Abstract
With geometric significance, the Euler Sombor index of a graph is defined as . It originates from the mathematical distance property and has been proven to have good chemical applications in octane isomers. In this paper, the minimum and maximum of the Euler Sombor index for unicyclic graphs with given girth, as well as the corresponding extremal graphs, are determined. As an application, the experimental values of this index for some benzenoid hydrocarbons and drug molecules were compared with the boiling point. Through regression analysis, it was further demonstrated that the Euler Sombor index has excellent predictability in the physicochemical properties of compounds.
MSC:
05C05; 05C09; 05C92
1. Introduction
Topological indices are used to indicate and predict the physical and chemical properties of compounds [1,2]. It has widespread applications in chemistry, mathematics, and other fields and has become one of the most active research areas in chemical graph theory [3,4,5]. Many types of topological indices have been studied, among which the most extensively investigated and widely applied is undoubtedly vertex-degree-based topological indices (see [6,7,8]).
A vertex-degree-based topological index (VDB topological index) of is as follows:
where is a pertinently chosen symmetric real function with and . In [9], motivated by geometrics, Gutman proposed a new concept for the Sombor index, which was defined by
where represents the degree of vertex u and takes over the edges of graph .
Since its proposal, this index has attracted considerable attention and research interest. Gutman [9] investigated the extremum of the Sombor index for any tree T and connected graphs. Das et al. [10] provided lower and upper bounds for the Sombor index and established some relationships between the Sombor index and the first and second Zagreb indices. Furthermore, the extremal values of this index among all molecular trees were determined by Deng and Tang [11]. In [12], the authors deduced several upper and lower bounds of the Sombor index for some uniform hypergraphs. Chen and Zhu [13] established the upper and lower bounds of the Sombor index for unicyclic graphs, as well as the extrema of chemical unicyclic graphs with a fixed girth. Cruz and Rada [14] obtained the minimal values of (chemical) unicyclic and bicyclic graphs for the Sombor index and presented an upper bound on this index for such graphs. In 2024, Das [15] completely solved these open problems and characterized the extremal graphs. In addition, the authors [16,17,18] also studied the relevant chemical properties and applications of the Sombor index. For more research on this index, refer to review paper [19,20,21,22].
Recently, a new type of Sombor index, i.e, the Euler Sombor index was proposed by Gutman [23] and Tang et al. [24], which is formulated as
Both articles explained that this index originates from an approximate expression of the circumference of an ellipse and is therefore a geometry-based invariant. Meanwhile, Gutman [23] determined some actual relations between the Euler Sombor index and the Sombor index. In [24], the authors determined the extremal values for this Euler Sombor index among all (molecular) trees and characterized the corresponding extremum graphs. More importantly, by studying the relevant chemical properties of octane isomers, such as the boiling point, acentric factor, entropy, SNar, HNar, etc., it has been demonstrated that the Euler Sombor index has excellent predictive applicability for physicochemical properties.
The purpose of our work was to investigate the extremum Euler Sombor index of unicyclic graphs with given girth l, with a focus on its applications for chemical and physical properties. In Section 2 and Section 3, the minimum and maximum Euler Sombor index of unicyclic graphs with given girth are identified and the corresponding extremum graphs are characterized. In Section 4, as an application of the Euler Sombor index, the experimental values of this index for some benzenoid hydrocarbons and drug molecules are compared with the boiling point. Through regression analysis, it is further demonstrated that the Euler Sombor index has excellent predictability of the physicochemical properties of compounds.
Now, we review some definitions and terms that will be used in the following text. In this article, we consider graphs as connected, simple, and undirected. A graph , where the vertex set and edge set are denoted by and , respectively. The degree of vertex v is denoted by or . In particular, a vertex v is called pendant if . The neighbors of vertex v, denoted by or , is the collection of vertices adjacent to v. An edge is called pendant if or . represents the subgraph from by deleting the edge . Meanwhile, represents the graph by joining a new edge from . Recall that a graph is unicyclic if . Let represent the set of unicyclic graphs with given girth l and an order of n. Clearly, if , then , where be the cycle of n vertices.
