Abstract
Clay minerals are promising candidates for caffeine removal due to their environmental friendliness and natural abundance. In this study, a commercially available bentonite was modified by Na+ exchange and characterized using Fourier transform infrared spectroscopy, X-ray diffractometry, scanning electron microscopy, zeta potential measurements, and specific surface area analysis. Caffeine adsorption was rapid, reaching equilibrium within 15 min. Adsorption isotherms for caffeine and its metabolites (theobromine, paraxanthine, and theophylline) in pure water were analyzed at 25.0 ± 0.5 °C using Langmuir and Freundlich models, both individually and in mixtures. Only caffeine exhibited favorable adsorption behavior, fitting the Langmuir equation, which allowed for the determination of a maximum adsorption capacity of 20 ± 3 mg/g, regardless of metabolite presence. The removal exceeded 85% of the caffeine from a 5.0 mg/L solution. The adsorption affinity of the studied compounds toward Na+-exchanged bentonite followed the order: caffeine >>> theobromine > paraxanthine ~ theophylline. The modified bentonite was then tested for caffeine removal from beverages and synthetic urine, achieving removal efficiencies exceeding 87%. To our knowledge, this is the first study investigating the effect of major caffeine metabolites on adsorption rates across different sample matrices, such as artificial urine, cola soda, soluble coffee, energy drinks, green tea, and yerba mate.
1. Introduction
Caffeine (CF) is the most widely consumed psychostimulant worldwide [1]. Because of its frequent detection, measurable concentrations, and stability, it serves as an indicator of emerging pollutants [2]. This substance is a naturally occurring alkaloid belonging to the xanthine class, found in over 60 plant species. The main sources of caffeine include coffee beans (Coffea arabica and Coffea robusta) and tea leaves (Camellia siniensis), as well as kola nuts (Cola acuminate), cocoa beans (Theobroma cacao), yerba mate (Ilex paraguariensis), and guarana berries (Paullinia cupana). Most intake sources are beverages such as coffee, tea, soda, and energy drinks. Products containing cocoa, analgesic medications, and dietary supplements also contribute lesser amounts to the diet [1,2].
In humans, caffeine is rapidly and completely absorbed in the gastrointestinal tract, and 99% of the quantity ingested is absorbed within 45 min into the bloodstream and becomes metabolized in the liver, where demethylation forms three major metabolites: 1,7-dimethylxanthine (paraxanthine, PX), 3,7-methylxanthine (theobromine, TB), and 1,3-methylxanthine (theophylline, TP) (Figure S1); with distributions of 84%, 12%, and 4%, respectively. Humans primarily excrete caffeine through the kidneys, with only 0.5%–2% of caffeine remaining unmetabolized in urine [3]. The effects of caffeine on humans can be positive, like an increase in alertness, attention, and cognition, or negative, like sleep disturbances, irritability, and anxiety. All three major metabolites are biologically active [3,4].
Caffeine accumulates in tissues of aquatic organisms, such as clams, fish, algae, and plants, after prolonged exposure to contaminated water. Residues at environmental concentrations (ng/L-mg/L) can harm aquatic organisms [5].
Various methods, such as advanced oxidative processes, bioremediation, membrane separation, and adsorption techniques, remove caffeine from aqueous media [6]. Among these processes, adsorption is the cheapest and easiest to operate. Adsorbents, such as activated carbon, polymeric resins, biopolymers, carbon-based materials (graphene and carbon nanotubes), agricultural waste (either as precursors for activated carbon or used directly), composites, biochar, soils, sediments, and aluminosilicate clay minerals, have been described to remove caffeine [6,7,8,9].
Clay minerals are useful for adsorption because they have a high surface area, high adsorption capacity, low cost, and ready availability. They can be used in their natural form or with modifications based on processes requiring less energy than the production of activated carbon [10]. In addition, they are also abundant in nature and, therefore, environmentally compatible [11]. Natural clay minerals remove organic cations and nonionic substances through mechanisms of ion exchange, hydrogen bonding, nonspecific van der Waals interactions, proton transfer reactions in the clay phase, and electron donor—electron acceptor interactions [12]. The adsorption of anionic compounds, as well as certain neutral compounds, necessitates surface chemical modification using polycations [13,14] or quaternary ammonium salts with long alkyl chains [11,15].
Bentonite is primarily composed of montmorillonite, a hydrated layered aluminosilicate mineral belonging to the monoclinic crystal system, with the following chemical formula: Al2O3•4SiO2•nH2O. It has a 2:1 layered structure, consisting of an aluminum–oxygen octahedral layer sandwiched by two silicon–oxygen tetrahedral layers [16]. Like other smectites, montmorillonite has permanent negative charges generated by isomorphic substitutions of Si4+ by Al3+ in the tetrahedral sheets and of Al3+ by Mg2+ in the octahedra. Cations such as Na+, Ca2+, and Mg2+ in the interlayer keep the electroneutrality and are easily exchangeable [11].
Among the aluminosilicates, the smectite group stands out. Within the category of organically modified smectites, research has explored the use of saponite modified with n-hexadecyltrimethylammonium bromide and/or 3-aminopropyltriethoxysilane for the adsorption and removal of caffeine from aqueous solutions [17]. Similarly, montmorillonite modified with benzylammonium and neostigmine has also been investigated for its effectiveness in caffeine adsorption from water [18]. Among calcined smectites, studies have reported the use of montmorillonite [19] and bentonites [20,21,22,23]. Additionally, studies have investigated the adsorption of caffeine using unmodified montmorillonites in water [24,25], in coffee extracts [26], and in tea extracts [27]: the latter two with the aim of decaffeination. To date, no studies have reported caffeine adsorption using bentonites without thermal modification nor the adsorption of caffeine metabolites by any aluminosilicate material.
Aiming to fill this knowledge gap, the present work describes a straightforward preparation of a homoionic Na+ bentonite for the adsorption of caffeine (CF) in the presence and absence of its three main demethylated metabolites—theobromine (TB), paraxanthine (PX), and theophylline (TP). The adsorbent material underwent characterization, and the influence of contact time, dosage, pH, and ionic strength on the caffeine’s adsorption rate was evaluated. Subsequently, the material was applied to samples of beverages, herbal products, and synthetic urine.
2. Materials and Methods
2.1. Reagents and Instrumentation
HPLC-grade methanol and ethanol were obtained from Merck KGaA (Darmstadt, Germany). CF, PX, TB, and TP solid standards were obtained from Merck KGaA (Darmstadt, Germany). All mobile phases were vacuum filtered by a 0.45 µm nylon filter. The ultrapure water (resistivity > 18 MΩ cm) used was deionized from a Simplicity 185 system from Millipore (Billerica, MA, USA).
Bentonite was obtained from Sigma-Aldrich (Lot MKCR 5849, CAS 1302-78-9), with 100% of the particles smaller than 74 μm and 99.75% smaller than 44 μm, with a mean particle size of 5.45 μm. The cation exchange capacity lies within a 90–120 cmol/kg range.
All reagents used in this work were of the analytical grade from Merck, Sigma, or Aldrich. Stock solutions of CF, TP, PX (1000 µg/mL), and TB (500 µg/mL) were prepared by dissolving the solid compounds in deionized water and stored at 4 °C. Working solutions of 0.5–100 µg/mL were prepared daily in ultrapure water by diluting the stocks.
Chromatographic experiments were made in a Dionex Ultimate 3000 Dual Micro LC system (Dionex Softron GmbH, Thermo Fisher Scientific, Germering, Germany) coupled to an MWD-3000 UV–VIS detector. System control, acquisition, and data processing were performed with Chromeleon 6.8 software. An Onyx Monolithic C18 chromatographic column (100 × 3.0 mm i.d) with an Onyx Monolithic C18 (5 × 4.6 mm i.d.) guard column was used. Peak identification was performed by comparing the retention times with the standards of pure compounds.
