Antioxidant, Antiglaucoma, Anticholinergic, and Antidiabetic Effects of Kiwifruit (Actinidia deliciosa) Oil: Metabolite Profile Analysis Using LC-HR/MS, GC/MS and GC-FID
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Kiwifruit (A. deliciosa) Oil
2.3. Polyphenolic Composition Using LC-HRMS Analysis
2.4. GC/MS and GC-FID Analyses of Kiwifruit Oil and Essential Oil Isolation
2.5. Reducing Ability Assays
2.6. Radical Scavenging Assays
2.7. Acetylcholinesterase Inhibition Assay
2.8. α-Amylase Inhibition Assay
2.9. hCA II Inhibition Assay
2.10. Determination of IC50 Value
2.11. Statistical Analysis
3. Results
3.1. Polyphenolic Composition of Kiwifruit Oil
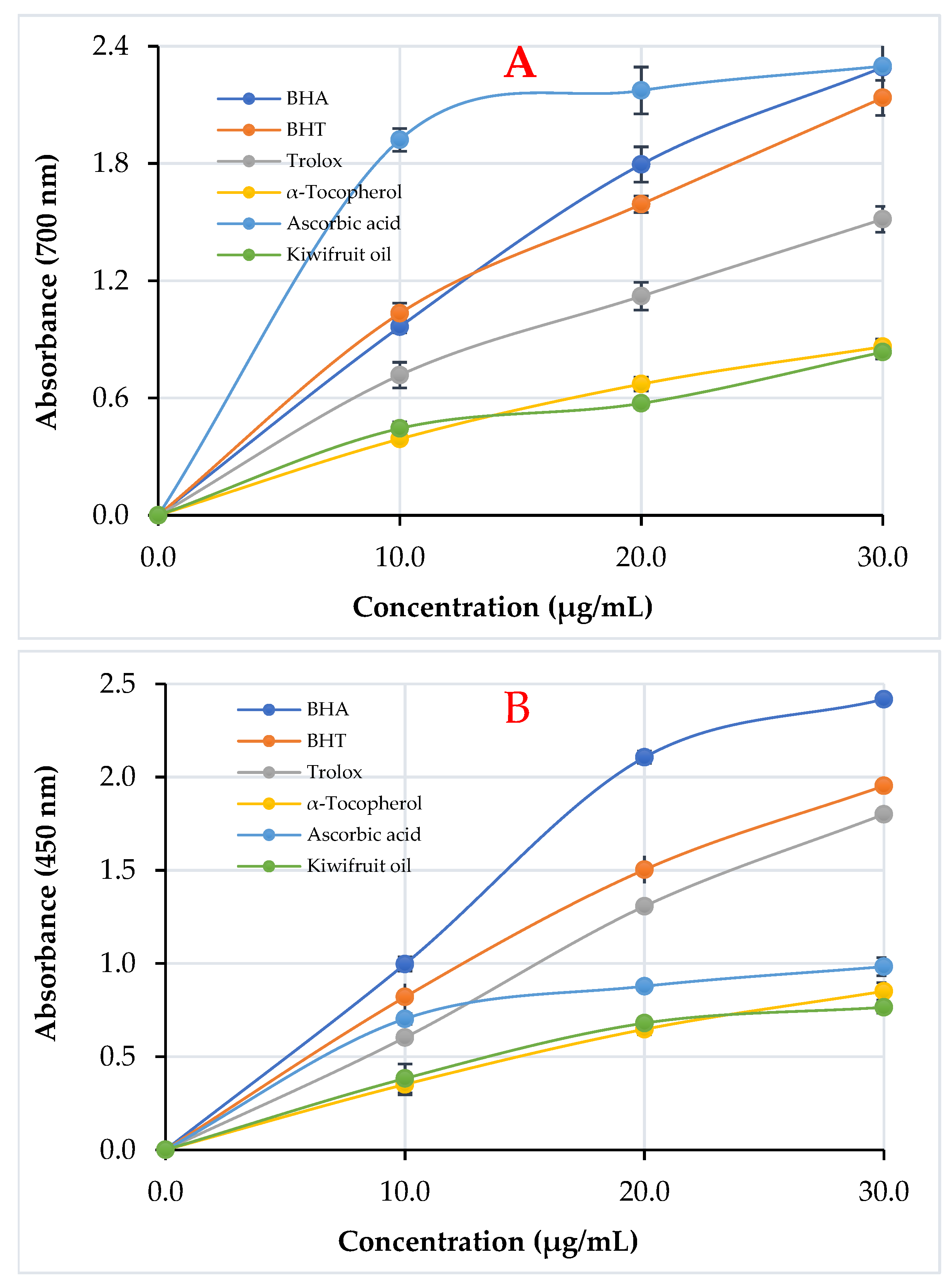
3.2. Reducing Ability of Kiwifruit Oil
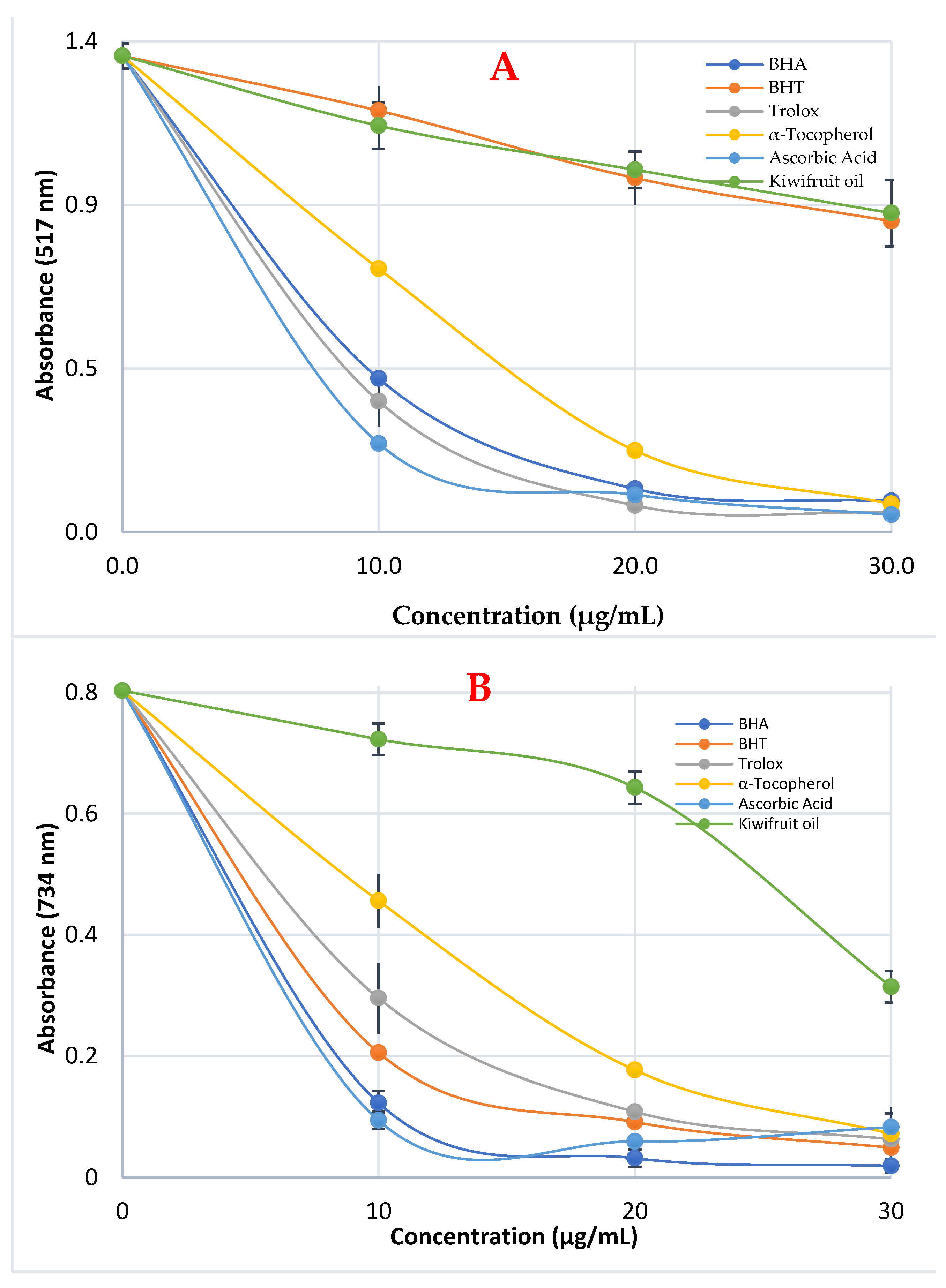
3.3. Radicals Scavenging Effect of Kiwifruit Oil
3.4. Enzymes Inhibition by Kiwifruit Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Ozler, E.; Topal, F.; Topal, M.; Sarıkaya, S.B.O. LC-HRMS profiling and phenolic content, cholinesterase, and antioxidant activities of Terminalia citrina. Chem. Biodivers. 2023, 20, e202201250. [Google Scholar] [CrossRef]
- Gulcin, I.; Sat, I.G.; Beydemir, S.; Kufrevioglu, O.I. Evaluation of the in vitro antioxidant properties of broccoli extracts (Brassica oleracea L.). Ital. J. Food Sci. 2004, 16, 17–30. [Google Scholar]
- Topal, M. The inhibition profile of sesamol against alpha-glycosidase and acetylcholinesterase enzyme. Int. J. Food Proper. 2019, 22, 1527–1535. [Google Scholar] [CrossRef]
- Topal, M. Secondary metabolites of ethanol extracts of Pinus sylvestris cones from eastern Anatolia and their antioxidant, cholinesterase and alpha-glucosidase activities. Rec. Nat. Prod. 2020, 14, 129–138. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food. 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Koksal, E.; Bursal, E.; Dikici, E.; Tozoglu, F.; Gulcin, I. Antioxidant activity of Melissa officinalis leaves. J. Med. Plants Res. 2011, 5, 217–222. [Google Scholar]
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Verasilp, S.; Trakhtenberg, S.; Gorinstein, S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food Res. Int. 2011, 44, 1671–1701. [Google Scholar] [CrossRef]
- Karagecili, H.; İzol, E.; Kirecci, E.; Gulcin, I. Determination of antioxidant, Anti-Alzheimer, antidiabetic, antiglaucoma and antimicrobial effects of zivzik pomegranate (Punica granatum)-A chemical profiling by LC-MS/MS. Life 2023, 13, 735. [Google Scholar] [CrossRef]
- Guan, R.; Van, L.Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhnong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Sever, B.; Turkes, C.; Altıntop, M.D.; Demir, Y.; Ciftci, G.A.; Beydemir, S. Novel metabolic enzyme inhibitors designed through the molecular hybridization of thiazole and pyrazoline scaffolds. Arch. Pharm. 2021, 354, e2100294. [Google Scholar] [CrossRef]
