Abstract
The flotation separation of scheelite from calcite is problematic, where sodium silicate modified by polyvalent metal ions has shown some advantages for selective depression. In this study, an Al-Na2SiO3 polymer was used as the depressant for the flotation separation of scheelite from calcite using a lead complex of benzohydroxamic acid (Pb-BHA) as the collector. Furthermore, a number of measurements were conducted to investigate the structure of the Al-Na2SiO3 polymer and its adsorption behavior with Pb-BHA complexes on the mineral surface. Flotation experiments indicated that the Al-Na2SiO3 polymer shows good selectivity for the flotation separation of scheelite from calcite at pH 8, where the optimum ratio of sodium silicate to aluminum sulfate was 2:1. Fourier-Transform Infrared (FTIR) and solution chemical analyses revealed that aluminum hydroxide complexes and the hydroxy moiety of silicic acid are able to self-assemble via condensation affording an Al-Na2SiO3 polymer, i.e., a composite aluminosilicate polymer. The zeta potential measurements and adsorption capacity measurements indicated that, upon adsorption of the Al-Na2SiO3 polymer and Pb-BHA complexes on the mineral surface, the Al-Na2SiO3 polymer had less influence on the adsorption of Pb-BHA complexes on the scheelite surface, while the opposite was true for calcite. Therefore, more Pb-BHA complexes and fewer Al-Na2SiO3 polymers were deposited on the scheelite surface, while fewer Pb-BHA complexes and more Al-Na2SiO3 polymers were adsorbed on the calcite surface. The selective separation of scheelite from calcite was attributed to the cooperative selectivity of the Pb-BHA complexes and Al-Na2SiO3 polymer.
1. Introduction
The flotation separation of scheelite from other calcium-containing minerals is problematic due to their similar physicochemical characteristics and flotation behavior [1,2,3,4,5,6]. Sodium silicate is commonly used in scheelite flotation to depress calcium-bearing gangue minerals and silicate minerals; however, the selectivity of sodium silicate for scheelite flotation is inadequate, whereby the floatability of scheelite is somewhat reduced [7,8,9]. Modification of sodium silicate with polyvalent metal ions, oxalic acid, sulfuric acid, ammonium salt, and some other reagents has been proven to be effective in enhancing the depression of gangue minerals with little effect on the desired minerals [7,10,11,12,13].
The selective adsorption of sodium silicate on minerals is significantly improved by modification with polyvalent metal ions, such as Fe(II), Pb(II), Cu(II), Mg(II), and Al(III) [10,12,14,15,16], especially for application in scheelite flotation. The Pb-benzohydroxamic acid (BHA) complex, a novel metal-organic collector, has shown specificity toward scheelite, contributing to the flotation separation of scheelite from other calcium minerals [4,17,18]. A simplified flotation process using Pb-BHA complexes was developed to replace the energy-inefficient Petrov’s process involving the use of a fatty acid to increase the recovery of scheelite and reduce the overall cost. In the new process, an Al-Na2SiO3 polymer was used to efficiently improve the quality of the concentrate. Compared to sodium silicate, the Al-Na2SiO3 polymer (sodium silicate modified by Al3+) showed better selective depression of scheelite and calcite during Pb-BHA flotation, leading to the separation of scheelite from calcite [19]. The improved selectivity of the Al-Na2SiO3 polymer was attributed to the addition of aluminum ions; however, the structure and active species of such Al-Na2SiO3 polymer in slurry and mineral surfaces remained unclear. In addition, in contrast to fatty acid flotation processes requiring large masses of sodium silicate (e.g., 1–3 kg/t), little amounts of Al-Na2SiO3 polymer (e.g., 0.1–0.3 kg/t) are enough and effective for Pb-BHA flotation, indicating that the combined utilization of Pb-BHA complexes and such an Al-Na2SiO3 polymer exhibits absolute specificity toward scheelite, thus contributing to efficient and clean scheelite flotation processes. However, the competition between the Al-Na2SiO3 polymer and Pb-BHA complex on the minerals surface remains unclear; their adsorption behavior on scheelite and calcite requires further investigation to uncover the mechanism behind the superior selectivity of the Al-Na2SiO3 polymer during Pb-BHA flotation.
In this study, the structure of the Al-Na2SiO3 polymer was studied in detail, and the competitive adsorption mechanism of the Al-Na2SiO3 polymer and Pb-BHA collectors on both scheelite and calcite was elucidated by some advanced experiments. Flotation experiments of pure minerals were conducted to explore the optimal conditions for the selective separation of scheelite from calcite using the Al-Na2SiO3 polymer. Fourier-Transform Infrared (FTIR) and solution chemical analyses of Na2SiO3 and the Al-Na2SiO3 polymer were conducted to clarify the structure of the polymer. Zeta potential and adsorption measurements were also performed to study the adsorption behavior of Al-Na2SiO3 and Pb-BHA complexes on the mineral surface. A model for the adsorption of the Al-Na2SiO3 polymer and Pb-BHA complexes on scheelite and calcite surfaces is proposed to explain the superior selectivity of the Al-Na2SiO3 polymer during Pb-BHA flotation.
2. Materials and Methods
2.1. Minerals and Reagents
Pure scheelite and calcite were sourced from the Shizhuyuan mine, Hunan, China. The powder X-ray diffraction data confirmed that the samples had a purity of >97%. The −74 μm fractions of scheelite and calcite were used for the experiments. The samples used for zeta potential measurements were further ground to −2 μm in an agate mortar.
Analytical grade lead nitrate and BHA were purchased from Guangfu, Tianjin, China. Sodium silicate (SS, Na2SiO3∙9H2O) was obtained from the Zhuzhou Flotation Reagents Factory, Hunan, China. Analytical grade aluminum sulfate (Al2(SO4)3) and terpineol were obtained from the Kemiou Chemical Research Institute, Tianjin, China. The pH was adjusted using NaOH or HCl stock solutions. Deionized water was used in all experiments.
