Dynamics of Dopamine and Other Monoamines Content in Rat Brain after Single Low-Dose Carbon Nuclei Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Carbon (12C) Nuclei Irradiation
2.3. Dark–Light Box
2.4. Morris Water Maze
2.5. Tissue Collection and High-Performance Liquid Chromatography
2.6. Data Processing
3. Results
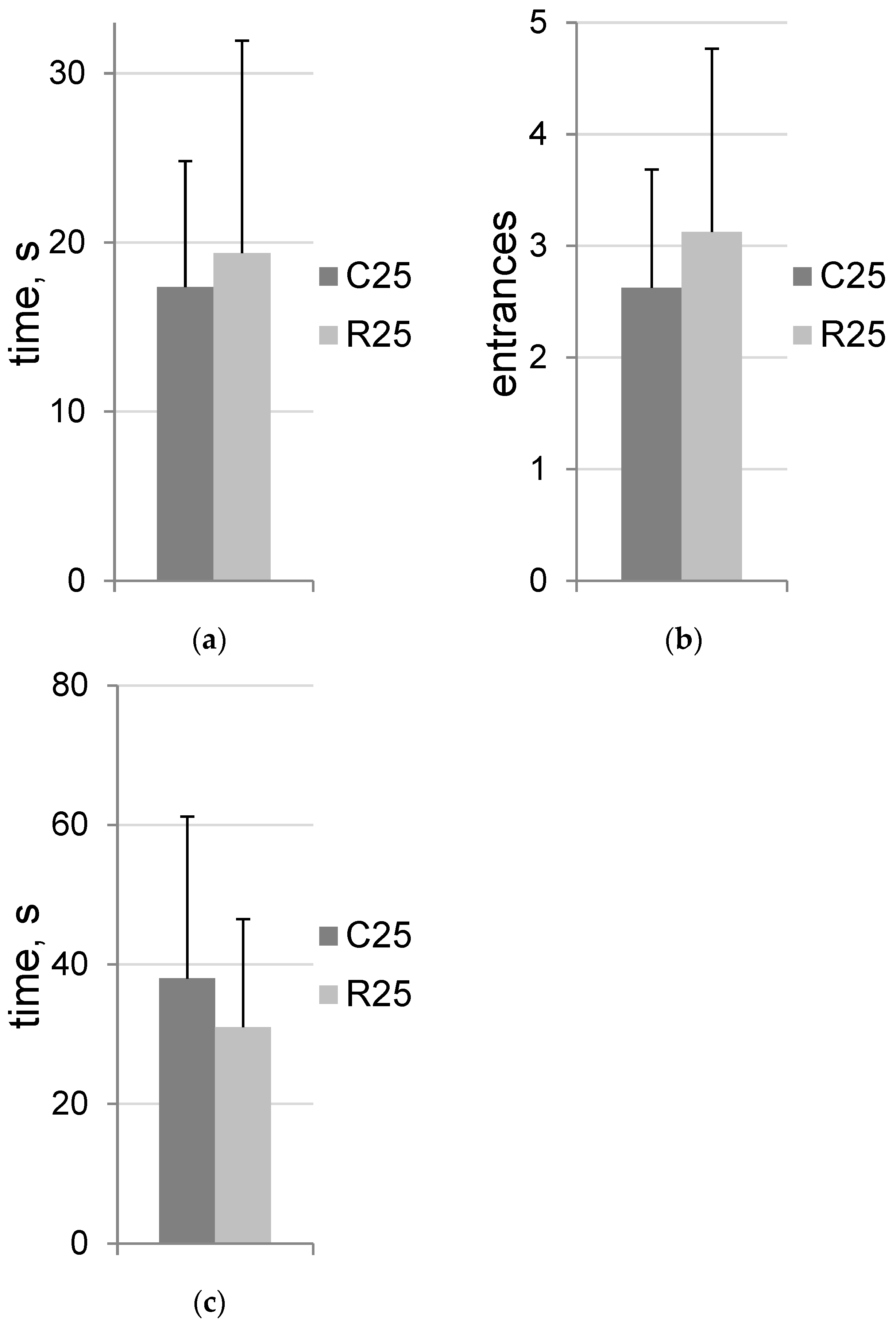
3.1. Irradiation Does Not Change Trait Anxiety
3.2. Irradiation Does Not Affect Spatial Training
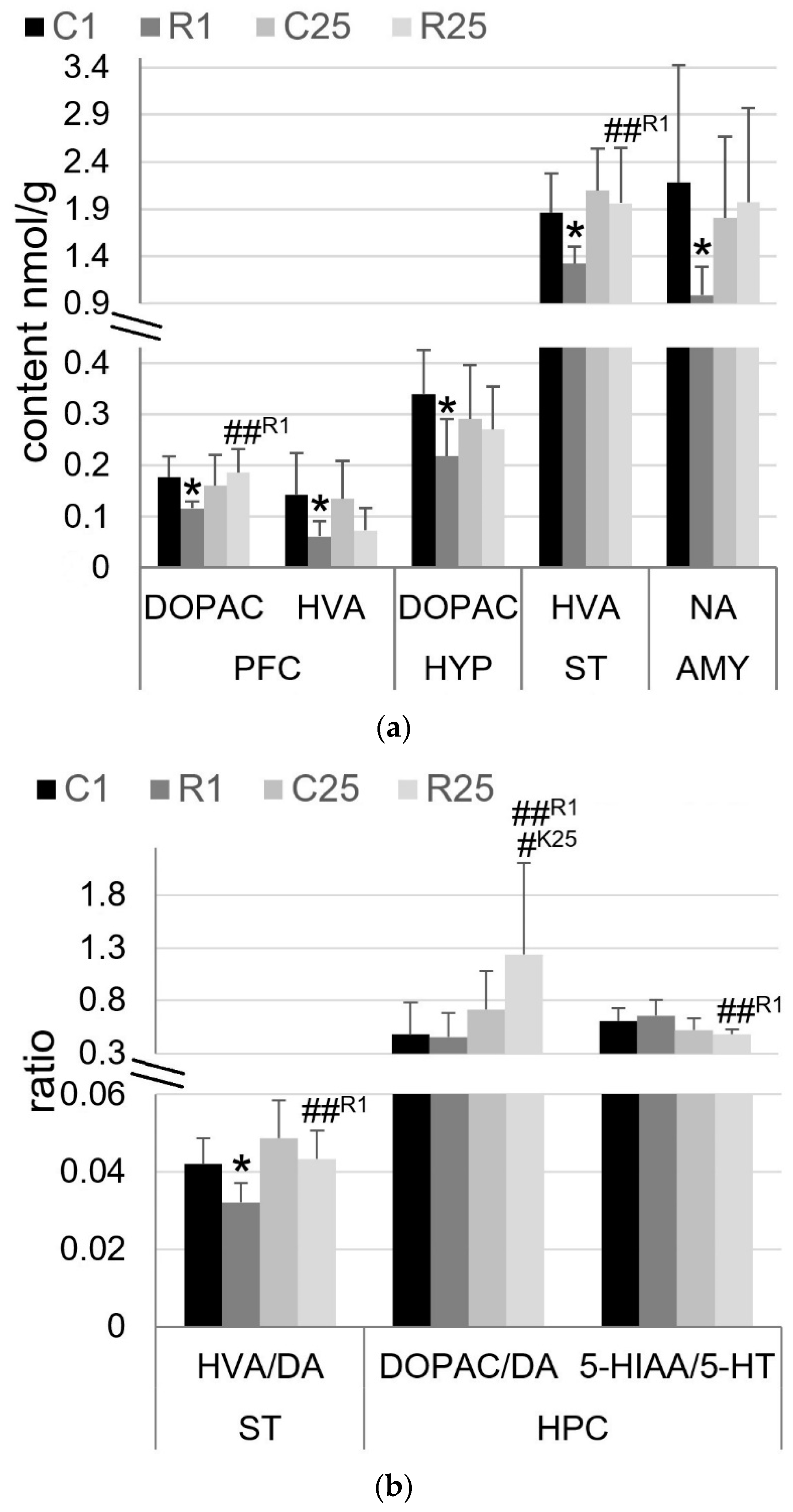
3.3. Irradiation Leads to Immediate and Transient Changes in Monoamine Metabolism, with the Exception of Hippocampus, Where It Appears Later
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeitlin, C.; Hassler, D.M.; Cucinotta, F.A.; Ehresmann, B.; Wimmer-Schweingruber, R.F.; Brinza, D.E.; Kang, S.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 2013, 340, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef] [PubMed]
- Lezniak, J.A.; Webber, W.R. The charge composition and energy spectra of cosmic-ray nuclei from 3000 MeV per nucleon to 50 GeV per nucleon. Astrophys. J. 1978, 223, 676–696. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Alp, M.; Sulzman, F.M.; Wang, M. Space radiation risks to the central nervous system. Life Sci. Space Res. 2014, 2, 54–69. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Rosi, S.; Costes, S.V. Central Nervous System Responses to Simulated Galactic Cosmic Rays. Int. J. Mol. Sci. 2018, 19, 3669. [Google Scholar] [CrossRef]
- Raber, J.; Fuentes Anaya, A.; Torres, E.R.S.; Lee, J.; Boutros, S.; Grygoryev, D.; Hammer, A.; Kasschau, K.D.; Sharpton, T.J.; Turker, M.S.; et al. Effects of Six Sequential Charged Particle Beams on Behavioral and Cognitive Performance in B6D2F1 Female and Male Mice. Front. Physiol. 2020, 11, 959. [Google Scholar] [CrossRef]
- Raber, J.; Yamazaki, J.; Torres, E.R.S.; Kirchoff, N.; Stagaman, K.; Sharpton, T.; Turker, M.S.; Kronenberg, A. Combined Effects of Three High-Energy Charged Particle Beams Important for Space Flight on Brain, Behavioral and Cognitive Endpoints in B6D2F1 Female and Male Mice. Front. Physiol. 2019, 10, 179. [Google Scholar] [CrossRef]
- Rabin, B.M.; Carrihill-Knoll, K.L.; Shukitt-Hale, B. Comparison of the Effectiveness of Exposure to Low-LET Helium Particles ((4)He) and Gamma Rays ((137)Cs) on the Disruption of Cognitive Performance. Radiat. Res. 2015, 184, 266–272. [Google Scholar] [CrossRef]
