1. Introduction
Lithium (Li) is a critical element with indispensable applications, particularly for energy storage systems and electric vehicles (EVs), both being key drivers of the increasing global demand for lithium. Lithium resources are primarily sourced from hard-rock ores and brine deposits, with supply projections indicating a growing deficit due to the rapid adoption of EVs and the rising need for battery-grade lithium [
1].
Key lithium-bearing minerals include spodumene, lepidolite, petalite, and zinnwaldite [
1]. Among these, spodumene is the most significant source, owing to its high lithium content (up to 8%, theoretically) and fluorine-free composition, which makes it particularly suitable for hydrometallurgical processing [
2,
3,
4].
Lithium is now recognized as a strategically important resource, with demand projected to grow 350% by 2030 compared to 2022 [
1]. Furthermore, according to the International Energy Agency (IEA)’s Global Critical Minerals Outlook [
5], total lithium demand is expected to increase from 165 kt in 2023 to 1326 kt by 2040, an eightfold rise.
While brine deposits are abundant in regions such as Argentina, Bolivia, Chile, China, and the United States, their geographic concentration can present supply chain vulnerabilities [
1]. In contrast, hard-rock lithium sources, particularly spodumene-bearing pegmatites, are more geographically diversified, offering improved supply resilience. Major spodumene-hosting pegmatite deposits are found in Australia, Canada, China, Zimbabwe, the United States, Brazil, Serbia, the Democratic Republic of Congo, and other regions such as Austria [
1,
6].
Spodumene exists in three distinct polymorphs: α-spodumene, which occurs naturally as a monoclinic structure with the highest density (3.27 g/cm
3) and is chemically inert and resistant to conventional extraction methods, and β-spodumene and γ-spodumene, exhibiting tetragonal and hexagonal crystal structures, respectively [
7]. To enable lithium recovery, high-temperature treatment is required to convert α-spodumene into the reactive β-phase through a two-step process: α → γ at 800–950 °C, followed by γ → β at 950–1100 °C [
1,
8,
9,
10,
11]. While the usual phase transition pathway is α → γ → β, studies have reported that the direct conversion of α → β is also possible, depending on factors such as particle fineness, the presence of amorphous phases, and the heating rate [
12]. This conversion results in the formation of large interstitial spaces in β- and γ-spodumene, allowing significant mobility of Li
+ ions, thereby enhancing lithium extraction [
13,
14].
The conventional method for extracting lithium from spodumene is sulfuric acid roasting. The acid roasting process for lithium extraction from spodumene has been known since 1950 and remains the dominant method [
15]. In this process, α-spodumene is first calcined to β-spodumene and then digested with concentrated sulfuric acid at 200–250 °C. In this digestion process, hydrogen ions (H
+) replace lithium ions (Li
+), resulting in a compound that easily dissolves in water, facilitating lithium recovery. While this method achieves high lithium recovery rates of up to 98% [
16], it has critical limitations. The calcination–sulfuric acid roasting–water leaching process is energy-intensive and generates large volumes of low-value, acidic residues, primarily acidic aluminosilicates (HAlSi
2O
6) and sodium sulfate (Na
2SO
4), along with significant levels of impurity ions (e.g., Fe
3+, Al
3+, Mg
2+, and Ca
2+), which require extensive reagent use (lime, sodium carbonate, and ion exchange resins) for purification [
17,
18,
19,
20].
To address these drawbacks, researchers have developed alternative, more sustainable methods for lithium extraction from both α- and β-spodumene. The Li extraction from β-spodumene, which first needs a phase transition from α to β, includes high-pressure leaching with reagents such as sodium sulfate (Na
2SO
4) [
18], sodium chloride (NaCl) [
19], sodium carbonate (Na
2CO
3) [
21,
22], nitric acid (HNO
3) [
23], and carbonic acid (H
2CO
3) [
20]; atmospheric leaching using phosphoric acid (H
3PO
4) [
24] and hydrofluoric acid (HF) [
25]; chlorination [
17]; and salt roasting with reagents such as calcium chloride (CaCl
2) [
26], sodium carbonate–sodium chloride (Na
2CO
3–NaCl) [
27], sodium fluoride (NaF) [
28], and sodium carbonate (Na
2CO
3) [
29]. Among these, the Metso-Outotec lithium hydroxide process exemplifies the industrial feasibility of high-pressure leaching, with a large-scale implementation planned at the Keliber Project in Finland [
22].
For bypassing the conventional high-temperature phase transformation to β-spodumene, several alternative methods have been developed to extract lithium directly from α-spodumene. These include salt roasting using reagents such as sodium carbonate (Na
2CO
3) [
30,
31,
32], sodium sulfate (Na
2SO
4) [
33,
34], sodium fluoride [
35,
36], and potassium fluoride (NaF) [
37,
38,
39], sodium hydroxide (NaOH) [
40,
41], ammonium bifluoride (NH
4HF
2) [
42], and calcium oxide (CaO) [
43,
44], followed by leaching; pretreatment-assisted leaching methods, including mechanical activation [
45] and alkaline decomposition with NaOH [
46,
47] or KOH [
48]; and direct leaching approaches such as high-pressure or atmospheric leaching using NaOH [
49,
50] or HF/H
2SO
4 mixtures [
51,
52,
53]. Emerging techniques like microwave-assisted calcination have also been investigated to enhance the α- to β-spodumene transformation more efficiently than conventional methods [
54,
55,
56]. Furthermore, bioleaching using microorganisms capable of producing organic or inorganic acids is being studied as a potential environmentally friendly route [
57,
58,
59].
This review critically evaluates alternative methods for lithium extraction from both α- and β-spodumene, aiming to overcome the limitations associated with conventional sulfuric acid digestion, including the high energy requirements, complex purification processes, and the generation of large volumes of low-value waste. Building upon previous studies that primarily describe individual processes, this review provides a comparative analysis of emerging technologies, highlighting their potential to simplify flowsheets, reduce reagent and energy consumption, and improve impurity management. Special attention is given to challenges such as reagent recycling, byproduct valorization, and the selective removal of impurities, which are crucial for developing more sustainable and economically viable lithium production pathways. Finally, future research opportunities are identified to support the advancement of cost-effective and environmentally responsible lithium supply chains.
2. Research Methodology
To compile this review, a comprehensive literature search was conducted using well-established academic databases, including ScienceDirect, SpringerLink, Wiley Online Library, Taylor & Francis Online, ACS Publications, MDPI, and Google Scholar. Only peer-reviewed journal articles, patents, industrial reports, and conference proceedings that were directly accessible through these platforms were considered. The primary focus was on lithium extraction from α- and β-spodumene, with particular emphasis on alternatives to the conventional sulfuric acid digestion route.
The search covered mainly literature published from 2011 to early 2025, while also including a few key earlier works to provide historical context and support foundational understanding. A wide range of keyword combinations was used to ensure thorough coverage, including lithium extraction from spodumene, α-spodumene, β-spodumene, phase transition, decrepitation, pretreatment, atmospheric leaching, high pressure leaching, salt roasting, sulfuric acid digestion, alkaline roasting, salt roasting, chlorination, mechanical activation, bioleaching, microwave-assisted calcination, direct lithium extraction, impurity removal, and Li precipitation. Articles were selected based on their relevance, originality, methodological clarity, and publication date. In total, the review includes 80 references, of which 42 were published from 2020 onward, offering a well-rounded overview of both the historical progression and recent innovations in spodumene processing.
3. Traditional Process for Extracting Lithium from Spodumene
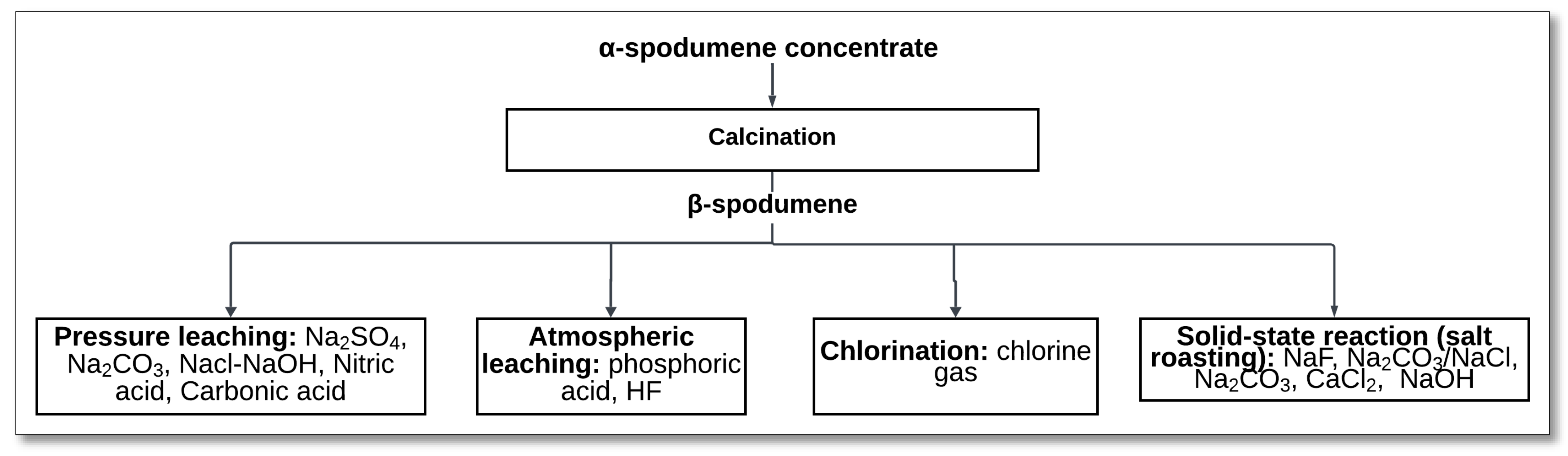
The conventional method for extracting lithium from spodumene concentrate is illustrated in
Figure 1. This well-established process begins with a phase transition of the mineral through calcination. During this step, α-spodumene undergoes a thermal treatment at temperatures between 1000 °C and 1100 °C for a duration of 0.5 to 2 h [
54]. This treatment converts the α-spodumene into its more reactive form, β-spodumene [
49,
60,
61,
62]. After the calcination, the β-spodumene is ground to increase its surface area, thereby enhancing its reactivity for the subsequent acid digestion stage. Next, the ground β-spodumene is mixed with concentrated sulfuric acid and subjected to a digestion process at 200–250 °C for approximately 30 to 60 min. This reaction, as shown in Equation (1), results in the formation of lithium sulfate along with aluminosilicate compounds as byproducts.
After digestion, the lithium sulfate is extracted by water leaching, followed by a solid–liquid (S/L) separation step to isolate the pregnant leach solution (PLS) containing the dissolved lithium ions.
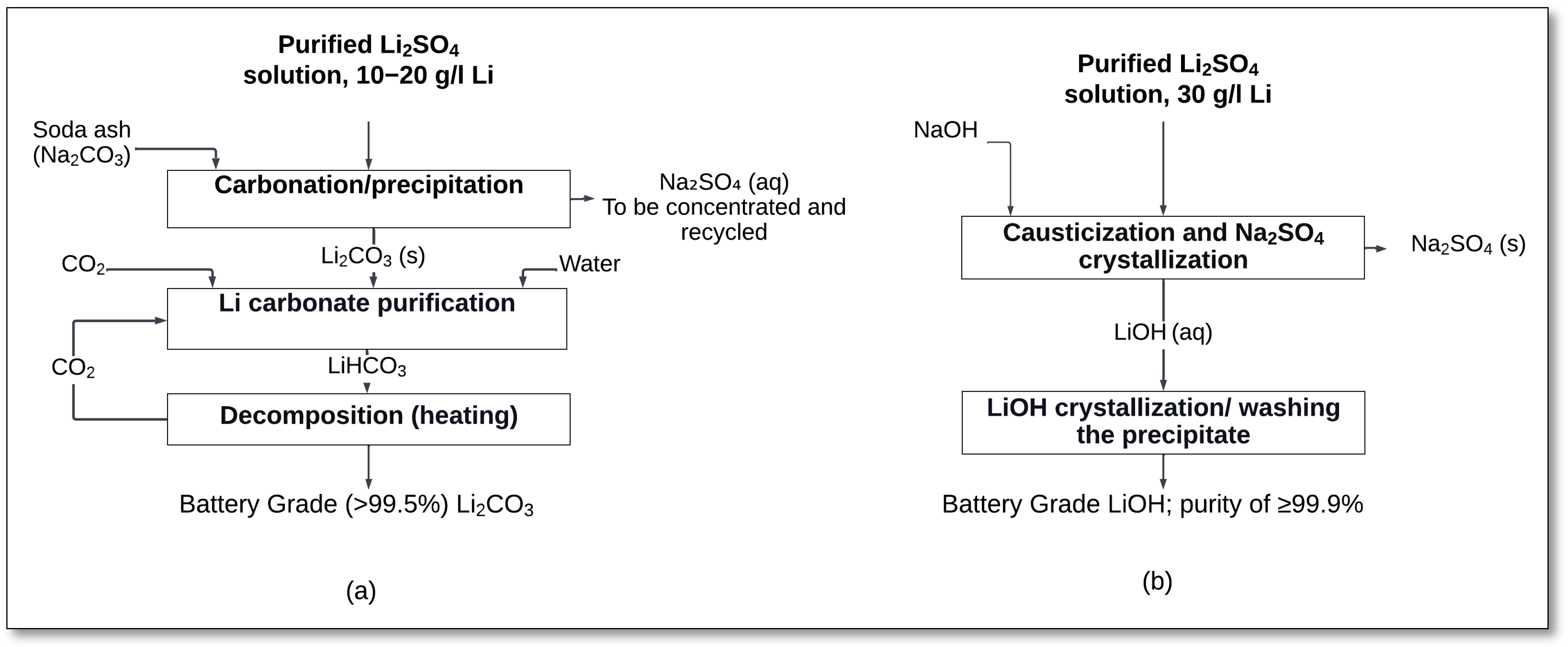
Impurity removal from the PLS is a crucial step in the process. Lime is added to precipitate iron and aluminum, while sodium carbonate is often used to remove calcium and magnesium. Further refinement can be achieved by using ion exchange resins to eliminate residual impurities. Once purified, the lithium sulfate solution can be further processed to produce high-purity lithium compounds, such as lithium carbonate or lithium hydroxide, which are used in battery production (
Figure 2) [
49,
60,
61,
62].
Figure 2a illustrates the production of lithium carbonate. The purified lithium sulfate solution (10–20 g/L Li) undergoes a carbonation/precipitation step with soda ash (Na
2CO
3), resulting in the formation of lithium carbonate, as per Equation (2).
The product is further purified through CO
2 carbonation and heating, whereby lithium carbonate reacts with CO
2 and water to form soluble lithium bicarbonate, allowing impurities to be removed. Upon heating, lithium bicarbonate decomposes back into high-purity lithium carbonate, achieving battery-grade purity (>99.5%) [
61].
In contrast,
Figure 2b outlines the production of lithium hydroxide via a causticization process. The purified lithium sulfate solution (approximately 30 g/L Li) reacts with sodium hydroxide, producing lithium hydroxide and sodium sulfate (Equation (3)), which is then separated and recycled [
60,
61].
The reaction results in the precipitation of sodium sulfate as a solid byproduct, which is filtered out, while lithium hydroxide remains in the solution. The lithium hydroxide solution is then subjected to crystallization and washing steps to obtain high-purity battery-grade lithium hydroxide (≥99.9%). This step ensures the removal of residual impurities, improving the final product’s quality for battery applications [
60,
61].
The main advantages of this process are that it is well-established and relatively easy to control, with consistent product quality. It also achieves a high lithium recovery rate, with extraction efficiencies reaching up to 98%, making it a highly effective method for lithium extraction [
16].
However, the sulfuric acid digestion process has several disadvantages. First, it leads to the extraction of a large quantity of impurities, such as iron, aluminum, magnesium, and calcium, complicating the purification of the leach liquor [
33]. As a result, additional purification steps are necessary, increasing both the cost and complexity of the process. Second, the process requires a large quantity of sulfuric acid, that not only adds to the cost but also complicates the management of waste products [
19,
49,
62].
To neutralize the acidic PLS in the purification stage, significant amounts of lime or limestone are required. In fact, the process produces approximately 0.8 tons of acidic leaching residue per ton of α-spodumene processed [
49]. These residues, which primarily consist of acidic aluminosilicate tailings (Equation (1)), have limited commercial value and are difficult to market or repurpose, creating disposal challenges. In addition, the production of low-value sulfate salts, such as Na
2SO
4 in the final LiOH or Li
2CO
3 production process (
Figure 2), adds to the waste management burden, further emphasizing the need for more sustainable and economically viable alternatives to sulfuric acid roasting [
61].