An orbital shaker from Marconi (Piracicaba, SP, Brazil), set at 25.0 ± 0.5 °C, was employed in the adsorption/desorption experiments. They were all performed in 15 mL polypropylene centrifuge tubes placed horizontally in the shaker. The dispersions were passed through 0.22 μm syringe filters. A Thermo Scientific Sorvall ST16R centrifuge was employed in all the centrifugation processes at 25 °C. A freeze dryer (Thermo Fisher Scientific, Waltham, MA, USA) was used in the last step of the adsorbent preparation.
Nitrogen adsorption–desorption analyses were conducted at 77K after degassing at 150 °C for 18 h using a Quantachrome Instruments Nova 1200e surface analyzer (Boynton Beach, FL, USA). The specific surface areas were measured using the Brunauer–Emmett–Teller (BET) method, and the pore volumes were calculated employing the Barrett–Joyner–Halenda (BHJ) method.
Adsorbent drying was performed in a ModulyoD freeze dryer (Thermo Fisher Scientific) at −45 °C and 500 μbar for five days. Scanning electron microscopy (SEM) was performed with a Fesem Jeol JSM-740 1 F instrument (Jeol Ltd, Tokyo, Japan), and samples were covered with a 10 nm layer of gold.
X-ray diffraction (XRD) experiments were performed in a Rigaku Miniflex instrument using a Cu Kα radiation source at voltage = 30 kV, current = 15 mA, scattering slit = 4.2°, and receiving slit = 0.3 mm. Continuous scan mode was used at scan speed = 0.300°/min, sampling width = 0.050°, and a range from 5.000 to 90.000°. The basal spacing (d001) was calculated using Bragg’s Law.
Zeta potential determination and zeta potential titration were performed in a Malvern Zetasizer Nano (Malvern, UK). Fourier transform infrared (FT-IR) spectra were obtained in a PerkinElmer Frontier spectrometer using KBr pellets over a spectral range from 4000 to 400 cm−1 with a resolution of 1 cm−1. The attribution of FT-IR bands was based on reference tables and spectral data.
2.2. Chromatographic Conditions
A green chromatographic method [4] was used for the mixtures of all analytes and samples. It comprised a gradient of ethanol (solvent B) in ultrapure water (solvent A) at a constant flow rate of 0.75 mL/min. The separation program consisted of isocratic elution at 6% B for up to 3 min, followed by a linear gradient from 6% to 10% B (v/v) over 2 min, then returning to 6% B (v/v) at 6 min, and finally keeping the isocratic column reconditioned until 10 min. The column was kept at 35 °C. The injection volume was 2 µL, and the chromatograms were recorded at 272 nm.
Isocratic elution with 20% (v/v) methanol in water was used to quantify CF, PX, and TP in adsorption studies containing only one of the compounds. For TB, isocratic elution was performed using 15% (v/v) methanol in water.
2.3. Preparation of Na+ Exchanged Bentonite (Na-BT)
Approximately 20 g of bentonite clay mineral (BT) was equilibrated with 25 mL of 1.0 mol/L NaCl solution per gram for 24 h, followed by centrifugation (9000× g, 5 min). The solid was repeatedly washed four times with deionized water, then separated by centrifugation. The supernatant was discarded between each washing. Afterward, the clay mineral was freeze-dried and ground to <234 µm, resulting in the sodium homoionic bentonite labeled Na-BT.
2.4. Sorption and Desorption Experiments
Unless specified, no controls for pH and ionic strength were established to investigate the adsorption kinetics and to construct the adsorption isotherms. Na-BT was just dispersed in the solutions, resulting in a pH of around 9.0, which was not altered after the adsorption experiments.
For adsorption experiments, duplicates of experiments were performed individually for TB, PX, TP, CF, and a mixture containing all four analytes, employing the batch equilibration approach. The range of initial concentrations used to construct the adsorption isotherms varied between 5.0 and 100 mg/L, which is within the linear dynamic range of the analytical method and is expected to be found in the investigated samples. Approximately 30 mg (weighted with a 0.01 mg precision) of adsorbent was weighed in 15 mL polypropylene centrifuge tubes. Next, 5 mL of solutions containing the analytes, with concentrations of between 5 and 100 mg L−1 were added to the tubes, which were closed and kept under agitation for 30 min at 25 °C on an orbital shaker at 220 rpm. After that, the solid phases were separated by centrifugation for 5 min at 9000× g. Then, 1 mL of the supernatant was withdrawn, buffered with 100 μL of 10 mmol/L phosphate buffer (pH 7.0), centrifuged at 17,000× g for 10 min, filtered through polyvinylidene difluoride (PVDF) syringe filters (0.22 µm, 13 mm), and analyzed by the HPLC method.
The adsorbed amount of CF or the metabolite at a given contact time (qt) was computed as follows:
where ci is the initial concentration, ct is the free concentration after the given contact time (t) with the adsorbent, V is the volume of solution dispersing the adsorbents, and m is the adsorbent’s mass. At equilibrium, qt turns to qe, and ct becomes ce as the free equilibrium concentration [11].
The removal percentage was calculated as follows:
The partition coefficient (Kd) was calculated as follows:
Desorption experiments were performed by adding 5 mL of deionized water to tubes containing the adsorbing material already used for sorption. They were shaken for 24 h at 25 °C on an orbital shaker at 220 rpm. After that time, the suspensions were centrifuged, and the supernatant phase was treated as in the sorption experiments and analyzed by the chromatographic method. Desorption in organic solvents was performed in 4 mL of pure ethanol, pure acetonitrile, or methanol containing either 20% of 0.10 mol/L formic acid or 20% of 0.10 mol/L ammonium hydroxide. The tubes were shaken for 2 h at 25 °C in the same conditions as the sorption experiments.
2.5. Adsorption Isotherms Fitting
The adsorption isotherms were fitted to the Langmuir [28] (Equation (4)) and Freundlich [29] (Equation (5)) models, with Origin 8.5 software (OriginLab). The Langmuir equation can be written as follows:
where qmax is the maximum amount of solute adsorbed per unit dose of adsorbent (mg/g), and KL is the Langmuir adsorption constant (L/g) [11,30].
The Freundlich equation is given by
where KF is the Freundlich empirical constant (mg/g)/(mg/L)n, and n is the dimensionless parameter associated with the energetic heterogeneity of the adsorption sites [11,30].
To evaluate the fittings, the coefficient of determination (R2) (Equation (6)) and the chi-squared (χ2) (Equation (7)) were calculated for each regression [30]:
where qe,exp is the experimental value of q, measured at equilibrium, qe,cal is the calculated or fitted value of q, and qe,mean is the mean value of experimental q.
2.6. Samples and Sample Preparations Used to Evaluate Caffeine Removal
Cola soda, energy drink, soluble coffee, green tea Sencha loose leaves, and yerba mate were purchased in local markets. Artificial urine was prepared according to the protocol developed by Sarigul et al. [31] (Table S2).
The soda and energy drink samples were degassed in an ultrasound bath and filtered with a polyvinylidene difluoride (PVDF) syringe filter (0.22 µm; 13 mm). The green tea and yerba mate samples were extracted according to Komes et al. [32]. A mass of 0.5 g was extracted with 50 mL of water at 60 °C. The leaves were put in disposable tea filters that were withdrawn after 5 min. The volumes were adjusted back to 50 mL in volumetric flasks. Soluble coffee samples (1 g) were dissolved in 50 mL of ultrapure water at room temperature. The resulting suspension was centrifuged at 9000× g for 5 min and filtered with a PVDF syringe filter (0.22 µm; 25 mm). Before HPLC injection, the samples were diluted with ultrapure water as necessary.