- Carr, H.; Alexander, T.C.; Groves, T.; Kiffer, F.; Wang, J.; Price, E.; Boerma, M.; Allen, A.R. Early effects of (16)O radiation on neuronal morphology and cognition in a murine model. Life Sci. Space Res. 2018, 17, 63–73. [Google Scholar] [CrossRef]
- Britten, R.A.; Jewell, J.S.; Duncan, V.D.; Davis, L.K.; Hadley, M.M.; Wyrobek, A.J. Spatial Memory Performance of Socially Mature Wistar Rats is Impaired after Exposure to Low (5 cGy) Doses of 1 GeV/n (48)Ti Particles. Radiat. Res. 2017, 187, 60–65. [Google Scholar] [CrossRef]
- Whoolery, C.W.; Yun, S.; Reynolds, R.P.; Lucero, M.J.; Soler, I.; Tran, F.H.; Ito, N.; Redfield, R.L.; Richardson, D.R.; Shih, H.Y.; et al. Multi-domain cognitive assessment of male mice shows space radiation is not harmful to high-level cognition and actually improves pattern separation. Sci. Rep. 2020, 10, 2737. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.E.; Younger, S.; Bertheau, E.; Fallgren, C.M.; Weil, M.M.; Raber, J. Effects of chronic exposure to a mixed field of neutrons and photons on behavioral and cognitive performance in mice. Behav. Brain Res. 2020, 379, 112377. [Google Scholar] [CrossRef]
- Chicheva, M.M.; Mal’tsev, A.V.; Kokhan, V.S.; Bachurin, S.O. The Effect of Ionizing Radiation on Cognitive Functions in Mouse Models of Alzheimer’s Disease. Dokl. Biol. Sci. 2020, 494, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S.; Matveeva, M.I.; Mukhametov, A.; Shtemberg, A.S. Risk of defeats in the central nervous system during deep space missions. Neurosci. Biobehav. Rev. 2016, 71, 621–632. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Shakhbazian, E.V.; Markova, N.A. Psycho-emotional status but not cognition are changed under the combined effect of ionizing radiations at doses related to deep space missions. Behav. Brain Res. 2019, 362, 311–318. [Google Scholar] [CrossRef]
- Rice, O.V.; Grande, A.V.; Dehktyar, N.; Bruneus, M.; Robinson, J.K.; Gatley, S.J. Long-term effects of irradiation with iron-56 particles on the nigrostriatal dopamine system. Radiat. Environ. Biophys. 2009, 48, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; DeCicco-Skinner, K.L.; Roma, P.G.; Hienz, R.D. Individual differences in attentional deficits and dopaminergic protein levels following exposure to proton radiation. Radiat. Res. 2014, 181, 258–271. [Google Scholar] [CrossRef]