4. Lithium Extraction from α-Spodumene
Several alternative methods have been investigated for extracting lithium directly from α-spodumene, aiming to overcome the limitations of conventional sulfuric acid roasting and high-temperature phase transformation. These methods focus on either modifying the crystal structure of α-spodumene or enhancing its chemical reactivity to facilitate lithium recovery under milder or more sustainable conditions. The main approaches include the following:
Salt roasting followed by leaching: This widely explored technique involves roasting α-spodumene with additives such as sodium carbonate, sodium sulfate, sodium fluoride, sodium hydroxide, calcium oxide, or ammonium bifluoride. The resulting products are subsequently leached using water, acids, or bases to recover lithium.
Pretreatment-assisted leaching: Strategies such as mechanical activation or alkaline decomposition (e.g., with NaOH or KOH) are employed to disrupt the spodumene structure before leaching. These methods enhance lithium availability while potentially lowering the energy input and acid consumption.
Direct high-pressure or atmospheric leaching: In these approaches, α-spodumene is treated directly with reagents like NaOH or HF/H2SO4 mixtures under elevated or atmospheric pressure, bypassing the need for thermal transformation to β-spodumene.
Microwave-assisted calcination: This emerging technology uses volumetric microwave heating, often with susceptors, to convert α- to β-spodumene more efficiently than conventional calcination, improving downstream leachability.
Bioleaching: A promising but still developing area, bioleaching explores the use of microorganisms (e.g., fungi or bacteria) that generate organic or inorganic acids to solubilize lithium from α-spodumene in environmentally benign conditions.
The following sections will present these alternative methods in detail, highlighting their reaction mechanisms, operating parameters, lithium extraction efficiencies, and associated advantages or challenges.
4.1. Salt Roasting
4.1.1. Carbonate-Assisted Calcination–Sulfuric Acid/Water Leaching
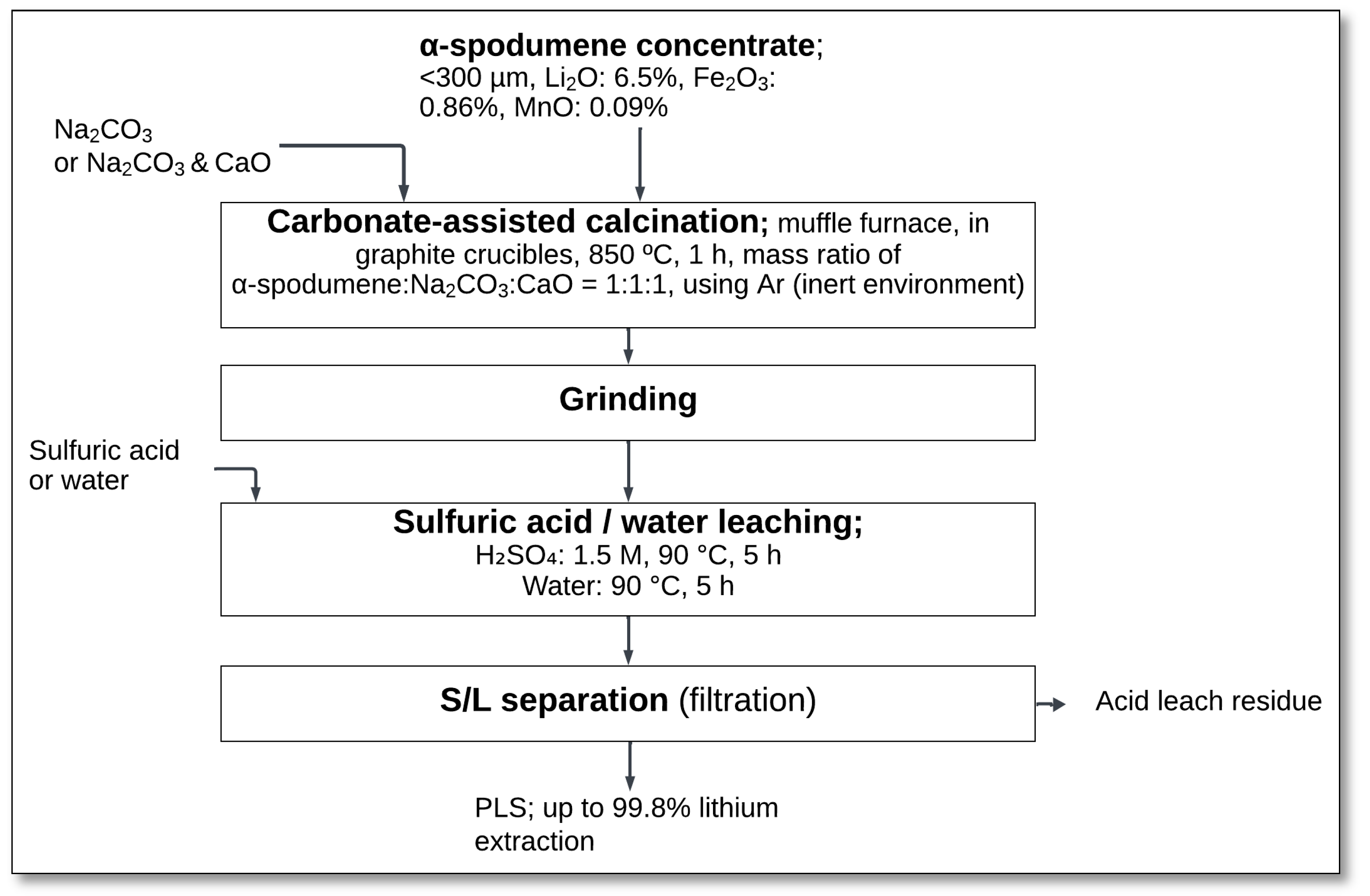
Maliachova et al. [
30] investigated lithium extraction using carbonate-assisted calcination followed by sulfuric acid or water leaching. Their process involved treating α-spodumene concentrate (<300 μm, containing 6.5% Li
2O) with sodium carbonate (Na
2CO
3) or a mixture of Na
2CO
3 and calcium oxide (CaO). The material was subjected to 850 °C for 1 h in a muffle furnace, using graphite crucibles under an inert argon atmosphere (
Figure 3). This process facilitated the reaction between spodumene and Na
2CO
3 to form lithium compounds that are more readily leachable.
Following calcination, the material was ground before sulfuric acid or water leaching. Sulfuric acid leaching was conducted at 1.5 M H
2SO
4, 90 °C, for 5 h, while water leaching was performed at 90 °C for 5 h (
Figure 1). The resulting slurry was then subjected to solid–liquid (S/L) separation through filtration, producing a PLS containing the extracted lithium ions.
X-ray diffraction (XRD) analysis by Maliachova et al. [
30] confirmed the formation of lithium silicate (Li
2SiO
3) upon Na
2CO
3 addition, which is insoluble in water but soluble in acid. The dominant phase of the calcined product in the presence of Na
2CO
3 was sodium aluminum silicate (Na
1.15Al
1.15Si
0.85O
4), while unreacted Na
2CO
3 was also detected. In spodumene–Na
2CO
3–CaO mixtures, lithium was present as a mixture of Li
2O/Li
2CO
3. The dominant phases of the calcined product in this case included a different type of sodium aluminum silicate (Na
1.55Al
1.55Si
0.45O
4) along with calcium silicate, while unreacted Na
2CO
3 was also observed.
The method achieved up to 99.8% lithium extraction efficiency when only Na
2CO
3 was added, using sulfuric acid as the leaching agent. Compared to conventional acid roasting, which requires 250 °C, this approach enabled leaching at significantly lower temperatures (90 °C). Additionally, the process eliminated the requirement for high-temperature β-spodumene conversion [
30].
In another work by Wang et al. [
31], a direct salt roasting method was developed to extract lithium from α-spodumene without converting it to the β-phase or using acid leaching. The process was begun by grinding the α-spodumene for 30 min to enhance reactivity, followed by mixing with Na
2CO
3 and Al
2O
3. This mixture was heated at 750 °C for 30 min, leading to the formation of Li
2CO
3 and NaAlSiO
4 while minimizing the formation of Li
2SiO
3. After cooling, the product was subjected to washing with water and filtration to dissolve Li
2CO
3, which was then isolated by evaporating the solution at 70 °C for 12 h. This route avoids using acid and the high-temperature β-phase conversion, achieving up to 90.4% lithium recovery. The study demonstrated an energy-efficient, environmentally friendlier approach for producing battery-grade Li
2CO
3 from spodumene using only solid reagents and a water-based separation.
4.1.2. Sodium Carbonate Roasting–NaOH Leaching
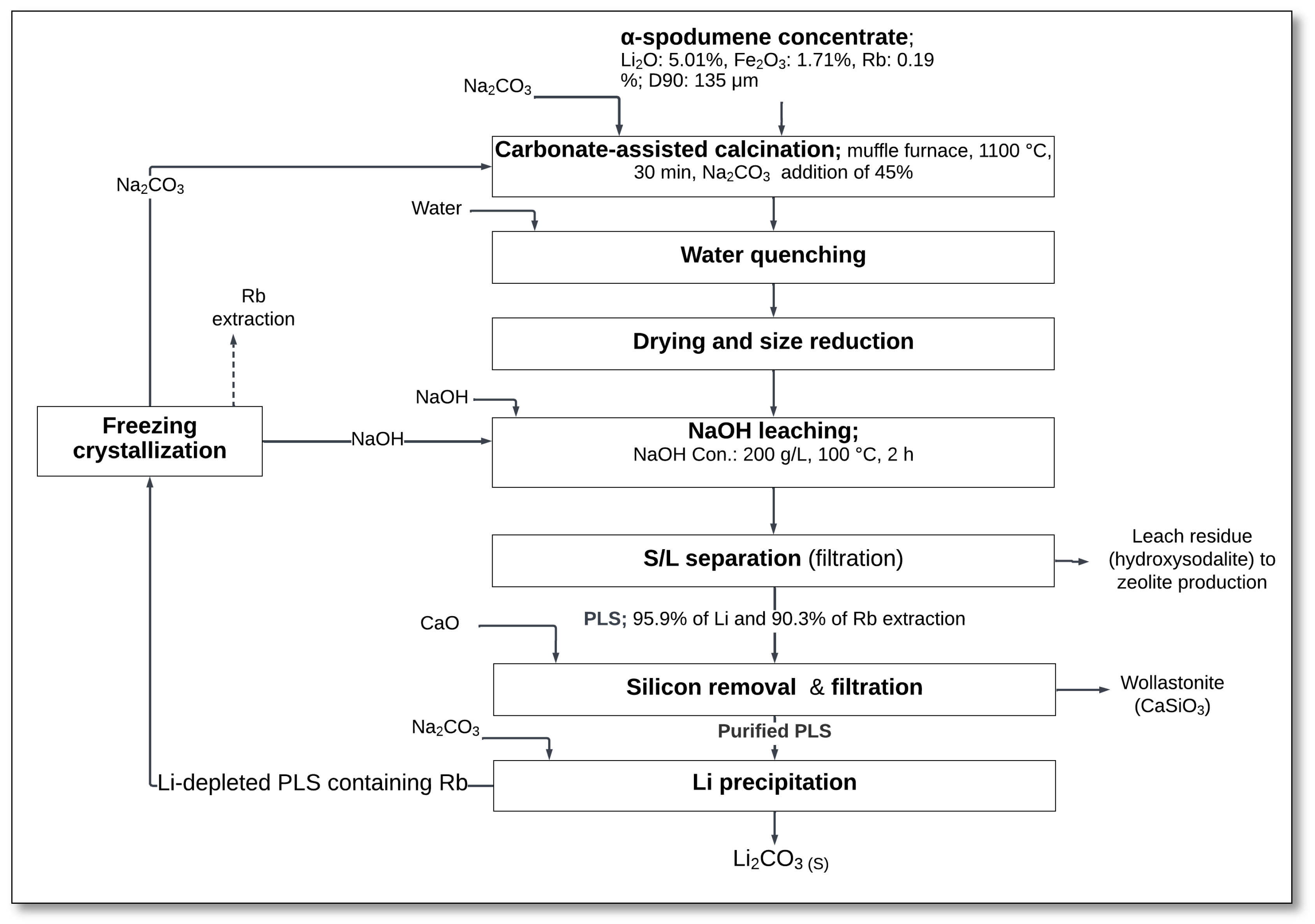
Zhou et al. [
32] investigated an alternative approach for lithium extraction from α-spodumene that involved high-temperature roasting with sodium carbonate, rapid water quenching, and selective NaOH leaching to enhance lithium and rubidium recovery (
Figure 4).
The process started with the mixing of α-spodumene concentrate with sodium carbonate, followed by calcination in a muffle furnace at 1100 °C for 30 min with Na
2CO
3 added at a 45% mass ratio. During this stage, spodumene reacted with Na
2CO
3, forming lithium silicate (Li
2SiO
3), sodium aluminum silicate (NaAlSiO
4), and sodium silicate (Na
2SiO
3) [
32]:
The calcined material was then subjected to water quenching, which disrupted the mineral structure, altered the phase composition of the roasted product, and enhanced its reactivity, ultimately improving lithium extraction efficiency.
After quenching, the material underwent NaOH leaching using a NaOH solution with a concentration of 200 g/L at 100 °C for 2 h. The leaching step facilitated the dissolution of lithium silicate, leading to the formation of lithium hydroxide (LiOH) and sodium silicate (Na
2SiO
3) [
32]:
Solid–liquid separation (S/L) was performed to recover a PLS containing lithium and rubidium. The lithium and rubidium extraction rates are reported to have reached 95.9% and 90.3%, respectively [
32].
Following leaching, silicon was removed from the pregnant leach solution through the addition of CaO, forming wollastonite (CaSiO
3). The purified PLS was then used for lithium precipitation, where Na
2CO
3 was added to precipitate lithium carbonate (Li
2CO
3):
The resulting lithium carbonate was recovered as a solid, while NaOH was regenerated and recycled back into the process. Additionally, rubidium could be extracted through further processing.
The method provides a single-step roasting and leaching approach that eliminates the need for high-pressure equipment or double roasting. Additionally, the residual hydroxysodalite produced during the process can be repurposed for zeolite production.
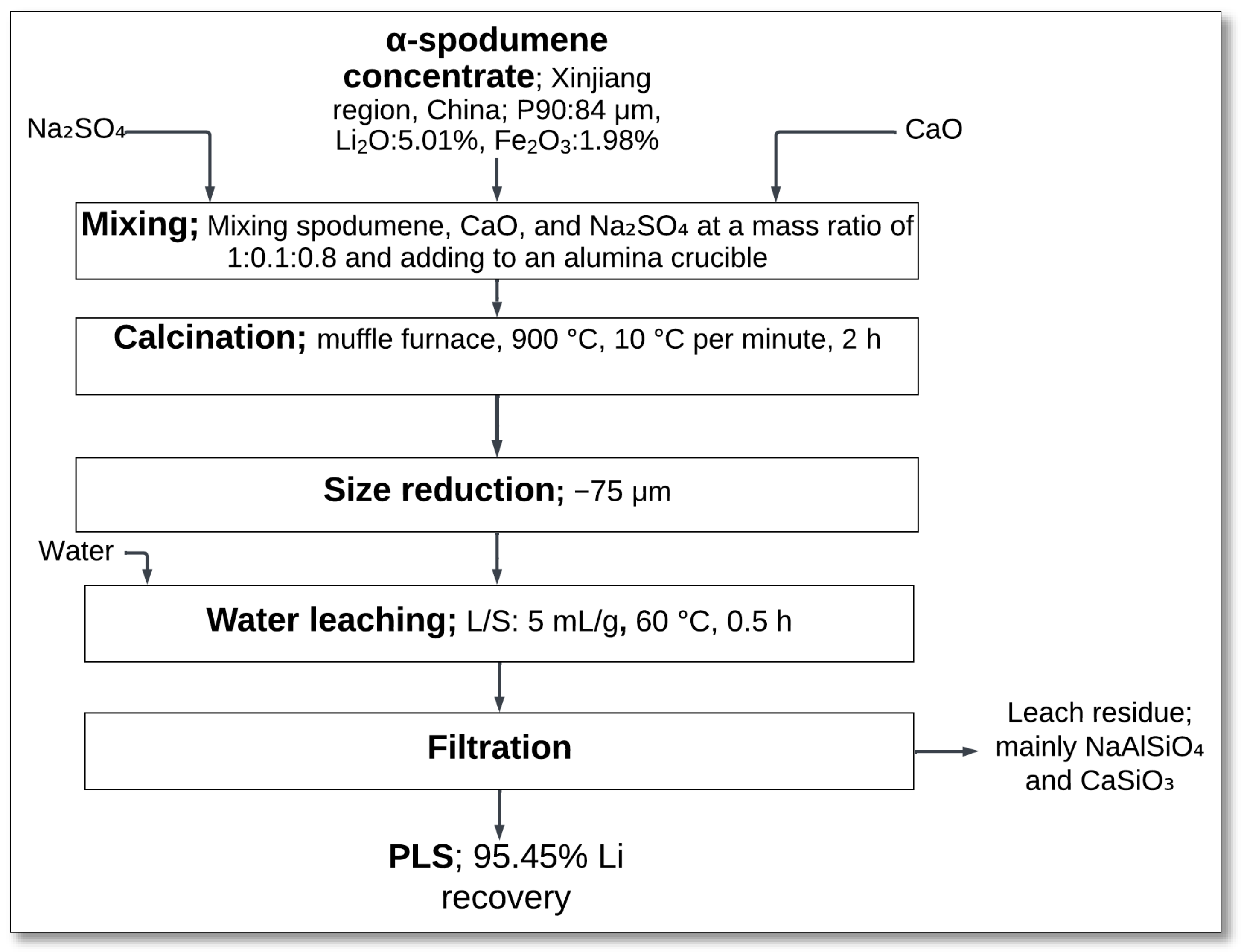
4.1.3. Na2SO4–CaO Salt Roasting–Water Leaching
The Na
2SO
4–CaO salt roasting method followed by water leaching is a low-temperature approach for lithium extraction from α-spodumene. Ni et al. [
33] investigated this method, focusing on phase transformation and lithium recovery efficiency.
