Titanite Ores of the Khibiny Apatite-Nepheline-Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
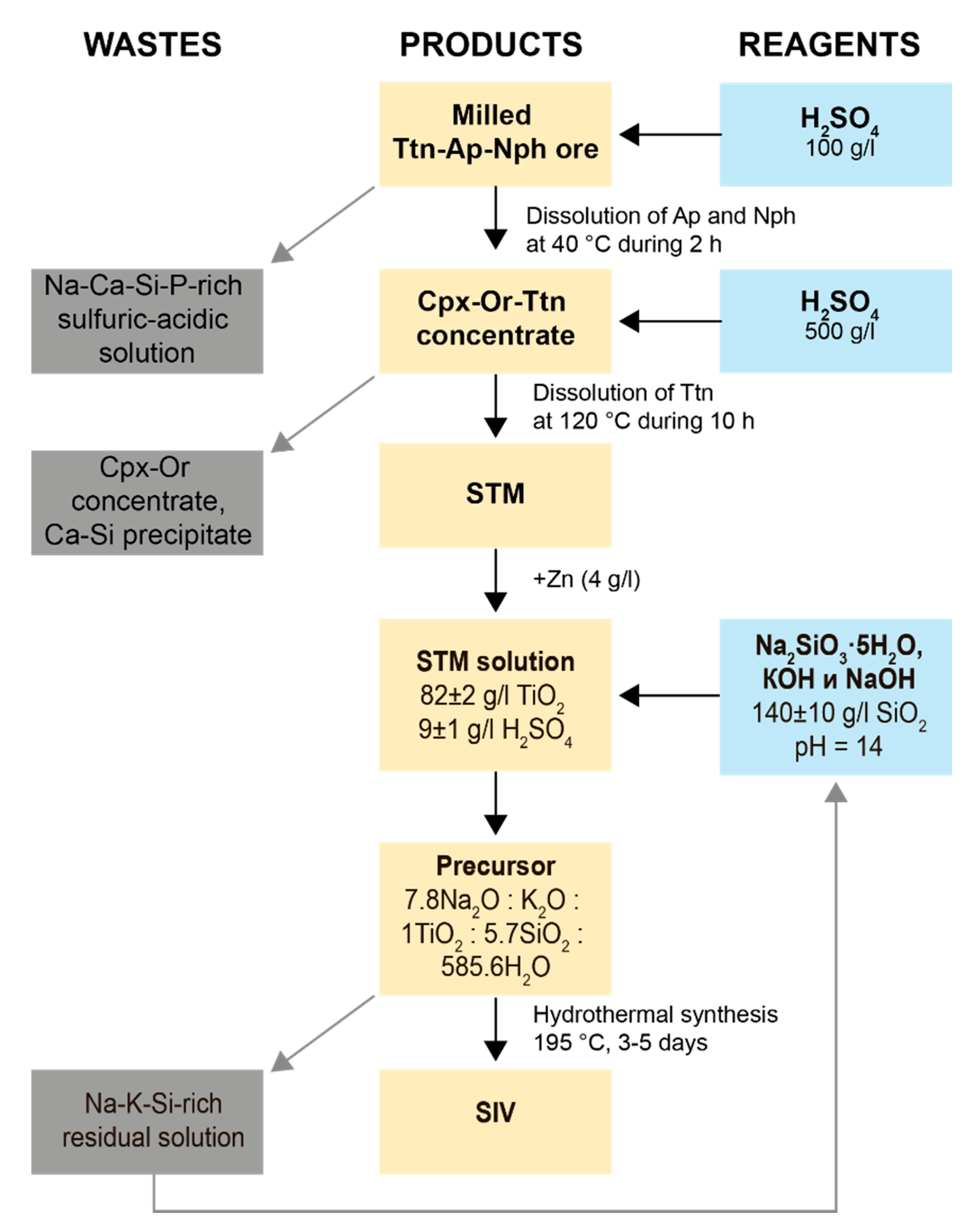
4.1. STA and STM Production
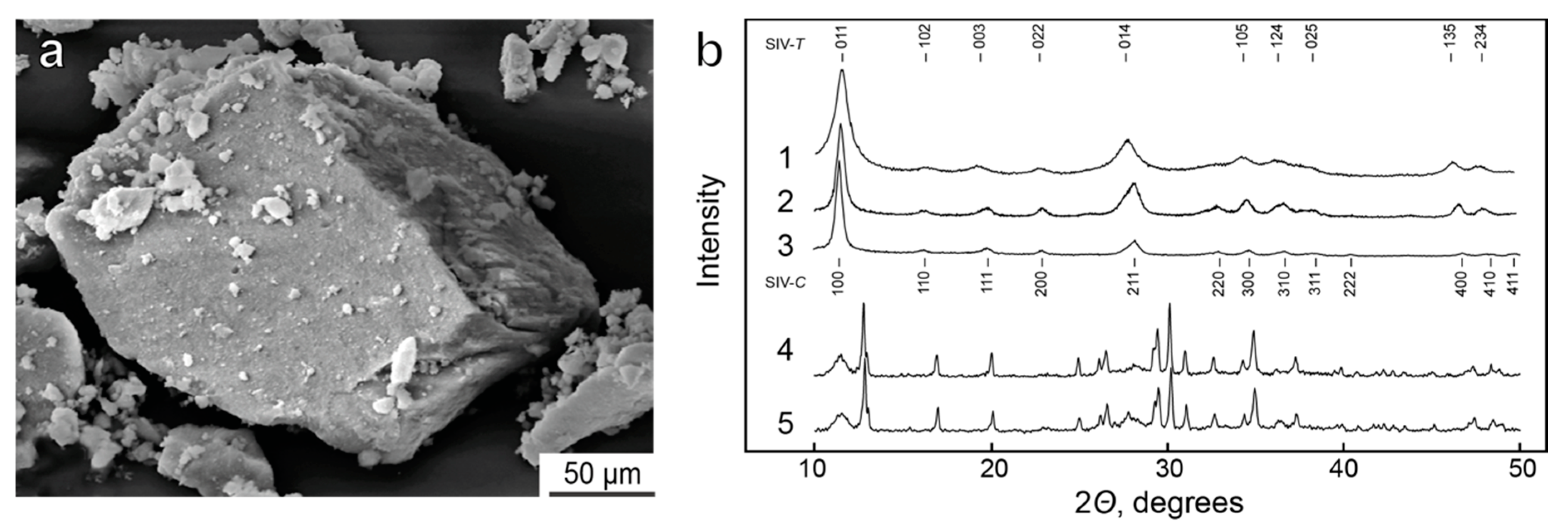
4.2. Synthetic Ivanyukite-Na (SIV) Synthesis
5. Discussion
6. Conclusions
- (1)
- Large lenses and layers of (apatite)-nepheline-titanite ore, up to 50 m thick, are widespread in apical parts of the most apatite deposits in the Khibiny massif. This ore can be selectively mined and processed as a new kind of titanium raw materials;
- (2)
- This ore can be processed by acidic cleaning only, without a conventional flotation stage. With a new approach, the yield of Ti-salts becomes 5–6 times higher than in the known process flow diagram of titanite recovery from apatite-nepheline ore with flotation followed by sulfuric-acidic processing of titanite concentrate;
- (3)
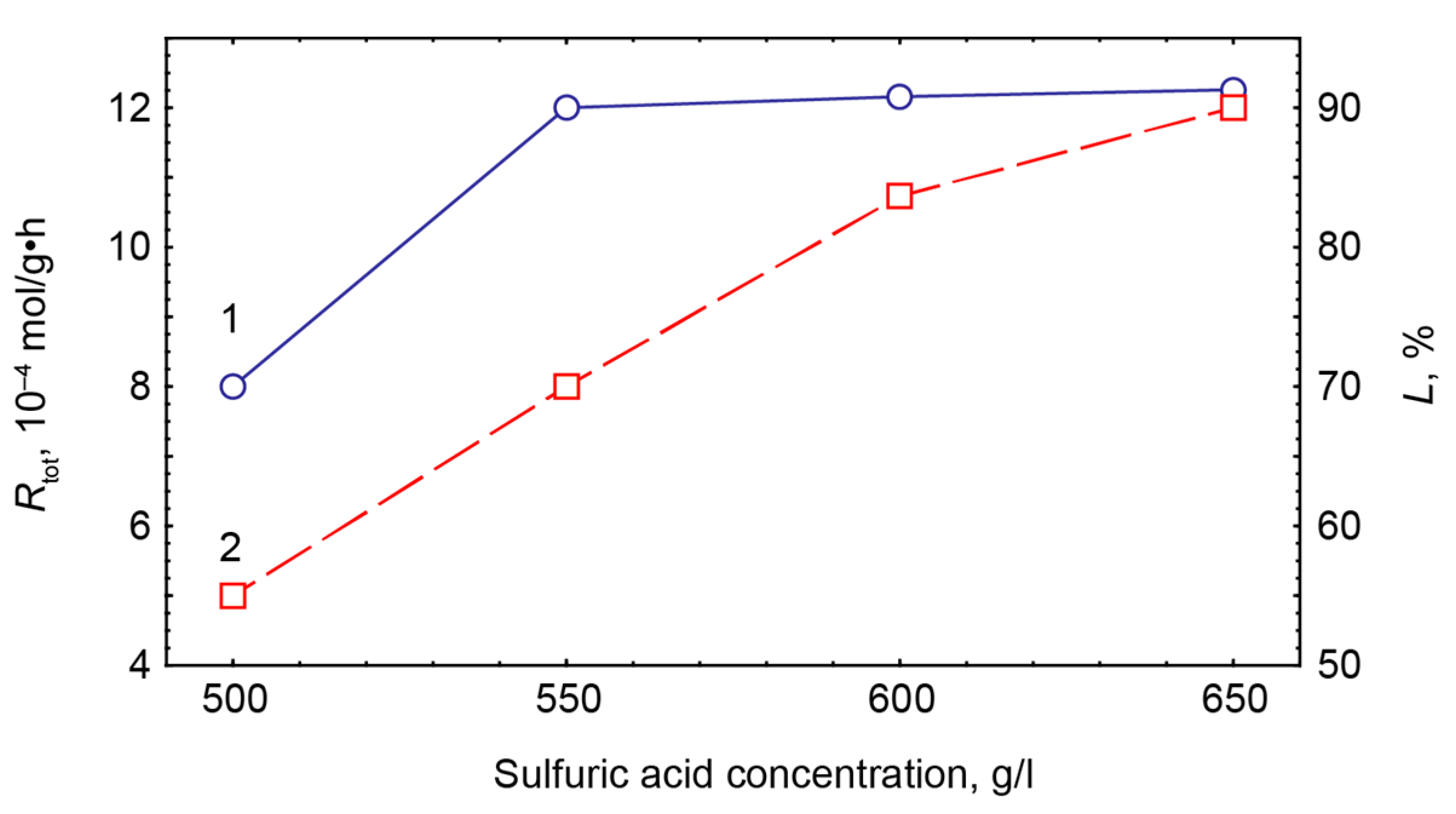
- Sulfuric-acidic processing of titanite ore enables to recover up to 90% Ti into the liquid phase that becomes a common precursor for hydrothermal synthesis of functional titanosilicates, in particular, synthetic analogues of the ivanyukite-group minerals. To prevent from ETS-4 crystallization, the rate of titanium cations hydrolysis should be reduced by partial transformation of Ti4+ into Ti3+;
- (4)
- Perspectives of SIV application include recovering of liquid radioactive wastes into Synroc-type titanate ceramics, selective or collective extraction of non-ferrous and, especially, precious metals from process solutions and effluents.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fedorov, S.G.; Nikolaev, A.I.; Brylyakov, Y.E.; Gerasimova, L.G.; Vasilieva, N.Y. Chemical Processing of Mineral Concentrates of the Kola Peninsula; The Kola Science Center Press: Apatity, Russia, 2003. (In Russian) [Google Scholar]
