Abstract
p53 is known as the guardian of the genome and plays various roles in DNA damage and cancer suppression. The p53 gene was found to express multiple p53 splice variants (isoforms) in a physiological, tissue-dependent manner. The various genes that up- and down-regulated p53 are involved in cell viability, senescence, inflammation, and carcinogenesis. Moreover, p53 affects the radioadaptive response. Given that several studies have already been published on p53, this review presents its role in the response to gamma irradiation by interacting with MDM2, NF-κB, and miRNA, as well as in the inflammation processes, senescence, carcinogenesis, and radiation adaptive responses. Finally, the potential of p53 as a biomarker is discussed.
1. Introduction
In 1979, p53 was identified by Arnold J. Levin et al. as a protein that binds to the large T antigen of the oncovirus simian virus (SV40) [1]. Almost at the same time, p53 was reported by Kress et al. [2] and Lane et al. [3]. In 1984, p53 was reported to be an oncogene [4,5,6,7]; however, this was a function of a mutant form of p53 found in cancer cells. In 1989, the p53 gene was reported to be a tumor suppressor gene [8,9]. Thus, p53 was called “The Guardian of the Genome” [10]. Ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related kinase (ATR) exist upstream of p53. In response to DNA strand breaks caused by ionizing radiation, ATM is activated by chromatin structural changes [11]. Following DNA damage by UV irradiation, ATR is activated by the interruption of DNA replication and transcription [12]. The p53 gene has a wide range of activities and induces the expresses of genes involved in DNA repair (ribonucleotide reductase regulatory subunit M2 (RRM2), ERCC excision repair 5 (ERCC5), FA complementation group C (FANCC), XPC complex subunit (XPC), X-ray repair cross complementing 5 (XRCC5), growth arrest and DNA damage inducible alpha (GADD45A), Pre-intermoult gene 1 (Pig1), etc.), cell cycle arrest (p21, 14-3-3σ, Riprimo, GADD45A, miR34, cyclin dependent kinase 2 (CDK2), cyclin E1 (CCNE1), etc.), apoptosis (GADD45A, BCL2 associated X (BAX), PIG3, p53 upregulated modulator of apoptosis (Puma), NADPH oxidase activator (NOXA; now known as phorbol-12-myristate-13-acetate-induced protein 1 [PMAIP1])), tumor necrosis factor (TNF), Killer/Dr5, FAS (known as cluster of differentiation 95 (CD95)), p53Alp, apoptotic peptidase activating factor 1 (APAF1), prolyl endopeptidase (PREP), p53-inducible death-domain-containing protein (PIDD), DNA damage-regulated autophagy modulator (DRAM), Fas ligand (FASL), mir34c, p53-regulated apoptosis-inducing protein 1 (tpip1), etc.), autophagy (DRAM, Sestrin 1, Sestrin 2, etc.), antioxidant function (Sestrin 1, Sestrin 2, glutathione peroxidase 1 (GPX1), etc.), senescence (resistance to audiogenic seizures (RAS), MYC proto-oncogene (MYC), PML nuclear body scaffold (PML), plasminogen activator inhibitor-1 (PAI1), p21,Genes of the SASP, mir34a, etc.), and heat shock protein (HSP70) [13,14,15,16,17,18,19,20,21].
The p53 gene accumulates in the cell nucleus after radiation exposure [22,23,24]. The expression of the p53 gene was shown to be induced by at least 10 mGy of total body irradiation of mice [21]. Currently, a search for “p53” reveals more than 100,000 articles, with more than 5000 reports per year since 2011. A search of “p53” and “radiation” resulted in more than 8000 papers, and approximately 300 papers have been published every year for the past 20 years. In this review, the role of p53 after ionizing irradiation will be presented with the latest data.
2. p53 Isoforms and Their Responses Response to Radiation
The TP53 gene is composed of 11 exons [25,26], the end-products of alternative usage of promoters (P1 and P2) and splicing of intron-2 and intron-9 (Figure 1A). The p53 protein comprises 393 amino acids and is classified into six domains (Figure 1B) [25,27]: (1) transcriptional activation domain (TAD) (residues 1–67), which can be classified into TAD I (residues 1–40) and TAD II (residues 41–67); (2) interaction with various proteins, including the proline-rich region (residues 67–98); (3) conservation of most p53, i.e., the central core domain (residues 98–303); (4) DNA-binding domain with >90% of p53 mutations causing cancer in humans, i.e., nuclear localization signal (residues 303–323); (5) tetramerization domain (residues 323–363); (6) C-terminal basic domain (residues 363–393) i.e., a nonspecific DNA-binding domain that recognizes and binds damaged DNA [25,27]. Twelve p53 protein isoforms, p53α, p53β, p53γ, Δ40p53α, Δ40p53β, Δ40p53γ, Δ133p53α, Δ133p53β, Δ133p53γ, Δ160p53α, Δ160p53 β, and Δ160p53γ have been identified. Depending on the cell type, p53 mRNA translation starts at different codons. Then, the mRNA transcribed from the proximal promoter (P1) initiates codon 1 and/or 40 translation, whereas that transcribed from the internal promoter (P2) initiates codon 133 and/or 160 translation. p63 and p73 are family members of p53 [28,29].
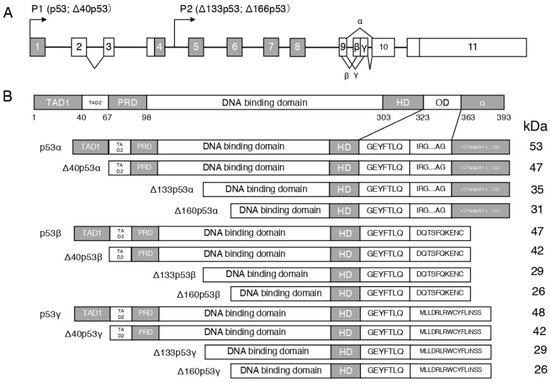
Figure 1.
Schema of the human p53 gene structure: (A): alternative splicing sites (α, β, and γ) and promoters (P1 and P2) are marked. (B): Schema of the human p53 protein isoforms can be expressed by the human p53 gene. TAD, transcription activation domain; PRD, proline-rich domain; HD, hinge domain; and OD, oligomerization domain.
By irradiation, the p53 isoform Δ113p53 (as Δ133p53 in human) is a p53 target gene that antagonizes the apoptotic activity of p53 via activation of bcl2L (as Bcl-xL in human) in zebrafish [30]. Restoration of ∆133p53 expression protects astrocytes from radiation-induced senescence, promotes DNA repair, and inhibits astrocyte-mediated neuroinflammation [31]. Different radiation doses in germ cells during Drosophila oogenesis play different roles depending on p53 isoforms (p53A and p53B) [32]. In the absence of stress, p53A is expressed to support normal oogenesis, and, in the early stages of oogenesis, p53A and p53B are involved in radiation-induced apoptosis, and both isoforms were involved in restoring the number of germline stem cells after high dose of irradiation [32]. p53A, but not p53B, was involved in the maintenance of germline stem cells’ function after high doses of irradiation [32]. It had been known that p63, but not p53, is involved in the irradiation-mediated response in human oocytes [33].
3. The Murine Double Minute 2 (MDM2)-p53 Pathway after Radiation
Some X-ray-transformed foci showed amplification and/or overexpression of MDM2, and other foci expressed mutant p53 [34] (Figure 2). In the ML-1 human myeloid leukemia cell line, the p53-regulated genes, namely cyclin-dependent kinase inhibitor 1A (CDKN1A, also known as: CDKN1, p21, WAF1, p21CIP1), GADD45A, and MDM2, were reported to be induced at doses between 2 and 50 cGy [35]. When haploinsufficiency is used in Mdm2- and Mdm4-lacking mice, the p53 gene function is activated in response to radiation-induced DNA damage in a p53-dependent manner [36]. Ionizing radiation phosphorylated Thr18 in wild-type (wt) p53-containing cancer cell lines, accompanied by an increased expression of p21 and MDM2. However, ultraviolet (UV) treatment showed a cell line-dependent effect [37]. It has been reported that RPS27, a ribosomal protein, and RPS27L, an RPS27-like protein, regulate DNA damage caused by radiation and other factors in the p53–MDM2 axis [38]. In a study of normal lips and actinic cheilitis (AC), a precancerous lesion, MDM2, and p21 were significantly correlated with p53 in normal lips; however, only MDM2 was correlated with p53 expression in AC. In this report, it was found that p53 was the only predictor of AC and a potential marker of the early malignancy of the lips [39]. In a study of cervical cancer radiotherapy, p53 and MDM2 were found in the remaining cancer cells after treatment, and DNA-dependent protein kinase (DNAPK), which is involved in DNA repair, was activated, resulting in radioresistance [40].
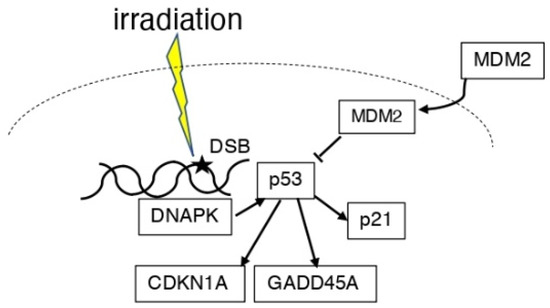
Figure 2.
Scheme of the association between p53 and MDM2 in irradiation. DNA damage induced by ionizing radiation induces p53. DNAPK can also induce p53 expression. MDM2 is present in the cytoplasm and nucleus and suppresses p53 function. P53 expresses p21, GADD45A, and CDKN1A.
4. The p53 and Nuclear Factor-KappaB (NF-κB) Pathway after Radiation
The regulation of the DNA damage response and immune response involves the activity of transcription factors such as p53 protein, activated protein 1 (AP-1), and NF-κB [41,42] (Figure 3). In mouse lymphoid cells and thymocytes, the activation of NF-κB and apoptosis after X-irradiation is p53 dependent [43]. When DNA damage occurs, ATM activates p53 in the cell nucleus, which in turn activates the NF-κB pathway via the cytoplasmic IκB complex (IKKα, IKKβ, and NEMO) [44]. Transcription of NF-κB and NF-κB target genes was shown to be involved in radioresistance in human keratinocytes in which TP53 is inactivated by fractionated ionizing radiation (2 Gy/fraction; total dose, 60 Gy) [45]. Even in p53-deficient tumor cells, the fungal metabolite gliotoxin inhibited NF-κB activation with high selectivity, activated c-Jun N-terminal kinase (JNK), released cytochrome c from mitochondria, and potently stimulated the caspase cascade, inducing the cleavage of caspase-9, -8, -7, and -3 [46]. Inhibiting NF-κB activation is a strategy to protect against radiotherapy in cancer. Inhibition of NF-κB signaling may trigger oncogenesis independent of initiating mutations in the Ha-ras gene or additional mutations in the p53 gene in skin cancer induced by gamma irradiation [47]. It has been reported that, in human umbilical vein endothelial cells (HUVECs), the protein levels of the mitogen-activated protein kinase (MAPK) pathway molecules, p38, p53, p21, and p27, were increased after gamma irradiation, and that the activation of the NF-κB pathway was also induced [48]. The PIDD is involved in caspase-2 activation and apoptosis in response to DNA damage and has an important role in NF-κB activation [49]. Irradiation was shown to activate or repress NF-κB and induce differential expression downstream genes depending on the expression status of the p53 gene in the cells [50]. NF-κB is a negative regulator of autophagy in mutant p53 (p53-R273H) cells [51]. p300 or p65 inhibition not only activated autophagy but also induced radiosensitivity in p53-R273H cells [51]. This finding indicates that autophagy may be regulated by NF-κB [51]. When mice were irradiated at a young age, p53 and NF-κB were activated at an older age, but not at a middle-aged age [52]. This finding indicates that exposure at a young age accelerated the senescence process [52]. Furthermore, attenuation of the p53 function promoted inflammation and carcinogenesis [53], and NF-κB activity plays a major role in this process [54,55,56]. If gamma irradiation (activating the p53 pathway) precedes TNF (activating the NF-κB pathway), the role of NF-κB is proapoptotic; if the stimuli are reversed, the role of NF-κB is antiapoptotic [57]. Radiation-induced p53 and NF-κB can be dependent or independent.
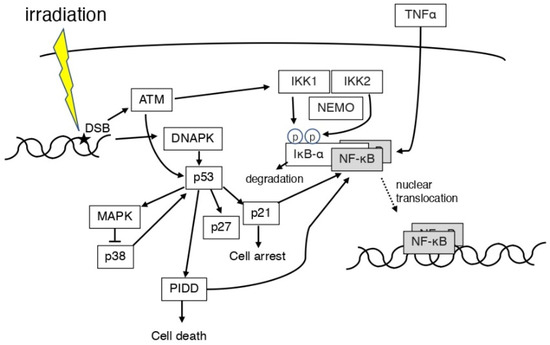
Figure 3.