- Aktas Anıl, D.; Ozturk Aydın, B.; Demir, Y.; Turkmenoglu, B. Design, synthesis, biological evaluation and molecular docking studies of novel 1H-1,2,3-Triazole derivatives as potent inhibitors of carbonic anhydrase, acetylcholinesterase and aldose reductase. J. Mol. Struct. 2022, 1257, 132613. [Google Scholar] [CrossRef]
- Yigit, M.; Yigit, B.; Taslimi, P.; Ozdemir, I.; Karaman, M.; Gulcin, I. Novel amine-functionalized benzimidazolium salts: Synthesis, characterization, bioactivity, and molecular docking studies. J. Mol. Struct. 2020, 1207, 127802. [Google Scholar] [CrossRef]
- Kalaycı, M.; Turkes, C.; Arslan, M.; Demir, Y.; Beydemir, S. Novel benzoic acid derivatives: Synthesis and biological evaluation as multitarget acetylcholinesterase and carbonic anhydrase inhibitors. Arch. Pharm. 2021, 354, e2000282. [Google Scholar] [CrossRef]
- Osmaniye, D.; Turkes, C.; Demir, Y.; Okay, Y.; Beydemir, S.; Kaplancıklı, Z.A. Design, synthesis, and biological activity of novel dithiocarbamate-methylsulfonyl hybrids as carbonic anhydrase inhibitors. Arch. Pharm. 2022, 355, e2200132. [Google Scholar] [CrossRef]
- Wong, C.Y.; Leong, K.H.; He, X.; Zheng, F.; Sun, J.; Wang, Z.; Heh, C.H.; Kong, K.W. Phytochemicals of six selected herbal plants and their inhibitory activities towards free radicals and glycation. Food Biosci. 2022, 46, 101557. [Google Scholar] [CrossRef]
- Durmaz, L.; Kiziltas, H.; Guven, L.; Karagecili, H.; Alwasel, S.; Gulcin, I. Antioxidant, antidiabetic, anticholinergic, and antiglaucoma effects of magnofluorine. Molecules 2022, 27, 50902. [Google Scholar] [CrossRef]
- Gulcin, I.; Bingol, Z.; Taslimi, P.; Goren, A.C.; Alwasel, S.H.; Tel, A.Z. Polyphenol contents, potential antioxidant, anticholinergic and antidiabetic properties of mountain mint (Cyclotrichium leucotrichum). Chem. Biodivers. 2022, 19, e202100775. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; Cindio, B.D.; Houghton, P.J.; Menichini, F.; et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem. 2009, 117, 174–180. [Google Scholar] [CrossRef]
- Istrefi, Q.; Turkes, C.; Arslan, M.; Demir, Y.; Nixha, A.R.; Beydemir, S.; Kufrevioglu, O.I. Sulfonamides incorporating ketene N,S-acetal bioisosteres as potent carbonic anhydrase and acetylcholinesterase inhibitors. Arch. Pharm. 2017, 353, e1900383. [Google Scholar] [CrossRef]
- Sever, B.; Turkes, C.; Altintop, M.D.; Demir, Y.; Beydemir, S. Thiazolyl-pyrazoline derivatives: In vitro and in silico evaluation as potential acetylcholinesterase and carbonic anhydrase inhibitors. Int. J. Biol. Macromol. 2020, 163, 1970–1988. [Google Scholar] [CrossRef]
- Cetin Cakmak, K.; Gulcin, I. Anticholinergic and antioxidant activities of usnic acid-an activity-structure insight. Toxicol. Rep. 2019, 6, 1273–1280. [Google Scholar] [CrossRef]
- Akbas, F.; Ozaydin, A.; Polat, E.; Onaran, I. Lucilia sericata larval secretions stimulating wound healing effects on rat dermal fibroblast cells. Rec. Nat. Prod. 2020, 14, 340–354. [Google Scholar] [CrossRef]