- Popova, E.A.; Zalessky, V.G.; Yakovenchuk, V.N.; Krivovichev, S.V.; Lushnikov, S.G. Ferroelectric phase transition and relaxer-like behavior of loparite-(Ce). Ferroelectrics 2014, 469, 130–137. [Google Scholar] [CrossRef]
- Men’shikov, Y.P.; Krivovichev, S.V.; Pakhomovsky, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Mikhailova, J.A.; Armbruster, T.; Selivanova, E.A. Chivruaiite, Ca4(Ti,Nb)5[(Si6O17)2 [(OH,O)5]·13-14H2O, a new mineral from hydrothermal veins of Khibiny and Lovozero alkaline massifs. Am. Mineral. 2006, 91, 922–928. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Krivovichev, S.V.; Men’shikov, Y.P.; Pakhomovsky, Y.A.; Ivanyuk, G.Y.; Armbruster, T.; Selivanova, E.A. Chivruaiite, a new mineral with ion-exchange properties. In Minerals as Advanced Materials I; Krivovichev, S.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 57–63. ISBN 978-3-540-77122-7. [Google Scholar]
- Kuznicki, S.M.; Bell, V.A.; Nair, S.; Hillhouse, H.W.; Jacubinas, R.M.; Braunbarth, C.M.; Toby, B.H.; Tsapatis, M. A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules. Nature 2001, 412, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Braunbarth, C.; Hillhouse, H.W.; Nair, S.; Tsapatis, M.; Burton, A.; Lobo, R.F.; Jacubinas, R.M.; Kuznicki, S.M. Structure of strontium ion-exchanged ETS-4 microporous molecular sieves. Chem. Mater. 2000, 12, 1857–1865. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Bogdanova, A.N. Kukisvumite, a new mineral from alkaline pegmatites of the Khibiny massif (Kola Peninsula). Mineral. Zhurnal 1991, 13, 63–67. (In Russian) [Google Scholar]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Selivanova, E.A.; Men’shikov, Y.P.; Korchak, J.A.; Krivovichev, S.V.; Spiridonova, D.V.; Zalkind, O.A. Punkaruaivite, LiTi2[Si4O11(OH)](OH)2·H2O, a new mineral species from hydrothermal assemblages, Khibiny and Lovozero alkaline massifs, Kola Peninsula, Russia. Can. Mineral. 2010, 48, 41–50. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Krivovichev, S.V.; Pakhomovsky, Y.A.; Selivanova, E.A.; Ivanyuk, G.Y. Microporous titanosilicates of the lintisite-kukisvumite group and their transformation in acidic solutions. In Minerals as Advanced Materials II; Krivovichev, S.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 229–238. ISBN 978-3-642-20017-5. [Google Scholar]
- Kalashnikova, G.O.; Selivanova, E.A.; Pakhomovsky, Y.A.; Zhitova, E.S.; Yakovenchuk, V.N.; Ivanyuk, G.Y.; Nikolaev, A.I. Synthesis of new functional materials by the self-assembly of titanosilicate nanolayers Ti2Si4O10(OH)4. Perspect. Mater. 2015, 10, 64–72. (In Russian) [Google Scholar]
- Dadachov, M.S.; Rocha, J.; Ferreira, A.; Lin, Z.; Anderson, M.W. Ab initio structure determination of layered sodium titanium silicate containing edge-sharing titanate chains (AM-4) Na3(Na,H)Ti2O2[Si2O6]2·2.2H2O. Chem. Commun. 1997, 24, 2371–2372. [Google Scholar] [CrossRef]
- Perez-Carvajal, J.; Lalueza, P.; Casado, C.; Téllez, C.; Coronas, J. Layered titanosilicates JDF-L1 and AM–4 for biocide applications. Appl. Clay Sci. 2012, 56, 30–35. [Google Scholar] [CrossRef]
- Men’shikov, Y.P.; Sokolova, E.V.; Egorov-Tismenko, Y.K.; Khomyakov, A.P.; Polezhaeva, L.I. Sitinakote Na2KTi4Si2O13(OH)·4H2O, a new mineral. Zap. RMO 1992, 1, 94–99. (In Russian) [Google Scholar]
- Clearfield, A.; Bortun, L.N.; Bortun, A.I. Alkali metal ion exchange by the framework titanium silicate M2Ti2O3SiO4·nH2O (M=H, Na). React. Funct. Polym. 2000, 43, 85–95. [Google Scholar] [CrossRef]
- Mann, N.R.; Todd, T.A. Removal of cesium from acidic radioactive tank waste by using Ionsiv IE-911. Sep. Sci. Technol. 2005, 39, 2351–2371. [Google Scholar] [CrossRef]
- Dyer, A.; Newton, J.; O’Brien, L.; Owens, S. Studies on a synthetic sitinakite-type silicotitanate cation exchanger: Part 1: Measurement of cation exchange diffusion coefficients. Micropor. Mesopor. Mater. 2009, 117, 304–308. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Nikolaev, A.P.; Selivanova, E.A.; Pakhomovsky, Y.A.; Korchak, J.A.; Spiridonova, D.V.; Zalkind, O.A.; Krivovichev, S.V. Ivanyukite-Na-T, ivanyukite-Na-C, ivanyukite-K, and ivanyukite-Cu: New microporous titanosilicates from the Khibiny massif (Kola Peninsula, Russia) and crystal structure of ivanyukite-Na-T. Am. Mineral. 2009, 94, 1450–1458. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Selivanova, E.A.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Spiridonova, D.V.; Krivovichev, S.V. First natural pharmacosiderite-related titanosilicates and their ion-exchange properties. In Minerals as Advanced Materials I; Krivovichev, S.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 27–35. ISBN 978-3-540-77122-7. [Google Scholar]
- Yakovenchuk, V.N.; Selivanova, E.A.; Krivovichev, S.V.; Pakhomovsky, Y.A.; Spiridonova, D.V.; Kasikov, A.G.; Ivanyuk, G.Y. Ivanyukite-group minerals: Crystal structure and cation-exchange properties. In Minerals as Advanced Materials II; Krivovichev, S.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 205–211. ISBN 978-3-642-20017-5. [Google Scholar]
- Britvin, S.N.; Gerasimova, L.G.; Ivanyuk, G.Y.; Kalashnikova, G.O.; Krzhizhanovskaya, M.G.; Krivovivhev, S.V.; Mararitsa, V.F.; Nikolaev, A.I.; Oginova, O.A.; Panteleev, V.N.; et al. Application of titanium-containing sorbents for treating liquid radioactive waste with the subsequent conservation of radionuclides in Synroc-type titanate ceramics. Theor. Found. Chem. Eng. 2016, 50, 598–606. [Google Scholar] [CrossRef]
- Britvin, S.N.; Lotnyk, A.; Kienle, L.; Krivovichev, S.V.; Depmeier, W. Layered hydrazinium titanate: Advanced reductive adsorbent and chemical toolkit for design of titanium dioxide nanomaterials. J. Am. Chem. Soc. 2011, 133, 9516–9525. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.S.; Coppens, M.O. Titano-silicates: Highlights on development, evolution and application in oxidative catalysis. In Catalysis 28; Spivey, J., Dooley, K.M., Han, Y.F., Eds.; Royal Society of Chemistry: London, UK, 2016; pp. 119–143. ISBN 1782626859. [Google Scholar]