2.2. Flotation Experiments
Pure mineral flotation tests were conducted in an XFG flotation machine at an impeller speed of 1600 rpm, with a plexiglass cell volume of 40 mL. The mineral suspension was prepared by adding 2.0 g of mineral to 30 mL of deionized water. Samples of mineral mixtures were prepared with 1.0 g of scheelite and 1.0 g of calcite. Pb(NO3)2 was mixed with BHA, referred to as Pb-BHA, before addition. Na2SiO3 was mixed with Al2(SO4)3, referred to as Al-Na2SiO3, before addition. The flotation experimental procedure is that previously described in the literature [19]. After 5 min of flotation, the floated and non-floated particles were collected, dried, and weighed, and the recovery was calculated.
2.3. Fourier-Transform Infrared (FTIR) Measurements
For the FTIR measurements, an Al-Na2SiO3 polymer with the desired mass ratio was prepared in a solution and agitated for 10 min at pH 8.0. The pulp was then centrifuged, the precipitate was washed at least three times with deionized water, and the sample was then dried in a vacuum oven at 40 °C. The FTIR measurements were conducted using a Bruker Alpha FTIR spectrometer at 20 °C in the range of 400–4000 cm−1.
2.4. Zeta Potential Measurements
Zeta potential measurements were conducted using a zeta potential analyzer (ZetaPlus, Bruker, Germany) at 20 °C. Colloid particle suspensions were prepared by adding the desired reagents to 40 mL of KCl background electrolyte (10−3 mol/L) and agitating for 3 min. Mineral suspensions containing 0.02 g of solids and 40 mL of a KCl background electrolyte (10−3 mol/L) were prepared in a beaker at a given pH and desired reagent concentration. After settling for 10 min, the supernatant liquor was used for zeta potential measurements.
2.5. Adsorption Capacity Determination
The adsorption capacity toward BHA was monitored using a total organic carbon analyzer (TOC-LCPH/CN, Shimadzu Corporation, Kyoto, Japan). The −74 μm fraction of pure scheelite or calcite (2.0 g) was conditioned in a 40 mL solution using the desired reagents for the flotation tests. After that, the conditioned pulp suspension was centrifuged for 15 min, and the supernatant solution was removed for analysis. The amount of BHA adsorbed on the scheelite and calcite particles was calculated as:
where Γ is the amount of BHA adsorbed on the mineral surface (mol/m2), C0 and C are the initial and residual concentrations (mol/L) of BHA, respectively, V is the volume of the solution (L), m is the amount (g) of particles per sample, and A is the specific surface area of the minerals (0.192 m2/g for scheelite and 0.335 m2/g for calcite, as determined by BET analysis).
3. Results and Discussion
3.1. Single Mineral Flotation Experiments
Pb-BHA complexes have proven to be selective for calcium mineral flotation, demonstrating good collecting ability toward scheelite. In Pb-BHA flotation experiments, although fluorite could not be collected, a high recovery of calcite was maintained across the tested pH range [4,18]. A depressant is necessary to enable the separation of scheelite from calcite; however, the use of Na2SiO3 was inefficient [19]. Different dosages of Al-Na2SiO3 polymer were added to investigate its effect on scheelite and calcite flotation, as shown in Figure 1. An atypical scheelite recovery curve was observed with the increasing depressant dosage, and a further detailed analysis of such a singular recovery curve will be conducted in the near future. From Figure 1, the recovery of calcite decreased rapidly with the increasing Al-Na2SiO3 polymer loading; at Al-Na2SiO3 polymer concentrations over 50 mg/L, the recovery of scheelite was higher than that of calcite, and thus, the separation of scheelite from calcite was realized. In particular, the optimum concentration of Al-Na2SiO3 polymer for scheelite flotation using Pb-BHA complexes as the collector was determined as 100 mg/L.
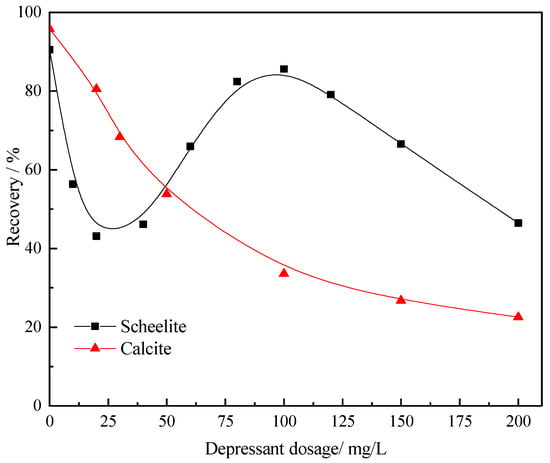
Figure 1.
Effect of Al-Na2SiO3 polymer dosage on scheelite and calcite flotation (pH = 9.0 ± 0.1, Clead ion = 3 × 10−4 mol/L, CBHA = 1.5 × 10−4 mol/L, Cterpineol = 12.5 μL/L).
Figure 2 shows the effect of the mass ratio of sodium silicate to aluminum sulfate on the flotation of scheelite and calcite, where the dosage of sodium silicate was fixed at 100 mg/L. The results demonstrate that the floatability of scheelite and calcite varies with an increasing aluminum ion concentration. The addition of aluminum ions significantly improves the selectivity toward scheelite and calcite; however, excess aluminum ions have a negative impact on scheelite flotation, as seen in Figure 2, from which the optimum ratio of sodium silicate to aluminum sulfate was determined to be 2:1.
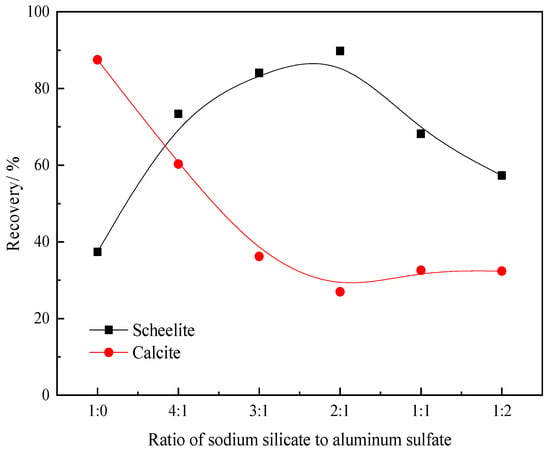
Figure 2.
