Abstract
The new mineral polyarsite, ideally Na7CaMgCu2(AsO4)4F2Cl, was discovered in high-temperature incrustations of the active Arsenatnaya fumarole at the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka, Russia. It is associated with aegirine, sanidine, ferrisanidine, hematite, halite, sylvite, cassiterite, evseevite, axelite, badalovite, johillerite, arsmirandite, aphthitalite, tridymite, potassic-magnesio-fluoro-arfvedsonite and litidionite. Polyarsite forms short-prismatic, equant or tabular crystals up to 0.15 mm across, their clusters up to 0.3 mm in size or crusts up to 0.5 mm across and up to 0.03 mm thick. Polyarsite is transparent, sky-blue to light blue, with vitreous lustre. It is brittle, no cleavage is observed and the fracture is uneven. Dcalc. = 3.592 g cm−3. Polyarsite is optically biaxial (+), α = 1.624 (4), β = 1.645 (4), γ = 1.682 (4) (589 nm), 2Vmeas. = 70 (10)°. The empirical chemical formula calculated based on 19 O+F+Cl apfu is Na7.04Ca1.00Mg0.92Cu2.06Fe3+0.06(As3.96S0.05)Σ4.01O16.28F1.66Cl1.06. Polyarsite is monoclinic, space group I2/m, a = 8.4323(4), b = 10.0974(4), c = 10.7099(6) Å, β = 90.822(4)°, V = 911.79(8) Å3 and Z = 2. The crystal structure was determined based on SCXRD data, R = 0.0391. Polyarsite demonstrates a novel structure type. The structure is based on the (1 0 1) heteropolyhedral layers formed by Cu2O8Cl dimers built by CuO4Cl tetragonal pyramids sharing common Cl vertex, AsO4 tetrahedra and MgO4F2 octahedra. Adjacent layers are linked via CaO8 cubes to form a pseudo-framework which hosts octahedrally coordinated Na cations. Polyarsite was named based on the Greek words πολύς, poly, “many” and due to belonging to arsenates: this arsenate contains many chemical components ordered between different positions in crystal structure.
1. Introduction
In this article, we describe the new mineral species polyarsite, a complex chloro-fluoro-arsenate with the ideal formula Na7CaMgCu2(AsO4)4F2Cl and a unique crystal structure. It was discovered in high-temperature incrustations of the active Arsenatnaya fumarole situated at the summit of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption 1975–1976, Tolbachik volcano, Kamchatka peninsula, Far-Eastern Region, Russia (55°41′ N, 160°14′ E, 1200 m asl). The Second scoria cone is a monogenetic volcano ca. 300 m high and about 0.1 km3 in volume formed in 1975 [1] and still, after a half of century, demonstrates strong fumarolic activity. The fumaroles located here belong to the oxidizing type due to the mixing of hot (up to 500 °C) volcanic gas with atmospheric oxygen. Some fumaroles are richly mineralized and show an outstanding mineral diversity and originality [2]. The largest and mineralogically richest of them is Arsenatnaya. This active fumarole was discovered by us in 2012 and soon became famous as one of the world-class mineral localities: at present day, >210 mineral species were identified reliably in its incrustations including 73 IMA-approved new minerals. We named this fumarole for the unusual abundance and diversity of arsenates which belong to the specific genetic type being high-temperature volcanic sublimates: 58 valid arsenate minerals are known here, including 40 new mineral species. The Arsenatnaya fumarole and the mineralization formed in this unique “natural laboratory” were characterized in general in [3,4,5].
Polyarsite is named from the Greek πολύς, poly, “many” and due to belonging to arsenates: this arsenate mineral contains many chemical components ordered between different positions in crystal structure, namely four species-defining metal cations (Na, Ca, Mg and Cu2+) and two species-defining additional halide anions (F− and Cl−). Both the new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification (CNMNC), IMA2019–058. The IMA-accepted symbol is Par. The type material is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, with the catalogue number 96700.
2. Methods
The chemical composition of polyarsite was studied in the Laboratory of Analytical Techniques of High Spatial Resolution, Dept. of Petrology, Moscow State University, using a JEOL JXA 8230 Superprobe instrument (Jeol, Japan). Electron microprobe analyses (EMPA) were carried out in WDS mode (20 kV and 20 nA; the electron beam was 3 μm in diameter). The probe standards used were as follows: albite (Na), wollastonite (Ca), chromite (Mg), Cu (Cu), magnetite (Fe), InAs (As), ZnS (S), fluorophlogopite (F) and atacamite (Cl). Contents of other chemical elements with atomic numbers higher than that of carbon are below their detection limits.