Sorption experiments with the samples were performed using 30 mg of Na-BT under agitation with 5 mL of the samples or sample extracts without dilution for 30 min at 25 °C on an orbital shaker at 220 rpm. Before HPLC injection, the samples were diluted with ultrapure water as necessary.
3. Results and Discussion
The present work focused on the direct use of a Na+-exchanged bentonite because transforming a 2:1 clay mineral into its homoionic form using a univalent cation is a critical preparatory step to ensure uniformity and reproducibility in adsorption studies. Natural clays typically contain a mixture of exchangeable cations (e.g., Ca2+, Mg2+, K+, Na+), which can lead to variable interlayer chemistry and inconsistent adsorption behavior due to differing hydration properties, layer charge compensations, and cation selectivity. Na+ offers several advantages because Na+ homoionic clay minerals exhibit greater hydration and swelling capacities, improving the access to internal surfaces and increasing the surface area for adsorption. In addition, Na+ is easily displaced by organic cations, making the modified clay mineral a more effective ion exchanger. Another reason for directly studying the homoionic bentonite is to minimize the competitive effects of Ca2+ and Mg2+, which may compete with the adsorbates or bind too tightly to the mineral matrix, reducing the adsorption capacity.
3.1. Characterization
3.1.1. Zeta Potential
The pH of a 1.0 mg/mL (0.1 wt%) dispersion in water (without ionic strength control) was 7.17, resulting in a zeta potential of −32 ± 4 mV at 25 °C, confirming the expected negative charge of the 2:1 clay mineral (Figure S2). The zeta potential–pH titration curve in the pH range from 2.15 to 11.8 varied from −34.5 to −1.52 mV, remaining approximately constant at −6.6 ± 0.3 mV in the pH range from 4.6 to 10.9 (Figure S3). The insensitivity to pH of the zeta potential or electrophoretic mobility of the Na-BT slurry was also observed by Goh et al. [33] and by Callaghan and Ottewill [34]. The titration from pH 7.11 to 2.28 exhibited a zeta potential variation from −32.7 to −39 mV. The little variation of mobility with the pH agrees with the fact that edge sites do not make a substantial contribution to the charge on clay minerals such as montmorillonites, consistent with the major role the permanent charges on the faces and interlamellar space play in the control of the electrokinetic properties of these materials. In all measurements, the zeta potential was negative, but the increase in its value from about −32 to −6.6 mV after exposing the dispersion to acidic medium (Figure S3b) suggests structural changes that can affect the density of permanent charges. No isoelectric point was identified, in agreement with the results found for other Na+-exchanged bentonites [35,36]. The variation of the zeta potential as a function of the studied Na-BT was unusual in the sense that a less negative potential at a lower pH was expected, as observed by Huang et al. [37] and Goh et al. [33].
3.1.2. Specific Surface Area
Figure 1 shows the N2 adsorption and desorption isotherms for Na-BT before and after caffeine adsorption (Na-BT+CF). The calculation of the specific surface area is based on the monolayer formation of gas molecules on the surface of the bentonite, and the principle of capillary condensation determines the presence of pores, pore volume, and pore size distribution [38]. According to the IUPAC classification of adsorption isotherms, both Na-BT, before and after caffeine adsorption (Figure 1), have the type IV, characteristic of mesoporous materials. There is a correlation between the shape of the hysteresis loop and the texture (e.g., pore size distribution, pore geometry, and connectivity) of a mesoporous material, with pore sizes of between 2.0 and 50.0 nm. According to the IUPAC empirical classification of hysteresis loops, both have type H3 hysteresis, which indicates a slit-shaped pore character [38,39]. This lack of change suggests that there were no structural changes in the pores after adsorption.
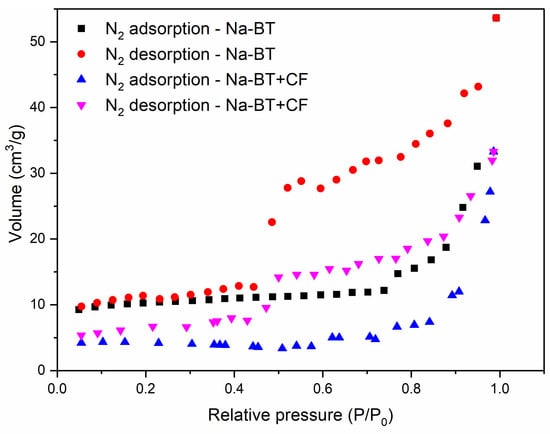
Figure 1.
Nitrogen adsorption and desorption isotherms for Na-BT and Na-BT+CF.
The specific surface area determined by N2 using the Brunauer–Emmett–Teller (BET) equation (SSAN2BET) was 38.749 m2/g for Na-BT. This value is consistent with the 40 m2/g values for Na+-dominated bentonites obtained by Kaufhold et al. [40] in similar conditions, though it is smaller than the 77.08 m2/g value obtained by Yang et al. [41] for their sodium bentonite. It is also smaller than the SSAN2BET obtained by Quintero-Jamarillo et al. [23] for their thermally modified sodium bentonite, which ranged from 57.1 to 83.1 m2/g. After caffeine adsorption, the SSAN2BET was reduced to 14.013 m2/g. This decrease can be explained by the caffeine occupying pores that N2 can no longer access. The value is similar to that observed by Oliveira et al. [21] for their polycationic bentonite containing Ca2+, Mg2+, and Na+. This hypothesis is corroborated by the decrease in pore volumes, computed by the Barrett–Joyner–Halenda (BJH) method, from 0.099 cm3/g before CF adsorption to 0.058 cm3/g after the adsorption.
3.1.3. FT-IR
The bands present in the FT-IR spectra of Na-BT are characteristic of bentonites, with montmorillonite as the dominant mineral (Figure 2): 3623 cm−1 corresponds to the stretching of the OH groups present in the lamellar structure; 3448.33 and 1638.23 cm−1 to the stretching and bending, respectively, of H–O–H from water molecules located in the interlayer region; 1047.23 cm−1 to Si–O stretching; 917.02 cm−1 to Al–Al–OH bending; 798.17 cm−1 may indicate the presence of quartz in the sample; 624.39 cm−1 corresponds to the out-of-plane vibrations of coupled Si–O and Al–O; and 523.59 and 467.18 cm−1 are attributed to the bending vibrations of Al–O–Si and Si–O–Si, respectively. The FT-IR spectrum after CF, PX, TB, and TP adsorption did not show significant changes, except a slight decrease in the signal intensity at 1638 cm−1, which may be associated with band broadening due to the overlap of signals related to the H–O–H bending of water molecules and the stretching of C=C and C=O bonds present in caffeine and metabolites (commonly observed in the region between 1650 and 1700 cm−1, respectively). This is consistent with the findings of Oliveira et al. [22] because the FT-IR spectra for their thermally modified bentonite before and after CF adsorption were very similar.
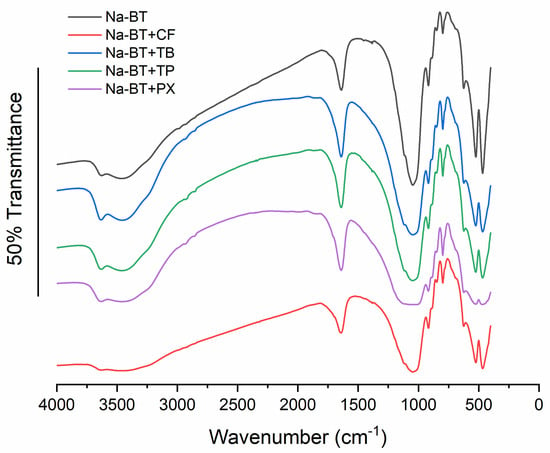
Figure 2.
FT-IR spectra of the Na-BT sorbent before and after adsorption of caffeine and its metabolites.
