Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) has emerged as a key cytokine in the pathogenesis of rheumatoid arthritis, an autoimmune disease distinguished by synovial inflammation and progressive joint destruction. GM-CSF orchestrates the activation, proliferation, and differentiation of myeloid cells (mainly macrophages and neutrophils) thereby sustaining the pro-inflammatory synovial milieu. Recent advances in monoclonal antibody immunotherapy have enabled selective inhibition of GM-CSF or its receptor. Clinical data on several monoclonal antibodies are presented, focusing on their pharmacodynamic properties and efficacy results documented in phase II and III clinical studies. Cumulative evidence supports GM-CSF inhibition as a compelling strategy for modulating inflammation and improving clinical outcomes in rheumatoid arthritis.
1. Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disorder characterized by persistent synovial inflammation, pannus formation, and destruction of articular cartilage and subchondral bone [1]. This disease arises from a multifactorial interplay of genetic risk factors, environmental triggers, and dysregulated immune responses that culminate in chronic joint inflammation and systemic comorbidities [2,3]. RA pathogenesis involves the aberrant activation of both innate and adaptive immune compartments, including macrophages, neutrophils, dendritic cells, Th1 and Th17 lymphocytes, and autoreactive B cells producing rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs) [4,5,6]. These immune populations generate a pathogenic cytokine milieu dominated by TNF-α, IL-1β, IL-6, IL-17, and granulocyte-macrophage colony-stimulating factor (GM-CSF), which sustains synovial inflammation and drives structural joint damage [7,8,9].
Among these mediators, GM-CSF has gained increasing attention as a central regulator of myeloid cell activation within the inflamed synovium [10,11]. GM-CSF is produced by activated T cells, fibroblast-like synoviocytes (FLS), endothelial cells, and macrophages in response to pro-inflammatory cues such as TNF-α and IL-1β [12,13]. Its effects are mediated through binding to the heterodimeric GM-CSF receptor (GM-CSFR), composed of a cytokine-specific α-chain (GM-CSFRα) and a signal-transducing common β-chain (GM-CSFRβc), functionally shared with the IL-3 and IL-5 receptor families [14,15]. Ligand engagement induces receptor dimerization and activates downstream signaling cascades, mainly JAK2-STAT5, MAPK/ERK, PI3K/Akt, and NF-κB pathways, resulting in enhanced survival, differentiation, and effector function of myeloid cells [16,17,18,19].
In RA, dysregulated GM-CSF signaling elicits the differentiation of circulating monocytes into pro-inflammatory macrophages that secrete TNF-α, IL-6, and IL-23, reinforcing a pathogenic axis with Th17 cells [20,21]. GM-CSF further augments neutrophil survival, oxidative burst, and degranulation, and enhances dendritic cell maturation and antigen presentation, thereby sustaining autoreactive T-cell activation and chronic synovitis [15,22]. These mechanisms collectively amplify a self-reinforcing inflammatory circuit that accelerates osteoclastogenesis, cartilage degradation, and bone erosion [22].
Despite the substantial therapeutic progress achieved with conventional and biologic disease-modifying antirheumatic drugs (DMARDs) have transformed RA management, 30–40% of patients display inadequate clinical responses or develop secondary resistance [23,24,25]. This therapeutic limitation has prompted investigation of alternative cytokine targets upstream of established inflammatory cascades. Blockade of the GM-CSF pathway offers a mechanistically rational strategy to interfere with early myeloid-driven inflammation. Neutralizing antibodies directed against GM-CSF (e.g., otilimab and namilumab) or GM-CSFRα (e.g., mavrilimumab) facilitate selective interruption of this signaling axis [26,27]. Preclinical models demonstrate that GM-CSF inhibition reduces synovial macrophage accumulation, pro-inflammatory cytokine production, and osteoclast differentiation, thereby preventing structural joint damage [15,28].
Translation of these findings into clinical settings has been supported by Phase II and III trials evaluating mavrilimumab, otilimab, and namilumab, which have shown significant improvements in some disease activity indices (e.g., DAS28 and ACR responses) and patient-reported outcomes, accompanied by favorable safety profiles [29,30]. Mild respiratory adverse events observed in some studies reflect the physiological role of GM-CSF in pulmonary macrophage homeostasis, underscoring the importance of balanced cytokine modulation [31]. Comparative analyses suggest that GM-CSF blockade might provide additive or complementary benefit, particularly in patients with myeloid-dominant disease or insufficient response to TNF-α inhibition.
This review synthesizes current mechanistic insights and clinical evidence regarding GM-CSF and GM-CSFR inhibition in RA, with the aim of critically assessing the therapeutic potential of targeting this pathway as an innovative strategy for durable immune modulation and improved disease control. Additionally, this review addresses the knowledge gap concerning the translational relevance of GM-CSF blockade, highlighting novel perspectives that have not yet been integrated into the current literature, thereby underscoring its potential to reshape future therapeutic paradigms in RA.
2. Fundamental Aspects of RA
RA is a chronic, systemic, autoimmune disease characterized by persistent synovial inflammation, progressive joint destruction, and many extra-articular manifestations [1]. It represents one of the most prevalent autoimmune disorders worldwide, affecting approximately 0.5 to 1% of the adult population [32], exhibiting a marked female predominance (3:1) with peak incidence occurring between 40 and 60 years [33]. The disease course is typically progressive and fluctuating, with periods of exacerbation and remission [34]. If inadequately treated, it can cause considerable functional impairment, functional disability, and premature mortality, predominantly attributable to cardiovascular complications and systemic inflammation [35,36].
The etiology of RA is multifactorial, involving a complex interplay of genetic, environmental, hormonal, and immunological factors [2]. Genetic susceptibility accounts for nearly half of the risk, and among genetic determinants, the strongest association has been established with alleles of the HLA-DRB1 gene encoding the so-called “shared epitope”, which influences antigen presentation to CD4+ T lymphocytes [37,38]. Other genetic loci, such as PTPN22, STAT4, and CTLA4, contribute to immune dysregulation and loss of tolerance [39,40,41]. Environmental factors, especially cigarette smoking, have been identified as major triggers in genetically predisposed individuals, enhancing protein citrullination and the generation of neoantigens that stimulate autoimmunity [42]. Additional environmental and microbial agents, including Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, have been implicated in the pathogenesis through similar mechanisms involving post-translational protein modifications [43,44]. Hormonal influences, like estrogen fluctuations and pregnancy-associated immune modulation, might further modify disease risk and activity [45].
The immunopathogenesis of RA is characterized by a loss of self-tolerance, resulting in the activation of autoreactive T and B lymphocytes (Figure 1) [46]. Autoreactive CD4+ T cells, including Th1 and Th17 subsets, infiltrate the synovial tissue, where they interact with local antigen-presenting cells (APCs) like dendritic cells and macrophages [47,48]. Th1 cells secrete IFN-γ, enhancing macrophage activation, while Th17 cells produce IL-17A, IL-17F, and IL-22, which synergize with TNF-α, IL-1β, and IL-6 to amplify inflammation and recruit neutrophils and monocytes to the synovium [49]. Regulatory T cell (Treg) dysfunction in RA further exacerbates this pro-inflammatory milieu, resulting in uncontrolled effector T cell activity [50]. Under these conditions, FLS adopt a pathogenic, hyperactivated phenotype, marked by increased proliferative capacity and resistance to programmed cell death, and increased production of matrix metalloproteinases (MMPs), such as MMP-1, MMP-3, and MMP-9, which mediate degradation of the cartilage extracellular matrix [51,52]. FLS also secrete chemokines such as CCL2, CCL5, and CXCL10, recruiting additional immune cells and sustaining local inflammation [53].
On the other hand, B cells contribute to RA pathogenesis via many mechanisms. They differentiate into plasma cells that secrete autoantibodies, most prominently RF and ACPAs [54]. These autoantibodies induce the formation of immune complexes that activate the classical complement pathway, generating C3a and C5a fragments that act as chemoattractants for neutrophils and monocytes, further amplifying synovial inflammation [55]. B cells also function as antigen-presenting cells, stimulating autoreactive T cells, and secrete pro-inflammatory cytokines like IL-6, TNF-α, and lymphotoxin, thereby perpetuating local immune activation [56,57].
Neutrophils contribute through the release of reactive oxygen species (ROS) and neutrophil extracellular traps (NETs), which not only damage synovial tissue but also provide citrullinated antigens that fuel ACPA production [58]. Monocytes and macrophages in the synovium adopt a pro-inflammatory M1 phenotype (Figure 1), secreting TNF-α, IL-1β, IL-6, and IL-12, which promote Th1/Th17 responses and enhance osteoclastogenesis [59].
Chronic inflammation drives synovial hyperplasia and the formation of pannus tissue, an invasive structure that erodes articular cartilage and subchondral bone [60]. Osteoclast differentiation is mainly mediated by receptor activator of nuclear factor κB ligand (RANKL), located at activated T cells, FLS, and synovial macrophages [61]. RANKL binds to its receptor RANK on osteoclast precursors, triggering NF-κB, NFATc1, and c-Fos signaling pathways, which culminate in osteoclast maturation and activation [62]. Other contributing modulators of osteoclastogenesis include TNF-α, IL-17, and M-CSF, further enhance bone resorption [63].
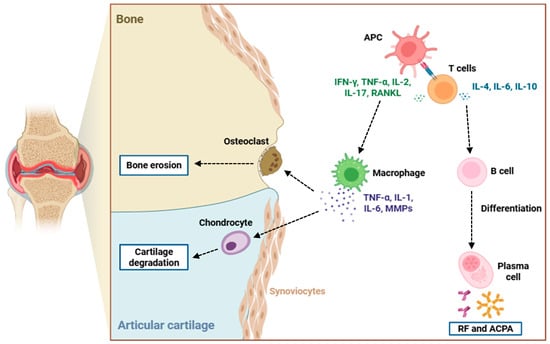
Figure 1.
Immunopathogenesis of bone and cartilage destruction in RA. This figure illustrates the cellular and molecular mechanisms driving joint damage in RA. APCs activate T cells, which release pro-inflammatory cytokines like IFN-γ, TNF-α, IL-2, IL-17, and RANKL, promoting macrophage activation and osteoclast differentiation. Activated macrophages secrete and release TNF-α, IL-1, IL-6, and MMPs, contributing to synovial inflammation, chondrocyte-mediated cartilage degradation, and osteoclast-induced bone erosion. Concurrently, T cells stimulate B cell differentiation into plasma cells that produce RF and ACPAs, further amplifying the autoimmune response. The interplay among these immune cells and cytokines leads to the progressive destruction of articular cartilage and bone typical of RA pathology. Abbreviations: APC (antigen-presenting cell), RF (rheumatoid factor), ACPA (anti-citrullinated protein antibody), IFN-γ (interferon-gamma), TNF-α (tumor necrosis factor-alpha), IL-1 (interleukin 1), IL-2 (interleukin 2), IL-4 (interleukin 4), IL-6 (interleukin 6), IL-10 (interleukin 10), IL-17 (interleukin 17), RANKL (receptor activator of nuclear factor kappa-B ligand), and MMPs (matrix metalloproteinases).
Clinically, RA typically presents as a symmetrical polyarthritis mainly involving the joints of the hands (metacarpophalangeal and proximal interphalangeal), wrists, and feet (metatarsophalangeal joints) [64,65]. The onset is typically insidious, characterized by articular pain, swelling, increased warmth, and prolonged morning stiffness persisting for more than one hour [66]. Fatigue, low-grade fever, and malaise are common symptoms [67]. As the disease advances, chronic synovitis leads to joint deformities including ulnar deviation, boutonnière and swan-neck deformities, and subluxations, leading to progressive loss of function [68]. Extra-articular manifestations occur in approximately 30–40% of patients and may include rheumatoid nodules, vasculitis, pleural and pericardial effusions, interstitial lung disease, peripheral neuropathy, scleritis, and hematological abnormalities (e.g., anemia and thrombocytosis) [69]. Cardiovascular involvement, particularly accelerated atherosclerosis, contributes significantly to the increased mortality observed in RA populations [70].