The Raman spectrum of a randomly oriented crystal of polyarsite was obtained using an EnSpectr R532 instrument (Chernogolovka, Russia) at the Dept. of Mineralogy, Lomonosov Moscow State University with a green laser (532 nm) at room temperature. The output power of the laser beam was about 13 mW. The spectrum was processed using the EnSpectr expert mode programme in the range from 100 to 4000 cm−1 with the use of a holographic diffraction grating with 1800 lines mm−1 and a resolution of 6 cm−1. Acquisition time for a single scan was 2000 ms and the signal was averaged over 2 scans. The diameter of the laser spot on the sample was about 10 μm. The backscattered Raman signal was collected with 40× objective.
The single-crystal X-ray diffraction (XRD) investigation was carried out at Dept. of Crystallography and Crystal Chemistry, Lomonosov Moscow State University. The data were obtained with an Xcalibur S diffractometer equipped with a CCD detector (Oxford Diffraction, Oxford, UK) (MoKα radiation). Data reduction was performed using CrysAlisPro Version 1.171.39.46 [6]. The crystal structure was solved by direct methods and refined with the use of the SHELX software package (version 2018/3) [7].
The powder XRD study of polyarsite was carried out at the Centre for X-Ray Diffraction Research of the Science Park of St. Petersburg State University. Diffraction data were collected with a Rigaku R-AXIS Rapid II (Rigaku Corporation, Tokyo, Japan) diffractometer equipped with cylindrical image plate detector using the Debye–Sсherrer geometry (d = 127.4 mm). The data were integrated using the software package Osc2Tab [8].
3. Results
3.1. Occurrence and General Appearance
Polyarsite belongs to the rarest minerals in incrustations of the Arsenatnaya fumarole. The specimens in which it was discovered were collected by us in July 2018 from the intermediate zone of Arsenatnaya, about 1.5 m below the surface. The temperatures measured with the use of a chromel-alumel thermocouple in these pockets immediately after the opening were 300–350 °C. The minerals associated with polyarsite are aegirine, sanidine, ferrisanidine, hematite, halite, sylvite, cassiterite, evseevite, axelite, badalovite, johillerite, arsmirandite, aphthitalite, tridymite, potassic-magnesio-fluoro-arfvedsonite and litidionite.
Polyarsite forms short-prismatic, equant or tabular crystals up to 0.15 mm across, well-shaped (Figure 1a,b) or, more typically, crude, distorted. Sometimes they are combined in clusters (Figure 1b and Figure 2) up to 0.3 mm in size. Some crystals of the mineral are skeletal (Figure 1a). Most commonly, polyarsite occurs as small (up to 0.5 mm across and up to 0.03 mm in thickness) crusts which overgrow aegirine, hematite, sanidine (Figure 1c and Figure 2) or badalovite.
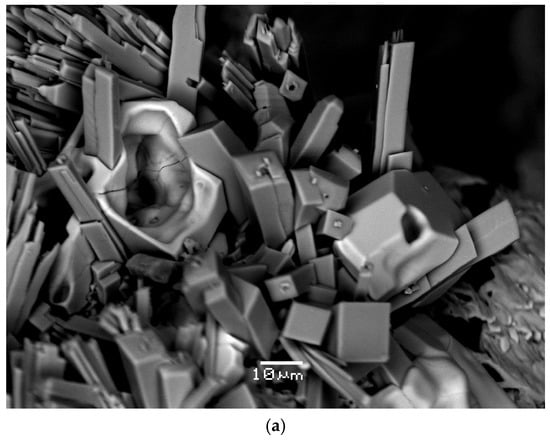
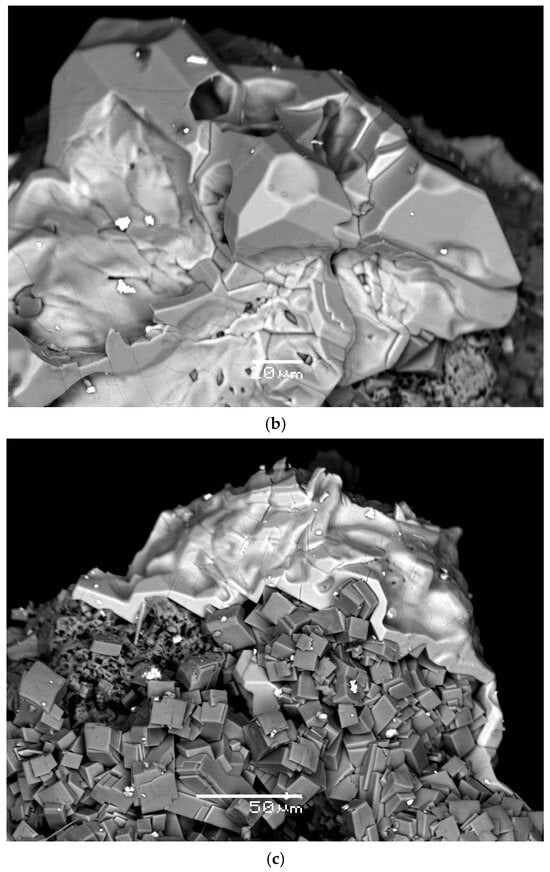
Figure 1.
