Association Between Severe Periodontitis and Cognitive Decline in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Periodontal Examination
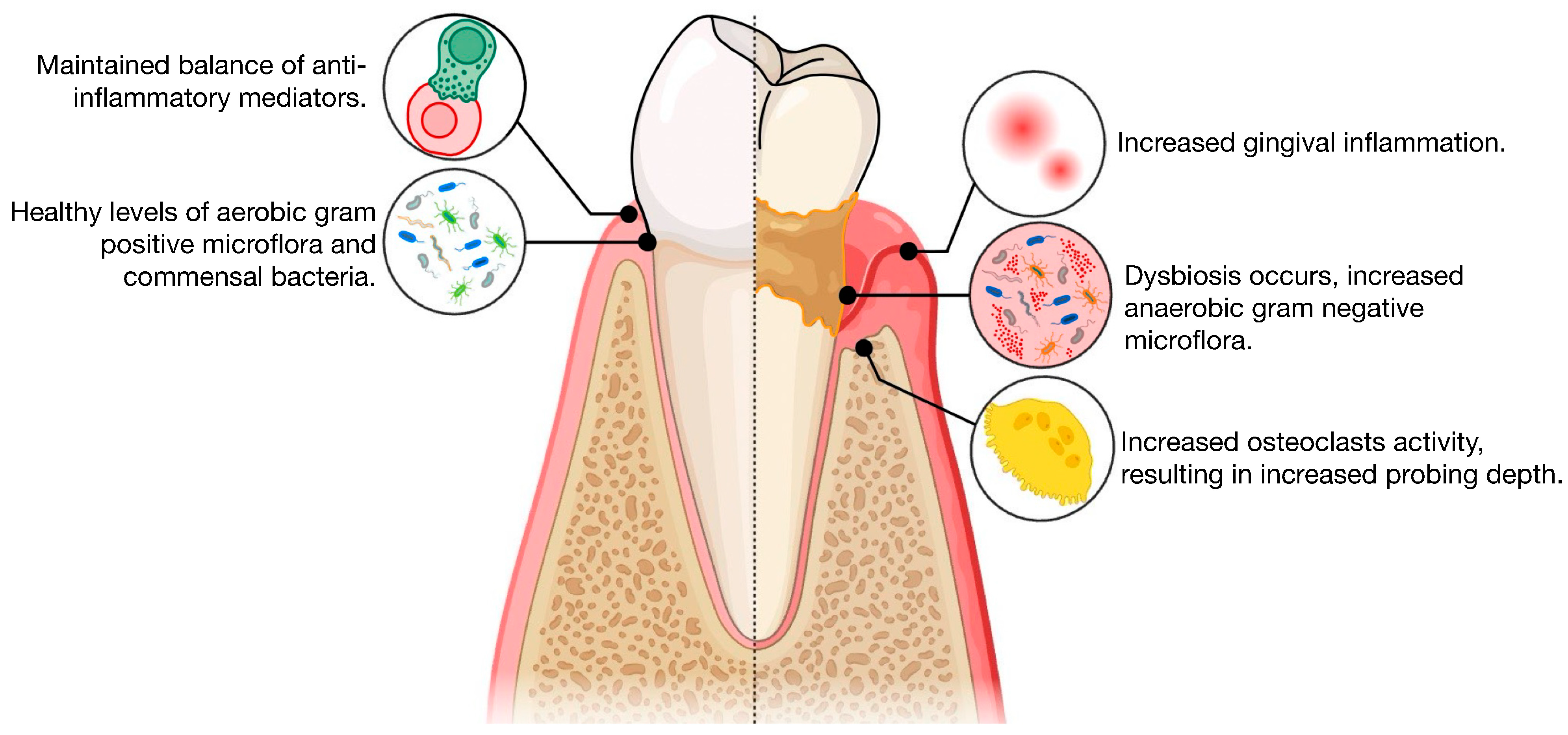
2.3. Definition of the Dependent Variable: Severe Periodontitis
2.4. Description of Independent Variable: Cognitive Function
2.5. The Effect Modifier Variable
2.6. The Potential Confounding Variables
2.7. Statistical Method
3. Results
4. Discussion
4.1. Limitation of the Research
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Razani, J.; Casas, R.; Wong, J.T.; Lu, P.; Alessi, C.; Josephson, K. Relationship between executive functioning and activities of daily living in patients with relatively mild dementia. Appl. Neuropsychol. 2007, 14, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Subjective Cognitive Decline—A Public Health Issue; Department of Health and Human Services, Alzheimer’s Disease and Healthy Aging; CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Ijaopo, E.O. Dementia-related agitation: A review of non-pharmacological interventions and analysis of risks and benefits of pharmacotherapy. Transl. Psychiatry 2017, 7, e1250. [Google Scholar] [CrossRef] [PubMed]
- Ampadu, J.; Morley, J.E. Heart failure and cognitive dysfunction. Int. J. Cardiol. 2015, 178, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. The complexities of diabetes in older persons. J. Am. Med. Dir. Assoc. 2016, 17, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Bouldin, E.D.; Greenlund, K.J.; McGuire, L.C. Comorbid chronic conditions among older adults with subjective cognitive decline, United States, 2015–2017. Innov. Aging 2020, 4, igz045. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.; Stephen, R.; Mäntylä, P.; Suominen, A.L.; Solomon, A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J. Am. Geriatr. Soc. 2022, 70, 2695–2709. [Google Scholar] [CrossRef]
- Chapple, I.L.; Genco, R. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S106–S112. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Hashioka, S.; Inoue, K.; Hayashida, M.; Wake, R.; Oh-Nishi, A.; Miyaoka, T. Implications of systemic inflammation and periodontitis for major depression. Front. Neurosci. 2018, 12, 483. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.; Gul, S.S.; Sha, A.; Chapple, I.L. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef]
- Chi, L.; Cheng, X.; Lin, L.; Yang, T.; Sun, J.; Feng, Y.; Liang, F.; Pei, Z.; Teng, W. Porphyromonas gingivalis-induced cognitive impairment is associated with gut dysbiosis, neuroinflammation, and glymphatic dysfunction. Front. Cell. Infect. Microbiol. 2021, 11, 755925. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Zhang, N.; Yang, X.; Zheng, X.; Li, L. Elevated serum alkaline phosphatase is associated with the presence and severity of dementia. J. Alzheimers Dis. 2019, 69, 465–473. [Google Scholar] [CrossRef]
