Applications of Artificial Intelligence in Transcatheter Aortic Valve Replacement: A Review of the Literature
Abstract
1. Introduction
2. Methods
2.1. Research Strategy
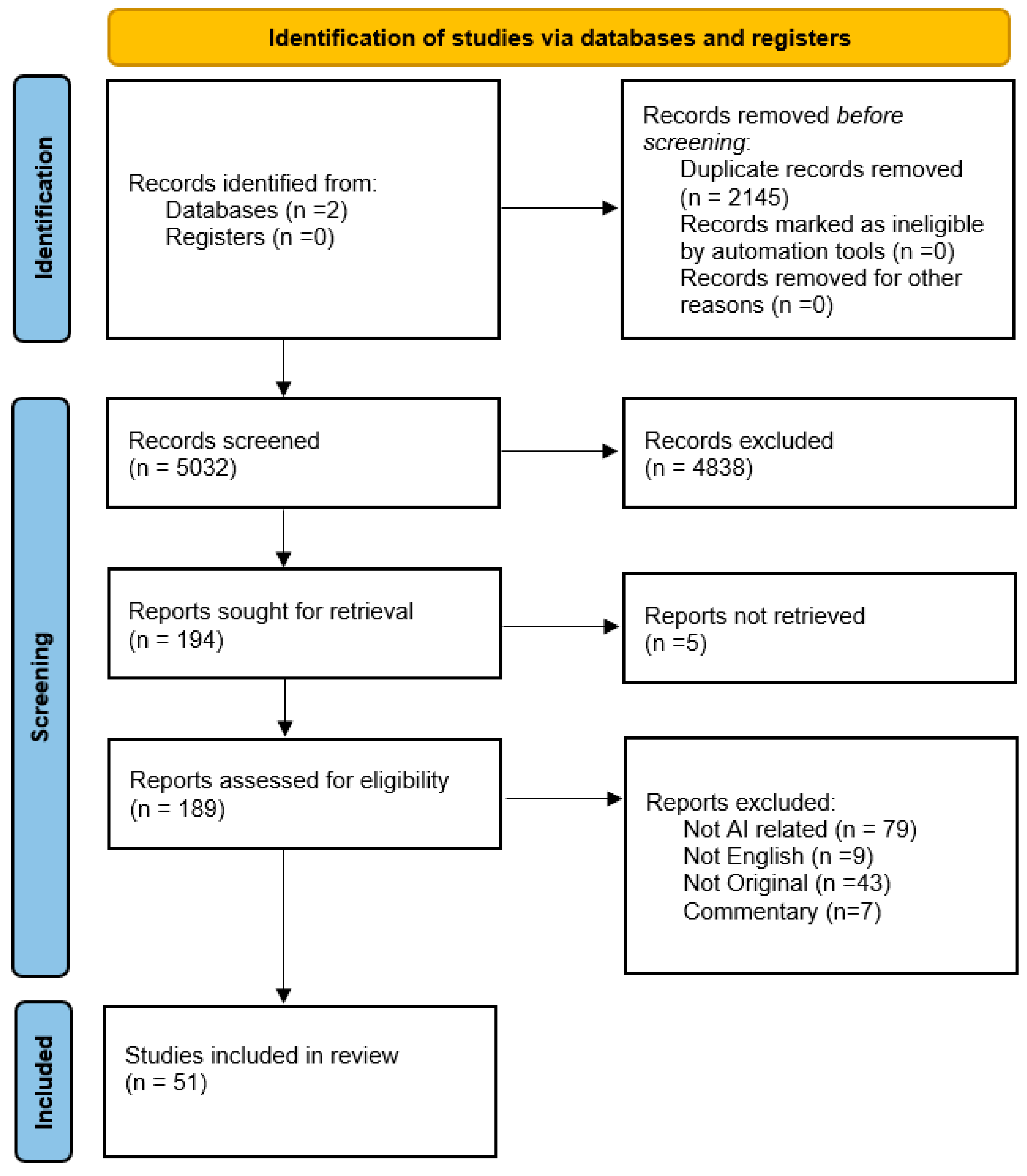
2.2. Eligibility Criteria, Screening, and Data Extraction
3. Results and Discussion
3.1. AI in Imaging and Pre-Procedural Planning
3.1.1. Automated CT Segmentation, Landmarking, and Measurement
3.1.2. Quantifying Calcification and Anatomic Complexity
3.1.3. Body Composition, Opportunistic Imaging Biomarkers, and CT-Derived Physiology
3.1.4. 3D/4D Analysis, Deformation Tracking, and Post-TAVI Assessment
3.2. Image Quality Optimization: AI Reconstruction, Coronary Rule-Out, and Protocols
3.2.1. AI-Enabled CT Reconstruction and Dose Reduction
3.2.2. Coronary Evaluation During TAVR Work-Up
3.3. Predictive and Decision-Making Models
3.3.1. Mortality Prediction: Single-Study Signals and Pooled Evidence
3.3.2. Complication Prediction: Conduction, Pacemaker, Bleeding, Stroke/CVE
| Author (Year) | Study Type | AI Method/Model | Data Source (CT/Echo/Clinical/Other) | Main Findings | Clinical Relevance/Impact |
|---|---|---|---|---|---|
| Wang et al. (2023) [2] | Multicenter retrospective imaging study | End-to-end deep learning (segmentation + landmarking) | CT (pre-TAVR aortic root, annulus, coronaries) | Automated pipeline achieved Acc 0.989, Sens 0.979, Spec 0.986 for anatomical risk factors; standardized outputs and faster analysis. | Reduces variability, accelerates pre-TAVR planning, and reliable risk-factor detection. |
| Skalidis et al. (2025) [3] | Narrative review | AI analytics + XR (VR/AR) visualization | ECG, Echo, CT; XR-assisted procedural planning | Synthesizes AI for sizing/simulation and XR for complex anatomy/MDT planning; highlights validation/standardization gaps. | Frames opportunities and barriers for clinical adoption; guides MDT workflows. |
| Windecker & Tomii (2025) [4] | Perspective/Commentary | CT strategy + AI-enabled planning principles | CT (lifetime valve care, redo-TAVR planning) | Discusses CT-based insights for redo-TAVR and lifetime management; need for standardized, reproducible imaging pathways. | Strategic guidance for longitudinal TAVR care planning. |
| Zhang et al. (2024) [5] | Review | Rule-based + ML approaches across pathway | ECG, digital auscultation, Echo, CT | AI augments screening and planning; bias/validation concerns emphasized. | Supports earlier detection and more consistent planning; underscores governance needs. |
| Watson et al. (2022) [6] | Review (cardiology AI) | Neural networks, computer vision | Imaging + EHR streams | Outlines gains in workflow efficiency and decision support; need for clinician–AI collaboration. | System-level rationale for adoption and training. |
| Henein et al. (2024) [7] | Focused review (TAVR) | Various supervised ML/DL | Pre/post-TAVR imaging + clinical data | AI predicts leaflet dysfunction, stroke, pacemaker, mortality; flags opacity/bias/privacy barriers. | Identifies promising applications with caveats for real-world use. |
| Zhang et al. (2022) [8] | Imaging reconstruction study | DL-based spiral CT 3D reconstruction | CT (pre-TAVI) | Improved SSIM/PSNR and ~90% correct valve sizing with high sensitivity/specificity. | Enhances image quality and valve sizing confidence. |
| Saitta et al. (2023) [9] | Retrospective imaging/technical validation | DL segmentation + landmark detection | CT (aortic root morphology) | Dice ~0.93; sub-millimeter landmark error (annulus/STJ); automated morphology extraction. | Reliable automated measurements for TAVI sizing/orientation. |
| Krüger et al. (2022) [10] | Technical pipeline/feasibility | Cascaded CNNs for segmentation and orientation | CT (aortic root analysis) | Runtime < 30 s; annulus diameter within ~2 mm of expert; robust orientation. | Near-real-time automated planning support. |
| Boeckling et al. (2025) [11] | Observational cohort (biomarkers) | Statistical and ML risk modeling | Serum ECM markers (incl. TIMP-1), hs-cTnT | TIMP-1 independently predicted 2-year mortality; combined with hs-cTnT outperformed STS-PROM. | Biomarker integration can refine TAVR risk stratification. |
| Bernhard et al. (2024) [12] | Observational imaging cohort | Feature extraction/ML on 4D-CT myocardium | 4D Cardiac CT | CT-derived myocardial metrics predicted reverse remodeling and clinical outcomes post-TAVR. | Adds tissue-level markers to prognostication. |