Finally, diagnosis is based on the synthesis of clinical assessment, serological testing, and imaging modalities [71]. The 2010 ACR/EULAR criteria integrate the number and distribution of affected joints, serological markers (e.g., RF and ACPA), acute-phase reactants such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), and symptom duration [72]. ACPA testing, mainly anti-cyclic citrullinated peptide (anti-CCP) antibodies, provides high specificity for RA and may precede clinical onset by several years [73]. Imaging modalities, including ultrasonography and magnetic resonance imaging (MRI), facilitate the detection of early synovitis, tenosynovitis, and erosions that are not apparent on conventional radiographs, thus facilitating early diagnosis [74].
3. Role of GM-CSF in RA
GM-CSF is a pleiotropic cytokine that plays a central role in the immunopathogenesis of RA. Its multifaceted actions on myeloid cell differentiation, activation, survival, and effector function make GM-CSF a critical driver of chronic synovial inflammation, pannus formation, and joint destruction [10,11]. In RA, GM-CSF acts not merely as a hematopoietic growth factor but also as a key pro-inflammatory mediator coordinating innate and adaptive immune responses. This section provides an in-depth exploration of the molecular and cellular mechanisms by which GM-CSF contributes to RA pathogenesis.
3.1. Molecular Signaling Mechanisms of GM-CSF
GM-CSF mediates its diverse biological effects mainly through GM-CSFR, a heterodimeric transmembrane complex composed of a ligand-specific α-chain (GM-CSFRα) and a common βc signaling subunit (GM-CSFRβc) shared with the IL-3 and IL-5 receptors [75]. The α-chain is a single-pass transmembrane protein that mediates ligand recognition [76]. Its extracellular domain comprises modular motifs typical of type I cytokine receptors, including a cytokine receptor homology domain (CHD) with conserved fibronectin type III-like subdomains. Although the α-chain lacks intrinsic signaling capability, it serves a structural function by capturing GM-CSF and presenting it in a conformation that facilitates βc subunit engagement [77]. On the other hand, the βc subunit functions as a multifunctional signaling component that mediates receptor dimerization, stability, and downstream signaling [78]. Its extracellular domain facilitates low-affinity ligand binding and heterodimerization with GM-CSFRα through fibronectin type III-like repeats that stabilize the receptor-ligand complex [79]. The transmembrane region promotes receptor oligomerization through helix-helix interactions, promoting the spatial alignment of cytoplasmic domains. The cytoplasmic tail, enriched in conserved tyrosine residues, provides docking sites for signaling molecules [80]. Ligand binding induces conformational rearrangements that bring βc cytoplasmic domains together, enabling recruitment and activation of JAK2, which triggers subsequent intracellular signaling cascades [81].
Activated JAK2 phosphorylates multiple conserved tyrosine residues within the cytoplasmic tail of the βc subunit, creating docking sites for several STAT proteins, mainly STAT5 [82]. STAT5 is subsequently phosphorylated, dimerize, and translocate to the nucleus, where they bind to promoter regions of some target genes to regulate transcription of critical effectors controlling cell survival (e.g., BCL2, MCL1, and BCL-XL), proliferation (e.g., cyclin D1 and c-Myc), and pro-inflammatory cytokine production (e.g., TNF-α, IL-1β, IL-6, and IL-23) [83,84,85]. This STAT5-dependent transcriptional program is critical for promoting the differentiation of monocytes into pro-inflammatory macrophages and dendritic cells within the synovial microenvironment [86].
Parallel to the JAK/STAT axis, GM-CSFR engagement activates PI3K/Akt signaling [87]. PI3K binds phosphorylated tyrosine residues on the receptor via its SH2 domains, catalyzing the conversion of PIP2 to PIP3 and recruiting Akt to the plasma membrane [88]. Akt undergoes phosphorylation by PDK1 and mTORC2, commencing a signaling cascade that promotes metabolic reprogramming, glycolytic flux, mitochondrial biogenesis, and inhibition of apoptotic pathways [89,90]. In parallel, GM-CSF receptor engagement stimulates the Ras/Raf/MEK/ERK (MAPK) pathway [91]. Upon activation, Ras engages Raf-1 kinase, promoting MEK1/2 phosphorylation and thus triggering ERK1/2 activation [92]. Nuclear translocation of ERK1/2 promotes phosphorylation of transcription factors such as Elk-1 and AP-1, driving the expression of inflammatory mediators (e.g., MMP-1, MMP-3, and MMP-13) and chemokines (CCL2, CCL3, and CXCL8) that orchestrate immune cell recruitment and synovial tissue remodeling [93,94,95].
Finally, GM-CSFR signaling activates the NF-κB pathway via phosphorylation and degradation of IκBα through the IKK complex [96]. Upon release, NF-κB translocates to the nucleus and drives expression of genes for pro-inflammatory cytokines, adhesion molecules, and survival factors, sustaining chronic inflammation [97]. Crosstalk between the JAK/STAT, PI3K/Akt, MAPK, and NF-κB pathways strengthens the transcriptional output, resulting in synergistic effects on myeloid cell activation, cytokine secretion, and tissue-destructive capacity.
3.2. Impact of GM-CSF on Immune Effector Cell Differentiation and Activation
In RA synovium, GM-CSF is synthesized by numerous resident and infiltrating cells (Figure 2), including FLS, Th17 lymphocytes, B cells, and endothelial cells, functioning via autocrine and paracrine signaling pathways to perpetuate synovial inflammation [98]. GM-CSF induces FLS to secrete IL-6, IL-8, and MMPs, which amplify leukocyte recruitment, promote angiogenesis, and drive cartilage degradation [99,100]. Molecularly, this involves activation of the GM-CSFR/JAK2/STAT5 axis in FLS, converging on NF-κB and AP-1 transcription factors, leading to increased expression of several pro-inflammatory mediators [101].
On the other hand, GM-CSF exerts pleiotropic effects on cells of the myeloid lineage (e.g., monocytes, macrophages, dendritic cells, and neutrophils) all of which play central roles in the pathogenesis of RA [102]. In macrophages, GM-CSF drives macrophage polarization toward a pro-inflammatory M1-like phenotype, marked by inducible nitric oxide synthase (iNOS) upregulation and augmented NO production, contributing to microbial killing and oxidative tissue stress [103]. Simultaneously, GM-CSF primes monocytes and macrophages to promote costimulatory molecules (CD40 and CD86) and chemokine receptors (CCR2 and CX3CR1), enhancing chemotactic responsiveness and sustaining immune cell retention within the synovium [104]. Moreover, GM-CSF augments ROS production, thus intensifying the inflammatory microenvironment and exacerbating oxidative injury to adjacent tissues [105].
GM-CSF-differentiated macrophages (GM-DMs) in the RA synovium secrete several chemokines (e.g., CCL22), which attract CD4+ T cells into the inflamed tissue (Figure 2). Upon recruitment, these T cells differentiate into Th1 and Th17 subsets, driven by the cytokine milieu enriched in IL-1β, IL-6, and IL-23 [20,106]. The interaction between GM-DMs and T cells creates a positive feedback loop that amplifies synovial inflammation and joint destruction [107]. Notably, GM-CSF upregulates CCL22 expression in macrophages through activation of the transcription factor IRF4, underscoring a pivotal axis in the pathogenesis of RA [20].
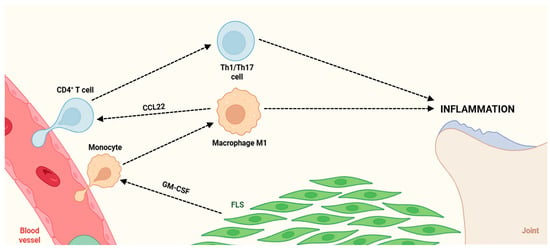
Figure 2.
Pathogenic crosstalk between immune cells and FLS drives chronic joint inflammation. Circulating monocytes extravasate from the blood vessel into the synovial tissue, where GM-CSF produced by FLS promotes their differentiation into pro-inflammatory M1 macrophages. Activated M1 macrophages secrete the chemokine CCL22, which recruits CD4+ T cells and promotes their differentiation into Th1/Th17 effector subsets, establishing a self-amplifying inflammatory loop which sustains chronic joint inflammation. Abbreviations: CD4 (cluster of differentiation 4), Th1 (T helper 1 cell), Th17 (T helper 17 cell), CCL22 (chemokine (C-C motif) ligand 22), FLS (fibroblast-like synoviocytes), and GM-CSF (granulocyte-macrophage colony-stimulating factor).
In addition to these functions, GM-CSF stimulates the secretion of several matrix metalloproteinases (MMP-1, MMP-9, and MMP-13), proteolytic enzymes that degrade critical components of the extracellular matrix, such as collagen and proteoglycans, thus driving cartilage erosion and joint destruction in pathological contexts [108]. At the transcriptional level, GM-CSF potentiates this phenotype through activation of IRF5 and NF-κB signaling pathways, which direct the expression of some pro-inflammatory cytokines, chemokines, and effector molecules, establishing a self-propagating inflammatory state within the synovial microenvironment [109,110].
In dendritic cells, GM-CSF enhances the antigen-presenting function by promoting upregulation of major histocompatibility complex (MHC) class II molecules as well as co-stimulatory receptors including CD80 and CD86, thereby increasing the capacity of dendritic cells to activate naïve and autoreactive T cells [111,112]. GM-CSF-stimulated dendritic cells produce elevated levels of pro-inflammatory cytokines including IL-1β, IL-6, and IL-23, which are critical drivers of Th17 cell differentiation, expansion, and functional polarization [113]. Simultaneously, these dendritic cells increase IL-12 production, supporting Th1 lineage commitment and reinforcing a multi-faceted Th cell-mediated inflammatory response [114]. This finding establishes a self-amplifying loop in which GM-CSF-producing Th17 cells further stimulate myeloid cells, thus exacerbating synovial inflammation and maintaining tissue damage [115]. The reciprocal activation between myeloid cells and autoreactive T cells functionally couples innate and adaptive immune responses, orchestrating the persistence of chronic inflammatory processes.
Neutrophils exposed to GM-CSF show delayed apoptosis, prolonging their lifespan within inflammatory sites, while concurrently exhibiting enhanced degranulation, releasing proteases and antimicrobial peptides that contribute to tissue damage [116]. GM-CSF also promotes formation of neutrophil extracellular traps (NETs), a process dependent in part on PI3K/Akt-mediated stabilization of the anti-apoptotic protein MCL1, as well as activation of ERK1/2 and p38 MAPK signaling cascades [117,118]. These NETs often contain citrullinated proteins, which function as potent autoantigens capable of breaking immune tolerance and driving the autoimmune response, a key pathophysiological process of RA [119].
Finally, GM-CSF signaling converges with various metabolic reprogramming pathways in immune cells, notably macrophages and dendritic cells. Engagement of the GM-CSFR triggers downstream activation of the JAK2/STAT5 and PI3K/Akt/mTOR signaling cascades, resulting in the transcriptional upregulation of critical metabolic enzymes [120]. This signaling promotes enhanced glycolysis through upregulation of hexokinase 2 (HK2) and phosphofructokinase-1 (PFK1), as well as increased glutaminolysis through induction of glutaminase (GLS), thus providing biosynthetic precursors and energy to support sustained pro-inflammatory cytokine production, such as TNF-α, IL-6, and IL-1β [121,122].
4. Therapeutic Targeting of GM-CSF
4.1. Overview of Monoclonal Antibody Strategies: GM-CSF Neutralization vs. Receptor Blockade
The GM-CSF signaling axis has emerged as a central mediator in the pathophysiology of RA, integrating both innate and adaptive immune mechanisms to drive synovial inflammation, tissue destruction, and pain sensitization. Its pleiotropic activity on myeloid lineage cells, particularly monocytes, macrophages, and neutrophils, positions GM-CSF as a key amplifier of pro-inflammatory cytokine networks, such as TNF-α, IL-1β, and IL-6, while simultaneously enhancing antigen presentation and osteoclast differentiation [102]. Consequently, therapeutic strategies that interfere with GM-CSF signaling have become a major focus in the development of novel biologic disease-modifying antirheumatic drugs (bDMARDs) [123].