3.1.4. XRD
The diffraction pattern obtained for BT follows the expected result for montmorillonite (Figure 3), which is the main component of bentonites, showing characteristic diffraction peaks at 2θ values of 6.72°, 20.22°, and 35.32° [37]. The peak near 30° is associated with the quartz in the sample. Hai and colleagues found a similar result for raw bentonite [16]. The insert in Figure 3 shows the diffraction pattern for BT compared to reference ones for quartz and montmorillonite [38]. The basal spacing (d001), which was determined on the first peak in the diffractogram of BT at 7.22°, corresponds to 12.23 Å. After treatment with NaCl, the first diffraction peak of Na-BT appeared at 7.12°, corresponding to a basal spacing of 12.40 Å. This modest increase in spacing can be attributed to the higher hydration energy of Na+ ions compared to divalent cations that may have originally occupied the interlayer space. Sodium ions strongly attract water molecules, and their high hydration energy promotes the incorporation of water into the interlayer region. As a result, the hydrated Na+ ions expand the interlayer spacing by pushing apart the tetrahedral–octahedral–tetrahedral layers.
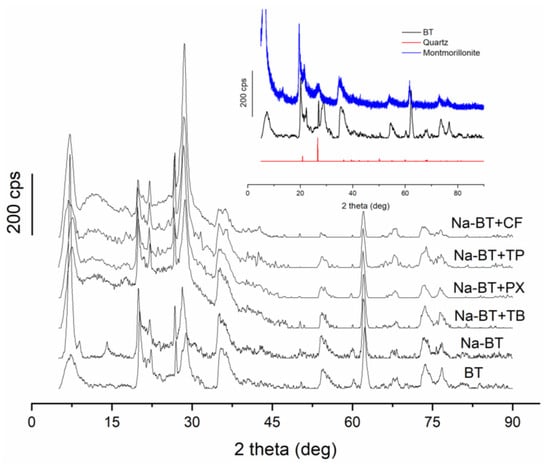
Figure 3.
XRD patterns of the raw bentonite (BT) and Na+-exchanged bentonite (Na-BT) before and after the adsorption of caffeine and metabolites. The inset compares the XRD pattern of the BT with quartz and montmorillonite [42].
After the adsorption of analytes, the first peak in the diffractograms and the d001 values were 7.45° (11.8560 Å) for Na-BT+TB, 7.60° (11.6223 Å) for Na-BT+PX, 6.80° (12.9877 Å) for Na-BT+TP, and 7.20° (12.4396 Å) for Na-BT+CF. The d001 values increased slightly after CF and TP adsorption, indicating the exchange of ions with the analyte molecules and/or water. On the other hand, the d001 values slightly decreased for PX and TB. Therefore, there is no clear trending effect on the Na-BT structure caused by the sorbed molecules.
3.1.5. Scanning Electron Microscopy
Micrographs obtained for Na-BT before and after caffeine adsorption are shown in Figure 4. Before adsorption, bentonite’s layers, pores, and irregular structure are easily observed. Yang et al. [41] found a similar lamellar structure in their sodium-modified bentonite. After adsorption, the clay mineral is much less rough with less visible pores, thus indicating the pores were occupied with caffeine, which is consistent with the findings of the BET and BJH measurements. Oliveira et al. [21] found similar results for caffeine adsorption in their thermally modified bentonite.
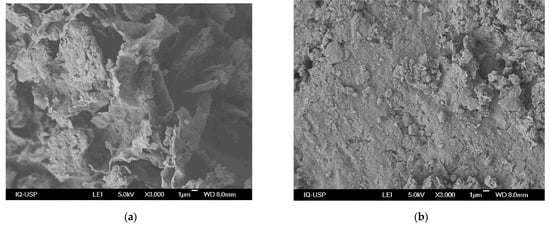
Figure 4.
Scanning electronic microscopy micrographs for Na-BT (a) before and (b) after caffeine adsorption.
3.2. Caffeine Adsorption
3.2.1. Effect of Contact Time
From 15 to 240 min, the adsorbed amount (qt) and removal rate from a 5 µg/mL (0.0255 mmol/L) caffeine solution were nearly constant (Figure 5a), showing that the adsorption was fast, suggesting it occurs predominantly at the faces and interlamellar spaces without slow diffusion into the stagnant solution inside the pores. Thus, it was not possible to derive a kinetic law from the experimental data. The contact time of 30 min was chosen for the rest of the studies to save time and provide more reproducible measurements than 15 min. Consistent with these results, Shiono et al. [27] achieved more than 99% caffeine adsorption within 5 min from a 5.2 mmol/L caffeine solution and within 20 min from the green tea extract with the same concentration onto pure montmorillonite. In another work, Shiono et al. [26] found that the equilibrium was approached within 5 min from a coffee extract’s 6.2 mmol/L caffeine concentration. Quintero-Jaramillo et al. [23] also observed rapid caffeine adsorption from 20 mg/L (0.102 mmol/L) solutions on their sodium-exchanged bentonites at several temperatures. The contact time was 120 min to ensure equilibrium conditions for all adsorbents. Their kinetic data were fitted better by the Elovich model. Oliveira et al. [22] found an adsorption equilibrium time of 40 h for their thermally modified Verde-lodo bentonite for 0.184 to 0.736 mmol/L (35.7 to 143 mg/L) caffeine solutions. One possible explanation for the discrepancy with the current study is the particle size of the bentonites used. The Verde-lodo bentonite had a medium size of 0.855 mm, while the Na-BT used in the present work has much finer particles.
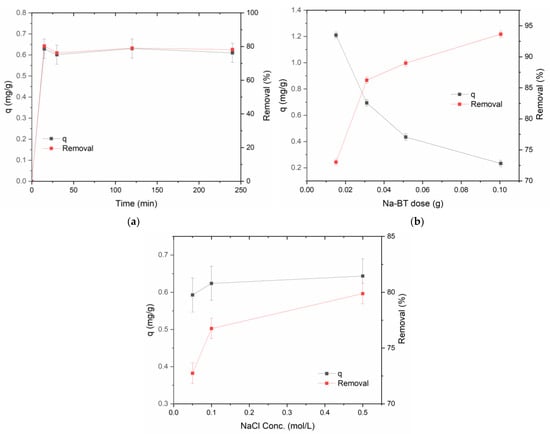
Figure 5.
The influence of (a) contact time; (b) adsorbent mass; and (c) ionic strength on the adsorbed amount (q) and the removal % of caffeine from a 5 mg/L solution. The experiments in A and C used 30 mg of Na-BT.
3.2.2. Effect of Na-BT Dose
The increase in Na-BT doses from 15 to 100 mg increased the adsorption of caffeine from a 5 µg/mL solution from 72 to 93%, as expected, since the number of active sites increases for larger adsorbent doses. On the other hand, there was a clear decrease in the adsorption capacity per unit dose of the adsorbent (q) from 1.2 to 0.25 mg/g (Figure 5b). This decrease in q values may be explained by the fact that, with the increased adsorbent dose, the fixed amount of caffeine is distributed to many more particles, so that each unit dose binds less caffeine. A dose of 30 mg was chosen for the rest of the studies because q is still high and provides more reproducible results than 15 mg.
3.2.3. Effect of Ionic Strength
For the adsorption of a 5 µg/mL caffeine solution, the NaCl concentration was raised to 0.5 mol/L, leading to a small increase in the amount (q) of sorbed CF and, consequently, an increase in the removal percentages (Figure 5c). However, considering the error bars in the q values, the variation is not significant, and it is not possible to conclude whether the main interaction is electrostatic (q diminishes with a rise in ionic strength) or covalent (q increases with a rise in ionic strength). Thus, the continuation of the studies was performed without an ionic strength control.