| Boninsegna et al. (2024) [13] | Validation vs. radiologists | Automated CTA measurements (AI) | CTA (9 vessel landmarks) | Within ±2 mm accuracy vs. humans; <2 min AI vs. >5 min readers; ±1 mm discrepancies near aortic valve. | Speeds reporting with acceptable accuracy; gains in workflow. |
| Tremamunno et al. (2025) [14] | Retrospective observational | Supervised ML (feature-selected models) | Planning CT + clinical | AI improved prediction of MACE during/after TAVR-planning CT vs. conventional. | Earlier identification of higher-risk candidates during planning. |
| Sun et al. (2024) [15] | Narrative/Topical review | Various DL tools (e.g., TAVI-PREP, 4TAVR) | CTA ± other modalities | Automated extraction of 22+ measures in ~2 min (high correlation), strong annulus metrics; needs broader external validation. | Supports standardization and speed; flags generalizability gaps. |
| Cadour & Dacher (2024) [16] | Commentary | Photon-counting CT + AI decision support | CCTA | Argues AI + photon-counting CCTA could obviate invasive CA in TAVR work-up for many. | Potentially reduces invasive testing burden. |
| Brendel et al. (2024) [17] | Feasibility/cohort | Photon-counting CT with AI support | CCTA in TAVR work-up | Improved CAD evaluation quality in TAVR candidates; feasibility shown. | Strengthens non-invasive CAD assessment pathway. |
| Zhang et al. (2024) [18] | Prospective/observational imaging | DL image reconstruction (DLR) | CT (pre-TAVI) | Improved image quality and diagnostic performance with reduced contrast and dose. | Safer imaging (lower dose/contrast) without sacrificing accuracy. |
| Rouhollahi et al. (2023) [19] | Technical tool/software package | Deep learning segmentation and reconstruction (digital twins) | CT (aortic stenosis) | CardioVision generated patient-specific digital twins and calcification maps. | Enables simulation, planning, and device testing in silico. |
| Pekař et al. (2024) [20] | Observational cohort | DL body composition | CT (L3 SMA, fat density) | Low skeletal muscle index and higher fat density predicted poorer survival after TAVR. | Opportunistic prognostics from routine CT. |
| van Erck et al. (2024) [21] | Observational cohort | DL assessment of muscle quality | CT (procedural scan) | Low muscle quality predicted 1-year mortality in severe AS. | Frailty proxy from CT to refine risk. |
| Zsarnoczay et al. (2025) [22] | Retrospective cohort | AI-derived LACI (automated LA/LV volumes) | CTA (automated volumetry) | LACI ≥ 43.7% independently predicted mortality over ~2 years, including preserved EF. | Adds independent prognostic signal beyond STS-PROM. |
| Paukovitsch et al. (2025) [23] | Observational cohort | Automated vBMD estimation (AI) | Opportunistic CT (thoracic vertebrae) | Thoracic vBMD AUC ~0.96 for osteoporosis; osteoporosis is linked to worse 1-year survival. | Frailty/osteoporosis screening within routine TAVR CT planning. |
| Weferling et al. (2022) [24] | Observational cohort | Automated epicardial fat quantification | CT (pre-TAVI) | Higher epicardial fat is associated with baseline AV block and higher PPM rates. | Biologic risk layer for conduction injury. |
| Dasi et al. (2023) [25] | Modeling/computational | AI hemodynamic modeling | Clinical and hemodynamic features | Predicted post-TAVR pressure gradients consistent with physiologic patterns. | Assists device selection and expectation setting. |
| Busto et al. (2023) [26] | Algorithm validation (post-TAVR) | Automated 4DCT quantification (AI) | 4DCT (prosthesis dynamics) | Automated prosthesis volume/area/displacement across cycle; aligned with valve size patterns. | Objective tracking for post-TAVR assessment. |
| Busto et al. (2025) [27] | Technical/observational | Automated 4DCT deformation analytics | 4DCT throughout cardiac cycle | Quantified deformation: potential link to long-term durability monitoring. | Foundation for durability surveillance post-implant. |
| Kojima et al. (2024) [28] | Prospective imaging cohort | Deep-learning reconstruction (DLR) at low kV | CTA (low-tube-voltage) | Improved CNR, lower noise; preserved annulus measurement fidelity vs. HIR/MBIR. | Enables dose reduction without losing sizing accuracy. |