Morphology of polyarsite: (a) two skeletal crystals (light grey) on aggregate of well-formed aegirine crystals; (b) crust composed by well-shaped and distorted, crude crystals; (c) crust on aegirine crystal crust with separate tiny cassiterite grains (bright white). SEM images, BSE mode. FOV width (mm): (a) 0.13; (b) 0.21; (c) 0.24.
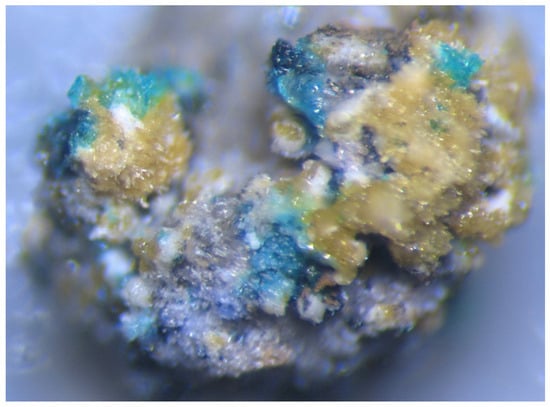
Figure 2.
Blue crude crystals, crystal clusters and crusts of polyarsite with yellow aegirine, colourless to white tridymite and sanidine and black hematite. FOV width: 1.7 mm. Photo: I.V. Pekov and A.V. Kasatkin.
3.2. Physical Properties and Optical Data
Polyarsite is sky-blue to light blue. Its streak is white streak and lustre is vitreous. The mineral is transparent. It is brittle, with no cleavage or parting and the fracture is uneven. The calculated density, using the program MINCALC recommended by the IMA CNMNC (based on the empirical formula and unit cell volume obtained from SCXRD data), is 3.592 g cm−3.
Polyarsite is optically biaxial (+), α = 1.624 (4), β = 1.645 (4), γ = 1.682 (4) (589 nm), 2V (meas.) = 70 (10)° and 2V (calc.) = 75°. Dispersion of optical axes is weak, r > v. Pleochroism is distinct: Z (turquoise-blue) > Y (light turquoise-blue) > X (pale bluish to colourless).
3.3. Raman Spectroscopy
Bands in the Raman spectrum of polyarsite (Figure 3) are assigned according to [9]. Bands between 930 and 760 cm−1 correspond to As5+–O stretching vibrations of the groups [AsO4]3−. Bands lower than 550 cm−1 are assigned to bending vibrations of AsO4 tetrahedra, stretching vibrations of Cu2+–O, Mg–O, Ca–O and Na–O and lattice modes. No bands with frequencies > 930 cm−1 that indicates that groups with O–H, C–H, C–O, N–H, N–O and B–O bonds are absent in polyarsite.
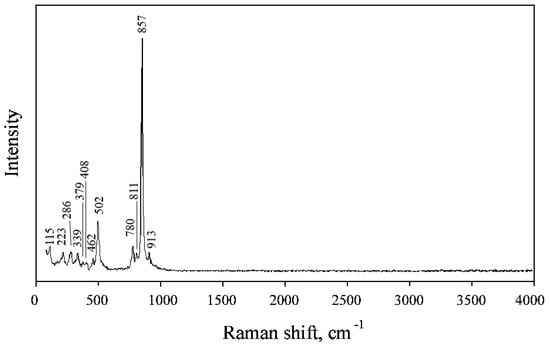
Figure 3.
The Raman spectrum of polyarsite.