Scheme of the association between p53 and NF-κB in irradiation. ATM and DNAPK are expressed by ionizing radiation causing DNA damage. PIDD is involved in the NF-κB expression. ROS and ATM induced IKK1 and IKK2. IκB-α degrades and activates NF-κB. TNFα may also activate NF-κB. p53 and NF-κB may be dependent or independent.
5. p53 and MicroRNAs (miRs) after Radiation
MicroRNAs (miRs) are 21–25 base-long, single-stranded RNA molecules that are involved in the post-transcriptional regulation of gene expression, cell division, differentiation, apoptosis, metabolism, and tumorigenesis in eukaryotes [58,59,60]. Radiation-induced DNA damage induces the expression of miR-34 by the p53 gene [61,62], and overexpression of miR-34b increases radiosensitivity [63] (Figure 4). MiR-34a remains stable in serum after irradiation and may be a new indicator of radiation injury, strongly suggesting that miR-34a may be a new indicator, mediator, and target of radiation injury, radiosensitivity, and radiation protection [64]. MCF-7 cells have higher γ-ray-induced p53 expression than HeLa cells; however, the induction of miR-34 shows the opposite pattern, suggesting that miR-34 induction may be cell type-specific [65]. In glioblastoma cells, after 60 Gy irradiation, miR-34a was highly expressed; however, p53 expression was decreased [66]. It is possible that miR-34a regulation induces apoptosis even in cells that have become radioresistant [66]. In human mammary epithelial cells, p38 MAPK-mediated p53 ser15 phosphorylation is essential for radiation-induced functional regulation of miR-34a transcription, and histone modifications also play an important role in miR-34a expression [67].
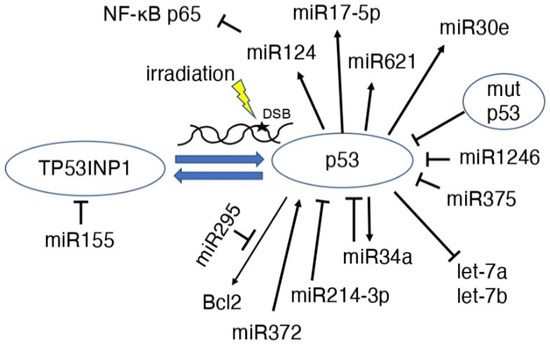
Figure 4.
Scheme of the association between p53 and miRNAs in irradiation MiRNAs induce p53 or suppress p53 function.
The expression of let-7a and let-7b, which are downstream of p53, was downregulated by radiation and correlated with changes in the expression of proteins in the p53-regulated apoptosis-promoting signaling pathway, which are important for radiation-induced cytotoxicity [68]. In other words, the p53 direct binds and activates genes, but let-7a and let-7b were repressed by p53. In prostate cancer cells, fractionated irradiation increased the expression of tumor suppressive miR-34a and let-7, which are not dependent on p53 alone [69].
MiR-295 is found at a site identified as a direct transcriptional target of p53 that binds to Bcl2 and may act to suppress apoptosis in tumor cells, making them radioresistant [70]. MiR-155 has been suggested to modulate radiation-induced aging by regulating the expression of tumor protein 53-induced nuclear protein 1 (TP53INP1) downstream of the p53 and p38 MAPK pathways [71]. MiR-375 expression was elevated in recurrent gastric cancer, and the overexpression of miR-375 resulted in the decreased expression of p53 protein [72]. In other words, miR-375 inhibits cell cycle arrest and apoptosis, making the cells radioresistant [72]. MiR-124 expression is induced by p53, and miR-124 suppresses NF-κB p65, leading to the suppression of MYC/BCL2 expression and cell survival in B-cell lymphoma [73]. MiR-17-5p was shown to cause p53-dependent apoptosis and contributes to radiosensitivity in irradiated betel nut chewing-oral squamous cell carcinoma OC3 cells [74]. Increased miR-372 activated p53 via suppression of the PDZ-binding kinase and increased radiosensitivity of tumor cells [75]. MiR-621 is also involved in the radiosensitivity of hepatocellular carcinoma through the p53 signaling pathway [76]. MiR-1246 targets and suppresses the p53 gene, which has anticancer effects on bladder cancer cells [77]. MiR-30e expression was upregulated in a p53-dependent manner after gamma-irradiation and strongly accelerated and increased the aging phenotype [78]. MiR-214-3p suppressed ATM/P53/P21 signaling when extracellular vehicles (EVs) derived from mesenchymal stem cells (MSCs) suppressed radiation-induced lung injury, resulting in senescence-associated secretory phenotype development [79]. The regulation of p53 by miRNAs has been reviewed previously [80].
6. p53 and Inflammation Induced by Radiation
Radiation exposure has been known to produce various immune molecules, such as cytokines, danger-associated molecular patterns (DAMPs), and cellular factors that cause inflammation [81]. The role of p53 in innate immunity and inflammatory responses has already been well established [82,83,84]. In CBA/Ca male mice, at post-irradiation with 4 Gy gamma rays, many NF-κB-positive cells were still present after 100 days, suggesting a persistent inflammation [85]. In the hematopoietic system of irradiated CBA/Ca mice, the microenvironment, a non-target of radiation, is enhanced by effect changes and inflammatory-type signals [86,87,88,89], namely, changes in oxygen- and nitrogen-free radical activity and cytokines such as TNFα and Fas-L, and cyclooxygenase (COX)-2 signaling, are observed [86,87,88,89]. Changes are thought to induce p53-mediated apoptosis [90]. The addition of NS-398, a nonsteroidal anti-inflammatory drug that selectively inhibits COX-2, significantly reduces cell death [91]. In irradiated mouse bone marrow cells, p53-gene-dependent apoptosis involves inflammatory mechanisms at 24 h during the apoptotic process [92]. Then, the codon 72 polymorphism in p53 has been reported to affect p53-mediated inflammatory responses at post-irradiation [93]. Macrophage activation after a radiation exposure is caused by radiation-induced apoptosis and may represent a direct p53-mediated pathway of macrophage activation [94]. In human monocytes and macrophages, inflammatory cytokine gene expression may be directly induced by p53- and ATM-dependent mechanisms [81]. p21 deletion in macrophages decreased macrophage inflammatory protein (MIP)-1, MIP-2, IL-1α, and IL-1β expressions, which are involved in the inflammation process [95]. Fetal radiation on day 12 (post-conception) releases inflammatory cytokines into the amniotic fluid on day 16 and subsequently induces p53-gene-dependent, apoptosis-related genes, resulting in malformations [96]. Irradiation of primary human retinal microvascular endothelial cells (RECs) rapidly induces the p38 mitogen-activated protein kinase (MAPK) stress kinase-mediated pathway and its downstream effectors (tumor suppressor, p53, intercellular adhesion molecule 1 (ICAM-1)), resulting in inflammatory responses [97]. In the radiation-induced brain injury mice, IκB-α expression was downregulated, accompanied by the upregulated NF-κB expression essential modulator (NEMO) and regulated auto-proteolysis of PIDD [98]. Irradiation activates cathepsin K (CSTK), nuclear factor of activated T-cells 5 (NFAT), Tartrate-Resistant Acid Phosphatase-5b (TRAP-5b), receptor activator of nuclear factor-kappa B ligand/Osteoprotegerin (Rank l/OPG), IL-1, IL-6, and TNFα genes in osteoclasts, induces inflammation and promotes osteoporosis [99]. Irradiation of patient-derived glioblastoma cells induces p53-dependent inflammation through NF-κB signaling [100]. In patients with glioblastoma undergoing radiation therapy, decreased Ki67 labeling index, O-methyl guanine methyl transferase (MGMT) methylation status, isocitrate dehydrogenase (IDH) mutation status, and absence of p53 overexpression have all also been associated with long-term survivors [101]. Radiation-induced pneumonitis in patients with lung cancer treated with radiotherapy is associated with dysregulation of p53 signaling by p53 and ATM polymorphisms [102], which is consistent with the association between miRNAs and p53 signaling during whole-thorax lung irradiation in mice [103]. In patients with Alzheimer’s disease treated with low doses of radiation, Helicobacter pylori increased the intestinal permeability through p53-dependent activation of the TLR4/Myd88 inflammatory pathway, causing metabolic dysfunction [104].
Recently, inflammation has been hypothesized to cause carcinogenesis [105]. Chronic inflammation is a major cancer predisposition factor [106,107,108,109]. Irradiation of mice lacking the p53 gene caused TGF-β-associated inflammation, which was robustly associated with claudin-low tumors [110]. Colorectal cancer in Mlh1-deficient mice is more likely to develop when exposed to radiation and simultaneously induced inflammation [111]. Naringin, a citrus flavonoid, suppresses radiation-induced cytotoxicity of the p53 pathway and inflammation in the NF-κB pathway [112], which is important in suppressing various radiation-induced cytotoxicity. In prostate cancer radiotherapy, transcript levels were elevated, primarily of DNA damage binding protein 2, p21, collagen, laminin, and integrins, as well as the upregulated p53 pathway [113]. Interstitial remodeling, extracellular matrix proteins, and focal adhesion pathways were also strongly upregulated, as was inflammation [113]. These indicate a clustering of apoptosis and programmed cell death, extracellular matrix organization, and inherent changes in immune regulation [113]. Deletion of lysine (K)-specific demethylase 6A (KDM6A) cooperates with p53 haploinsufficiency to promote the activation of the cytokine–chemokine pathway and M2 macrophages polarization, ultimately causing bladder cancer [114].
7. p53 and Radiation Induced Carcinogenesis
Loss or mutation of p53 is known to be involved in radiation-induced carcinogenesis [115,116,117]. Radiation-induced mutations at the thymidine kinase locus were 30-fold more common in p53 mutant cells than in p53 wild-type cells, and loss of heterozygosity was more common [118]. Secondary sarcomas arising in the irradiated region of the primary tumor after radiotherapy showed somatic inactivating mutations in one allele of TP53 and were systematically associated with defects in the other allele [119]. Furthermore, induction of p16 has a stronger relationship to carcinogenesis than loss of p53 [120]. Mutations in p53 may not be directly induced by radiation but may arise in cells several generations after irradiation [121]. We have reported that changes in T-cell surface markers occur in a delayed manner when mice are exposed to whole body irradiation, and this phenomenon was observed earlier in p53 heterozygotes [122]. X-irradiated cells undergo delayed apoptosis, which may not be a result of nonfunctioning of the acute TP53 damage response pathway but rather X-ray-induced genomic instability that occurs in the remote descendants of irradiated cells [123].
Hepatocellular carcinoma caused by alpha particles has been related to point mutation at codon 249 of p53 [124,125,126]. Electron and neon ion beam-induced rat skin cancers, but not alpha-induced liver cancer, showed alterations in critical binding regions in exons 5–8 of the p53 gene [124,127]. Structural genetic abnormalities, including mutations in p53 (exons 5–8), RAS (codons 12, 13, 61), and Gsα (codons 201 and 227), and, less frequently, in receptor tyrosine kinases such as RET and NTRK1, have been found in various sporadic thyroid tumors in adults [128]; however, no p53 gene mutations have been found in thyroid cancer from the Chernobyl nuclear accident [129,130]. In human colorectal cancer, tripartite motif containing 23 has been reported to physically bind p53 and promote p53 ubiquitination, thereby promoting tumor growth [131].
In mice, mTOR signaling is activated through the p53-Fbxw7 pathway early after irradiation, and the suppression of Fbxw7 and mTOR suppressed carcinogenesis [132]. Whole-body irradiation of p53 wild-type mice resulted in an acute DNA damage response that activated p53 in the bone marrow; however, cells from the bone marrow have a reduced ability to compete with tumor-forming cells in the thymus and promote radiation-induced lymphoma in the thymus via noncell autonomous mechanisms [133]. Although p53 suppresses the development of spontaneous tumors expressing Kras, in the context of exposure to ionizing radiation, regardless of low-LET or high-LET, extra copies of p53 may conversely not protect against radiation-induced lymphoma and may promote Kras mutant lung cancer [134]. In Mdm2-S394A mice (a mouse model in which ATM phosphorylation at serine residue 394 of Mdm2 is abolished), the Mdm2 response was that the remains destabilized after irradiation, eliminating the need for p53 mutations in Myc-driven carcinogenesis [135]. Akt, a DNA damage effector kinase, phosphorylates Mdm2 and regulates the p53 response to oxidative stress without affecting the p53-mediated effects of ionizing radiation in mice [136]. The mouse model of radiation-induced thymic lymphoma is a well-known model. In p53 wild-type mice, genetic inhibition of Notch1 signaling suppressed the formation of radiation-induced thymic lymphoma and plays an important role in promoting multistep carcinogenesis of thymic lymphoma [137].