| Shao et al. (2025) [29] | Protocol/imaging cohort | Dual DLR combination (AI-IR) | CT on 8 cm detector scanner | ≈50% dose reduction with equal/better quality for valve and access assessment. | Safer scanning for elderly/comorbid TAVR population. |
| Vaitkus et al. (2014) [30] | Protocol optimization | Low-kV strategy (not AI) | CCTA + aorto-iliac CTA | 70–100 kV protocols preserved quality and enabled large dose savings; accurate annulus sizing. | Baseline for later AI-DLR dose reductions. |
| Li et al. (2025) [31] | Prospective cohort | AI Iterative Reconstruction (AI-IR) | Low-dose aortic CTA (access assessment) | AI-IR produced higher signal/contrast with least noise at lower dose. | Improves safety and confidence for access planning. |
| Mehier et al. (2024) [32] | Diagnostic cohort | DL classification/CAD exclusion | CCTA in TAVR work-up | Sensitivity 100% and NPV 100% for >50% stenosis; PPV ~39%. | If negative, it may obviate invasive angiography pre-TAVR. |
| Baeßler et al. (2023) [33] | Topical review | Multiple AI tools along pipeline | CCTA (CT-FFR, perfusion, risk scores) | AI assists acquisition→analysis; supports dose reduction and decision-making. | Consolidates best practices for CCTA-led work-up. |
| Agasthi et al. (2021) [34] | Retrospective cohort | Gradient Boosting Machine | Clinical and procedural variables | Outperformed TAVI-2/CoreValve scores for 1-year mortality prediction. | Sharper long-term risk identification. |
| Zisiopoulou et al. (2024) [35] | Pilot cohort | Supervised ML (with ASA, CFS) | Clinical pre-procedural data | AUC ~0.80 for 1-year mortality vs. EuroSCORE II ~0.66; stratified LOS and costs. | Simple clinical metrics enable accessible risk stratification. |
| Sazzad et al. (2024) [36] | Systematic review and meta-analysis | Aggregate across ML/DL approaches | Multi-study, multi-modality | Pooled AUC ~0.79 for mortality; exceeds conventional risk scores. | Evidence base for adopting ML prognostics. |
| Sulaiman et al. (2025) [37] | Systematic review | Multiple ML models | Clinical and imaging across studies | ML consistently superior for survival, pacemaker, MACE; urges external validation. | Synthesizes breadth of applications and gaps. |
| Zaka et al. (2025) [38] | Systematic review and meta-analysis | ML vs. traditional methods | Aggregated cohorts (~30k pts) | ML AUC ≈ 0.79 vs. ~0.68 for conventional scores (in-hospital/30d/1y mortality). | Confirms superiority across time horizons. |
| Lachmann et al. (2022) [39] | Methodologic cohort | Pre-trained CNN (VGG-16) features; unsupervised clustering | Doppler outflow velocity profiles (AS) | Unsupervised separation of flow phenotypes from VGG-16 features. | Supports AI-aided phenotyping beyond imaging morphology. |
| Toggweiler et al. (2024) [40] | Software feasibility | Fully automated AI planning software | CTA (multi-center) | End-to-end planning outputs; feasibility in clinical workflows. | Streamlines planning and standardizes measurements. |
| Shojaei et al. (2025) [41] | Systematic review and meta-analysis | Multiple ML/DL | 43 studies; >360k TAVR pts | Mortality AUC ~0.78; conduction AUC ~0.75; imaging/biomarkers improve models. | Comprehensive evidence of AI’s prognostic value. |
| Yannakula et al. (2025) [42] | Narrative review | Real-time intra-procedural AI guidance | IVUS/OCT/fluoro/echo (concepts) | Landmark detection, device positioning concepts; integration/regulatory hurdles. | Future path for live guidance. |
| Vasileios et al. (2025) [44] | Clinical cohort (no prior LBBB) | ML classifiers (XGBoost, etc.) and LLMs | Clinical pre-implant variables | Persistent LBBB ~15%; XGBoost best; GPT-4 competitive via reasoning prompts. | Risk-flagging to tailor device choice/implant depth/monitoring. |
| Okuno et al. (2021) [45] | Multicenter cohort | DL autoencoder (rare-event prediction) | Clinical and imaging (TAVR) | AUC ~0.79 for 30-day cerebrovascular events despite low incidence. | Enables CVE risk flagging and preventive strategies. |
| Zheng et al. (2021) [46] | CTA DL study | DL feature extraction/classification | CTA (pre-TAVI and complications) | Identified CT image markers predictive of complications beyond standard review. | Sharper risk stratification from CTA. |
