Abstract
Adenosine triphosphate (ATP) is a multifunctional small molecule, necessary for all modern Earth life, which must be a component of the last universal common ancestor (LUCA). However, the relatively complex structure of ATP causes doubts about its accessibility on prebiotic Earth. In this paper, based on previous studies on the synthesis of ATP components, a plausible prebiotic pathway yielding this key molecule is constructed, which relies on terrestrial volcanism to provide the required materials and suitable conditions.
1. Introduction
Composed of adenine, ribose, and a triphosphate group, adenosine triphosphate (ATP) is among the most important molecules to Earth life. It is the universal “energy currency”, one of the units of RNA, the activator of amino acids in protein synthesis, and the most prevalent protein cofactor [1,2]. ATP is also an important regulator, through working as the substrate of regulatory phosphorylation and controlling biomacromolecule aggregation as a hydrotrope [3,4]. Bearing these fundamental functions, ATP should have existed in the last universal common ancestor (LUCA).
However, due to the relatively complex structure and the demand for relatively rare material (phosphorus, in particular), the prebiotic synthesis of ATP is a multi-step process that needs to overcome various obstacles. Therefore, the availability of ATP on primitive Earth is questionable, and some theories suggested that ATP may not be the first player of its current roles during the origin of life. Thioester has been suggested to work as an energy reservoir and supported the expansion of primitive metabolic networks [5,6]. Abundant metal compounds such as FeS can be used as coenzymes for early proteins [7]. Inorganic phosphates were suggested to be the prebiotic phosphorylating agents [8]. ATP may not even be the unit of the earliest informational polymer; nucleic acid analogs such as depsipeptide nucleic acids are attractive challengers to the RNA-first scenario [9].
As a necessary piece of the puzzle, the role of ATP played in the origin of life is fundamental and intriguing. Although the de novo synthesis of ATP has not been reported, the origin of its components, including ribose, adenine, and the triphosphate group has been extensively studied. In this paper, based on previous knowledge, we investigate what environments on primitive Earth may enable the steps in ATP synthesis and try to construct a pathway by which they can collaborate to produce ATP. Based on this premise, we briefly discuss how ATP is involved in the origin of life.
2. Source of Materials Required for ATP Synthesis
Like most other biological molecules, ATP is composed elementally of hydrogen (H), carbon (C), nitrogen (N), oxygen (O), and phosphorus (P). The first four elements are abundant in the solar system (5.13 × 106, 7.60 × 105, 5.53 × 104, and 7.53 × 106 atoms per 106 atoms of Si), while P is relatively less (8.34 × 103 atoms per 106 atoms of Si) but still not rare [10]. In addition to being in sufficient amounts, these elements also need to exist in proper forms and environments for biomolecular synthesis to take place. For example, inert diamonds, graphite, and carbonaceous minerals are not good carbon substrates for prebiotic reactions. Fortunately, there are multiple sources of hydrogen, carbon, oxygen, and nitrogen that exit in volatile forms, which are more likely to participate in biomolecular synthesis reactions. For instance, N2, CO2, and H2O are major components of inferred primitive atmospheres of rocky planets like Earth, Mars, and Venus [11]. H2O also makes up the ocean of Earth, primitive Mars [12], and Jupiter’s satellite, Europa [13]. NH3 exists in the atmosphere of Jupiter and Saturn [14], their satellites like Titan [15], the ocean (companioning with H2O) of Uranus and Neptune [16], and Pluto’s surface [17]. Simple organics like CH4 are also common in the solar system. Massive quantities of CH4 form oceans on ice giant planets and Titan [16]. As will be shown, these substances are capable of producing important molecules such as aldehydes and cyanides, which could be the foundation of ribose and nucleobase synthesis.
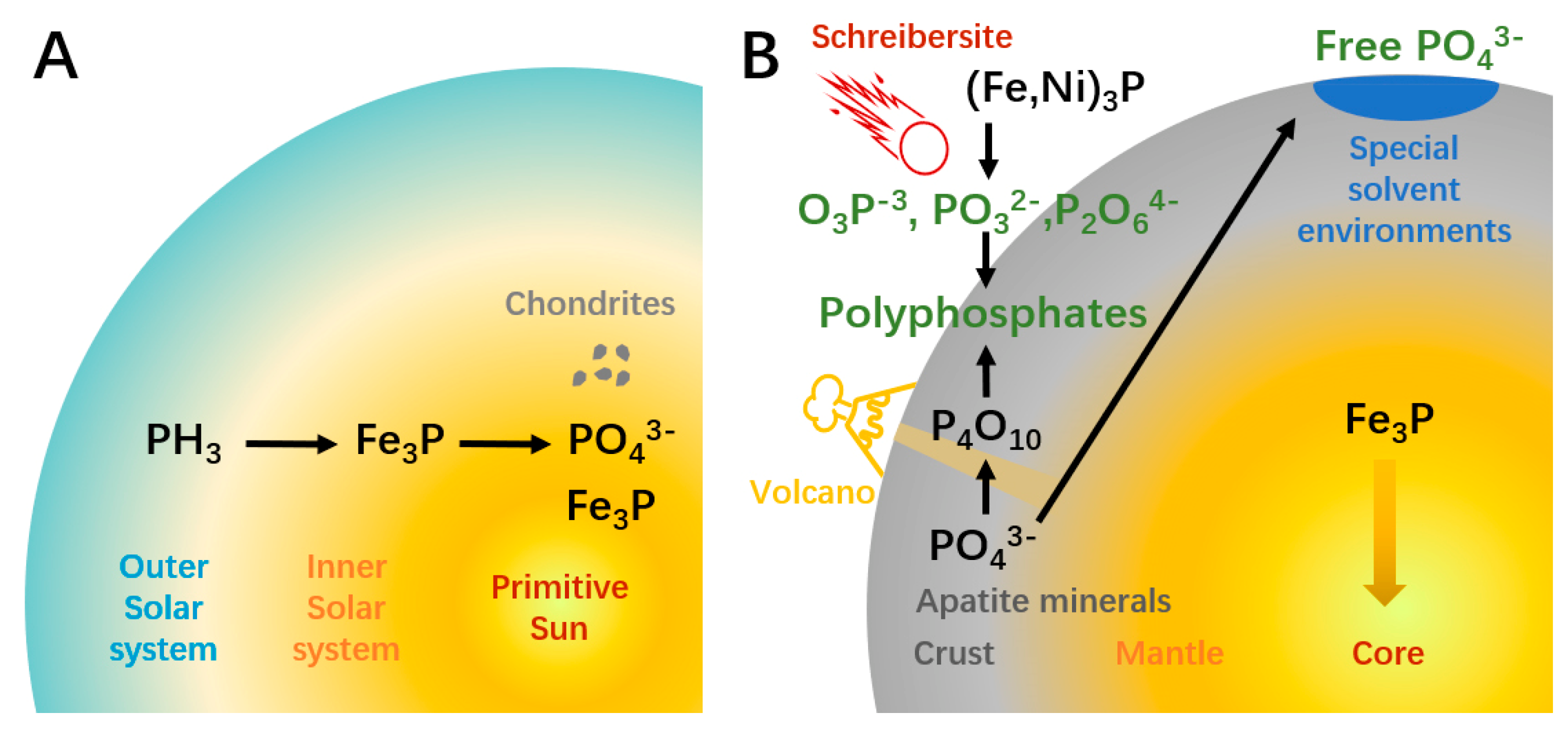
Different from the other four elements, reactive phosphorus is less available for the prebiotic synthesis of organophosphorus compounds like ATP. This obstacle to the origin of life is the so-termed “phosphorus problem”. The root of the problem can be traced to the formation of solar galaxies (Figure 1A). In the nebula stage of the solar system, phosphorus mainly existed as PH3 gas. The siderophile property induces PH3 tending to react with iron and generated the earliest phosphorus mineral schreibersite ((Fe,Ni)3P), which removed almost gaseous phosphorus in the inner solar system in around 10,000 years [18]. In the modern solar system, abiotic PH3 can only be found in gas giant planets. Therefore, unlike other biogenic elements that mainly exist in volatile forms, phosphorus must be “extracted” from minerals before participating in the synthesis of biomolecules. During the system evolution, mineral particles accumulate into larger objects like chondrites [19]. The thermal metamorphism and equilibration of chondrite components change the redox state, oxidizing part of reduced phosphorus to phosphate minerals. On planetary celestial bodies such as Earth, the complex geological process caused further differentiation and uneven distribution of phosphorus (Figure 1B). The siderophile property induced a large amount of phosphorus to sink to Earth’s core with iron. It is estimated that the core contains 80% of the total phosphorus on Earth. In the core and the metal-saturated deep mantle (below 250 km), phosphorus mainly exists as P0 or P1−, while phosphorus left in the upper silicate mantle is oxidized (P5+). Near the surface of the earth, phosphorus manifests as an incompatible element during the repetitive mantle melting. As a result, most crust phosphorus exists in the apatite mineral group (Ca5(PO4)3OH) [19]. The solubility of this mineral is low (~10−6 M), which means that the phosphate ions (H2PO4− or HPO42−, depending on pH) are hard to extract from minerals. Moreover, in organic phosphorylation products, phosphorus exists in the form of R-OPO3H−, which means that metaphosphate (PO3−) is the reactive species in phosphorylation. To form metaphosphate from phosphate, the removal of H2O or OH−, a dehydration process is needed, which is incompatible with the aqueous solutions required for most prebiotic reactions [20].