The process started with the mixing of α-spodumene concentrate with calcium oxide (CaO) and sodium sulfate (Na
2SO
4) in a mass ratio of 1:0.1:0.8 (
Figure 5). The mixture was then placed in an alumina crucible and subjected to calcination at 900 °C for 2 h. During this process, spodumene reacted with the additives to form lithium sodium sulfate (LiNaSO
4), sodium aluminum silicate (NaAlSiO
4), and calcium silicate (CaSiO
3) [
33]:
Following calcination, the material underwent size reduction to achieve a particle size of less than 75 μm (P100). Water leaching was then performed at 60 °C for 30 min with a liquid-to-solid (L/S) ratio of 5 mL/g. The lithium sodium sulfate formed during roasting was highly water-soluble and dissolved into the leach solution, producing a PLS containing the extracted lithium [
33].
Solid–liquid separation (S/L) was carried out through filtration to recover the leachate, yielding a lithium extraction efficiency of 95.45% [
33]. The leach residue primarily consisted of sodium aluminum silicate and calcium silicate, as these phases remained undissolved during the water leaching process.
This method demonstrated an effective approach to reducing the phase transition temperature of α-spodumene while ensuring high lithium recovery. The introduction of CaO played a crucial role in facilitating phase transformation at lower temperatures, leading to improved leachability of lithium compounds. The process also eliminated the need for aggressive acid leaching, reducing reagent consumption and environmental impact. An additional advantage of this process is the possibility to recycle the Na2SO4 generated after lithium carbonate or lithium hydroxide recovery, further enhancing its sustainability.
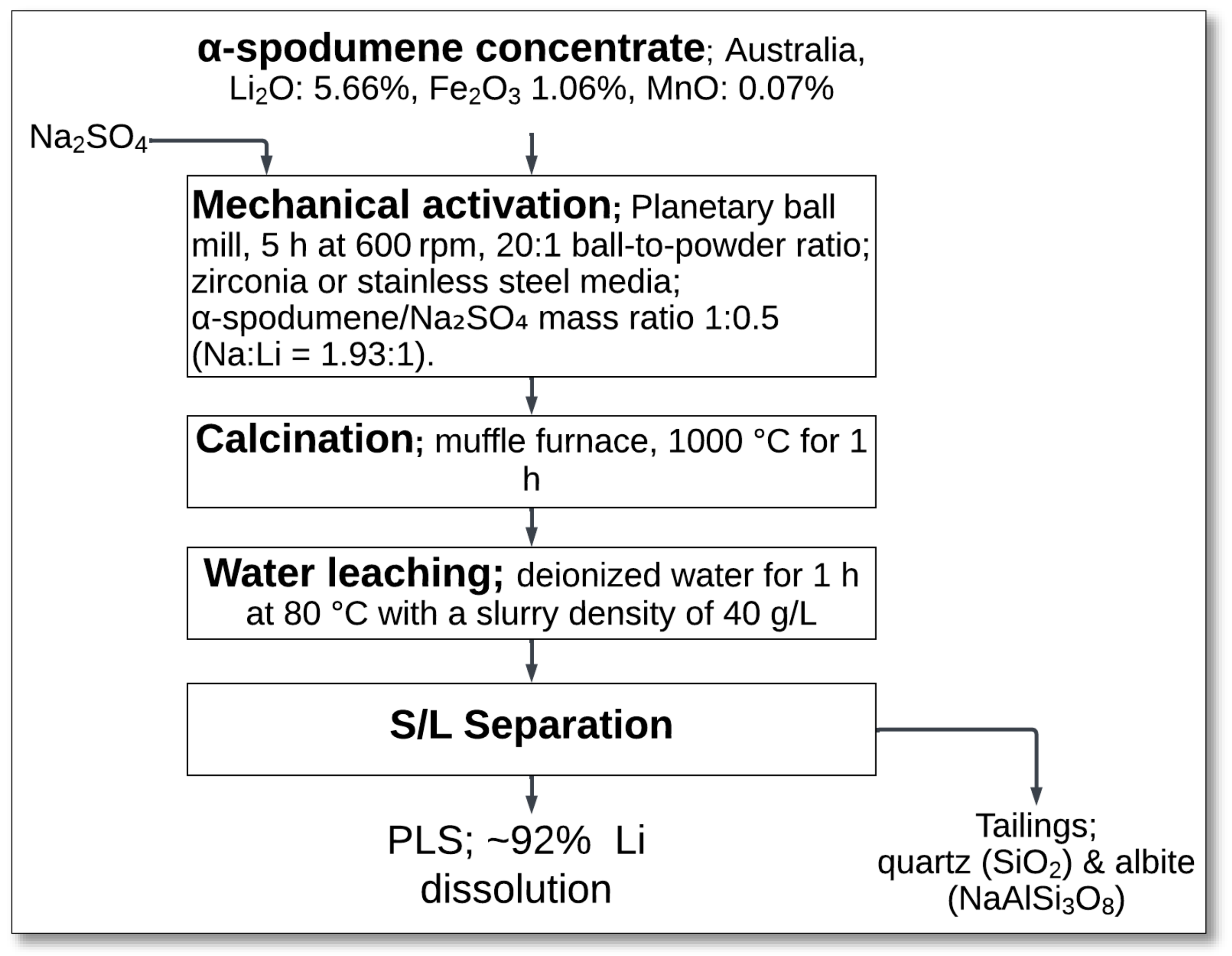
4.1.4. Mechanical Activation (Spodumene–Na2SO4 Mixture)–Calcination–Water Leaching
The mechanical activation of spodumene with sodium sulfate (Na
2SO
4), followed by calcination and water leaching, offers an alternative approach for lithium extraction. Setoudeh et al. [
34] investigated the impact of mechanical activation on phase transformations and lithium recovery.
The process started with the mechanical activation of a mixture of α-spodumene and Na
2SO
4 in a planetary ball mill for 5 h at 600 rpm, with a ball-to-powder weight ratio of 20:1, using either zirconia or stainless-steel media (
Figure 6). The mass ratio of spodumene to Na
2SO
4 was maintained at 1:0.5, corresponding to a Na/Li molar ratio of 1.93:1 when accounting for the sodium content in the ore. Mechanical activation reduced the particle size and induced lattice distortions, enhancing the subsequent reactivity of the materials [
34].
Following milling, the mixture underwent calcination at 1000 °C for 1 h in a muffle furnace. During this stage, spodumene reacted with Na
2SO
4 to form lithium sodium sulfate (LiNaSO
4), sodium aluminum silicate (NaAlSiO
4), and silica (SiO
2) [
34]:
After calcination, the material was subjected to water leaching at 80 °C for 1 h, using deionized water at a slurry density of 40 g/L. During this stage, the water-soluble LiNaSO
4 dissolved into the leach solution, leading to lithium extraction. The solid–liquid (S/L) separation process was then carried out via filtration, yielding a PLS with ~92% lithium dissolution efficiency [
34].
The remaining solid residue mainly consisted of quartz and albite, as these phases are insoluble in water. The process demonstrated the effect of mechanical activation on enhancing the reactivity of spodumene and reducing the overall energy requirements for lithium extraction. The choice of milling media influenced the final phase composition, zirconia providing better energy transfer for particle activation than stainless steel. However, both approaches yielded similar lithium recoveries after calcination and water leaching [
34].
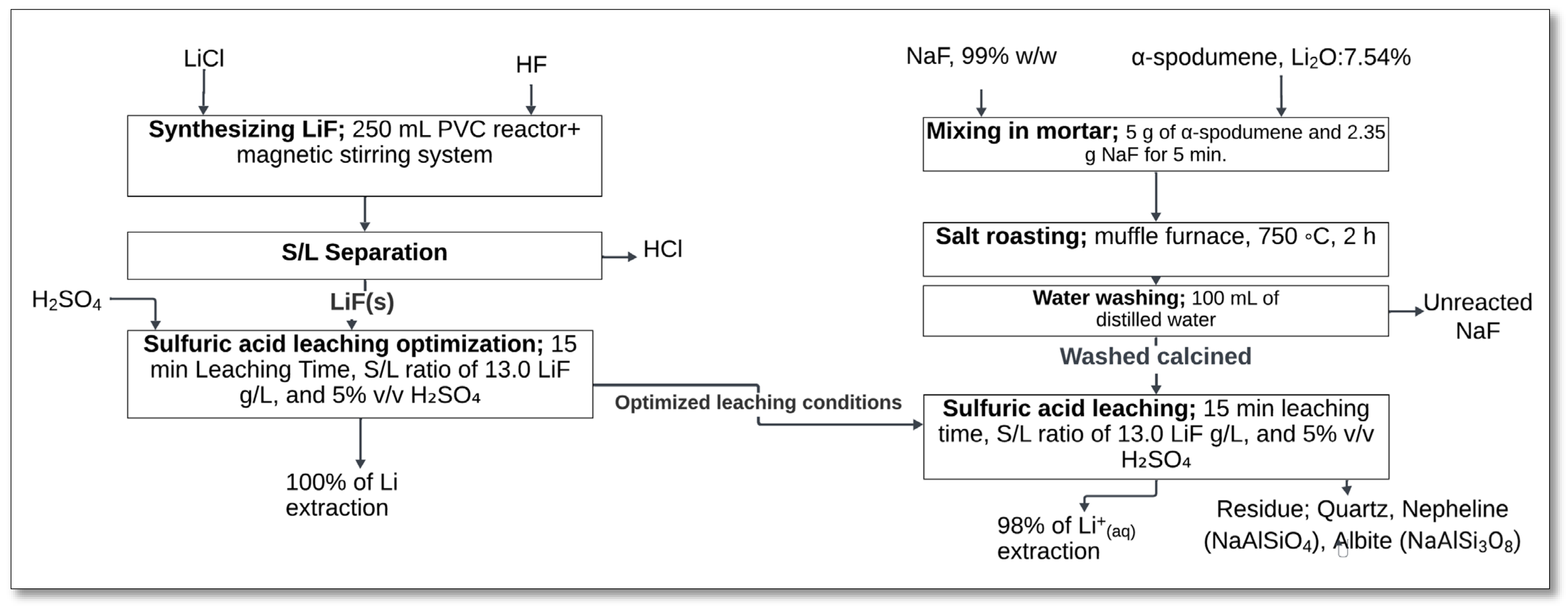
4.1.5. NaF and KF Roasting–Sulfuric Acid Leaching
The roasting of α-spodumene with sodium fluoride (NaF) followed by sulfuric acid leaching is an alternative lithium extraction method. Resentera et al. [
35] investigated this process, focusing on fluorination reactions and the dissolution of lithium fluoride (LiF) in sulfuric acid.
The process started by mixing α-spodumene with NaF in a mortar at a spodumene-to-NaF mass ratio of 5:2.35 (
Figure 7). The mixture was then subjected to salt roasting in a muffle furnace at 750 °C for 2 h. During this stage, spodumene reacted with NaF to form LiF and a mixture of sodium aluminosilicate phases, including nepheline (NaAlSiO
4) and albite (NaAlSi
3O
8) [
35]:
Following roasting, the calcined product underwent water washing with 100 mL of distilled water to remove unreacted NaF. The washed calcine was then subjected to sulfuric acid leaching at optimized conditions: 15 min of leaching, a solid–liquid (S/L) ratio of 13.0 g/L LiF, and 5%
v/
v H
2SO
4. In this stage, LiF reacted with sulfuric acid to form lithium ions (Li
+) in solution, along with sulfate (SO
42−) and hydrogen fluoride (HF) [
35]:
Solid–liquid (S/L) separation was performed via filtration to obtain a PLS containing the extracted lithium. The final lithium extraction efficiency reached 98% [
35]. The primary leach residue consisted of quartz, nepheline, and albite, which remained undissolved in the acid medium.
To address the low solubility of LiF formed in spodumene fluorination, Rosales et al. [
38] developed a three-stage process for acid-free lithium extraction from α-spodumene using aluminum sulfate [Al
2(SO
4)
3]. In Stage 1, a mixture of α-spodumene and potassium fluoride dihydrate (KF·2H
2O) was roasted at 750 °C for 40 min, yielding a calcine containing LiF and KAlSi
2O
6. In Stage 2, the calcine was leached with water at 25 °C for 30 min to remove unreacted KF, leaving behind LiF and aluminosilicate. In Stage 3, the water-leached residue was treated with Al
2(SO
4)
3 solution (262.16 g/L) at 45 °C for 60 min, effectively dissolving LiF through the formation of soluble Al–F complexes. This step achieved 92% lithium recovery, producing a final leach solution (pH ~3) containing 4.95 g/L Li, 20.76 g/L Al, 13.61 g/L F, and minimal impurities. The method avoided the use of HF or H
2SO
4, minimized corrosion risks, and simplified downstream purification, positioning it as an environmentally friendly and scalable route for lithium recovery from fluorinated spodumene.
In another work, Rosales et al. [
39] proposed a simple two-step method for extracting lithium from α-spodumene using potassium fluoride (KF) as a fluorinating agent. The process involves salt roasting of α-spodumene with KF at 779 °C and a KF-to-spodumene mass ratio of 0.56:1, resulting in the formation of LiF and KAlSi
2O
6 (leucite). Following calcination, a two-stage leaching process was applied: water leaching to remove excess KF, followed by acid leaching (10% H
2SO
4) to recover lithium. Lithium extraction reached 94 ± 3%, with minimal dissolution of LiF during the water leach due to the common ion effect. The study used Design of Experiments (DOE) and Artificial Neural Networks (ANNs) to optimize process parameters, supported by thermodynamic modeling and product characterization via XRD and SEM–EDS (Scanning Electron Microscope—Energy-Dispersive Spectroscopy). This method offers an efficient and scalable alternative to acid roasting, eliminating the need for β-phase conversion.
Fluoride-based salt roasting processes offer an alternative to conventional methods by reducing water and acid consumption while enabling the conversion of LiF into more soluble lithium compounds based on market demand. However, a major drawback is the handling and environmental impact of fluoride compounds. The formation of lithium fluoride (LiF), which is poorly soluble in water, necessitates additional steps for its conversion into commercially valuable lithium products. Furthermore, these processes can generate hazardous fluoride-containing emissions, requiring strict environmental controls and neutralization measures to mitigate associated risks.