- Sengoku, T.; Morita, K.; Sakuma, S.; Motoyama, Y.; Goto, T. Possible inhibitory mechanism of FK506 (tacrolimus hydrate) ointment for atopic dermatitis based on animal models. Eur. J. Pharmacol. 1999, 379, 183–189. [Google Scholar] [CrossRef]
- Patino-Bayona, W.R.; Plazas, E.; Bustos-Cortes, J.J.; Prieto-Rodriguez, J.A.; Patino-Ladino, O.J. Essential oils of three Hypericum species from colombia: Chemical composition, insecticidal and repellent activity against Sitophilus zeamais motsch. (coleoptera: Curculionidae). Rec. Nat. Prod. 2021, 15, 111–121. [Google Scholar] [CrossRef]
- Suzgec-Selcuk, S.; Ozek, T.; Ozek, G.; Yur, S.; Goger, F.; Gurdal, M.B.; Toplan, G.G.; Mericli, A.H.; Baser, K.H.C. The leaf and the gall volatiles of Salvia fruticosa miller from turkey: Chemical composition and biological activities. Rec. Nat. Prod. 2021, 15, 10–24. [Google Scholar] [CrossRef]
- Ha, C.T.T.; Thuy, D.T.T.; Nam, V.Q.; Tam, N.K.B.; Setzer, W.N. Composition and antimicrobial activity of essential oils from leaves and twigs of Magnolia hookeri var. Longirostrata, D.X.LI & R. Z. Zhou and Magnolia insignis wall. in Ha Giang province of Vietnam. Rec. Nat. Prod. 2021, 15, 207–212. [Google Scholar]
- Salinas, M.; Bec, N.; Calva, J.; Ramirez, J.; Andrade, J.M.; Vidari, G.; Larroque, C. Chemical composition and anticholinesterase activity of the essential oil from the Ecuadorian plant Salvia pichinchensis benth. Rec. Nat. Prod. 2020, 14, 276–285. [Google Scholar] [CrossRef]
- Topal, F.; Topal, M.; Gocer, H.; Kalın, P.; Kocyigit, U.M.; Gulcin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Guclu, K.; Ozyurek, M.; Esin Karademir, S.; Ercag, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Gocer, H.; Menzek, A.; Gulcin, I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch. Pharm. 2012, 345, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 2952. [Google Scholar] [CrossRef]
- Savikin, K.; Zivkovic, J.; Alimpic, A.; Zdunic, G.; Jankovic, T.; Duletic-Lausevic, S.; Menkovic, N. Activity guided fractionation of pomegranate extract and its antioxidant, antidiabetic and antineurodegenerative properties. Ind. Crops Prod. 2018, 113, 142–149. [Google Scholar] [CrossRef]
- Xiao, Z.; Storms, R.; Tsang, A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal. Biochem. 2006, 351, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, U.M.; Budak, Y.; Gurdere, M.B.; Tekin, S.; Koprulu, T.K.; Erturk, F.; Ozcan, K.; Gulcin, I.; Ceylan, M. Synthesis, characterization, anticancer, antimicrobial and carbonic anhydrase inhibition profiles of novel (3aR,4S,7R,7aS)-2-(4-((E)-3-(3-aryl)acryloyl) phenyl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione derivatives. Bioorg. Chem. 2017, 70, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 67, 248–254. [Google Scholar] [CrossRef]