Two primary monoclonal antibody (mAb) strategies have been developed to target the GM-CSF pathway in RA: ligand neutralization and receptor blockade [124]. Ligand neutralization employs antibodies directed against the soluble GM-CSF molecule itself, effectively sequestering this cytokine and preventing it from binding to its receptor on several target cells [125]. This approach is exemplified by agents like otilimab and namilumab, which demonstrate high-affinity binding to GM-CSF and inhibit its interaction with GM-CSFRα. Ligand neutralization might also modulate systemic GM-CSF-dependent immune functions, including hematopoiesis and myeloid cell survival [126,127].
Receptor blockade, in contrast, targets the GM-CSF receptor itself, usually via mAbs directed against GM-CSFRα [128]. Mavrilimumab represents a prototypical agent of this approach, targeting GM-CSFRα on immune innate cells (monocytes, macrophages, and neutrophils) to block GM-CSF interaction and abrogate ensuing receptor-dependent signaling cascades. Receptor blockade offers theoretical advantages in mechanistic specificity by selectively acting on GM-CSFRα-expressing cells, thereby mitigating off-target systemic effects and enabling more localized modulation of myeloid activation within inflamed tissues [129]. Moreover, this strategy may ensure prolonged suppression of GM-CSF signaling, as it prevents both locally synthesized and circulating GM-CSF from triggering downstream receptor-dependent pathways [129].
Both strategies share the common objective of interrupting the pathological GM-CSF axis to reduce synovial inflammation and tissue damage, but their mechanistic distinctions have important implications for pharmacokinetics, pharmacodynamics, and clinical application. Ligand-neutralizing antibodies necessitate plasma concentrations to bind circulating GM-CSF and achieve ligand sequestration, whereas receptor-blocking antibodies depend on receptor occupancy and target-cell distribution, factors that might influence dosing strategies and the onset of clinical efficacy [130]. Numerous preclinical models suggest that receptor blockade may provide a faster and more potent inhibition of local inflammatory responses, while ligand neutralization may offer broader systemic modulation of GM-CSF-dependent pathways.
The clinical development of GM-CSF-targeted therapies indicates that their efficacy and safety might vary according to patient-specific factors such as disease duration, serological status, baseline inflammatory activity, and pulmonary comorbidities. Elucidating these mechanistic differences is essential to refine patient selection and reduce side effects. Overall, the distinction between ligand neutralization and receptor blockade represents a therapeutic paradigm shift in RA, providing therapeutic options beyond TNF-α, IL-6, and B cell-directed biologics for patients unresponsive to current treatments.
4.2. Neutralization of GM-CSF Pathway in RA: Preclinical Efficacy of Monoclonal Antibodies
Preclinical studies investigating blockade of GM-CSF signaling in RA models remain limited but provide reproducible evidence of therapeutic potential (Table 1). The mAb CAM-3003, directed against GM-CSFR, demonstrated robust anti-inflammatory activity in the mouse collagen-induced arthritis (CIA) model, where treatment significantly reduced disease severity and progression, blocked synovial inflammation, and protected against cartilage and bone damage, concomitant with decreased TNF-α and IL-1β expression in joint tissue [131]. Other studies confirmed that GM-CSF pathway inhibition attenuated CIA severity, reduced circulating Ly-6Chigh inflammatory monocytes, and reduced synovial immune cell infiltration [132], with a dose-dependent reduction in arthritis severity associated with decreased F4/80+ synovial macrophages and diminished joint pathology [133]. Preclinical evidence in non-rodent species is limited; however, a protein-engineered anti-GM-CSFRα antibody (574D04) in cynomolgus monkeys demonstrated in vivo pharmacologic activity, as pretreatment with 574D04 dose-dependently suppressed GM-CSF-induced leukocyte margination and leukocytosis at doses of 1–10 mg/kg [134], supporting blockade of GM-CSF signaling. These findings highlight GM-CSF/GM-CSFR inhibition as a promising immunomodulatory strategy in RA, although preclinical data, particularly in non-human primates, remain limited.

Table 1.
Preclinical evaluation of several mAbs targeting GM-CSFR in preclinical arthritis models. Abbreviations: GM-CSFR (granulocyte-macrophage colony-stimulating factor receptor), mAb (monoclonal antibody), CIA (collagen-induced arthritis), GM-CSF (granulocyte-macrophage colony-stimulating factor), TNF-α (tumor necrosis factor-alpha), IL-1β (interleukin-1 beta), and Ly-6Chigh (lymphocyte antigen 6C high-expressing monocytes).
4.3. Monoclonal Antibodies Against GM-CSF/GM-CSFR in RA: Clinical Trial Insights
GM-CSF and GM-CSFR have emerged as key therapeutic targets in RA, given their central role in synovial inflammation and joint damage. Numerous mAbs targeting this pathway have progressed through early- to late-phase clinical trials, demonstrating varying degrees of efficacy and safety.
Otilimab showed mixed results across trials. In the phase III ContRAst 3 study of 549 RA patients, 90 mg and 150 mg doses did not achieve significant ACR20 responses at week 12 compared with placebo, whereas sarilumab 200 mg demonstrated superior efficacy [28] (NCT04134728). In the contRAst 1 (1537 patients) and contRAst 2 (1625 patients) clinical trials, otilimab met primary endpoints with ACR20 responses, although secondary outcomes were lower than tofacitinib [135] (NCT03980483,NCT03970837). Long-term extension (contRAst X, ~3000 patients) showed a well-tolerated safety profile, with mostly moderate side events and no new safety signals [126] (NCT04333147). Phase IIa studies with weekly subcutaneous 180 mg demonstrated trends toward decreased synovitis and osteitis, though differences were not statistically significant [136] (NCT02799472).
Namilumab demonstrated more consistent efficacy. Phase II trials showed significant reductions in DAS28-CRP and improvements in ACR20/50/70 responses, with early effects visible by week 2 and MRI-confirmed reductions in synovitis, bone erosion, and bone marrow edema [137] (NCT02379091,NCT02393378). Gimsilumab and MOR103 were generally well tolerated in Phase I studies, with supportive safety for further development [138,139] (NCT01357759,NCT01023256). Plonmarlimab has finished a Phase I study, with results yet to be reported [NCT03794180], while early-phase data for TJ003234 suggest acceptable safety and GM-CSF inhibition [NCT04457856].
Mavrilimumab consistently improved DAS28-CRP and ACR responses in numerous Phase II trials. The EARTH Phase IIa study (233 patients) showed dose-dependent efficacy and early onset of improvement [140] (NCT01050998). In the EARTH EXPLORER 1 Phase IIb study, biweekly administration of 150 mg resulted in ACR20/50/70 responses, along with marked reductions in DAS28-CRP [141,142] (NCT01706926). Long-term open-label extension confirmed durable efficacy and a manageable safety profile, with no pulmonary toxicity [143,144] (NCT01712399). Pharmacokinetic analyses show dose-proportional kinetics and a half-life of approximately 13 days [143].
Finally, Table 2 provides a comparative overview of all anti-GM-CSF mAbs currently evaluated for the treatment of RA, summarizing their clinical efficacy, safety profiles, and key trial outcomes. This comparison allows for a direct assessment of therapeutic potential, dosing strategies, and long-term tolerability across the different agents.

Table 2.
Therapeutic targeting of GM-CSF and GM-CSFR in RA: clinical efficacy, safety, and mechanistic insights from phase I–III trials. Abbreviations: GM-CSF (granulocyte-macrophage colony-stimulating factor), RA (rheumatoid arthritis), bDMARDs (biologic disease-modifying antirheumatic drugs), CDAI (clinical disease activity index), HAQ-DI (health assessment questionnaire disability index), ACR20 (American College of Rheumatology 20% improvement criteria), RAMRIS (rheumatoid arthritis magnetic resonance imaging score), RAMRIQ (rheumatoid arthritis magnetic resonance imaging quantitative score), OMERACT (outcome measures in rheumatology), DAS28-CRP (disease activity score in 28 joints with C-reactive protein), ACR50 (American College of Rheumatology 50% improvement criteria), ACR70 (American College of Rheumatology 70% improvement criteria), MRI (magnetic resonance imaging), DAS28 (disease activity score in 28 joints), GM-CSFR (granulocyte-macrophage colony-stimulating factor receptor), DMARD (disease-modifying antirheumatic drug), and ACR/EULAR (American College of Rheumatology/European League Against Rheumatism).
5. Next-Generation Strategies for GM-CSF-Targeted Therapies in RA
5.1. Long-Term Safety and Immunovigilance
Although current clinical evidence supports the safety of GM-CSF mAbs in the medium term, long-term immunological consequences remain insufficiently characterized. GM-CSF is crucial for the maturation and functional competence of alveolar macrophages and plays an essential role in innate immune defense mechanisms, particularly in modulating several intracellular pathogens and fungal infections [146,147]. Prolonged pharmacovigilance through open-label extension studies and post-marketing registries will be required to detect delayed side events, including pulmonary alveolar proteinosis (PAP), uncommon opportunistic infections, and immunohematologic perturbations.
Evidence from cohorts of RA, systemic sclerosis, and COVID-19 patients treated with anti-GM-CSF agents indicates a non-trivial incidence of lower respiratory tract infections, including bacterial pneumonia [148] and invasive pulmonary aspergillosis [149]. This risk appears to be particularly elevated in individuals with pre-existing structural lung disease or concomitant corticosteroid therapy [148,149]. Importantly, subclinical surfactant accumulation and early radiographic markers of alveolar macrophage dysfunction have been documented, suggesting that pulmonary toxicity might develop insidiously before overt clinical manifestations [150,151].
The potential development of secondary PAP is of particular relevance, as GM-CSF is indispensable for surfactant catabolism and alveolar homeostasis [152]. While no definitive PAP cases have been confirmed in long-term trials to date, isolated reports of PAP-like radiological patterns underscore the need for proactive pulmonary monitoring. Moreover, hematological alterations such as monocytopenia, impaired dendritic cell differentiation, and reduced circulating myeloid precursors have been documented in pharmacodynamic studies, raising the possibility of broader myelopoietic suppression with chronic dosing [153,154].
Another important avenue of investigation involves elucidating the mechanisms underlying vaccine-induced immune responses under conditions of GM-CSF blockade. GM-CSF is a critical immunoregulatory cytokine that coordinates the interplay between innate and adaptive immune pathways by driving the differentiation, maturation, and activation of antigen-presenting cells, enhancing antigen processing and presentation, and facilitating T-cell priming and clonal expansion [155]. Consequently, pharmacologic blockade of GM-CSF signaling could theoretically attenuate vaccine responsiveness by impairing antigen presentation dynamics or downstream T-cell-mediated effector functions.
This potential immunomodulatory effect warrants comprehensive evaluation, particularly with respect to vaccines that rely on robust antigen presentation and T-cell activation, such as mRNA-based and polysaccharide formulations [156,157]. Future research should incorporate standardized and quantitative immunogenicity assays (such as measurement of neutralizing antibody titers, assessment of memory B-cell responses, and functional T-cell assays) to accurately determine the degree of post-vaccination seroprotection in patients undergoing prolonged GM-CSF inhibition. Longitudinal studies will be essential to delineate the temporal dynamics of immune recovery following treatment cessation and to establish evidence-based vaccination guidelines for this patient population [158].
5.2. Precision Medicine and Predictive Biomarkers
The pathogenesis of RA suggests that therapeutic response to GM-CSF inhibition will vary across patient subgroups. The development of predictive biomarkers to guide patient selection represents a critical objective. Emerging studies indicate that patients exhibiting a myeloid-dominant synovial phenotype (with enrichment of GM-CSF-responsive macrophages and monocytes) may derive greater clinical benefit [159,160]. Likewise, elevated serum concentrations of CCL17, a downstream effector chemokine triggered by GM-CSF signaling, along with increased expression of CSF2RA and CSF2RB, may serve as mechanistic biomarkers for predicting therapeutic response [161]. In parallel, accumulating preclinical data and early-phase clinical observations provide a mechanistic rationale for the co-administration of GM-CSF inhibitors with other immunomodulatory agents, such as JAK inhibitors (tofacitinib) [NCT03970837], with the aim of enhancing the depth and durability of clinical response. These combinatorial strategies are under investigation as a means to overcome cytokine network redundancy and compensatory signaling pathways that may attenuate the efficacy of GM-CSF blockade when used as monotherapy.