- Du, H.; Fang, M.; Chen, J.; Pang, W. Synthesis and characterization of a novel layered titanium silicate JDF-L1. J. Mater. Chem. 1996, 6, 1827–1830. [Google Scholar] [CrossRef]
- Kim, S.N.; Kim, J.; Kim, H.Y.; Cho, H.Y.; Ahn, W.S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- Vilela, S.; Salcedo-Abraira, P.; Colinet, I.; Salles, F.; de Koning, M.; Joosen, M.; Serre, C.; Horcajada, P. Nanometric MIL-125-NH2 Metal–Organic Framework as a Potential Nerve Agent Antidote Carrier. Nanomaterials 2017, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- Milyutin, V.V.; Nekrasova, N.A.; Yanicheva, N.Y.; Kalashnikova, G.O.; Ganicheva, Y.Y. Sorption of cesium and strontium radionuclides onto crystalline alkali metal titanosilicates. Radiochemistry 2017, 59, 65–69. [Google Scholar] [CrossRef]
- Nikolaev, A.I.; Larichkin, F.D.; Gerasimova, L.G.; Glushchenko, Y.G.; Novoseltseva, V.D.; Maslova, M.V.; Nikolaeva, O.A. Titanium and Titanium Compounds: Resources, Production, and Markets—Current State and Trends; The Kola Science Center Press: Apatity, Russia, 2011. (In Russian) [Google Scholar]
- Gerasimova, L.G.; Maslova, M.V.; Nikolaev, A.I. Investigation of Non-Equilibrium Chemical Processes in Raw-Material Technologies; LKM Press: Moscow, Russia, 2014. (In Russian) [Google Scholar]
- Maslova, M.V.; Gerasimova, L.G.; Nikolaev, A.I. Treatment of apatite nepheline ore enrichment waste. Mod. App. Sci. 2015, 9, 81–92. [Google Scholar]
- Arzamastsev, A.A.; Arzamastseva, L.V.; Travin, A.V.; Belyatsky, B.V.; Shamatrina, A.M.; Antonov, A.V.; Larionov, A.N.; Rodionov, N.V.; Sergeev, S.A. Duration of formation of magmatic system of polyphase Paleozoic alkaline complexes of the central Kola: U-Pb, Rb-Sr, Ar-Ar data. Dokl. Earth Sci. 2007, 413, 432–436. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Men’shikov, Y.P. Khibiny; Wall, F., Ed.; Laplandia Minerals: Apatity, Russia, 2005; ISBN 5900395480. [Google Scholar]