Effect of the ratio of sodium silicate to aluminum sulfate on scheelite and calcite flotation (pH = 8.0 ± 0.1, Css = 100 mg/L, Clead ion = 3 × 10−4 mol/L, CBHA = 1.5 × 10−4 mol/L, Cterpineol = 12.5 μL/L).
Figure 3 shows the effect of the Al-Na2SiO3 polymer on the scheelite and calcite flotation versus the pH. The results indicate that the recovery of scheelite after treatment with the Al-Na2SiO3 polymer was >80% at pH 7–9; however, that of calcite was only 20–30% under these conditions. In particular, at the optimal pH of 8, the recoveries of scheelite and calcite were 89.8% and 27%, respectively.
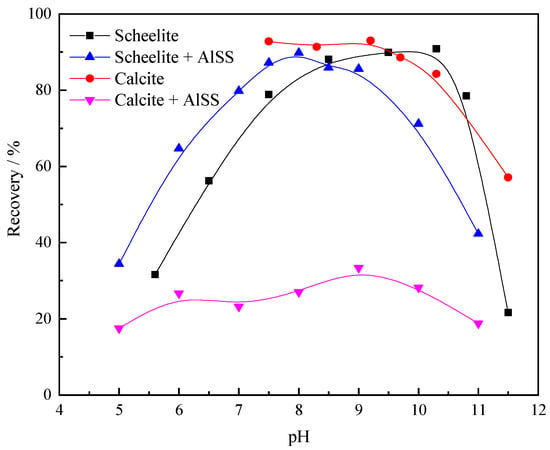
Figure 3.
Effect of the Al-Na2SiO3 polymer on scheelite and calcite flotation with the varying pH (CAlss = 100 mg/L, Clead ion = 3 × 10−4 mol/L, CBHA = 1.5 × 10−4 mol/L, Cterpineol = 12.5 μL/L).
Flotation tests of a mixture of scheelite and calcite minerals were conducted under the optimal conditions determined from the above experiments. As shown in Table 1, a concentrate with 66.10% WO3 was obtained with a WO3 recovery of 79.81%, demonstrating that the Al-Na2SiO3 polymer shows good selectivity for the separation of scheelite from calcite.

Table 1.
Flotation results from mixed minerals.
3.2. Structure of the Al-Na2SiO3 Polymer
3.2.1. FTIR Spectroscopy
In order to gain detailed information on the structure of the Al-Na2SiO3 polymer, the FTIR spectra of Na2SiO3 and Al-Na2SiO3 polymer were recorded, as shown in Figure 4. As for sodium silicate, the bands at 3324.73 and 3396.63 cm−1 are associated with hydroxyl stretching vibrations of sodium silicate molecules, the band at 1646.20 cm−1 corresponds to the bending vibration of hydroxyl groups, that at 990.71 cm−1 is assigned to the stretching vibration of the Si–O–Si bonds of SiO22−, and the band at 460.92 cm−1 is attributed to the bending vibration of the Si–O–Si bonds of SiO44− [20,21,22]. Compared to the FTIR spectrum of sodium silicate, the Al-Na2SiO3 polymer spectrum shows some differences. New bands are observed at 2520.54 and 586.26 cm−1, while the bands at 2954.46, 2328.51, 1454.20, and 835.94 cm−1 have disappeared, and the rest of absorption bands have shifted to different degrees, suggesting that the sodium silicate structure has changed upon the addition of aluminum ions. The new bands at 2520.54 and 586.26 cm−1 are attributed to O–Al bonding and Al–OH bending vibration, respectively, while the bands at 1105.02 and 445.14 cm−1 are attributed to the vibration of Si–O–Al bonds [23,24,25,26,27]. These bands indicate that a chemical reaction has occurred between the aluminum ions and sodium silicate, where the aluminum ions have combined with the oxygen atoms of sodium silicate to form Si–O–Al bonds.
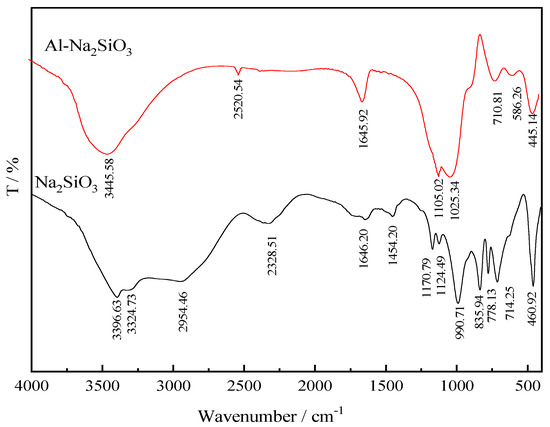
Figure 4.
Fourier-Transform Infrared (FTIR) spectra of Na2SiO3 and the Al-Na2SiO3 polymer.
3.2.2. Self-Assembly of Sodium Silicate and Aluminum Ions in Solution
Sodium silicate is an alkali salt that undergoes severe hydrolysis in aqueous solution. Marinakis and Shergold [28] calculated the distribution of sodium silicate species in solution according to the following equilibria using the equilibrium constants determined by Lagerstrom [29]:
SiO2 (s,amorphous) + 2H2O ⇌ Si(OH)4 log K = −2.7
Si(OH)4 ⇌ SiO(OH)3− + H+ log K = −9.46
SiO(OH)3− ⇌ SiO2(OH)22− + H+ log K = −12.56
Based on Equations (2)–(4), the distribution of the hydrolysis species from sodium silicate at an initial concentration of 1 × 10−4 mol/L was calculated, as presented in Figure 5. In aqueous solution, sodium silicates present three major species: a neutral polyprotic Si(OH)4 species prevailing at pH < 9.4, a mono-deprotonated conjugate base, SiO(OH)3−, which is dominant in the pH range of 9.4–12.6, and a di-deprotonated conjugate base, SiO2(OH)22−, which exists at pH > 12.6.