Comprehensive multi-omics profiling of synovial tissue, in conjunction with machine-learning-guided clustering, may allow the classification of patients into distinct GM-CSF-high and GM-CSF-low inflammatory endotypes. Prospective clinical trials incorporating adaptive enrichment designs will be necessary to confirm the validity of this biomarker-guided strategy and to optimize the risk-benefit profile at the individual level. Numerous advances in precision medicine (e.g., spatial transcriptomics, single-cell profiling, and circulating immune signatures) are expected to further refine stratification frameworks and support the rational pairing of GM-CSF inhibitors with targeted co-therapies tailored to mechanistic disease endotypes [162,163]. Well-controlled trials will also be essential to determine the optimal sequencing, dosing, and safety profile of these combination regimens.
5.3. Combination and Sequencing Strategies
Single-cytokine inhibition has limitations in some RA patients due to cytokine redundancy and compensatory inflammatory pathways [164]. The combination of GM-CSF inhibitors with other targeted agents (e.g., IL-6 receptor antagonists, TNF-α inhibitors, and JAK inhibitors) might provide synergistic efficacy, especially in patients with treatment-refractory disease. Combination therapy may also allow for dose reduction in individual agents, thereby potentially mitigating long-term toxicity [165].
Rational sequencing strategies must also be evaluated [166]. For instance, numerous patients with inadequate response to TNF-α inhibitors but evidence of persistent myeloid activation might transition effectively to GM-CSF blockade rather than to another TNF-α inhibitor. Clinical trials that integrate immunophenotyping and pharmacodynamic endpoints will be vital to resolving optimal treatment algorithms for real-world practice.
5.4. Extra-Articular Implications and Comorbidity Management
RA is a multisystem disease, and targeting GM-CSF may yield benefits beyond joint-specific inflammation. GM-CSF is implicated in the pathogenesis of RA-associated interstitial lung disease (RA-ILD) [146], one of the most severe and fatal manifestations of RA. Given that GM-CSF regulates lung-resident macrophage homeostasis and fibroblast activation, inhibition of this pathway may have therapeutic potential in patients with RA-ILD [167].
Future trials should include dedicated pulmonary endpoints, such as forced vital capacity trajectory, diffusion capacity metrics, and high-resolution computed tomography scoring. Moreover, GM-CSF blockade might alleviate systemic manifestations of RA such as fatigue, anemia of chronic disease, and symptoms driven by several myeloid cytokine networks. Incorporating validated quality-of-life measures could clarify broader patient-reported benefits.
5.5. Biomarker-Driven Patient Selection
The incorporation of biomarker-guided patient selection into GM-CSF-targeted therapeutic strategies holds the potential to transform RA management from empirically derived, population-based interventions toward a precision medicine paradigm [10]. Putative biomarkers (such as circulating GM-CSF concentrations, synovial expression of GM-CSF and its receptor, transcriptional signatures indicative of myeloid cell activation, specific monocyte/macrophage phenotypes, and cytokine networks) might help identify patients whose disease pathogenesis is predominantly driven by GM-CSF-mediated pathways, thus predicting therapeutic responsiveness [11]. Other approaches, such as imaging biomarkers (e.g., PET tracers indicative of macrophage activation) and pharmacogenomic variants affecting drug metabolism or immunogenicity, serve as complementary tools for patient stratification [168,169,170].
The integration of these biomarkers as enrichment or stratification variables in early-phase and adaptive clinical trials might improve signal detection, reduce unnecessary exposure in non-responders, and support the development of many companion diagnostics [171]. However, rigorous prospective validation across diverse RA populations, together with the standardization of analytical methodologies, is essential to ensure reproducibility, clinical utility, and equitable implementation in routine clinical practice [172].
5.6. Pediatric and Global Health Applications
The role of GM-CSF in juvenile idiopathic arthritis (JIA), especially systemic JIA and polyarticular JIA subsets, remains understood. Considering that myeloid activation is a key feature of systemic juvenile idiopathic arthritis (sJIA) and macrophage activation syndrome [173,174], GM-CSF inhibition merits investigation in pediatric populations exhibiting severe disease phenotypes.
6. Conclusions
Targeting GM-CSF represents a promising therapeutic strategy in the management of RA, reflecting the growing emphasis on cytokine-directed immunotherapy. Accumulating preclinical and early-phase clinical evidence supports the pathogenic role of GM-CSF in promoting synovial inflammation, joint destruction, and systemic immune dysregulation in RA. Although mAbs targeting GM-CSF or its receptor have demonstrated encouraging efficacy and acceptable safety profiles in phase I-II trials, phase III studies have so far yielded inconclusive results, preventing definitive conclusions regarding their long-term clinical utility. Accordingly, further research is needed to improve the evidence base, refine patient stratification, clarify long-term safety, and assess the potential of combination strategies to maximize therapeutic benefit.
This review advances the existing literature by providing an analysis that connects GM-CSF biology with emerging biomarker-driven and precision-medicine frameworks. Specifically, this review highlights how molecular, cellular, and clinical biomarkers may help identify patient subgroups most likely to benefit from GM-CSF inhibition and discusses the potential incorporation of GM-CSF-targeted agents into treatment algorithms. By linking therapeutic mechanisms with individualized disease profiling, this review not only summarizes current evidence but also outlines a forward-looking roadmap for the clinical translation of GM-CSF blockade in RA.
Importantly, several key questions remain unresolved and require systematic investigation. First, although short- to mid-term safety data indicate an adequate safety profile, long-term pharmacovigilance is essential to fully assess risks related to sustained GM-CSF suppression, including infection, malignancy, and alterations in tissue homeostasis. Second, reproducible criteria for patient stratification (mainly based on biomarker signatures, disease stage, or comorbid immune pathways) are still lacking and will be critical to prevent overtreatment and ensure optimal therapeutic allocation. Third, the role of GM-CSF inhibitors within combination regimens, either alongside conventional DMARDs or other targeted biologics, remains unexplored and might uncover synergistic or complementary effects.
Overall, therapies targeting GM-CSF represent a significant step forward in the development of personalized treatment strategies for RA, yet their successful translation into routine clinical practice will require the systematic resolution of these outstanding issues through rigorously designed long-term studies incorporating biomarker-based stratification and combination therapy frameworks.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACPA | Anti-citrullinated protein antibody |
| ACR | American College of Rheumatology |
| ACR20 | American College of Rheumatology 20% improvement criteria |
| ACR50 | American College of Rheumatology 50% improvement criteria |
| ACR70 | American College of Rheumatology 70% improvement criteria |
| Akt | Protein kinase B |
| anti-CCP | Anti-cyclic citrullinated peptide antibody |
| AP-1 | Activator protein 1 |
| APC | Antigen-presenting cell |
| bDMARD | Biologic disease-modifying antirheumatic drug |
| BCL2 | B-cell lymphoma 2 |
| BCL-XL | B-cell lymphoma-extra large |
| C3a | Complement component 3a |
| C5a | Complement component 5a |
| CCL17 | Chemokine (C-C motif) ligand 17 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CCL22 | Chemokine (C-C motif) ligand 22 |
| CCL3 | Chemokine (C-C motif) ligand 3 |
| CCL5 | C-C motif chemokine ligand 5 |
| CCR2 | C-C chemokine receptor type 2 |
| CD4 | Cluster of differentiation 4 |
| CD40 | Cluster of differentiation 40 |
| CD80 | Cluster of differentiation 80 |
| CD86 | Cluster of differentiation 86 |
| CDAI | Clinical disease activity index |
| c-Fos | Cellular FOS proto-oncogene |
| CHD | Cytokine receptor homology domain |
| CIA | Collagen-induced arthritis |
| CRP | C-reactive protein |
| CTLA4 | Cytotoxic T-lymphocyte antigen 4 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| CXCL8 | Chemokine (C-X-C motif) ligand 8 |
| CX3CR1 | CX3C chemokine receptor 1 |
| DAS28 | Disease activity score in 28 joints |
| DAS28-CRP | Disease activity score in 28 joints with C-reactive protein |
| DCE-MRI | Dynamic contrast-enhanced MRI |
| DMARD | Disease-modifying antirheumatic drug |
| Elk-1 | ETS-like protein 1 |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ESR | Erythrocyte sedimentation rate |
| EULAR | European League Against Rheumatism |
| FLS | Fibroblast-like synoviocyte |
| GLS | Glutaminase |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GM-CSFR | Granulocyte-macrophage colony-stimulating factor receptor |
| GM-CSFRα | Granulocyte-macrophage colony-stimulating factor receptor alpha subunit |
| GM-CSFRβc | Granulocyte-macrophage colony-stimulating factor receptor β common subunit |
| GM-DM | GM-CSF-differentiated macrophage |
| HAQ-DI | Health assessment questionnaire disability index |
| HK | Hexokinase 2 |
| HLA-DRB1 | Human leukocyte antigen-DR beta 1 |
| IFN-γ | Interferon gamma |
| IgG1 | Immunoglobulin G1 |
| IKK | IκB Kinase |
| IL-12 | Interleukin 12 |
| IL-17 | Interleukin 17 |
| IL-17A | Interleukin 17A |
| IL-17F | Interleukin 17F |
| IL-1β | Interleukin 1 beta |
| IL-22 | Interleukin 22 |
| IL-23 | Interleukin 23 |
| IL-3 | Interleukin 3 |
| IL-5 | Interleukin 5 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| iNOS | Inducible nitric oxide synthase |
| IRF4 | Interferon regulatory factor 4 |
| IRF5 | Interferon regulatory factor 5 |
| IκBα | Inhibitor of kappa B alpha |
| i.v. | Intravenous injection |
| JAK2 | Janus kinase |
| JAK2 | Janus kinase 2 |
| JIA | Juvenile idiopathic arthritis |
| Ly-6Chigh | Lymphocyte antigen 6C high-expressing monocytes |
| M1 | Classically activated macrophage phenotype |
| mAb | Monoclonal antibody |
| MAPK | Mitogen-activated protein kinase |
| MAPK/ERK | Mitogen-activated protein kinase/extracellular signal-regulated kinase |