- Davis, C.M.; DeCicco-Skinner, K.L.; Hienz, R.D. Deficits in Sustained Attention and Changes in Dopaminergic Protein Levels following Exposure to Proton Radiation Are Related to Basal Dopaminergic Function. PLoS ONE 2015, 10, e0144556. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Lebedeva-Georgievskaya, K.B.; Kudrin, V.S.; Bazyan, A.S.; Maltsev, A.V.; Shtemberg, A.S. An investigation of the single and combined effects of hypogravity and ionizing radiation on brain monoamine metabolism and rats’ behavior. Life Sci. Space Res. 2019, 20, 12–19. [Google Scholar] [CrossRef]
- Shtemberg, A.S.; Bazian, A.S.; Lebedeva-Georgievskaya, K.B.; Matveeva, M.I.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M.; Kokhan, V.S. Effects of exposure to high-energy protons on rat’s behavior and underlying neurochemical mechanisms. Aerosp. Environ. Med. 2013, 47, 54–60. [Google Scholar]
- Matveeva, M.I.; Shtemberg, A.S.; Timoshenko, G.N.; Krasavin, E.A.; Narkevich, V.B.; Klodt, P.M.; Kudrin, V.S.; Bazyan, A.S. The effects of irradiation by 12C carbon ions on monoamine exchange in several rat brain structures. Neurochem. J. 2013, 7, 303–307. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Matveeva, M.I.; Bazyan, A.S.; Kudrin, V.S.; Mukhametov, A.; Shtemberg, A.S. Combined effects of antiorthostatic suspension and ionizing radiation on the behaviour and neurotransmitters changes in different brain structures of rats. Behav. Brain Res. 2017, 320, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Belov, O.V.; Belokopytova, K.V.; Bazyan, A.S.; Kudrin, V.S.; Narkevich, V.B.; Ivanov, A.A.; Severiukhin, Y.S.; Timoshenko, G.N.; Krasavin, E.A. Exposure to (12)C particles alters the normal dynamics of brain monoamine metabolism and behaviour in rats. Phys. Med. 2016, 32, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S. Some aspects of the effect of combined irradiation by gamma-rays and carbon nuclei (12C) on the serotonergic system in rat brain. J. Biomed 2020, 16, 68–72. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Anokhin, P.K.; Belov, O.V.; Gulyaev, M.V. Cortical Glutamate/GABA Imbalance after Combined Radiation Exposure: Relevance to Human Deep-Space Missions. Neuroscience 2019, 416, 295–308. [Google Scholar] [CrossRef]