2. The Minimal Euler Sombor Index in
Lemma 1.
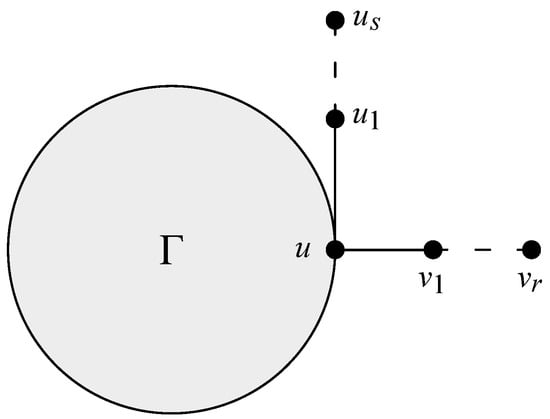
Let be as shown in Figure 1, where , and is a subgraph of . Suppose that . Then, .

Figure 1.
Graph of .
Proof.
Assume that and , where . Thus, we have . Now, three possibilities occur according to the values of and .
Case 1. If . Since , combining the structures of and , we obtain
Case 2. If . Similar to Case 1, it can be concluded that
Case 3. If , it follows that
Therefore, based on the above three cases, we can conclude that . The lemma is proven. □
Lemma 2.
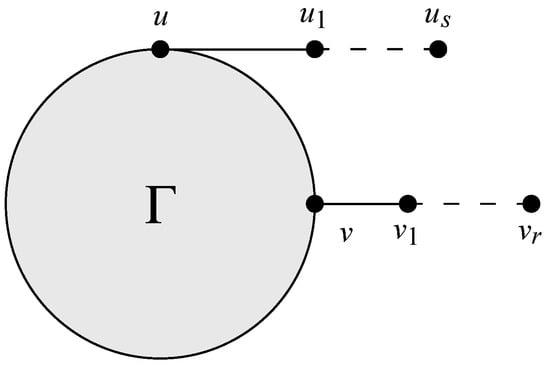
Let be as shown in Figure 2, where , and . Assume that . Then, we have .

Figure 2.
Graph of .
Proof.
We assume that and . If , then , . We can use this for the following three cases.
Case 1. . Since for and 2, we deduce that
Case 2. . It follows that
Case 3. . If , we immediately have
Similarly, if , then we have
Consequently, the above discussion implies that . This completes the proof. □
Let , and is obtained by attaching the path to the cycle of via the vertex . Now, we present the main results.
Theorem 1.
Let . Then,
equality holds if and only if .
Proof.
Firstly, for and , by direct calculation, we have
and
Next, we consider the case . Suppose is the unique cycle in . Consequently, , and there is at least a vertex , such that .
If there are at least two vertices in whose degrees are greater than or equal to 3, then Lemmas 1 and 2 can be applied to obtain
If there is exactly one vertex in whose degree is greater than or equal to 3, without loss of generality, let . If , according to Lemma 1, we obtain
If , by Lemma 1, we deduce that
and equality holds when . Therefore, from the above discussion, the theorem holds. □
3. The Maximal Euler Sombor Index in
Let be a unicylic graph obtained by attaching pendant edges to the vertex . In particular, is denoted as .
Lemma 3.
Let , where . Then, .
Proof.
Since is increasing with , we have
Similarly, it can be concluded that
Consequently, we obtain , and Lemma 3 is finished. □
Lemma 4.
Let , where . Then, .
Proof.
Because and are increasing and decreasing with , respectively, we deduce that
Similarly, we obtain
Therefore, we have , and the proof is completed. □
Lemma 5.
Let , and T is a component of , where and . If , then the edge is contracted to u, and joining a pendant edge is denoted it by . Thus, .