3.4. Chemical Composition
The chemical data for polyarsite in wt.% are reported in Table 1.

Table 1.
Chemical composition (in wt.%) of polyarsite.
The empirical formula calculated on the basis of 19 (O+F+Cl) atoms per formula unit (apfu) is Na7.04Ca1.00Mg0.92Cu2.06Fe3+0.06(As3.96S0.05)Σ4.01O16.28F1.66Cl1.06.
The ideal formula is Na7CaMgCu2(AsO4)4F2Cl which requires Na2O 22.1, CaO 5.7, MgO 4.1, CuO 16.2, As2O5 46.9, F 3.9, Cl 3.6, –O=(F,Cl) −2.5, total 100 wt.%.
A low value of the Gladstone–Dale compatibility index [10] confirms the correctness of the obtained data: 1 − (Kp/Kc) = −0.030 (rated as excellent).
3.5. X-Ray Crystallography and Crystal Structure Determination
The XRD data of polyarsite obtained from powder sample (for CoKα) are given in Table 2. Unit cell parameters refined from the powder data are as follows: a = 8.436(2), b = 10.097(3), c = 10.717(4) Å, β = 90.86(2)° and V = 912.8(8) Å3.

Table 2.
Powder X-ray diffraction data (d in Å) of polyarsite.
Crystal data, data collection information and structure refinement details for polyarsite are reported in Table 3. Coordinates and equivalent thermal displacement parameters of atoms are given in Table 4, selected interatomic distances in Table 5 and bond valence calculations in Table 6.

Table 3.
Crystal data, data collection information and single-crystal structure refinement details for polyarsite.

Table 4.
Coordinates and equivalent displacement parameters (Ueq, in Å2) of atoms and site multiplicities (Q) for polyarsite.

Table 5.
Selected interatomic distances (Å) in the structure of polyarsite.

Table 6.
Bond valence calculations for polyarsite.
4. Discussion
Polyarsite is unique in terms of crystal structure: its structure type is novel. The structure of polyarsite (Figure 4a) is based on the (1 0 1) heteropolyhedral layers formed by CuO4Cl elongated tetragonal pyramids, AsO4 tetrahedra and MgO4F2 octahedra (Figure 4b). According to [13], five-fold coordinated Cu2+ ions preferably occupy an apically strongly elongated square pyramids, whereas electrostatic calculations slightly favour an elongated trigonal bipyramid compared with a compressed square pyramid. Two CuO4Cl pyramids share common Cl vertex to form Cu2O8Cl dimer which shares all eight oxygen vertices with eight AsO4 tetrahedra. Each As-centred tetrahedron is linked via two oxygen vertices with Cu-centred polyhedra belonging to adjacent dimers and one vertex with Mg-centred octahedron. MgO4F2 octahedra share all oxygen vertices with AsO4 tetrahedra. Adjacent Cu-Mg-As-O-F-Cl layers are linked via CaO8 cubes to form a pseudo-framework in which each CaO8 cube shares four common edges with four As-centred tetrahedra (two tetrahedra belonging to one layer and two of the neighbouring one) and two edges with CuO4Cl tetragonal pyramids from adjacent layers (Figure 5).
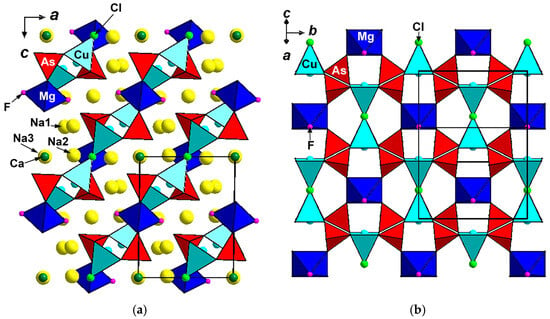
Figure 4.
The crystal structure of polyarsite projected along the b axis (a) and heteropolyhedral layer in this structure (b). The unit cell is outlined.
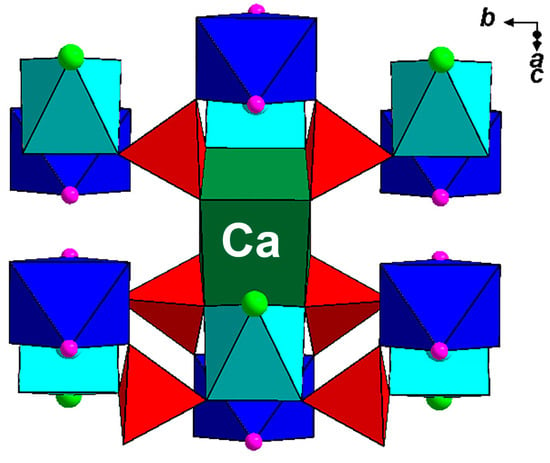
Figure 5.