- Ivanyuk, G.; Yakovenchuk, V.; Pakhomovsky, Y.; Kalashnikov, A.; Mikhailova, J.; Goryainov, P. Self-Organization of the Khibiny Alkaline Massif (Kola Peninsula, Russia). In Earth Sciences; Ahmad Dar, I., Ed.; INTECH: London, UK, 2012; pp. 131–156. ISBN 978-953-307-861-8. [Google Scholar]
- Ivanyuk, G.Y.; Konopleva, N.G.; Pakhomovsky, Y.A.; Yakovenchuk, V.N.; Mikhailova, J.A.; Bazai, A.V. Titanite of the Khibiny Alkaline Massif (Kola Peninsula). Zap. RMO 2016, 3, 36–55. (In Russian) [Google Scholar]
- Kalinkin, A.M. Physicochemical processes in mechanical activation of titanium- and calcium-containing minerals. Russ. J. Appl. Chem. 2007, 80, 1613–1620. [Google Scholar] [CrossRef]
- Lazareva, I.V.; Gerasimova, L.G.; Maslova, M.V.; Okhrimenko, R.F. Interaction of titanite with sulfuric acid solution. Russ. J. Appl. Chem. 2006, 79, 18–21. [Google Scholar] [CrossRef]
- Melikhov, I.V. Ways of using crystallization to produce solid products with specified properties. Chem. Ind. 1997, 7, 488–500. (In Russian) [Google Scholar]
- Ji, Z.; Yilmaz, B.; Warzywoda, J.; Sacco, A., Jr. Hydrothermal synthesis of titanosilicate ETS-10 using Ti(SO4)2. Micropor. Mezpor. Mat. 2005, 81, 1–10. [Google Scholar] [CrossRef]
- Motov, D.L. Physico-Chemistry and Sulphate Technology of Titanium-Rare Metal Raw Materials. Part 2; The Kola Science Center Press: Apatity, Russia, 2002. (In Russian) [Google Scholar]
- Gerasimova, L.G.; Nikolaev, A.I.; Kuz’mich, J.V.; Ivanyuk, G.Y.; Yakovenchuk, V.N.; Schukina, E.S. Patent RU-2539303: Method of Titanium-Silicon Sodium-Containing Composition Obtaining. U.S. Patent RU2539303C1, 20 January 2015. [Google Scholar]
- Gerasimova, L.G.; Maslova, M.V.; Nikolaev, A.I. Synthesis of new nano-porous titanosilicates using ammonium oxysulphotitanite. J. Glass Phys. Chem. 2013, 39, 846–855. [Google Scholar] [CrossRef]
