Clinical and Virological Response to Convalescent Plasma in a Chronic Lymphocytic Leukemia Patient with COVID-19 Pneumonia
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| CCP | COVID-19 convalescent plasma |
References
- Khoury, E.; Nevitt, S.; Madsen, W.R.; Turtle, L.; Davies, G.; Palmieri, C. Differences in Outcomes and Factors Associated With Mortality Among Patients With SARS-CoV-2 Infection and Cancer Compared With Those Without Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2210880. [Google Scholar] [CrossRef] [PubMed]
- FDA Statement. Coronavirus (COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron (accessed on 12 June 2022).
- FDA Updates Sotrovimab Emergency Use Authorization. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization (accessed on 26 April 2022).
- Focosi, D.; Franchini, M.; Pirofski, L.A.; Burnouf, T.; Paneth, N.; Joyner, M.J.; Casadevall, A. COVID-19 Convalescent Plasma and Clinical Trials: Understanding Conflicting Outcomes. Clin. Microbiol. Rev. 2022, e0020021. [Google Scholar] [CrossRef]
- Libster, R.; Pérez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.; Gebo, K.; Shoham, S.; Bloch, E.; Lau, B.; Shenoy, A.; Mosnaim, G.; Gniadek, T.; Fukuta, Y.; Patel, B.; et al. Early Outpatient Treatment for Covid-19 with Convalescent Plasma. N. Engl. J. Med. 2021, 386, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Millat-Martinez, P.; Gharbharan, A.; Alemany, A.; Rokx, C.; Geurtsvankessel, C.; Papageourgiou, G.; van Geloven, N.; Jordans, C.; Groeneveld, G.; Swaneveld, F.; et al. Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients. Nat. Commun. 2022, 13, 2583. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Franchini, M. Potential use of convalescent plasma for SARS-CoV-2 prophylaxis and treatment in immunocompromised and vulnerable populations. Expert Rev. Vaccines 2021, 21, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Hueso, T.; Pouderoux, C.; Péré, H.; Beaumont, A.L.; Raillon, L.A.; Ader, F.; Chatenoud, L.; Eshagh, D.; Szwebel, T.A.; Martinot, M.; et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood 2020, 136, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). Clinical Memorandum Re: EUA 26382. Product: COVID-19 Convalescent Plasma; Issued on 27 December 2021; US Food and Drug Administration (FDA): Silver Spring, MD, USA, 2021. Available online: https://www.fda.gov/media/141477/download (accessed on 12 June 2022).
- Rockett, R.J.; Basile, K.; Maddocks, S.; Fong, W.; Agius, J.E.; Johnson-Mackinnon, J.; Arnott, A.; Chandra, S.; Gall, M.; Draper, J.L.; et al. Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use. N. Engl. J. Med. 2021, 386, 1477–1479. [Google Scholar] [CrossRef]
- Huygens, S.; Oude Munnink, B.; Gharbharan, A.; Koopmans, M.; Rijnders, B. High incidence of sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant. MedRxiv 2022. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Müller-Tidow, C.; Janssen, M.; Schäkel, U.; Gall, J.; Leo, A.; Stelmach, P.; Krisam, J.; Baumann, L.; Stermann, J.; Merle, U.; et al. A randomized controlled clinical trial demonstrates that plam from convalescent and vaccinated donors improves outcome o COVID-19 in patients with hematological disease, cancer or immunosuppression. In Proceedings of the European Hematology Association, Vienna, Austria; Available online: https://library.ehaweb.org/eha/2022/eha2022-congress/357146/carsten.mller-tidow.a.randomized.controlled.clinical.trial.demonstrates.that.html?f=menu%3D6%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D3%2Ace_id%3D2233%2Aot_id%3D26862%2Amarker%3D1751%2Afeatured%3D17676 (accessed on 12 June 2022).
- Focosi, D.; Franchini, M.; Joyner, M.J.; Casadevall, A.; Sullivan, D.J. Analysis of anti-Omicron neutralizing antibody titers in plasma from pre-Omicron convalescents and vaccinees. medRxiv 2021. [Google Scholar] [CrossRef]
- Rijnders, B.J.A.; Huygens, S.; Mitjà, O. Evidence-based dosing of convalescent plasma for COVID-19 in future trials. Clin. Microbiol. Infect. 2022, 28, 667–671. [Google Scholar] [CrossRef]
- CoronaVirus 2019-nCoV—Comunicazioni dell’unità di Crisi Aziendale. Precisazioni Sulle Terapie per il Trattamento del Covid. Available online: https://www.uslnordovest.toscana.it/igea/attachments/article/3447/Precisazioni%20terapie%20antiCovid_08062022_clean.pdf (accessed on 13 January 2022).