- Jain, A.; Batista, E.L., Jr. Serum alkaline phosphatase as a marker of chronic periodontitis: A clinical and biochemical study. J. Clin. Diagn. Res. 2010, 4, 2511–2516. [Google Scholar]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.D.M.E.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part 1. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar]
- Strauss, E.; Sherman, E.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- National Center for Health Statistics. (National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 20 October 2024).
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Rasaei, N.; Ghadiri, A.; Peighan, M.; Rekabi, A.; Atashkar, N. Evaluation of alkaline phosphatase in gingival crevicular fluid and saliva of patients with periodontitis and healthy individuals. J. Fam. Med. Prim. Care 2022, 11, 6983–6987. [Google Scholar] [CrossRef]
- Sanikop, S.; Patil, S.; Agrawal, P. Gingival crevicular fluid alkaline phosphatase as a potential diagnostic marker of periodontal disease. J. Indian Soc. Periodontol. 2012, 16, 513–518. [Google Scholar]
- Koppolu, P.; Sirisha, S.; Mishra, A.; Deshpande, K.; Lingam, A.S.; Alotaibi, D.H.; Alwahibi, M.S.; Penela, S. Alkaline phosphatase and acid phosphatase levels in saliva and serum of patients with healthy periodontium, gingivitis, and periodontitis before and after scaling with root planing: A clinico-biochemical study. Saudi J. Biol. Sci. 2021, 28, 380–385. [Google Scholar] [CrossRef]
- Perinetti, G.; Paolantonio, M.; Femminella, B.; Serra, E.; Spoto, G. Gingival crevicular fluid alkaline phosphatase activity reflects periodontal healing/recurrent inflammation phases in chronic periodontitis patients. J. Periodontol. 2008, 79, 1200–1207. [Google Scholar] [CrossRef]
- Wadia, R. Periodontitis and cognitive decline. Br. Dent. J. 2023, 235, 405. [Google Scholar] [CrossRef]
- Sung, C.E.; Huang, R.Y.; Cheng, W.C.; Kao, T.W.; Chen, W.L. Association between periodontitis and cognitive impairment: Analysis of national health and nutrition examination survey (NHANES) III. J. Clin. Periodontol. 2019, 46, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Marruganti, C.; Baima, G.; Aimetti, M.; Grandini, S.; Sanz, M.; Romandini, M. Periodontitis and low cognitive performance: A population-based study. J. Clin. Periodontol. 2023, 50, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chang, S.; Pi, X.; Hua, F.; Jiang, H.; Liu, C.; Du, M. The effect of periodontitis on dementia and cognitive impairment: A meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 6823. [Google Scholar] [CrossRef]
- Pike, A.F.; Kramer, N.I.; Blaauboer, B.J.; Seinen, W.; Brands, R. An alkaline phosphatase transport mechanism in the pathogenesis of Alzheimer’s disease and neurodegeneration. Chem.-Biol. Interact. 2015, 226, 30–39. [Google Scholar] [CrossRef]
- Díaz-Hernández, M.; Gómez-Ramos, A.; Rubio, A.; Gómez-Villafuertes, R.; Naranjo, J.R.; Miras-Portugal, M.T.; Avila, J. Tissue-nonspecific alkaline phosphatase promotes the neurotoxicity effect of extracellular tau. J. Biol. Chem. 2010, 285, 32539–32548. [Google Scholar] [CrossRef]