- Parihar, V.K.; Allen, B.D.; Caressi, C.; Kwok, S.; Chu, E.; Tran, K.K.; Chmielewski, N.N.; Giedzinski, E.; Acharya, M.M.; Britten, R.A.; et al. Cosmic radiation exposure and persistent cognitive dysfunction. Sci. Rep. 2016, 6, 34774. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.M.; Baulch, J.E.; Klein, P.M.; Baddour, A.A.D.; Apodaca, L.A.; Kramar, E.A.; Alikhani, L.; Garcia, C., Jr.; Angulo, M.C.; Batra, R.S.; et al. New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose-Rate, Neutron Radiation. eNeuro 2019, 6, ENEURO.0094-19. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Mariasina, S.; Pikalov, V.A.; Abaimov, D.A.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Neurokinin-1 Receptor Antagonist Reverses Functional CNS Alteration Caused by Combined gamma-rays and Carbon Nuclei Irradiation. CNS Neurol. Disord. Drug Targets 2022, 21, 278–289. [Google Scholar] [CrossRef]
- Hurlemann, R.; Walter, H.; Rehme, A.K.; Kukolja, J.; Santoro, S.C.; Schmidt, C.; Schnell, K.; Musshoff, F.; Keysers, C.; Maier, W.; et al. Human amygdala reactivity is diminished by the beta-noradrenergic antagonist propranolol. Psychol. Med. 2010, 40, 1839–1848. [Google Scholar] [CrossRef]
- Morilak, D.A.; Barrera, G.; Echevarria, D.J.; Garcia, A.S.; Hernandez, A.; Ma, S.; Petre, C.O. Role of brain norepinephrine in the behavioral response to stress. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 1214–1224. [Google Scholar] [CrossRef]
- McCall, J.G.; Siuda, E.R.; Bhatti, D.L.; Lawson, L.A.; McElligott, Z.A.; Stuber, G.D.; Bruchas, M.R. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife 2017, 6, e18247. [Google Scholar] [CrossRef] [PubMed]
- Galvez, R.; Mesches, M.H.; McGaugh, J.L. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol. Learn. Mem. 1996, 66, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Tully, K.; Li, Y.; Tsvetkov, E.; Bolshakov, V.Y. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc. Natl. Acad. Sci. USA 2007, 104, 14146–14150. [Google Scholar] [CrossRef] [PubMed]
- Tully, K.; Bolshakov, V.Y. Emotional enhancement of memory: How norepinephrine enables synaptic plasticity. Mol. Brain 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Piper, W.T.; Branigan, L.A.; Vazey, E.M.; Aston-Jones, G.; Lin, L.; LeDoux, J.E.; Sears, R.M. A brainstem-central amygdala circuit underlies defensive responses to learned threats. Mol. Psychiatry 2020, 25, 640–654. [Google Scholar] [CrossRef]
- Haley, G.E.; Yeiser, L.; Olsen, R.H.; Davis, M.J.; Johnson, L.A.; Raber, J. Early effects of whole-body (56)Fe irradiation on hippocampal function in C57BL/6J mice. Radiat. Res. 2013, 179, 590–596. [Google Scholar] [CrossRef]