- Verpoorte, J.A.; Mehta, S.; Edsall, J.T. Esterase activities of human carbonic anhydrases B and C. J. Biol. Chem. 1967, 242, 4221–4229. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Kiziltas, H. Determination of LC-HRMS profiling, antioxidant activity, cytotoxic effect and enzyme inhibitory properties of Satureja avromanica using in vitro and in silico methods. Proceses. Biochem. 2022, 116, 157–172. [Google Scholar] [CrossRef]
- Ceylan, R.; Zengin, G.; Guler, G.O.; Aktumsek, A. Bioactive constituents of Lathyrus czeczottianus and ethyl acetate and water extracts and their biological activities: An endemic plant to Turkey. S. Afr. J. Bot. 2020, 143, 306–311. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations-A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Mollica, A.; Scioli, G.; Della Valle, A.; Cichelli, A.; Novellino, E.; Bauer, M.; Kamysz, W.; Lorent-Martinez, E.J.; Fernindez-de Cordova, M.L.; Castillo-Lopez, R.; et al. Phenolic analysis and in vitro biological activity of red wine, pomace and grape seeds oil derived from Vitis vinifera L. Cv. montepulciano d’abruzzo. Antioxidants 2021, 10, 1704. [Google Scholar] [CrossRef]
- Dougnon, G.; Ito, M. Essential oil from the leaves of Chromolaena odorata, and sesquiterpene caryophyllene oxide induce sedative activity in mice. Pharmaceuticals 2021, 14, 651. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr. Cancer Drug Targets 2009, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.A.; Hibbert, D.B.; Gulcin, I.; Demirci Cekic, S.; Guclu, K.; Ozyurek, M.; Esin Celik, S.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species. Pure Appl. Chem. 2022, 94, 87–144. [Google Scholar] [CrossRef]
- Raafat, K.; Al Haj, M. Modulators of diabetic neuropathy and inflammation from Saponaria officinalis: Isolation of active phytochemicals and potential mechanisms of action. J. Tradit. Complement. Med. 2023, 13, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Mechchate, H.; Es-safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of catechin, epicatechin, and rutin: Optimization of a novel complete antidiabetic formulation using a mixture design approach. J. Nutr. Biochem. 2021, 88, 108520. [Google Scholar] [CrossRef] [PubMed]
- Nyane, N.A.; Tlaila, T.B.; Malefane, T.G.; Ndwandwe, D.E.; Owira, P.M.O. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur. J. Pharmacol. 2017, 803, 103–111. [Google Scholar] [CrossRef]
- Gulcin, I.; Topal, F.; Ozturk Sarikaya, S.B.; Bursal, E.; Bilsel, G.; Goren, A.C. Polyphenol contents and antioxidant properties of medlar (Mespilus germanica L.). Rec. Nat. Prod. 2011, 5, 158–175. [Google Scholar]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Ceylan, R.; Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Jugreet, S.; Cakır, O.; Quelbani, R.; Paksoy, M.Y.; Yılmaz, M.A. Enzyme inhibition and antioxidant functionality of eleven Inula species based on chemical components and chemometric insights. Biochem. Syst. Ecol. 2021, 95, 104225. [Google Scholar] [CrossRef]