| MCL1 | Myeloid cell leukemia 1 |
| M-CSF | Macrophage colony-stimulating factor |
| MEK1/2 | Mitogen-activated protein kinase kinase 1/2 |
| MHC | Major histocompatibility complex |
| MHC-II | Major histocompatibility complex type II |
| MMP | Matrix metalloproteinase |
| MMP-1 | Matrix metalloproteinase 1 |
| MMP-13 | Matrix metalloproteinase 13 |
| MMP-3 | Matrix metalloproteinase 3 |
| MMP-9 | Matrix metalloproteinase 9 |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger ribonucleic acid |
| mTOR | Mechanistic target of rapamycin |
| mTORC2 | Mechanistic target of rapamycin complex 2 |
| NET | Neutrophil extracellular trap |
| NFATc1 | Nuclear factor of activated T-cells, cytoplasmic 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| OMERACT | Outcome measures in rheumatology clinical trials |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PET | Positron emission tomography |
| PFK1 | Phosphofructokinase 1 |
| PI3K | Phosphoinositide 3-kinase |
| PI3K/Akt | Phosphoinositide 3-kinase/protein kinase B |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PK | Pharmacokinetics |
| PTPN22 | Protein tyrosine phosphatase non-receptor type 22 |
| RA | Rheumatoid arthritis |
| RA-ILD | Rheumatoid arthritis-associated interstitial lung disease |
| Raf-1 | Rapidly accelerated fibrosarcoma 1 |
| RAMRIS | Rheumatoid arthritis magnetic resonance imaging score |
| RAMRIQ | Rheumatoid arthritis magnetic resonance imaging quantitative |
| RANK | Receptor activator of nuclear factor κB |
| RANKL | Receptor activator of nuclear factor κB ligand |
| Ras | Rat sarcoma |
| RF | Rheumatoid factor |
| ROS | Reactive oxygen species |
| s.c. | Subcutaneous injection |
| SH2 | Src homology 2 |
| sJIA | Systemic juvenile idiopathic arthritis |
| SOCS3 | Suppressor of cytokine signaling 3 |
| STAT | Signal transducer and activator of transcription |
| STAT4 | Signal transducer and activator of transcription 4 |
| STAT5 | Signal transducer and activator of transcription 5 |
| Th | T helper lymphocyte |
| Th1 | T helper 1 cell |
| Th17 | T helper 17 cell |
| TNF-α | Tumor necrosis factor alpha |
| Treg | Regulatory T cell |
References
- Gao, Y.; Zhang, Y.; Liu, X. Rheumatoid arthritis: Pathogenesis and therapeutic advances. MedComm 2024, 5, e509. [Google Scholar] [CrossRef]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar] [CrossRef]
- Edilova, M.I.; Akram, A.; Abdul-Sater, A.A. Innate immunity drives pathogenesis of rheumatoid arthritis. Biomed. J. 2021, 44, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H. Adaptive immunity in the joint of rheumatoid arthritis. Immunol. Med. 2022, 45, 1–11. [Google Scholar] [CrossRef]
- Brzustewicz, E.; Bryl, E. The role of cytokines in the pathogenesis of rheumatoid arthritis—Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 2015, 76, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef]
- Fujimoto, S.; Niiro, H. Pathogenic Role of Cytokines in Rheumatoid Arthritis. J. Clin. Med. 2025, 14, 6409. [Google Scholar] [CrossRef]
- Fuentelsaz-Romero, S.; Cuervo, A.; Estrada-Capetillo, L.; Celis, R.; García-Campos, R.; Ramírez, J.; Sastre, S.; Samaniego, R.; Puig-Kröger, A.; Cañete, J.D. GM-CSF Expression and Macrophage Polarization in Joints of Undifferentiated Arthritis Patients Evolving to Rheumatoid Arthritis or Psoriatic Arthritis. Front. Immunol. 2021, 11, 613975. [Google Scholar] [CrossRef]
- Su, J.; Hu, W.; Ding, Y.; Zhang, P.; Li, T.; Liu, S.; Xing, L. Serum GM-CSF level is a predictor of treatment response to tocilizumab in rheumatoid arthritis patients: A prospective observational cohort study. Arthritis Res. Ther. 2024, 26, 130. [Google Scholar] [CrossRef]
- Shiomi, A.; Usui, T.; Mimori, T. GM-CSF as a therapeutic target in autoimmune diseases. Inflamm. Regen. 2016, 36, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.C.; Achuthan, A.A.; Hamilton, J.A. GM-CSF: A Promising Target in Inflammation and Autoimmunity. Immunotargets Ther. 2020, 9, 225–240. [Google Scholar] [CrossRef]
- Woodcock, J.M.; McClure, B.J.; Stomski, F.C.; Elliott, M.J.; Bagley, C.J.; Lopez, A.F. The human granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor exists as a preformed receptor complex that can be activated by GM-CSF, interleukin-3, or interleukin-5. Blood 1997, 90, 3005–3017. [Google Scholar] [CrossRef]
- Hansen, G.; Hercus, T.R.; McClure, B.J.; Stomski, F.C.; Dottore, M.; Powell, J.; Ramshaw, H.; Woodcock, J.M.; Xu, Y.; Guthridge, M.; et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 2008, 134, 496–507. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Nagenborg, J.; Jin, H.; Ruder, A.V.; Temmerman, L.; Mees, B.; Schalkwijk, C.; Müller-Klieser, D.; Berg, T.; Goossens, P.; Donners, M.M.P.C.; et al. GM-CSF-activated STAT5A regulates macrophage functions and inflammation in atherosclerosis. Front. Immunol. 2023, 14, 1165306. [Google Scholar] [CrossRef]
- Larbi, A.; Douziech, N.; Fortin, C.; Linteau, A.; Dupuis, G.; Fulop, T., Jr. The role of the MAPK pathway alterations in GM-CSF modulated human neutrophil apoptosis with aging. Immun. Ageing 2005, 2, 6. [Google Scholar] [CrossRef]
- Han, N.-R.; Park, H.-J.; Moon, P.-D. Resveratrol Downregulates Granulocyte-Macrophage Colony-Stimulating Factor-Induced Oncostatin M Production through Blocking of PI3K/Akt/NF-κB Signal Cascade in Neutrophil-like Differentiated HL-60 Cells. Curr. Issues Mol. Biol. 2022, 44, 541–549. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, M.; Zhao, Y.; Xu, H.; Feng, F.; Bai, Z.; Zhang, K.; Fu, L.; Wang, F.; Cheng, Y.; et al. GM-CSF potentiates macrophages to retain an inflammatory feature from their circulating monocyte precursors in rheumatoid arthritis. J. Transl. Med. 2025, 23, 883. [Google Scholar] [CrossRef]
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.X.; Rezaei, A.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front. Immunol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Hamilton, J.A. GM-CSF in inflammation. J. Exp. Med. 2020, 217, e20190945. [Google Scholar] [CrossRef]
- Domańska-Poboża, J.; Wisłowska, M. Evolving strategies in the treatment of rheumatoid arthritis: A historical perspective. Reumatologia 2025, 63, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; McDermott, G.C.; Juge, P.A.; Chang, S.H.; Vanni, K.M.; Qian, G.; Bade, K.J.; Mueller, K.T.; Kowalski, E.N.; Saavedra, A.A.; et al. Disease-modifying antirheumatic drugs and risk of incident interstitial lung disease among patients with rheumatoid arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2024, 69, 152561. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Crotti, C.; Agape, E.; Becciolini, A.; Biggioggero, M.; Favalli, E.G. Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects. Drugs 2019, 79, 1741–1755. [Google Scholar] [CrossRef]
- Bykerk, V.P. The efficacy and safety of targeting GM-CSF in arthritis. Lancet Rheumatol. 2020, 2, e648–e650. [Google Scholar] [CrossRef]
- Taylor, P.C.; Weinblatt, M.E.; McInnes, I.B.; Atsumi, T.; Strand, V.; Takeuchi, T.; Bracher, M.; Brooks, D.; Davies, J.; Goode, C.; et al. Anti-GM-CSF otilimab versus sarilumab or placebo in patients with rheumatoid arthritis and inadequate response to targeted therapies: A phase III randomised trial (contRAst 3). Ann. Rheum. Dis. 2023, 82, 1527–1537. [Google Scholar] [CrossRef]
- Weinblatt, M.E.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Vencovsky, J.; Guo, X.; White, W.I.; Ryan, P.C.; Godwood, A.; Albulescu, M.; et al. A Randomized Phase IIb Study of Mavrilimumab and Golimumab in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 49–59. [Google Scholar] [CrossRef]
- Buckley, C.D.; Simón-Campos, J.A.; Zhdan, V.; Becker, B.; Davy, K.; Fisheleva, E.; Gupta, A.; Hawkes, C.; Inman, D.; Layton, M.; et al. Efficacy, patient-reported outcomes, and safety of the anti-granulocyte macrophage colony-stimulating factor antibody otilimab (GSK3196165) in patients with rheumatoid arthritis: A randomised, phase 2b, dose-ranging study. Lancet Rheumatol. 2020, 2, e677–e688. [Google Scholar] [CrossRef]
- Genovese, M.C.; Buckley, C.D.; Saurigny, D.; Schett, G.; Davy, K.; Gupta, A.; Smith, J.E.; Patel, J.; Tak, P.P. Targeting GM-CSF in rheumatological conditions: Risk of PAP—Authors’ reply. Lancet Rheumatol. 2021, 3, e473–e474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, X.; Liu, S.; Wang, Q.; Wang, Y.; Hou, S.; Wang, J.; Zhang, Y. Global, regional, and national epidemiology of rheumatoid arthritis among people aged 20–54 years from 1990 to 2021. Sci. Rep. 2025, 15, 10736. [Google Scholar] [CrossRef] [PubMed]
- Maranini, B.; Bortoluzzi, A.; Silvagni, E.; Govoni, M. Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 499. [Google Scholar] [CrossRef]
- Scott, D.L.; Steer, S. The course of established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2007, 21, 943–967. [Google Scholar] [CrossRef]
- Rawla, P. Cardiac and vascular complications in rheumatoid arthritis. Reumatologia 2019, 57, 27–36. [Google Scholar] [CrossRef]
- Wu, D.; Luo, Y.; Li, T.; Zhao, X.; Lv, T.; Fang, G.; Ou, P.; Li, H.; Luo, X.; Huang, A.; et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front. Immunol. 2022, 13, 1051082. [Google Scholar] [CrossRef]
- Dedmon, L.E. The genetics of rheumatoid arthritis. Rheumatology 2020, 59, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, T.; Olesińska, M.; Paradowska-Gorycka, A. Current Understanding of an Emerging Role of HLA-DRB1 Gene in Rheumatoid Arthritis–From Research to Clinical Practice. Cells 2020, 9, 1127. [Google Scholar] [CrossRef]
- Mustelin, T.; Bottini, N.; Stanford, S.M. The Contribution of PTPN22 to Rheumatic Disease. Arthritis Rheumatol. 2019, 71, 486–495. [Google Scholar] [CrossRef]
- Bravo-Villagra, K.M.; Muñoz-Valle, J.F.; Baños-Hernández, C.J.; Cerpa-Cruz, S.; Navarro-Zarza, J.E.; Parra-Rojas, I.; Aguilar-Velázquez, J.A.; García-Arellano, S.; López-Quintero, A. STAT4 Gene Variant rs7574865 Is Associated with Rheumatoid Arthritis Activity and Anti-CCP Levels in the Western but Not in the Southern Population of Mexico. Genes 2024, 15, 241. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, S.; Yuan, X.; Shu, Z.; Li, S.; Sun, X.; Xiao, J.; Liu, H. Association between CTLA-4 gene polymorphism and risk of rheumatoid arthritis: A meta-analysis. Aging 2021, 13, 19397–19414. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, L.; Olsson, T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a028944. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Oduro, P.K.; Sun, T.; Chen, H.; Yi, Y.; Zeng, W.; Wang, Q.; Leng, L.; Yang, L.; et al. The Relationship Between Porphyromonas Gingivalis and Rheumatoid Arthritis: A Meta-Analysis. Front. Cell Infect. Microbiol. 2022, 12, 956417. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Zhang, R.D.; Musonye, H.A.; Zhao, H.Y.; He, Y.S.; Zhao, C.N.; He, T.; Tian, T.; Gao, Z.X.; Fang, Y.; et al. Hormonal and reproductive factors in relation to the risk of rheumatoid arthritis in women: A prospective cohort study with 223 526 participants. RMD Open 2024, 10, e003338. [Google Scholar] [CrossRef]