8. p53 and Senescence Induced by Radiation in Normal Cells
A hallmark of senescence is that it causes a steady arrest of cell proliferation [138]. It is also important in stopping dysfunctional cells’ proliferation, and two tumor suppressor pathways, namely, p53 [138,139,140] and p16/Rb [138,141], have important roles. Alternatively, there is an MDM2-mediated hypoxia-inducible factor 1α (HIF1α) senescence is found, which is not mediated by the p53 gene [142]. Irradiated bone marrow-derived mesenchymal stem cells were observed to exhibit aging phenotypes, such as elevated levels of aging-related genes p53/p21 but without changes in p16 [143]. In normal human embryo cells, X-irradiation caused sustained TP53 phosphorylation at Ser15 and TP53 protein accumulation, resulting in the induction of CDKN1A (p21 (Waf1/Cip1)) and CDKN2A (p16), which was preceded by the expression of senescence-associated-β-gal [144]. p16, with or without p53, causes senescence in mice after prolonged irradiation [145]. After an ionizing irradiation of normal human fibroblasts, the same distribution of foci containing p53 phosphorylated at serine 15 along with large foci containing phosphorylated ATM and γ-H2AX also cause senescence-like growth arrest, indicative of 53-dependent irreversible G1 arrest [146]. However, telomere shortening is not necessarily required for radiation-induced senescence-like growth arrest [144,146,147]. Although telomere shortening involves the activation of the pRB and p53 pathways, the absence of telomere instability and the defects in the G2 chromosome repair in patients with cancer aged over 80 years of age irradiated with 50 Gy or more of gamma radiation correlates with longevity [148]. Exposure of mouse bone marrow cells to 4 Gy of ionizing radiation induces hematopoietic cell senescence in a p53–p21(Cip1/Waf1)-dependent manner [149]. Continuous irradiation of primary human umbilical vein endothelial cells (HUVECs) with low dose rate gamma radiation rate (4.1 mGy/h) results in senescence via the p53/p21 pathway [150]. Radiation-induced senescence in HUVECs also involves nuclear factor kappa B essential modulator (NEMO) [151]. Suppression of the human papillomavirus (HPV) type 18 E6/E7 gene in HeLa cells by bovine papillomavirus E2 transcriptional regulatory protein leads to reactivation of the dormant p53 and p105 (Rb) tumor suppressor pathways, telomerase inhibition, and deep growth arrest [152]. Irradiation of immortalized cells with 60Co gamma radiation increased the mutated p53 gene and decreased the sdiI/p21 and MDM2 expressions [153]. The sdiI/p21 gene significantly reduces the DNA synthesis capacity, indicating that the p53 cascade may play an important role in human cell immortalization [153]. The promoter methylation of p21 (Waf1/Cip1) is implicated in the aging process by directly repressing its expression in middle-aged fibroblasts and blocking the radiation-induced DNA damage signaling pathway by p53 [154]. Ku80 loss results in increased susceptibility to ionizing radiation, and replicative senescence in ku80(-/-) cells is caused by a p53-dependent cell cycle response to the damaged DNA [155]. In normal fibroblasts, ATM-dependent signaling pathways in response to ionizing radiation-induced DNA damage are not required for the activation of p38 MAPK or stress-induced premature senescence [156]. Mouse embryo fibroblasts deficient of the c-Jun proto-oncogene (c-Jun-/-MEF) undergo p53-dependent premature senescence in conventional culture [157]. Although CK2α and CK2α’ are involved in senescence, the former was responsible for the progression of radiation-induced stem cell senescence [158]. SOCS1 sufficiently induced p53-dependent senescence in fibroblasts after irradiation [159]. Nerve-injury-induced protein 1 (Ninjurin1, Ninj1) is a target of p53 and causes p53 mRNA translation and p53-dependent premature aging in vitro and in vivo [160]. Insulin-like growth factor I (IGF-1) is a key regulator of IR-induced accelerated aging, requiring a pathway upstream of both p53 and p21/waf1 that requires intact mammalian target of rapamycin (mTOR) activity [161].
9. p53 and Senescence Induced by Radiation in Cancer Cells
Radiation-induced senescence is observed in cancer cells via the p53 gene; however, p53-null lung cancer cell line H1299 has radiation-induced senescence due to p21waf overexpression [162,163]. Furthermore, the overexpression of this cell surface proteoglycan syndecan 1 (SDC1) in senescent cells after irradiation of human breast cancer is the result of autocrine action in TGF-β via the Smad pathway and the transcription factor Sp1 and does not involve classical aging pathways, such as p53 or p38 MAPK-NF-kB [164]. Overexpression of thioredoxin-1 (TRX), which is observed in many primary human cancers, in HT-1080 fibrosarcoma cells, increased the cyclin D1, p53, and p21 expression, with cyclin D1 overexpression being particularly and directly responsible for increased cell senescence and radiosensitivity [165]. In response to fractionated radiotherapy, glioblastoma multiforme cell lines showed senescence in wild-type p53 cells but not in mutant p53 cells [166]. In breast cancer cells treated by 17-beta-estradiol after radiation injury, it does not affect p53 activation but decreases cyclin E binding to p21(waf1/cip1), and downstream Rb hyperphosphorylation persists due to functional p21(waf1/cip1) inactivation, resulting in continued senescence [167]. In MCF-7 mammospheric cells, higher expression of the post-irradiation DNA single-strand break repair (SSBR) protein human AP endonuclease 1 (Ape1) results in a more active SSBR pathway, higher telomerase activity, lower p21 protein expression, and a significantly reduced senescence tendency [168]. In human breast cancer MCF7 cells, low doses of radiation inhibit doxorubicin-induced replicative senescence by suppressing p38-dependent p53 phosphorylation and activating ERK1/2, without genomic damage [169]. In prostate cancer cells, Nutlin-3 activates p53, making it an effective radiosensitizer—an effect completely attributable to the increased induction of p53-dependent cellular senescence [170]. Laryngeal squamous cell carcinoma cells with wild-type p53 showed significantly increased radiosensitivity, cell cycle arrest, and senescence by Nutlin-3 [171]. In human non-small cell lung cancer cells [172] and esophageal squamous cancer [173], Nutlin-3 activates p53 in senescence. Breast cancer cell irradiation induced 53BP1 and poly (ADP-ribose) polymerase inhibitor-accelerated senescence [174]. Specific matrix metalloproteases (MMPs) inhibitors markedly inhibit the growth of malignant lung epithelial cells in the presence of aging fibroblasts [175]. DNAPK inhibition sufficiently causes accelerated aging in irradiated human cancer cells [176]. Regulated in development and DNA damage responses (REDD1) is dramatically suppressed when the NF-κB or p53 genes are deleted, making it a protective factor in radiation-induced osteoblast premature senescence [177]. The C-X-C motif chemokine receptor (CXCR) 2 is transactivated by p53 and is a major mediator of oncogene-induced senescence [178]. Allosteric inhibitors of the CXCR4 receptor block metastasis-associated cell activity stimulated by secretions from irradiated lung epithelial cells and may be effective as cancer therapy when combined with radiation therapy [179]. Treatment for cancer cells with imidazoacridinone C-1311 (SymadexTM) prior to irradiation causes senescence in the presence of p53 and apoptosis without p53 [180]. A combination of radiotherapy and drug therapy is also important. Irradiation of p53 wild-type non-small cell lung cancer cells in vitro may induce an senescence-associated secretory phenotype (SASP), which expressed CD63 and generates extracellular vesicles with DNA:RNA hybrids and LINE-1 retrotransposons, causing an abscopal phenomenon [181].
10. p53 in the Radioadaptive Response (RAR)
The radioadaptive response (RAR) is a phenomenon in which low-dose radiation is administered in advance to induce resistance to subsequent high-dose radiation [182]. In 1984, Olivieri first reported the phenomenon of the radiation adaptive response in vitro [182]. The p53 gene plays an important role in RAR [183,184,185,186]; however, it has reported that in some cases RAR occur without dependence on p53. [187]. One mechanism of RAR is associated with survivin [188]. In mammalian cells, priming doses of less than 100 mGy have been used [189,190]; however, in vivo studies have reported higher priming doses of 150–600 mGy [191], 300 mGy [192], and 1 Gy [193]. RAR is observed at a priming dose of 20 mGy in the presence of p53 [184]. We have not yet published data in the presence of p53, a priming dose of 20 mGy followed by a 3 Gy extended lifespan more than 3 Gy alone.
RAR has also been observed in reduced fetal malformations [192,194,195,196,197,198,199,200], in which p53 plays an important role [194,196,200]. RARs are found in normal cells but not in tumor cells [201]. In other words, RAR does not occur when the p53 gene is abnormal [201]. Interestingly, in the RAR in mouse thymocytes, apoptosis-related expression was lower in males than in females [202]. The reason for this finding is that, in males, phosphoserine-389-TRP53 mediates caspase-3, whereas, in females, there is activation of phosphoserine-18-TRP53 and caspase-3 as well as overexpression of Dlc1 and Fis1 [202]. In cells expressing p53, low-dose irradiation with high-LET radiation has been shown to cause RAR, which is characterized by a low mutation frequency at the hypoxanthine-guanine phosphoribosyl transferase locus and a fast DNA repair rate [203]. In individuals living in areas with high natural radiation levels (>5.0 mGy/year), the p53 pathway (CDKN1A (p21), MDM2, TNF receptor associated factor 4 (TRAF4), TNF receptor superfamily member 10b (TNFRSF10B), apoptotic peptidase activating factor 1 (APAF1), phorbol-12-myristate-13-acetate-induced protein (PMAIP1), GADD45B, etc.) is overexpressed in the blood, which explains the phenomenon of RAR. Immune responses, cell–cell communication, and gap junctions also play important roles in RAR [204]. Ligase1 and p53 levels in the blood of operating room personnel are higher than those in the control group due to the anesthetic gas, and, when blood samples from this group were irradiated, the response differed from that of the control group. These results are also not limited to radiation, as exposure to the low concentrations of chemicals can cause RAR, which may be one of the biological defenses [205]. Guéguen Y. et al. provided a detailed review of the adaptive response [206].
11. p53 as a Biomarker after Irradiation
Measurement of p53 protein expression or p53 antibodies in the serum was potentially useful for the early detection of lung cancer [207,208,209,210]. The mRNA regulation and metabolism by p53 in response to radiation exposure has identified important mRNAs and metabolites as potential p53 targets [211]. After irradiation with 4 Gy, 326 mRNAs were significantly altered, i.e., 269 and 57 were increased and decreased, respectively [211].
Although p53 expression increased with age [52], it is not a suitable parameter for the early detection of lung cancer in former uranium miners [212]. When human peripheral blood was irradiated with X-rays, genes associated with chemokines (platelet factor 4 [PF4], G protein subunit gamma 11 [GNG11] and C-C motif chemokine receptor 4 [CCR4]) were independently expressed from the p53 gene at 0.05 Gy, whereas genes involved in p53 gene signaling (damage-specific DNA binding protein 2 [DDB2], apoptosis enhancing nuclease [AEN] and CDKN1A [p21]) were expressed at 1 Gy [213].
Furthermore, 147 metabolites were significantly expressed: 45 were increased and 102 were decreased. Particularly, gamma-glutamyl transferase 1 [GGT1], Phospholipase A2G [PLA2], post-transcriptional gene silencing [PTGS], glutathione peroxidase 6 [GPX6], aldolase, fructose-bisphosphate A [ALDOA], acyl-CoA synthetase short chain family member 2 [ACSS2], aldehyde dehydrogenase 3 family member A1 [ALDH3A1], and gamma-glutamyl transferase 6 [GGT6], involved in nitrogen metabolism, glutathione metabolism, glycolysis, or glycogenesis, and arachidonic acid metabolism, were suggested to be regulated by p53 [211]. DDB2, AEN, TP53 regulated inhibitor of apoptosis (TRIAP1), and TRAF4, which are all downstream of p53, are potential biomarkers within the first 24 h after radiation exposure [214].
12. Conclusions
The roles of p53 isoforms are still unknown, although they have already been identified. Specifically, only a few reports demonstrated the association between radiation and isoforms.