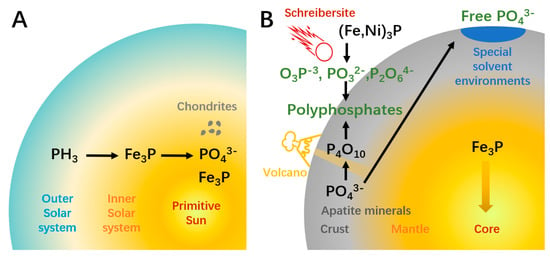
Figure 1.
The source and potential solution of the phosphorus problem. (A) In the nebula that formed the solar system, phosphorus mainly exists in the form of PH3. The heavy element Fe was enriched in the inner solar system which reacted with PH3 to form Fe3P. Part of Fe3P was oxidized to phosphate minerals (PO43−) during the formation of celestial bodies like chondrite. (B) On the newly formed earth, 80% of the phosphorus combined with Fe and sunk to the core. Crust phosphorus mainly exists in the form of insoluble apatite minerals, which is difficult for participating in prebiotic reactions. Lakes or pools of formamide, carboxylic acids, or carbonate solutions can extract PO43− from minerals. Volcanic activity can generate polyphosphates, which is a class of active phosphorylation reagents. Schreibersites that fall to Earth can react with water and generate hypophosphite, phosphite, and hypophosphate, which are active phosphorylation reagents and can be further oxidized to produce polyphosphates.
Solutions to the phosphorus problem can be classified into two categories. The first possible solution is enhancing the extraction of phosphorus from minerals, which depends on special solvent environments (Figure 1B). Changing the solvent is a common way to enhance solubility in modern chemistry. Formamide can release phosphate from various minerals [21]. The eutectic solvent consisting of choline chloride and urea enables the high yields of phosphorylated nucleosides [22]. However, it is unlikely that these substances ever reached sufficient concentrations and purity on Earth to be used as prevalent solvents [20]. Other studies used chelation and condensing agents to promote the dissolution and reaction of inert minerals. Carboxylic acids can elevate mineral solubility by chelate cations [23]. Despite the successful examples, for the organic chelating agent to be effective, its concentration is presumed to need to be 10−2 M, which may have been difficult to achieve on primitive Earth [20]. A more recent version of this solution uses carbonate, a possibly more abundant substance on prebiotic Earth, as the condensing agent, which suggests a high concentration of phosphate can be found in carbonate-rich lakes [24].
The second solution to the phosphorus problem is finding possible reactive phosphorus (Figure 1B). Polyphosphates are ideal phosphorylation reagents. In addition to higher solubility, unlike reactions using phosphates, phosphorylation using polyphosphates does not require the removal of water or hydroxyl groups, which means it can occur in aquatic conditions [20]. Trimetaphosphate could be the most studied polyphosphate, which can phosphorylate sugars, glyceric acid, amino acids, and nucleosides under moderate conditions [25,26]. A weakness of the polyphosphate-based pathway is that the corresponding minerals are rare on Earth. Volcanic fumarole may have been a major source of polyphosphate on primitive Earth. Yamagata et al. showed that a high temperature of over 1000 °C can transform phosphate minerals to P4O10; the latter can release trimetaphosphate and other polyphosphates when reacting with water [27]. The half-life of polyphosphates ranges from days to years [28,29], implying a need for constant but mild volcanic activity to enable a stable supply without damaging surrounding organic matters. A “living” example of this environment is the active volcano Mount Usu, active since 1663 [30], where polyphosphate has been detected in the gas released [27]. This kind of condition appears in different geological eras and may be more prevalent on the young Hadean-Archaean Earth.
Another attractive option is phosphide, which may preserve in meteorite mineral schreibersite [(Fe,Ni)3P]. This reduced phosphorus mineral reacts with water and produces phosphorous compounds of varying degrees of oxidation, including soluble hypophosphite, phosphite, and hypophosphate [31]. Further oxidation can also produce polyphosphates such as hypophosphate and triphosphate. Phosphite (PO32−) is a major product of the reaction between schreibersite or Fe3P and water. As a strong electrophile, PO32− can react with the OH group of the organics to phosphorylate the latter [31]. This reaction has been verified in the phosphorylation of glycerol and nucleosides in aqueous solution [31,32] and liquid sulfur dioxide [33], suggesting the potential role of extraterrestrial phosphorus in the origin of life. Phosphite is stable in the reducing environment of primitive Earth, with a half-life between 108 and 1010 years [34]. A study of the composition of rocks from different ages found that phosphite is only present in the oldest 3.52 Ga-years-old early Archean samples, which may be rooted in the enstatite chondrites during the bombardment that occurred about 4.5–3.8 Ga [35]. The disappearance of phosphite in samples younger than 3.4 Ga implies a time window for its availability.
The two solutions to the phosphorus problem imply that the element available to the origin of life on Earth may depend on volcanic activity or random meteorite impacts rather than conventional atmospheric or aquatic components. In the context of substance supply, phosphorus seems to be the bottleneck of the pathway to ATP. As seen above, the substance needed for ATP synthesis may be available on primitive Earth. In light of these potential substance sources, the next section will discuss how they might support plausible pathways to ATP.
3. Plausible Pathways to ATP
Composed of ribose, nucleobase, and phosphate group, the structure of nucleotides determines that their synthesis must go through multiple steps, whether in biotic or abiotic style. The related reactions have been summarized in recent excellent reviews [36,37,38]. We briefly introduce the representative reactions and discuss how might they cascade together to eventually lead to ATP. Although not necessarily a precondition, the synthesis of the parts of ATP will be discussed first.
3.1. Adenine Synthesis
Adenine was the first nucleobase to be synthesized abiotically, which was obtained in 1960 through heating ammonium cyanide to below 100 °C [39]. Despite the reactant (up to 11 M) and the low yields (about 0.5%), the pioneering work confirmed that adenine can be obtained from simple small molecules. Later studies revealed the key polymerization processes of HCN in this kind of reaction [37]. Although the generation of HCN may have been common on primitive Earth, which can be achieved by the electrical discharges in the N2-CO2 atmosphere or supplied by the meteorite impacts, the high concentration (over 1 M) demanded by these early studies is difficult to achieve due to its instability and high reactivity [40]. Therefore, the preservation and concentration of HCN are necessary conditions for nucleobase synthesis using it as a starting material.
One potential way to stabilize HCN is to store it in less reactive salts. Recently, Sasselov et al. proposed a plausible scheme for the prebiotic cyanide chemistry: HCN generated in the atmosphere washed into mineral-rich shallow waters, at depths beyond the reach of UV, HCN reacts with Fe2+ to form ferrocyanide and exists stably [41]. When water was evaporated, ferrocyanide formed mixed salts with Ca and Mg, and precipitated, which could be seen as a concentration step. These accumulated cyanide salts can be converted to CaCN2, KCN, NaCH, and Mg3N2 under intense heat like volcanic activity and large impacts. When encountering near neutral solutions, they generate HCN, NH3, and H2CN2. Under mid-range UV irradiation, the reactions among these feedstocks and other prebiotic compounds like SO2 could be an efficient way to generate the precursor of nucleobases, amino acids, and lipids.