- Qadir, A.; Khan, N.; Mir Najib Ullah, S.N.; Ali, A.; Gupta, D.K.; Khan, S.A. GC–MS analysis of phytoconstituents present in methanolic extract of Actinidia deliciosa L. fruits and its antioxidant activity. J. Indian Chem. Soc. 2022, 99, 100566. [Google Scholar] [CrossRef]
- Sicari, V.; Pellicano, T.M.; Giuffre, A.M.; Loizzo, M.R.; Poiana, M. Effect of packaging materials on the quality of kiwifruits (Actinidia deliciosa cv. Hayward). J. Food Meas. Charact. 2019, 13, 3033–3039. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.l.; Li, J.Y.; Liang, Y.J.L.; Yang, R.Q.; Liu, J.Y.; Ma, Z.; Wu, L. Evaluation of biochemical components and antioxidant capacity of different kiwifruit (Actinidia spp.) genotypes grown in China. Biotechnol. Biotechnol. Equip. 2018, 32, 558–565. [Google Scholar] [CrossRef]
- Zhu, C.; Chou, O.; Lee, F.Y.; Wang, Z.; Barrow, J.C.; Dunshea, R.F.; Suleria, A.R.H. Characterization of phenolics in rejected kiwifruit and their antioxidant potential. Processes 2021, 9, 781. [Google Scholar] [CrossRef]
- Naoom, A.Y.; Kang, W.; Ghanem, N.F.; Abdel-Daim, M.M.; El-Demerdash, F.M. Actinidia deliciosa as a complemental therapy against nephropathy and oxidative stress in diabetic rats. Food Sci. Hum. Wellness 2023, 12, 1981–1990. [Google Scholar] [CrossRef]
- Bursal, E.; Gulcin, I. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Gulcin, I.; Elmastas, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil-A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Kim, Y.E.; Cho, C.H.; Kang, H.; Heo, H.J.; Cho, Y.S.; Kim, D.O. Kiwifruit of Actinidia eriantha cv. Bidan has in vitro antioxidative, anti-inflammatory and immunomodulatory effects on macrophages and splenocytes isolated from male BALB/c mice. Food Sci. Biotechnol. 2018, 27, 1503–1511. [Google Scholar] [CrossRef]
- Lee, I.; Im, S.; Jin, C.R.; Heo, H.J.; Cho, Y.S.; Baik, M.Y.; Kim, D.O. Effect of maturity stage at harvest on antioxidant capacity and total phenolics in kiwifruits (Actinidia spp.) grown in Korea. Hortic. Environ. Biotechnol. 2015, 56, 841–848. [Google Scholar] [CrossRef]
- Collins, A.R.; Harrington, V.; Drew, J.; Melvin, R. Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis 2003, 24, 511–515. [Google Scholar] [CrossRef]
- Brevik, A.; Gaivao, I.; Medin, T.; Jorgenesen, A.; Piasek, A.; Elilasson, J.; Karlsen, A.; Blomhoff, R.; Veggan, T.; Duttaroy, A.K.; et al. Supplementation of a western diet with golden kiwifruits (Actinidia chinensis var.’Hort 16A’:) effects on biomarkers of oxidation damage and antioxidant protection. Nutr. J. 2011, 10, 54. [Google Scholar] [CrossRef]
- Gulcin, I.; Kaya, R.; Goren, A.C.; Akıncıoglu, H.; Topal, M.; Bingol, Z.; Cetin Cakmak, K.; Ozturk Sarikaya, S.B.; Durmaz, L.; Alwasel, S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (Cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Proper. 2019, 22, 1511–1526. [Google Scholar] [CrossRef]