This review described the association between p53 and radiation. MDM2 has already been known to regulate p53. Regarding the NF-κB, several reports revealed that it may be p53-dependent. Although miRs are expected as biomarkers, various types and roles of miRs are still unclear. Although miR34 and let-7 have been widely associated with p53 and miRs after irradiation, their association with other miRs types is also diverse.
Inflammatory findings persist after radiation exposure in atomic bomb survivors [215,216]. Moreover, inflammation is a cause of carcinogenesis [105]. Senescence after radiation exposure is observed in normal and cancer cells and occurs when the cell cycle is stopped. Therefore, the role of p53 in these phenomena is significant.
This review described the role of p53 in radiation. The number of publications on p53, including those on DNA damage due to various mutagenic agents, is too numerous to mention even for related genes. p53 has many roles and continues to play various roles in the future. Hopefully, this review will contribute to further research.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Linzer, D.; Levine, A.J. Characterization of a 54K Dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 1979, 17, 43–52. [Google Scholar] [CrossRef]
- Kress, M.; May, E.; Cassingena, R.; May, P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J. Virol. 1979, 31, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.P.; Crawford, L.V. T antigen is bound to a host protein in SY40-transformed cells. Nature 1979, 278, 261–263. [Google Scholar] [CrossRef]
- Givol, D.; Oren, M.; Gruss, P.; Givol, D.; Oren, M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature 1984, 312, 646–649. [Google Scholar] [CrossRef]
- Rudge, K.; Jenkins, J.R.; Currie, G.A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature 1984, 312, 651–654. [Google Scholar] [CrossRef]
- Weinberg, R.A.; Rotter, V.; Parada, L.F.; Land, H.; Wolf, D. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature 1984, 312, 649–651. [Google Scholar] [CrossRef]
- Wolf, D.; Harris, N.; Rotter, V. Reconstitution of p53 expression in a nonproducer Ab-MuLV-transformed cell line by transfection of a functional p53 gene. Cell 1984, 38, 119–126. [Google Scholar] [CrossRef]
- Finlay, C.A.; Hinds, P.W.; Levine, A.J. The p53 proto-oncogene can act as a suppressor of transformation. Cell 1989, 57, 1083–1093. [Google Scholar] [CrossRef]
- Baker, S.J.; Fearon, E.R.; Nigro, J.M.; Hamilton, S.R.; Preisinger, A.C.; Jessup, J.M.; van Tuinen, P.; Ledbetter, D.H.; Barker, D.F.; Nakamura, Y.; et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 1989, 244, 217–221. [Google Scholar] [CrossRef]
- Lane, D.P. Cancer. p53, guardian of the genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef]
- Morgan, S.E.; Kastan, M.B. Foundations in Cancer Research p53 and ATM: Cell Cycle, Cell Death, and Cancer. Adv. Cancer Res. 1997, 71, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Westphal, C.H. Cell-cycle signaling: Atm displays its many talents. Curr. Biol. 1997, 7, R789–R792. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J.; Bhaumik, D. Two faces of p53: Aging and tumor suppression. Nucleic Acids Res. 2007, 35, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Y.; St Clair, D.K. ROS and p53: A versatile partnership. Free Radic Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Menendez, D.; Inga, A.; Resnick, M.A. The expanding universe of p53 targets. Nat. Rev. Cancer 2009, 9, 724–737. [Google Scholar] [CrossRef]
- Borrás, C.; Gómez-Cabrera, M.C.; Viña, J. The dual role of p53: DNA protection and antioxidant. Free Radic Res. 2011, 45, 643–652. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of p53 in glucose metabolism. Curr. Opin. Cell Biol. 2010, 22, 186–191. [Google Scholar] [CrossRef]
- Olovnikov, I.A.; Kravchenko, J.E.; Chumakov, P.M. Homeostatic functions of the p53 tumor suppressor: Regulation of energy metabolism and antioxidant defense. Semin. Cancer Biol. 2008, 19, 32–41. [Google Scholar] [CrossRef]
- Agoff, S.N.; Hou, J.; Linzer, D.I.; Wu, B. Regulation of the human hsp70 promoter by p53. Science 1993, 259, 84–87. [Google Scholar] [CrossRef]
- Hussain, S.P.; Amstad, P.; He, P.; Robles, A.; Lupold, S.; Kaneko, I.; Ichimiya, M.; Sengupta, S.; Mechanic, L.; Okamura, S.; et al. p53-Induced Up-Regulation of MnSOD and GPx but not Catalase Increases Oxidative Stress and Apoptosis. Cancer Res. 2004, 64, 2350–2356. [Google Scholar] [CrossRef]
- MacCallum, D.E.; Hall, P.A.; Wright, E.G. The Trp53 Pathway Is Induced In Vivo by Low Doses of Gamma Radiation. Radiat. Res. 2001, 156, 324–327. [Google Scholar] [CrossRef]
- Ghosh, J.C.; Suzuki, K.; Kodama, S.; Watanabe, M. Effects of Protein Kinase Inhibitors on the Accumulation Kinetics of p53 Protein in Normal Human Embryo Cells following X-irradiation. J. Radiat. Res. 1999, 40, 23–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aloni-Grinstein, R.; Schwartz, D.; Rotter, V. Accumulation of wild-type p53 protein upon gamma-irradiation induces a G2 arrest-dependent immunoglobulin kappa light chain gene expression. EMBO J. 1995, 14, 1392–1401. [Google Scholar] [CrossRef]
- Fritsche, M.; Haessler, C.; Brandner, G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene 1993, 8, 307–318. [Google Scholar] [PubMed]
- Kim, S.; An, S.S. Role of p53 isoforms and aggregations in cancer. Medicine 2016, 95, e3993. [Google Scholar] [CrossRef] [PubMed]
- Joruiz, S.M.; Bourdon, J. p53 Isoforms: Key Regulators of the Cell Fate Decision. Cold Spring Harb. Perspect. Med. 2016, 6, a026039. [Google Scholar] [CrossRef] [PubMed]
- Joerger, A.C.; Fersht, A.R. Structural Biology of the Tumor Suppressor p53. Annu. Rev. Biochem. 2008, 77, 557–582. [Google Scholar] [CrossRef]
- Osada, M.; Ohba, M.; Kawahara, C.; Ishioka, C.; Kanamaru, R.; Katoh, I.; Ikawa, Y.; Nimura, Y.; Nakagawara, A.; Obinata, M.; et al. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat. Med. 1998, 4, 839–843. [Google Scholar] [CrossRef]
- Kaghad, M.; Bonnet, H.; Yang, A.; Creancier, L.; Biscan, J.C.; Valent, A.; Minty, A.; Chalon, P.; Lelias, J.M.; Dumont, X.; et al. Monoallelically Expressed Gene Related to p53 at 1p36, a Region Frequently Deleted in Neuroblastoma and Other Human Cancers. Cell 1997, 90, 809–819. [Google Scholar] [CrossRef]
- Jun, C.; Sok, M.N.; Changqing, C.; Zhenhai, Z.; Bourdon, J.C.; Lane, D.P.; Peng, J. p53 isoform Δ113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 2009, 23, 278–290. [Google Scholar]
- Turnquist, C.; Beck, J.A.; Horikawa, I. Radiation-induced astrocyte senescence is rescued by Δ133p53. Neuro Oncol. 2019, 21, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Nguyen, T.; Lee, E.M.; Castro-Aceituno, V.; Wagle, R.; Lee, K.S.; Choi, J.; Song, Y.H. Role of p53 isoforms in the DNA damage response during Drosophila oogenesis. Sci. Rep. 2019, 9, 11473. [Google Scholar] [CrossRef] [PubMed]
- Coutandin, D.; Osterburg, C.; Srivastav, R.K.; Sumyk, M.; Kehrloesser, S.; Gebel, J.; Tuppi, M.; Hannewald, J.; Schäfer, B.; Salah, E.; et al. Quality control in oocytes by p63 is based on a spring-loaded activation mechanism on the molecular and cellular level. Elife 2016, 5, e13909. [Google Scholar] [CrossRef] [PubMed]
- Krolewski, B.; Little, J. Alterations of mdm2 gene in x-ray transformed mouse 10t1/2 cell clones. Int. J. Oncol. 1995, 6, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Amundson, S.A.; Lee, R.A.; Koch-Paiz, C.A.; Bittner, M.L.; Meltzer, P.; Trent, J.M.; Fornace, A.J., Jr. Differential Responses of Stress Genes to Low Dose-Rate γ Irradiation. Mol. Cancer Res. 2003, 1, 445. [Google Scholar]
- Terzian, T.; Wang, Y.; Van Pelt, C.S.; Box, N.F.; Travis, E.L.; Lozano, G. Haploinsufficiency of Mdm2 and Mdm4 in Tumorigenesis and Development. Mol. Cell. Biol. 2007, 27, 5479–5485. [Google Scholar] [CrossRef]
- Jabbur, J.R.; Tabor, A.D.; Cheng, X.; Wang, H.; Uesugi, M.; Lozano, G.; Zhang, W. Mdm-2 binding and TAFII31 recruitment is regulated by hydrogen bond disruption between the p53 residues Thr18 and Asp21. Oncogene 2002, 21, 7100–7113. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, Y.; He, H.; Sun, Y. Ribosomal protein S27-like and S27 interplay with p53-MDM2 axis s a target, a substrate, and a regulator. Oncogene 2010, 30, 1798–1811. [Google Scholar] [CrossRef]
- Martínez, A.; Brethauer, U.; Borlando, J.; Spencer, M.L.; Rojas, I.G. Epithelial expression of p53, mdm-2 and p21 in normal lip and actinic cheilitis. Oral Oncol. 2007, 44, 878–883. [Google Scholar] [CrossRef]
- Beskow, C.; Skikuniene, J.; Holgersson, A.; Nilsson, B.; Lewensohn, R.; Kanter, L.; Viktorsson, K. Radioresistant cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs, Ku70 and Ku86. Br. J. Cancer 2009, 101, 816–821. [Google Scholar] [CrossRef]
- Magné, N.; Toillon, R.A.; Bottero, V.; Didelot, C.; Houtte, P.V.; Gérard, J.P.; Peyron, J.F. NF-κB modulation and ionizing radiation: Mechanisms and future directions for cancer treatment. Cancer Lett. 2006, 231, 158–168. [Google Scholar] [CrossRef]
- Habraken, Y.; Piette, J. NF-κB activation by double-strand breaks. Biochem. Pharmacol. 2006, 72, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Yamada, Y.; Tatsuka, M.; Niwa, O.; Yamamoto, K.; Suzuki, F. Down-regulation of nuclear factor kappaB is required for p53-dependent apoptosis in X-ray-irradiated mouse lymphoma cells and thymocytes. Cancer Res. 1999, 59, 6038–6041. [Google Scholar] [PubMed]
- Jonak, K.; Kurpas, M.; Szoltysek, K.; Janus, P.; Abramowicz, A.; Puszynski, K. A novel mathematical model of ATM/p53/NF- κB pathways points to the importance of the DDR switch-off mechanisms. BMC Syst. Biol. 2016, 10, 75. [Google Scholar] [CrossRef]
- Xufeng, C.; Binghui, S.; Liqun, X.; Khaletzkiy, A.; Chu, D.; Wong, J.Y.; Li, J.J. Activation of nuclear factor κb in radioresistance of TP53-inactive human keratinocytes. Cancer Res. 2002, 62, 1213–1221. [Google Scholar]
- Baust, H.; Schoke, A.; Brey, A.; Gern, U.; Los, M.; Schmid, R.M.; Röttinger, E.M.; Seufferlein, T. Evidence for radiosensitizing by gliotoxin in HL-60 cells: Implications for a role of NF-κB independent mechanisms. Oncogene 2003, 22, 8786–8796. [Google Scholar] [CrossRef][Green Version]