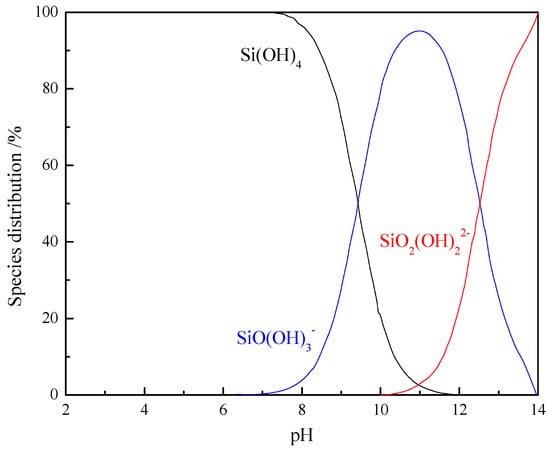
Figure 5.
Speciation distribution of silicate anions as a function of the pH (C = 1 × 10−4 mol/L).
The sodium silicate species in aqueous solution may polymerize into polysilicic acid at a certain concentration [30,31]. In acidic solution, Si(OH)4 may combine with H+ to form H5SiO4+. In neutral or alkaline solution, Si(OH)4 may lose H+ to form H3SiO4−, favoring the polymerization reaction between Si(OH)4 and H3SiO4− according to Figure 6 [31]:
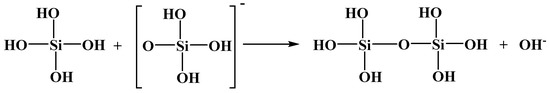
Figure 6.
The polymerization reaction between Si(OH)4 and H3SiO4−.
H6Si2O7 may react with OH− to form H5Si2O7−, and then, a polymerization reaction between H6Si2O7 and H5Si2O7− proceeds to furnish a molecule with a higher degree of polymerization.
When aluminum salts enter the aqueous solution, the aluminum ions undergo a step-by-step hydrolysis reaction at pH > 3.0; at low aluminum concentrations (i.e., less than 0.001 mol/L), the majority of hydrolyzed aluminum exists as mononuclear aluminum hydroxide complexes [32,33,34,35]. The hydrolysis reactions proceed as per the equilibrium constants shown in the following equilibria [33,36,37,38,39]:
Al3+ + H2O ⇌ AlOH2+ + H+ log K = −4.99
Al3+ + 2H2O⇌Al(OH)2+ + H+ log K = −10.0
Al3+ + 3H2O ⇌ Al(OH)3(aq) + H+ log K = −9.35
Al3+ + 4H2O ⇌ Al(OH)4− + H+ log K = −23.0
Based on Equations (5)–(8), the distribution of the hydrolysis species of aluminum ions at an initial concentration of 1 × 10−4 mol/L was calculated and plotted in Figure 7. In aqueous solution, Al3+ and mononuclear aluminum hydroxide complexes Al(OH)2+ and Al(OH)2+ are predominant over the pH range of 3–6, while Al(OH)3(aq) species prevail across the pH range of 6–9 and Al(OH)4− predominates at pH > 9.
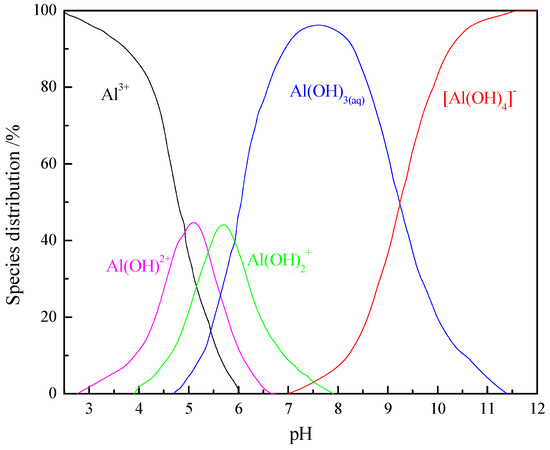
Figure 7.
Speciation distribution of monomer aluminum as a function of the pH (C = 1 × 10−4 mol/L).
When aluminum ions are added to a solution of sodium silicate, a dehydration condensation reaction occurs between the aluminum hydroxide complexes and the hydroxy moiety of silicic acid or polysilicic acid, forming a new composite aluminosilicate polymer [40,41,42]. Taking the mononuclear hydrolysis species of silicic acid and aluminum as an example, under neutral and alkalescent conditions, Si(OH)4 and Al(OH)3 are the dominant species in solution. Such dehydration condensation reaction is speculated to proceed as Figure 8:
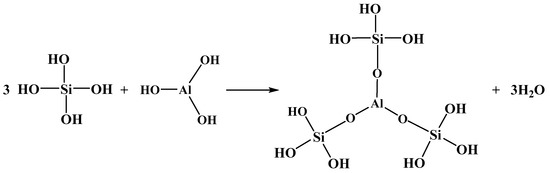
Figure 8.
The dehydration condensation reaction between Si(OH)4 and Al(OH)3.
Under alkaline conditions, H3SiO4− and Al(OH)4− are the dominant species in solution; thus, the dehydroxylation condensation reaction is speculated to proceed as Figure 9:
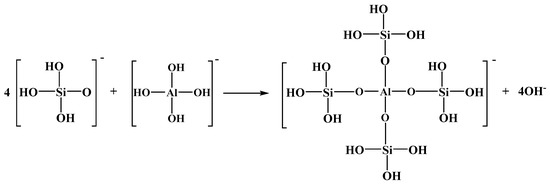
Figure 9.
The dehydroxylation condensation reaction between H3SiO4− and Al(OH)4−.
After the condensation reaction, a new composite aluminosilicate polymer is formed, which contains Si–O–Al bonds in its structure, in agreement with the FTIR results from Figure 4. From the analysis of the above solution, when aluminum ions are added to the solution of sodium silicate, the aluminum hydroxide complexes and hydroxyl moiety of silicic acid or polysilicic acid self-assemble by dehydration or dehydroxylation condensation reactions to form an Al-Na2SiO3 polymer. Compared to Na2SiO3, the Al-Na2SiO3 polymer has a different structure with different physical and/or chemical properties. Indeed, the Al-Na2SiO3 polymer presents better selective adsorption for scheelite and calcite.