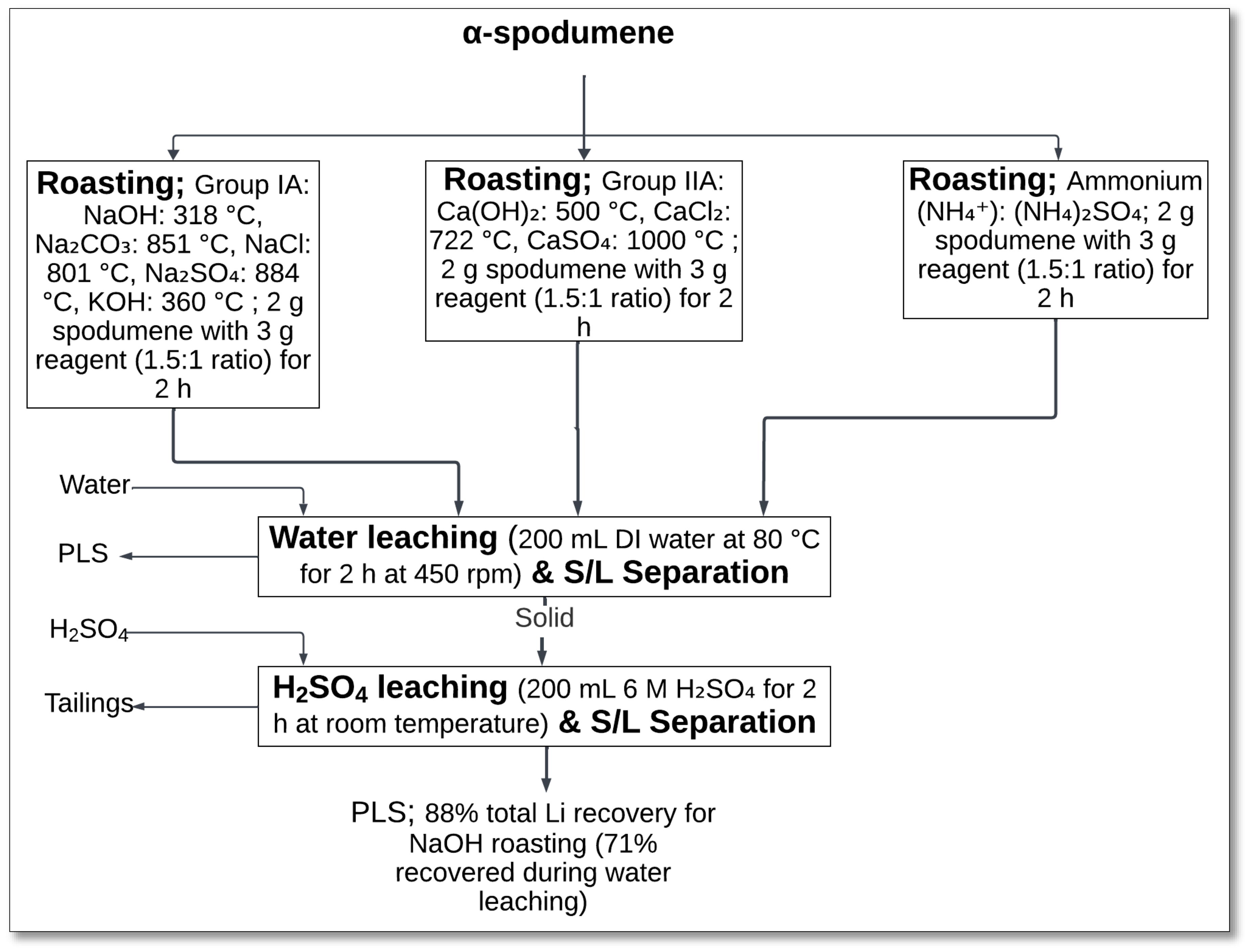
4.1.6. NaOH, Na2CO3, Na2SO4, and KOH Roasting–Water and Sulfuric Acid Leaching
The salt roasting method, followed by sequential water and sulfuric acid leaching, has been explored for lithium extraction from α-spodumene. Han et al. [
40] investigated the effectiveness of various reagents under different roasting conditions, aiming to optimize lithium recovery.
In this method, α-spodumene was roasted with different alkaline reagents at various temperatures. The study categorized the roasting agents into three groups: Group IA included NaOH (318 °C), Na
2CO
3 (851 °C), Na
2SO
4 (884 °C), NaCl (801 °C), and KOH (360 °C); Group IIA consisted of Ca(OH)
2 (500 °C), CaCl
2 (722 °C), and CaSO
4 (1000 °C); and ammonium salts, represented by (NH
4)
2SO
4, were found to be ineffective due to poor phase transformation (
Figure 8). Each roasting experiment used a spodumene-to-reagent ratio of 1.5:1 for a roasting duration of 2 h [
40].
Following roasting, the calcined material underwent water leaching at 80 °C for 2 h while stirred at 450 rpm. During this step, lithium compounds formed during roasting dissolve in water, leaving behind solid residues. The remaining solid was then subjected to an additional sulfuric acid leaching step (6M H2SO4, room temperature, 2 h) to extract residual lithium. The PLS was subsequently analyzed for its lithium content.
Among the tested reagents, NaOH roasting at about 320 °C resulted in the highest lithium recovery (88% total, 71% in water leaching), whereas other reagents exhibited lower efficiencies. Calcium-based reagents were found to be the least effective due to insufficient reactions with spodumene [
40].
The primary chemical reaction during NaOH roasting involved the transformation of α-spodumene into sodium aluminate, sodium silicate, and lithium sodium silicate:
During water leaching, lithium dissolved into the aqueous phase, while aluminum remained as a solid residue. The final sulfuric acid leaching step ensured complete lithium recovery but also led to the dissolution of silicon, forming colloidal silica gel, which posed filtration challenges [
40].
Further process optimizations, such as microwave-assisted roasting and direct LiOH production from water leach solutions, are suggested to improve lithium extraction efficiency [
40]. This method demonstrated the effectiveness of NaOH roasting for lithium recovery, provided that silica gel formation was adequately managed.
Building on the shift toward acid-free lithium extraction, Subasinghe and Rezaee [
41] introduced a two-step alkaline roasting–leaching process for direct lithium recovery from α-spodumene. The method started with roasting α-spodumene with NaOH at a 1.5:1 ratio and 325 °C for 2 h, forming water-soluble lithium-bearing phases. This was followed by a first leaching stage under mild conditions (10% S/L, 23 °C, 1 min), recovering ~75% of lithium. The solid residue was subjected to a second roasting (1:1 ratio, 325 °C, 10 min), then re-leached using a dilute slurry (1% S/L, 200 rpm, 23 °C, 10 min) to extract another ~24% lithium. Together, the two-stage process achieved ~99% total lithium recovery. The process operated at relatively low temperatures, avoided acid use, and allowed rapid extraction and simplified purification, offering a scalable and environmentally friendly alternative to conventional spodumene processing.
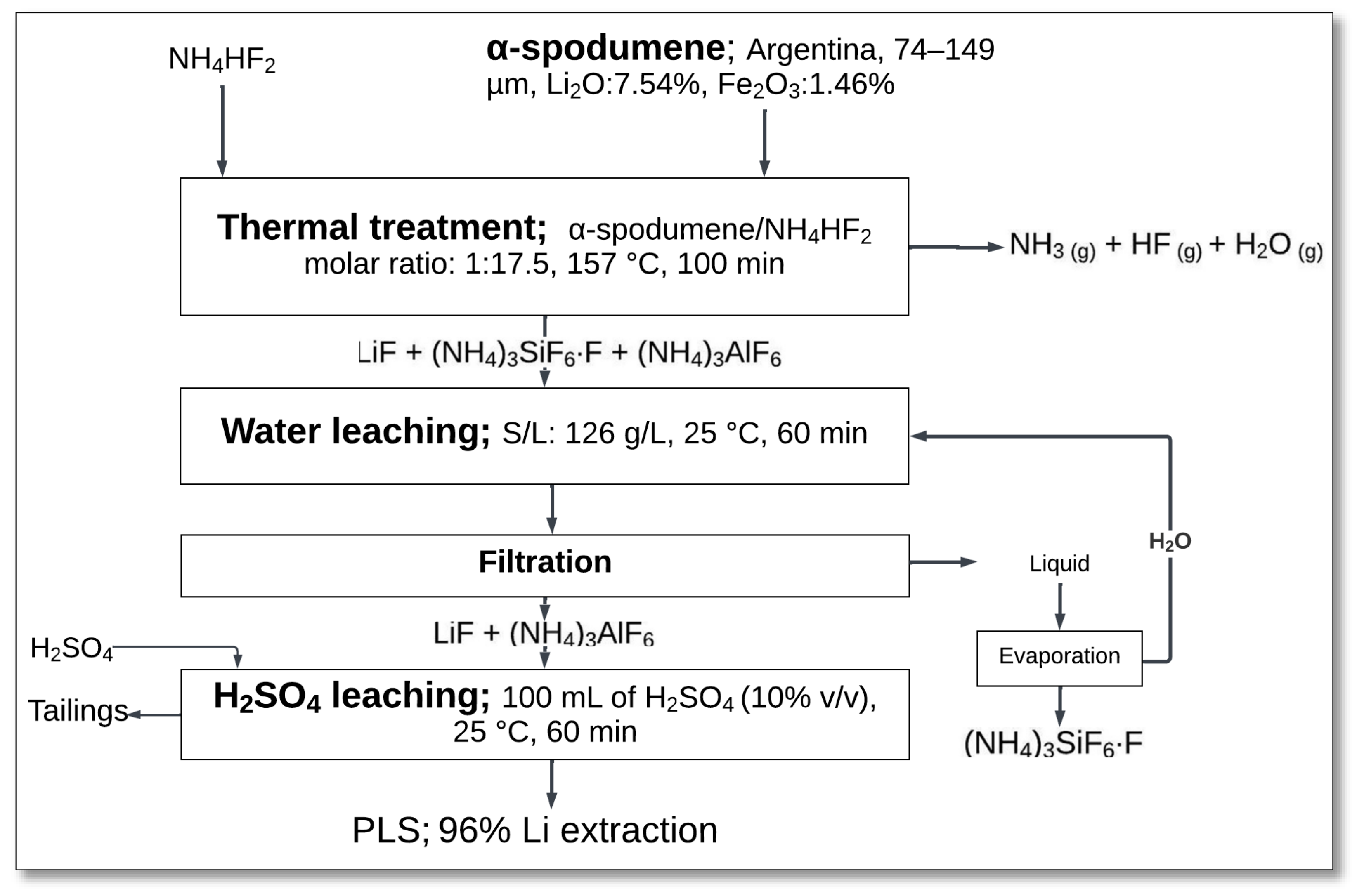
4.1.7. NH4HF2 Roasting–Water and Sulfuric Acid Leaching
The salt roasting of α-spodumene with ammonium bifluoride (NH
4HF
2) provides an alternative approach for lithium extraction at relatively low temperatures. This method involves a thermal treatment stage, followed by sequential water and sulfuric acid leaching. Resentera et al. [
42] investigated the efficiency of this process and the optimization parameters.
The process started with the thermal reaction between α-spodumene and NH
4HF
2 at a molar ratio of Li
2O in α-spodumene to NH
4HF
2 of 1:17.5 at a temperature of 157 °C for 100 min (
Figure 9). During this step, NH
4HF
2 decomposed, releasing ammonia (NH
3), hydrogen fluoride (HF), and water vapor, while forming lithium fluoride (LiF), ammonium fluoroaluminate, and ammonium fluorosilicate.
After thermal treatment, the solid product underwent water leaching at 25 °C with a solid-to-liquid ratio of 126 g/L for 60 min. This step selectively removed the silicon-containing compounds, yielding a filtrate rich in (NH
4)
3SiF
6⋅F, while LiF and (NH
4)
3AlF
6 remained in the solid phase. The remaining residue was subjected to sulfuric acid leaching using 100 mL of 10% H
2SO
4 at 25 °C for 60 min, effectively converting LiF into soluble lithium sulfate (Li
2SO
4). This leaching sequence yielded high lithium extraction efficiency, achieving approximately 96% lithium recovery [
42].
Despite its efficiency, the process required a substantial excess of NH4HF2 (17.5 times the molar amount of spodumene), leading to high reagent consumption and increased solid waste generation. Additionally, the formation of ammonium-based byproducts would necessitate further processing or disposal strategies to ensure environmental sustainability. Nevertheless, this method presents an alternative to conventional lithium extraction techniques by lowering energy requirements and offering selective dissolution pathways for lithium recovery.
In another work by Resentera et al. [
63], a low-temperature alternative was proposed for lithium extraction from α-spodumene using ammonium bifluoride (NH
4HF
2), avoiding the need for high-temperature phase transformation. The process began with thermal treatment of α-spodumene with NH
4HF
2 (1:17.5 molar ratio) at 165 °C for 120 min, releasing NH
3 and H
2O vapors. The resulting mixture, primarily containing LiF, (NH
4)
3AlF
6, and (NH
4)
3SiF
7, was then leached with water to dissolve the silicon compounds and unreacted NH
4HF
2. After filtration and drying, the remaining solid, mainly LiF/(NH
4)
3AlF
6, underwent acid leaching using 6% (
v/
v) sulfuric acid at 25 °C for 24 min and a 30 g/L solid-to-liquid ratio. This final step achieved 93% lithium extraction and 99% aluminum dissolution. The work integrated Response Surface Methodology (RSM) and Artificial Neural Networks (ANNs) for process modeling and optimization, leading to an energy-efficient and effective approach to lithium recovery from α-spodumene.
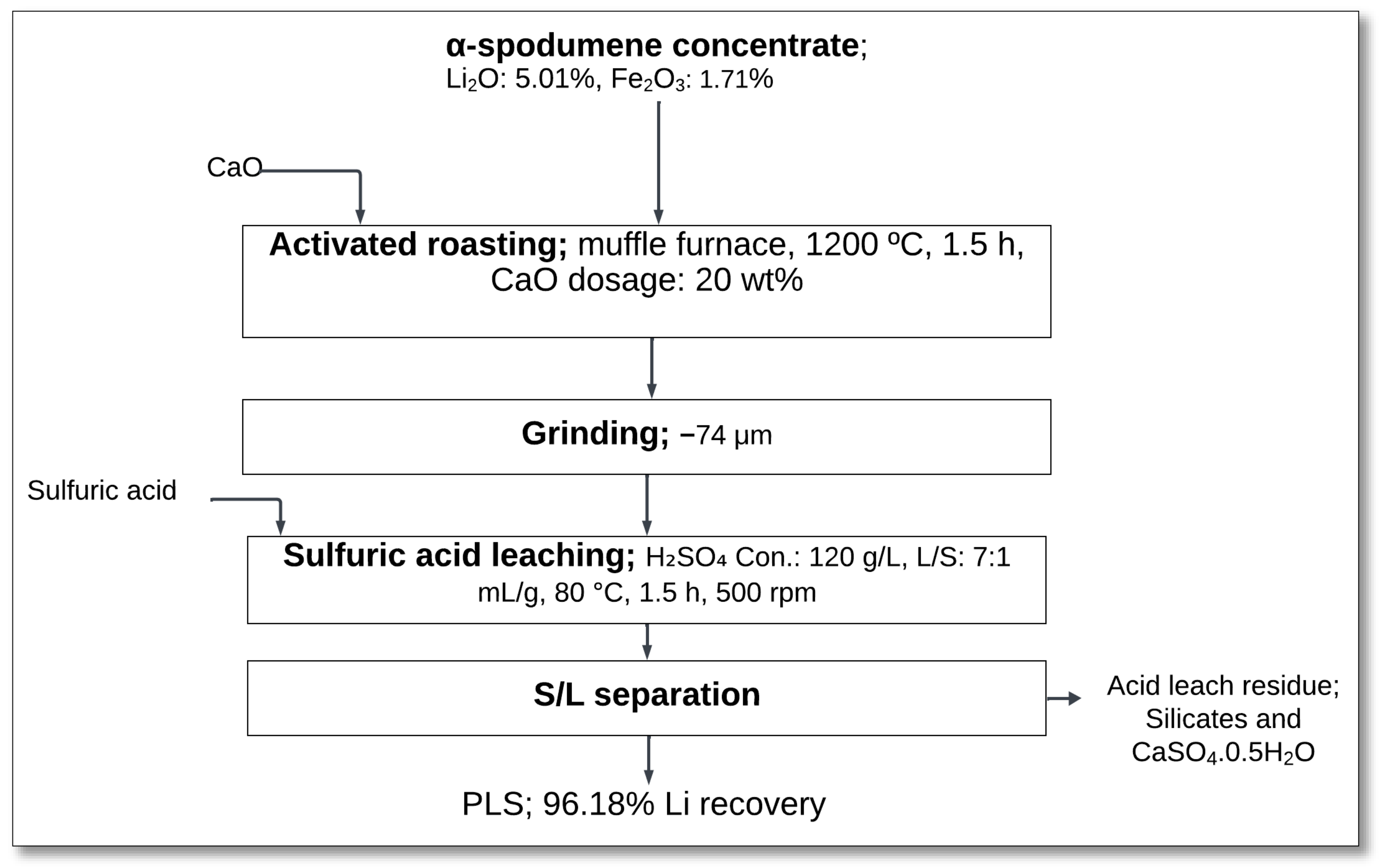
4.1.8. CaO Roasting–Sulfuric Acid Leaching
The CaO roasting–sulfuric acid leaching process has been investigated as an alternative lithium extraction method to improve lithium recovery from α-spodumene. Zhang et al. [
43] studied this approach to enhance lithium dissolution efficiency while reducing the consumption of sulfuric acid.
The process begun with activated roasting, where α-spodumene concentrate (Li
2O: 5.01%) was mixed with 20 wt% CaO and heated in a muffle furnace at 1200 °C for 1.5 h (
Figure 10). The addition of CaO promoted the transformation of α-spodumene to β-spodumene, improving its reactivity for leaching. During roasting, CaO reacted with SiO
2 to form CaSiO
3, which facilitated lithium extraction:
Following roasting, the material was ground to −74 µm using a vibration grinder to increase the surface area and enhance leaching efficiency.
The roasted product was then subjected to sulfuric acid leaching, with a solution of 120 g/L H
2SO
4 at 80 °C for 1.5 h at a L/S ratio of 7:1 mL/g, and stirred at 500 rpm. The leaching reactions involved the dissolution of lithium and aluminum phases:
After leaching, the slurry underwent solid–liquid (S/L) separation via filtration, yielding a PLS with 96.18% lithium recovery. The remaining residue primarily consisted of silicates and CaSO
4·0.5H
2O [
43].