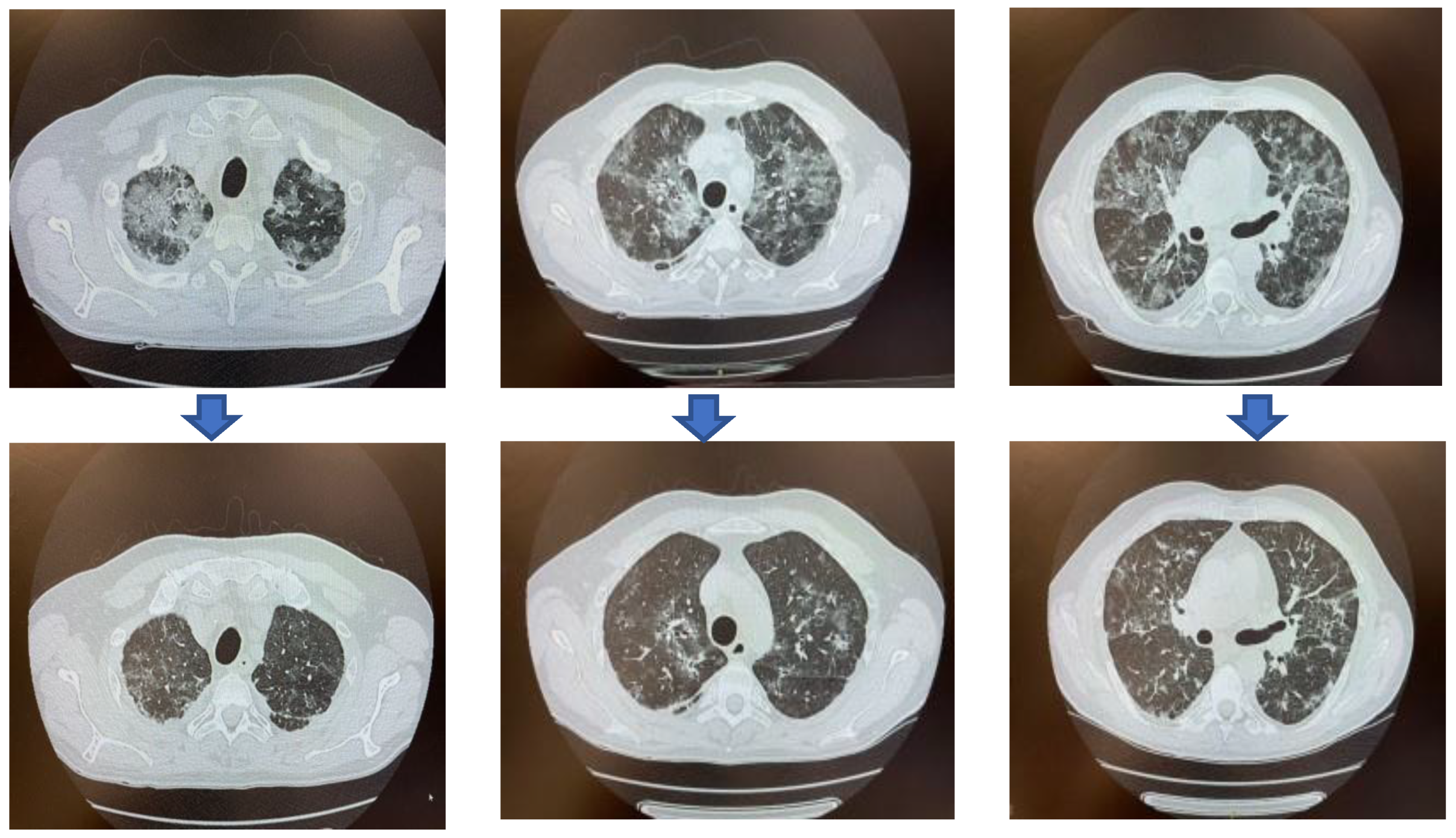
| Jan 30 | April 25 | April 30 | May 5 | May 9 | May 14 | May 20 | May 26 | May 31 | June 4 | Jun 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E-gene probe (Ct) | 19.6 | 24.8 | 27 | 26.8 | 27.7 | 37.0 | 36.8 | neg | neg | neg | |
| N2-gene probe (Ct) | 21.6 | 27.4 | 29.3 | 29.7 | 30.6 | 39.0 | 38.9 | 42.5 | 42 | neg | |
| lymphocytes/mcl | 600 | 100 | 300 | n.a. | 600 | n.a. | 1400 | n.a. | n.a. | n.a. | n.a. |
| high-sensitivity C-reactive protein (mg/dL) | 4.31 | 15.78 | 4.49 | n.a. | 0.58 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| D-dimer (ng/mL) | 694 | 2777 | 1366 | n.a. | 1247 ng/mL | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belcari, G.; Conti, A.; Mazzoni, A.; Lanza, M.; Mazzetti, P.; Focosi, D. Clinical and Virological Response to Convalescent Plasma in a Chronic Lymphocytic Leukemia Patient with COVID-19 Pneumonia. Life 2022, 12, 1098. https://doi.org/10.3390/life12071098
Belcari G, Conti A, Mazzoni A, Lanza M, Mazzetti P, Focosi D. Clinical and Virological Response to Convalescent Plasma in a Chronic Lymphocytic Leukemia Patient with COVID-19 Pneumonia. Life. 2022; 12(7):1098. https://doi.org/10.3390/life12071098
Chicago/Turabian StyleBelcari, Giovanni, Alberto Conti, Alessandro Mazzoni, Maria Lanza, Paola Mazzetti, and Daniele Focosi. 2022. "Clinical and Virological Response to Convalescent Plasma in a Chronic Lymphocytic Leukemia Patient with COVID-19 Pneumonia" Life 12, no. 7: 1098. https://doi.org/10.3390/life12071098
APA StyleBelcari, G., Conti, A., Mazzoni, A., Lanza, M., Mazzetti, P., & Focosi, D. (2022). Clinical and Virological Response to Convalescent Plasma in a Chronic Lymphocytic Leukemia Patient with COVID-19 Pneumonia. Life, 12(7), 1098. https://doi.org/10.3390/life12071098