- van Hogerlinden, M.; Auer, G.; Toftgard, R. Inhibition of Rel/Nuclear Factor-[kappa]B signaling in skin results in defective DNA damage-induced cell cycle arrest and Ha-ras- and p53-independent tumor development. Oncogene 2002, 21, 4969. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Segaran, R.C.; Chan, L.Y.; Aladresi, A.A.M.; Chinnathambi, A.; Alharbi, S.A.; Sethi, G.; Tang, F.R. Gamma Radiation-Induced Disruption of Cellular Junctions in HUVECs Is Mediated through Affecting MAPK/NF-κB Inflammatory Pathways. Oxid. Med. Cell Longev. 2019, 2019, 1486232. [Google Scholar] [CrossRef]
- Janssens, S.; Tinel, A.; Lippens, S.; Tschopp, J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell 2005, 123, 1079–1092. [Google Scholar] [CrossRef]
- Zając, G.; Marek, R.; Barbara, Ł.S.; Krzysztof, P.; Piotr, W. Activation of the atypical NF-κB pathway induced by ionizing radiation is not affected by the p53 status. Acta Biochim. Pol. 2022, 69, 205–210. [Google Scholar] [CrossRef]
- Zhu, Y.; Zuo, W.; Shen, X.; Liu, Y.; Zhao, Y.; Xiong, Y.; Cao, H.; Wang, Y.; Liang, Z. NF-κB is involved in the regulation of autophagy in mutant p53 cells in response to ionizing radiation. Cell Death. Discov. 2021, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Okazaki, R.; Yoshida, Y. Senescence-associated secretory phenotype and activation of NF-κB in splenocytes of old mice exposed to irradiation at a young age. Dev. Comp. Immunol. 2021, 122, 104124. [Google Scholar] [CrossRef] [PubMed]
- Wellenstein, M.D.; Coffelt, S.B.; Duits, D.E.M.; van Miltenburg, M.H.; Slagter, M.; de Rink, I.; Henneman, L.; Kas, S.M.; Prekovic, S.; Hau, C.S. Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature 2019, 572, 538–542. [Google Scholar] [CrossRef]
- Gasparian, A.V.; Burkhart, C.A.; Purmal, A.A.; Brodsky, L.; Pal, M.; Saranadasa, M.; Bosykh, D.A.; Commane, M.; Guryanova, O.A.; Pal, S.; et al. Curaxins: Anticancer compounds that simultaneously suppress NF-κB and activate p53 by targeting FACT. Sci. Transl. Med. 2011, 3, 95ra74. [Google Scholar] [CrossRef] [PubMed]
- Gurova, K.V.; Hill, J.E.; Guo, C.; Prokvolit, A.; Burdelya, L.G.; Samoylova, E.; Khodyakova, A.V.; Ganapathi, R.; Ganapathi, M.; Tararova, N.D.; et al. Small Molecules That Reactivate p53 in Renal Cell Carcinoma Reveal a NF-κB-Dependent Mechanism of p53 Suppression in Tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 17448–17453. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, A.V.; Komarova, E.A. p53 and the Carcinogenicity of Chronic Inflammation. Cold Spring Harb. Perspect. Med. 2016, 6, a026161. [Google Scholar] [CrossRef]
- Puszynski, K.; Bertolusso, R.; Lipniacki, T. Crosstalk between p53 and nuclear factor-B systems: Pro- and anti-apoptotic functions of NF-B. IET Syst. Biol. 2009, 3, 356–367. [Google Scholar] [CrossRef]
- Adams, B.D.; Parsons, C.; Walker, L.; Zhang, W.C.; Slack, F.J. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017, 127, 761–771. [Google Scholar] [CrossRef]
- Bajan, S.; Hutvagner, G. Regulation of miRNA Processing and miRNA Mediated Gene Repression in Cancer. Microrna 2014, 3, 10–17. [Google Scholar] [CrossRef]
- Bajan, S.; Hutvagner, G. RNA-Based Therapeutics: From Antisense Oligonucleotides to miRNAs. Cells 2020, 9, 137. [Google Scholar] [CrossRef]
- Zenz, T.; Mohr, J.; Eldering, E.; Kater, A.P.; Bühler, A.; Kienle, D.; Winkler, D.; Dürig, J.; van Oers, M.H.; Mertens, D.; et al. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood 2009, 113, 3801–3808. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H. p53 Enters the MicroRNA World. Cancer Cell 2007, 12, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Balca-Silva, J.; Sousa Neves, S.; Goncalves, C.; Abrantes, A.M.; Casalta-Lopes, J.; Botelho, M.F.; Sarmento-Ribeiro, A.B.; Silva, H.C. Effect of miR-34b Overexpression on the Radiosensitivity of Non-small Cell Lung Cancer Cell Lines. Anticancer Res. 2012, 32, 1603–1609. [Google Scholar] [PubMed]
- Liu, C.; Zhou, C.; Gao, F.; Cai, S.; Zhang, C.; Zhao, L.; Zhao, F.; Cao, F.; Lin, J.; Yang, Y.; et al. MiR-34a in Age and Tissue Related Radio-Sensitivity and Serum miR-34a as a Novel Indicator of Radiation Injury. Int. J. Biol. Sci. 2011, 7, 221–233. [Google Scholar] [CrossRef]
- Mert, U.; Ozgür, E.; Tiryakioglu, D.; Dalay, N.; Gezer, U. Induction of p53-inducible microRNA miR-34 by gamma radiation and bleomycin are different. Front. Genet. 2012, 3, 220. [Google Scholar] [CrossRef]
- Sasaki, A.; Udaka, Y.; Tsunoda, Y.; Yamamoto, G.; Tsuji, M.; Oyamada, H.; Oguchi, K.; Mizutani, T. Analysis of p53 and miRNA Expression after Irradiation of Glioblastoma Cell Lines. Anticancer Res. 2012, 32, 4709–4713. [Google Scholar]
- Wang, B.; Li, D.; Kovalchuk, O. p53 Ser15 phosphorylation and histone modifications contribute to IR-induced miR-34a transcription in mammary epithelial cells. Cell Cycle 2013, 12, 2073–2083. [Google Scholar] [CrossRef]
- Saleh, A.D.; Savage, J.E.; Cao, L.; Soule, B.P.; Ly, D.; DeGraff, W.; Harris, C.C.; Mitchell, J.B.; Simone, N.L. Cellular stress induced alterations in microRNA let-7a and let-7b expression are dependent on p53. PLoS ONE 2011, 6, e24429. [Google Scholar] [CrossRef]
- John-Aryankalayil, M.; Palayoor, S.T.; Makinde, A.Y.; Cerna, D.; Simone, C.B.; Falduto, M.T.; Magnuson, S.R.; Coleman, C.N. Fractionated Radiation Alters Oncomir and Tumor Suppressor miRNAs in Human Prostate Cancer Cells. Radiat. Res. 2012, 178, 105–117. [Google Scholar] [CrossRef]
- Zheng, G.X.; Ravi, A.; Calabrese, J.M.; Medeiros, L.A.; Kirak, O.; Dennis, L.M.; Jaenisch, R.; Burge, C.B.; Sharp, P.A. A Latent Pro-Survival Function for the Mir-290-295 Cluster in Mouse Embryonic Stem Cells. PLoS Genet. 2011, 7, e1002054. [Google Scholar] [CrossRef]
- Wang, Y.; Scheiber, M.N.; Neumann, C.; Calin, G.A.; Zhou, D. MicroRNA Regulation of Ionizing Radiation-Induced Premature Senescence. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xing, R.; Zhang, X.; Dong, W.; Zhang, J.; Yan, Z.; Li, W.; Cui, J.; Lu, Y. miR-375 targets the p53 gene to regulate cellular response to ionizing radiation and etoposide in gastric cancer cells. DNA Repair. 2013, 12, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Kim, J.; Nam, J.; Sun, H.; Lee, Y.H.; Lee, T.J.; Aguiar, R.C.; Kim, S.W. MicroRNA-124 links p53 to the NF-κB pathway in B-cell lymphomas. Leukemia 2015, 29, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wu, A.T.; Liu, S. MicroRNA-17-5p regulated apoptosis-related protein expression and radiosensitivity in oral squamous cell carcinoma caused by betel nut chewing. Oncotarget 2016, 7, 51482–51493. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, J.; Liu, G.; Wang, F.G.; Ju, Z.S.; Zhou, D.; Wang, R.Y. MicroRNA-372 enhances radiosensitivity while inhibiting cell invasion and metastasis in nasopharyngeal carcinoma through activating the PBK-dependent p53 signaling pathway. Cancer Med. 2019, 8, 712–728. [Google Scholar] [CrossRef]
- Shao, Y.; Song, X.; Jiang, W.; Chen, Y.; Ning, Z.; Gu, W.; Jiang, J. MicroRNA-621 Acts as a Tumor Radiosensitizer by Directly Targeting SETDB1 in Hepatocellular Carcinoma. Mol. Ther. 2019, 27, 355–364. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Wu, S.; Qu, J.; Yuan, H.; Zhou, Y.; Lu, Q. MicroRNA-1246 regulates the radio-sensitizing effect of curcumin in bladder cancer cells via activating P53. Int. Urol. Nephrol. 2019, 51, 1771–1779. [Google Scholar] [CrossRef]
- Sohn, D.; Peters, D.; Piekorz, R.P.; Budach, W.; Jänicke, R.U. miR-30e controls DNA damage-induced stress responses by modulating expression of the CDK inhibitor p21WAF1/CIP1 and caspase-3. Oncotarget 2016, 7, 15915–15929. [Google Scholar] [CrossRef]
- Lei, X.; He, N.; Zhu, L.; Zhou, M.; Zhang, K.; Wang, C.; Huang, H.; Chen, S.; Li, Y.; Liu, Q.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via miRNA-214-3p. Antioxid. Redox Signal. 2021, 35, 849–862. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Zhao, Y.; Feng, Z. MicroRNA Control of p53. J. Cell. Biochem. 2017, 118, 7–14. [Google Scholar] [CrossRef]
- Candéias, S.M.; Testard, I. The many interactions between the innate immune system and the response to radiation. Cancer Lett. 2015, 368, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Takaoka, A.; Hayakawa, S.; Yanai, H.; Stoiber, D.; Negishi, H.; Kikuchi, H.; Sasaki, S.; Imai, K.; Shibue, T.; et al. Integration of interferon-α/β signalling to p53 responses in tumour suppression and antiviral defence. Nature 2003, 424, 516–523. [Google Scholar] [CrossRef]
- Muñoz-Fontela, C.; Macip, S.; Martínez-Sobrido, L.; Brown, L.; Ashour, J.; García-Sastre, A.; Lee, S.W.; Aaronson, S.A. Transcriptional role of p53 in interferon-mediated antiviral immunity. J. Exp. Med. 2008, 205, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Dharel, N.; Kato, N.; Muroyama, R.; Taniguchi, H.; Otsuka, M.; Wang, Y.; Jazag, A.; Shao, R.X.; Chang, J.H.; Adler, M.K.; et al. Potential contribution of tumor suppressor p53 in the host defense against hepatitis C virus. Hepatology 2008, 47, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Coates, P.J.; Lorimore, S.A.; Wright, E.G. The in vivo expression of radiation-induced chromosomal instability has an inflammatory mechanism. Radiat. Res. 2012, 177, 18–24. [Google Scholar] [CrossRef]
- Coates, P.; Robinson, J.; Lorimore, S.A.; Wright, E.G. Ongoing activation of p53 pathway responses is a long-term consequence of radiation exposure in vivo and associates with altered macrophage activities. J. Pathol. 2008, 214, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Coates, P.J.; Rundle, J.K.; Lorimore, S.A.; Wright, E.G. Indirect Macrophage Responses to Ionizing Radiation: Implications for Genotype-Dependent Bystander Signaling. Cancer Res. 2008, 68, 450–456. [Google Scholar] [CrossRef]
- Lorimore, S.A.; Chrystal, J.A.; Robinson, J.I.; Coates, P.J.; Wright, E.G. Chromosomal Instability in Unirradiated Hemaopoietic Cells Induced by Macrophages Exposed In vivo to Ionizing Radiation. Cancer Res. 2008, 68, 8122–8126. [Google Scholar] [CrossRef]
- Lorimore, S.A.; Mukherjee, D.; Robinson, J.I.; Chrystal, J.A.; Wright, E.G. Long-lived inflammatory signaling in irradiated bone marrow is genome dependent. Cancer Res. 2011, 71, 6485–6491. [Google Scholar] [CrossRef]
- McAllister, K.A.; Lorimore, S.A.; Wright, E.G.; Coates, P.J. In Vivo Interactions between Ionizing Radiation, Inflammation and Chemical Carcinogens Identified by Increased DNA Damage Responses. Radiat. Res. 2012, 177, 584–593. [Google Scholar] [CrossRef]
- Futaki, N.; Takahashi, S.; Yokoyama, M.; Arai, I.; Higuchi, S.; Otomo, S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins 1994, 47, 55–59. [Google Scholar] [CrossRef]