Proof.
Suppose that and , where . Since increases with , we have
Therefore, the lemma holds true. □
Lemma 6.
Let ; and are the pendant edges joining to u and v, where and . Assume that ; then, .
Proof.
Assume that and . If , then , . We consider two cases according to whether or not.
Case 1. . Since , we have
Through direct calculations, it can be concluded that
Consequently, based on the above two equations and Lemma 3, we have
Case 2. . Note that and . From Case 1, for , . This together with Lemma 4 implies that
Therefore, the proof is finished. □
With the help of the above two lemmas, we can deduce the maximal Euler Sombor index in .
Theorem 2.
Let . Then,
and equality occurs when .
Proof.
Let be the unique cycle in . Then, there is at least one vertex that meets with . If there are at least two vertices in whose degrees are greater than or equal to 3, then according to Lemmas 5 and 6, we deduce that
If there is exactly one vertex in whose degrees are greater than or equal to 3, and , then, by Lemma 5, we have
If , we have
Therefore, considering the above cases, the theorem holds. □
4. Chemical Applications of the Euler Sombor Index
There are various types of topological indices, and they have grown rapidly in recent years. One important role of topological indices is to predict the physical and chemical properties of molecules, such as the melting point, boiling point, etc. However, current research on topological indices mostly focuses on mathematical calculations, and it is very important to calculate topological indices through experiments and compare them with chemical and physical properties. Recently, Tang et al. [24] determined the relationship between the Euler Sombor index and the structure performance for octane. As an important compound, the study of the index and properties of benzene hydrocarbons is also of great significance. Therefore, in this section, we fill this gap.
Firstly, some typical benzenoid hydrocarbons (BHs) were considered (Figure 3). The experimental values of the Euler Sombor index (EP), Sombor index (SO), Elliptic Sombor index (ESO), forgotten topological index (F), symmetric division degree index (SDD), first (M1) and second (M2) Zagreb indices, and sum connectivity index (SCI), as well as the boiling point (BP) of benzenoid hydrocarbons, are shown in Table 1. In particular, |V(BH)| represents the size of molecules (the number of vertices) in BHs.
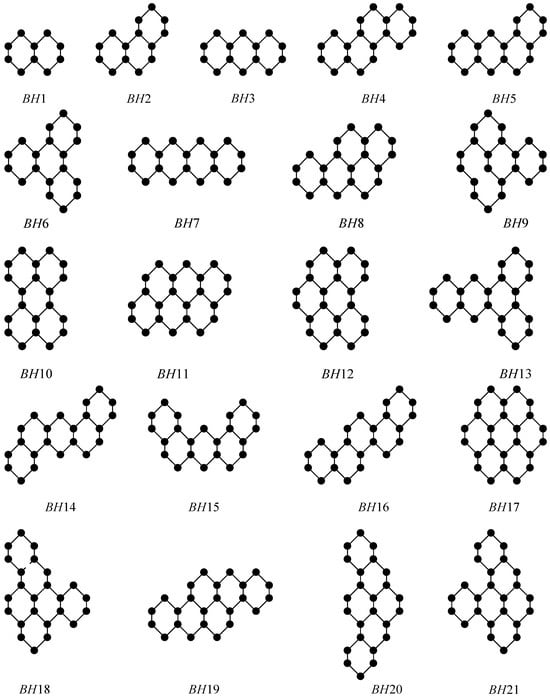
Figure 3.
Molecular graphs of some BHs.

Table 1.
Experimental values of some existing indices for BHs with BP.
To eliminate the influence of molecular size on the prediction model, we adopted the method of dividing the obtained index value with the number of vertices and then compared different graphs of BHs to obtain the true prediction potentiality. From Table 1, we constructed the scatter chart among BP and values of BHs, as shown in Figure 4. Furthermore, we obtained the correlation coefficient (R) of various indices through regression analysis, as shown in Table 2. Therefore, the above results imply that the Euler Sombor index has important applicability in predicting the boiling point of BHs.