Compared to HCN, HCN-polymer is more stable and has diverse hydrolysis patterns under different conditions, and thus is considered to be a plausible source of many biotic molecules. The formation of HCN-polymer does not necessarily depend on the high concentration of HCN. At a temperature below −20 °C, an HCN tetramer (DAMN) was generated from a dilute solution (0.1 to 0.001 M). A subsequent room temperature melting step produced adenine, albeit in very low yields [42]. Based on this finding, the Miller group carried out a series of long-term experiments, in which 0.1 M NH4CN or a mixture of HCN and NH3 were frozen from 2 months to 27 years [43,44,45]. Cyanide polymer, adenine, guanine, and other compounds were found in the melt materials. Heating the reaction mixture with acid (6 N HCl) or alkali (0.01 M phosphate, pH = 8) solution can significantly enhance the production of adenine to 2.9 and 1.2%, though the acid hydrolysis also degenerates half of the generated adenine [45]. These studies showed a “cold” origin of nucleobases, in which the products can be stored for longer periods and accumulated in larger quantities to sustain subsequent reactions. Interestingly, HCN-polymers can also form under an alkaline hydrothermal environment. Heat 0.15 M HCN over 100 °C, pH = 10 generated stable polymers [46,47]. Theoretically, under such an environment, HCN polymers can be used to synthesize nucleobases, and further experimental research is required [48].
Formamide which is generated during HCN hydrolysis was intriguing because of its potential to reach high concentration and boiling point, which means it can suffer high-temperature reactions. Heating formamide between 140 and 200 °C gave high yields of purines [37,49]. Several minerals can change the composition and yield of the products: adding CaCO3, TiO2, phosphate minerals, and iron sulfide minerals during heating facilitate the production of adenine [50,51]. Ultraviolet light also has such an effect [52]. A disadvantage of formamide is its high hygroscopicity, which means that formamide mainly exists in the mixture with water. Nucleobases cannot be effectively produced in formamide solutions with more than 10% water content [51]. Some special conditions can remove water from formamide. At a temperature between the freezing point of formamide (2.25 °C) and water, formamide froze and sank to the bottom of the water body. When the temperature continues to drop below 0 °C, the top water layer also froze, which may be removed by a following sublimation or ablation process [53]. Purification of formamide could also be achieved in a fractional distillation-like manner with a suitable temperature gradient from 10 to 70 °C [54].
Beyond HCN-related materials, adenine can also be obtained from the combinations of N2, ammonia, methane, CO, and water, which demand high energy input such as electron beam, electric discharge, laser-driven plasma, or high-temperature plasma [37]. In some of these processes, HCN is also generated and worked as an intermediate product [55]. Adenine and other nucleobases can also be derived from more complex starting materials such as urea and amino acid derivatives, as summarized by Yadav et al. [37]. These pathways should still essentially be traced back to the aforementioned simple small molecules and are not discussed in this paper.
Among the five nucleobases used in current life, adenine seems to be the most readily abiotically synthesized through the HCN-based pathways, which may be the reason for its more widespread use in life. Adenine has a half-life of 1 year at 100 °C in solution and can be extended to decades at lower temperatures [56]. If out of the water environment, the half-life of dry adenine can be over 106 years, enabling geological time scale accumulation [57].
3.2. Ribose Synthesis
The formose reaction discovered in 1861 has long been of interest as a plausible prebiotic sugar synthesis pathway [58]. In the canonical formose reaction (in an alkaline solution environment, catalyzed by the minerals), formaldehyde or other low-carbon aldol polymerize and generates a complex mixture of mono- and polysaccharides. The materials and conditions required for the reaction to occur are plausible: formaldehyde can be generated in the photochemical and electrochemical reactions from CO2 in the primitive atmosphere, or delivered by meteorites or comets [59]. The alkaline solution environment can be provided by hydrothermal vents. However, using formose reaction as the ribose source to synthesize ATP needs to solve several problems [37]. First, while the reaction demands a relatively high concentration of formaldehyde (about 0.1 M), it is easily converted to formate and methanol in solution. Second, the kinetics and products of the reaction are complex. The composition of the reaction product is greatly affected by the reaction time, excessive reaction time reduces the yield of sugars; and in any case, ribose is always a small part of the product. Third, the aimed product, ribose, is also unstable, with a half-life of less than one hour at 100 °C. Therefore, for the accumulation of ribose based on formose reaction, an environment with an adequate supply of formaldehyde as well as the ability to enhance ribose production, selection/purification, and preservation would be ideal.
Among the possible sources of formaldehyde on primitive Earth, the photochemical reaction in the atmosphere may be the most prevalent one. Under the action of hydrogen and light, CO2 was reduced to methane in several steps. According to a pioneer model, formaldehyde produced in the atmosphere was rained into the ocean and accumulates to 10−3 M over millions of years [60]. Volcanic activity can facilitate this process, which provides large amounts of CO2 and hydrogen, and simultaneously induce electrics—the energy source of electrochemical synthesis of formaldehyde. Formaldehyde from atmospheric sources should be more abundant in shallow waters, such as terrestrial hot spring systems and intertidal zone near volcanoes. In the opposite direction, deep-sea hydrothermal vents are “hot” areas in the origin of life studies because of their ability to produce abundant organic matter. Theoretical and simulation studies suggested that hydrothermal vents can generate formaldehyde through methanediol reduction and dehydration [61], or catalyze formose reaction in CaCO3-based chemical gardens [62], though there is no conclusive evidence that these processes occur near modern hydrothermal vents.
After ensuring the source of raw materials, the next issue is enhancing ribose production. Several minerals could be helpful. Borate and silicate minerals can stabilize sugars by binding them with released ions, which not only stabilize the sugar but may also modulate the reaction process [63,64]. However, the availability of borate ions in primitive Earth is questionable, due to the scarcity of the element in the earth’s crust. The selectivity of silicate on ribose is controversial. Moreover, these processes may result in complex that cannot undergo further reactions or so-called “dead-end” complex [37]. The participation of phosphors also helps to enhance ribose formation. Hydroxyapatite was reported to enhance ribose production, but it is still not a major product [65]. Using phosphorylated aldehydes as starting materials, products that are less complex than the conventional formose reaction can be obtained. Mixing glycolaldehyde-2-phosphate with formaldehyde in 2 M NaOH, up to 15% ribose 2,4-diphosphate can be generated at room temperature [66].
Purifying or selecting ribose from complex products could be another solution. Recently, Zhao and Wang’s experiments showed that metal-doped-clays (MDC), which can be widely speared on the primitive Earth, is a new solution [67]. They found that clays including kaolin, montmorillonite, and mica which doped Cu2+, Ca2+, or Fe2+ have a significant preference for the retention of ribose in the formose reaction products. This effect can separate ribose from the alkaline reaction solution thereby enriching and protecting it to a certain extent. Silica mineral surface is also able to absorb and protect ribose and prefers the furanose form [68].
Beyond the formose reaction, the “glyoxylate scenario” is another possible prebiotic pathway to ribose. Instead of formaldehyde, the reaction started with glyoxylate and its dimmer dihydroxyfumarate (DHF) and generate ketoses and sugar acids in a more “clean” way. The experiment showed that the glyoxylate scenario is feasible under lithium or cesium catalysis, at pH ≈ 8–9, 4 °C [69]. Whether this more specific approach to producing ribose precursors can be implemented on primitive Earth requires more investigation. Glyoxylate may be easily generated by the reductive conversion of CO2, but the prebiotic synthesis of DHF is only theoretical. Moreover, the catalysts currently used are not abundant on Earth, more common materials will provide a higher probability that the scenario occurred on primitive Earth.