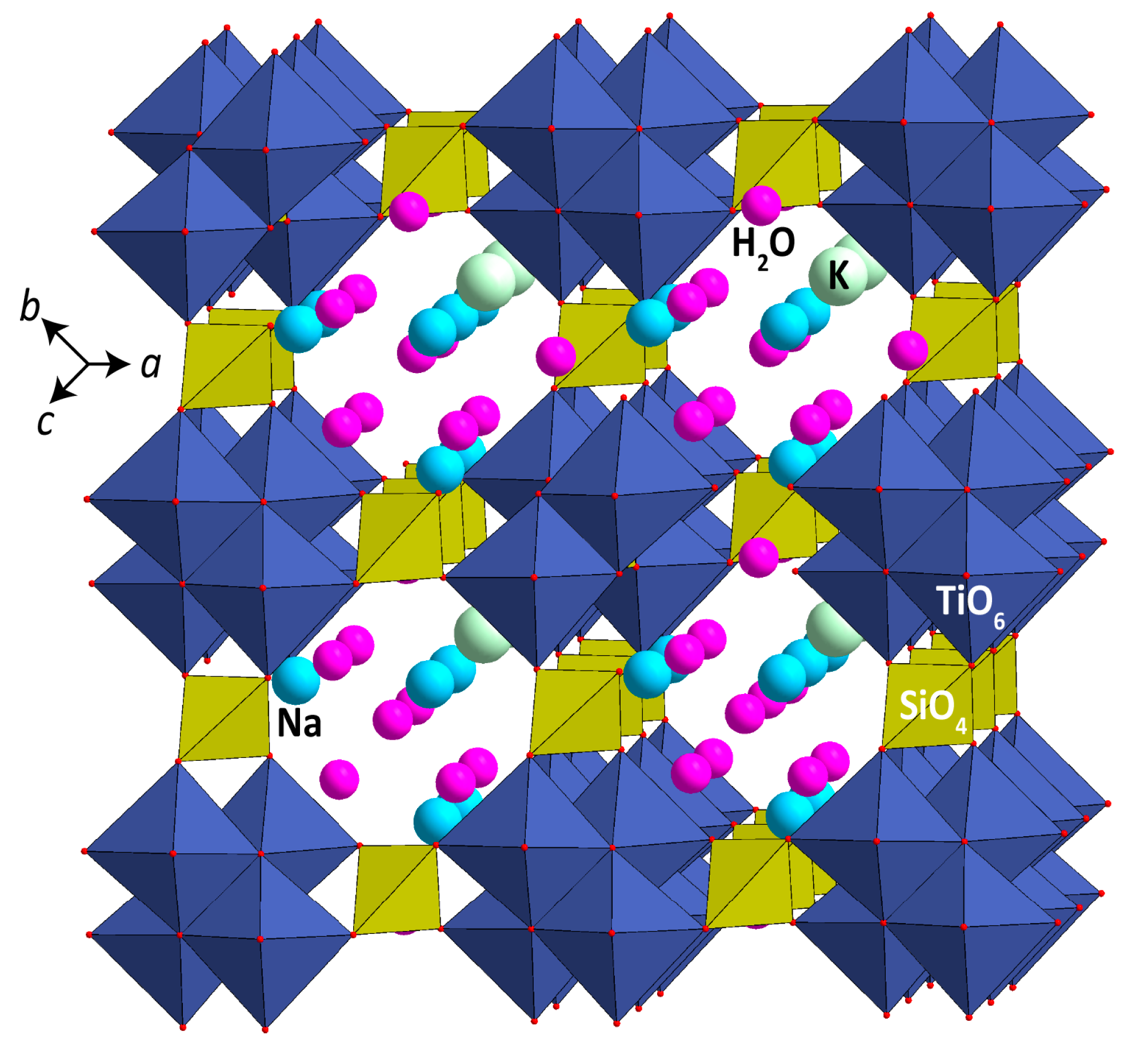
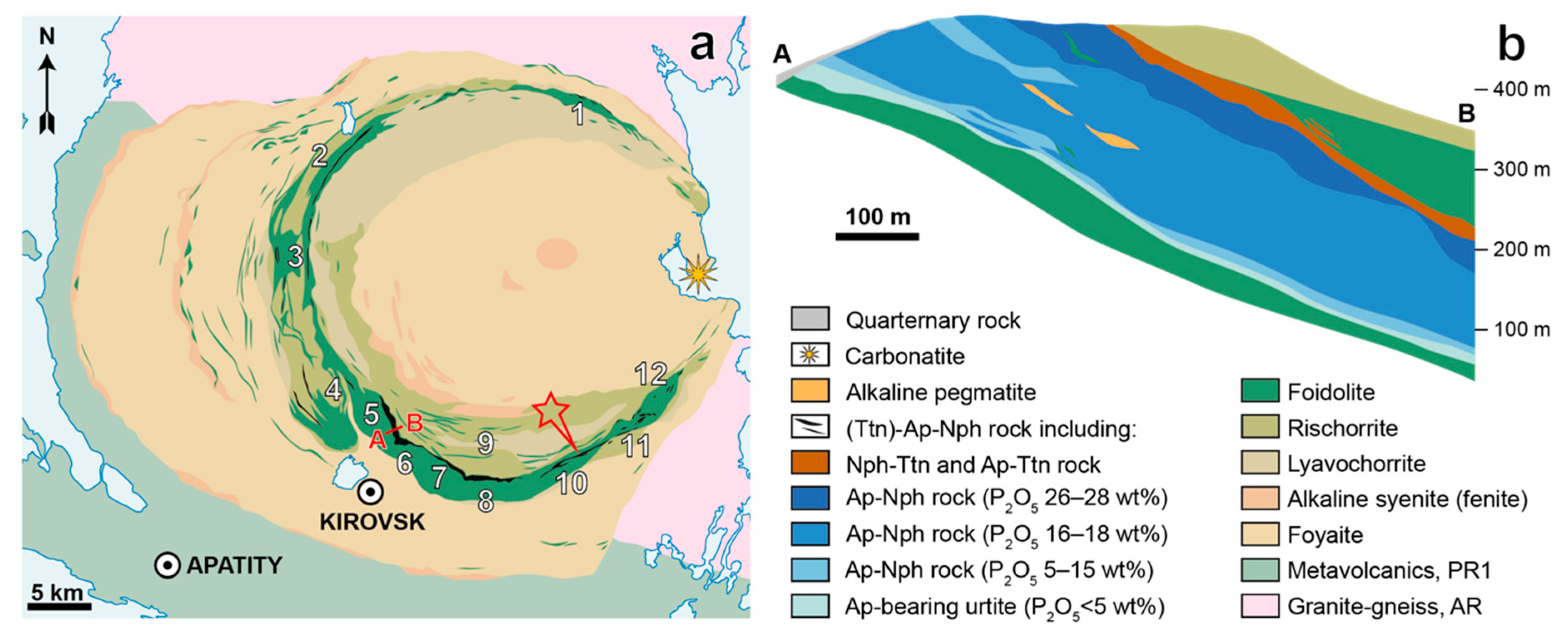
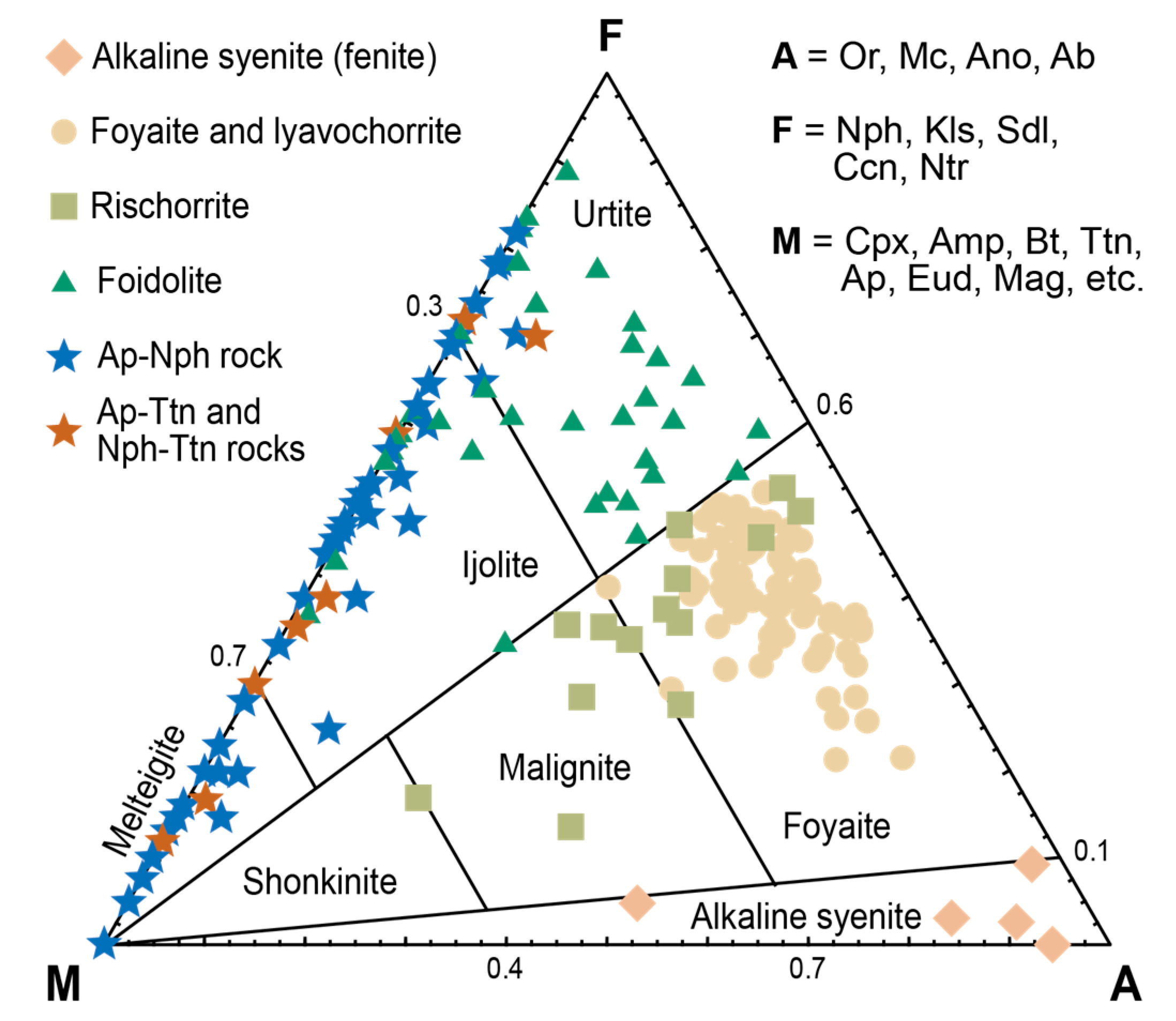
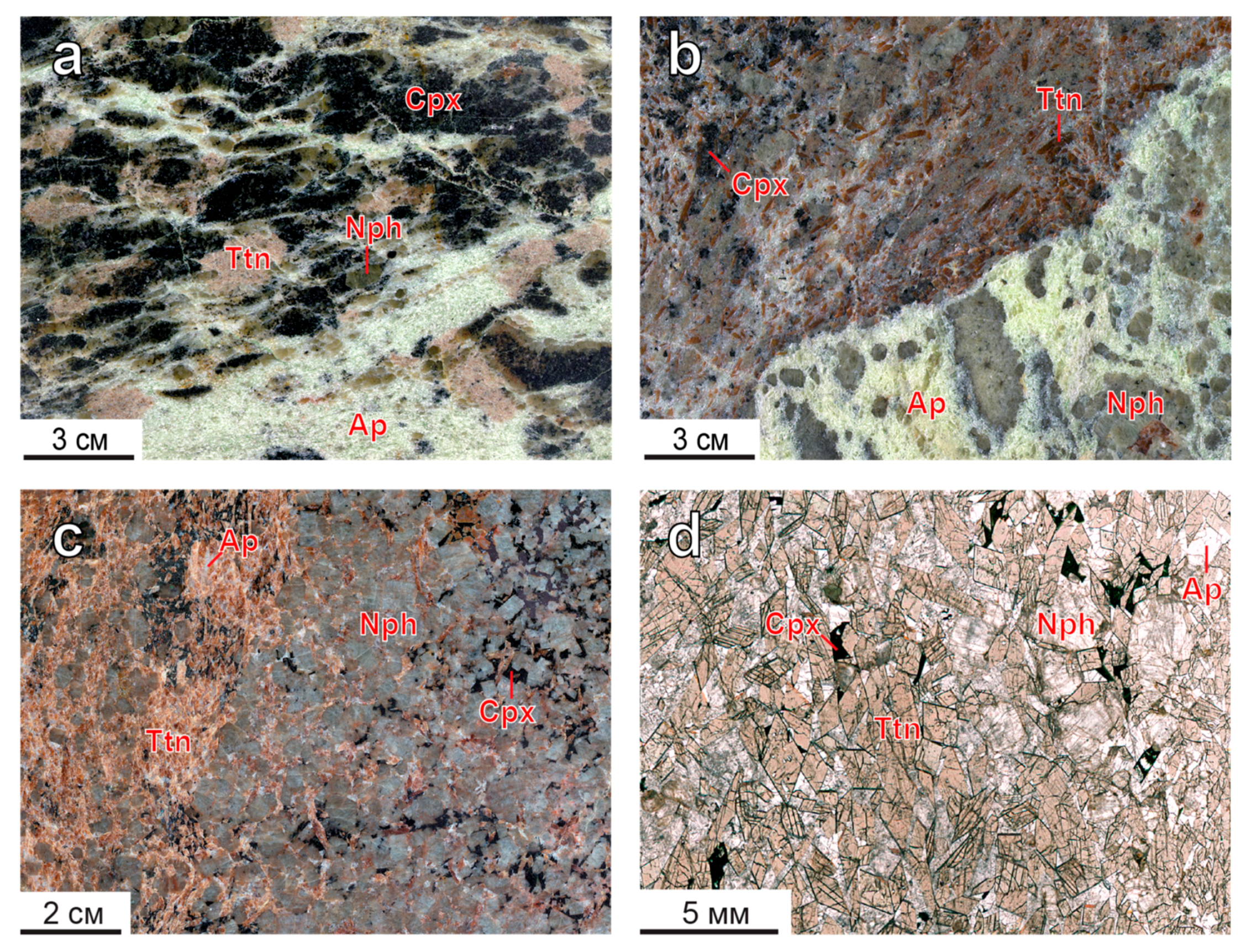
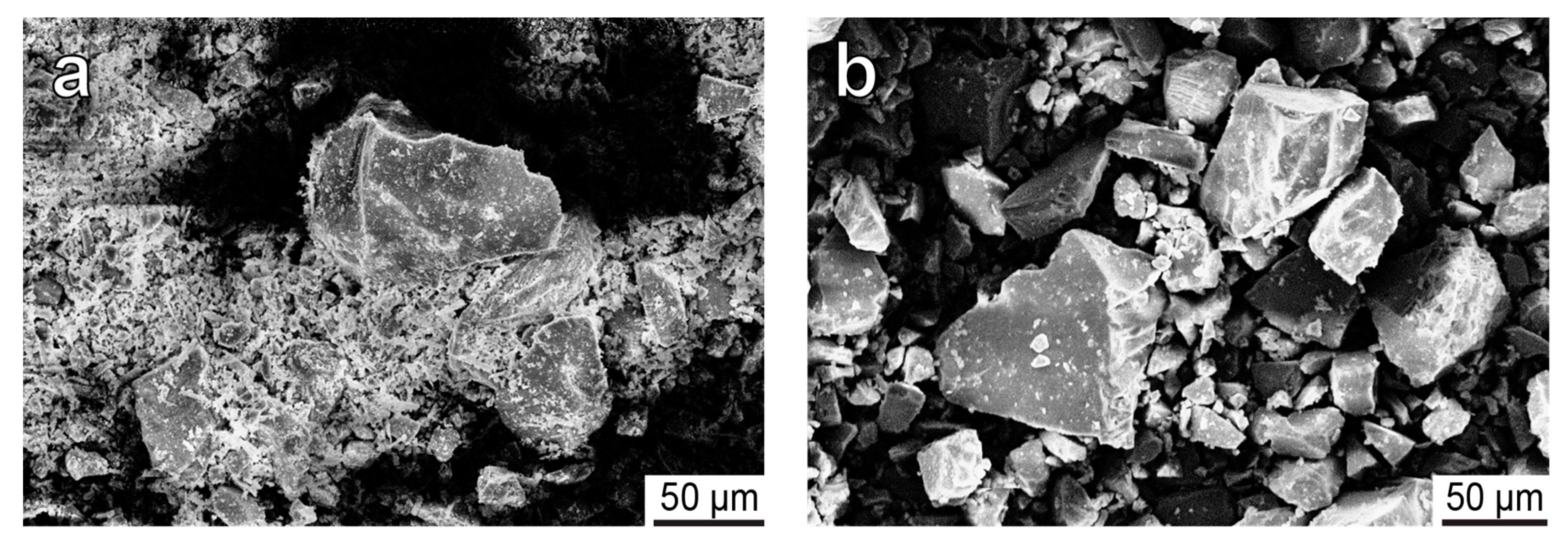
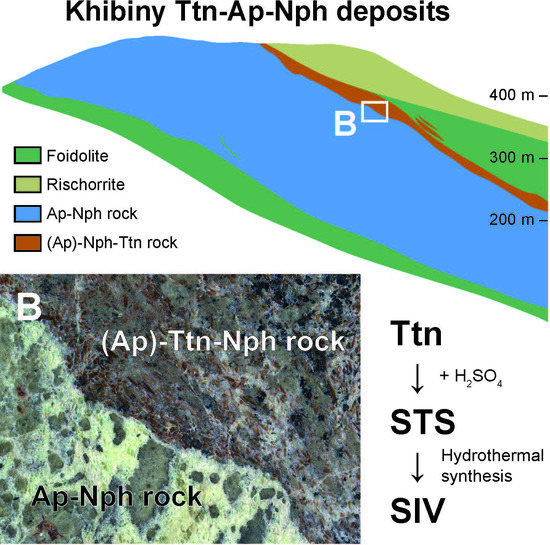
| Deposit | Measured Ttn Resources, kt | Average Ttn Content in (Ttn)-Ap-Nph ore, wt % | Fraction of Ap-Ttn and Nph-Ttn Ores, vol % |
|---|---|---|---|
| Partomchorr | 59,615 | 6.9 | 13.6 |
| Kuelporr | 995 | 5.2 | 4.3 |
| Kukisvumchorr | 17,550 | 4.2 | 6.9 |
| Yuksporr | 23,340 | 4.4 | 8.1 |
| Apatite Circus | 3840 | 3.2 | 1.9 |
| Rasvumchorr | 13,395 | 4.0 | 2.1 |
| Eveslogchorr | 45 | 8.9 | 27.7 |
| Koashva | 38,780 | 4.7 | 8.4 |
| Niorkpakhk | 1595 | 2.4 | 0.0 |
| OleniyRuchei | 12,075 | 3.1 | 0.0 |
| Constituent | n | Mean | Min | Max | SD |
|---|---|---|---|---|---|
| Na2O | 15 | 0.61 | b.d. | 1.18 | 0.30 |
| Al2O3 | 15 | 0.24 | b.d. | 0.66 | 0.17 |
| SiO2 | 15 | 30.31 | 29.61 | 32.38 | 0.62 |
| K2O | 15 | 0.01 | b.d. | 0.20 | 0.05 |
| CaO | 15 | 27.16 | 25.22 | 28.04 | 0.95 |
| TiO2 | 15 | 38.93 | 37.28 | 42.86 | 1.30 |
| V2O3 | 15 | 0.02 | b.d. | 0.15 | 0.04 |
| MnO | 15 | 0.01 | b.d. | 0.06 | 0.02 |
| FeO | 15 | 0.88 | 0.33 | 1.29 | 0.29 |
| SrO | 15 | 0.32 | b.d. | 0.53 | 0.15 |
| ZrO2 | 15 | 0.14 | b.d. | 0.43 | 0.15 |
| Nb2O5 | 15 | 0.38 | b.d. | 1.35 | 0.41 |
| La2O3 | 15 | 0.05 | b.d. | 0.17 | 0.07 |
| Ce2O3 | 15 | 0.30 | b.d. | 0.68 | 0.16 |
| Nd2O3 | 15 | 0.04 | b.d. | 0.26 | 0.08 |
| Experiment | Conditions | Ore Composition, wt % | ||
|---|---|---|---|---|
| TiO2 | Al2O3 | P2O5 | ||
| 1 | Н2SO4, 80 g/L, S:L = 1:3, 4 h, 18 °C | 30.5 | 0.97 | 4.23 |
| 2 | Н2SO4, 80 g/L, S:L = 1:4, 2h, 50 °С | 28.0 | 2.69 | 1.00 |
| 3 | 1 stage: Н2SO4, 80 g/L, S:L = 1:3, 4 h, 18 °C; 2 stage: Н2SO4, 100 g/L, S:L = 1:4, 2 h, 50 °С | 32.0 | 0.27 | 1.15 |
| 4 | НCl, 50 g/L, S:L = 1:4, 2 h, 50 °С | 31.5 | 1.62 | 0.21 |