This method presents several advantages, including the promotion of the α-to-β spodumene conversion, the formation of CaSiO3, which enhances lithium recovery, and lower sulfuric acid consumption (1.05 kg H2SO4/kg ore) compared to conventional acid methods (1.47 kg H2SO4/kg ore). Additionally, CaO can replace expensive reagents like sulfuric acid and sodium carbonate, reducing the risk of sodium contamination in lithium battery electrolytes. By eliminating soda ash from lithium extraction, this method enhances the overall purity of lithium carbonate production. However, optimization of CaO dosage and roasting conditions remains essential to maximize efficiency and minimize unwanted side reactions.
4.1.9. Mechanical Activation–CaO Roasting–Water Leaching
The mechanical activation of spodumene, followed by CaO roasting and water leaching, has been explored as an alternative lithium extraction method, particularly focusing on direct lithium hydroxide production. Braga et al. [
44] investigated this approach to improve lithium recovery while reducing the reliance on expensive reagents such as sulfuric acid and sodium carbonate.
The process started with the mechanical activation of α-spodumene concentrate (Li
2O: 5.8%) through grinding with lime (CaO) in a stainless-steel rod mill (
Figure 11). The spodumene/lime mass ratio was set at 1:2, and the mixture was further processed into 32 mm briquettes. The briquetted material underwent calcination at 1050 °C for 30 min, during which spodumene reacted with lime to form lithium aluminate (LiAlO
2) and calcium silicate (Ca
2SiO
4):
After calcination, the material was ground to a particle size (d80) of −100 μm to enhance reactivity during leaching. The roasted material was then subjected to water leaching at 90 °C for 240 min with a solid-to-liquid ratio of 10%. This step dissolved the lithium compounds, facilitating their recovery.
Following the initial solid–liquid (S/L) separation, a second re-leaching step at 90 °C for 20 min further extracted lithium from the solid residues. A final filtration step separated the lithium-rich solution from the remaining CaO·Al2O3 and CaO·SiO2 residues. The process achieved 84% lithium extraction efficiency, producing LiOH as the final product.
This method presents an alternative for lithium extraction by enabling direct LiOH production, reducing the dependency on expensive chemical reagents like sulfuric acid and sodium carbonate. The elimination of sulfuric acid would significantly lower the environmental footprint, making this process a promising route for sustainable lithium recovery.
4.2. Pretreatment–Atmospheric Leaching
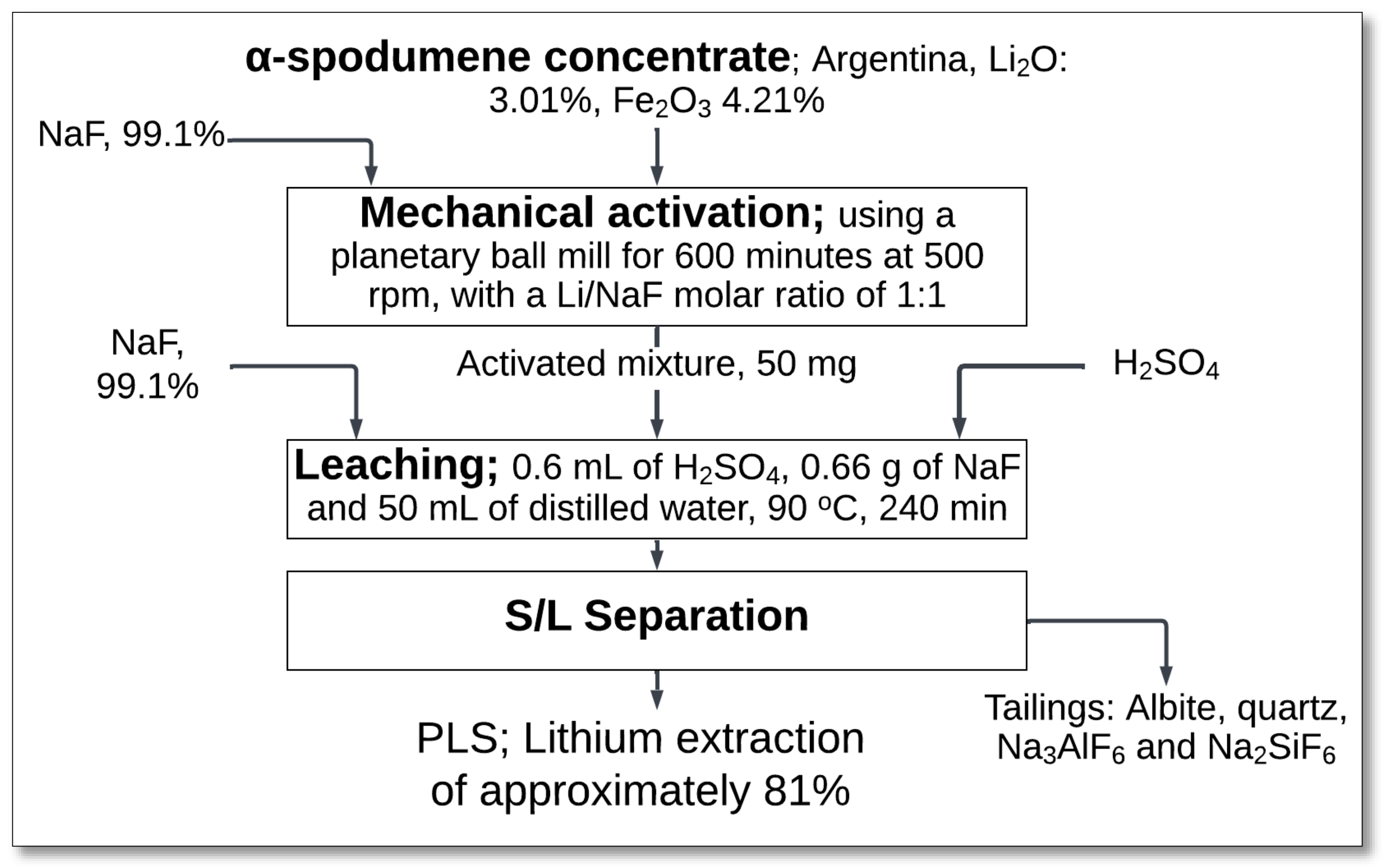
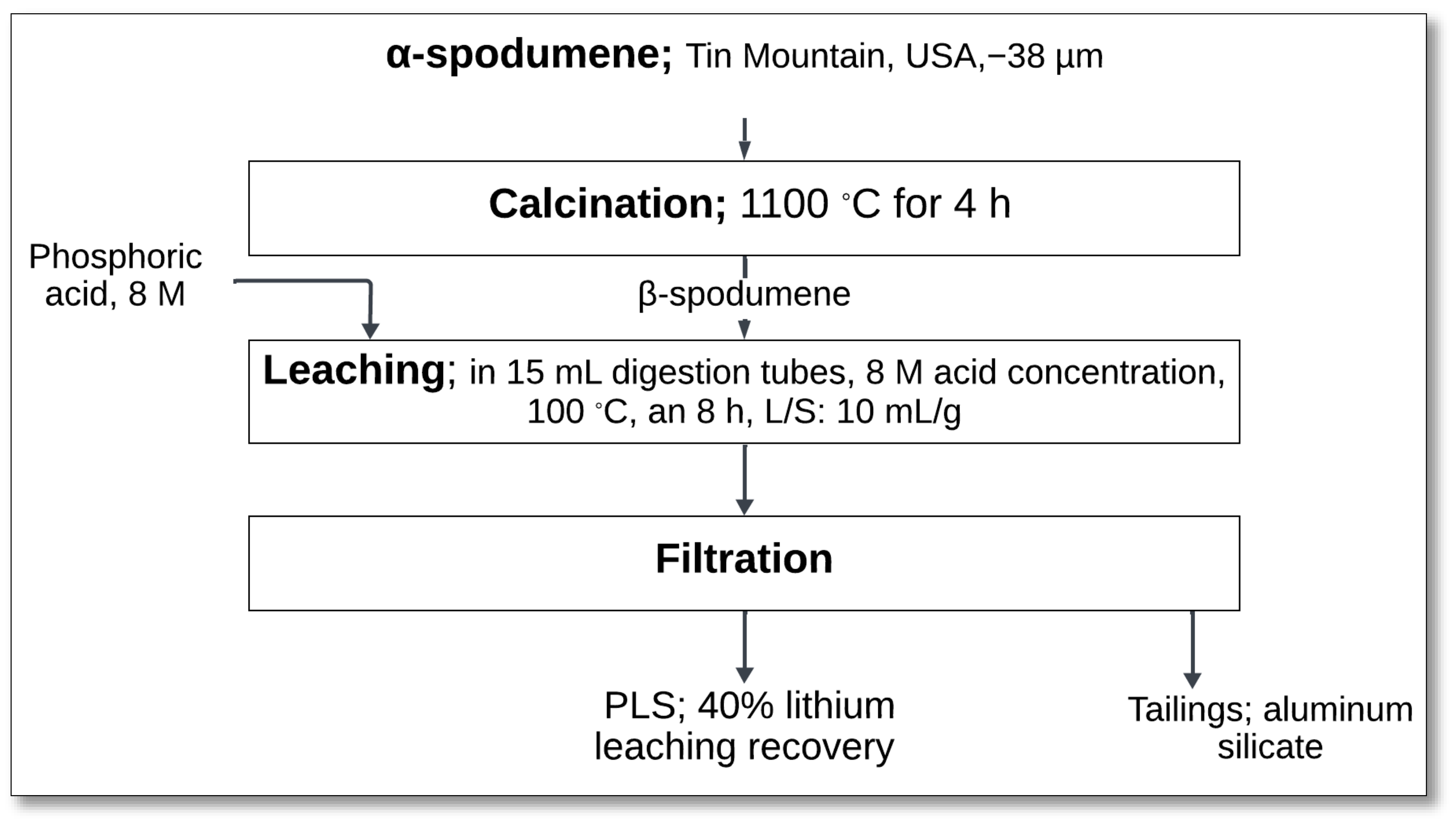
4.2.1. Mechanical Activation (Spodumene–NaF Mixture)–Atmospheric Leaching
The mechanical activation of spodumene with sodium fluoride (NaF) followed by atmospheric leaching has been investigated as an alternative approach for lithium extraction, particularly from low-grade spodumene concentrate. Rosales et al. [
45] explored this method to enhance lithium recovery efficiency while minimizing energy consumption.
The process started with mechanical activation in a planetary ball mill, where α-spodumene concentrate is ground with NaF at a molar ratio of 1:1 (
Figure 12). The milling process was carried out for 600 min at 500 rpm, which facilitated lattice distortion, reduced crystallite size, and increased surface area, improving the material’s reactivity during leaching.
The activated spodumene–NaF mixture underwent subsequent leaching in 0.6 mL of H
2SO
4, with 0.66 g of NaF and 50 mL of distilled water at 90 °C for 240 min. During this stage, NaF reacted with sulfuric acid, forming sodium sulfate (Na
2SO
4) and hydrogen fluoride (HF):
Simultaneously, spodumene underwent dissolution in the presence of HF, releasing lithium ions:
After leaching, solid–liquid (S/L) separation was performed to obtain a PLS, achieving a lithium extraction efficiency of about 81%. The remaining tailings primarily consisted of quartz, albite, sodium fluoroaluminates (Na
3AlF
6), and sodium fluorosilicates (Na
2SiF
6), which may have further technological applications [
45].
This method offers advantages over conventional leaching processes, as fluoride compounds can effectively break Si-O bonds, enhancing the dissolution of refractory minerals. Additionally, the process operates under lower energy conditions, reducing overall processing costs. However, the selective fluorination approach necessitates prior concentration of minerals to avoid excessive gangue dissolution, which could lead to lithium compound co-precipitation. NaF also plays a role in promoting surface ionic exchange in the mineral structure, further facilitating dissolution [
45].
Despite these benefits, the process presents challenges such as the formation of silica-based residues, which can impact downstream processing and waste management. Optimization of parameters such as milling time, NaF dosage, and acid concentration is necessary to maximize lithium recovery while minimizing unwanted side reactions.
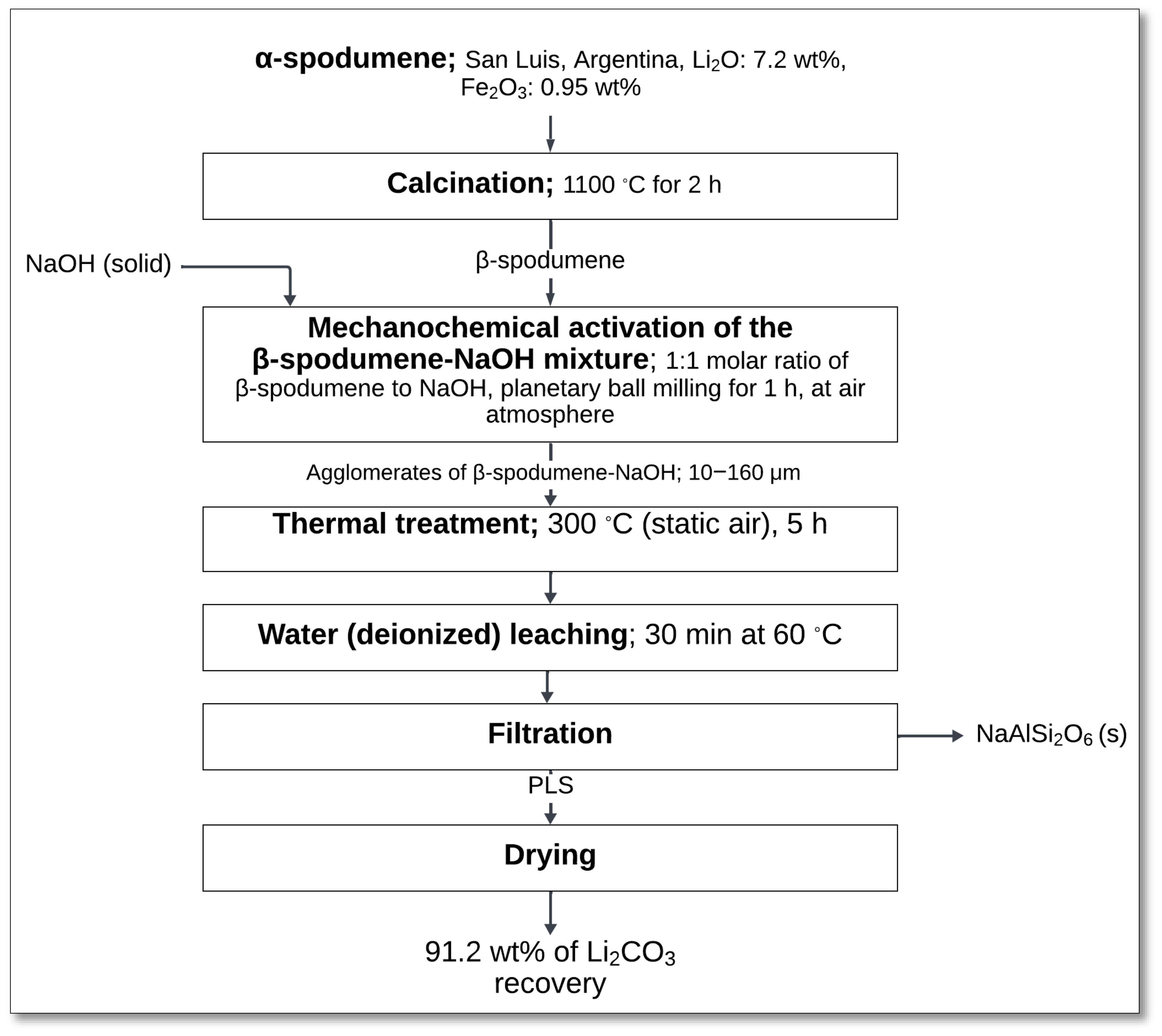
4.2.2. NaOH Decomposition and Acid Leaching
The NaOH decomposition and acid leaching method, as studied by Qiu et al. [
46], provides an alternative approach for lithium extraction from α-spodumene without requiring high-temperature calcination. This process consists of two main stages: alkaline treatment using a NaOH solution and acid leaching with H
2SO
4.
The process starts with alkaline treatment in a 250 mL zirconium autoclave, where α-spodumene (−74 μm) was reacted with a 25 wt% NaOH solution under vigorous stirring (500 rpm) at 250 °C for 24 h (
Figure 13). The NaOH-to-ore mass ratio was maintained at 1.5:1. During this step, α-spodumene reacted with NaOH, converting lithium into Li
2SiO
3, a more soluble lithium metasilicate compound:
The solid–liquid separation (centrifugation) followed, where the alkaline residue retained 79.7% of the lithium as Li
2SiO
3, while only 7.6% of the lithium dissolved into the liquid phase [
46].
Next, the solid residue underwent acid leaching using 0.75 mol/L H
2SO
4 at ambient temperature for 1 h with a H
2SO
4-to-Li
2O molar ratio of 1.9:1. This step dissolved Li
2SiO
3, releasing lithium into the solution:
After another solid–liquid separation (filtration), the PLS contained 78.7% of the lithium.