No matter how ribose was generated on the primitive Earth, the short half-life in minutes in aquatic environments is an obstacle to its participation in the synthesis of biomolecules [70]. The alkaline and normally hot condition in formose reaction is especially harmful to ribose. Adsorption of ribose to the solid phase can extend its half-life to hours, a limited effect [68]. Low temperature can preserve ribose solution for decades [70], but it does not seem to be compatible with volcanic environments. It may be a more plausible scenario that ribose is used to synthesize nucleosides as soon as possible after production.
3.3. Adenosine Synthesis and Phosphorylation
Early studies tried the synthesis of nucleosides by heating the mixture of ribose and nucleobases. This simple method works in adenosine under the catalysis of MgCl2 and trimetaphosphate, achieving a yield of about 4% [71]. Phosphorylated ribose is the substrate of the biological synthesis of nucleosides and performed better in abiotic reactions. The attempts to synthesize nucleosides with phosphorylated ribose have been partly successful in adenosine. A 15% yield of adenosine-2′-phosphate was obtained by heating the dilute solution of ribose-1,2-cyclic phosphate and adenine to dryness at 85 °C in the presence of calcium chloride [72]. However, this kind of method cannot produce other nucleosides efficiently [73]. Several environmental factors, such as silica surface, kaolinite, and aqueous microdroplet, were reported to facilitate the condensation of the parts of nucleosides and nucleotides, but inconsistent results of the constitution or structure of the products from different methods appeared in some studies, suggested that they should be regarded with caution [37]. Moreover, this kind of method also cannot produce pyrimidine nucleosides.
Beyond condensing ribose (or phosphorylated ribose) and nucleobase, there are alternative methods to produce nucleosides. By constructing a cytidine bit-by-bit on a phosphorylated ribose with cyanamide, Sanchez and Orgel achieved the synthesis of pyrimidine nucleosides firstly, though the yield of the target product β-cytosine was 5% [74]. The key intermediate in the reaction, amino-oxazoline, inspired other studies, represented by the Sutherland groups. In their system, amino-oxazoline originates from HCN and H2O, and then produces pyrimidine nucleosides [75]. This pathway is also a part of the HCN-based reaction network, which produces multiple amino acids and lipid precursors [76]. In an altered route, instead of amino-oxazoline, 2-thiooxazole was synthesized first by glycoaldehyde and thiocyanic under heating and dehydration at 80 °C [77]. Interestedly, this sulfur-dependent scenario can generate both purine and pyrimidine nucleotides, through experiencing acidic or basic conditions. However, the final products are generated by heating with phosphate in a urea/formamide mixture, this condition may not be prevalent on primitive Earth.
If it is assumed that the phosphorus problem can be solved on the primitive Earth as mentioned above, the phosphorylation of nucleobase should be available. Several different phosphorylation reagents have been tested and summarized by Kitadai and Maruyama [36]. Basically, adenosine monophosphate (AMP) can be generated from adenosine by heating with phosphate or polyphosphates in dry or aquatic conditions. Heating the adenosine solution containing urea, MgSO4, K2CO3, or NH4OH; and the analogs of schreibersite (Fe3P and Fe2NiP) also generate AMP [32]. The earliest abiotic synthesis of ATP in the context of the origin of life was performed in 1963, in which ethyl metaphosphate and adenosine dilute solution (less than 1 mM) was treated with UV irradiation at 40 °C, which generated 0.5% ATP, 0.2% ADP, and 0.1% ATP in one hour [78]. The subsequent studies showed that trimetaphosphate is arguably the most successful phosphorylation reagent for ATP synthesis. Kept the solution containing 0.05 M adenosine and trimetaphosphate at room temperature and pH 8-12 for ten days can produce 91.8% AMP and 3% ATP. High pH (12-14) promotes the production of ATP (9.9%) but degrade AMP (8.5%) [79]. Dehydration and metal ions promote the phosphorylation reaction. Under the catalyzing of Ni ions and four rounds of wet-dry cycles at 37 °C in two weeks, the mixture of 0.02 M adenosine and 0.2 M sodium trimetaphosphate generates different adenosine phosphates including 13% ATP [80]. The reaction catalyzed by Ag ions reached a 23.7% ATP yield, though this rare element cannot participate in prebiotic reactions on a large scale. ATP can also be generated in a single aqueous phase or solid phase reactions, albeit less efficiently. Certainly, ATP can be generated from AMP and ADP, with similar conditions in AMP formation [36]. The difficulties and solutions in ATP synthesis are summarized in Table 1.

Table 1.
The difficulties and solutions in ATP synthesis.
3.4. A Terrestrial Volcanism-Dependent Pathway to ATP
As so far, we summarized the possible steps in ATP abiotic synthesis and discussed the environments that may support them. Terrestrial volcanism could be an important contributor to linking these steps together to construct a pathway connecting the basic components of primitive Earth and ATP. During volcano activity, the generated large amounts of hydrocarbons, CO, and CO2 altered the local atmospheric composition. Combined with triggered lightning, the atmospheric environment around active volcanoes should be suitable for HCN and formaldehyde production, which serve as the source substances of nucleobase and ribose. Polyphosphates, especially trimetaphosphate from milder volcanic activity enable phosphorylation reactions. The geothermal effect can promote reactions that require heating.
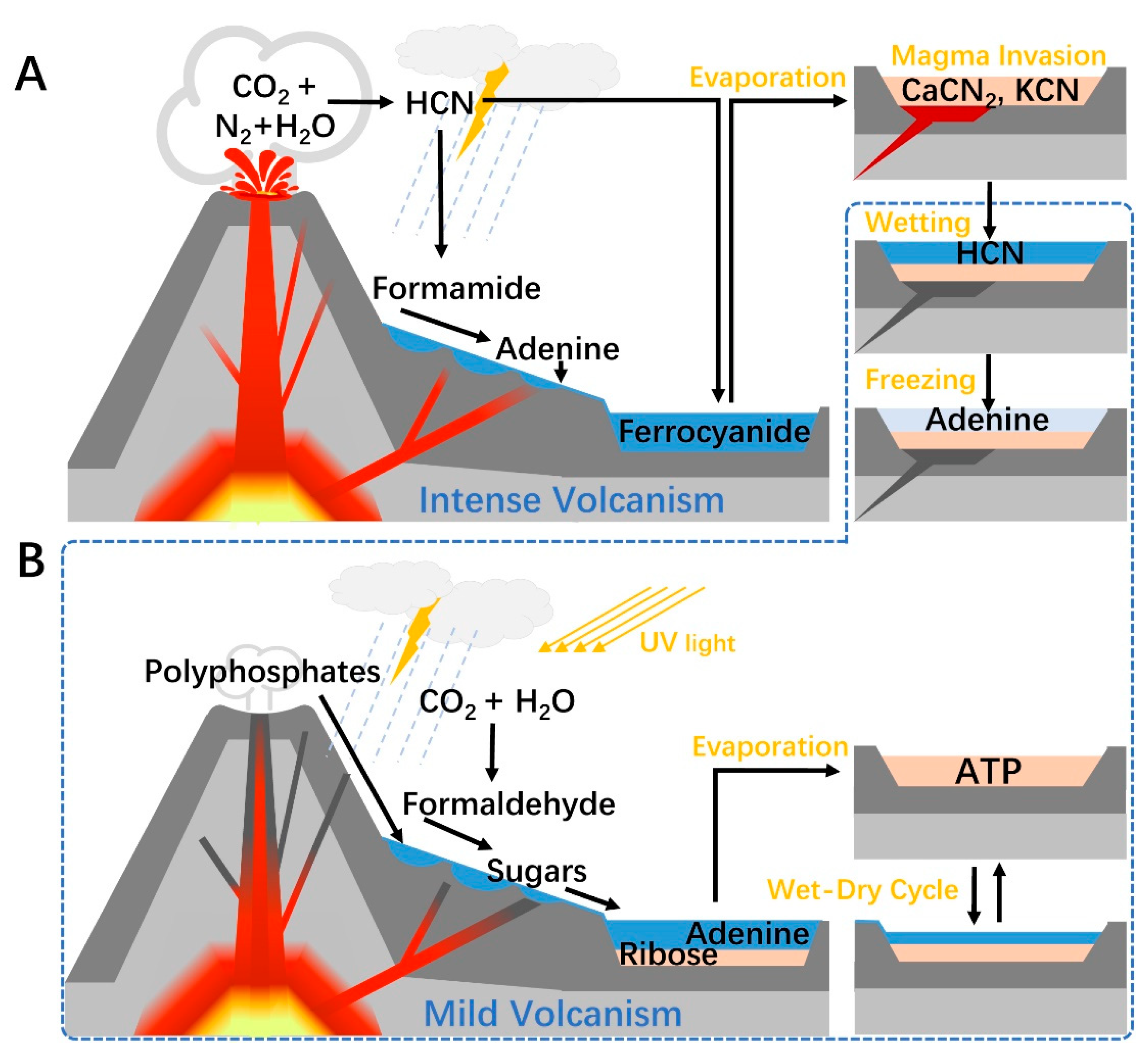
Centering on volcanic activity, a plausible pathway for prebiotic ATP synthesis could be constructed. In the first stage, the volcano is highly active (Figure 2A), large amounts of HCN and formamide were produced in the surrounding atmosphere and washed into lakes or lagoons by rain and rivers. A suitable temperature gradient may form on the slope of the volcano, where the water was gradually evaporated to obtain concentrated formamide. By mineral catalysis, the remained formamide can yield adenine which can be preserved in the dry basin. This process may also trigger the HCN preservation scenario supposed by Sasselov et al. [41], if magma invades the basin, though it can also degrade the already formed adenine. In the second stage, the volcanic activity turns mild (Figure 2B). Cyanide-rich basin generated in the previous stage was again filled with water and released HCN. Freezing of water bodies in winter can induce the polymerization of HCN and produce adenine. A terrestrial hydrothermal environment may also appear near the volcano, which can enable the hot polymerization of HCN. Formose reaction may occur in temporary ponds formed by rainfall or hydrothermal processes that adjacent to volcanic vents, under mineral catalysis and geothermal heating. Temporary runoff from the surface of the volcanic body washed down reaction products, which can prevent overreaction that could lead to ribose degradation. In this process, polyphosphates can be mixed into the water stream and ribose may also be phosphorylated. Small-grained minerals and clays in water channels may separate ribose from other materials and carried away by the current. When this current flushes into a pond or basin containing adenine, the substrates for ATP synthesis are assembled, which will be condensed during the drying process driven by geothermal or sunlight. ATP can be accumulated during wet-dry cycles. The half-life of ATP at room temperature solution ranges from years to decades depending on the pH and ions [81], which could be longer in freezing or dry conditions. Therefore, ATP may have existed relatively stable on primitive Earth, waiting for interacting with other molecules.