- Howe, A.; Kiffer, F.; Alexander, T.C.; Sridharan, V.; Wang, J.; Ntagwabira, F.; Rodriguez, A.; Boerma, M.; Allen, A.R. Long-Term Changes in Cognition and Physiology after Low-Dose (16)O Irradiation. Int. J. Mol. Sci. 2019, 20, 188. [Google Scholar] [CrossRef]
- Kiffer, F.; Alexander, T.; Anderson, J.E.; Groves, T.; Wang, J.; Sridharan, V.; Boerma, M.; Allen, A.R. Late Effects of (16)O-Particle Radiation on Female Social and Cognitive Behavior and Hippocampal Physiology. Radiat. Res. 2019, 191, 278–294. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y. Heavier ions with a different linear energy transfer spectrum kill more cells due to similar interference with the Ku-dependent DNA repair pathway. Radiat. Res. 2014, 182, 458–461. [Google Scholar] [CrossRef]
- Thorre, K.; Chaouloff, F.; Sarre, S.; Meeusen, R.; Ebinger, G.; Michotte, Y. Differential effects of restraint stress on hippocampal 5-HT metabolism and extracellular levels of 5-HT in streptozotocin-diabetic rats. Brain Res. 1997, 772, 209–216. [Google Scholar] [CrossRef]
- Shtemberg, A.S.; Kokhan, V.S.; Matveeva, M.I.; Lebedeva-Georgievskaia, K.V.; Timoshenko, G.N.; Molokanov, A.G.; Krasavin, E.A.; Narkevich, V.B.; Klodt, P.M.; Bazian, A.S. The impact of high-energy protons in Bragg peak on the behavior of rats and exchange of monoamines in some brain structures. Neurochem. J. 2015, 9, 78–85. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal 2013, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Lewis, V.; Przedborski, S.; Kostic, V.; Suber, F.; Fahn, S.; Cadet, J.L. Partial attenuation of chronic fluphenazine-induced changes in regional monoamine metabolism by D-alpha-tocopherol in rat brain. Brain Res. Bull. 1991, 26, 251–258. [Google Scholar] [CrossRef]
- Loghin, F.; Chagraoui, A.; Asencio, M.; Comoy, E.; Speisky, H.; Cassels, B.K.; Protais, P. Effects of some antioxidative aporphine derivatives on striatal dopaminergic transmission and on MPTP-induced striatal dopamine depletion in B6CBA mice. Eur. J. Pharm. Sci. 2003, 18, 133–140. [Google Scholar] [CrossRef]
- Liu, M.; Bachstetter, A.D.; Cass, W.A.; Lifshitz, J.; Bing, G. Pioglitazone Attenuates Neuroinflammation and Promotes Dopaminergic Neuronal Survival in the Nigrostriatal System of Rats after Diffuse Brain Injury. J. Neurotrauma 2017, 34, 414–422. [Google Scholar] [CrossRef]