Lithium was then concentrated by evaporation, followed by precipitation using a 225 g/L Na
2CO
3 solution in a jacketed glass reactor at 90 °C for 1 h. This step yielded Li
2CO
3 with 86.3% purity, achieving an overall lithium recovery of 53.2% from the alkaline residue:
This process eliminates the need for high-temperature calcination, reducing energy consumption and avoiding complex roasting steps. It also provides an efficient pathway for lithium recovery through direct α-spodumene conversion, offering a viable alternative to conventional acid roasting.
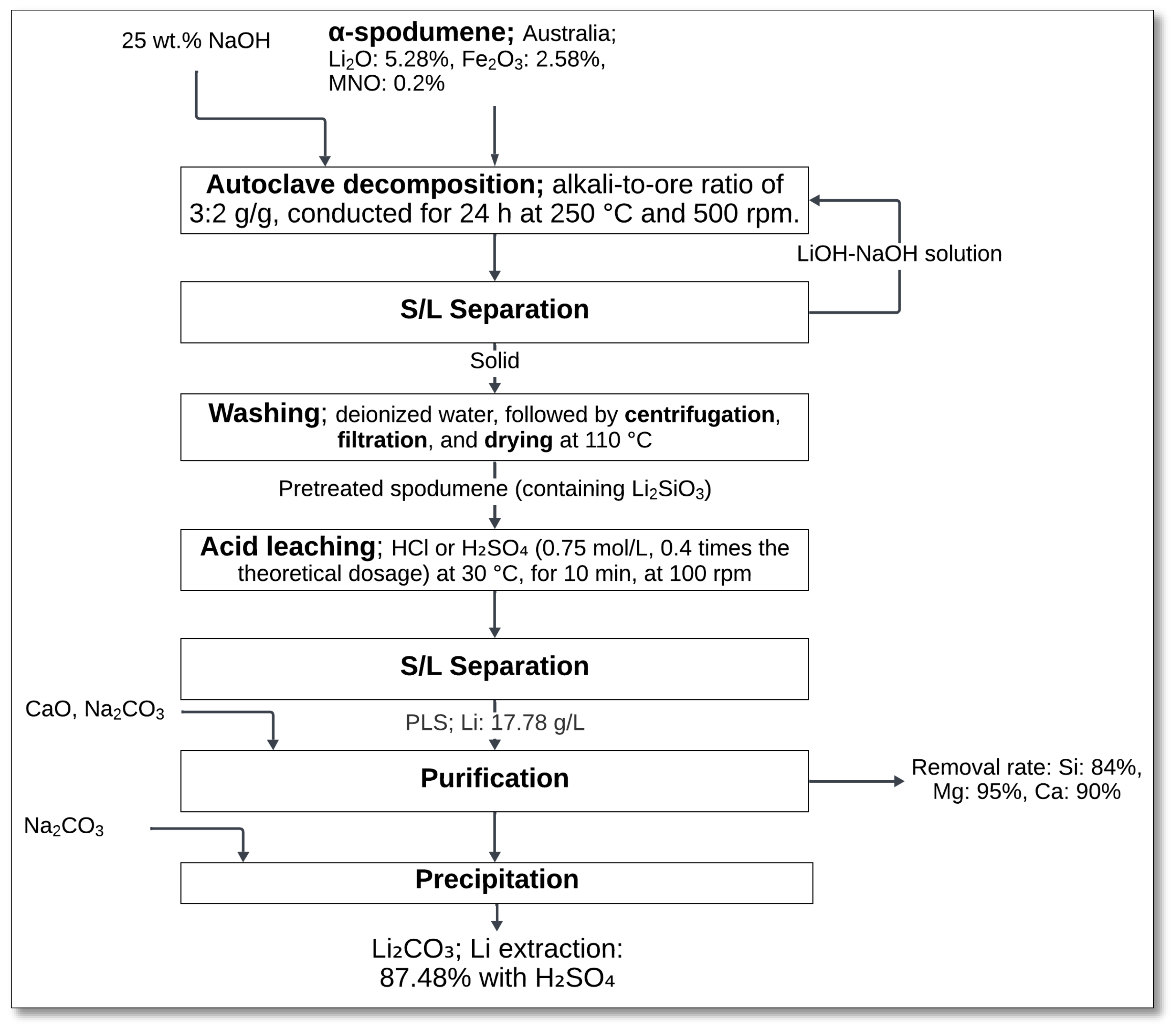
In a related study, Zhu et al. [
47] explored a similar NaOH decomposition and acid leaching approach, emphasizing ambient-temperature acid leaching as a means of reducing energy consumption. In their method, α-spodumene was treated with 25 wt% NaOH at an NaOH-to-ore mass ratio of 3:2, 250 °C for 24 h, and 500 rpm stirring speed (
Figure 14). This process aimed to transform α-spodumene into a more reactive form to enhance its leachability.
Following solid–liquid separation, the obtained solid residue underwent washing with deionized water, centrifugation, filtration, and drying at 110 °C. The pretreated spodumene, containing Li
2SiO
3, was then subjected to acid leaching using HCl or H
2SO
4 (0.75 mol/L) at 30 °C for 10 min while being stirred (100 rpm). The reaction mechanism for lithium extraction is as follows:
After a second solid–liquid separation, the pregnant leach solution contained 17.78 g/L lithium, which was then purified before lithium carbonate precipitation. The final lithium carbonate product was obtained by letting the purified lithium sulfate solution react with sodium carbonate [
47].
This study demonstrated an efficient, low-temperature pretreatment method for enhancing lithium leachability while reducing the energy-intensive steps seen in conventional processes. Additionally, the process achieved a high removal efficiency for Si (84%), Mg (95%), and Ca (90%), improving impurity control and downstream processing.
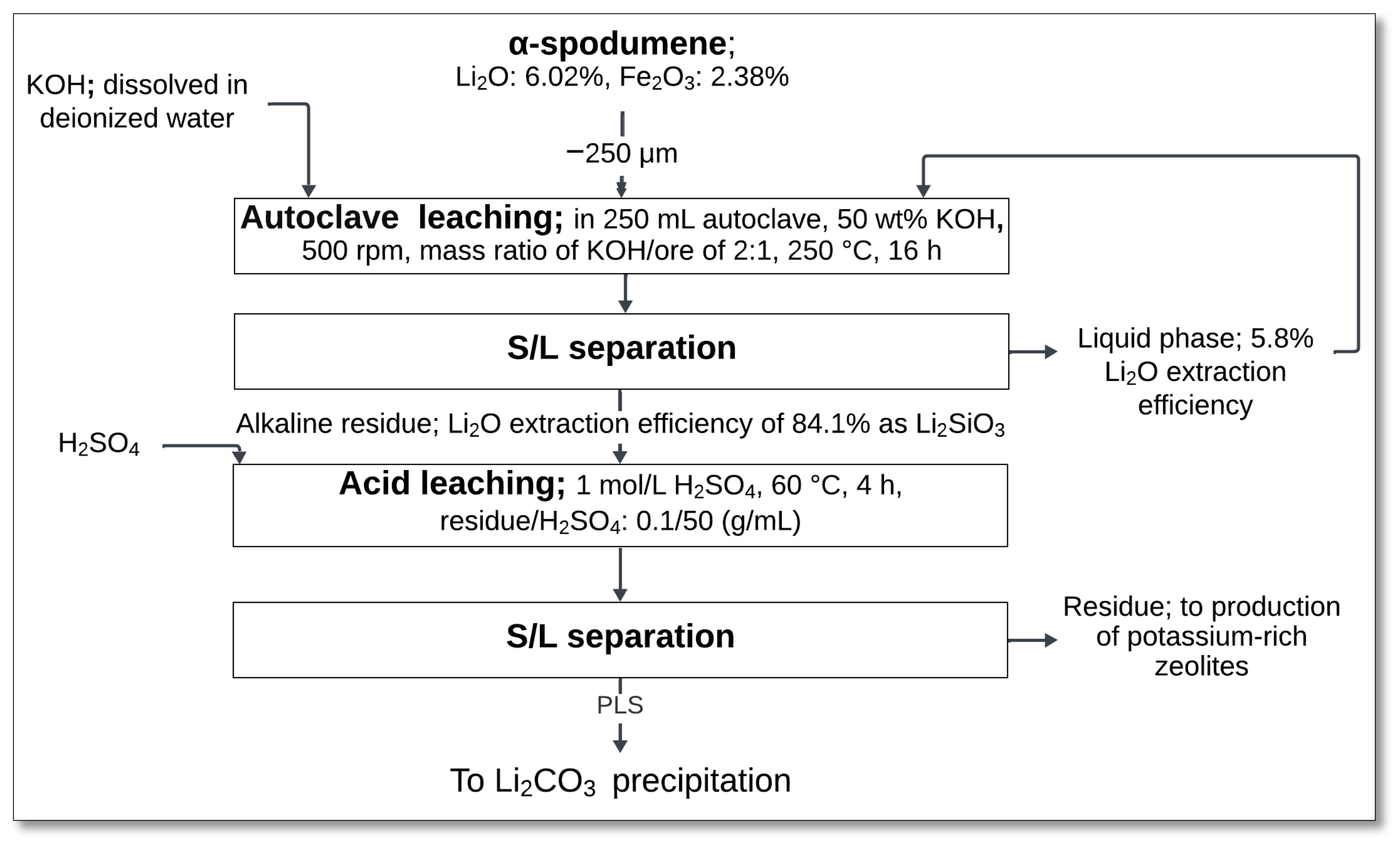
4.2.3. KOH Decomposition and Acid Leaching
The decomposition of α-spodumene using potassium hydroxide (KOH), followed by acid leaching, provides an alternative approach for lithium extraction without requiring high-temperature calcination. Qiu et al. [
48] investigated this method as a less energy-intensive alternative to conventional roasting techniques. This process consists of two main stages: alkaline treatment using KOH and subsequent acid leaching with sulfuric acid (H
2SO
4).
In the first stage, α-spodumene concentrate was subjected to an alkaline treatment in a 250 mL autoclave containing 50 wt% KOH solution. The reaction took place at 250 °C under vigorous stirring (500 rpm) for 16 h, maintaining a KOH-to-ore mass ratio of 2:1 (
Figure 15). This treatment facilitated the transformation of spodumene into lithium metasilicate (Li
2SiO
3) and potassium aluminosilicate (KAlSiO
4), with water as a byproduct:
Following alkaline digestion, solid–liquid (S/L) separation via centrifugation was performed. The alkaline residue contained 84.1% of the lithium as Li2SiO3, while only 5.8% of the lithium was dissolved in the liquid phase.
The solid residue underwent acid leaching using 1 mol/L H
2SO
4 at 60 °C for 4 h with a residue-to-H
2SO
4 ratio of 0.1:50 (g/mL). This step dissolved Li
2SiO
3, releasing lithium into the solution:
After a second S/L separation by filtration, the PLS was obtained, which can be further processed for lithium carbonate (Li2CO3) precipitation. The remaining residue, mainly composed of potassium-rich zeolites, may have additional applications.
This method offers several advantages over traditional roasting–acid leaching techniques. It eliminates the need for high-temperature calcination and avoids concentrated sulfuric acid roasting, simplifying the separation process. However, optimizing process parameters such as KOH concentration, temperature, and leaching duration is crucial to maximizing lithium recovery while minimizing unwanted byproducts [
48].
4.3. Direct Leaching
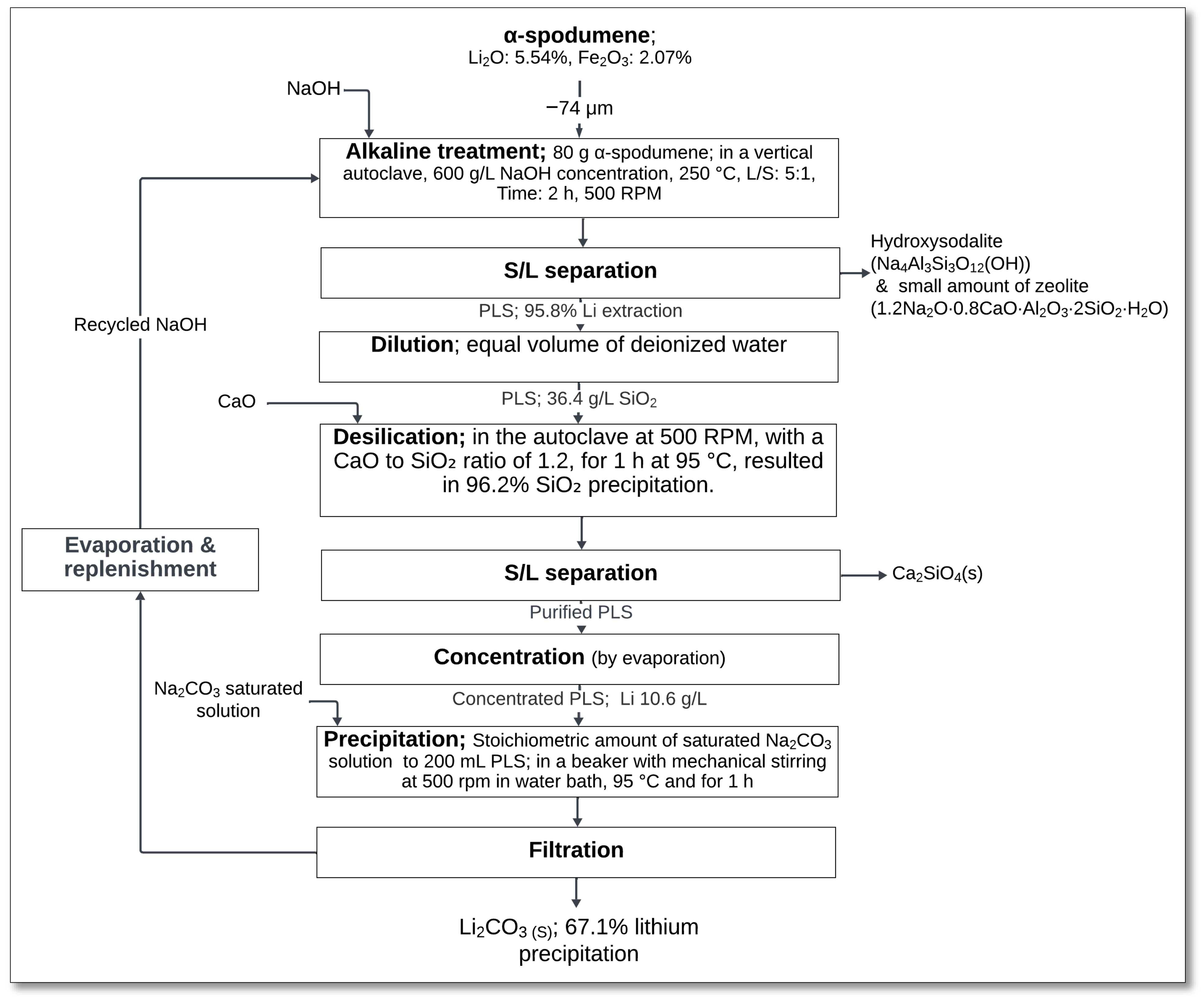
4.3.1. High-Pressure NaOH Leaching
High-pressure NaOH leaching has been explored as an alternative to conventional acid leaching for lithium extraction from α-spodumene. Xing et al. [
49] investigated this process, which involves alkaline treatment, desilication, and lithium precipitation to achieve high lithium recovery while minimizing the environmental impact.
The process was begun with an alkaline treatment in a vertical autoclave, where α-spodumene (−74 μm) was reacted with 600 g/L NaOH solution at 250 °C for 2 h, with a solid-to-liquid ratio (L/S) of 5:1 and stirring at 500 rpm (
Figure 16). During this step, spodumene reacted with NaOH, forming sodium aluminosilicate hydrate (Na
4Al
3Si
3O
12(OH)), sodium silicate (Na
2SiO
3), and hydroxide ions in solution:
After solid–liquid (S/L) separation, the pregnant leach solution underwent dilution with an equal volume of deionized water to optimize silica precipitation. The diluted PLS was subjected to desilication in an autoclave at 95 °C for 1 h, where silica was precipitated by adding CaO at a CaO-to-SiO
2 molar ratio of 1.2:1, achieving 96.2% silica removal:
Following desilication, another S/L separation was performed to obtain a purified lithium-containing solution. Lithium was then concentrated through evaporation, followed by precipitation using a saturated Na
2CO
3 solution (200 mL) in a beaker with mechanical stirring at 500 rpm in a water bath at 95 °C for 1 h:
Finally, the precipitated lithium carbonate (Li2CO3) underwent filtration, achieving an overall lithium precipitation efficiency of 67.1%, with the remaining lithium being recirculated. The total lithium extraction efficiency reached 95.8% (Li precipitation 67.1%, the rest was circulated).
This method offers multiple advantages, including lower energy consumption, no gas emissions, and the synthesis of hydroxysodalite zeolite (Na8(AlSiO4)6(OH)2·nH2O) as a byproduct, which has industrial applications. Additionally, the production of calcium silicate (CaSiO3) further enhances the economic feasibility of the process. Compared to conventional acid leaching, NaOH high-pressure leaching could provide a more sustainable and efficient alternative for lithium recovery from spodumene.