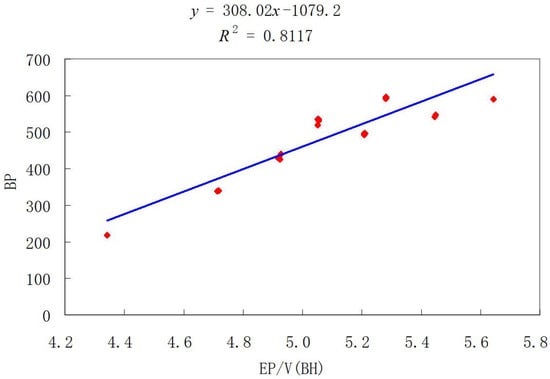
Figure 4.
Linear fitting of with BP for some BHs.

Table 2.
The correlation coefficient of some existing indices for BHs with BP.
We conducted the above-mentioned research on some drug designs and mainly examined the ten chemical drugs listed in Table 3, whose molecular structures can be viewed online and are thus omitted here. The experimental values of the Euler Sombor index (EP), Sombor index (SO), Elliptic Sombor index (ESO), and boiling point (BP) of some drugs are shown in Table 3.

Table 3.
Experimental values of three types of Sombor index for drug molecules with BP.
Similar to the operation of BHs, we eliminated the influence of molecular size on the prediction model by dividing the obtained index value with the number of vertices. Therefore, we constructed the scatter chart among BP and values of these drug molecules, as shown in Figure 5. Moreover, we obtained the correlation coefficient (R) of three types of Sombor index through regression analysis, as shown in Table 4. Clearly, the Euler Sombor index has a good ability to distinguish drug molecules and can be widely used in compound identification and drugs design.
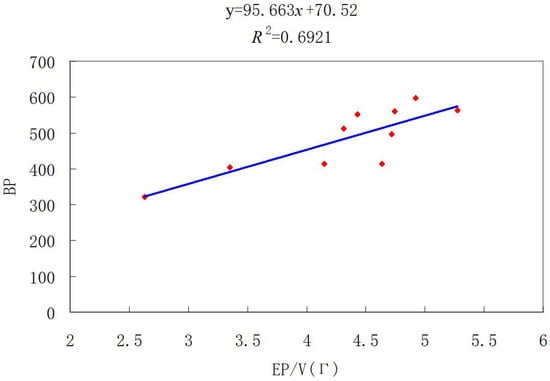
Figure 5.
Linear fitting of with BP for some drug molecules.

Table 4.
The correlation coefficient of three types of Sombor index for drug molecules with BP.
5. Conclusions
In this paper, the minimum and maximum of the Euler Sombor index for unicyclic graphs with given girth were determined and the corresponding extremal graphs were characterized. In addition, by experimental comparison, it was confirmed that the Euler Sombor index can describe the physicochemical properties of benzene hydrocarbons and some drug structures. Through regression analysis, Figure 4 and Figure 5 show that both of the correlation coefficients (R) of the Euler Sombor index () for benzenoid hydrocarbons and drug molecules with boiling point (BP) are greater than 0.8. This indicates that this index has good predictive ability for the physicochemical properties of compounds, especially those containing benzene rings. Therefore, it is of great significance for the design of new drugs and the development of new chemical materials.