| Experiment | Pre-Reaction with Zn | TiO2:SiO2, Moles | рН | Т °С | Time, Day |
|---|---|---|---|---|---|
| 1 | + | 1:4 | 11.5 | 200 | 3 |
| 2 | + | 1:5 | 12.5 | 190 | 3 |
| 3 | + | 1:4 | 12.5 | 200 | 5 |
| 4 | – | 1:4 | 11.5 | 200 | 5 |
| 5 | – | 1:5 | 12.5 | 200 | 3 |
| Experiment | Modal Composition | Surface Properties | ||
|---|---|---|---|---|
| S, m2/g | V, cm3/g | Dav, nm | ||
| 1 | SIV-T + SIV-C | 148.0 ± 0.9 | 0.68 | 18.4 ± 0.2 (3.4–68.0) |
| 2 | SIV-T + SIV-C | 158 ± 1 | 0.73 | 18.6 ± 0.1 (2.9–65.4) |
| 3 | SIV-C | 143.3 ± 0.6 | 0.75 | 20.9 ± 0.1 (4.3–75.0) |
| 4 | 70% ETS-4 + 30%-SIV-T | 81.0 ± 0.4 | 0.18 | 8.9 ± 0.1 (2.1–54.3) |
| 5 | 65% ETS-4 + 30% SIV-T + 5% TiO2 | 85.1 ± 0.5 | 0.22 | 10.3 ± 0.1 (1.8–58.2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimova, L.G.; Nikolaev, A.I.; Maslova, M.V.; Shchukina, E.S.; Samburov, G.O.; Yakovenchuk, V.N.; Ivanyuk, G.Y. Titanite Ores of the Khibiny Apatite-Nepheline-Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis. Minerals 2018, 8, 446. https://doi.org/10.3390/min8100446
Gerasimova LG, Nikolaev AI, Maslova MV, Shchukina ES, Samburov GO, Yakovenchuk VN, Ivanyuk GY. Titanite Ores of the Khibiny Apatite-Nepheline-Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis. Minerals. 2018; 8(10):446. https://doi.org/10.3390/min8100446
Chicago/Turabian StyleGerasimova, Lidia G., Anatoly I. Nikolaev, Marina V. Maslova, Ekaterina S. Shchukina, Gleb O. Samburov, Victor N. Yakovenchuk, and Gregory Yu. Ivanyuk. 2018. "Titanite Ores of the Khibiny Apatite-Nepheline-Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis" Minerals 8, no. 10: 446. https://doi.org/10.3390/min8100446
APA StyleGerasimova, L. G., Nikolaev, A. I., Maslova, M. V., Shchukina, E. S., Samburov, G. O., Yakovenchuk, V. N., & Ivanyuk, G. Y. (2018). Titanite Ores of the Khibiny Apatite-Nepheline-Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis. Minerals, 8(10), 446. https://doi.org/10.3390/min8100446