In another work, a high-pressure leaching process utilizing a combination of NaOH and CaO was explored for lithium extraction from α-spodumene concentrate [
50]. The process started with autoclave leaching at 250 °C for 6 h, using a NaOH concentration of 400 g/L and a CaO-to-ore ratio of 0.5:1 (wt%) (
Figure 17). The solid-to-liquid ratio was maintained at 7 mL/g, with a stirring speed of 400 rpm [
50]. During this stage, spodumene underwent chemical transformation, forming sodium–calcium hydrosilicate (NaCaHSiO
4), lithium hydroxide (LiOH), and sodium aluminate (NaAl(OH)
4):
After solid–liquid separation, the PLS underwent lithium precipitation using sodium phosphate (Na
3PO
4) at 65 °C for 2 h, with a PO
43−/Li
+ molar ratio of 1.3:3.3. This reaction facilitated the selective precipitation of lithium phosphate (Li
3PO
4), while sodium hydroxide was regenerated [
50]:
Following precipitation, the lithium phosphate underwent acid dissolution and electrodialysis to remove impurities before final conversion to lithium carbonate (Li
2CO
3). The purified lithium solution was then let to react with sodium carbonate (Na
2CO
3) at 80 °C, yielding lithium carbonate as the final product [
50]:
This process achieved a high lithium extraction efficiency of about 93%. It involved harsh reaction conditions, however, and the use of a large dosage of leaching agents. Additionally, obtaining high-purity lithium carbonate can be challenging due to sodium contamination in the leachate when using conventional carbonation methods. However, the approach demonstrated potential for industrial application due to its relatively low energy consumption compared to traditional calcination–acid roasting processes.
The LieNA
® process, developed by Lithium Australia in collaboration with ANSTO Minerals, offers an innovative alternative to conventional spodumene conversion methods. Unlike high-temperature conversion, LieNA
® operates via a chemical phase transformation in solution, where fine spodumene is reacted with caustic soda in an autoclave to form synthetic lithium sodalite. Lithium is then recovered from this intermediate by weak acid leaching through an ion-exchange reaction, enabling lithium extraction without aggressive roasting steps [
64,
65,
66].
The LieNA® caustic digestion approach is particularly designed for fine spodumene, which is typically considered a low-value waste stream in traditional processing. This is a key advantage allowing for the recovery of lithium from current tailings discharges and historic storage facilities. This strategy could not only improve resource utilization but also enhance the environmental sustainability of lithium production by enabling the valorization of materials that would otherwise remain unprocessed.
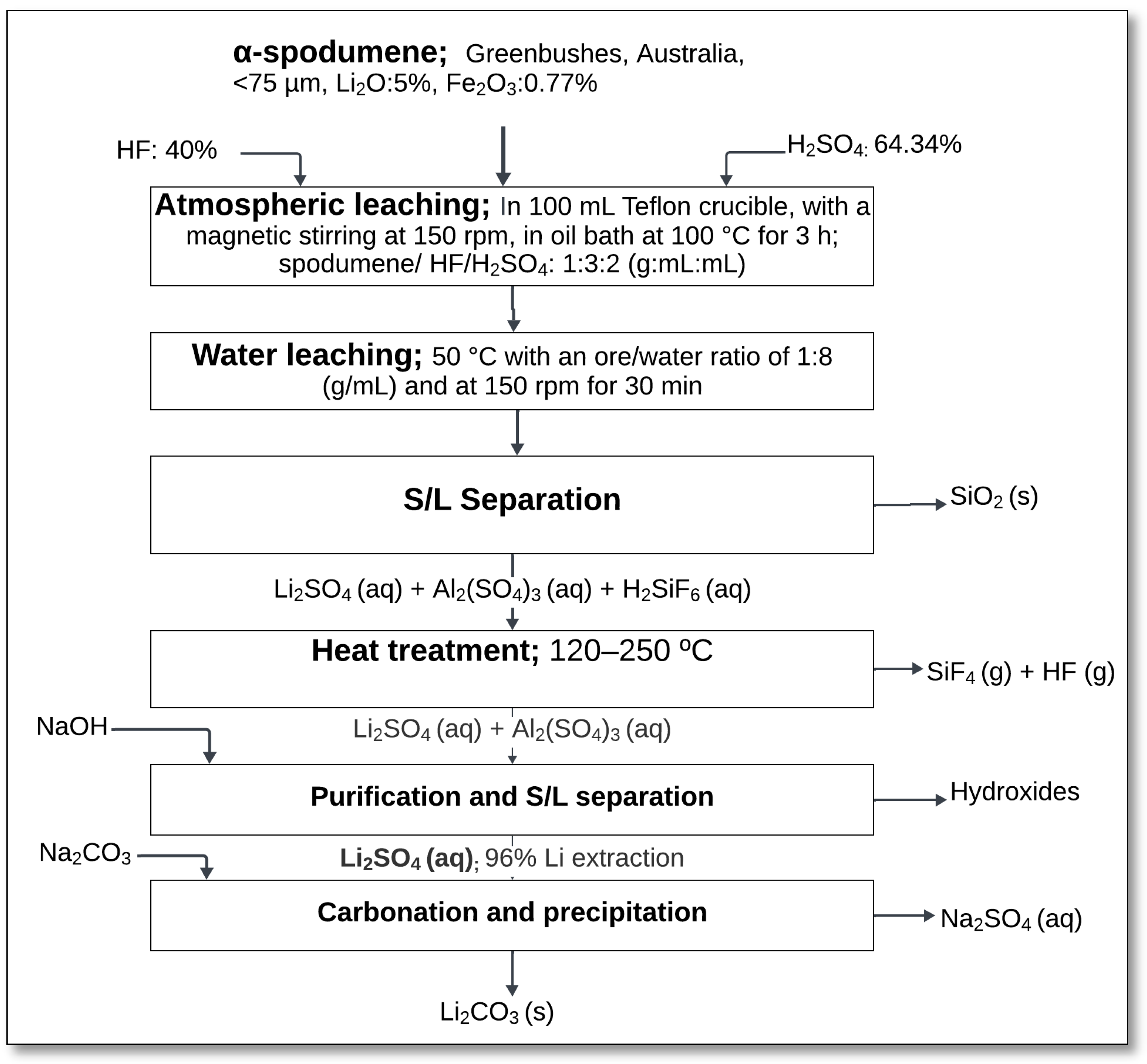
4.3.2. Atmospheric HF/H2SO4 Acid Leaching
Atmospheric leaching of α-spodumene using hydrofluoric acid and sulfuric acid mixtures has been investigated as a direct alternative to conventional thermal treatment processes. This method offers the potential to extract lithium without the need for roasting to transform α-spodumene into its β polymorph, thus reducing energy consumption. Guo et al. [
51,
52] studied the efficiency of this approach by performing leaching experiments at atmospheric pressure in a Teflon-lined reactor. In their process, α-spodumene from the Greenbushes deposit (<75 µm, Li
2O: 5%) reacted with a HF/H
2SO
4 mixture at a mass ratio of 1:3:2 (spodumene/HF/H
2SO
4), under continuous stirring at 150 rpm and a temperature of 100 °C for 3 h (
Figure 18).
The reaction (Equation (35)) led to the decomposition of α-spodumene and subsequent formation of soluble lithium sulfate (Li
2SO
4) and insoluble residues containing silicon and aluminum species.
Following leaching, the slurry was subjected to solid–liquid separation, with the filtrate containing dissolved lithium sulfate and aluminum sulfate species. The residue primarily consisted of silica (SiO
2) and fluorosilicates. A subsequent heat treatment step at 120–250 °C released volatile silicon tetrafluoride (SiF
4) and hydrogen fluoride (HF), while the lithium remained in solution as Li
2SO
4. Further purification and precipitation stages included neutralization with NaOH and carbonation with Na
2CO
3 to recover lithium carbonate (Li
2CO
3). The lithium extraction efficiencies reached up to 96% under the optimized leaching conditions [
52].
The HF/H2SO4 leaching method presents several advantages, including its ability to directly process α-spodumene without prior thermal activation, thus reducing overall energy demand. It also offers high lithium recovery and operates under atmospheric pressure, simplifying equipment requirements. Despite these benefits, significant drawbacks remain. The method requires large amounts of acid, and generates substantial amounts of fluoride-containing residues, raising concerns regarding environmental safety and waste management. Furthermore, the presence of fluorides increases the complexity of downstream purification processes.
Further research is necessary to address these limitations, particularly in optimizing reagent consumption, minimizing the formation of refractory residues, and developing environmentally friendly treatment methods for the fluoride-rich tailings.
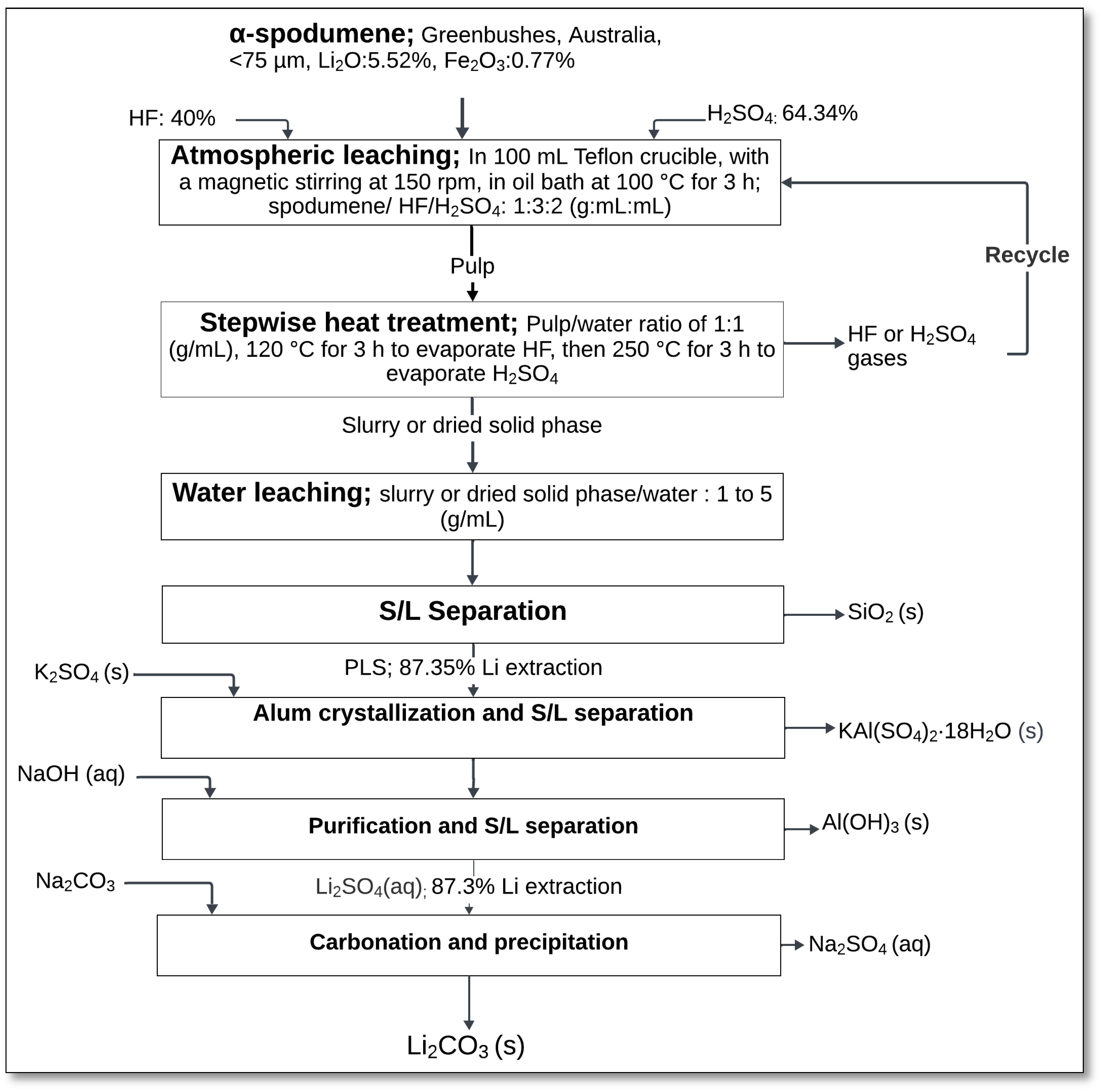
In another study, Guo et al. [
53] proposed an optimized method for lithium extraction from α-spodumene, combining atmospheric HF/H
2SO
4 leaching with a stepwise heat treatment to enhance fluorine removal and minimize lithium losses. The acid leaching process was the same as in their previous work [
52]. Following acid leaching, a stepwise heat treatment was applied to remove volatile fluorides and improve lithium recovery. The slurry from acid leaching was heated at 120 °C for 3 h to remove HF, followed by heating at 250 °C for an additional 3 h to evaporate H
2SO
4. This two-step thermal process effectively minimized lithium losses caused by the formation of insoluble fluorides, such as cryolithionite (Na
3Li
3Al
2F
12), which otherwise trap lithium in solid residues (
Figure 19).
After thermal treatment, the solid residue was subjected to water leaching at a solid-to-water ratio of 1:5 (g/mL). This step solubilized lithium and aluminum sulfates, while silica (SiO
2) remained as an insoluble residue. The solution then underwent alum crystallization by adding K
2SO
4, resulting in the formation of potassium alum (KAl(SO
4)
2·18H
2O), followed by neutralization with NaOH to precipitate aluminum hydroxide (Al(OH)
3). Finally, lithium carbonate (Li
2CO
3) was recovered by adding Na
2CO
3 to induce carbonation and precipitation [
53].
This integrated process achieved a lithium extraction efficiency of up to 87.3%, while reducing fluorine content in the solution. The approach also mitigated the formation of hazardous fluoride-containing residues and allowed for the recycling of HF and H
2SO
4 vapors [
53].
As an alternative to high-temperature calcination and acid baking, Kuang et al. [
67] introduced a fluorine-based chemical approach for lithium extraction from α-spodumene. In this process, α-spodumene was first treated with hydrofluoric acid (HF) at 400 K, which disrupted its silicate structure by breaking Li–O, Al–O, and Si–O bonds, forming soluble compounds like Li
3AlF
6 and AlF
3. These intermediates were then converted to Li
2SO
4 and Al
2(SO
4)
3 through reaction with sulfuric acid, either by direct HF addition or via in situ HF generation from fluorite and H
2SO
4. The resulting sulfates were subsequently recovered through water leaching, enabling efficient lithium separation. Analytical techniques, including XRD, SEM, and FTIR confirmed complete structural decomposition and formation of the desired products. The method achieved 95.7% lithium recovery, offering a low-temperature, high-efficiency extraction route suitable for spodumene ores, particularly lower-grade deposits.
4.4. Microwave-Aided Calcination of α-Spodumene
The conventional calcination of α-spodumene is recognized as one of the most energy-intensive steps in the lithium extraction process. This phase transition from α- to β-spodumene typically requires temperatures between 1000–1100 °C, making it the bottleneck of the economic viability of lithium production from spodumene ores [
54,
55]. In recent studies, microwave-assisted heating has emerged as an alternative technology promising cleaner, faster, and more energy-efficient calcination, with reduced greenhouse gas emissions [
55,
56].
Unlike conventional heating, which relies on external energy sources to transfer heat to the material’s surface, microwave heating generates heat internally, within the material, in a volumetric way. This leads to faster heating rates and energy savings [
68,
69]. Materials respond to microwave energy differently based on their dielectric properties and are typically classified into absorbers, transparent materials, and conductors [
69,
70,
71]. Materials capable of directly absorbing microwaves undergo rapid heating, while those that are transparent require pre-heating or hybrid techniques to improve energy absorption [
56].
Microwave calcination demonstrates significant advantages. In materials that strongly absorb microwaves, the treatment duration can be reduced to as little as 1% of the time required by conventional methods. However, spodumene is generally transparent to microwaves at ambient temperatures, making it challenging to heat directly from room temperature. To address this limitation, hybrid microwave heating methods are employed [
56]. These methods use susceptors like silicon carbide (SiC), which strongly absorb microwaves and transfer heat to spodumene by conduction and convection. Once the spodumene reaches a critical temperature (approximately 634 °C), it becomes capable of directly absorbing microwave energy, promoting further rapid heating [
56].
During the initial stages of microwave-assisted calcination, α-spodumene undergoes an exothermic conversion to γ-spodumene, followed by an endothermic transformation into β-spodumene [
56]. The heat generated by γ-spodumene formation enhances microwave absorption, which can lead to localized melting if not controlled. It has been observed that β-spodumene begins to absorb microwaves at a lower temperature compared to α-spodumene, which translates in an acceleration of the temperature increase [
56].