3.3. Effect of the Al-Na2SiO3 Polymer on the Adsorption of Pb-BHA Complexes on the Mineral Surface
The Al-Na2SiO3 polymer adsorbs on the surface of scheelite and calcite by chemisorption, where the oxygen atoms of Al-Na2SiO3 bind the calcium cations on the scheelite and calcite surface. Al-Na2SiO3 exhibits weak chemisorption on the scheelite surface, while it is adsorbed more powerfully on the calcite surface [19]. The Pb-BHA complex is a metal-organic collector, where the metal is the functional group that recognizes the W–O bonds on the scheelite surface. This approach is significantly different from traditional anion collectors that react with Ca sites on the mineral surface [17]. The adsorption diagram of silicic acid and Pb-BHA on the scheelite or calcite surface is shown in Figure 10. Silicic acid binds the calcium cations of the mineral surface; however, the Pb-BHA complexes recognize the oxygen bonds on the mineral surface, and thus, the adsorption of silicic acid (or Al-Na2SiO3 polymer) and Pb-BHA occurs at different sites. This mechanism is very different from traditional fatty acid flotation, where the fatty acid and silicic acid both react with the calcium cations on the mineral surface in a competitive manner [7,9,11,16].
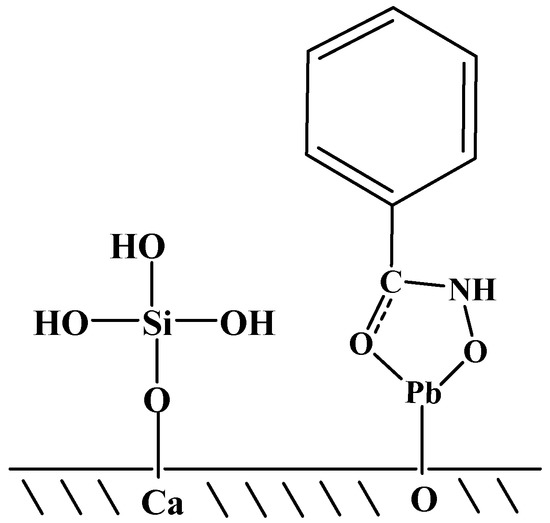
Figure 10.
Adsorption diagram for silicic acid and a Pb- benzohydroxamic acid (BHA) complex on the scheelite surface.
3.3.1. Zeta Potential Measurements
The zeta potential results for Na2SiO3, Al-Na2SiO3 polymer, and Pb-BHA complex are shown in Figure 11. The Pb-BHA complex colloid exhibits a strong positive charge in the pH range of 6–10, and thus, it tends to adsorb on the negatively charged scheelite surfaces by electrostatic interactions [17]. In contrast, colloidal silicic acid and the Al-Na2SiO3 polymer have a negative charge, and the charge on the Al-Na2SiO3 polymer is more negative at about 20 mV in the pH range of 8–11, which may be attributed to the larger number of hydroxyl groups on the Al-Na2SiO3 polymer due to the bridging action of aluminum ions.
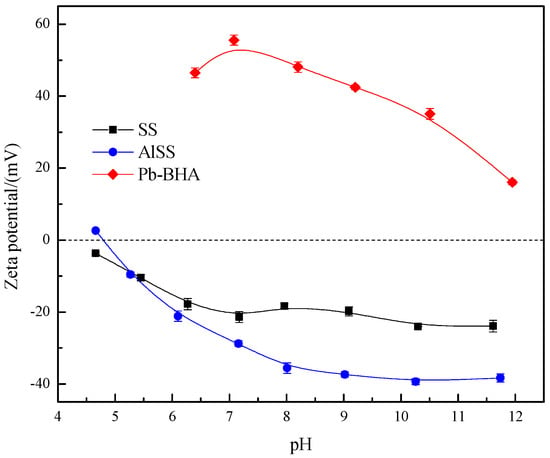
Figure 11.
Zeta potential plots for Na2SiO3, Al-Na2SiO3 polymer, and Pb-BHA complex (CSS = CAlSS = 100 mg/L, Clead ion = 3 × 10−4 mol/L, CBHA = 1.5 × 10−4 mol/L).
Compared to Na2SiO3, Al-Na2SiO3 adsorbs to a larger extent on the calcite surface than on scheelite [19]; therefore, the Al-Na2SiO3 polymer will also present a different influence on the adsorption of Pb-BHA complexes on the scheelite and calcite surfaces. The isoelectric point (IEP) of scheelite and calcite is circa 1.3 and 9.6, respectively; therefore, in the pH range of 7–9, the scheelite particles are negatively charged but calcite is always positively charged [8,43,44,45]. Figure 12 shows the zeta potential of scheelite and calcite at different concentrations of Pb-BHA after treatment with Na2SiO3 and the Al-Na2SiO3 polymer. The zeta potential of scheelite and calcite increases with the Pb-BHA dosage, indicating the adsorption of Pb-BHA on the minerals. From Figure 12a, the zeta potential of the scheelite treated with Al-Na2SiO3 increased remarkably compared to that with Na2SiO3, indicating that more Pb-BHA was adsorbed on the scheelite surface after treatment with the Al-Na2SiO3 polymer than with Na2SiO3. However, the opposite occurred for calcite, as seen in Figure 12b. Therefore, the Al-Na2SiO3 polymer had less influence on the adsorption of Pb-BHA complexes on the scheelite surface, while an obvious influence was observed for the calcite surface.