- Ligaj, M.; Kobus-Cisowska, J.; Szczepaniak, O.; Szulc, P.; Kikut-Ligaj, D.; Mikołajczak-Ratajczak, A.; Bykowski, P.; Szymanowska, D.; Przeor, M.; Polewski, K.; et al. Electrochemical screening of genoprotective and antioxidative effectiveness of Origanum vulgare L. and its functionality in the prevention of neurodegenerative disorders. Talanta 2021, 223, 121749. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Cho, C.H.; Baik, M.Y.; Park, S.K.; Heo, J.H.; Cho, Y.S.; Kim, D.O. Effects of freeze-drying on antioxidant and anticholinesterase activities in various cultivars of kiwifruit (Actinidia spp.). Food Sci. Biotechnol. 2017, 26, 221–228. [Google Scholar] [CrossRef]
- Arachchige, S.P.G.; Abeysekera, W.P.K.M.; Ratnasooriya, W.D. Antiamylase, anticholinesterases, antiglycation, and glycation reversing potential of bark and leaf of ceylon cinnamon (Cinnamomum zeylanicum Blume) in vitro. Evid. Based Complement. Alternat. Med. 2017, 2017, 5076029. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Han, Q.; Fu, R.; Ni, Y. Inhibition of α-amylase activity by insoluble and soluble dietary fibers from kiwifruit (Actinidia deliciosa). Food Biosci. 2021, 42, 101057. [Google Scholar] [CrossRef]
- Li, H.Y.; Yuan, Q.; Yang, Y.L.; Han, Q.H.; He, J.L.; Zhao, L.; Zhang, Q.; Liu, S.X.; Lin, D.-R.; Wu, D.T.; et al. Phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of different kiwifruits. Molecules 2018, 23, 2957. [Google Scholar] [CrossRef]
- Turkes, C.; Arslan, M.; Demir, Y.; Cocaj, L.; Nixha, A.R.; Beydemir, S. Synthesis, biological evaluation and in silico studies of novel N-substituted phthalazine sulfonamide compounds as potent carbonic anhydrase and acetylcholinesterase inhibitors. Bioorg. Med. 2019, 89, 103004. [Google Scholar]
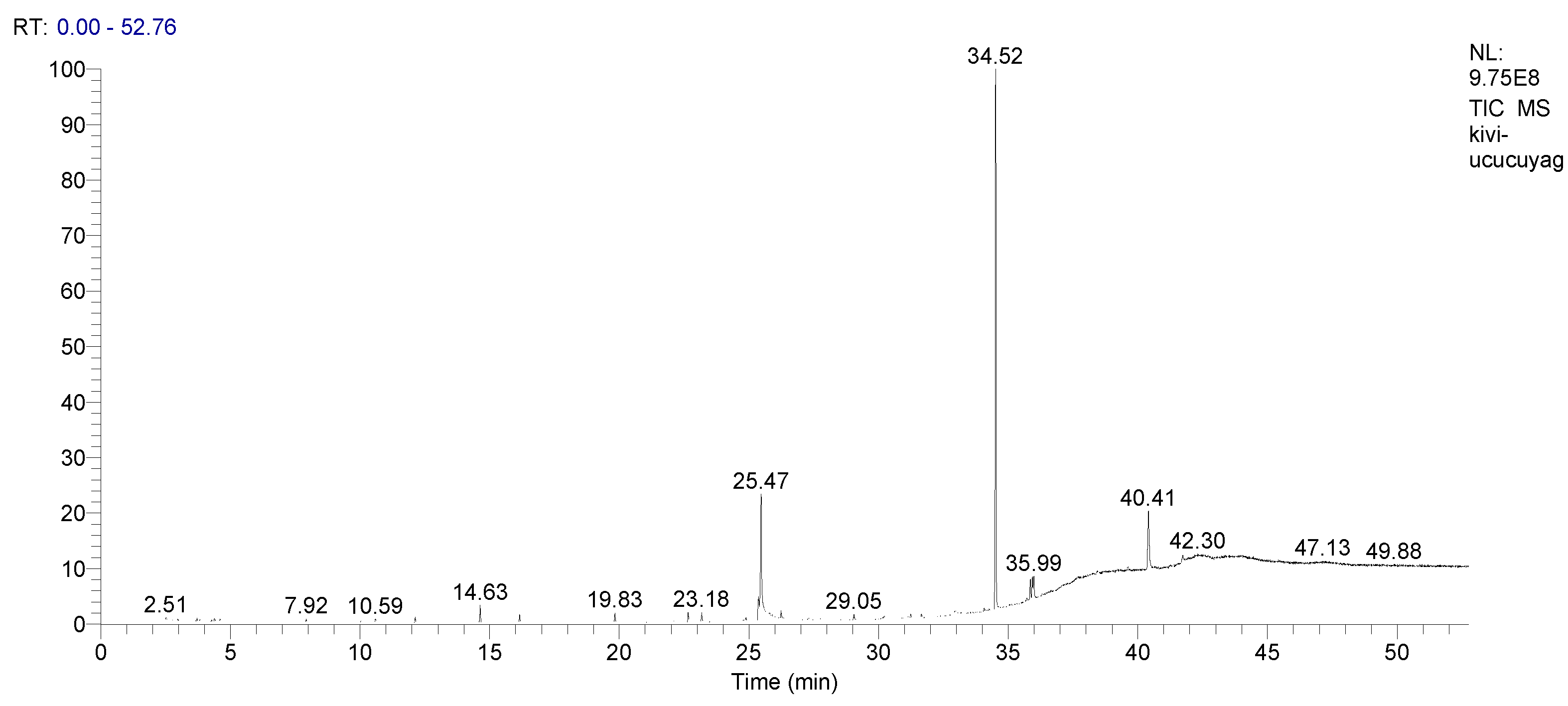

| Phenolic Compounds | Molecular Formula | m/z | Ionization Mode | Linear Range | Linear Regression Equation | LOD/LOQ | R² | Recovery | U% | Phenolics |
|---|---|---|---|---|---|---|---|---|---|---|
| Ascorbic acid | C6H8O6 | 175.0248 | Negative | 0.5–10 | y = 0.00347x − 0.00137 | 0.39/1.29 | 0.9988 | 96.20 | 3.94 | 4.57 |
| Epigallocatechin | C15H14O7 | 307.0812 | Positive | 0.3–5 | y = 0.00317x + 0.000443 | 0.17/0.57 | 0.9947 | 102.22 | 3.09 | 12.89 |
| Chlorogenic acid | C16H18O9 | 353.0878 | Negative | 0.05–10 | y = 0.00817x + 0.000163 | 0.02/0.06 | 0.9994 | 96.68 | 3.58 | 0.61 |
| Fumaric acid | C4H4O4 | 115.0037 | Negative | 0.1–10 | y = 0.00061x − 0.0000329 | 0.05/0.17 | 0.9991 | 97.13 | 2.88 | <LOD |
| Verbascoside | C29H36O15 | 623.1981 | Negative | 0.1–10 | y = 0.00758x + 0.000563 | 0.03/0.1 | 0.9995 | 96.19 | 2.93 | 0.25 |
| Orientin | C21H20O11 | 447.0933 | Negative | 0.1–10 | y = 0.00757x + 0.000347 | 0.01/0.03 | 0.9993 | 96.22 | 3.67 | 0.43 |
| Caffeic acid | C9H8O4 | 179.0350 | Negative | 0.3–10 | y = 0.0304x + 0.00366 | 0.08/0.27 | 0.9993 | 94.51 | 3.74 | 0.38 |