- Mukherjee, D.; Coates, P.J.; Rastogi, S.; Lorimore, S.A.; Wright, E.G. Radiation-induced bone marrow apoptosis, inflammatory bystander-type signaling and tissue cytotoxicity. Int. J. Radiat. Biol. 2013, 89, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.K.; Leu, J.J.; Zhou, Y.; Devarajan, K.; Nedelko, T.; Klein-Szanto, A.; Hollstein, M.; Murphy, M.E. The Codon 72 Polymorphism of p53 Regulates Interaction with NF-κB and Transactivation of Genes Involved in Immunity and Inflammation. Mol. Cell. Biol. 2011, 31, 1201–1213. [Google Scholar] [CrossRef][Green Version]
- Lorimore, S.A.; Coates, P.J.; Scobie, G.E.; Milne, G.; Wright, E.G. Inflammatory-type responses after exposure to ionizing radiation in vivo: A mechanism for radiation-induced bystander effects. Oncogene 2001, 20, 7085–7095. [Google Scholar] [CrossRef]
- Merched, A.J.; Chan, L. Absence of p21Waf1/Cip1/Sdi1 Modulates Macrophage Differentiation and Inflammatory Response and Protects Against Atherosclerosis. Circulation 2004, 110, 3830–3841. [Google Scholar] [CrossRef] [PubMed]
- Derradji, H.; Bekaert, S.; De Meyer, T.; Jacquet, P.; Abou-El-Ardat, K.; Ghardi, M.; Arlette, M.; Baatout, S. Ionizing radiation-induced gene modulations, cytokine content changes and telomere shortening in mouse fetuses exhibiting forelimb defects. Dev. Biol. 2008, 322, 302–313. [Google Scholar] [CrossRef]
- Toutounchian, J.J.; Steinle, J.J.; Makena, P.S.; Waters, C.M.; Wilson, M.W.; Haik, B.G.; Miller, D.D.; Yates, C.R. Modulation of radiation injury response in retinal endothelial cells by quinic acid derivative KZ-41 involves p38 MAPK. PLoS ONE 2014, 9, e100210. [Google Scholar] [CrossRef]
- Dong, X.; Luo, M.; Huang, G.; Zhang, J.; Tong, F.; Cheng, Y.; Cai, Q.; Dong, J.; Wu, G.; Cheng, J. Relationship between irradiation-induced neuro-inflammatory environments and impaired cognitive function in the developing brain of mice. Int. J. Radiat. Biol. 2015, 91, 224–239. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Q.; Yuan, Y.; Xin, N.; He, K.; Huang, Y.; Tang, H.; Gong, P. Sema3a inhibits the differentiation of Raw264.7 cells to osteoclasts under 2Gy radiation by reducing inflammation. PLoS ONE 2018, 13, e0200000. [Google Scholar] [CrossRef]
- Ham, S.W.; Jeon, H.Y.; Jin, X.; Kim, E.J.; Kim, J.K.; Shin, Y.J.; Lee, Y.; Kim, S.H.; Lee, S.Y.; Seo, S.; et al. TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death Differ. 2019, 26, 409–425. [Google Scholar] [CrossRef]
- Madhugiri, V.S.; Moiyadi, A.V.; Shetty, P.; Gupta, T.; Epari, S.; Jalali, R.; Subeikshanan, V.; Dutt, A.; Sasidharan, G.M.; Roopesh Kumar, V.R.; et al. Analysis of Factors Associated with Long-Term Survival in Patients with Glioblastoma. World. Neurosurg. 2021, 149, e758–e765. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Bi, N.; Ji, W.; Tan, W.; Zhao, L.; Yu, D.; Wu, C.; Wang, L.; Lin, D. Association of P53 and ATM polymorphisms with risk of radiation-Induced pneumonitis in lung cancer patients treated with radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1402–1407. [Google Scholar] [CrossRef]
- Rogers, C.J.; Kyubwa, E.M.; Lukaszewicz, A.I.; Yamada-Hanff, J.; Starbird, M.A.; Miller, T.A.; Phelps, A.A.; Wallack, S.; Mahendra, S.; Thrall, K.; et al. Identification of miRNA Associated with Reduced Survival after Whole-Thorax Lung Irradiation in Non-Human Primates. Radiat. Res. 2021, 196, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Shen, L.; Zhou, M.; Luo, J.; Yu, Z.; Qu, C.; Lei, R.; Lei, M.; Huang, R. Helicobacter pylori and Alzheimer’s Disease-Related Metabolic Dysfunction: Activation of TLR4/Myd88 Inflammation Pathway from p53 Perspective and a Case Study of Low-Dose Radiation Intervention. ACS. Chem. Neurosci. 2022, 13, 1065–1081. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N. Reexamining the role of tissue inflammation in radiation carcinogenesis: A hypothesis to explain an earlier onset of cancer. Int. J. Radiat. Biol. 2021, 97, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.W.; Karin, M.A. cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef]
- Coussens, L.M.; de Visser, K.E.; Eichten, A. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Balkwill, F.; Coussens, L.M. Cancer: An inflammatory link. Nature 2004, 431, 405–406. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zena, W. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Fredlund, E.; Zhao, W.; Perou, C.M.; Balmain, A.; Mao, J.H.; Barcellos-Hoff, M.H. Murine microenvironment metaprofiles associate with human cancer etiology and intrinsic subtypes. Clin. Cancer Res. 2013, 19, 1353–1362. [Google Scholar] [CrossRef]
- Morioka, T.; Miyoshi-Imamura, T.; Blyth, B.J.; Kaminishi, M.; Kokubo, T.; Nishimura, M.; Kito, S.; Tokairin, Y.; Tani, S.; Murakami-Murofushi, K.; et al. Ionizing radiation, inflammation, and their interactions in colon carcinogenesis in Mlh1-deficient mice. Cancer Sci. 2015, 106, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Manna, K.; Das, U.; Das, D.; Kaminishi, M.; Kokubo, T.; Nishimura, M.; Kito, S.; Tokairin, Y.; Tani, S.; Murakami-Murofushi, K.; et al. Naringin inhibits gamma radiation-induced oxidative DNA damage and inflammation, by modulating p53 and NF-κB signaling pathways in murine splenocytes. Cancer Sci. 2015, 49, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.P.; Caramia, F.; Gamell, C.; Paul, P.J.; Arnau, G.M.; Neeson, P.J.; Williams, S.G.; Haupt, Y. The Transcriptional Landscape of Radiation-Treated Human Prostate Cancer: Analysis of a Prospective Tissue Cohort. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, K.; Ikeda, K.; Nakata, Y.; Yamasaki, N.; Ueda, T.; Kanai, A.; Sentani, K.; Sera, Y.; Hayashi, T.; Koizumi, M.; et al. Kdm6a Deficiency Activates Inflammatory Pathways, Promotes M2 Macrophage Polarization, and Causes Bladder Cancer in Cooperation with p53 Dysfunction. Clin. Cancer Res. 2020, 26, 2065–2079. [Google Scholar] [CrossRef]
- Brachman, D.G.; Hallahan, D.E.; Beckett, M.A.; Yandell, D.W.; Weichselbaum, R.R. p53 gene mutations and abnormal retinoblastoma protein in radiation-induced human sarcomas. Cancer Res. 1991, 51, 6393–6396. [Google Scholar]
- Lee, J.M.; Abrahamson, J.L.; Kandel, R.; Donehower, L.A.; Bernstein, A. Susceptibility to radiation-carcinogenesis and accumulation of chromosomal breakage in p53 deficient mice. Oncogene 1994, 9, 3731–3736. [Google Scholar]
- Iwamoto, K.S.; Fujii, S.; Kurata, A.; Suzuki, M.; Hayashi, T.; Ohtsuki, Y.; Okada, Y.; Narita, M.; Takahashi, M.; Hosobe, S.; et al. p53 mutations in tumor and non-tumor tissues of Thorotrast recipients: A model for cellular selection during radiation carcinogenesis in the liver. Carcinogenesis 1999, 20, 1283–1291. [Google Scholar] [CrossRef][Green Version]
- Wiese, C.; Gauny, S.S.; Liu, W.C.; Cherbonnel-Lasserre, C.L.; Kronenberg, A. Different Mechanisms of Radiation-induced Loss of Heterozygosity in Two Human Lymphoid Cell Lines from a Single Donor. Cancer Res. 2001, 61, 1129–1137. [Google Scholar]
- Gonin-Laurent, N.; Gibaud, A.; Huygue, M.; Lefèvre, S.H.; Le Bras, M.; Chauveinc, L.; Sastre-Garau, X.; Doz, F.; Lumbroso, L.; Chevillard, S.; et al. Specific TP53 mutation pattern in radiation-induced sarcomas. Carcinogenesis 2006, 27, 1266–1272. [Google Scholar] [CrossRef]
- Po, Z.; De-Wen, W.; Guomin, L.; Yabing, G.; Xiaoying, L. Abnormal location of p16 protein and overexpression of p53 protein in human radiation-induced skin cancer. J. Environ. Pathol. Toxicol. Oncol. 1995, 14, 25–28. [Google Scholar]
- Selvanayagam, C.S.; Davis, C.M.; Cornforth, M.N.; Ullrich, R.L. Latent Expression of p53 Mutations and Radiation-induced Mammary Cancer. Cancer Res. 1995, 55, 3310–3317. [Google Scholar] [PubMed]
- Igari, K.; Igari, Y.; Okazaki, R.; Kato, F.; Ootsuyama, A.; Norimura, T. The Delayed Manifestation of T-Cell Receptor (TCR) Variants in X-Irradiated Mice Depends on Trp53 Status. Radiat. Res. 2006, 166, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.S.; Mayhugh, B.M.; McDowell, B.; Chin-Sinex, H.; Smith, M.L.; Dynlacht, J.R.; Spandau, D.F.; Lewis, D.A. A Radiation-Induced Acute Apoptosis Involving TP53 and BAX Precedes the Delayed Apoptosis and Neoplastic Transformation of CGL1 Human Hybrid Cells. Radiat. Res. 2005, 163, 614–622. [Google Scholar] [CrossRef]
- Hollstein, M.; Bartsch, H.; Wesch, H.; Kure, E.H.; Mustonen, R.; Mühlbauer, K.R.; Spiethoff, A.; Wegener, K.; Wiethege, T.; Müller, K.M. p53 gene mutation analysis in tumors of patients exposed to alpha-particles. Carcinogenesis 1997, 18, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Kennedy, C.H.; Amstad, P.; Lui, H.; Lechner, J.F.; Harris, C.C. Radon and lung carcinogenesis: Mutability of p53 codons 249 and 250 to 238Pu alpha-particles in human bronchial epithelial cells. Carcinogenesis 1997, 18, 121–125. [Google Scholar] [CrossRef]
- Andersson, M.; Jönsson, M.; Nielsen, L.L.; Vyberg, M.; Visfeldt, J.; Storm, H.H.; Wallin, H. Mutations in the tumor suppressor gene p53 in human liver cancer induced by alpha-particles. Cancer Epidemiol. Biomark. Prev. 1995, 4, 765–770. [Google Scholar]
- Jin, Y.; Burns, F.J.; Garte, S.J.; Hosselet, S. Infrequent alterations of the p53 gene in rat skin cancers induced by ionizing radiation. Carcinogenesis 1996, 17, 873–876. [Google Scholar] [CrossRef]
- Rabes, H.M. Gene rearrangements in radiation-induced thyroid carcinogenesis. Med. Pediatr. Oncol. 2001, 36, 574–582. [Google Scholar] [CrossRef]
- Suchy, B.; Waldmann, V.; Klugbauer, S.; Rabes, H.M. Absence of RAS and p53 mutations in thyroid carcinomas of children after Chernobyl in contrast to adult thyroid tumours. Br. J. Cancer. 1998, 77, 952–955. [Google Scholar] [CrossRef]
- Hillebrandt, S.; Streffer, C.; Demidchik, E.P.; Biko, J.; Reiners, C. Polymorphisms in the p53 gene in thyroid tumours and blood samples of children from areas in Belarus. Mutat. Res. 1997, 381, 201–207. [Google Scholar] [CrossRef]
- Han, Y.; Tan, Y.; Zhao, Y.; Zhang, Y.; He, X.; Yu, L.; Jiang, H.; Lu, H.; Tian, H. TRIM23 overexpression is a poor prognostic factor and contributes to carcinogenesis in colorectal cancer. J. Cell. Mol. Med. 2020, 24, 5491–5500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Wang, Z.; Li, X.; Louie, A.; Wei, G.; Mao, J.H. Temporal mTOR inhibition protects Fbxw7-deficient mice from radiation-induced tumor development. Aging 2013, 5, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Castle, K.D.; Moding, E.J.; Blum, J.M.; Williams, N.; Luo, L.; Ma, Y.; Borst, L.B.; Kim, Y.; Kirsch, D.G. Acute DNA damage activates the tumour suppressor p53 to promote radiation-induced lymphoma. Nat. Commun. 2015, 6, 8477. [Google Scholar] [CrossRef]