- Bhide, A.; Sen, A. Interactions with amyloid beta peptide and acetylcholinesterase increase alkaline phosphatase activity. Phys. Chem. Chem. Phys. 2023, 25, 21149–21153. [Google Scholar] [CrossRef]
- Liccardo, D.; Marzano, F.; Carraturo, F.; Guida, M.; Femminella, G.D.; Bencivenga, L.; Agrimi, J.; Addonizio, A.; Melino, I.; Valletta, A.; et al. Potential bidirectional relationship between periodontitis and Alzheimer’s disease. Front. Physiol. 2020, 11, 683. [Google Scholar] [CrossRef]
- Kantarci, A.; Tognoni, C.M.; Yaghmoor, W.; Marghalani, A.; Stephens, D.; Ahn, J.Y.; Carreras, I.; Dedeoglu, A. Microglial response to experimental periodontitis in a murine model of Alzheimer’s disease. Sci. Rep. 2020, 10, 18561. [Google Scholar] [CrossRef]
- Rubio, M.D.C.; Rudzinski, J.J.; Ramos, C.; Lifshitz, F.; Friedman, S.M.; Nicolosi, L.N. Cognitive impairment related to arterial stiffness in cardiovascular disease patients with severe periodontitis. Acta Odontol. Latinoam. 2020, 33, 200–208. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qiu, W.; Zhu, X.; Li, X.; Xie, Z.; Carreras, I.; Dedeoglu, A.; Van Dyke, T.; Han, Y.W.; Karimbux, N.; et al. The periodontal pathogen Fusobacterium nucleatum exacerbates Alzheimer’s pathogenesis via specific pathways. Front. Aging Neurosci. 2022, 14, 912709. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Bubba, V.; Murasecco, I.; Pigliautile, M.; Monastero, R.; Cecchetti, R.; Scamosci, M.; Bastiani, P.; Mecocci, P.; ReGAL 2.0 Study Group. Serum alkaline phosphatase is elevated and inversely correlated with cognitive functions in subjective cognitive decline: Results from the ReGAl 2.0 project. Aging Clin. Exp. Res. 2021, 33, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Kellett, K.A.; Williams, J.; Vardy, E.R.; Smith, A.D.; Hooper, N.M. Plasma alkaline phosphatase is elevated in Alzheimer’s disease and inversely correlates with cognitive function. Int. J. Mol. Epidemiol. Genet. 2011, 2, 114. [Google Scholar]
- Grenier, D.; Chen, H.; Ben Lagha, A.; Fournier-Larente, J.; Morin, M.P. Dual Action of Myricetin on Porphyromonas gingivalis and the Inflammatory Response of Host Cells: A Promising Therapeutic Molecule for Periodontal Diseases. PLoS ONE 2015, 10, e0131758. [Google Scholar] [CrossRef]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef]
- Miklossy, J. Alzheimer’s disease-A neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J. Neuroinflamm. 2011, 8, 90. [Google Scholar] [CrossRef]
- Yan, C.; Diao, Q.; Zhao, Y.; Zhang, C.; He, X.; Huang, R.; Li, Y. Fusobacterium nucleatum infection-induced neurodegeneration and abnormal gut microbiota composition in Alzheimer’s disease-like rats. Front. Neurosci. 2022, 16, 884543. [Google Scholar] [CrossRef]
- Johansson, A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011, 3, 242–259. [Google Scholar] [CrossRef]