| Luteolin-7-rutinoside | C27H30O15 | 593.1512 | Negative | 0.1–10 | y = 0.00879x + 0.000739 | 0.01/0.03 | 0.9988 | 93.05 | 3.06 | 0.27 |
| Luteolin-7-glycoside | C21H20O11 | 447.0933 | Negative | 0.1–7 | y = 0.0162x + 0.00226 | 0.01/0.03 | 0.9961 | 96.31 | 4.14 | 0.48 |
| Rutin | C27H30O16 | 609.1461 | Negative | 0.05–10 | y = 0.00329x − 0.00005576 | 0.01/0.03 | 0.999 | 96.97 | 3.07 | 4.54 |
| Rosmarinic acid | C18H16O8 | 359.0772 | Negative | 0.05–10 | y = 0.00717x − 0.0003067 | 0.01/0.03 | 0.9992 | 99.85 | 3.77 | 0.19 |
| Hyperoside | C21H20O12 | 463.0882 | Negative | 0.05–10 | y = 0.0072x − 0.00003096 | 0.01/0.03 | 0.9995 | 96.62 | 3.46 | 0.45 |
| Apigenin 7-glycoside | C21H20O10 | 431.0984 | Negative | 0.3–7 | y = 0.0246x + 0.00306 | 0.01/0.03 | 0.9962 | 96.07 | 2.86 | 0.93 |
| Ellagic acid | C14H6O8 | 300.9990 | Negative | 0.05–10 | y = 0.0085x − 0.000612 | 0.03/1 | 0.9994 | 101.49 | 3.59 | 0.04 |
| Quercitrin | C21H20O11 | 447.0933 | Negative | 0.05–10 | y = 0.0179 + 0.0003331 | 0.01/0.03 | 0.999 | 97.00 | 4.20 | 0.10 |
| Quercetin | C15H10O7 | 301.0354 | Negative | 0.1–10 | y = 0.0509x + 0.00467 | 0.01/0.03 | 0.9978 | 96.41 | 3.78 | 2.36 |
| Herniarin | C10H8O3 | 177.0546 | Positive | 0.1–7 | y = 0.309x + 0.0266 | 0.01/0.03 | 0.9983 | 92.92 | 2.95 | 1.44 |
| Salicylic acid | C7H6O3 | 137.0244 | Negative | 0.3–10 | y = 0.0361x + 0.00245 | 0.01/0.03 | 0.9982 | 92.88 | 3.89 | 4.84 |
| Naringenin | C15H12O5 | 271.0612 | Negative | 0.1–10 | y = 0.0281x + 0.00182 | 0.01/0.03 | 0.9995 | 86.65 | 1.89 | 3.62 |
| Luteolin | C15H10O6 | 285.0405 | Negative | 0.1–10 | y = 0.117x + 0.00848 | 0.01/0.03 | 0.9981 | 96.98 | 4.20 | 5.49 |
| Apigenin | C15H10O5 | 269.0456 | Negative | 0.3–10 | y = 0.104x + 0.0199 | 0.01/0.03 | 0.9998 | 81.55 | 3.42 | 74.24 |
| Hispidulin | C16H12O6 | 301.0707 | Positive | 0.05–10 | y = 0.02614x + 0.0003114 | 0.01/0.03 | 0.9993 | 98.36 | 2.87 | 1.99 |
| Isosakuranetin | C16H14O5 | 285.0769 | Negative | 0.05–10 | y = 0.0235x + 0.000561 | 0.01/0.03 | 0.9992 | 96.56 | 3.41 | 0.08 |
| Penduletin | C18H16O7 | 343.0823 | Negative | 0.3–10 | y = 0.0258x + 0.00253 | 0.01/0.03 | 0.9991 | 83.43 | 3.20 | 1.31 |
| CAPE | C17H16O4 | 283.0976 | Negative | 0.3–7 | y = 0.255x + 0.0477 | 0.01/0.03 | 0.9964 | 94.42 | 3.13 | 2.17 |
| Chrysin | C15H10O4 | 253.0506 | Negative | 0.05–7 | y = 0.0964x − 0.0002622 | 0.01/0.03 | 0.999 | 87.92 | 3.24 | >LOQ |
| Quillaic acid | C30H46O5 | 485.3273 | Negative | 0.05–10 | y = 0.00781x − 0.0001318 | 0.01/0.03 | 0.9992 | 90.29 | 2.56 | 4.57 |
| Caryophyllene oxide | C15H24O | 221.1900 | Positive | 0.3–7 | y = 0.00151x + 0.00692 | 0.10/0.50 | 0.9909 | 96.87 | 4.05 | 12.89 |
| Essential Oils | RT (min) | Formula | Contents (%) |
|---|---|---|---|
| Cetal | 16.15 | C17H34O | 0.91 |
| Stenol | 19.83 | C18H38O | 0.97 |
| Palmitic acid | 22.65 | C16H32O2 | 1.54 |
| Linoleic acid | 25.37 | C18H32O2 | 2.67 |