| Kurmanaliyev et al. (2025) [47] | Cohort (n = 224) | Random Forest and class imbalance handling; SHAP | Clinical, imaging, and labs | Early 30-day complications predicted; key features: femoral diameter, annulus angle, calcification. | Pre-procedural triage for safety planning. |
| Bamford et al. (2024) [48] | State-of-the-art review | Multiple ML/DL modalities | Screening→planning→procedure | AI stethoscopes, ECG rule-out tools; automated TAVI sizing/placement; early robotics. | Broad overview of maturing use-cases in valve disease. |
| Kwiecinski et al. (2023) [49] | Multicenter registry | Supervised ML (various) | Clinical ± imaging | Improved 1-year mortality discrimination vs. conventional scores. | Supports ML adoption for routine outcomes. |
| Jacquemyn et al. (2025) [50] | Systematic review and meta-analysis | ML prognostics (multiple methods) | Aggregated literature | Strong performance, but reproducibility/reporting limitations impede translation. | Roadmap for better reporting/validation. |
| Herrero-Brocal et al. (2025) [51] | Program evaluation | AI-supported telemonitoring model (TeleTAVI) | Remote monitoring data | Enabled early discharge with close follow-up; feasibility and safety were described. | Resource optimization and patient monitoring. |
| Scuoppo et al. (2025) [52] | Modeling/in silico | Statistical shape modeling and ML | CT (aortic root with calcification) | Generated virtual TAVI cohort resembling real anatomies; accurate sizing and gradient estimation. | Accelerates testing and planning with synthetic anatomies. |
3.3.3. Multimodal and Biomarker-Integrated Risk
3.4. Emerging Directions: XR, Digital Twins, Virtual Cohorts, and Real-Time Guidance
3.4.1. XR-Assisted Planning and Team Decision-Making
3.4.2. Virtual Cohorts and In Silico Evaluation
3.4.3. Toward Intra-Procedural AI
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zhang, S.; Zhang, X.; Xiong, J.; Han, X.; Wu, Z.; Zhao, D.; Li, Y.; Xu, Y.; Chen, D. Fast Virtual Stenting for Thoracic Endovascular Aortic Repair of Aortic Dissection Using Graph Deep Learning. IEEE J. Biomed. Health Inform. 2025, 29, 4374–4387. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Niu, G.; Chen, Y.; Zhou, Z.; Feng, D.; Zhang, Y.; Wu, Y.; Song, G.; Zhang, H.; Zhou, D.; et al. Development and Validation of a Deep Learning-Based Fully Automated Algorithm for Pre-TAVR CT Assessment of the Aortic Valvular Complex and Detection of Anatomical Risk Factors: A Retrospective, Multicentre Study. EBioMedicine 2023, 96, 104794. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, I.; Sayah, N.; Benamer, H.; Amabile, N.; Laforgia, P.; Champagne, S.; Hovasse, T.; Garot, J.; Garot, P.; Akodad, M. Artificial Intelligence and Extended Reality in TAVR: Current Applications and Challenges. Trends Cardiovasc. Med. 2025; in press. [Google Scholar] [CrossRef]
- Windecker, S.; Tomii, D. Planning for the Future: CT-Based Insights into Redo-TAVR and Lifetime Aortic Valve Care. JACC Cardiovasc. Interv. 2025, 18, 1186–1189. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Zhang, E.; Wu, Y. Artificial Intelligence in the Screening, Diagnosis, and Management of Aortic Stenosis. Rev. Cardiovasc. Med. 2024, 25, 31. [Google Scholar] [CrossRef]
- Watson, X.; D’Souza, J.; Cooper, D.; Markham, R. Artificial Intelligence in Cardiology: Fundamentals and Applications. Intern. Med. J. 2022, 52, 912–920. [Google Scholar] [CrossRef]
- Henein, M.; El-Baz, A.; Benjamin, M.M.; Rabbat, M.G. Artificial Intelligence in Transcatheter Aortic Valve Replacement: Its Current Role and Ongoing Challenges. Diagnostics 2024, 14, 261. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, Y.; Lv, J.; Li, J.; Liu, J. Artificial Intelligence-Based Spiral CT 3D Reconstruction in Transcatheter Aortic Valve Implantation. Comput. Math. Methods Med. 2022, 2022, 5794681. [Google Scholar] [CrossRef]
- Saitta, S.; Sturla, F.; Gorla, R.; Oliva, O.A.; Votta, E.; Bedogni, F.; Redaelli, A. A CT-Based Deep Learning System for Automatic Assessment of Aortic Root Morphology for TAVI Planning. Comput. Biol. Med. 2023, 163, 107147. [Google Scholar] [CrossRef]
- Krüger, N.; Meyer, A.; Tautz, L.; Hüllebrand, M.; Wamala, I.; Pullig, M.; Kofler, M.; Kempfert, J.; Sündermann, S.; Falk, V.; et al. Cascaded Neural Network-Based CT Image Processing for Aortic Root Analysis. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 507–519. [Google Scholar] [CrossRef]