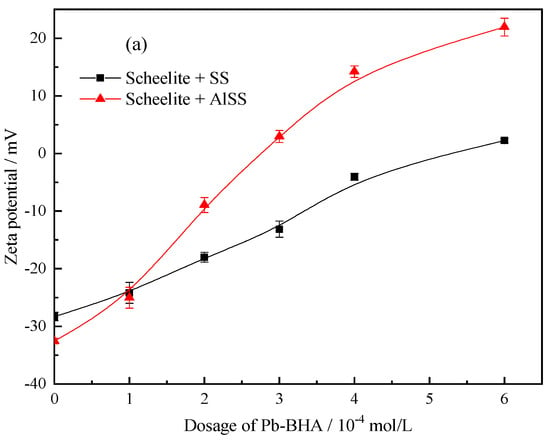
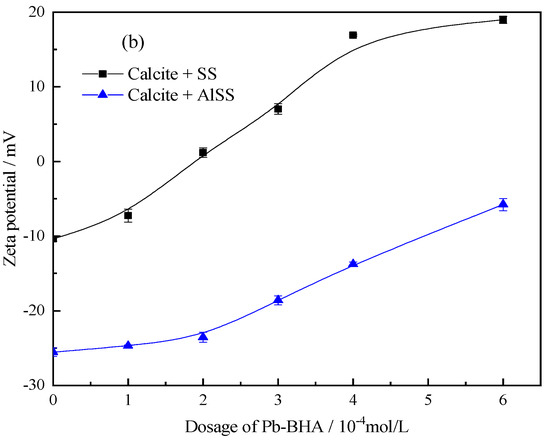
Figure 12.
Zeta potential plots for (a) scheelite and (b) calcite at different concentrations of Pb-BHA after treatment with Na2SiO3 and the Al-Na2SiO3 polymer (pH = 8.0 ± 0.1, CPb:CBHA = 2, CSS = CAlSS = 100 mg/L).
3.3.2. Adsorption Capacity
Figure 13 shows the adsorption capacity of Pb-BHA on the scheelite and calcite surface at different concentrations of Na2SiO3 and Al-Na2SiO3 polymer. The amount of Pb-BHA adsorbed on scheelite was more than that of calcite without Na2SiO3 or the Al-Na2SiO3 polymer, which is attributed to the specificity of Pb-BHA toward scheelite. Additionally, the adsorption curve shows some peaks with the increasing depressant dosage; however, herein, we focused on the different influence of Na2SiO3 and Al-Na2SiO3 polymer on the adsorption of Pb-BHA on scheelite and calcite. The results from Figure 13 indicate that the Al-Na2SiO3 polymer had less influence on the adsorption of Pb-BHA complexes on the scheelite surface, however, a significant influence was true for calcite, consistent with the zeta potential measurement results.
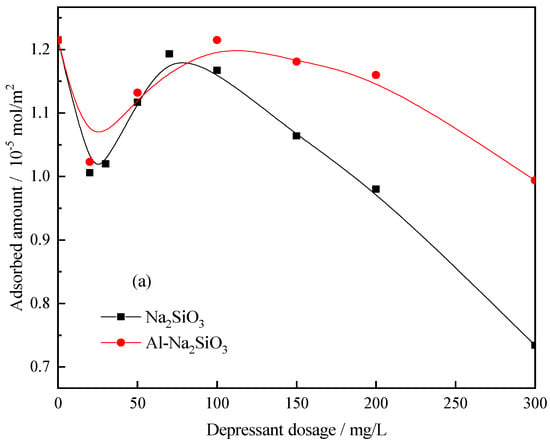
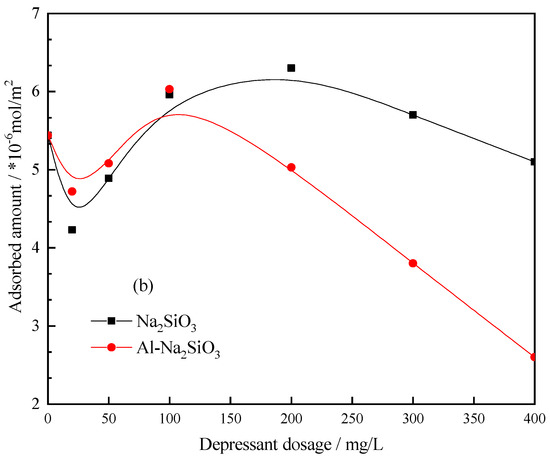
Figure 13.
Pb-BHA amount adsorbed on the (a) scheelite and (b) calcite surface at different concentrations of Na2SiO3 and Al-Na2SiO3 polymer (pH = 8.0 ± 0.1, CPb = 3 × 10−4 mol/L, CBHA = 1.5 × 10−4 mol/L).
3.4. Adsorption Model of the Al-Na2SiO3 Polymer and Pb-BHA Complexes on the Surface of Scheelite and Calcite
Based on the above analysis results, an adsorption model of the Al-Na2SiO3 polymer and Pb-BHA complexes on the scheelite and calcite surface was established to explain the superior selectivity of the Al-Na2SiO3 polymer toward scheelite and calcite during Pb-BHA flotation, as shown in Figure 14. The typical cleavage planes of scheelite expose more O atoms overall, leading to a negatively charged surface due to the dominant WO42− species, and thus, the strongly positively charged Pb-BHA complexes can react with the O atoms of the scheelite surface more easily. In turn, the usual cleavage planes of calcite expose more Ca atoms; such dominant Ca2+ species result in a positively charged surface in the pH range of 7–9, and thus, the Pb-BHA complexes have more difficulty to adsorb on the calcite surface. This leads to the selectivity of the Pb-BHA complex as the collector during scheelite and calcite flotation. On the other hand, the Al-Na2SiO3 polymer has a larger negative charge than Na2SiO3 and thus, it is more difficult for the Al-Na2SiO3 polymers to adsorb on the negatively charged scheelite surface by electrostatic interactions, resulting in weaker chemisorption of the Al-Na2SiO3 polymer on the scheelite, which in turn leads to a lower amount of Al-Na2SiO3 polymers adsorbed on the scheelite surface. However, the opposite is true for calcite, resulting in a larger more amount of Al-Na2SiO3 polymers adsorbed on the calcite surface, rendering the surface strongly hydrophilic. This leads to the selectivity of the Al-Na2SiO3 polymer as the depressant during scheelite and calcite flotation. When selective flotation of scheelite from calcite is conducted using Pb-BHA complexes as the collector and Al-Na2SiO3 polymers as the depressant, their selectivity is fully exploited and reinforced by each other. More Pb-BHA complexes and fewer Al-Na2SiO3 polymers are adsorbed on the scheelite surface, rendering it strongly hydrophobic and floating to the surface of the slurry. Fewer Pb-BHA complexes and more Al-Na2SiO3 polymers are deposited on the calcite surface, rendering it strongly hydrophilic and sinking to the bottom of the slurry. Thus, the selective separation of scheelite from calcite is achieved through the cooperative selectivity of Pb-BHA complexes and Al-Na2SiO3 polymers.