- Shuai, Z.; Zheng, S.; Wang, K.; Wang, J.; Leung, P.S.C.; Xu, B. Reestablish immune tolerance in rheumatoid arthritis. Front. Immunol. 2022, 13, 1012868. [Google Scholar] [CrossRef]
- Carlé, C.; Degboe, Y.; Ruyssen-Witrand, A.; Arleevskaya, M.I.; Clavel, C.; Renaudineau, Y. Characteristics of the (Auto)Reactive T Cells in Rheumatoid Arthritis According to the Immune Epitope Database. Int. J. Mol. Sci. 2023, 24, 4296. [Google Scholar] [CrossRef]
- van Loosdregt, J.; Rossetti, M.; Spreafico, R.; Moshref, M.; Olmer, M.; Williams, G.W.; Kumar, P.; Copeland, D.; Pischel, K.; Lotz, M.; et al. Increased autophagy in CD4+ T cells of rheumatoid arthritis patients results in T-cell hyperactivation and apoptosis resistance. Eur. J. Immunol. 2016, 46, 2862–2870. [Google Scholar] [CrossRef]
- Luo, P.; Wang, P.; Xu, J.; Hou, W.; Xu, P.; Xu, K.; Liu, L. Immunomodulatory role of T helper cells in rheumatoid arthritis: A comprehensive research review. Bone Jt. Res. 2022, 11, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Kotschenreuther, K.; Deng, S.; Kofler, D.M. Regulatory T cells in rheumatoid arthritis: Functions, development, regulation, and therapeutic potential. Cell. Mol. Life Sci. 2022, 79, 533. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Tsaltskan, V.; Firestein, G.S. Targeting fibroblast-like synoviocytes in rheumatoid arthritis. Curr. Opin. Pharmacol. 2022, 67, 102304. [Google Scholar] [CrossRef]
- Brescia, A.C.; Simonds, M.M.; Sullivan, K.E.; Rose, C.D. Secretion of pro-inflammatory cytokines and chemokines and loss of regulatory signals by fibroblast-like synoviocytes in juvenile idiopathic arthritis. Proteom. Clin. Appl. 2017, 11, 1600088. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Schett, G.; Steffen, U. Autoantibodies in Rheumatoid Arthritis: Historical Background and Novel Findings. Clin. Rev. Allergy Immunol. 2022, 63, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Banda, N.K.; Hyatt, S.; Antonioli, A.H.; White, J.T.; Glogowska, M.; Takahashi, K.; Merkel, T.J.; Stahl, G.L.; Mueller-Ortiz, S.; Wetsel, R.; et al. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J. Immunol. 2012, 188, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Youinou, P.; Mackenzie, L.; Katsikis, P.; Merdrignac, G.; Isenberg, D.A.; Tuaillon, N.; Lamour, A.; Le Goff, P.; Jouquan, J.; Drogou, A.; et al. The relationship between CD5-expressing B lymphocytes and serologic abnormalities in rheumatoid arthritis patients and their relatives. Arthritis Rheum. 1990, 33, 339–348. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Grimstad, K.; Thorarinsdottir, K.; Forslind, K.; Glinatsi, D.; Leu Agelii, M.; Aranburu, A.; Sundell, T.; Jonsson, C.A.; Camponeschi, A.; et al. Correlation of Professional Antigen-Presenting Tbet+CD11c+ B Cells With Bone Destruction in Untreated Rheumatoid Arthritis. Arthritis Rheumatol. 2024, 76, 1263–1277. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Wu, R. Neutrophils in Rheumatoid Arthritis Synovium: Implications on Disease Activity and Inflammation State. J. Inflamm. Res. 2025, 18, 4741–4753. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, K.; Jiang, P.; Zhao, J.; Shan, Y.; Shi, Y.; Zhao, F.; Chang, C.; Li, Y.; Zhou, M.; et al. Macrophage polarization in rheumatoid arthritis: Signaling pathways, metabolic reprogramming, and crosstalk with synovial fibroblasts. Front. Immunol. 2024, 15, 1394108. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Chu, Y.; Xie, J.; Ding, W.; Wang, F. Tissue factor expression in rheumatoid synovium: A potential role in pannus invasion of rheumatoid arthritis. Acta Histochem. 2013, 115, 692–697. [Google Scholar] [CrossRef]
- Tanaka, S. RANKL is a therapeutic target of bone destruction in rheumatoid arthritis. F1000Research 2019, 8, F1000, Faculty Rev-533. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.Á.; et al. The RANK–RANKL–OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef]
- Rolph, D.; Das, H. Transcriptional Regulation of Osteoclastogenesis: The Emerging Role of KLF2. Front. Immunol. 2020, 11, 937. [Google Scholar] [CrossRef]
- DI Matteo, A.; Emery, P. Rheumatoid arthritis: A review of the key clinical features and ongoing challenges of the disease. Panminerva Med. 2024, 66, 427–442. [Google Scholar] [CrossRef]
- Zimba, O.; Baimukhamedov, C.; Kocyigit, B.F. Late-onset rheumatoid arthritis: Clinical features, diagnostic challenges, and treatment approaches. Rheumatol. Int. 2025, 45, 152. [Google Scholar] [CrossRef]
- Pavlov-Dolijanovic, S.; Bogojevic, M.; Nozica-Radulovic, T.; Radunovic, G.; Mujovic, N. Elderly-Onset Rheumatoid Arthritis: Characteristics and Treatment Options. Medicina 2023, 59, 1878. [Google Scholar] [CrossRef]
- Chmielewski, G.; Majewski, M.S.; Kuna, J.; Mikiewicz, M.; Krajewska-Włodarczyk, M. Fatigue in Inflammatory Joint Diseases. Int. J. Mol. Sci. 2023, 24, 12040. [Google Scholar] [CrossRef]
- Debreova, M.; Culenova, M.; Smolinska, V.; Nicodemou, A.; Csobonyeiova, M.; Danisovic, L. Rheumatoid arthritis: From synovium biology to cell-based therapy. Cytotherapy 2022, 24, 365–375. [Google Scholar] [CrossRef]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Vrabie, C.D.; Tanasescu, R. Extra-articular Manifestations in Rheumatoid Arthritis. Maedica 2010, 5, 286–291. [Google Scholar]
- Sanghavi, N.; Ingrassia, J.P.; Korem, S.; Ash, J.; Pan, S.; Wasserman, A. Cardiovascular Manifestations in Rheumatoid Arthritis. Cardiol. Rev. 2024, 32, 146–152. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef]
- Aggarwal, R.; Liao, K.; Nair, R.; Ringold, S.; Costenbader, K.H. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009, 61, 1472–1483. [Google Scholar] [CrossRef]
- Kgoebane, K.; Ally, M.M.T.M.; Duim-Beytell, M.C.; Suleman, F.E. The role of imaging in rheumatoid arthritis. SA J. Radiol. 2018, 22, 1316. [Google Scholar] [CrossRef]
- Hercus, T.R.; Thomas, D.; Guthridge, M.A.; Ekert, P.G.; King-Scott, J.; Parker, M.W.; Lopez, A.F. The granulocyte-macrophage colony-stimulating factor receptor: Linking its structure to cell signaling and its role in disease. Blood 2009, 114, 1289–1298. [Google Scholar] [CrossRef]
- Weng, S.; Zhang, D.-E. The GM-CSF Receptor Alpha Chain (CSF2RA) Functions As a Novel Ligand-Independent Tumor Suppressor in t(8;21) AML. Blood 2015, 126, 3589. [Google Scholar] [CrossRef]
- Mirza, S.; Walker, A.; Chen, J.; Murphy, J.M.; Young, I.G. The Ig-like domain of human GM-CSF receptor alpha plays a critical role in cytokine binding and receptor activation. Biochem. J. 2010, 426, 307–317. [Google Scholar] [CrossRef]
- Pundavela, J.; Hall, A.; Dinglasan, S.A.; Choi, K.; Rizvi, T.A.; Trapnell, B.C.; Wu, J.; Ratner, N. Granulocyte-Macrophage Colony Stimulating Factor Receptor Contributes to Plexiform Neurofibroma Initiation. Cancers 2025, 17, 905. [Google Scholar] [CrossRef]
- Carr, P.D.; Gustin, S.E.; Church, A.P.; Murphy, J.M.; Ford, S.C.; Mann, D.A.; Woltring, D.M.; Walker, I.; Ollis, D.L.; Young, I.G. Structure of the complete extracellular domain of the common beta subunit of the human GM-CSF, IL-3, and IL-5 receptors reveals a novel dimer configuration. Cell 2001, 104, 291–300. [Google Scholar] [CrossRef]
- Zsiros, V.; Katz, S.; Doczi, N.; Kiss, A.L. Endocytosis of GM-CSF receptor β is essential for signal transduction regulating mesothelial-macrophage transition. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1450–1462. [Google Scholar] [CrossRef]
- Zhao, Y.; Wagner, F.; Frank, S.J.; Kraft, A.S. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J. Biol. Chem. 1995, 270, 13814–13818. [Google Scholar] [CrossRef]
- Liu, R.Y.; Fan, C.; Garcia, R.; Jove, R.; Zuckerman, K.S. Constitutive activation of the JAK2/STAT5 signal transduction pathway correlates with growth factor independence of megakaryocytic leukemic cell lines. Blood 1999, 93, 2369–2379. [Google Scholar] [CrossRef]
- Kimura, A.; Rieger, M.A.; Simone, J.M.; Chen, W.; Wickre, M.C.; Zhu, B.M.; Hoppe, P.S.; O’Shea, J.J.; Schroeder, T.; Hennighausen, L. The transcription factors STAT5A/B regulate GM-CSF-mediated granulopoiesis. Blood 2009, 114, 4721–4728. [Google Scholar] [CrossRef]
- Rumore-Maton, B.; Elf, J.; Belkin, N.; Stutevoss, B.; Seydel, F.; Garrigan, E.; Litherland, S.A. M-CSF and GM-CSF regulation of STAT5 activation and DNA binding in myeloid cell differentiation is disrupted in nonobese diabetic mice. Clin. Dev. Immunol. 2008, 2008, 769795. [Google Scholar] [CrossRef]
- Vázquez Marrero, V.R.; Dresler, M.; Haggadone, M.D.; Lu, A.; Shin, S. GM-CSF engages multiple signaling pathways to enhance pro-inflammatory cytokine responses in human monocytes during Legionella infection. Infect. Immun. 2025, 93, e0056524. [Google Scholar] [CrossRef]
- López-Navarro, B.; Simón-Fuentes, M.; Ríos, I.; Schiaffino, M.T.; Sanchez, A.; Torres-Torresano, M.; Nieto-Valle, A.; Castrejón, I.; Puig-Kröger, A. Macrophage re-programming by JAK inhibitors relies on MAFB. Cell Mol. Life Sci. 2024, 81, 152. [Google Scholar] [CrossRef]
- Boyer, S.; Lee, H.J.; Steele, N.; Zhang, L.; Sajjakulnukit, P.; Andren, A.; Ward, M.H.; Singh, R.; Basrur, V.; Zhang, Y.; et al. Multiomic characterization of pancreatic cancer-associated macrophage polarization reveals deregulated metabolic programs driven by the GM-CSF-PI3K pathway. Elife 2022, 11, e73796. [Google Scholar] [CrossRef]
- Jücker, M.; Feldman, R.A. Identification of a new adapter protein that may link the common beta subunit of the receptor for granulocyte/macrophage colony-stimulating factor, interleukin (IL)-3, and IL-5 to phosphatidylinositol 3-kinase. J. Bol. Chem. 1995, 270, 27817–27822. [Google Scholar] [CrossRef]
- Mafi, S.; Mansoori, B.; Taeb, S.; Sadeghi, H.; Abbasi, R.; Cho, W.C.; Rostamzadeh, D. mTOR-Mediated Regulation of Immune Responses in Cancer and Tumor Microenvironment. Front. Immunol. 2022, 12, 774103. [Google Scholar] [CrossRef]
- de Carvalho Oliveira, V.; Tatsiy, O.; McDonald, P.P. Phosphoinositol 3-kinase-driven NET formation involves different isoforms and signaling partners depending on the stimulus. Front. Immunol. 2023, 14, 1042686. [Google Scholar] [CrossRef]
- Kolonics, A.; Apáti, A.; Jánossy, J.; Brózik, A.; Gáti, R.; Schaefer, A.; Magócsi, M. Activation of Raf/ERK1/2 MAP kinase pathway is involved in GM-CSF-induced proliferation and survival but not in erythropoietin-induced differentiation of TF-1 cells. Cell Signal. 2001, 13, 743–754. [Google Scholar] [CrossRef]