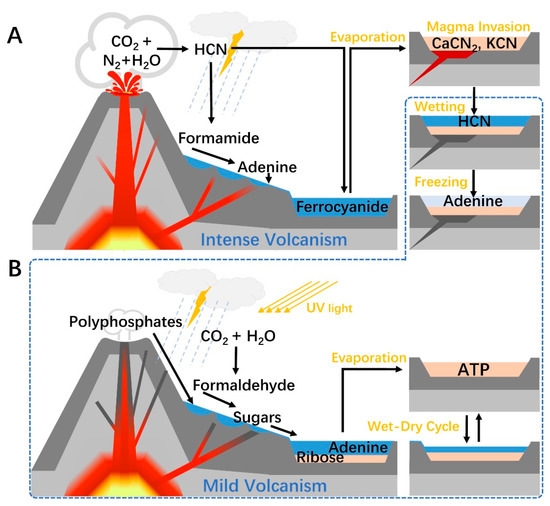
Figure 2.
A terrestrial volcanism-dependent pathway for prebiotic ATP synthesis. (A) Adenine synthesis. During intense volcanism, HCN is generated in the atmosphere around an active volcano and then partially hydrolyzed to formamide. As the mixture of formamide and water washed down the slope, the increasing temperature gradient, created by geothermal activity, removed most of the water by fractionation. Adenine is produced in the hot formamide pond and is washed into cooler water. Alternatively, after HCN enters a mineral-rich pool, it forms ferrocyanide with ferrous ions and is converted into soluble cyanide salts after suffering the intense heat caused by magma invasion. During mild volcanism, cyanide salts revert to HCN when encountering water, and the latter polymerizes under a freezing condition to give adenine. (B) Ribose and ATP synthesis. Formaldehyde is produced in the atmosphere by photochemical and electrochemical reactions, then washed into the ponds around the volcano along with the polyphosphates from the fumarole. Formose reaction occurred in these pounds, then the generated ribose may be separated from the sugars by minerals along the way downstream. Finally, the substrates are assembled in a pool or lake at the foot of the volcano, and ATP can be synthesized during wet-dry cycles.
4. Discussion
Because of the fundamentality and multiplicity of its roles, ATP has long been a focus of studies in various fields of life sciences. The previous content showed the possible pathway to ATP on primitive Earth. However, whether ATP assumed its present functions from the moment it was synthesized is still debatable. As a complex system, modern organisms are constructed by thousands of molecules, from simple H2O to genome DNA, ATP also depends on other molecules to function, which should also be the case in prebiotic chemical evolution.
As a component of RNA and cofactor of protein, ATP needs to cooperate with other nucleotides and amino acids to form functional polymers. HCN and its derivative acrylonitrile have been supposed to be sources of pyrimidine nucleotides, amino acids, and lipid precursors [76]. The related reactions may be occurred in different places with suitable conditions and the products are gathered through the water system. The wet-dry cycle environment in which ATP is produced is also appropriate for the condensation of nucleotides and amino acids. If ATP is present when the peptide is formed, it may bind to the latter and help them fold. This effect may have picked out some of the earliest structured peptides [82,83]. As evidence, ATP binds some oldest protein structures and conserved sequence fragments and is the most prevalent cofactor in protein structure space [2]. in vitro selection experiments using ATP as the bait in random sequence peptides obtained items with a similar structure or sequence motif to modern ATP binding proteins [84,85]. The inheritance of these proteins remains a problem because how RNA coding proteins and self-replication still does not have a solution. Recent studies showing the preference for interactions between different oligonucleotides and amino acids [86], as well as the modified nucleotides which are easier to be ligated [87], have shed new light on these problems. Moreover, the homochirality of RNA and protein, which is a significant issue in the origin of life, may also be achieved during the replication of RNA and ribozyme catalyzed peptide synthesis [88,89,90].
Interestingly, the in vitro selected peptides have ATPase activity, which is connected to the role of ATP as an energy currency. In modern organisms, the energy from ATP phosphate bonds hydrolysis is generally channeled to certain energy-consuming processes through specific proteins with ATPase activity. In addition to the ATP binding domain, these proteins normally have other domains to specifically bind substrates of the reaction that need to be powered. These proteins not only promote energy release but more importantly also enables precise control of energy flow. In the early stage of chemical evolution, these processes are unlikely to involve protein enzymes. Without the controller, the energy sources may be more extensive than the specific use of ATP. That is, the role of ATP as energy currency may be established as its binding proteins became dominant. Other simpler compounds such as pyrophosphate and acetyl phosphate may be more widely used in the prebiotic reactions [91]. Similarly, other simpler phosphorylation reagents may also have been widely used before the advent of kinases.
The recently identified hydrotrope function of ATP depends on its concentration at the millimolar level. This function may only be available and significant in the membrane-enclosed vesicles. In modern life, the membrane is a necessary condition for the stable supply of large amounts of ATP. ATP synthase is driven by the proton gradient inside and outside the membrane. The complex structure of ATP synthase implies its later origination. Moreover, the main function of hydrotrope is inhibiting biomacromolecule aggregation. If not confined in vesicles, it should also be difficult for macromolecules to reach concentrations where they can aggregate.
In summary, based on previous findings on the synthesis of ATP components, we constructed a terrestrial volcanism-dependent ATP synthesis pathway. Some other reactions that may play an important role in the prebiotic synthesis of ATP were also briefly described. After appearing on Earth, ATP may have first served as the component of macromolecules and driven energy-consuming processes together with other high-energy molecules. As enzyme-catalyzed reactions took over the original abiotic ones, ATP became the main direct energy source. Perhaps no later than the emergence of the enzyme system, the membranes that encapsulate it also appeared. To avoid the harmful aggregation of macromolecules in the crowded primitive cell, ATP got a new role as a hydrotrope. Beyond the fundamental functions in the origin of life, in modern organisms, ATP plays a role in antiaging [92], and may have an application in precision medicine by inspiring the identification of prognostic biomarkers and treatments for breast cancer [93].