3.2.4. Effect of Solution Initial pH
Adsorption onto 30 mg of Na-BT dispersed in 10 mM ammonium acetate buffer with pHs of 6, 7, or 8 from 5.0 and 50 mg/L initial caffeine concentrations resulted in more than 99.6% removal, whereas at a pH of around 9.0 (not buffered), the removal was about 83%. Caffeine is predominantly neutral at this pH range [43,44], and the surface adsorption sites are negatively charged. An explanation for this behavior is not clear, but as the pH decreases, the silanols and aluminols become more and more protonated and so can form H-bonds with the caffeine molecules. Other articles have reported little effect of the pH except a small decrease in the adsorption percentage at higher pHs, consistent with the findings of the present work [23,27].
3.3. Partition Coefficient (Kd)
The partition coefficient (Equation (3)) was computed for CF, TB, TP, and PX for the initial concentrations ranging from 5 to 100 mg/L for each compound individually or in mixtures of the four compounds (Figure S4). These experiments were performed without a pH control. In all the scenarios, the affinity order based on the Kd values was consistently CF >>>> TB > PX > TP. In competitive conditions, TB retained a higher affinity than TP and PX, particularly at lower concentrations. Nevertheless, even under competitive conditions, the Kd for CF was approximately six times greater than that for TB at initial concentrations of below 40 mg/L. One possible explanation is that CF remains a neutral molecule across a broad pH range, whereas approximately 50% of PX and TB exist in their anionic forms at pH 9.0, given that their pKa values lie between 8.6 to 8.8 (Figure S1). As a result, they are likely subject to electrostatic repulsion from the permanent negatively charged Na-BT. TB is in an intermediate ionization state, with only about 10% existing in its anionic form at pH 9.0, due to its pKa of 10.0. It is well established that the deprotonation of nitrogen atoms at positions 1, 3, and 7 (Figure S1) is favored by the delocalization of the negative charge [44,45].
3.4. Adsorption Isotherms
The adsorption isotherms of each compound individually and in mixtures of the four compounds (Figure 6) suggest that only for CF the experimental data fitted well to the Langmuir equation (Equation (4)), resulting in a qmax = 20 ± 3 mg/g (0.102 mmol/g) and KL = 0.05 ± 0.02 L/g, with R2 = 0.95 and reduced chi-squared (χ2) = 0.70, for the experiment with CF (no competition with metabolites) in the solution (Table 1). In competition with the other compounds, no significant variation was observed, as the fitted parameters were qmax = 19 ± 2 mg/g (0.097 mmol/g) and KL = 0.08 ± 0.02 L/g, with R2 = 0.94 and reduced chi-squared (χ2) = 1.00, thus suggesting that the adsorption of CF onto Na-BT is not affected by the demethylated metabolites.
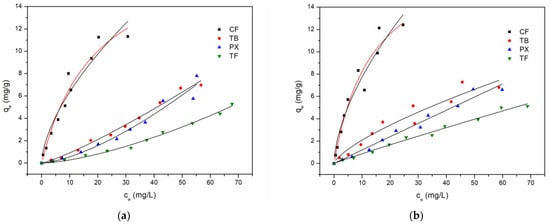
Figure 6.
The adsorption isotherms (25.0 ± 0.5 °C) of CF, TB, PX, and TP onto Na-BT, fitted by Langmuir (caffeine, red line) and Freundlich (black lines) models, for (a) each analyte separately and for (b) a mixture containing all four compounds. The initial concentrations varied from 5.0 to 100 mg L−1.

Table 1.
Freundlich parameters fitted for adsorption isotherms of CF, TB, PX, and TP individually and mixed at initial concentrations ranging from 5 to 100 mg/L. Langmuir parameters fitted for the adsorption of CF.
The Langmuir parameters obtained in the present study were lower than those reported for other aluminosilicate adsorbents (Table 2). However, this comparison should be interpreted with caution, as the fitted parameters are highly dependent on experimental conditions such as the initial concentration range, adsorbent dose, particle size, contact time, and pH. For calcined bentonites, the qmax values ranged from 0.21 to 0.41mmol/g (41 to 80 mg/g) [20,21,23]. Although calcination leads to enhanced adsorption capacity, its preparation demands much more energy consumption and is less environmentally friendly than a cation exchange. In addition, calcination, an underestimated extrapolation of the Langmuir qmax, may explain the lower values found in the present work. From the isotherms (Figure 6), it is possible to observe that the experimental measurements were not made at the saturation range, different from the works by Oliveira et al. (2019) [21], Lenzi et al. (2020) [20], and Quintero-Jaramillo et al. (2024) [23]. For instance, our maximum concentration studied was 100 mg/L (0.51 mmol/L), whereas the work by Oliveira et al. (2019) [21] used 6.0 mmol/L (1170 mg/L) (Table 2).

Table 2.
A comparison of other aluminosilicate adsorbents adjusted by the Langmuir isotherm for caffeine.
The Freundlich equation (Equation (5)) fitted the data satisfactorily (R2 > 0.90) (Table 1), confirming the affinity order suggested by the Kd values, with a much higher affinity of CF for Na-BT. The adsorption isotherms of TB, PX, and TP as individual compounds exhibited a concave–upward shape, indicating weak interactions (Figure 6a). This shape suggests a cooperative interaction in which the weakly sorbed molecule enhances the affinity of the sorbent towards the adsorbate due to adsorbate–adsorbate interactions. This hypothesis is reinforced by the adsorption isotherms of the four mixed compounds (Figure 6b). In this case, the adsorption of CF is not affected in comparison with the individual solution, although the isotherm shapes of TB, TX, and TP assumed slightly concave (TB) or linear shapes (PX and TP), with a significant increase in KF values (Table 1). The decrease of the 1/n values indicates an enhanced affinity in comparison to the parameters found with the individual compounds.
3.5. Desorption Experiments
The test tubes of Na-BT after the sorption of 50 µg/mL solutions were shaken for 24 h at 25 °C with deionized water on an orbital shaker at 220 rpm (Section 2.4). The free concentration in the supernatant implies that only 6.5% of the CF is desorbed (Table S1), independent of the presence or absence of the metabolites. TB was the second compound that was most retained, with about 29% desorption. Regarding TP and PX, desorptions from 35 to 54% were obtained, and no conclusive trend among these compounds was observed, consistent with the adsorption studies.
Aiming to use Na-BT as a sorbent for dispersive solid phase extraction (SPE) [49], the desorption experiments were repeated by adsorbing the compounds from 5 mg/L solutions, followed by elution with 4 mL of ethanol, acetonitrile, or methanol containing 20% of 0.1 M formic acid or 20% of 0.1 M ammonium hydroxide (Section 2.4), aiming to disrupt hydrogen bonding, dipole–dipole, induced dipole–dipole, and van der Waals interactions. The desorption rate was no higher than 15% in any solvent, so we concluded that the adsorption is predominantly irreversible, especially for CF, and that the Na-BT is unsuitable for developing a dispersive SPE method for CF and its metabolites. The low degree of desorption implies the limited reusability of the material because, as new experiments are conducted, fewer available adsorption sites are left to sorb new molecules.