- Schallenberg, M.; Charalambous, P.; Thanos, S. GM-CSF regulates the ERK1/2 pathways and protects injured retinal ganglion cells from induced death. Exp. Eye Res. 2009, 89, 665–677. [Google Scholar] [CrossRef]
- Parajuli, B.; Sonobe, Y.; Kawanokuchi, J.; Doi, Y.; Noda, M.; Takeuchi, H.; Mizuno, T.; Suzumura, A. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J. Neuroinflamm. 2012, 9, 268. [Google Scholar] [CrossRef]
- Hu, N.; Qiu, Y.; Dong, F. Role of Erk1/2 signaling in the regulation of neutrophil versus monocyte development in response to G-CSF and M-CSF. J. Biol. Chem. 2015, 290, 24561–24573. [Google Scholar] [CrossRef]
- Rodriguez, R.M.; Suarez-Alvarez, B.; Lavín, J.L.; Ascensión, A.M.; Gonzalez, M.; Lozano, J.J.; Raneros, A.B.; Bulnes, P.D.; Vidal-Castiñeira, J.R.; Huidobro, C.; et al. Signal Integration and Transcriptional Regulation of the Inflammatory Response Mediated by the GM-/M-CSF Signaling Axis in Human Monocytes. Cell Rep. 2019, 29, 860–872.e5. [Google Scholar] [CrossRef]
- Ebner, K.; Bandion, A.; Binder, B.R.; de Martin, R.; Schmid, J.A. GMCSF activates NF-kappaB via direct interaction of the GMCSF receptor with IkappaB kinase beta. Blood 2003, 102, 192–199. [Google Scholar] [CrossRef]
- Guerrero, P.; Bono, C.; Sobén, M.; Guiu, A.; Cheng, Q.J.; Gil, M.L.; Yáñez, A. GM-CSF receptor expression determines opposing innate memory phenotypes at different stages of myelopoiesis. Blood 2024, 143, 2763–2777. [Google Scholar] [CrossRef]
- Cook, A.D.; Louis, C.; Robinson, M.J.; Saleh, R.; Sleeman, M.A.; Hamilton, J.A. Granulocyte macrophage colony-stimulating factor receptor α expression and its targeting in antigen-induced arthritis and inflammation. Arthritis Res. Ther. 2016, 18, 287. [Google Scholar] [CrossRef]
- Yoshitomi, H. Regulation of Immune Responses and Chronic Inflammation by Fibroblast-Like Synoviocytes. Front. Immunol. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Tu, J.; Hong, W.; Zhang, P.; Wang, X.; Körner, H.; Wei, W. Ontology and Function of Fibroblast-Like and Macrophage-Like Synoviocytes: How Do They Talk to Each Other and Can They Be Targeted for Rheumatoid Arthritis Therapy? Front. Immunol. 2018, 9, 1467. [Google Scholar] [CrossRef]
- Xiang, C.; Li, H.; Tang, W. Targeting CSF-1R represents an effective strategy in modulating inflammatory diseases. Pharmacol. Res. 2023, 187, 106566. [Google Scholar] [CrossRef]
- Zhan, Y.; Lew, A.M.; Chopin, M. The Pleiotropic Effects of the GM-CSF Rheostat on Myeloid Cell Differentiation and Function: More Than a Numbers Game. Front. Immunol. 2019, 10, 2679. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Lotfi, N.; Zhang, G.X.; Esmaeil, N.; Rostami, A. Evaluation of the effect of GM-CSF blocking on the phenotype and function of human monocytes. Sci. Rep. 2020, 10, 1567. [Google Scholar] [CrossRef] [PubMed]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S., Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 2013, 39, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Hirota, K.; Sakaguchi, S. Synovial Tissue Inflammation Mediated by Autoimmune T Cells. Front. Immunol. 2019, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Fuhler, G.M.; Cadwallader, K.A.; Knol, G.J.; Chilvers, E.R.; Drayer, A.L.; Vellenga, E. Disturbed granulocyte macrophage-colony stimulating factor priming of phosphatidylinositol 3,4,5-trisphosphate accumulation and Rac activation in fMLP-stimulated neutrophils from patients with myelodysplasia. J. Leukoc. Biol. 2004, 76, 254–262. [Google Scholar] [CrossRef]
- Zhang, Y.; McCluskey, K.; Fujii, K.; Wahl, L.M. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J. Immunol. 1998, 161, 3071–3076. [Google Scholar] [CrossRef]
- Schreck, R.; Baeuerle, P.A. NF-kappa B as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol. Cell. Biol. 1990, 10, 1281–1286. [Google Scholar] [CrossRef]
- Weiss, M.; Blazek, K.; Byrne, A.J.; Perocheau, D.P.; Udalova, I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediat. Inflamm. 2013, 2013, 245804. [Google Scholar] [CrossRef]
- Helft, J.; Böttcher, J.P.; Chakravarty, P.; Zelenay, S.; Huotari, J.; Schraml, B.U.; Goubau, D.; Reis e Sousa, C. Alive but Confused: Heterogeneity of CD11c(+) MHC Class II(+) Cells in GM-CSF Mouse Bone Marrow Cultures. Immunity 2016, 44, 3–4. [Google Scholar] [CrossRef]
- Mashima, H.; Zhang, R.; Kobayashi, T.; Hagiya, Y.; Tsukamoto, H.; Liu, T.; Iwama, T.; Yamamoto, M.; Lin, C.; Nakatsuka, R.; et al. Generation of GM-CSF-producing antigen-presenting cells that induce a cytotoxic T cell-mediated antitumor response. Oncoimmunology 2020, 9, 1814620. [Google Scholar] [CrossRef]
- Korniotis, S.; Saichi, M.; Trichot, C.; Hoffmann, C.; Amblard, E.; Viguier, A.; Grondin, S.; Noel, F.; Mattoo, H.; Soumelis, V. GM-CSF-activated human dendritic cells promote type 1 T follicular helper cell polarization in a CD40-dependent manner. J. Cell Sci. 2022, 135, jcs260298. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, B.C.; Corthay, A.; Øynebråten, I. High production of IL-12 by human dendritic cells stimulated with combinations of pattern-recognition receptor agonists. npj Vaccines 2024, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hashimoto, M.; Ito, Y.; Matsuura, M.; Ito, H.; Tanaka, M.; Watanabe, H.; Kondoh, G.; Tanaka, A.; Yasuda, K.; et al. Autoimmune Th17 Cells Induced Synovial Stromal and Innate Lymphoid Cell Secretion of the Cytokine GM-CSF to Initiate and Augment Autoimmune Arthritis. Immunity 2018, 48, 1220–1232.e5. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, K.; Roesler, J.; Emmendoerffer, A.; Elsner, J.; Welte, K. Functional features of neutrophils induced by G-CSF and GM-CSF treatment: Differential effects and clinical implications. Leukemia 1997, 11, 466–478. [Google Scholar] [CrossRef]
- Chao, J.R.; Wang, J.M.; Lee, S.F.; Peng, H.W.; Lin, Y.H.; Chou, C.H.; Li, J.C.; Huang, H.M.; Chou, C.K.; Kuo, M.L.; et al. mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol. Cell. Biol. 1998, 18, 4883–4898. [Google Scholar] [CrossRef]
- He, K.; Liu, X.; Hoffman, R.D.; Shi, R.Z.; Lv, G.Y.; Gao, J.L. G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors. FEBS Open Bio 2022, 12, 1268–1285. [Google Scholar] [CrossRef]
- Chen, J.; Cao, Y.; Xiao, J.; Hong, Y.; Zhu, Y. The emerging role of neutrophil extracellular traps in the progression of rheumatoid arthritis. Front. Immunol. 2024, 15, 1438272. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Thiruppathi, M.; Elshabrawy, H.A.; Alharshawi, K.; Kumar, P.; Prabhakar, B.S. GM-CSF: An immune modulatory cytokine that can suppress autoimmunity. Cytokine 2015, 75, 261–271. [Google Scholar] [CrossRef]
- Singh, P.; González-Ramos, S.; Mojena, M.; Rosales-Mendoza, C.E.; Emami, H.; Swanson, J.; Morss, A.; Fayad, Z.A.; Rudd, J.H.; Gelfand, J.; et al. GM-CSF Enhances Macrophage Glycolytic Activity In Vitro and Improves Detection of Inflammation In Vivo. J. Nucl. Med. 2016, 57, 1428–1435. [Google Scholar] [CrossRef]
- Sun, H.W.; Wu, W.C.; Chen, H.T.; Xu, Y.T.; Yang, Y.Y.; Chen, J.; Yu, X.J.; Wang, Z.; Shuang, Z.Y.; Zheng, L. Glutamine Deprivation Promotes the Generation and Mobilization of MDSCs by Enhancing Expression of G-CSF and GM-CSF. Front. Immunol. 2021, 11, 616367. [Google Scholar] [CrossRef]
- Guo, X.; Wang, S.; Godwood, A.; Close, D.; Ryan, P.C.; Roskos, L.K.; White, W.I. Pharmacodynamic biomarkers and differential effects of TNF- and GM-CSF-targeting biologics in rheumatoid arthritis. Int. J. Rheum. Dis. 2019, 22, 646–653. [Google Scholar] [CrossRef]
- Senolt, L. Emerging therapies in rheumatoid arthritis: Focus on monoclonal antibodies. F1000Research 2019, 8, F1000, Faculty Rev-1549. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Weinblatt, M.E.; Taylor, P.C.; McInnes, I.B.; Atsumi, T.; Strand, V.; Takeuchi, T.; Bracher, M.; Brooks, D.; Curtis, P.; Gupta, A.; et al. Long-term safety and efficacy of anti-GM-CSF otilimab in patients with rheumatoid arthritis: Long-term extension of three phase 3 randomised trials (contRAst X). BMJ Open 2025, 15, e088869. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, T.W.; Batalov, A.; Stoilov, R.; Lloyd, E.; Wagner, T.; Saurigny, D.; Souberbielle, B.; Esfandiari, E. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Corbera-Bellalta, M.; Alba-Rovira, R.; Muralidharan, S.; Espígol-Frigolé, G.; Ríos-Garcés, R.; Marco-Hernández, J.; Denuc, A.; Kamberovic, F.; Pérez-Galán, P.; Joseph, A.; et al. Blocking GM-CSF receptor α with mavrilimumab reduces infiltrating cells, pro-inflammatory markers and neoangiogenesis in ex vivo cultured arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 2022, 81, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Feist, E.; Sleeman, M.A.; Wang, B.; White, B.; Magrini, F. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: A randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann. Rheum. Dis. 2011, 70, 1542–1549. [Google Scholar] [CrossRef]
- Davda, J.P.; Hansen, R.J. Properties of a general PK/PD model of antibody-ligand interactions for therapeutic antibodies that bind to soluble endogenous targets. MAbs 2010, 2, 576–588. [Google Scholar] [CrossRef]
- Cook, A.D.; Braine, E.L.; Campbell, I.K.; Rich, M.J.; Hamilton, J.A. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): Requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001, 3, 293–298. [Google Scholar] [CrossRef]
- Cook, A.D.; Turner, A.L.; Braine, E.L.; Pobjoy, J.; Lenzo, J.C.; Hamilton, J.A. Regulation of systemic and local myeloid cell subpopulations by bone marrow cell-derived granulocyte-macrophage colony-stimulating factor in experimental inflammatory arthritis. Arthritis Rheum. 2011, 63, 2340–2351. [Google Scholar] [CrossRef]
- Greven, D.E.; Cohen, E.S.; Gerlag, D.M.; Campbell, J.; Woods, J.; Davis, N.; van Nieuwenhuijze, A.; Lewis, A.; Heasmen, S.; McCourt, M.; et al. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 1924–1930. [Google Scholar] [CrossRef]
- Minter, R.R.; Cohen, E.S.; Wang, B.; Liang, M.; Vainshtein, I.; Rees, G.; Eghobamien, L.; Harrison, P.; Sims, D.A.; Matthews, C.; et al. Protein engineering and preclinical development of a GM-CSF receptor antibody for the treatment of rheumatoid arthritis. Br. J. Pharmacol. 2013, 168, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.M.; van der Heijde, D.; Strand, V.; Atsumi, T.; McInnes, I.B.; Takeuchi, T.; Taylor, P.C.; Bracher, M.; Brooks, D.; Davies, J.; et al. Anti-GM-CSF otilimab versus tofacitinib or placebo in patients with active rheumatoid arthritis and an inadequate response to conventional or biologic DMARDs: Two phase 3 randomised trials (contRAst 1 and contRAst 2). Ann. Rheum. Dis. 2023, 82, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Berkowitz, M.; Conaghan, P.G.; Peterfy, C.; Davy, K.; Fisheleva, E.; Gupta, A.; Inman, D.; Janiczek, R.; Layton, M.; et al. MRI of the joint and evaluation of the granulocyte-macrophage colony-stimulating factor-CCL17 axis in patients with rheumatoid arthritis receiving otilimab: A phase 2a randomised mechanistic study. Lancet Rheumatol. 2020, 2, e666–e676. [Google Scholar] [CrossRef]