- Haubek, D. The highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans: Evolutionary aspects, epidemiology and etiological role in aggressive periodontitis. APMIS Suppl. 2010, 130, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.P.; Ho, Y.S.; Leung, W.K.; Goto, T.; Chang, R.C. Systemic inflammation linking chronic periodontitis to cognitive decline. Brain Behav. Immun. 2019, 81, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; McKiernan, M.; Gunsolley, J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: Longitudinal cohort study of initially healthy adolescents. J. Clin. Microbiol. 2007, 45, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, N.; Capristo, E.; Taveira, T.H.; Mingrone, G.; Wu, W.C. Cognitive function in individuals with normal weight obesity: Results from the Third National Health and Nutrition Examination Survey (NHANES III). J. Alzheimers Dis. 2018, 65, 125–135. [Google Scholar] [CrossRef]
- Seo, S.W.; Gottesman, R.F.; Clark, J.M.; Hernaez, R.; Chang, Y.; Kim, C.; Ha, K.H.; Guallar, E.; Lazo, M. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology 2016, 86, 1136–1142. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale (WAIS–III), 3rd ed.; Psychological Corporation: New York, NY, USA, 1987. [Google Scholar]
- Said-Sadier, N.; Sayegh, B.; Farah, R.; Abbas, L.A.; Dweik, R.; Tang, N.; Ojcius, D.M. Association between periodontal disease and cognitive impairment in adults. Int. J. Environ. Res. Public Health 2023, 20, 4707. [Google Scholar] [CrossRef]
- Tsukasaki, M. RANKL and osteoimmunology in periodontitis. J. Bone Miner. Metab. 2021, 39, 82–90. [Google Scholar] [CrossRef]
- López Roldán, A.; García Giménez, J.L.; Alpiste Illueca, F. Impact of periodontal treatment on the RANKL/OPG ratio in crevicular fluid. PLoS ONE 2020, 15, e0227757. [Google Scholar] [CrossRef]
- Costa, L.C.; Fonseca MA, D.; Pinheiro AD, R.; Aguiar TR, D.S.; Machado, A.N.; Quinelato, V.; Bonato, L.L.; Aguiar, D.P.; Vieira, T.; Almeida FL, D.; et al. Chronic Periodontitis and RANKL/OPG Ratio in Peri-Implant Mucosae Inflammation. Braz. Dent. J. 2018, 29, 14–22. [Google Scholar] [CrossRef]
- Mousa, S.O.; Abd El-Hafez, A.H.; Abu El-Ela, M.A.; Mourad, M.A.; Saleh, R.N.; Sayed, S.Z. RANK/RANKL/OPG axis genes relation to cognitive impairment in children with transfusion-dependent thalassemia: A cross-sectional study. BMC Pediatr. 2022, 22, 435. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Lin, Y.T.; Chen, C.S.; Chiu, Y.W.; Tsai, J.C.; Kuo, P.L.; Hsu, Y.L.; Ljunggren, Ö.; Fellström, B.; Kuo, M.C. Associations of Bone Turnover Markers with Cognitive Function in Patients Undergoing Hemodialysis. Dis. Markers 2020, 2020, 8641749. [Google Scholar] [CrossRef] [PubMed]
- Gil Montoya, J.A.; Barrios, R.; Sanchez-Lara, I.; Ramos, P.; Carnero, C.; Fornieles, F.; Montes, J.; Santana, S.; Luna, J.D.D.; Gonzalez-Moles, M.A. Systemic inflammatory impact of periodontitis on cognitive impairment. Gerodontology 2020, 37, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, M.J.; Geerlings, M.I.; Meijer, J.; Kiliaan, A.; Ruitenberg, A.; Van Swieten, J.C.; Stijnen, T.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Inflammatory proteins in plasma and the risk of dementia. Arch. Neurol. 2004, 61, 668–672. [Google Scholar] [CrossRef]