An area less covered above is the possible contribution of extraterrestrial materials in ATP synthesis. In addition to the reactive phosphorus, ribose [94], nucleobases [95], and other abundant organics [96] have also been identified in meteorites, which may be involved in the prebiotic synthesis of ATP. The possible mechanism for how these compounds were generated in space was also reported recently [97]. The volcanism-based ATP synthesis pathway proposed in this paper may also be achieved beyond Earth. Primitive Mars is supposed to be somehow similar to primitive Earth. Primitive Mars was once geologically active, and Olympus Mons, the highest mountain in the solar system, was also created by volcanism [98]. Volcanic lightning can produce HCN in the carbon dioxide-nitrogen Martian atmosphere [99]. Geological activity may also be a source of formaldehyde [100]. In addition, Martian phosphate minerals have higher dissolution rates and phosphate release rates than common Earth phosphate minerals, which is conducive to the occurrence of phosphorus-related reactions [101]. Based on these substances, Earth-like planets such as Mars may be able to generate ATP according to the pathway we proposed. On the contrary, on smaller celestial bodies that cannot maintain geological activity and atmosphere, other ways are needed to produce ATP.
Author Contributions
H.-Y.Z. defined the scope of the paper. X.-Y.C. wrote the main manuscript text and prepared Figure 1. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31870837), the Scientific Research Grant of Ningbo University (215-432000282), and the Ningbo Top Talent Project (215-432094250).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing financial interests.
References
- Ji, H.-F.; Kong, D.-X.; Shen, L.; Chen, L.-L.; Ma, B.-G.; Zhang, H.-Y. Distribution patterns of small-molecule ligands in the protein universe and implications for origin of life and drug discovery. Genome Biol. 2007, 8, R176. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.-Y.; Zhang, H.-Y. Cofactors as molecular fossils to trace the origin and evolution of proteins. ChemBioChem 2020, 21, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Malinovska, L.; Saha, S.; Wang, J.; Alberti, S.; Krishnan, Y.; Hyman, A.A. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Xu, Y.-Y.; Tong, X.-Y.; Wang, G.; Zhang, H.-Y. The legend of ATP: From origin of life to precision medicine. Metabolites 2022, 12, 461. [Google Scholar] [CrossRef]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segrè, D. Remnants of an ancient metabolism without phosphate. Cell 2017, 168, 1126–1134.e9. [Google Scholar] [CrossRef] [PubMed]
- Goldford, J.E.; Hartman, H.; Marsland, R.; Segrè, D. Environmental boundary conditions for the origin of life converge to an organo-sulfur metabolism. Nat. Ecol. Evol. 2019, 3, 1715–1724. [Google Scholar] [CrossRef]
- Raanan, H.; Poudel, S.; Pike, D.H.; Nanda, V.; Falkowski, P.G. Small protein folds at the root of an ancient metabolic network. Proc. Natl. Acad. Sci. USA 2020, 117, 7193–7199. [Google Scholar] [CrossRef]
- Pasek, M.A. Thermodynamics of prebiotic phosphorylation. Chem. Rev. 2020, 120, 4690–4706. [Google Scholar] [CrossRef]
- Fialho, D.M.; Karunakaran, S.C.; Greeson, K.W.; Martínez, I.; Schuster, G.B.; Krishnamurthy, R.; Hud, N.V. Depsipeptide nucleic acids: Prebiotic formation, oligomerization, and self-assembly of a new proto-nucleic acid candidate. J. Am. Chem. Soc. 2021, 143, 13525–13537. [Google Scholar] [CrossRef]
- Palme, H.; Lodders, K.; Jones, A. Solar system abundances of the elements. In Treatise on Geochemistry, 2nd ed.; Davis, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 15–36. [Google Scholar]
- Sossi, P.A.; Burnham, A.D.; Badro, J.; Lanzirotti, A.; Newville, M.; O’Neill, H.S.C. Redox state of Earth’s magma ocean and its venus-like early atmosphere. Sci. Adv. 2020, 6, eabd1387. [Google Scholar] [CrossRef]
- Villanueva, G.L.; Mumma, M.J.; Novak, R.E.; Käufl, H.U.; Hartogh, P.; Encrenaz, T.; Tokunaga, A.; Khayat, A.; Smith, M.D. Strong water isotopic anomalies in the Martian atmosphere: Probing current and ancient reservoirs. Science 2015, 348, 218–221. [Google Scholar] [CrossRef]
- Vu, T.H.; Hodyss, R.; Choukroun, M.; Johnson, P.V. Chemistry of frozen sodium–magnesium–sulfate–chloride brines: Implications for surface expression of Europa’s ocean composition. Astrophys. J. Lett. 2016, 816, L26. [Google Scholar] [CrossRef]
- Keane, T.C. Mechanism for the coupled photochemistry of ammonia and acetylene: Implications for giant planets, comets and interstellar organic synthesis. Orig. Life Evol. Biosph. 2017, 47, 223–248. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.M.; Kamp, L.W.; Matson, D.L.; Irwin, P.G.J.; Baines, K.H.; Boryta, M.D.; Leader, F.E.; Jaumann, R.; Smythe, W.D.; Sotin, C.; et al. Saturn’s Titan: Surface change, ammonia, and implications for atmospheric and tectonic activity. Icarus 2009, 199, 429–441. [Google Scholar] [CrossRef]
- Gibb, B.C. A Chemist’s guide to the Solar system. Nat. Chem. 2015, 7, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Dalle Ore, C.M.; Cruikshank, D.P.; Protopapa, S.; Scipioni, F.; McKinnon, W.B.; Cook, J.C.; Grundy, W.M.; Schmitt, B.; Stern, S.A.; Moore, J.M.; et al. Detection of ammonia on Pluto’s surface in a region of geologically recent tectonism. Sci. Adv. 2019, 5, eaav5731. [Google Scholar] [CrossRef]
- Pasek, M.A. Phosphorus volatility in the early Solar nebula. Icarus 2019, 317, 59–65. [Google Scholar] [CrossRef]
- Walton, C.R.; Shorttle, O.; Jenner, F.E.; Williams, H.M.; Golden, J.; Morrison, S.M.; Downs, R.T.; Zerkle, A.; Hazen, R.M.; Pasek, M. Phosphorus mineral evolution and prebiotic chemistry: From minerals to microbes. Earth Sci. Rev. 2021, 221, 103806. [Google Scholar] [CrossRef]
- Pasek, M.A.; Gull, M.; Herschy, B. Phosphorylation on the early Earth. Chem. Geol. 2017, 475, 149–170. [Google Scholar] [CrossRef]
- Schreiber, U.; Locker-Grütjen, O.; Mayer, C. Hypothesis: Origin of life in deep-reaching tectonic faults. Orig. Life Evol. Biosph. 2012, 42, 47–54. [Google Scholar] [CrossRef]
- Gull, M.; Zhou, M.; Fernández, F.M.; Pasek, M.A. Prebiotic phosphate ester syntheses in a deep eutectic solvent. J. Mol. Evol. 2014, 78, 109–117. [Google Scholar] [CrossRef]
- Schwartz, A.W. Prebiotic phosphorylation-nucleotide synthesis with apatite. Biochim. Biophys. Acta 1972, 281, 477–480. [Google Scholar] [CrossRef]
- Toner, J.D.; Catling, D.C. A Carbonate-rich lake solution to the phosphate problem of the origin of life. Proc. Natl. Acad. Sci. USA 2020, 117, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Gull, M. Prebiotic phosphorylation reactions on the early Earth. Challenges 2014, 5, 193–212. [Google Scholar] [CrossRef]
- Gan, D.; Ying, J.; Zhao, Y. Prebiotic chemistry: The role of trimetaphosphate in prebiotic chemical evolution. Front. Chem. 2022, 10, 941228. [Google Scholar] [CrossRef]
- Yamagata, Y.; Watanabe, H.; Saitoh, M.; Namba, T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 1991, 352, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Miller, S.L. Are polyphosphates or phosphate esters prebiotic reagents? J. Mol. Evol. 1995, 41, 639–702. [Google Scholar] [CrossRef] [PubMed]
- McBeath, T.M.; Lombi, E.; McLaughlin, M.J.; Bünemann, E.K. Polyphosphate-fertilizer solution stability with time, temperature, and PH. Z. Nutr. Soil. Sc. 2007, 170, 387–391. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Usuzan. Available online: https://www.data.jma.go.jp/svd/vois/data/tokyo/STOCK/souran_eng/volcanoes/015_usuzan.pdf (accessed on 15 June 2022).