The location of the CaO8 cube, sharing common edges with AsO4 tetrahedra and CuO4Cl tetragonal pyramids, between adjacent Cu-Mg-As-O-F-Cl layers in the structure of polyarsite. For legend see Figure 4.
Three crystallographically non-equivalent octahedrally coordinated Na sites are localized in cavities of the pseudo-framework. The Na(1) sites centre strongly distorted Na(1)O4FCl octahedra, located in the [0 1 0] channels inside the pseudo-framework; these octahedra share common Cl vertices and form isolated from each other dimers [Na2O8F2Cl] (Figure 6a). The Na(2) sites are located between F- anions and occupy distorted Na(2)O4FCl octahedra connected with each other via common Cl–O(2)–O(2) faces forming dimers. Two adjacent dimers share common Cl vertex to form four-polyhedral cluster. These clusters are linked via common F vertices forming ribbons [Na4O12F2Cl]∞ running along the b axis (Figure 6b). Na(3)-centred octahedra Na(3)O4F2 isolated from each other are located between CaO8 cubes (Figure 6c). The structural fragments built by Na-centred octahedra share edges and faces and form a complicated cationic motif (Figure 6d).
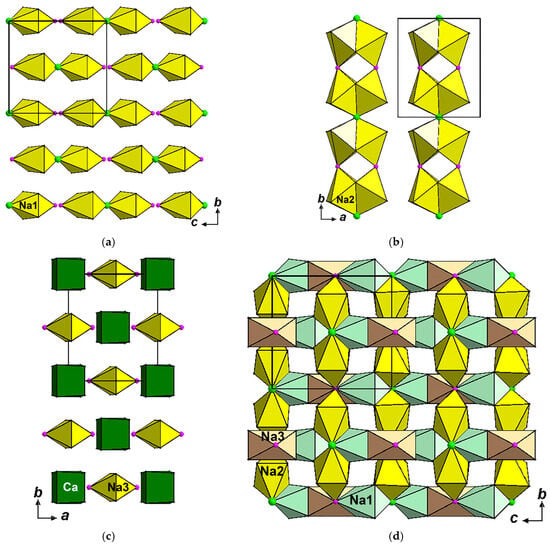
Figure 6.
Arrangement of Na-centred octahedra in the crystal structure of polyarsite: Na(1)-centred octahedra (a), Na(2)-centred octahedra (b), Na(3)-centred octahedra with CaO8 cubes (c) and the motif formed by all Na-centred octahedra (d).
Only two arsenate minerals with both species-defining F and Cl are known, namely axelite Na14Cu7(AsO4)8F2Cl2 [14] and lehmannite Na18Cu12TiO8(AsO4)8FCl5 [15,16]. These fluoro-chloro-arsenates were recently discovered in the same Arsenatnaya fumarole and both are Na- and Cu-rich, like polyarsite Na7CaMgCu2(AsO4)4F2Cl; however, they strongly differ from polyarsite and from one another in chemistry and crystal structure.
This mineral association forms at temperatures not lower than 350 °C. Polyarsite can be deposited directly from fumarole gas as a volcanic sublimate, but we assume that it is more likely to be formed as a result of the interaction of hot gas with basaltic scoria, which could be the source of Ca and Mg that demonstrate in fumarolic systems very low volatility at temperatures up to 500 °C [17].
