procedural-efficiency advantage was observed, confirming ADR safety but not superiority. The trial validated ADR feasibility in experienced centers. |
| Domain/Representative Studies | Scope and Setting | Dominant Techniques | Key Interpretive Outcomes |
|---|---|---|---|
| Hybrid Strategy Registries [11,13,19,20] | Large multicenter registries across the US and Europe implementing the hybrid algorithm. | Dynamic integration of AWE, ADR, and retrograde methods with real-time switching. | Demonstrated reproducibly high success (~85–90%) and low complication rates (<4%) across diverse centers. Confirmed the hybrid algorithm as the benchmark contemporary framework. |
| Antegrade Dissection/Re-entry (ADR) [21,22,27] | Multicenter registries assessing ADR role and temporal trends. | CrossBoss/Stingray and STAR techniques used as primary, secondary, or bailout strategies. | ADR yields moderately lower success and slightly higher perforation/MACE than wire escalation but remains indispensable for long, complex, or ambiguous-cap lesions. Use of ADR has declined with improved retrograde proficiency. |
| Retrograde Approaches [2,12,23] | High-volume centers in the US, Japan, and Korea. | Septal and epicardial collateral tracking, reverse CART, IVUS-guided crossing. | Retrograde adoption increased technical success in complex CTOs without significantly raising periprocedural mortality. Long-term follow-up shows higher re-occlusion risk, underscoring the need for meticulous stent optimization. |
| Temporal and Geographic Trends [17,24,29] | Continental registries from Europe, Latin America, and the Middle East. | Predominantly hybrid approaches; ADR and retrograde adapted variably by region. | Success rates of 80–90% achieved globally, confirming transferability of hybrid methodology. Resource-limited settings reported slightly lower success but comparable safety. |
| In-stent CTO PCI [28] | Pooled analysis of 4 major registries. | AWE and imaging-guided wiring predominant. | Immediate outcomes similar to de novo CTOs; however, higher long-term revascularization rates indicate inferior durability of ISR CTO PCI. |
| Enabling Technologies and Imaging [12,14] | National and expert-center registries. | Dual access, IVUS, atherectomy, microcatheters, CrossBoss/Stingray. | Increasing use of ≥3 enabling tools markedly improved procedural success but modestly raised perforation rates, highlighting the balance between efficacy and complexity. |
| Population-Based Evidence [15,16] | National datasets from the US VA system and New York State. | Standard real-world CTO PCI, operator-discretional technique. | Demonstrated mortality benefit of successful CTO PCI versus failed attempts and confirmed influence of operator and institutional volume on outcomes. |
| Study Type/Setting | Technical Success (%) | Procedural Success (%) | In-Hospital MACE (%) | Perforation (%) | Tamponade (%) | In-Hospital Mortality (%) |
|---|---|---|---|---|---|---|
| Randomized trials (n = 2) [10,25] | 87–91 | 83–86 | 2–4 | 1–7 | ≤0.5 | ≤0.3 |
| Multicenter hybrid registries (n = 6) [11,13,17,19,20,21] | 85–91 | 83–90 | 1.7–3.6 | 1–3 | ≤1 | ≤0.3 |
| ADR-focused analyses (n = 2) [22,27] | 75–78 (ADR) vs. 88–89 (non-ADR) | 73–76 (ADR) vs. 87–88 (non-ADR) | 3.7 vs. 1.6–1.9 | 3–9 (ADR) vs. 4 (non-ADR) | ≤0.5 | ≤0.7 |
| Retrograde strategy studies (n = 3) [12,23,26] | 78–87 | 80–85 | 2–4 | 2–5 | ≤1 | ≤0.3 |
| National/large-scale registries (n = 4) [14,15,16,17] | 56–89 | 67–89 | 2–5 | 1–4 | ≤1 | 0.1–0.9 |
| Developing-region cohorts (n = 2) [24,29] | 70–83 | 70–82 | 3–3.5 | ≤1 | 0.4–0.9 | 0.3–1.0 |
| Overall weighted mean | ≈86 | ≈84 | ≈2.8 | ≈2.6 | ≈0.6 | ≈0.4 |
| Strategy | Mean Technical Success (%) | Mean Procedural Success (%) | Typical MACE (%) | Typical Perforation (%) | Distinguishing Features |
|---|---|---|---|---|---|
| AWE | 90–93 | 85–90 | 1.5–2.5 | 1–2 | High success in less complex lesions; baseline strategy in most cases. |
| ADR | 75–86 | 72–85 | 3–5 | 3–9 | Used as secondary strategy; greater radiation, contrast, and perforation risk. |
| RWE | 85–88 | 80–85 | 2–4 | 2–4 | Effective for proximal cap ambiguity or long lesions; requires dual access. |
| RDR | 78–83 | 75–80 | 3–6 | 5–7 | Highest fluoroscopy and contrast use; reserved for complex and refractory occlusions. |
| Hybrid (Integrated AWE + ADR + Retrograde) | 86–91 | 83–89 | 1.5–3.5 | 2–3 | Combines approaches within one procedure; consistent success across registries. |
| Enabling Techniques (IVUS, atherectomy, dual access) | +10–15 improvement in success vs. baseline | – | ↓ complications when used systematically | – | Improves planning and wire control; associated with 83–84% national success in BCIS data [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosu, A.-M.; Tanasescu, M.-D.; Badea, T.-G.; Radu, E.-S.; Cismas, E.-G.; Minca, A.; Popa, O.-A.; Tomescu, L.-F. Hybrid Strategies for CTO PCI: A Systematic Review and Meta-Analysis of Antegrade and Retrograde Techniques. Life 2025, 15, 1739. https://doi.org/10.3390/life15111739
Rosu A-M, Tanasescu M-D, Badea T-G, Radu E-S, Cismas E-G, Minca A, Popa O-A, Tomescu L-F. Hybrid Strategies for CTO PCI: A Systematic Review and Meta-Analysis of Antegrade and Retrograde Techniques. Life. 2025; 15(11):1739. https://doi.org/10.3390/life15111739
Chicago/Turabian StyleRosu, Andrei-Mihnea, Maria-Daniela Tanasescu, Theodor-Georgian Badea, Emanuel-Stefan Radu, Eduard-George Cismas, Alexandru Minca, Oana-Andreea Popa, and Luminita-Florentina Tomescu. 2025. "Hybrid Strategies for CTO PCI: A Systematic Review and Meta-Analysis of Antegrade and Retrograde Techniques" Life 15, no. 11: 1739. https://doi.org/10.3390/life15111739
APA StyleRosu, A.-M., Tanasescu, M.-D., Badea, T.-G., Radu, E.-S., Cismas, E.-G., Minca, A., Popa, O.-A., & Tomescu, L.-F. (2025). Hybrid Strategies for CTO PCI: A Systematic Review and Meta-Analysis of Antegrade and Retrograde Techniques. Life, 15(11), 1739. https://doi.org/10.3390/life15111739