Author Contributions
Writing—original draft, Z.S.; Writing—review & editing, Z.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hunan Province Natural Science Foundation (2025JJ70485).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gutman, I.; Polansky, O.E. Mathematical Concepts in Organic Chemistry; Springer: Berlin, Germany, 1986. [Google Scholar]
- Devillers, J.; Balaban, A.T. (Eds.) Topological Indices and Related Descriptors in QSAR and QSPR; Gordon & Breach: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Ghorbani, M.; Vaziri, Z.; Ravandi, R.A.; Shang, Y. The symmetric division Szeged index: A novel tool for predicting physical and chemical properties of complex networks. Heliyon 2025, 11, e42280. [Google Scholar] [CrossRef]
- Zuo, X.; Jahanbani, A.; Shooshtari, H. On the atom-bond sum-connectivity index of chemical graphs. J. Mol. Struct. 2024, 1296, 136849. [Google Scholar]
- Gao, W. The minimum ABC index of chemical trees. Discret. Appl. Math. 2024, 348, 132–143. [Google Scholar]
- Nithya, P.; Elumalai, S.; Balachandran, S. Smallest ABS index of unicyclic graphs with given girth. J. Appl. Math. Comput. 2023, 69, 3675–3692. [Google Scholar]
- Song, R.; Liu, S.; Ou, J.; Cui, J. On bicyclic graphs with maximal Graovac-Ghorbani index. MATCH Commun. Math. Comput. Chem. 2024, 91, 513–532. [Google Scholar]
- Wei, P.; Jia, W.; Belardo, F.; Liu, M. Maximal Lanzhou index of trees and unicyclic graphs with prescribed diameter. Appl. Math. Comput. 2025, 48, 129116. [Google Scholar] [CrossRef]
- Gutman, I. Geometric approach to degree-based topological indices: Sombor indices. MATCH Commun. Math. Comput. Chem. 2021, 86, 11–16. [Google Scholar]
- Das, K.C.; Cevik, A.S.; Cangul, I.N.; Shang, Y. On Sombor index. Symmetry 2021, 13, 140. [Google Scholar]
- Deng, H.; Tang, Z.; Wu, R. Molecular trees with extremal values of Sombor indices. Int. J. Quant. Chem. 2021, 121, e26622. [Google Scholar]
- Wang, X.; Wang, M. Sombor index of uniform hypergraphs. AIMS Math. 2024, 9, 30174–30185. [Google Scholar]
- Chen, M.; Zhu, Y. Extremal unicyclic graphs of Sombor index. Appl. Math. Comput. 2024, 463, 128374. [Google Scholar] [CrossRef]
- Cruz, R.; Rada, J. Extremal values of the Sombor index in unicyclic and bicyclic graphs. J. Math. Chem. 2021, 59, 1098–1116. [Google Scholar] [CrossRef]
- Das, K.C. Open problems on Sombor index of unicyclic and bicyclic graphs. Appl. Math. Comput. 2024, 473, 128647. [Google Scholar] [CrossRef]
- Gutman, I. Some basic properties of Sombor indices. Open J. Discr. Appl. Math. 2021, 4, 1–3. [Google Scholar]
- Redžepović, I. Chemical applicability of Sombor indices. J. Serb. Chem. Soc. 2021, 86, 445–457. [Google Scholar]
- Du, Z.; You, L.; Liu, H.; Huang, F. The Sombor index and coindex of chemical graphs. Polycycl. Aromat. Comp. 2024, 44, 2942–2965. [Google Scholar]
- Liu, H.; Gutman, I.; You, L.; Huang, Y. Sombor index: Review of extremal results and bounds. J. Math. Chem. 2022, 66, 771–798. [Google Scholar]
- Oboudi, M.R. The mean value of Sombor index of graphs. MATCH Commun. Math. Comput. Chem. 2023, 89, 733–740. [Google Scholar]
- Rada, J.; Rodríguez, J.M.; Sigarreta, J.M. On integral Sombor indices. Appl. Math. Comput. 2023, 452, 128036. [Google Scholar]
- Wei, P.; Liu, M. Note on Sombor index of connected graphs with given degree sequence. Discret. Appl. Math. 2023, 330, 51–55. [Google Scholar]
- Gutman, I. Relating sombor and Euler indices. Vojnoteh. Glas. 2024, 72, 1–12. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Y.; Deng, H. The Euler Sombor index of a graph. Int. J. Quantum Chem. 2024, 124, e27387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).