3.6. Applications
3.6.1. Cola Soda, Energy Drink, Soluble Coffee, Green Tea, and Yerba Mate
Equilibrium concentrations after adsorption, removal rates, and sample concentrations of the analytes in cola soda, energy drink, soluble coffee, and extract concentrations of green tea and yerba mate samples are in Table 3. The chromatograms of the samples before and after the treatment with Na-BT are shown in Figure S5. The only analyte found in cola soda was caffeine, with a concentration of 97.4 μg/mL. After adsorption with Na-BT, no caffeine was detected, consistent with the qmax of 20 mg/g estimated by the Langmuir equation (Equation (4)). In the energy drink, only caffeine was found (263.5 ± 0.6 µg/mL); in this case, the removal was 87.4%, which was consistent with the high initial CF concentration. Interestingly, this 87.4% removal implies an experimental qmax = 38.3 mg/g. In soluble coffee, the paraxanthine removal rate was only 9.0%, and for caffeine, the removal rate was 42.1%. This low percentage may be explained by the high initial CF concentration (791 ± 2 µg/mL) in the extract, much higher than the 100 µg/mL used as the maximum initial concentration of the analytes in the adsorption isotherms. Based on this initial concentration and the removal percentage, it is possible to infer an experimental qmax = 55.5 mg/g for CF, thus confirming that the qmax determined by adsorption isotherms using a maximum initial concentration of 100 mg/L in pure water produced an underestimated maximum adsorption capacity in the nonlinear regression analysis. For green tea and yerba mate, the caffeine removal rates in the extracts were 99.6% and 93.7%, respectively. These results were consistent with the adsorption isotherm fitted by the Langmuir equation. The theobromine removal rates were 78.9% and 56.0%, respectively. Theophylline was not found in any of the samples, as expected [4].

Table 3.
The equilibrium concentrations after adsorption, the removal rates, and the sample concentrations of the analytes in cola soda, an energy drink, soluble coffee, and extract concentrations of green tea and yerba mate.
3.6.2. Artificial Urine
For artificial urine (Table S2), spike concentrations of 5 and 10 µg/mL were chosen for being of the same magnitude as in real urine [4,50]. The removal of all compounds was >88.3% (Table 4), and the equilibrium concentrations for CF were <LOD of the analytical method (0.10 μg/mL), indicating that the high saline concentrations in urine do not worsen the adsorption capacity and indeed enhance it, thus suggesting that hydrophobic interaction plays an important role in retention. In addition, at the urine pH of around 6, the neutral form of the metabolites predominates in the solution [44], decreasing the electrostatic repulsion that occurred at the higher pHs at which the adsorption experiments in pure water were performed.

Table 4.
Removal percentages found from spiked urine samples following the adsorption conditions described in Section 2.4.
3.7. Molecular Modeling
Initially, molecular modeling required the geometric optimization of the compounds under study. For caffeine and its metabolites, optimization was performed using Density Functional Theory (DFT) calculations with the B3LYP functional, which combines local and non-local functional components and incorporates a Hartree–Fock exchange term, enhancing the calculation accuracy. B3LYP is widely used for organic molecules [51]. A 6-31G* basis set was employed, appropriate for large systems and enabling the reliable qualitative analysis of geometry, interaction distances, and adsorption energies, using the GAMESS (General Atomic and Molecular Electronic Structure System, version 2020) and LAMMPS Molecular Dynamics Simulator software packages (version 2024).
The hybrid B3LYP functional (Becke, 3-parameter, Lee–Yang–Parr) combined with the 6-31G* basis set is widely adopted for studies involving the adsorption of organic molecules onto inorganic surfaces such as clays. This combination offers a balance between computational efficiency and accuracy, making it well suited for modeling non-covalent interactions, including hydrogen bonding and electrostatic forces, which are predominant in the adsorption of caffeine onto bentonite [52].
The B3LYP method, part of DFT, is well known for its ability to accurately describe hydrogen bonding, electrostatic interactions, and charge transfer effects and is frequently employed in adsorption studies of organic molecules on inorganic surfaces [53].
The 6-31G* basis set, a medium-sized set that includes polarization functions (*), allows for a more accurate description of the electronic distribution around the heavy atoms and functional groups in caffeine, such as carbonyl and amine groups. This basis provides a good compromise between computational costs and accuracy, making it suitable for moderately sized systems such as cluster-modeled clay–organic complexes [54].
The clay structure (Figure 7) was constructed with the assistance of the CHARMM-GUI software (version 2024), considering a solvated and homoionic environment. The interlayer spacings were adjusted to match those experimentally determined by X-ray diffraction (XRD).
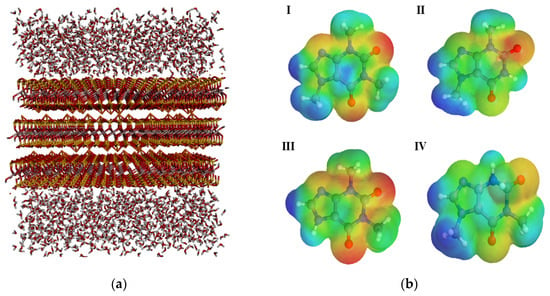
Figure 7.
Geometrically optimized structures illustrating adsorption on the (a) bentonite surface of (b) the molecules: (I) caffeine; (II) theophylline; (III) theobromine; (IV) paraxanthine. Regions of higher and lower electronegativity are represented by warm and cool colors, respectively.
Since this is an adsorption phenomenon, electrostatic interactions are crucial for understanding the mechanisms observed empirically. The theoretical calculations revealed a considerable difference in the electrostatic interaction of caffeine compared to its metabolites, which, in turn, exhibited subtle differences that nonetheless affect the desorption process, as shown in Table 5.

Table 5.
Free energies in solvent and electrostatic interaction energies obtained from theoretical calculations using the 6-31G* basis set within a DFT framework and the B3LYP functional.
Molecules with more negative electrostatic interactions experience a repulsive effect from the lamellae, which hinders the adsorption process and facilitates the desorption process. The metabolites, in their deprotonated (negative) form, exhibit a deformation in their geometry when compared to the caffeine molecule, which has a geometry closer to a planar structure. This, in turn, interferes with the adsorbent–adsorbate interaction (Figure 8) by increasing the steric hindrance at the active site of the clay.
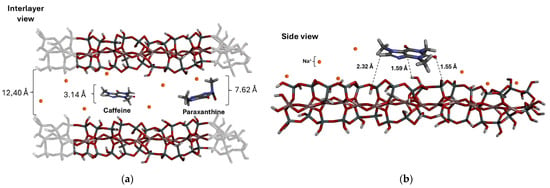
Figure 8.
Representation obtained through molecular docking showing the interaction between the caffeine (neutral) and paraxanthine (anionic) molecules and the bentonite lamellae: (a) interlayer view and (b) side view.
The comparison of the energy of the complete system with the energies of the separated parts, as shown by Equation (8), provides strong indications of the type of interaction occurring between the compounds studied.
As observed in Figure 8, the interaction between the lamellae of bentonite and caffeine occurs via hydrogen bonds, with interatomic distances typically under 3.0 Å and binding energies ranging from −5 to −20 kcal/mol [55]. The metabolites exhibited interatomic distances greater than 3.0 Å (TB = 5.1 Å; PX = 6.7 Å; TF = 5.9 Å), leading to van der Waals interactions, but these were weakened by steric hindrance and electrostatic repulsion [56].
4. Conclusions
Na+-exchanged bentonite was an efficient adsorbent for removing caffeine from aqueous solutions, including complex matrices such as beverages and artificial urine, despite having poor desorption rates, leading to scarce reusability without further treatment. Consequently, the produced Na-BT offers a promising alternative for sample decaffeination and wastewater treatment. The adsorption of the demethylated metabolites was less efficient than that of caffeine and more sensitive to pH. This was particularly evident in alkaline solutions, where anionic forms of PX, TB, and TP were prevalent, resulting in reduced adsorption due to electrostatic repulsion and steric hindrance, as confirmed by both the experimental data and the molecular modeling. The efficient removal of CF and metabolites (>88%) from synthetic, slightly acidic urine samples reinforces the potential applicability of Na-BT for wastewater treatment, as well as the need for pH adjustment to the acidic/neutral range.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/min15060573/s1. Figure S1: Chemical structures of caffeine, theobromine, paraxanthine, and theophylline with their respective pKa values. Figure S2: Three measurements of the zeta potential of a 1.0 mg/mL Na-BT dispersion using water as the dispersant (Refraction Index = 1.330, Viscosity = 0.8872 cP, Dielectric constant = 78.5) at 25.0 °C, resulting in a mean zeta potential = −31,7 mV and zeta deviation = 3.89 mV. Figure S3: Zeta potential titrations at 25 °C of a 1.0 mg/mL Na-BT dispersion in water from (a) pH 7.17 (dispersion initial pH) to 2.28 with 0.10 M HCl and (b) a new aliquot acidulated to pH 2.0 and then titrated to pH 12 with 0.4 M NaOH. Figure S4: Kd values at different initial concentrations for each analyte separately and for a mixture containing all four compounds. Figure S5: Chromatograms of the samples before and after the treatment with Na-BT: (a) cola soda; (b) yerba mate; (c) green tea; (d) soluble coffee; (e) energy drink; (f) artificial urine after 10 µg/mL spike; (g) artificial urine after 5 µg/mL spike. Table S1: Desorption percentages from Na-BT in water after adsorption of caffeine and metabolites (50 mg/L) using each compound individually or a mixture of the four compounds. Table S2: Composition of synthetic urine.