| Linoleoyl chloride | 25.47 | C18H31ClO | 20.28 |
| Squalene | 34.52 | C30H50 | 53.04 |
| Total | 79.41 |
| Antioxidants | Fe3+ Reducing * | Cu2+ Reducing * | Fe3+-TPTZ Reducing * | |||
|---|---|---|---|---|---|---|
| λ700 | r2 | λ450 | r2 | λ593 | r2 | |
| BHA | 2.292 ± 0.012 | 0.9993 | 2.418 ± 0.018 | 0.9887 | 1.172 ± 0.014 | 0.9605 |
| BHT | 2.136 ± 0.090 | 0.9957 | 1.953 ± 0.045 | 0.9998 | 0.690 ± 0.008 | 0.9645 |
| Trolox | 1.514 ± 0.066 | 0.9963 | 1.800 ± 0.096 | 0.9974 | 1.180 ± 0.032 | 0.9732 |
| α-Tocopherol | 0.862 ± 0.038 | 0.9996 | 0.851 ± 0.046 | 0.9994 | 0.918 ± 0.011 | 0.9904 |
| Ascorbic acid | 2.298 ± 0.086 | 0.9659 | 0.983 ± 0.048 | 0.9822 | 1.257 ± 0.024 | 0.9869 |
| Kiwifruit oil | 0.835 ± 0.035 | 0.9723 | 0.765 ± 0.031 | 0.9978 | 0.583 ± 0.017 | 0.9525 |
| Antioxidants | DPPH• Scavenging | ABTS•+ Scavenging | ||
|---|---|---|---|---|
| IC50 | r2 | IC50 | r2 | |
| BHA | 6.86 | 0.9949 | 6.35 | 0.9746 |
| BHT | 49.50 | 0.9957 | 12.60 | 0.9995 |
| Trolox | 6.03 | 0.9925 | 16.50 | 0.9775 |
| α-Tocopherol | 7.70 | 0.9961 | 18.72 | 0.9347 |
| Ascorbic acid | 5.82 | 0.9668 | 11.74 | 0.9983 |
| Kiwifruit oil | 48.55 | 0.9977 | 77.00 | 0.9890 |
| Enzymes | Kiwifruit (A. deliciosa) Oil | Standard Inhibitors | ||
|---|---|---|---|---|
| IC50 | r2 | IC50 | r2 | |
| α-Amylase 1 | 421.02 | 0.9080 | 7.54 | 0.9074 |
| Acetylcholinesterase 2 | 12.80 | 0.9680 | 8.82 | 0.9836 |
| Carbonic anhydrase II 3 | 505.83 | 0.9249 | 9.96 | 0.9930 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozden, E.M.; Bingol, Z.; Mutlu, M.; Karagecili, H.; Köksal, E.; Goren, A.C.; Alwasel, S.H.; Gulcin, İ. Antioxidant, Antiglaucoma, Anticholinergic, and Antidiabetic Effects of Kiwifruit (Actinidia deliciosa) Oil: Metabolite Profile Analysis Using LC-HR/MS, GC/MS and GC-FID. Life 2023, 13, 1939. https://doi.org/10.3390/life13091939
Ozden EM, Bingol Z, Mutlu M, Karagecili H, Köksal E, Goren AC, Alwasel SH, Gulcin İ. Antioxidant, Antiglaucoma, Anticholinergic, and Antidiabetic Effects of Kiwifruit (Actinidia deliciosa) Oil: Metabolite Profile Analysis Using LC-HR/MS, GC/MS and GC-FID. Life. 2023; 13(9):1939. https://doi.org/10.3390/life13091939
Chicago/Turabian StyleOzden, Eda Mehtap, Zeynebe Bingol, Muzaffer Mutlu, Hasan Karagecili, Ekrem Köksal, Ahmet C. Goren, Saleh H. Alwasel, and İlhami Gulcin. 2023. "Antioxidant, Antiglaucoma, Anticholinergic, and Antidiabetic Effects of Kiwifruit (Actinidia deliciosa) Oil: Metabolite Profile Analysis Using LC-HR/MS, GC/MS and GC-FID" Life 13, no. 9: 1939. https://doi.org/10.3390/life13091939
APA StyleOzden, E. M., Bingol, Z., Mutlu, M., Karagecili, H., Köksal, E., Goren, A. C., Alwasel, S. H., & Gulcin, İ. (2023). Antioxidant, Antiglaucoma, Anticholinergic, and Antidiabetic Effects of Kiwifruit (Actinidia deliciosa) Oil: Metabolite Profile Analysis Using LC-HR/MS, GC/MS and GC-FID. Life, 13(9), 1939. https://doi.org/10.3390/life13091939