- Furutama, D.; Matsuda, S.; Yamawaki, Y.; Hatano, S.; Okanobu, A.; Memida, T.; Oue, H.; Fujita, T.; Ouhara, K.; Kajiya, M.; et al. IL-6 induced by periodontal inflammation causes neuroinflammation and disrupts the blood–brain barrier. Brain Sci. 2020, 10, 679. [Google Scholar] [CrossRef]

| Covariate | No Severe Periodontitis N = 4340 (92.95%) | Severe Periodontitis N = 329 (7.05%) | Total N= 4669 | p-Value | |
|---|---|---|---|---|---|
| Sex | male | 1987 (45.48%) | 229 (69.60%) | 2216 (47.46%) | <0.001 |
| female | 2353 (54.22%) | 100 (30.40%) | 2453 (52.54%) | ||
| Smoking | no | 2413 (55.60%) | 127 (38.60%) | 2540 (54.40%) | <0.001 |
| yes | 1927 (44.40%) | 202 (61.04%) | 2129 (45.60%) | ||
| Poverty Level | <138% | 1343 (30.94%) | 129 (39.21%) | 1472 (31.53%) | <0.001 |
| 138–399% | 1529 (35.23%) | 129 (39.21%) | 1658 (35.51%) | ||
| 400%+ | 1468 (33.82%) | 71 (21.58%) | 1539 (32.96%) | ||
| Diabetes | No | 3686 (84.99%) | 279 (84.80%) | 3965 (84.98%) | 0.927 |
| Yes | 651 (15.01%) | 50 (15.20%) | 701 (15.02%) | ||
| Any disease | No | 2372 (54.81%) | 196 (59.94%) | 2568 (55.17%) | 0.072 |
| Yes | 1956 (45.19%) | 131 (40.06%) | 2087 (44.83%) | ||
| Cont. Variable | No Severe Periodontitis Mean (SD) | Severe Periodontitis Mean (SD) | p-Value |
|---|---|---|---|
| Cognitive Function | 46.3 (17.3) | 42.1 (15.1) | 0.006 |
| Serum Alkaline Phosphatase | 67.1 (27.4) | 70.9 (23.0) | 0.016 |
| Age in years | 53.8 (14.9) | 56.9 (11.9) | <0.001 |
| Covariate | Composite | ||||
|---|---|---|---|---|---|
| Odds Ratio | Confidence Interval | p Value | |||
| Lower | Upper | ||||
| Cognitive Function | 0.98 | 0.96 | 0.99 | 0.018 | |
| Alkaline Phosphatase Cognition (interaction term) | 1.1 | 1.0 | 1.2 | <0.001 | |
| Age | 0.94 | 0.90 | 0.99 | 0.035 | |
| Sex (male is the reference) | 0.32 | 0.18 | 0.57 | <0.001 | |
| Diabetes Status (no diabetes is the reference) | 1.3 | 0.66 | 2.6 | 0.422 | |
| Smoking Status (no smoking is the reference) | 0.93 | 0.53 | 1.6 | 0.816 | |
| Income (below 138% FPL is the reference) | 138–399% FPL | 0.63 | 0.35 | 1.1 | 0.111 |
| 400% FPL | 0.58 | 0.30 | 1.1 | 0.111 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brahmbhatt, Y.; Alqaderi, H.; Chinipardaz, Z. Association Between Severe Periodontitis and Cognitive Decline in Older Adults. Life 2024, 14, 1589. https://doi.org/10.3390/life14121589
Brahmbhatt Y, Alqaderi H, Chinipardaz Z. Association Between Severe Periodontitis and Cognitive Decline in Older Adults. Life. 2024; 14(12):1589. https://doi.org/10.3390/life14121589
Chicago/Turabian StyleBrahmbhatt, Yash, Hend Alqaderi, and Zahra Chinipardaz. 2024. "Association Between Severe Periodontitis and Cognitive Decline in Older Adults" Life 14, no. 12: 1589. https://doi.org/10.3390/life14121589
APA StyleBrahmbhatt, Y., Alqaderi, H., & Chinipardaz, Z. (2024). Association Between Severe Periodontitis and Cognitive Decline in Older Adults. Life, 14(12), 1589. https://doi.org/10.3390/life14121589