Author Contributions
Conceptualization, J.C.M.; methodology, D.M.B.G. and L.V.; validation, D.M.B.G.; formal analysis, D.M.B.G. and J.C.M.; investigation, D.M.B.G. and L.V.; resources, J.C.M.; data curation, D.M.B.G.; writing—original draft preparation, D.M.B.G. and L.V.; writing—review and editing, J.C.M.; supervision, J.C.M.; project administration, J.C.M.; funding acquisition, J.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants 306674/2021-1 from the National Council for Scientific and Technological Development (CNPq) and 2024/16458-0 from The São Paulo Research Foundation (FAPESP). D.M.B.G. acknowledges CNPq for an MSc fellowship (131226/2022-3).
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors would like to thank the laboratories of Pedro Vidinha (IQ-USP), Cassiana Seimi Nomura (IQ-USP), and Denise Petri (IQ-USP) and their students for their support on the usage of dry-freezing and surface analyzing equipment. The present work was carried out with the support of the Institute of Chemistry and its Analytical Center—Code CAIQUSP/100.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CF | caffeine |
| PX | paraxanthine |
| TB | theobromine |
| TP | theophylline |
| BT | bentonite |
| Na-BT | Na+-exchanged bentonite |
| SSAN2BET | specific surface area determined by N2 using the Brunauer–Emmett–Teller (BET) equation |
| GAMESS | General Atomic and Molecular Electronic Structure System software. |
References
- Heckman, M.A.; Weil, J.; de Mejia, E.G. Caffeine (1,3,7-Trimethylxanthine) in Foods: A Comprehensive Review on Consumption, Functionality, Safety, and Regulatory Matters. J. Food Sci. 2010, 75, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Lor, E.; Rousis, N.I.; Zuccato, E.; Bade, R.; Baz-Lomba, J.A.; Castrignanò, E.; Causanilles, A.; Hernández, F.; Kasprzyk-Hordern, B.; Kinyua, J.; et al. Estimation of Caffeine Intake from Analysis of Caffeine Metabolites in Wastewater. Sci. Total Environ. 2017, 609, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef] [PubMed]
- Goldner, D.M.B.; do Nascimento, F.H.; Masini, J.C. A Green Liquid Chromatography Method for Simultaneous Quantification of Caffeine and Its Three Major Metabolites in Urine, Drinks, and Herbal Products. Sep. Sci. Plus 2024, 7, e202400098. [Google Scholar] [CrossRef]
- Li, S.; He, B.; Wang, J.; Liu, J.; Hu, X. Risks of Caffeine Residues in the Environment: Necessity for a Targeted Ecopharmacovigilance Program. Chemosphere 2020, 243, 125343. [Google Scholar] [CrossRef]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Caffeine Removal from Aqueous Media by Adsorption: An Overview of Adsorbents Evolution and the Kinetic, Equilibrium and Thermodynamic Studies. Sci. Total Environ. 2021, 767, 144229. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Orfanos, A.G.; Manariotis, I.D.; Tatarchuk, T.; Sellaoui, L.; Bonilla-Petriciolet, A.; Mittal, A.; Núñez-Delgado, A. Removal of Caffeine, Nicotine and Amoxicillin from (Waste)Waters by Various Adsorbents. A Review. J. Environ. Manag. 2020, 261, 110236. [Google Scholar] [CrossRef]
- Fakioğlu, M.; Kalpaklı, Y. Mechanism and Behavior of Caffeine Sorption: Affecting Factors. RSC Adv. 2022, 12, 26504–26513. [Google Scholar] [CrossRef]
- Quintero-Jaramillo, J.A.; Carrero-Mantilla, J.I.; Sanabria-Gonzalez, N.R. A Review of Caffeine Adsorption Studies onto Various Types of Adsorbents. Sci. World J. 2021, 2021, 9998924. [Google Scholar] [CrossRef]
- Danish, M.; Birnbach, J.; Mohamad Ibrahim, M.N.; Hashim, R.; Majeed, S.; Tay, G.S.; Sapawe, N. Optimization Study of Caffeine Adsorption onto Large Surface Area Wood Activated Carbon through Central Composite Design Approach. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100594. [Google Scholar] [CrossRef]
- Masini, J.C.; Abate, G. Guidelines to Study the Adsorption of Pesticides onto Clay Minerals Aiming at a Straightforward Evaluation of Their Removal Performance. Minerals 2021, 11, 1282. [Google Scholar] [CrossRef]
- Borisover, M.; Davis, J.A. Adsorption of Inorganic and Organic Solutes by Clay Minerals. In Natural and Engineered Clay Barriers; Tournassat, C., Steefel, C.I., Bourg, I.C., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 6, pp. 33–70. [Google Scholar]
- Leite, S.T.; do Nascimento, F.H.; Masini, J.C. Fe(III)-Polyhydroxy Cations Supported onto K10 Montmorillonite for Removal of Phosphate from Waters. Heliyon 2020, 6, e03868. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, F.H.; Masini, J.C. Vermiculite Modified with Fe3+ Polyhydroxy Cations Is a Low-Cost and Highly Available Adsorbent for the Removal of Phosphate Ions. Minerals 2022, 12, 1033. [Google Scholar] [CrossRef]
- Teixeira, N.A.; Amorim Batista, L.F.; Schneider de Mira, P.; Scremin Miyazaki, D.M.; Grassi, M.T.; Zawadzki, S.F.; Abate, G. Modified Vermiculite as a Sorbent Phase for Stir-Bar Sorptive Extraction. Anal. Chim. Acta 2025, 1347, 343798. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; Wang, J. Experimental Study on the Heat Treatment Reaction Process of Bentonite. Sci. Rep. 2024, 14, 16649. [Google Scholar] [CrossRef]
- Marçal, L.; De Faria, E.H.; Nassar, E.J.; Trujillano, R.; Martín, N.; Vicente, M.A.; Rives, V.; Gil, A.; Korili, S.A.; Ciuffi, K.J. Organically Modified Saponites: SAXS Study of Swelling and Application in Caffeine Removal. ACS Appl. Mater. Interfaces 2015, 7, 10853–10862. [Google Scholar] [CrossRef]
- Okada, T.; Oguchi, J.; Yamamoto, K.I.; Shiono, T.; Fujita, M.; Iiyama, T. Organoclays in Water Cause Expansion That Facilitates Caffeine Adsorption. Langmuir 2015, 31, 180–187. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shiono, T.; Matsui, Y.; Yoneda, M. Changes the structure and caffeine adsorption property of calcined montmorillonite. Int. J. GEOMATE 2016, 11, 2186–2990. [Google Scholar] [CrossRef]
- Lenzi, G.G.; Fuziki, M.E.K.; Fidelis, M.Z.; Fávaro, Y.B.; Ribeiro, M.A.; Chaves, E.S.; Lenzi, E.K. Caffeine Adsorption onto Bentonite Clay in Suspension and Immobilized. Braz. Arch. Biol. Technol. 2020, 63, e20180637. [Google Scholar] [CrossRef]
- Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G.A. Equilibrium and Kinetic Studies of Caffeine Adsorption from Aqueous Solutions on Thermally Modified Verde-Lodo Bentonite. Appl. Clay Sci. 2019, 168, 366–373. [Google Scholar] [CrossRef]