Author Contributions
Conceptualization, I.V.P., N.V.Z. and D.Y.P.; methodology, I.V.P., N.V.Z., A.A.A. and S.N.B.; fieldworks I.V.P., A.A.A., A.G.T., E.G.S. and E.S.Z.; investigation, I.V.P., N.V.Z., A.A.A., D.I.B., M.F.V., V.O.Y., S.N.B. and A.G.T.; writing—original draft preparation, I.V.P. and N.V.Z.; writing—I.V.P., N.V.Z. and M.F.V.; visualization, N.V.Z. and V.O.Y.; supervision, I.V.P. and D.Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work in part of mineralogical studies, crystal chemical analysis and crystal structure solution was supported by the Russian Science Foundation, grant no. 25-17-00005 (I.V.P., N.V.Z., M.F.V. and D.Y.P.). The powder XRD study was performed at the Centre for X-Ray Diffraction Research of the Science Park of St. Petersburg State University within the framework of project 125021702335-5.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fedotov, S.A.; Markhinin, Y.K. (Eds.) The Great Tolbachik Fissure Eruption; Cambridge University Press: New York, NY, USA, 1983. [Google Scholar]
- Pekov, I.V.; Agakhanov, A.A.; Zubkova, N.V.; Koshlyakova, N.N.; Shchipalkina, N.V.; Sandalov, F.D.; Yapaskurt, V.O.; Turchkova, A.G.; Sidorov, E.G. Oxidizing-type fumaroles of the Tolbachik volcano, a mineralogical and geochemical unique. Russ. Geol. Geophys. 2020, 61, 675–688. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Yapaskurt, V.O.; Belakovskiy, D.I.; Lykova, I.S.; Vigasina, M.F.; Sidorov, E.G.; Pushcharovsky, D.Y. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. I. Yurmarinite, Na7(Fe3+,Mg,Cu)4(AsO4)6. Mineral. Mag. 2014, 78, 905–917. [Google Scholar] [CrossRef]
- Pekov, I.V.; Koshlyakova, N.N.; Zubkova, N.V.; Lykova, I.S.; Britvin, S.N.; Yapaskurt, V.O.; Agakhanov, A.A.; Shchipalkina, N.V.; Turchkova, A.G.; Sidorov, E.G. Fumarolic arsenates—A special type of arsenic mineralization. Eur. J. Mineral. 2018, 30, 305–322. [Google Scholar] [CrossRef]
- Shchipalkina, N.V.; Pekov, I.V.; Koshlyakova, N.N.; Britvin, S.N.; Zubkova, N.V.; Varlamov, D.A.; Sidorov, E.G. Unusual silicate mineralization in fumarolic sublimates of the Tolbachik volcano, Kamchatka, Russia—Part 1: Neso-, cyclo-, ino- and phyllosilicates. Eur. J. Mineral. 2020, 32, 101–119. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysAlisPro Software System, Version 1.171.37.34; Agilent Technologies UK Ltd.: Oxford, UK, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing the X-Ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zap. Ross. Mineral. Obs. 2017, 146, 104–107. (In Russian) [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Mandarino, J.A. The Gladstone–Dale compatibility of minerals and its use in selecting mineral species for further study. Can. Mineral. 2007, 45, 1307–1324. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Struct. Sci. 2015, 71, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Brese, N.E.; O`Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Reinen, D. Cu2+, a chameleon in coordination chemistry. Comments Inorg. Chem. 1983, 2, 227–246. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Agakhanov, A.A.; Yapaskurt, V.O.; Belakovskiy, D.I.; Britvin, S.N.; Sidorov, E.G.; Kutyrev, A.V.; Pushcharovsky, D.Y. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. XIX. Axelite, Na14Cu7(AsO4)8F2Cl2. Mineral. Mag. 2023, 87, 109–117. [Google Scholar] [CrossRef]
- Britvin, S.N.; Pekov, I.V.; Yapaskurt, V.O.; Koshlyakova, N.N.; Göttlicher, J.; Krivovichev, S.V.; Turchkova, A.G.; Sidorov, E.G. Polyoxometalate chemistry at volcanoes: Discovery of a novel class of polyoxocuprate nanoclusters in fumarolic minerals. Sci. Rep. 2020, 10, 6345. [Google Scholar] [CrossRef] [PubMed]
- Pekov, I.V.; Britvin, S.N.; Yapaskurt, V.O.; Koshlyakova, N.N.; Polekhovsky, Y.S.; Göttlicher, J.; Chukanov, N.V.; Vigasina, M.F.; Krivovichev, S.V.; Turchkova, A.G.; et al. Arsmirandite, Na18Cu12Fe3+O8(AsO4)8Cl5, and lehmannite, Na18Cu12TiO8(AsO4)8FCl5, new mineral species from fumarole exhalations of the Tolbachik volcano, Kamchatka, Russia. Zap. Ross. Mineral. Obs. 2020, 149, 1–17. [Google Scholar] [CrossRef]
- Symonds, R.B.; Reed, M.H. Calculation of multicomponent chemical equilibria in gas-solid-liquid systems; calculation methods, thermo-chemical data, and applications to studies of high-temperature volcanic gases with examples from Mount St. Helens. Am. J. Sci. 1993, 293, 758–864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.