- Taylor, P.C.; Saurigny, D.; Vencovsky, J.; Takeuchi, T.; Nakamura, T.; Matsievskaia, G.; Hunt, B.; Wagner, T.; Souberbielle, B.; NEXUS Study Group. Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: A randomized, controlled trial. Arthritis Res. Ther. 2019, 21, 101. [Google Scholar]
- Kivitz, A.; Hazan, L.; Hoffman, K.; Wallin, B. FRI0209 MORAb-022, an Anti-Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) Monoclonal Antibody (MAB): RESULTS of the First Study in Patients with Mild-to-Moderate Rheumatoid Arthritis (RA); BMJ Publishing Group Ltd.: London, UK, 2016. [Google Scholar]
- Behrens, F.; Tak, P.P.; Østergaard, M.; Stoilov, R.; Wiland, P.; Huizinga, T.W.; Berenfus, V.Y.; Vladeva, S.; Rech, J.; Rubbert-Roth, A.; et al. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: Results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann. Rheum. Dis. 2015, 74, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Weinblatt, M.E.; McInnes, I.B.; Porter, D.; Barbarash, O.; Vatutin, M.; Szombati, I.; Esfandiari, E.; Sleeman, M.A.; Kane, C.D.; et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1445–1452. [Google Scholar] [CrossRef]
- Burmester, G.R.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Korkosz, M.; Vencovsky, J.; Rubbert-Roth, A.; Mysler, E.; Sleeman, M.A.; Godwood, A.; et al. Efficacy and safety of mavrilimumab, A fully human Gm–CSFR-Alpha monoclonal antibody in patients with rheumatoid arthritis: Primary results from the Earth Explorer 1 Study. Ann. Rheum. Dis. 2015, 74, S78. [Google Scholar] [CrossRef]
- Burmester, G.R.; McInnes, I.B.; Kremer, J.; Miranda, P.; Korkosz, M.; Vencovsky, J.; Rubbert-Roth, A.; Mysler, E.; Sleeman, M.A.; Godwood, A.; et al. A randomised phase IIb study of mavrilimumab, a novel GM-CSF receptor alpha monoclonal antibody, in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.C.; Wu, C.Y.; Roskos, L.K.; Godwood, A.; Close, D.; Wang, B. AB0445 Exposure–Efficacy Analysis of Mavrilimumab in Rheumatoid Arthritis: Modeling and Simulation of Phase II Clinical Data. Ann. Rheum. Dis. 2015, 74, 1043. [Google Scholar] [CrossRef]
- Burmester, G.R.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Vencovský, J.; Godwood, A.; Albulescu, M.; Michaels, M.A.; Guo, X.; Close, D.; et al. Mavrilimumab, a Fully Human Granulocyte-Macrophage Colony-Stimulating Factor Receptor α Monoclonal Antibody: Long-Term Safety and Efficacy in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 679–689. [Google Scholar] [CrossRef]
- Weinblatt, M.; McInnes, I.; Kremer, J.; Miranda, P.; Vencovský, J.; Godwood, A.; Albulescu, M.; Close, D.; Burmester, G. Earth explorer 2, a phase IIb exploratory study evaluating efficacy and safety of mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor-alpha monoclonal antibody, and the tumor necrosis factor antagonist golimumab in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, S717–S718. [Google Scholar]
- Chen, Y.; Li, F.; Hua, M.; Liang, M.; Song, C. Role of GM-CSF in lung balance and disease. Front. Immunol. 2023, 14, 1158859. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; Pitts, K.; Wang, T.; Lee, E.; Buchbinder, E.; Dougan, M.; Armstrong, D.G.; Paine, R., 3rd; Ragsdale, C.E.; Boyd, T.; et al. Recombinant GM-CSF for diseases of GM-CSF insufficiency: Correcting dysfunctional mononuclear phagocyte disorders. Front. Immunol. 2023, 13, 1069444. [Google Scholar] [CrossRef]
- McCormick, T.S.; Hejal, R.B.; Leal, L.O.; Ghannoum, M.A. GM-CSF: Orchestrating the Pulmonary Response to Infection. Front. Pharmacol. 2022, 12, 735443. [Google Scholar] [CrossRef]
- Wishart, A.L.; Pechacek, J.; Rosen, L.B.; Desai, J.V.; Zarakas, M.A.; Webb, T.; Pittaluga, S.; Seyedmousavi, A.; Hohl, T.M.; Kuhns, D.B.; et al. Neutralizing GM-CSF autoantibodies impair neutrophil antifungal effector function in a patient with aspergillosis. J. Infect. 2025, 91, 106588. [Google Scholar] [CrossRef]
- Ataya, A.; Knight, V.; Carey, B.C.; Lee, E.; Tarling, E.J.; Wang, T. The Role of GM-CSF Autoantibodies in Infection and Autoimmune Pulmonary Alveolar Proteinosis: A Concise Review. Front. Immunol. 2021, 12, 752856. [Google Scholar] [CrossRef]
- Campo, I.; Carey, B.C.; Paracchini, E.; Kadija, Z.; De Silvestri, A.; Rodi, G.; De Amici, M.; Torre, C.; Zorzetto, M.; Griese, M.; et al. Inhaled recombinant GM-CSF reduces the need for whole lung lavage and improves gas exchange in autoimmune pulmonary alveolar proteinosis patients. Eur. Respir. J. 2024, 63, 2301233. [Google Scholar] [CrossRef]
- Bonfield, T.L.; Kavuru, M.S.; Thomassen, M.J. Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin. Immunol. 2002, 105, 342–350. [Google Scholar] [CrossRef]
- Campo, I.; Meloni, F.; Gahlemann, M.; Sauter, W.; Ittrich, C.; Schoelch, C.; Trapnell, B.C.; Gupta, A. An exploratory study investigating biomarkers associated with autoimmune pulmonary alveolar proteinosis (aPAP). Sci. Rep. 2022, 12, 8708. [Google Scholar] [CrossRef]
- Chen, W.; Feng, X.; Yao, L.K.; Li, X.; Yang, Z.M.; Qin, X.Y.; Li, Y.; Qiu, Y. Exogenous GM-CSF therapy for autoimmune pulmonary alveolar proteinosis: A systematic literature review. Front. Med. 2025, 12, 1552566. [Google Scholar]
- Chen, H.; Gao, N.; Fan, D.; Wu, J.; Zhu, J.; Li, J.; Wang, J.; Chen, Y.; An, J. Suppressive effects on the immune response and protective immunity to a JEV DNA vaccine by co-administration of a GM-CSF-expressing plasmid in mice. PLoS ONE 2012, 7, e34602. [Google Scholar] [CrossRef] [PubMed]
- Parmiani, G.; Castelli, C.; Pilla, L.; Santinami, M.; Colombo, M.P.; Rivoltini, L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann. Oncol. 2007, 18, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.W.; Chueh, H.Y.; Tsai, C.C.; Lin, C.T.; Qiu, J.T. Novel GM-CSF-based vaccines: One small step in GM-CSF gene optimization, one giant leap for human vaccines. Hum. Vaccin. Immunother. 2016, 12, 3020–3028. [Google Scholar] [CrossRef]
- Henrickson, S.E.; Ruffner, M.A.; Kwan, M. Unintended Immunological Consequences of Biologic Therapy. Curr. Allergy Asthma Rep. 2016, 16, 46. [Google Scholar] [CrossRef]
- Zhang, F.; Jonsson, A.H.; Nathan, A.; Millard, N.; Curtis, M.; Xiao, Q.; Gutierrez-Arcelus, M.; Apruzzese, W.; Watts, G.F.M.; Weisenfeld, D.; et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 2023, 623, 616–624. [Google Scholar] [CrossRef]
- Hanlon, M.M.; Smith, C.M.; Canavan, M.; Neto, N.G.B.; Song, Q.; Lewis, M.J.; O’Rourke, A.M.; Tynan, O.; Barker, B.E.; Gallagher, P.; et al. Loss of synovial tissue macrophage homeostasis precedes rheumatoid arthritis clinical onset. Sci. Adv. 2024, 10, eadj1252. [Google Scholar] [CrossRef]
- Achuthan, A.; Cook, A.D.; Lee, M.C.; Saleh, R.; Khiew, H.W.; Chang, M.W.; Louis, C.; Fleetwood, A.J.; Lacey, D.C.; Christensen, A.D.; et al. Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J. Clin. Investig. 2016, 126, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Bluett, J. Towards Personalized Medicine in Rheumatoid Arthritis. Open Access Rheumatol. 2024, 16, 89–114. [Google Scholar] [CrossRef]
- Momoi, Y.; Kumagai, S.; Nishikawa, H. Immunogenomic precision medicine: A personalized approach based on immunogenomic cancer evolution. Int. Immunol. 2025, 37, 517–537. [Google Scholar] [CrossRef]
- Silva, R.C.M.C.; Travassos, L.H.; Dutra, F.F. The dichotomic role of single cytokines: Fine-tuning immune responses. Cytokine 2024, 173, 156408. [Google Scholar] [CrossRef]
- Chang, C.J.; Chen, Y.H.; Huang, K.W.; Cheng, H.W.; Chan, S.F.; Tai, K.F.; Hwang, L.H. Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology 2007, 45, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shi, Y.; Li, Q.; Luo, L.; Li, X.; Luo, Z.; Lu, Y.; Zhang, J.; Jiang, M.; Qin, B.; et al. Rational administration sequencing of immunochemotherapy elicits powerful anti-tumor effect. J. Control Release 2022, 341, 769–781. [Google Scholar] [CrossRef]
- Kwon, O.C.; Lee, E.J.; Chang, E.J.; Youn, J.; Ghang, B.; Hong, S.; Lee, C.K.; Yoo, B.; Kim, Y.G. IL-17A+GM-CSF+ Neutrophils Are the Major Infiltrating Cells in Interstitial Lung Disease in an Autoimmune Arthritis Model. Front. Immunol. 2018, 9, 1544. [Google Scholar] [CrossRef]
- O’Rielly, D.D.; Rahman, P. Pharmacogenetics of rheumatoid arthritis: Potential targets from susceptibility genes and present therapies. Pharmgenom. Pers. Med. 2010, 3, 15–31. [Google Scholar]
- Gent, Y.Y.; Voskuyl, A.E.; Kloet, R.W.; van Schaardenburg, D.; Hoekstra, O.S.; Dijkmans, B.A.; Lammertsma, A.A.; van der Laken, C.J. Macrophage positron emission tomography imaging as a biomarker for preclinical rheumatoid arthritis: Findings of a prospective pilot study. Arthritis Rheum. 2012, 64, 62–66. [Google Scholar] [PubMed]
- Bruijnen, S.T.G.; Verweij, N.J.F.; Gent, Y.Y.J.; Huisman, M.C.; Windhorst, A.D.; Kassiou, M.; van de Ven, P.M.; Lammertsma, A.A.; Hoekstra, O.S.; Voskuyl, A.E.; et al. Imaging disease activity of rheumatoid arthritis by macrophage targeting using second generation translocator protein positron emission tomography tracers. PLoS ONE 2019, 14, e0222844. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; van der Helm-van Mil, A.H.; Knevel, R.; Huizinga, T.W.; Haney, D.J.; Shen, Y.; Ramanujan, S.; Cavet, G.; Centola, M.; Hesterberg, L.K.; et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res. 2012, 64, 1794–1803. [Google Scholar] [CrossRef]
- Syversen, S.W.; Landewe, R.; van der Heijde, D.; Bathon, J.M.; Boers, M.; Bykerk, V.P.; Fitzgerald, O.; Gladman, D.D.; Garnero, P.; Geusens, P.; et al. Testing of the OMERACT 8 draft validation criteria for a soluble biomarker reflecting structural damage in rheumatoid arthritis: A systematic literature search on 5 candidate biomarkers. J. Rheumatol. 2009, 36, 1769–1784. [Google Scholar] [CrossRef] [PubMed]
- Piper, C.; Pesenacker, A.M.; Bending, D.; Thirugnanabalan, B.; Varsani, H.; Wedderburn, L.R.; Nistala, K. T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheumatol. 2014, 66, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Malengier-Devlies, B.; Bernaerts, E.; Ahmadzadeh, K.; Filtjens, J.; Vandenhaute, J.; Boeckx, B.; Burton, O.; De Visscher, A.; Mitera, T.; Berghmans, N.; et al. Role for Granulocyte Colony-Stimulating Factor in Neutrophilic Extramedullary Myelopoiesis in a Murine Model of Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2022, 74, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).