- Oliveira, M.F.; De Souza, V.M.; Da Silva, M.G.C.; Vieira, M.G.A. Fixed-Bed Adsorption of Caffeine onto Thermally Modified Verde-Lodo Bentonite. Ind. Eng. Chem. Res. 2018, 57, 17480–17487. [Google Scholar] [CrossRef]
- Quintero-Jaramillo, J.A.; Carrero, J.I.; Sanabria-González, N.R. Caffeine Adsorption on a Thermally Modified Bentonite: Adsorbent Characterization, Experimental Design, Equilibrium and Kinetics. Colloids Interfaces 2024, 8, 26. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shiono, T.; Yoshimura, R.; Matsui, Y.; Yoneda, M. Influence of Hydrophilicity on Adsorption of Caffeine onto Montmorillonite. Adsorpt. Sci. Technol. 2018, 36, 967–981. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shiono, T.; Matsui, Y.; Yoneda, M. Interaction of Caffeine with Montmorillonite. Part. Sci. Technol. 2017, 37, 325–332. [Google Scholar] [CrossRef]
- Shiono, T.; Yamamoto, K.; Yotsumoto, Y.; Yoshida, A. Caffeine Adsorption of Montmorillonite in Coffee Extracts. Biosci. Biotechnol. Biochem. 2017, 81, 1591–1597. [Google Scholar] [CrossRef]
- Shiono, T.; Yamamoto, K.; Yotsumoto, Y.; Kawai, J.; Imada, N.; Hioki, J.; Naganuma, H.; Eguchi, T.; Kurihara, M.; Yoshida, A.; et al. Selective Decaffeination of Tea Extracts by Montmorillonite. J. Food Eng. 2017, 200, 13–21. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ. A New Artificial Urine Protocol to Better Imitate Human Urine. Sci. Rep. 2019, 9, 20159. [Google Scholar] [CrossRef]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green Tea Preparation and Its Influence on the Content of Bioactive Compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Goh, R.; Leong, Y.K.; Lehane, B. Bentonite Slurries-Zeta Potential, Yield Stress, Adsorbed Additive and Time-Dependent Behaviour. Rheol. Acta 2011, 50, 29–38. [Google Scholar] [CrossRef]
- Callaghan, I.C.; Ottewill, R.H. Interparticle Forces in Montmorillonite Gels. Faraday Discuss. Chem. Soc. 1974, 57, 110–118. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O. The Effect of Different Molecular Weight of Poly(Ethylene Glycol) on the Electrokinetic and Rheological Properties of Na-Bentonite Suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 93–99. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S. Electrokinetic and Rheological Properties of Na-Bentonite in Some Electrolyte Solutions. Microporous Mesoporous Mater. 2009, 117, 331–338. [Google Scholar] [CrossRef]
- Huang, W.; Leong, Y.K.; Chen, T.; Au, P.I.; Liu, X.; Qiu, Z. Surface Chemistry and Rheological Properties of API Bentonite Drilling Fluid: PH Effect, Yield Stress, Zeta Potential and Ageing Behaviour. J. Pet. Sci. Eng. 2016, 146, 561–569. [Google Scholar] [CrossRef]
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Clay Materials for Environmental Remediation; Springer International Publishing: Cham, Switerland, 2015; ISBN 9783319167114. [Google Scholar]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Klinkenberg, M.; Siegesmund, S.; Ufer, K. N2-BET Specific Surface Area of Bentonites. J. Colloid Interface Sci. 2010, 349, 275–282. [Google Scholar] [CrossRef]
- Yang, D.; Cheng, F.; Chang, L.; Wu, D. Sodium Modification of Low Quality Natural Bentonite as Enhanced Lead Ion Adsorbent. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129753. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Lichtenberg, D.; Bergmann, F.; Neiman, Z. Tautomeric Forms and Ionisation Processes in Xanthine and Its N-Methyl Derivatives. J. Chem. Soc. C 1971, 1676–1682. [Google Scholar] [CrossRef]
- Cavalieri, L.F.; Fox, J.J.; Stone, A.; Chang, N. On the Nature of Xanthine and Substituted Xanthines in Solution. J. Am. Chem. Soc. 1954, 76, 1119–1122. [Google Scholar] [CrossRef]
- Blanco, M.; Valverde, I. Electrophoretic Behaviour of Pharmacologically Active Alkylxanthines. J. Chromatogr. A 2002, 950, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.L.; Ovejero, G.; Rodríguez, A.; Álvarez, S.; García, J. Study of Natural Clay Adsorbent Sepiolite for the Removal of Caffeine from Aqueous Solutions: Batch and Fixed-Bed Column Operation. Water Air Soil. Pollut. 2013, 224, 1466. [Google Scholar] [CrossRef]
- Cabrera-Lafaurie, W.A.; Román, F.R.; Hernández-Maldonado, A.J. Transition Metal Modified and Partially Calcined Inorganic-Organic Pillared Clays for the Adsorption of Salicylic Acid, Clofibric Acid, Carbamazepine, and Caffeine from Water. J. Colloid Interface Sci. 2012, 386, 381–391. [Google Scholar] [CrossRef]
- Sakuma, H.; Tamura, K.; Hashi, K.; Kamon, M. Caffeine Adsorption on Natural and Synthetic Smectite Clays: Adsorption Mechanism and Effect of Interlayer Cation Valence. J. Phys. Chem. C 2020, 124, 25369–25381. [Google Scholar] [CrossRef]
- Zarpon, L.; Abate, G.; dos Santos, L.B.O.; Masini, J.C. Montmorillonite as an Adsorbent for Extraction and Concentration of Atrazine, Propazine, Deethylatrazine, Deisopropylatrazine and Hydroxyatrazine. Anal. Chim. Acta 2006, 579, 81–87. [Google Scholar] [CrossRef]
- Zambonin, C.G.; Aresta, A.; Palmisano, F. Determination of Methylxanthines in Urine by Liquid Chromatography with Diode Array UV Detection. J. Pharm. Biomed. Anal. 2004, 36, 621–624. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Cygan, R.T. Molecular Simulation of Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 405–423. [Google Scholar] [CrossRef]
- Johnson, E.R.; Otero-De-La-Roza, A. Adsorption of Organic Molecules on Kaolinite from the Exchange-Hole Dipole Moment Dispersion Model. J. Chem. Theory Comput. 2012, 8, 5124–5131. [Google Scholar] [CrossRef] [PubMed]
- Cancade, M.; Thiebault, T.; Mignon, P. Selective Adsorption of Organic Micro-Pollutants by Smectite Clays Revealed from Atomistic Simulations. Int. J. Mol. Sci. 2023, 24, 14781. [Google Scholar] [CrossRef]
- Zhang, I.Y.; Wu, J.; Xu, X. Extending the Reliability and Applicability of B3LYP. Chem. Commun. 2010, 46, 3057–3070. [Google Scholar] [CrossRef]
- Min, F.; Wang, L.; Chen, J.; Liu, C.; Ren, B.; Zhang, L.; Zhu, Y. Molecular Simulation in Surface Hydration of Clay Minerals: A Review of Theory and Applications. Miner. Miner. Mater. 2022, 1, 3. [Google Scholar] [CrossRef]
- Tantardini, C. When Does a Hydrogen Bond Become a van Der Waals Interaction? A Topological Answer. J. Comput. Chem. 2019, 40, 937–943. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).