- Laatikainen, L.M.; Sharp, T.; Bannerman, D.M.; Harrison, P.J.; Tunbridge, E.M. Modulation of hippocampal dopamine metabolism and hippocampal-dependent cognitive function by catechol-O-methyltransferase inhibition. J. Psychopharmacol. 2012, 26, 1561–1568. [Google Scholar] [CrossRef]
- Krishna, G.; Beitchman, J.A.; Bromberg, C.E.; Currier Thomas, T. Approaches to Monitor Circuit Disruption after Traumatic Brain Injury: Frontiers in Preclinical Research. Int. J. Mol. Sci. 2020, 21, 588. [Google Scholar] [CrossRef]
- Meythaler, J.M.; Brunner, R.C.; Johnson, A.; Novack, T.A. Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: A pilot double-blind randomized trial. J. Head Trauma Rehabil. 2002, 17, 300–313. [Google Scholar] [CrossRef]
- Shtemberg, A.S.; Lebedeva-Georgievskaia, K.V.; Matveeva, M.I.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M.; Bazian, A.S. Effect of space flight factors simulated in ground-based experiments on the behavior, discriminant learning, and exchange of monoamines in different brain structures of rats. Izv. Akad. Nauk. Seriia Biol./Ross. Akad. Nauk. 2014, 41, 168–175. [Google Scholar] [CrossRef]
- Peng, S.; Yang, B.; Duan, M.Y.; Liu, Z.W.; Wang, W.F.; Zhang, X.Z.; Ren, B.X.; Tang, F.R. The Disparity of Impairment of Neurogenesis and Cognition After Acute or Fractionated Radiation Exposure in Adolescent BALB/c Mice. Dose Response 2019, 17, 1559325818822574. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokhan, V.S.; Ustyugov, A.A.; Pikalov, V.A. Dynamics of Dopamine and Other Monoamines Content in Rat Brain after Single Low-Dose Carbon Nuclei Irradiation. Life 2022, 12, 1306. https://doi.org/10.3390/life12091306
Kokhan VS, Ustyugov AA, Pikalov VA. Dynamics of Dopamine and Other Monoamines Content in Rat Brain after Single Low-Dose Carbon Nuclei Irradiation. Life. 2022; 12(9):1306. https://doi.org/10.3390/life12091306
Chicago/Turabian StyleKokhan, Viktor S., Alexey A. Ustyugov, and Vladimir A. Pikalov. 2022. "Dynamics of Dopamine and Other Monoamines Content in Rat Brain after Single Low-Dose Carbon Nuclei Irradiation" Life 12, no. 9: 1306. https://doi.org/10.3390/life12091306
APA StyleKokhan, V. S., Ustyugov, A. A., & Pikalov, V. A. (2022). Dynamics of Dopamine and Other Monoamines Content in Rat Brain after Single Low-Dose Carbon Nuclei Irradiation. Life, 12(9), 1306. https://doi.org/10.3390/life12091306