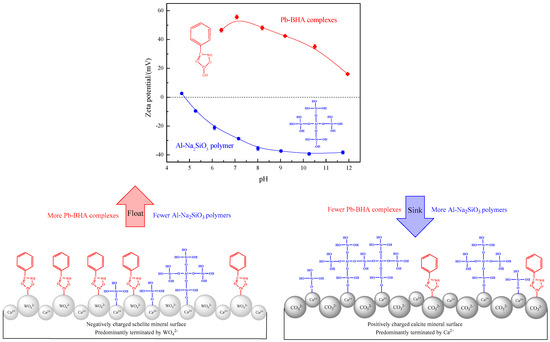
Figure 14.
Adsorption model for the Al-Na2SiO3 polymer and Pb-BHA complexes on the scheelite and calcite surface.
In addition, based on the reverse zeta potential of Pb-BHA complexes and silicic acid colloid (or Al-Na2SiO3 polymer), we believe that a moderate amount of silicic acid colloid could make the zeta potential of the mineral surface more negative, which may benefit the adsorption of Pb-BHA complexes by electrostatic forces, thus providing an explanation for the increased flotation recovery and adsorbed amount curve. Certainly, this hypothesis needs further detailed studies for its clarification.
4. Conclusions
The Al-Na2SiO3 polymer showed good selectivity for the flotation separation of scheelite from calcite using Pb-BHA complexes as the collector at pH 8, where the optimum ratio of sodium silicate to aluminum sulfate was determined as 2:1. When aluminum ions were added to the solution of sodium silicate, the aluminum hydroxide complexes and the hydroxy moieties of silicic acid or polysilicic acid self-assembled via condensation, affording an Al-Na2SiO3 polymer, which is a new composite aluminosilicate polymer containing Si–O–Al bonds in its structure. The Pb-BHA complexes adsorbed more easily on the scheelite surface than on the calcite surface. On the other hand, the Al-Na2SiO3 polymer adsorbed with difficulty on the scheelite surface, but easily on the calcite surface. Upon adsorption of the Al-Na2SiO3 polymer and Pb-BHA complexes on the mineral surface, the Al-Na2SiO3 polymer exhibited low influence on the adsorption of Pb-BHA complexes on the scheelite surface, while the opposite was observed for calcite, which is attributed to the cooperative selectivity of Pb-BHA complexes and Al-Na2SiO3 polymers. More Pb-BHA complexes and fewer Al-Na2SiO3 polymers were adsorbed on the scheelite surface, while fewer Pb-BHA complexes and more Al-Na2SiO3 polymers were deposited on the calcite surface, leading to the selective separation of scheelite from calcite.
Author Contributions
Conceptualization, H.H. and W.S.(Wei Sun); Methodology, Y.H.; Software, C.Z.; Data curation, R.W. and W.S. (Wenjuan Sun); Writing—original draft, Z.W.; Writing—review & editing, Z.W., J.W. and Z.G.; Formal Analysis, L.W., L.S. and R.L.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 51804340), Innovation Driven Plan of Central South University (No. 2015CX005), National 111 Project (No. B14034), and the Collaborative Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources, Key Laboratory of Hunan Province for Clean and Efficient Utilization of Strategic Calcium-containing Mineral Resources (No. 2018TP1002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, C.; Lü, Y. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. II. The mechanism of the interaction between phosphate modifiers and minerals. Int. J. Miner. Process. 1983, 10, 219–235. [Google Scholar]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Hu, Y.H.; Sun, W.; Li, X.D.; Cao, C.G.; Liu, R.Q.; Yue, T.; Meng, X.S.; Guo, Y.Z.; Wang, J.J.; et al. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Sampaio, J.A. Development studies for the recovery of Brazilian scheelite fines by froth flotation. In Production and Processing of Fine Particles; Plumpton, A.J., Ed.; Pergamon: Amsterdam, The Netherlands, 1988; pp. 209–217. [Google Scholar]
- Liu, C.; Feng, Q.; Zhang, G.; Chen, W.; Chen, Y. Effect of depressants in the selective flotation of scheelite and calcite using oxidized paraffin soap as collector. Int. J. Miner. Process. 2016, 157, 210–215. [Google Scholar] [CrossRef]
- Mercade, V. Effect of polyvalent metal-silicate hydrosols on the flotation of calcite. Trans. SME/AIME 1981, 268, 1842–1846. [Google Scholar]
- Yang, Y.; Xu, L.; Tian, J.; Liu, Y.; Han, Y. Selective flotation of ilmenite from olivine using the acidified water glass as depressant. Int. J. Miner. Process. 2016, 157, 73–79. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Xu, H.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodium silicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2016, 52, 567–573. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghani, A. Effect of sodium silicate on the reverse anionic flotation of a siliceous–phosphorus iron ore. Sep. Purif. Technol. 2016, 164, 28–33. [Google Scholar] [CrossRef]
- Deng, R.; Yang, X.; Hu, Y.; Ku, J.; Zuo, W.; Ma, Y. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Han, H.S.; Hu, Y.H.; Sun, W.; Li, X.D.; Chen, K.F.; Zhu, Y.G.; Nguyen, A.V.; Tian, M.J.; Wang, L.; Yue, T.; et al. Novel catalysis catalysis mechanisms of benzohydroxamic acid adsorption by lead ions and changes in the-surface of scheelite particles. Miner. Eng. 2018, 119, 11–22. [Google Scholar] [CrossRef]