- Pasek, M.A.; Dworkin, J.P.; Lauretta, D.S. A Radical pathway for organic phosphorylation during schreibersite corrosion with implications for the origin of life. Geochim. Cosmochim. Acta 2007, 71, 1721–1736. [Google Scholar] [CrossRef]
- Gull, M.; Mojica, M.A.; Fernández, F.M.; Gaul, D.A.; Orlando, T.M.; Liotta, C.L.; Pasek, M.A. Nucleoside phosphorylation by the mineral schreibersite. Sci. Rep. 2015, 5, 17198. [Google Scholar] [CrossRef]
- Sydow, C.; Seiband, C.; Siegle, A.F.; Trapp, O. Phosphorylation in liquid sulfur dioxide under prebiotically plausible conditions. Commun. Chem. 2022, 5, 143. [Google Scholar] [CrossRef]
- Pasek, M.A. Rethinking early earth phosphorus geochemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 853–858. [Google Scholar] [CrossRef]
- Pasek, M.A.; Harnmeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc. Natl. Acad. Sci. USA 2013, 110, 10089–10094. [Google Scholar] [CrossRef]
- Kitadai, N.; Maruyama, S. Origins of building blocks of life: A review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, R.; Krishnamurthy, R. Chemistry of abiotic nucleotide synthesis. Chem. Rev. 2020, 120, 4766–4805. [Google Scholar] [CrossRef]
- Green, N.J.; Xu, J.; Sutherland, J.D. Illuminating life’s origins: UV photochemistry in abiotic synthesis of biomolecules. J. Am. Chem. Soc. 2021, 143, 7219–7236. [Google Scholar] [CrossRef] [PubMed]
- Oró, J. Synthesis of adenine from ammonium cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412. [Google Scholar] [CrossRef]
- Shapiro, R. The Prebiotic role of adenine: A critical analysis. Orig. Life Evol. Biosph. 1995, 25, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Sasselov, D.D.; Grotzinger, J.P.; Sutherland, J.D. The origin of life as a planetary phenomenon. Sci. Adv. 2020, 6, eaax3419. [Google Scholar] [CrossRef]
- Sanchez, R.; Ferris, J.; Orgel, L.E. Conditions for purine synthesis: Did prebiotic synthesis occur at low temperatures? Science 1966, 153, 72–73. [Google Scholar] [CrossRef]
- Levy, M. Prebiotic synthesis of adenine and amino acids under Europa-like conditions. Icarus 2000, 145, 609–613. [Google Scholar] [CrossRef]
- Miyakawa, S.; James Cleaves, H.; Miller, S.L. The cold origin of life: A. implications based on the hydrolytic stabilities of hydrogen cyanide and formamide. Orig. Life Evol. Biosph. 2002, 32, 195–208. [Google Scholar] [CrossRef]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The cold origin of life: B. implications based on pyrimidines and purines produced from frozen ammonium cyanide solutions. Orig. Life Evol. Biosph. 2002, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Gálvez-Martínez, S.; Mateo-Martí, E.; Colín-García, M. A Lizardite–hcn interaction leading the increasing of molecular complexity in an alkaline hydrothermal scenario: Implications for origin of life studies. Life 2021, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Colín-García, M. Characterization of HCN-derived thermal polymer: Implications for chemical evolution. Processes 2020, 8, 968. [Google Scholar] [CrossRef]
- LaRowe, D.E.; Regnier, P. Thermodynamic potential for the abiotic synthesis of adenine, cytosine, guanine, thymine, uracil, ribose, and deoxyribose in hydrothermal systems. Orig. Life Evol. Biosph. 2008, 38, 383–397. [Google Scholar] [CrossRef]
- Yamada, H.; Hirobe, M.; Higashiyama, K.; Takahashi, H.; Suzuki, K.T. Reaction mechanism for purine ring formation as studied by 13C-15N coupling. Tetrahedron Lett. 1978, 19, 4039–4042. [Google Scholar] [CrossRef]
- Saladino, R. A possible prebiotic synthesis of purine, adenine, cytosine, and 4(3H)-pyrimidinone from formamide implications for the origin of life. Bioorg. Med. Chem. 2001, 9, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Neri, V.; Crestini, C.; Costanzo, G.; Graciotti, M.; Di Mauro, E. Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals. J. Am. Chem. Soc. 2008, 130, 15512–15518. [Google Scholar] [CrossRef]
- Barks, H.L.; Buckley, R.; Grieves, G.A.; Di Mauro, E.; Hud, N.V.; Orlando, T.M. Guanine, adenine, and hypoxanthine production in UV-irradiated formamide solutions: Relaxation of the requirements for prebiotic purine nucleobase formation. Chem. Eur. J. Chem. Biol. 2010, 11, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L.; Chalmers, J.H.; Cleaves, H.J. Is Formamide a geochemically plausible prebiotic solvent? Phys. Chem. Chem. Phys. 2016, 18, 20085–20090. [Google Scholar] [CrossRef] [PubMed]
- Niether, D.; Afanasenkau, D.; Dhont, J.K.G.; Wiegand, S. Accumulation of formamide in hydrothermal pores to form prebiotic nucleobases. Proc. Natl. Acad. Sci. USA 2016, 113, 4272–4277. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Pietrucci, F.; Saitta, A.M.; Knížek, A.; Kubelík, P.; Ivanek, O.; Shestivska, V.; Civiš, S. Formation of nucleobases in a Miller–Urey reducing atmosphere. Proc. Natl. Acad. Sci. USA 2017, 114, 4306–4311. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Miller, S.L. The Stability of the RNA bases: Implications for the origin of life. Proc. Natl. Acad. Sci. USA 1998, 95, 7933–7938. [Google Scholar] [CrossRef]
- Rosenberg, E. Purine and pyrimidines in sediments from the experimental Mohole. Science 1964, 146, 1680–1681. [Google Scholar] [CrossRef]
- Butlerow, A. Bildung einer zuckerartigen substanz durch synthese. Ann. Chem. Pharm. 1861, 120, 295–298. [Google Scholar] [CrossRef]
- Cody, G.D.; Heying, E.; Alexander, C.M.O.; Nittler, L.R.; Kilcoyne, A.L.D.; Sandford, S.A.; Stroud, R.M. Establishing a molecular relationship between chondritic and cometary organic solids. Proc. Natl. Acad. Sci. USA 2011, 108, 19171–19176. [Google Scholar] [CrossRef]
- Pinto, J.P.; Gladstone, G.R.; Yung, Y.L. Photochemical production of formaldehyde in Earth’s primitive atmosphere. Science 1980, 210, 183–185. [Google Scholar] [CrossRef]
- Inaba, S. Primary formation path of formaldehyde in hydrothermal vents. Orig. Life Evol. Biosph. 2018, 48, 1–22. [Google Scholar] [CrossRef]
- Omran, A. Plausibility of the formose reaction in alkaline hydrothermal vent environments. Orig. Life Evol. Biosph. 2020. [Google Scholar] [CrossRef]
- Ricardo, A.; Carrigan, M.A.; Olcott, A.N.; Benner, S.A. Borate minerals stabilize ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef]
- Vázquez-Mayagoitia, Á.; Horton, S.R.; Sumpter, B.G.; Šponer, J.; Šponer, J.E.; Fuentes-Cabrera, M. On the stabilization of ribose by silicate minerals. Astrobiology 2011, 11, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Usami, K.; Okamoto, A. Hydroxyapatite: Catalyst for a one-pot pentose formation. Org. Biomol. Chem. 2017, 15, 8888–8893. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Pitsch, S.; Kittaka, A.; Wagner, E.; Wintner, C.E.; Eschenmoser, A.; Ohlofjgewidmet, G. Chemistry of a-aminonitriles. Aldomerization of glycolaldehyde phosphate to rac-hexose 2, 4, 6-triphosphates and (in presence of formaldehyde) rac-pentose 2, 4-diphosphates: Rac-allose 2, 4, 6-triphosphate and rac-ribose 2, 4-diphosphate are the main reaction products. Helv. Chim. Acta 1990, 73, 1410–1468. [Google Scholar] [CrossRef]