- Boeckling, F.; Rasper, T.; Zanders, L.; Pergola, G.; Cremer, S.; Mas-Peiro, S.; Vasa-Nicotera, M.; Leistner, D.; Dimmeler, S.; Kattih, B. Extracellular Matrix Proteins Improve Risk Prediction in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2025, 14, 37296. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, B.; Schütze, J.; Leib, Z.L.; Spano, G.; Berto, M.B.; Bakula, A.; Tomii, D.; Shiri, I.; Brugger, N.; De Marchi, S.; et al. Myocardial Analysis from Routine 4D Cardiac-CT to Predict Reverse Remodeling and Clinical Outcomes after Transcatheter Aortic Valve Implantation. Eur. J. Radiol. 2024, 175, 111425. [Google Scholar] [CrossRef]
- Boninsegna, E.; Piffer, S.; Simonini, E.; Romano, M.; Lettieri, C.; Colopi, S.; Barai, G. CT Angiography Prior to Endovascular Procedures: Can Artificial Intelligence Improve Reporting? Phys. Eng. Sci. Med. 2024, 47, 643–649. [Google Scholar] [CrossRef]
- Tremamunno, G.; Vecsey-Nagy, M.; Schoepf, U.J.; Zsarnoczay, E.; Aquino, G.J.; Kravchenko, D.; Laghi, A.; Jacob, A.; Sharma, P.; Rapaka, S.; et al. Artificial Intelligence Improves Prediction of Major Adverse Cardiovascular Events in Patients Undergoing Transcatheter Aortic Valve Replacement Planning CT. Acad. Radiol. 2025, 32, 702–711. [Google Scholar] [CrossRef]
- Sun, S.; Yeh, L.; Imanzadeh, A.; Kooraki, S.; Kheradvar, A.; Bedayat, A. The Current Landscape of Artificial Intelligence in Imaging for Transcatheter Aortic Valve Replacement. Curr. Radiol. Rep. 2024, 12, 113–120. [Google Scholar] [CrossRef]
- Cadour, F.; Dacher, J.N. When Artificial Intelligence Meets Photon-Counting Coronary CT Angiography to Reduce the Need for Invasive Coronary Angiography in TAVR Candidates. Diagn. Interv. Imaging 2024, 105, 243–244. [Google Scholar] [CrossRef]
- Brendel, J.M.; Walterspiel, J.; Hagen, F.; Kübler, J.; Paul, J.F.; Nikolaou, K.; Gawaz, M.; Greulich, S.; Krumm, P.; Winkelmann, M. Coronary Artery Disease Evaluation during Transcatheter Aortic Valve Replacement Work-up Using Photon-Counting CT and Artificial Intelligence. Diagn. Interv. Imaging 2024, 105, 273–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Cheng, Y.; Wang, Z.; Li, Z.; Li, J.; Shuai, T. Deep Learning Image Reconstruction for Transcatheter Aortic Valve Implantation Planning: Image Quality, Diagnostic Performance, Contrast Volume and Radiation Dose Assessment. Acad. Radiol. 2024, 31, 2268–2280. [Google Scholar] [CrossRef]
- Rouhollahi, A.; Willi, J.N.; Haltmeier, S.; Mehrtash, A.; Straughan, R.; Javadikasgari, H.; Brown, J.; Itoh, A.; de la Cruz, K.I.; Aikawa, E.; et al. CardioVision: A Fully Automated Deep Learning Package for Medical Image Segmentation and Reconstruction Generating Digital Twins for Patients with Aortic Stenosis. Comput. Med. Imaging Graph. 2023, 109, 102289. [Google Scholar] [CrossRef] [PubMed]
- Pekař, M.; Jiravský, O.; Novák, J.; Branny, P.; Balušík, J.; Daniš, D.; Hečko, J.; Kantor, M.; Prosecky, R.; Blaha, L.; et al. Sarcopenia and Adipose Tissue Evaluation by Artificial Intelligence Predicts the Overall Survival after TAVI. Sci. Rep. 2024, 14, 8842. [Google Scholar] [CrossRef] [PubMed]
- van Erck, D.; Moeskops, P.; Schoufour, J.D.; Weijs, P.J.M.; Scholte op Reimer, W.J.M.; van Mourik, M.S.; Planken, R.N.; Vis, M.M.; Baan, J.; Išgum, I.; et al. Low Muscle Quality on a Procedural Computed Tomography Scan Assessed with Deep Learning as a Practical Useful Predictor of Mortality in Patients with Severe Aortic Valve Stenosis. Clin. Nutr. ESPEN 2024, 63, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Zsarnoczay, E.; Varga-Szemes, A.; Schoepf, U.J.; Rapaka, S.; Pinos, D.; Aquino, G.J.; Fink, N.; Vecsey-Nagy, M.; Tremamunno, G.; Kravchenko, D.; et al. Predicting Mortality after Transcatheter Aortic Valve Replacement Using AI-Based Fully Automated Left Atrioventricular Coupling Index. J. Cardiovasc. Comput. Tomogr. 2025, 19, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Paukovitsch, M.; Fechner, T.; Felbel, D.; Moerike, J.; Rottbauer, W.; Klömpken, S.; Brunner, H.; Kloth, C.; Beer, M.; Sekuboyina, A.; et al. Opportunistic Computed Tomography (CT) Assessment of Osteoporosis in Patients Undergoing Transcatheter Aortic Valve Replacement (TAVR). Arch. Osteoporos. 2025, 20, 100. [Google Scholar] [CrossRef]