- Moding, E.J.; Min, H.D.; Castle, K.D.; Ali, M.; Woodlief, L.; Williams, N.; Ma, Y.; Kim, Y.; Lee, C.L.; Kirsch, D.G. An extra copy of p53 suppresses development of spontaneous Kras-driven but not radiation-induced cancer. CI Insight 2016, 1, e86698. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.I.; Roderick, J.E.; Gannon, H.S.; Kelliher, M.A.; Jones, S.N. Mdm2 Phosphorylation Regulates Its Stability and Has Contrasting Effects on Oncogene and Radiation-Induced Tumorigenesis. Cell Rep. 2016, 16, 2618–2629. [Google Scholar] [CrossRef]
- Chibaya, L.; Karim, B.; Zhang, H.; Jones, S.N. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2003193118. [Google Scholar] [CrossRef]
- Lee, C.L.; Brock, K.D.; Hasapis, S.; Zhang, D.; Sibley, A.B.; Qin, X.; Gresham, J.S.; Caraballo, I.; Luo, L.; Daniel, A.R.; et al. Whole-Exome Sequencing of Radiation-Induced Thymic Lymphoma in Mouse Models Identifies Notch1 Activation as a Driver of p53 Wild-Type Lymphoma. Cancer Res. 2021, 81, 3777–3790. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Salama, R.; Sadaie, M.; Hoare, M.; Narita, M. Cellular senescence and its effector programs. Genes Dev. 2014, 28, 99–114. [Google Scholar] [CrossRef]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell. Biol. 2015, 16, 393–405. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti-Stella, A.; Mariani, E.; Kurelac, I.; Maresca, A.; Caratozzolo, M.F.; Iommarini, L.; Carelli, V.; Eusebi, L.H.; Guido, A.; Cenacchi, G.; et al. Gamma rays induce a p53-independent mitochondrial biogenesis that is counter-regulated by HIF1α. Cell Death Dis. 2013, 4, e663. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, Y.; Wang, J.; Zhai, J.; He, F.; Zhu, G. Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling. Am. J. Physiol. Cell Physiol. 2020, 318, C1005–C1017. [Google Scholar] [CrossRef]
- Suzuki, K.; Mori, I.; Nakayama, Y.; Miyakoda, M.; Kodama, S.; Watanabe, M. Radiation-Induced Senescence-like Growth Arrest Requires TP53 Function but not Telomere Shortening. Radiat. Res. 2001, 155, 248–253. [Google Scholar] [CrossRef]
- Le, O.N.; Rodier, F.; Fontaine, F.; Coppe, J.P.; Campisi, J.; DeGregori, J.; Laverdiere, C.; Kokta, V.; Haddad, E.; Beausejour, C.M. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell 2010, 9, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, K.; Kodama, S.; Watanabe, M. Interstitial chromatin alteration causes persistent p53 activation involved in the radiation-induced senescence-like growth arrest. Biochem. Biophys. Res. Commun. 2006, 340, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Elmore, L.W.; Jackson-Cook, C.; Demasters, G.; Povirk, L.F.; Holt, S.E.; Gewirtz, D.A. p53-Dependent accelerated senescence induced by ionizing radiation in breast tumour cells. Int. J. Radiat. Biol. 2005, 81, 445–458. [Google Scholar] [CrossRef]
- Sharma, G.G.; Hall, E.J.; Dhar, S.; Gupta, A.; Rao, P.H.; Pandita, T.K. Telomere stability correlates with longevity of human beings exposed to ionizing radiations. Oncol. Rep. 2003, 10, 1733–1736. [Google Scholar] [CrossRef]
- Meng, A.; Wang, Y.; Van Zant, G.; Zhou, D. Ionizing Radiation and Busulfan Induce Premature Senescence in Murine Bone Marrow Hematopoietic Cells. Cancer Res 2003, 63, 5414–5419. [Google Scholar]
- Yentrapalli, R.; Azimzadeh, O.; Barjaktarovic, Z.; Sarioglu, H.; Wojcik, A.; Harms-Ringdahl, M.; Atkinson, M.J.; Haghdoost, S.; Tapio, S. Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma radiation. Proteomics 2013, 13, 1096–1107. [Google Scholar] [CrossRef]
- Dong, X.; Tong, F.; Qian, C.; Zhang, R.; Dong, J.; Wu, G.; Hu, Y. NEMO Modulates Radiation-Induced Endothelial Senescence of Human Umbilical Veins Through NF-κB Signal Pathway. Radiat. Res. 2015, 183, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, E.C.; Dimaio, D. Induced senescence in HeLa cervical carcinoma cells containing elevated telomerase activity and extended telomeres. Cell Growth Differ. 2001, 12, 525–534. [Google Scholar] [PubMed]
- Itjima, M.; Mihara, K.; Kondo, T.; Tsuji, T.; Ishioka, C.; Namba, M. Mutation in p53 and de-regulation of p53-related gene expression in three human cell lines immortalized with 4-nitroquinoline 1-oxide or 60Co gamma rays. Int. J. Cancer 1996, 66, 698–702. [Google Scholar] [CrossRef]
- Zheng, Q.H.; Ma, L.W.; Zhu, W.G.; Zhang, Z.Y.; Tong, T.J. p21Waf1/Cip1 plays a critical role in modulating senescence through changes of DNA methylation. J. Cell. Biochem. 2006, 98, 1230–1248. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Vogel, H.; Willerford, D.M.; Sands, A.T.; Platt, K.A.; Hasty, P. Analysis of ku80-Mutant Mice and Cells with Deficient Levels of p53. Mol. Cell. Biol. 2000, 20, 3772–3780. [Google Scholar] [CrossRef][Green Version]
- Naka, K.; Tachibana, A.; Ikeda, K.; Motoyama, N. Stress-induced Premature Senescence in hTERT-expressing Ataxia Telangiectasia Fibroblasts. J. Biol. Chem. 2004, 279, 2030–2037. [Google Scholar] [CrossRef]
- MacLaren, A.; Black, E.J.; Clark, W.; Gillespie, D.A. c-Jun-Deficient Cells Undergo Premature Senescence as a Result of Spontaneous DNA Damage Accumulation. Mol. Cell. Biol. 2004, 24, 9006–9018. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Jang, D.J. Protein Kinase CK2 Regulates Cytoskeletal Reorganization during Ionizing Radiation-Induced Senescence of Human Mesenchymal Stem Cells. Cancer Res. 2009, 69, 8200–8207. [Google Scholar] [CrossRef]
- Calabrese, V.; Mallette, F.A.; Deschênes-Simard, X.; Ramanathan, S.; Gagnon, J.; Moores, A.; Ilangumaran, S.; Ferbeyre, G. SOCS1 Links Cytokine Signaling to p53 and Senescence. Mol. Cell 2009, 36, 754–767. [Google Scholar] [CrossRef]
- Cho, S.J.; Rossi, A.; Jung, Y.S.; Yan, W.; Liu, G.; Zhang, J.; Zhang, M.; Chen, X. Ninjurin1, a target of p53, regulates p53 expression and p53-dependent cell survival, senescence, and radiation-induced mortality. Proc. Natl. Acad. Sci. USA 2013, 110, 9362–9367. [Google Scholar] [CrossRef]
- Ronald Allan, M.P.; Regina, M.D. Inhibition of IGF-1R prevents ionizing radiation-induced primary endothelial cell senescence. PLoS ONE 2013, 8, e78589. [Google Scholar] [CrossRef]
- Neise, D.; Sohn, D.; Stefanski, A.; Goto, H.; Inagaki, M.; Wesselborg, S.; Budach, W.; Stühler, K.; Jänicke, R.U. The p90 ribosomal S6 kinase (RSK) inhibitor BI-D1870 prevents gamma irradiation-induced apoptosis and mediates senescence via RSK- and p53-independent accumulation of p21WAF1/CIP1. Cell Death Dis. 2013, 4, e859. [Google Scholar] [CrossRef]
- Wang, Y.; Blandino, G.; Givol, D. Induced p21waf expression in H1299 cell line promotes cell senescence and protects against cytotoxic effect of radiation and doxorubicin. Oncogene 1999, 18, 2643–2649. [Google Scholar] [CrossRef]
- Liakou, E.; Mavrogonatou, E.; Pratsinis, H.; Rizou, S.; Evangelou, K.; Panagiotou, P.N.; Karamanos, N.K.; Gorgoulis, V.G.; Kletsas, D. Ionizing radiation-mediated premature senescence and paracrine interactions with cancer cells enhance the expression of syndecan 1 in human breast stromal fibroblasts: The role of TGF-beta. Aging 2016, 8, 1650–1669. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.S.; Cho, E.W.; Kim, J.S.; Moon, M.S.; Yum, J.J.; Kim, K.C.; Kim, I.G. Thioredoxin overexpression in HT-1080 cells induced cellular senescence and sensitization to gamma radiation. FEBS Lett. 2005, 579, 4055–4062. [Google Scholar] [CrossRef][Green Version]
- Quick, Q.A.; Gewirtz, D.A. An accelerated senescence response to radiation in wild-type p53 glioblastoma multiforme cells. J. Neurosurg. 2006, 105, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Toillon, R.A.; Magne, N.; Laios, I.; Castadot, P.; Kinnaert, E.; Van Houtte, P.; Desmedt, C.; Leclercq, G.; Lacroix, M. Estrogens decrease [gamma]-ray-induced senescence and maintain cell cycle progression in breast cancer cells independently of p53. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1187. [Google Scholar] [CrossRef]
- Karimi-Busheri, F.; Rasouli-Nia, A.; Mackey, J.R.; Weinfeld, M. Senescence evasion by MCF-7 human breast tumor-initiating cells. Breast Cancer Res. 2010, 12, R31. [Google Scholar] [CrossRef]
- Shin, J.S.; Sang, H.W.; Jin, D.H.; Park, I.C.; Lee, H.C.; Hong, S.W.; Yoo, D.H.; Hong, S.I.; Lee, W.J.; Lee, M.S.; et al. Low doses of ionizing radiation suppress doxorubicin-induced senescence-like phenotypes by activation of ERK1/2 and suppression of p38 kinase in MCF7 human breast cancer cells. Int. J. Oncol. 2010, 36, 1445–1452. [Google Scholar] [CrossRef]
- Lehmann, B.D.; McCubrey, J.A.; Jefferson, H.S.; Paine, M.S.; Chappell, W.H.; Terrian, D.M. A Dominant Role for p53-Dependent Cellular Senescence in Radiosensitization of Human Prostate Cancer Cells. Cell Cycle 2007, 6, 595–605. [Google Scholar] [CrossRef]
- Arya, A.K.; El-Fert, A.; Jones, T.M.; Boyd, M.T.; Devling, T.; Eccles, R.M.; Aslam, M.A.; Rubbi, C.P.; Vlatkovic, N.; Fenwick, J.; et al. Nutlin-3, the small-molecule inhibitor of MDM2, promotes senescence and radiosensitises laryngeal carcinoma cells harbouring wild-type p53. Br. J. Cancer 2010, 103, 186–195. [Google Scholar] [CrossRef]
- Luo, H.; Yount, C.; Lang, H.; Yang, A.; Riemer, E.C.; Lyons, K.; Vanek, K.N.; Silvestri, G.A.; Schulte, B.A.; Wang, G.Y. Activation of p53 with Nutlin-3a radiosensitizes lung cancer cells via enhancing radiation-induced premature senescence. Lung Cancer 2013, 81, 167–173. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Guo, J.; Song, H.; Zhu, H.; Di, X.; Min, H.; Wang, Y.; Chen, G.; Dai, W.; Ma, J.; et al. Nutlin-3, an Antagonist of MDM2, Enhances the Radiosensitivity of Esophageal Squamous Cancer with Wild-Type p53. Pathol. Oncol. Res. 2017, 24, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Efimova, E.V.; Mauceri, H.J.; Kron, S.J.; Weichselbaum, R.R.; Golden, D.W.; Labay, E.; BindokasI, V.P.; Darga, T.E.; Chakraborty, C.; Barreto-Andrade, J.C.; et al. Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors. Cancer Res. 2010, 70, 6277–6282. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Kletsas, D. Human lung fibroblasts prematurely senescent after exposure to ionizing radiation enhance the growth of malignant lung epithelial cells in vitro and in vivo. Int. J. Oncol. 2011, 39, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.; Jackson, S.; Cullinane, C.; Natoli, A.; Neilsen, P.M.; Callen, D.F.; Maira, S.M.; Hackl, W.; McArthur, G.A.; Solomon, B.; et al. Inhibition of DNA-dependent protein kinase induces accelerated senescence in irradiated human cancer cells. Mol. Cancer Res. 2011, 9, 1696–1707. [Google Scholar] [CrossRef]