- Zhao, Z.-R.; Wang, X. A Plausible prebiotic selection of ribose for RNA—Formation, dynamic isolation, and nucleotide synthesis based on metal-doped-clays. Chem 2021, 7, 3292–3308. [Google Scholar] [CrossRef]
- Georgelin, T.; Jaber, M.; Fournier, F.; Laurent, G.; Costa-Torro, F.; Maurel, M.-C.; Lambert, J.-F. Stabilization of ribofuranose by a mineral surface. Carbohydr. Res. 2015, 402, 241–244. [Google Scholar] [CrossRef]
- Sagi, V.N.; Punna, V.; Hu, F.; Meher, G.; Krishnamurthy, R. Exploratory experiments on the chemistry of the “glyoxylate scenario”: Formation of ketosugars from dihydroxyfumarate. J. Am. Chem. Soc. 2012, 134, 3577–3589. [Google Scholar] [CrossRef]
- Larralde, R.; Robertson, M.P.; Miller, S.L. Rates of decomposition of ribose and other sugars: Implications for chemical evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 8158–8160. [Google Scholar] [CrossRef]
- Fuller, W.D.; Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis: VI. Synthesis of purine nucleosides. J. Mol. Biol. 1972, 67, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Benner, S.A. Prebiotic stereoselective synthesis of purine and noncanonical pyrimidine nucleotide from nucleobases and phosphorylated carbohydrates. Proc. Natl. Acad. Sci. USA 2017, 114, 11315–11320. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, J. A Prebiotic synthesis of canonical pyrimidine and purine ribonucleotides. Astrobiology 2019, 19, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis: V. Synthesis and photoanomerization of pyrimidine nucleosides. J. Mol. Biol. 1970, 47, 531–543. [Google Scholar] [CrossRef]
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef]
- Stairs, S.; Nikmal, A.; Bučar, D.-K.; Zheng, S.-L.; Szostak, J.W.; Powner, M.W. Divergent prebiotic synthesis of pyrimidine and 8-oxo-purine ribonucleotides. Nat. Commun. 2017, 8, 15270. [Google Scholar] [CrossRef]
- Ponnamperuma, C.; Sagan, C.; Mariner, R. Synthesis of adenosine triphosphate under possible primitive earth conditions. Nature 1963, 199, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Etaix, E.; Orgel, L.E. Phosphorylation of nucleosides in aqueous solution using trimetaphosphate: Formation of nucleoside triphosphates. J. Carbohydr. Nucleosides Nucleotides 1978, 5, 91–110. [Google Scholar]
- Cheng, C.; Fan, C.; Wan, R.; Tong, C.; Miao, Z.; Chen, J.; Zhao, Y. Phosphorylation of adenosine with trimetaphosphate under simulated prebiotic conditions. Orig. Life Evol. Biosph. 2002, 32, 219–224. [Google Scholar] [CrossRef]
- Stockbridge, R.B.; Wolfenden, R. The Intrinsic reactivity of ATP and the catalytic proficiencies of kinases acting on glucose, N-acetylgalactosamine, and homoserine. J. Biol. Chem. 2009, 284, 22747–22757. [Google Scholar] [CrossRef]
- Tokuriki, N.; Tawfik, D.S. Protein dynamism and evolvability. Science 2009, 324, 203–207. [Google Scholar] [CrossRef]
- Noor, E.; Flamholz, A.I.; Jayaraman, V.; Ross, B.L.; Cohen, Y.; Patrick, W.M.; Gruic-Sovulj, I.; Tawfik, D.S. Uniform binding and negative catalysis at the origin of enzymes. Protein Sci. 2022, 31, e4381. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Kim, K.M.; Caetano-Anollés, D. The Phylogenomic roots of modern biochemistry: Origins of proteins, cofactors and protein biosynthesis. J. Mol. Evol. 2012, 74, 1–34. [Google Scholar] [CrossRef]
- Kang, S.-K.; Chen, B.-X.; Tian, T.; Jia, X.-S.; Chu, X.-Y.; Liu, R.; Dong, P.-F.; Yang, Q.-Y.; Zhang, H.-Y. ATP selection in a random peptide library consisting of prebiotic amino acids. Biochem. Biophys. Res. Commun. 2015, 466, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Seligmann, H. First arrived, first served: Competition between codons for codon-amino acid stereochemical interactions determined early genetic code assignments. Sci. Nat. 2020, 107, 20. [Google Scholar] [CrossRef]
- Zhou, L.; O’Flaherty, D.K.; Szostak, J.W. Template-directed copying of RNA by non-enzymatic ligation. Angew. Chem. Int. Ed. 2020, 59, 15682–15687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, W. The Origin of biological homochirality along with the origin of life. PLoS Comput. Biol. 2020, 16, e1007592. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.-Y.; Zhang, H.-Y. Protein homochirality may be derived from primitive peptide synthesis by RNA. Astrobiology 2021, 21, 628–635. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Ying, J.; Liu, Y.; Zhang, G.; Zhao, Y. Selection of amino acid chirality induced by cyclic dipeptide synthesis in plausible prebiotic conditions. Front. Astron. Space Sci. 2022, 9, 794932. [Google Scholar] [CrossRef]
- Pinna, S.; Kunz, C.; Halpern, A.; Harrison, S.A.; Jordan, S.F.; Ward, J.; Werner, F.; Lane, N. A Prebiotic basis for ATP as the universal energy currency. PLoS Biol. 2022, 20, e3001437. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Wang, G.; Zhang, H.-Y. ATP as an anti-aging agent: Beyond the energy reservoir. Drug Discov. Today 2021, 26, 2783–2785. [Google Scholar] [CrossRef]
- Tong, X.-Y.; Liao, X.; Gao, M.; Lv, B.-M.; Chen, X.-H.; Chu, X.-Y.; Zhang, Q.-Y.; Zhang, H.-Y. Identification of NUDT5 inhibitors from approved drugs. Front. Mol. Biosci. 2020, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Ogawa, N.O.; Glavin, D.P.; Dworkin, J.P.; Abe, C.; Nakamura, T. Extraterrestrial ribose and other sugars in primitive meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Takano, Y.; Furukawa, Y.; Koga, T.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H. Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Naraoka, H.; Furukawa, Y.; Glavin, D.P.; Dworkin, J.P.; Tachibana, S. Extraterrestrial hexamethylenetetramine in meteorites—A precursor of prebiotic chemistry in the inner Solar system. Nat. Commun. 2020, 11, 6243. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Jiménez-Serra, I.; Puente-Sánchez, F.; Aguirre, J. The emergence of interstellar molecular complexity explained by interacting networks. Proc. Natl. Acad. Sci. USA 2022, 119, e2119734119. [Google Scholar] [CrossRef]
- NASA Confirms Thousands of Massive, Ancient Volcanic Eruptions on Mars. Available online: https://www.nasa.gov/feature/goddard/2021/nasa-confirms-thousands-of-massive-ancient-volcanic-eruptions-on-mars (accessed on 16 September 2022).
- Segura, A. Nitrogen fixation on early mars by volcanic lightning and other sources. Geophys. Res. Lett. 2005, 32, L05203. [Google Scholar] [CrossRef]
- Peplow, M. Formaldehyde claim inflames martian debate. Nature 2005, news050221-15. [Google Scholar] [CrossRef]
- Adcock, C.T.; Hausrath, E.M.; Forster, P.M. Readily available phosphate from minerals in early aqueous environments on mars. Nat. Geosci. 2013, 6, 824–827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).