- Weferling, M.; Rolf, A.; Fischer-Rasokat, U.; Liebetrau, C.; Renker, M.; Choi, Y.H.; Hamm, C.W.; Dey, D.; Kim, W.K. Epicardial Fat Volume Is Associated with Preexisting Atrioventricular Conduction Abnormalities and Increased Pacemaker Implantation Rate in Patients Undergoing Transcatheter Aortic Valve Implantation. Int. J. Cardiovasc. Imaging 2022, 38, 1399–1406. [Google Scholar] [CrossRef]
- Dasi, A.; Lee, B.; Polsani, V.; Yadav, P.; Dasi, L.P.; Thourani, V.H. Predicting Pressure Gradient Using Artificial Intelligence for Transcatheter Aortic Valve Replacement. JTCVS Tech. 2023, 23, 5–17. [Google Scholar] [CrossRef]
- Busto, L.; Veiga, C.; González-Nóvoa, J.A.; Campanioni, S.; Juan-Salvadores, P.; Jiménez Díaz, V.A.; Baz, J.A.; Alba-Castro, J.L.; Kütting, M.; Íñiguez, A. Automatic Assessment of Transcatheter Aortic Valve Implantation Results on Four-Dimensional Computed Tomography Images Using Artificial Intelligence. Bioengineering 2023, 10, 1206. [Google Scholar] [CrossRef]
- Busto, L.; Veiga, C.; González-Nóvoa, J.A.; Campanioni, S.; Martínez, C.; Juan-Salvadores, P.; Jiménez, V.; Suárez, S.; López-Campos, J.Á.; Segade, A.; et al. Automated Transcatheter Heart Valve 4DCT-Based Deformation Assessment throughout the Cardiac Cycle: Towards Enhanced Long-Term Durability. Int. J. Med. Inform. 2025, 203, 105991. [Google Scholar] [CrossRef]
- Kojima, T.; Yamasaki, Y.; Matsuura, Y.; Mikayama, R.; Shirasaka, T.; Kondo, M.; Kamitani, T.; Kato, T.; Ishigami, K.; Yabuuchi, H. The Feasibility of Deep Learning-Based Reconstruction for Low-Tube-Voltage CT Angiography for Transcatheter Aortic Valve Implantation. J. Comput. Assist. Tomogr. 2024, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Zhou, Y.; Li, J.; Zhang, G.; Li, K.; Ding, Z.; Zhou, R.; Zhang, J.; Zheng, X.; Du, Y. Transcatheter Aortic Valve Implantation (TAVI) Planning CT on 8-Cm Detector Scanners: Proper Dose Control by Combined Use of Two Deep-Learning Reconstruction Algorithms. J. Appl. Clin. Med. Phys. 2025, 26, e70224. [Google Scholar] [CrossRef] [PubMed]
- Vaitkus, P.T.; Wang, D.D.; Greenbaum, A.; Guerrero, M.; O’Neill, W. Assessment of a Novel Software Tool in the Selection of Aortic Valve Prosthesis Size for Transcatheter Aortic Valve Replacement. J. Invasive Cardiol. 2014, 26, 328–332. [Google Scholar]
- Li, Q.; Liu, D.; Li, K.; Li, J.; Zhou, Y. Artificial Intelligence Iterative Reconstruction Algorithm Combined with Low-Dose Aortic CTA for Preoperative Access Assessment of Transcatheter Aortic Valve Implantation: A Prospective Cohort Study. J. Imaging Inform. Med. 2025, 1–15. [Google Scholar] [CrossRef]
- Mehier, B.; Mahmoudi, K.; Veugeois, A.; Masri, A.; Amabile, N.; Del Giudice, C.; Paul, J.F. Diagnostic Performance of Deep Learning to Exclude Coronary Stenosis on CT Angiography in TAVI Patients. Int. J. Cardiovasc. Imaging 2024, 40, 981–990. [Google Scholar] [CrossRef]
- Baeßler, B.; Götz, M.; Antoniades, C.; Heidenreich, J.F.; Leiner, T.; Beer, M. Artificial Intelligence in Coronary Computed Tomography Angiography: Demands and Solutions from a Clinical Perspective. Front. Cardiovasc. Med. 2023, 10, 1120361. [Google Scholar] [CrossRef] [PubMed]
- Agasthi, P.; Ashraf, H.; Pujari, S.H.; Girardo, M.E.; Tseng, A.; Mookadam, F.; Venepally, N.R.; Buras, M.; Khetarpal, B.K.; Allam, M.; et al. Artificial Intelligence Trumps TAVI2-SCORE and CoreValve Score in Predicting 1-Year Mortality Post-Transcatheter Aortic Valve Replacement. Cardiovasc. Revascularization Med. 2021, 24, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zisiopoulou, M.; Berkowitsch, A.; Redlich, L.; Walther, T.; Fichtlscherer, S.; Leistner, D.M. Personalised Preinterventional Risk Stratification of Mortality, Length of Stay and Hospitalisation Costs in Transcatheter Aortic Valve Implantation Using a Machine Learning Algorithm: A Pilot Trial. Open Heart 2024, 11, 2540. [Google Scholar] [CrossRef] [PubMed]
- Sazzad, F.; Ler, A.A.L.; Furqan, M.S.; Tan, L.K.Z.; Leo, H.L.; Kuntjoro, I.; Tay, E.; Kofidis, T. Harnessing the Power of Artificial Intelligence in Predicting All-Cause Mortality in Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2024, 11, 1343210. [Google Scholar] [CrossRef]
- Sulaiman, R.; Atick Faisal, M.A.; Hasan, M.; Chowdhury, M.E.H.; Bensaali, F.; Alnabti, A.; Yalcin, H.C. Machine Learning for Predicting Outcomes of Transcatheter Aortic Valve Implantation: A Systematic Review. Int. J. Med. Inform. 2025, 197, 105840. [Google Scholar] [CrossRef]