- Li, X.H.; Ha, C.T.; Fu, D.; Xiao, M. REDD1 protects osteoblast cells from gamma radiation-induced premature senescence. PLoS ONE 2012, 7, e36604. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.; Xu, B.; Hu, H.; Wei, Z.; Liu, Q.; Zhang, X.; Ding, X.; Wang, Y.; Zhao, M.; et al. Chemokine receptor CXCR2 is transactivated by p53 and induces p38-mediated cellular senescence in response to DNA damage. Aging Cell 2013, 12, 1110–1121. [Google Scholar] [CrossRef]
- Feys, L.; Descamps, B.; Vanhove, C.; Vral, A.; Veldeman, L.; Vermeulen, S.; De Wagter, C.; Bracke, M.; De Wever, O. Radiation-induced lung damage promotes breast cancer lung-metastasis through CXCR4 signaling. Oncotarget 2015, 6, 26615–26632. [Google Scholar] [CrossRef]
- Skwarska, A.; Ramachandran, S.; Dobrynin, G.; Leszczynska, K.B.; Hammond, E.M. The imidazoacridinone C-1311 induces p53-dependent senescence or p53-independent apoptosis and sensitizes cancer cells to radiation. Oncotarget 2017, 8, 31187–31198. [Google Scholar] [CrossRef]
- Tesei, A.; Arienti, C.; Bossi, G.; Santi, S.; De Santis, I.; Bevilacqua, A.; Zanoni, M.; Pignatta, S.; Cortesi, M.; Zamagni, A.; et al. TP53 drives abscopal effect by secretion of senescence-associated molecular signals in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 89. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Bodycote, J.; Wolff, S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 1984, 223, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Ohnishi, K.; Asakawa, I.; Kondo, N.; Nakagawa, H.; Yonezawa, M.; Tachibana, A.; Matsumoto, H.; Ohnishi, T. Radiation response of apoptosis in C57BL/6N mouse spleen after whole-body irradiation. Int. J. Radiat. Biol. 2001, 77, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Ootsuyama, A.; Norimura, T. TP53 and TP53-Related Genes Associated with Protection from Apoptosis in the Radioadaptive Response. Radiat. Res. 2007, 167, 51–57. [Google Scholar] [CrossRef]
- Horie, K.; Kubo, K.; Yonezawa, M. p53 Dependency of Radio-adaptive Responses in Endogenous Spleen Colonies and Peripheral Blood-cell Counts in C57BL Mice. Comp. Study 2002, 43, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Involvement of p53-Dependent Apoptosis in Radiation Teratogenesis and in the Radioadaptive Response in the Late Organogenesis of Mice. J. Radiat. Res. 2001, 42, 1–10. [Google Scholar] [CrossRef]
- Ahmed, K.M.; Nantajit, D.; Fan, M.; Murley, J.S.; Grdina, D.J.; Li, J.J. Coactivation of ATM/ERK/NF-κB in the low-dose radiation-induced radioadaptive response in human skin keratinocytes. Free Radic Biol. Med. 2009, 46, 1543–1550. [Google Scholar] [CrossRef]
- Murley, J.S.; Miller, R.C.; Weichselbaum, R.R.; Grdina, D.J. TP53 Mutational Status and ROS Effect the Expression of the Survivin-Associated Radio-Adaptive Response. Radiat. Res. 2017, 188, 659–670. [Google Scholar] [CrossRef]
- Shadley, J.D. Chromosomal Adaptive Response in Human Lymphocytes. Radiat. Res. 1994, 138, S9–S12. [Google Scholar] [CrossRef]
- Sasaki, M.S. On the Reaction Kinetics of the Radioadaptive Response in Cultured Mouse Cells. Int. J. Radiat. Biol. 1995, 68, 281–291. [Google Scholar] [CrossRef]
- Takahashi, A.; Kondo, N.; Inaba, H.; Uotani, K.; Kiyohara, Y.; Ohnishi, K.; Ohnishi, T. Radiation-induced apoptosis in SCID mice spleen after low dose irradiation. Adv. Space Res. 2003, 31, 1569–1573. [Google Scholar] [CrossRef]
- Wang, B.; Ohyama, H.; Shang, Y.; Tanaka, K.; Aizawa, S.; Yukawa, O.; Hayata, I. Adaptive Response in Embryogenesis: V. Existence of Two Efficient Dose-Rate Ranges for 0.3 Gy of Priming Irradiation to Adapt Mouse Fetuses. Radiat. Res. 2004, 161, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Mitchel, R.E.; Jackson, J.S.; McCann, R.A.; Boreham, D.R. The Adaptive Response Modifies Latency for Radiation-Induced Myeloid Leukemia in CBA/H Mice. Radiat. Res. 1999, 152, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Vares, G.; Wang, B.; Shang, Y.; Ohyama, H.; Tanaka, K.; Nakajima, T.; Nenoi, M.; Hayata, I. Adaptive response in embryogenesis: VI. Comparative microarray analysis of gene expressions in mouse fetuses. Int. J. Radiat. Biol. 2009, 85, 70–86. [Google Scholar] [CrossRef]
- Wang, B.; Ohyama, H.; Shang, Y.; Fujita, K.; Tanaka, K.; Nakajima, T.; Aizawa, S.; Yukawa, O.; Hayata, I. Adaptive Response in Embryogenesis: IV. Protective and Detrimental Bystander Effects Induced by X Radiation in Cultured Limb Bud Cells of Fetal Mice. Radiat. Res. 2004, 161, 9–16. [Google Scholar] [CrossRef]
- Wang, B.; Ohyama, H.; Haginoya, K.; Odaka, T.; Itsukaichi, H.; Yukawa, O.; Yamada, T.; Hayata, I. Adaptive Response in Embryogenesis. III. Relationship to Radiation-Induced Apoptosis and Trp53 Gene Status. Radiat. Res. 2000, 154, 277–282. [Google Scholar] [CrossRef]
- Wang, B.; Ohyama, H.; Haginoya, K.; Odaka, T.; Itsukaichi, H.; Nose, M.; Nakajima, T.; Yukawa, O.; Yamada, T.; Hayata, I. Adaptive Response in Embryogenesis: II.Retardation of Postnatal Development of Prenatally Irradiated Mice. Radiat. Res. 1999, 152, 119–123. [Google Scholar] [CrossRef]
- Wang, B.; Ohyama, H.; Nose, T.; Itsukaichi, H.; Nakajima, T.; Yukawa, O.; Odaka, T.; Tanaka, K.; Kojima, E.; Yamada, T.; et al. Adaptive Response in Embryogenesis: I. Dose and Timing of Radiation for Reduction of Prenatal Death and Congenital Malformation during the Late Period of Organogenesis. Radiat. Res. 1998, 150, 120–122. [Google Scholar] [CrossRef]
- Okazaki, R.; Ootsuyama, A.; Norimura, T. Radioadaptive Response for Protection against Radiation-Induced Teratogenesis. Radiat. Res. 2005, 163, 266–270. [Google Scholar] [CrossRef]
- Boreham, D.R.; Dolling, J.A.; Somers, C.; Quinn, J.; Mitchel, R.E. The Adaptive Response and Protection against Heritable Mutations and Fetal Malformation. Dose Response 2006, 4, 317–326. [Google Scholar] [CrossRef]
- Jiang, H.; Li, W.; Li, X.; Cai, L.; Wang, G. Low-dose radiation induces adaptive response in normal cells, but not in tumor cells: In vitro and in vivo studies. J. Radiat. Res 2008, 49, 219–230. [Google Scholar] [CrossRef] [PubMed]
- López-Nieva, P.; Malavé, M.; González-Sánchez, L.; Fernández-Piqueras, J.; Fernández-Navarro, P.; Santos, J. Transcriptomic analysis reveals sex-specific differences in the expression of Dcl1 and Fis1 genes in the radio-adaptive response of thymocytes to TRP53-mediated apoptosis. BMC Genom. 2016, 17, 698. [Google Scholar] [CrossRef] [PubMed]
- Varès, G.; Wang, B.; Tanaka, K.; Kakimoto, A.; Eguchi-Kasai, K.; Nenoi, M. Mutagenic adaptive response to high-LET radiation in human lymphoblastoid cells exposed to low doses of heavy-ion radiation. Mutat. Res. 2011, 712, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Das, B. Global transcriptome profile reveals abundance of DNA damage response and repair genes in individuals from high level natural radiation areas of Kerala coast. PLoS ONE 2017, 12, e0187274. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, P.M.; Fardid, R.; Zare, T.; Kargar, S.F.; Moslen-Shirazi, M.A.; Behzad, B.A. Assessment of Adaptive Response of Gamma Radiation in the Operating Room Personnel Exposed to Anesthetic Gases by Measuring the Relative Gene Expression Changes Ku80, Ligase1 and P53. J. Biomed. Phys Eng. 2020, 10, 225–234. [Google Scholar] [CrossRef][Green Version]
- Guéguen, Y.; Bontemps, A.; Ebrahimian, T.G. Adaptive responses to low doses of radiation or chemicals: Their cellular and molecular mechanisms. Cell. Mol. Life Sci. 2018, 76, 1255–1273. [Google Scholar] [CrossRef]
- Schlichtholz, B.; Trédaniel, J.; Lubin, R.; Zalcman, G.; Hirsch, A.; Soussi, T. Analyses of p53 antibodies in sera of patients with lung carcinoma define immunodominant regions in the p53 protein. Br. J. Cancer 1994, 69, 809–816. [Google Scholar] [CrossRef][Green Version]
- Luo, J.C.; Zehab, R.; Anttila, S.; Ridanpaa, M.; Husgafvel-Pursiainen, K.; Vainio, H.; Carney, W.; De Vivo, I.; Milling, C.; Brandt-Rauf, P.W. Detection of Serum p53 Protein in Lung Cancer Patients. J. Occup. Med. 1994, 36, 155–160. [Google Scholar] [CrossRef]
- Lubin, R.; Zalcman, G.; Bouchet, L.; Tredanel, J.; Legros, Y.; Cazals, D.; Hirsch, A.; Soussi, T. Serum p53 antibodies as early markers of lung cancer. Nat. Med. 1995, 1, 701–702. [Google Scholar] [CrossRef]
- Brandt-Rauf, P.W.; Smith, S.; Hemminki, K.; Koskinen, H.; Vainio, H.; Niman, H.; Ford, J. Serum oncoproteins and growth factors in asbestosis and silicosis patients. Int. J. Cancer 1992, 50, 881–885. [Google Scholar] [CrossRef]
- Huang, R.; Liu, X.; Li, H.; Zhou, Y.; Zhou, P.K. Integrated analysis of transcriptomic and metabolomic profiling reveal the p53 associated pathways underlying the response to ionizing radiation in HBE cells. Cell. Biosci. 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Popp, W.; Plappert, U.; Müller, W.; Rehn, B.; Schneider, J.; Braun, A.; Bauer, P.C.; Vahrenholz, C.; Presek, P.; Brauksiepe, A.; et al. Biomarkers of genetic damage and inflammation in blood and bronchoalveolar lavage fluid among former German uranium miners: A pilot study. Radiat. Environ. Biophys. 2000, 39, 275–282. [Google Scholar] [CrossRef] [PubMed]
- El-Saghire, H.; Thierens, H.; Monsieurs, P.; Michaux, A.; Vandevoorde, C.; Baatout, S. Gene set enrichment analysis highlights different gene expression profiles in whole blood samples X-irradiated with low and high doses. Int. J. Radiat. Biol. 2013, 89, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, S.; Jia, M.; Li, X.; Li, L.; Wang, Q.; Qi, Z.; Zhou, P.; Li, Y.; Wang, Z. Early Biomarkers Associated with P53 Signaling for Acute Radiation Injury. Life 2022, 12, 99. [Google Scholar] [CrossRef]
- Hayashi, T.; Morishita, Y.; Khattree, R.; Misumi, M.; Sasaki, K.; Hayashi, I.; Yoshida, K.; Kajimura, J.; Kyoizumi, S.; Imai, K.; et al. Evaluation of systemic markers of inflammation in atomic-bomb survivors with special reference to radiation and age effects. FASEB J. 2012, 26, 4765–4773. [Google Scholar] [CrossRef]
- Hayashi, T.; Kusunoki, Y.; Hakoda, M.; Morishita, Y.; Kubo, Y.; Maki, M.; Kasagi, F.; Kodama, K.; Macphee, D.G.; Kyoizumi, S. Radiation dose-dependent increases in inflammatory response markers in A-bomb survivors. Int. J. Radiat. Biol. 2003, 79, 129–136. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).