- Han, H.S.; Liu, W.L.; Hu, Y.H.; Sun, W.; Li, X.D. A novel flotation scheme: Selective flotation of tungsten minerals from calcium minerals using Pb-BHA complexes in Shizhuyuan. Rare Met. 2017, 36, 533–540. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Du, J.; Cormack, A.N. The medium range structure of sodium silicate glasses: A molecular dynamics simulation. J. Non-Cryst. Solids 2004, 349, 66–79. [Google Scholar] [CrossRef]
- Neuefeind, J.; Liss, K.D. Bond angle distribution in amorphous germania and silica. Berichte der Bunsengesellschaft/Phys. Chem. Chem. Phys. 1996, 100, 1341–1349. [Google Scholar] [CrossRef]
- Poulsen, H.F.; Neuefeind, J.; Neumann, H.B.; Schneider, J.R.; Zeidler, M.D. Amorphous silica studied by high energy X-ray diffraction. J. Non-Cryst. Solids 1995, 188, 63–74. [Google Scholar] [CrossRef]
- Chen, X.; Niu, Z.; Wang, J.; Zhu, G.R.; Zhou, M. Effect of sodium polyacrylate on mechanical properties and microstructure of metakaolin-based geopolymer with different SiO2/Al2O3 ratio. Ceram. Int. 2018, 44, 18173–18180. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, Q.; Qiu, G.; Peng, B.; Guo, M.; Zhang, M. Synthesis of an alumina enriched Al2O3-SiO2 aerogel: Reinforcement and ambient pressure drying. J. Non-Cryst. Solids 2017, 471, 160–168. [Google Scholar] [CrossRef]
- Zagrajczuk, B.; Dziadek, M.; Olejniczak, Z.; Sulikowski, B.; Cholewa-Kowalska, K.; Laczka, M. Structural investigation of gel-derived materials from the SiO2Al2O3 system. J. Mol. Struct. 2018, 1167, 23–32. [Google Scholar] [CrossRef]
- Hernandez, C.; Pierre, A.C. Evolution of the Texture and Structure of SiO2-Al2O3 Xerogels and Aerogels as a Function of the Si to Al Molar Ratio. J. Sol-Gel Sci. Technol. 2001, 20, 227–243. [Google Scholar] [CrossRef]
- Orlović, A.; Janaćković, D.; Skala, D. Alumina/silica aerogel with zinc chloride alkylation catalyst: Influence of supercritical drying conditions and aerogel structure on alkylation catalytic activity. Catal. Commun. 2002, 3, 119–123. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Shergold, H.L. Influence of sodium silicate addition on the adsorption of oleic acid by fluorite, calcite and barite. Int. J. Miner. Process. 1985, 14, 177–193. [Google Scholar] [CrossRef]
- Qi, G.W.; Klauber, C.; Warren, L.J. Mechanism of action of sodium silicate in the flotation of apatite from hematite. Int. J. Miner. Process. 1993, 39, 251–273. [Google Scholar] [CrossRef]
- Bai, S.; Naren, G.; Nakano, M.; Okaue, Y.; Yokoyama, T. Effect of polysilicic acid on the precipitation of calcium carbonate. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 54–58. [Google Scholar] [CrossRef]
- Shimada, K.; Tarutani, T. The Kinetics of the Polymerization of Silicic Acid. Bull. Chem. Soc. Jpn. 1980, 53, 3488–3491. [Google Scholar] [CrossRef]
- Benezeth, P.; Palmer, D.A.; Wesolowski, D.J. The aqueous chemistry of aluminum. A new approach to high-temperature solubility measurements. Geothermics 1997, 26, 465–481. [Google Scholar] [CrossRef]
- Bi, S.; An, S.; Tang, W.; Yang, M.; Qian, H.; Wang, J. Modeling the distribution of aluminum speciation in acid soil solution equilibria with the mineral phase alunite. Environ. Geol. 2001, 41, 25–36. [Google Scholar] [CrossRef]
- Huang, C.; Stumm, W. Specific adsorption of cations on hydrous γ-Al2O3. J. Colloid Interface Sci. 1973, 43, 409–420. [Google Scholar] [CrossRef]
- Martell, A.E.; Hancock, R.D.; Smith, R.M.; Motekaitis, R.J. Coordination of Al(III) in the environment and in biological systems. Coord. Chem. Rev. 1996, 149, 311–328. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Schecher, W.D. The chemistry of aluminum in the environment. Environ. Geochem. Health 1990, 12, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Kawalec-Pietrenko, B.; Rybarczyk, P. Al(III) and Cu(II) simultaneous foam separation: Physicochemical problems. Chem. Pap. 2014, 68, 890–898. [Google Scholar] [CrossRef]
- Robert, E. The Hydrolysis of Cations; Wiley: Hoboken, NJ, USA, 1976; pp. 2385–2387. [Google Scholar]
- Van Benschoten, J.E.; Edzwald, J.K. Chemical aspects of coagulation using aluminum salts—I. Hydrolytic reactions of alum and polyaluminum chloride. Water Res. 1990, 24, 1519–1526. [Google Scholar] [CrossRef]
- ller, R.K. The effect of surface aluminosilicate ions on the properties of colloidal silica. J. Colloid Interface Sci. 1976, 55, 25–34. [Google Scholar]
- Ma, J. Preparation and Flocculation Mechansim of Modified Silica Sol; Nanjing Forestry University: Nanjing, China, 2004. [Google Scholar]
- Deren, K. Study on Preparation and Flocculating Mechanism of the Poly Aluminum Ferric Silicate-Sulfate Complex Flocculant; Harbin Institute of Technology: Harbin, China, 2006. [Google Scholar]
- Gao, Z.; Sun, W.; Hu, Y. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, Y.; Sun, W.; Drelich, J.W. Surface-Charge Anisotropy of Scheelite Crystals. Langmuir 2016, 32, 6282–6288. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z. Interactions of amphoteric amino phosphoric acids with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 87–94. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).