- Zaka, A.; Mustafiz, C.; Mutahar, D.; Sinhal, S.; Gorcilov, J.; Muston, B.; Evans, S.; Gupta, A.; Stretton, B.; Kovoor, J.; et al. Machine-Learning versus Traditional Methods for Prediction of All-Cause Mortality after Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-Analysis. Open Heart 2025, 12, e002779. [Google Scholar] [CrossRef]
- Lachmann, M.; Rippen, E.; Rueckert, D.; Schuster, T.; Xhepa, E.; Von Scheidt, M.; Pellegrini, C.; Trenkwalder, T.; Rheude, T.; Stundl, A.; et al. Harnessing Feature Extraction Capacities from a Pre-Trained Convolutional Neural Network (VGG-16) for the Unsupervised Distinction of Aortic Outflow Velocity Profiles in Patients with Severe Aortic Stenosis. Eur. Heart J. Digit. Health 2022, 3, 153–168. [Google Scholar] [CrossRef]
- Toggweiler, S.; Wyler von Ballmoos, M.C.; Moccetti, F.; Douverny, A.; Wolfrum, M.; Imamoglu, Z.; Mohler, A.; Gülan, U.; Kim, W.K. A Fully Automated Artificial Intelligence-Driven Software for Planning of Transcatheter Aortic Valve Replacement. Cardiovasc. Revascularization Med. 2024, 65, 25–31. [Google Scholar] [CrossRef]
- Shojaei, S.; Mousavi, A.; Kazemian, S.; Armani, S.; Maleki, S.; Fallahtafti, P.; Arashlow, F.T.; Daryabari, Y.; Naderian, M.; Alkhouli, M.; et al. Artificial Intelligence in Risk Stratification and Outcome Prediction for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. J. Pers. Med. 2025, 15, 302. [Google Scholar] [CrossRef]
- Yannakula, V.K.; Alluri, A.A.; Samuel, D.; Popoola, S.A.; Barake, B.A.; Alabbasi, A.; Ahmed, A.S.; Cortes Bandy, D.A.; Jesi, N.J. The Role of Artificial Intelligence in Providing Real-Time Guidance During Interventional Cardiology Procedures: A Narrative Review. Cureus 2025, 17, e83464. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Lombardo, M.; Baravelli, M.; Trotta, G.; Sommese, C.; Anzà, C. Exercise Stress Echocardiography with Tissue Doppler Imaging in Risk Stratification of Mild to Moderate Aortic Stenosis. Int. J. Cardiovasc. Imaging 2015, 31, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Vasileios, C.; Giorgos, F.; Antonios, M.; Anna, K. AI-Based Prediction of Left Bundle Branch Block Risk Post-TAVI Using Pre-Implantation Clinical Parameters. Future Cardiol. 2025, 21, 489–494. [Google Scholar] [CrossRef]
- Okuno, T.; Overtchouk, P.; Asami, M.; Tomii, D.; Stortecky, S.; Praz, F.; Lanz, J.; Siontis, G.C.M.; Gräni, C.; Windecker, S.; et al. Deep Learning-Based Prediction of Early Cerebrovascular Events after Transcatheter Aortic Valve Replacement. Sci. Rep. 2021, 11, 18754. [Google Scholar] [CrossRef]
- Zheng, X.; Qian, Z.; Wang, X.; Zhang, Z.; Liu, L. CT Image Feature Diagnosis on the Basis of Deep Learning Algorithm for Preoperative Patients and Complications of Transcatheter Aortic Valve Implantation. J. Healthc. Eng. 2021, 2021, 9734612. [Google Scholar] [CrossRef]
- Kurmanaliyev, A.; Sutiene, K.; Braukylienė, R.; Aldujeli, A.; Jurenas, M.; Kregzdyte, R.; Braukyla, L.; Zhumagaliyev, R.; Aitaliyev, S.; Zhanabayev, N.; et al. An Integrative Machine Learning Model for Predicting Early Safety Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Medicina 2025, 61, 374. [Google Scholar] [CrossRef]
- Bamford, P.; Abdelrahman, A.; Malkin, C.J.; Cunnington, M.S.; Blackman, D.J.; Ali, N. Artificial Intelligence in Heart Valve Disease: Diagnosis, Innovation and Treatment. A State-of-the-Art Review. Br. J. Cardiol. 2024, 31, 92–97. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Dabrowski, M.; Nombela-Franco, L.; Grodecki, K.; Pieszko, K.; Chmielak, Z.; Pylko, A.; Hennessey, B.; Kalinczuk, L.; Tirado-Conte, G.; et al. Machine Learning for Prediction of All-Cause Mortality after Transcatheter Aortic Valve Implantation. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 768–777. [Google Scholar] [CrossRef]
- Jacquemyn, X.; Van Onsem, E.; Dufendach, K.; Brown, J.A.; Kliner, D.; Toma, C.; Serna-Gallegos, D.; Sá, M.P.; Sultan, I. Machine-Learning Approaches for Risk Prediction in Transcatheter Aortic Valve Implantation: Systematic Review and Meta-Analysis. J. Thorac. Cardiovasc. Surg. 2025, 169, 1460–1470.e15. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Brocal, M.; Samper, R.; Riquelme, J.; Pineda, J.; Bordes, P.; Torres-Mezcua, F.; Valencia, J.; Torres-Saura, F.; Manso, M.G.; Ajo, R.; et al. Early Discharge Programme after Transcatheter Aortic Valve Implantation Based on Close Follow-up Supported by Telemonitoring Using Artificial Intelligence: The TeleTAVI Study. Eur. Heart J. Digit. Health 2025, 6, 73–81. [Google Scholar] [CrossRef]
- Scuoppo, R.; Castelbuono, S.; Cannata, S.; Gentile, G.; Agnese, V.; Bellavia, D.; Gandolfo, C.; Pasta, S. Generation of a Virtual Cohort of TAVI Patients for in Silico Trials: A Statistical Shape and Machine Learning Analysis. Med. Biol. Eng. Comput. 2025, 63, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, A.; Zito, E.; Pierucci, N.; Matteucci, A.; La Fazia, V.M. A Talk with ChatGPT: The Role of Artificial Intelligence in Shaping the Future of Cardiology and Electrophysiology. J. Pers. Med. 2025, 15, 205. [Google Scholar] [CrossRef] [PubMed]
| Algorithm Type | Representative Models/Methods | Main Application | Data Type Used | Performance Highlights/Key Findings |
|---|---|---|---|---|
| Deep Learning (DL) | CNNs, U-Net, VGG-16, Autoencoders | Automated CT segmentation, 3D/4D reconstruction, landmark detection | CT/4D-CT | Accuracy up to 0.98; sub-mm precision; reduced analysis time (<30 s); improved valve accuracy (~90%) |
| Machine Learning (ML) | Random Forest, Gradient Boosting XGBoost, SVM | Prediction of mortality, conduction abnormalities complications (stroke, pacemaker, bleeding) | Clinical +Imaging+ Biomarker data | AUC 0.78–0.90; superior to EuroSCORE II/STS-PROM in short-and long term prediction |
| Hybrid/Multimodal Models | Combined ML+ imaging/ biomarker integration | Risk stratification and outcome prediction using clinical+ CT+ biomarker data | Clinical+ Imaging+ Biomarkers data | TIMP-1 + hs-cTnT model outperformed STS-PROM for 2-year mortality enhanced prognostic power |
| Explainable ML models | SHAP-enhanced Random Forest, feature selection | Identification of drivers of early complication (bleeding, vascular injury) | Clinical+ Imaging | Highlighted key anatomical predictors (femoral diameter, annular angle, calcification) |
| Digital Twin & Simulation Models | CardioVision, Statistical Shape Modeling | Patient-specific 3D reconstruction and procedural simulation | CT Angiography | 40–50% dose reduction with preserved or improved diagnostic accuracy |
| LLMs/NLP Models | GPT-4, reasoning-prompted models | Structural clinical prediction, post-TAVI conduction outcomes | Clinical Variables | Comparable or superior to classical ML for LBBB prediction; supports reasoning-based risk forecasting |
| XR/Augmented Reality Integration | AI-assisted VR/AR visualization | Procedural planning and Heart Team simulation | CT + XR data | Enhanced MDT communication and complex anatomy assessment; requires further validation |
| AI Reconstruction Algorithms | Deep Learning Reconstruction (DLR), AI Iterative Reconstruction (AI-IR) | Image quality enhancement, radiation dose reduction | CT Angiography | 40-50% dose reduction with preserved or improved diagnostic accuracy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakirian, F.; Afendoulis, D.; Mavroudis, A.; Aghayan, S.; Drakopoulou, M.; Synetos, A.; Tsalamandris, S.; Tsioufis, K.; Vlachakis, P.; Toutouzas, K. Applications of Artificial Intelligence in Transcatheter Aortic Valve Replacement: A Review of the Literature. Life 2025, 15, 1724. https://doi.org/10.3390/life15111724
Tsakirian F, Afendoulis D, Mavroudis A, Aghayan S, Drakopoulou M, Synetos A, Tsalamandris S, Tsioufis K, Vlachakis P, Toutouzas K. Applications of Artificial Intelligence in Transcatheter Aortic Valve Replacement: A Review of the Literature. Life. 2025; 15(11):1724. https://doi.org/10.3390/life15111724
Chicago/Turabian StyleTsakirian, Flora, Dimitrios Afendoulis, Andreas Mavroudis, Svetlana Aghayan, Maria Drakopoulou, Andreas Synetos, Sotirios Tsalamandris, Konstantinos Tsioufis, Panayotis Vlachakis, and Konstantinos Toutouzas. 2025. "Applications of Artificial Intelligence in Transcatheter Aortic Valve Replacement: A Review of the Literature" Life 15, no. 11: 1724. https://doi.org/10.3390/life15111724
APA StyleTsakirian, F., Afendoulis, D., Mavroudis, A., Aghayan, S., Drakopoulou, M., Synetos, A., Tsalamandris, S., Tsioufis, K., Vlachakis, P., & Toutouzas, K. (2025). Applications of Artificial Intelligence in Transcatheter Aortic Valve Replacement: A Review of the Literature. Life, 15(11), 1724. https://doi.org/10.3390/life15111724