Studies have shown that using a hybrid microwave system, an optimal lithium recovery of 97% could be achieved at a microwave power of 2.0 kW, comparable to conventional heating processes [
56]. Furthermore, microwave calcination resulted in lower impurity recoveries (Fe, Na, and Ca) during leaching, simplifying downstream purification. Microwave power influences not only the rate of phase transformation but also the overall leachability of spodumene. The transformation from α- to γ- and β-spodumene under microwave heating typically occurs around 900 °C [
55].
In summary, microwave-aided calcination presents a promising alternative for spodumene processing, offering improved energy efficiency, reduced environmental impact, and potentially lower processing costs compared to traditional heating methods.
4.5. Bioleaching
Bioleaching has been investigated as an eco-friendly alternative for lithium extraction from α-spodumene. Rezza et al. [
57] evaluated heterotrophic fungi including
Penicillium purpurogenum,
Aspergillus niger, and
Rhodotorula rubra, isolated from spodumene-rich environments. Two different media were tested: M
1, containing glucose, ammonium sulfate, and high concentrations of magnesium (Mg
2+), iron (Fe
2+), and potassium (K
+); and M
2, containing less nutrients to promote microbial adaptation to low-nutrient environments. The assays were conducted at 30 °C under continuous shaking at 200 rpm for up to 30 days [
57]. The results showed that
R. rubra achieved the highest lithium bioaccumulation (16.7 mg per 100 g of dry weight) and the highest lithium concentration in solution (1.53 ppm), followed by
P. purpurogenum and
A. niger. Organic acids such as gluconic and citric acids, along with exopolymers produced by the fungi, enhanced lithium solubilization. Microbial adaptation to nutrient-limited conditions in M
2 played a key role in improving lithium extraction efficiency.
In another study, Zhao et al. [
58] investigated lithium bioleaching from α-spodumene and lepidolite using two bacterial strains,
Raoultella sp.
Z107 and
Bacillus mucilaginosus (
BM). Ore samples from Xinjiang, China, were incubated with a silicate leaching medium at 30 °C for 30 days.
Raoultella sp.
Z107 showed higher lithium extraction, achieving 1.32 mg/L from lepidolite and 0.36 mg/L from spodumene, compared to
BM’s 0.77 mg/L and 0.26 mg/L, respectively.
Z107’s superior performance was linked to its higher organic acid production (up to 11 g/L lactic acid), promoting mineral dissolution, while
BM produced more extracellular polysaccharides (EPS), aiding metal complexation.
Z107 also lowered pH to 4.02, enhancing leaching. Structural analyses confirmed greater surface corrosion and crystallinity loss, especially in lepidolite.
Wang et al. [
59] studied the bioleaching of lithium from lithium silicate ores (α-spodumene, lepidolite, and elbaite) using
Acidithiobacillus ferrooxidans (
AF) and
Bacillus mucilaginosus (
BM).
AF showed higher lithium recoveries (3.43% for spodumene, 9.06% for lepidolite, and 1.06% for elbaite) than
BM (0.39%, 1.39%, and 0.22%, respectively) after 60 days at 30 °C.
AF’s enhanced performance was due to bio-sulfuric acid and organic acid production, promoting acidolysis and lattice disruption, while
BM relied on passive mechanisms. Lithium recovery was influenced by ore structure, with lepidolite (layered) showing better results than spodumene (chain) and elbaite (ring). The study highlighted the potential of bacterial bioleaching for environmentally friendly lithium extraction.
Despite the promising results observed in various bioleaching studies, lithium extraction rates from α-spodumene and other lithium silicate ores remain relatively low compared to conventional hydrometallurgical processes. Factors such as the mineral’s crystal structure, microbial metabolic pathways, and leaching conditions significantly influence bioleaching efficiency. While autotrophic and heterotrophic microorganisms, including Penicillium purpurogenum, Raoultella sp., and Acidithiobacillus ferrooxidans, have shown potential in enhancing lithium solubilization through organic acid production, EPS secretion, and acidolysis, the extraction efficiencies achieved so far are insufficient for industrial application. Further optimization of microbial strains, nutrient conditions, and process parameters is necessary to improve leaching rates and selectivity. Nevertheless, these studies highlight the potential of biotechnological approaches as environmentally friendly alternatives for lithium recovery from refractory minerals like α-spodumene.
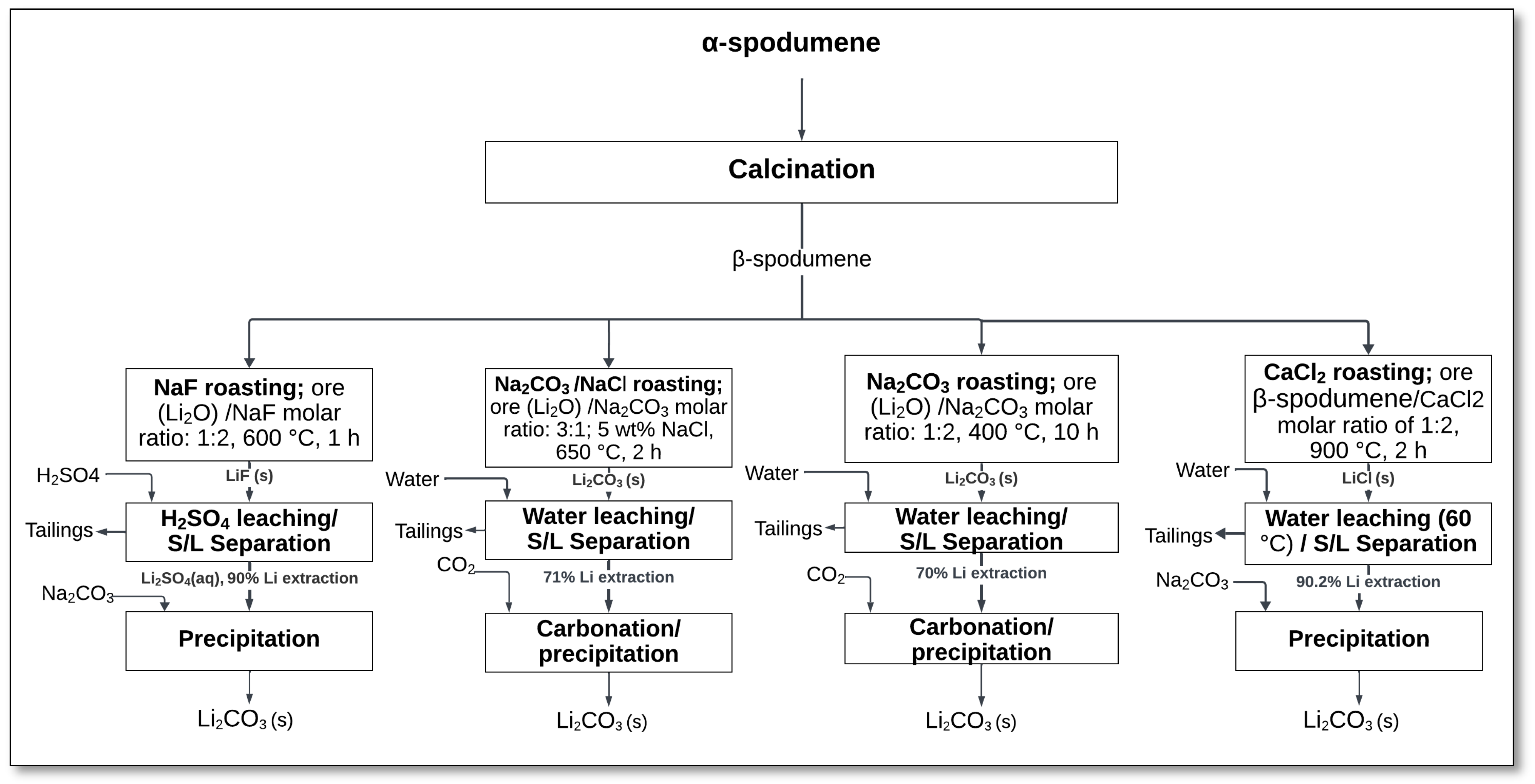
5. Lithium Extraction from β-Spodumene
Several alternative methods have been explored for lithium extraction from β-spodumene, as illustrated in
Figure 20. These methods include pressure leaching, atmospheric leaching, chlorination, and salt roasting, each employing different reagents and operating conditions:
Pressure leaching: This method utilizes reagents such as sodium sulfate, sodium carbonate, sodium chloride, sodium hydroxide, nitric acid, and carbonic acid to extract lithium under elevated pressure conditions.
Atmospheric leaching: Lithium extraction is achieved at atmospheric pressure using acids such as phosphoric acid or hydrofluoric acid, which help break down the β-spodumene structure.
Chlorination: This process involves the use of chlorine gas to react with β-spodumene, forming lithium chloride, which can be further processed.
Salt roasting: This method involves heating β-spodumene with various salts, including sodium fluoride, sodium carbonate and sodium chloride, calcium chloride, and sodium hydroxide, to enhance lithium extraction.
Figure 20.
Alternative methods for lithium extraction from β-spodumene.
Figure 20.
Alternative methods for lithium extraction from β-spodumene.
The following sections will explain these alternative methods in detail, outlining their processes, advantages, and challenges.
5.1. Pressure Leaching
5.1.1. Sodium Sulfate
Kuang et al. [
18] demonstrated that sodium sulfate pressure leaching is an effective alternative method for extracting lithium from β-spodumene. This process utilizes sodium sulfate and additives such as calcium oxide or sodium hydroxide to achieve high lithium recovery while minimizing environmental impacts. The approach aims to create a closed-loop system for lithium extraction and recovery, thereby reducing waste and enhancing resource efficiency.
The process started with the calcination of α-spodumene at 1100 °C for 1 h in a rotary kiln, achieving a conversion rate of 97% (
Figure 21). The resulting β-spodumene was then subjected to pressure leaching in an aqueous sodium sulfate solution with an additive (CaO or NaOH) under controlled conditions: a temperature of 230 °C, a reaction time of 3 h, a liquid-to-solid (L/S) ratio of 7.5 mL/g, and a particle size (D90) of 39 μm. During leaching, lithium ions in β-spodumene exchanged with sodium ions from the sodium sulfate solution, effectively leaching lithium into the solution, in accordance with Equation (36).
This method achieved high lithium extraction rates, with 93.3% recovery using CaO and 90.7% using NaOH as the additives. The PLS can be further purified to precipitate lithium as lithium carbonate. The leaching residue primarily consisted of analcime (NaAlSi2O6·H2O), which has potential applications in the glass-ceramic industry, offering opportunities for byproduct valorization. The presence of quartz (SiO2) in the residue confirmed the selectivity of the leaching process.
This method aligns with sustainability objectives by utilizing sodium sulfate as a byproduct of the lithium precipitation process in the traditional process, reducing waste, and providing a pathway toward a closed-loop lithium extraction process. The operational flow and key outcomes are illustrated in
Figure 21. XRD analysis of the leaching residue confirmed the formation of analcime as a stable and reusable byproduct [
18].
5.1.2. Sodium Carbonate
Chen et al. [
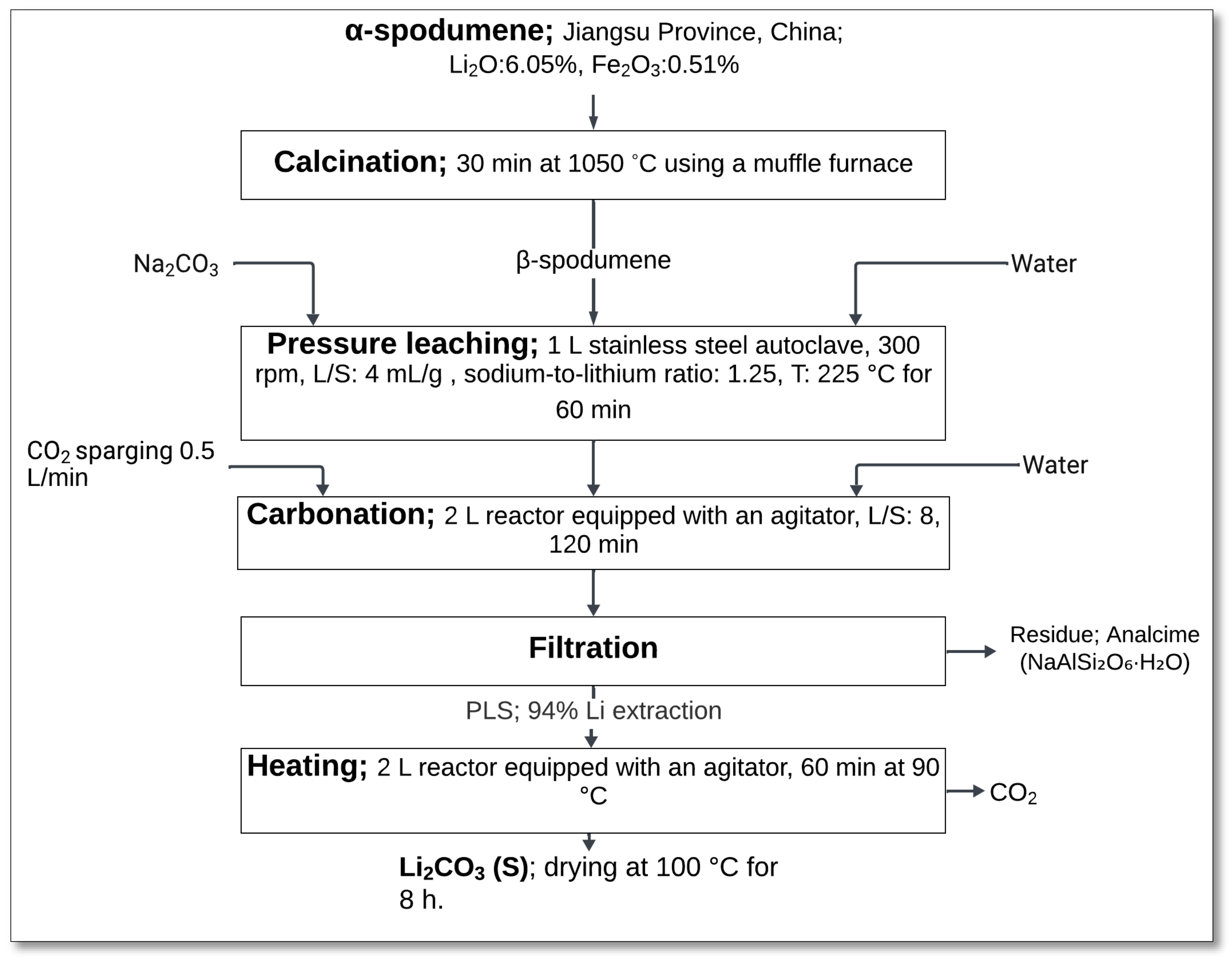
21] introduced a sodium carbonate autoclave process as an efficient method for extracting lithium from β-spodumene. This process eliminates the formation of sodium sulfate impurities and simplifies the overall lithium extraction process. By utilizing sodium carbonate as the leaching agent, this method achieved high lithium conversion efficiency while minimizing the environmental impact.
The process started with the calcination of α-spodumene at 1050 °C for 30 min in a muffle furnace, converting it to the β-phase (
Figure 22). Subsequently, β-spodumene was subjected to pressure leaching in a 1 L stainless steel autoclave using an aqueous sodium carbonate solution. The key operating parameters included an agitation speed of 300 rpm, a L/S (liquid-to-solid) ratio of 4 mL/g, a sodium-to-lithium ratio of 1.25:1, a temperature of 225 °C, and a reaction time of 60 min. During this step, lithium in β-spodumene reacted with sodium carbonate, forming lithium carbonate in the solution while generating analcime and quartz as solid residues, as represented in Equation (37) [
21].
After pressure leaching, the solution underwent carbonation in a 2 L reactor equipped with an agitator at a L/S ratio of 8 for 120 min. Carbon dioxide sparging (0.5 L/min) facilitated the conversion of lithium carbonate to lithium bicarbonate (LiHCO
3), as demonstrated by Equation (38).
The resulting slurry was then filtered, and the bicarbonate solution was heated at 90 °C for 60 min to regenerate lithium carbonate, releasing CO
2 and water in the process, in accordance with Equation (39).
The final lithium carbonate product was dried at 100 °C for 8 h, achieving a purity of 99.6%, a value superior to that obtained by the sulfuric acid method [
21].
The sodium carbonate process demonstrated significant advantages, including a lithium carbonate conversion efficiency exceeding 94% and the formation of high-purity lithium carbonate. Additionally, the process produced analcime as a byproduct, which has potential applications in the glass-ceramic industry. These outcomes, along with the operational simplicity and minimal impurity levels in the final product, make this method a promising alternative for sustainable lithium extraction.
The Metso-Outotec lithium hydroxide process is a high-pressure leaching method that utilizes sodium carbonate, commonly known as soda ash, for lithium extraction from spodumene concentrates. This process has been selected for implementation in the Keliber Project in Finland, where it is set to be launched in 2025 on a large scale, as reported online on the Lithium Project page by Sibanye-Stillwater. The method is gaining prominence due to its simplicity, efficiency, and environmental advantages [
72,
73].
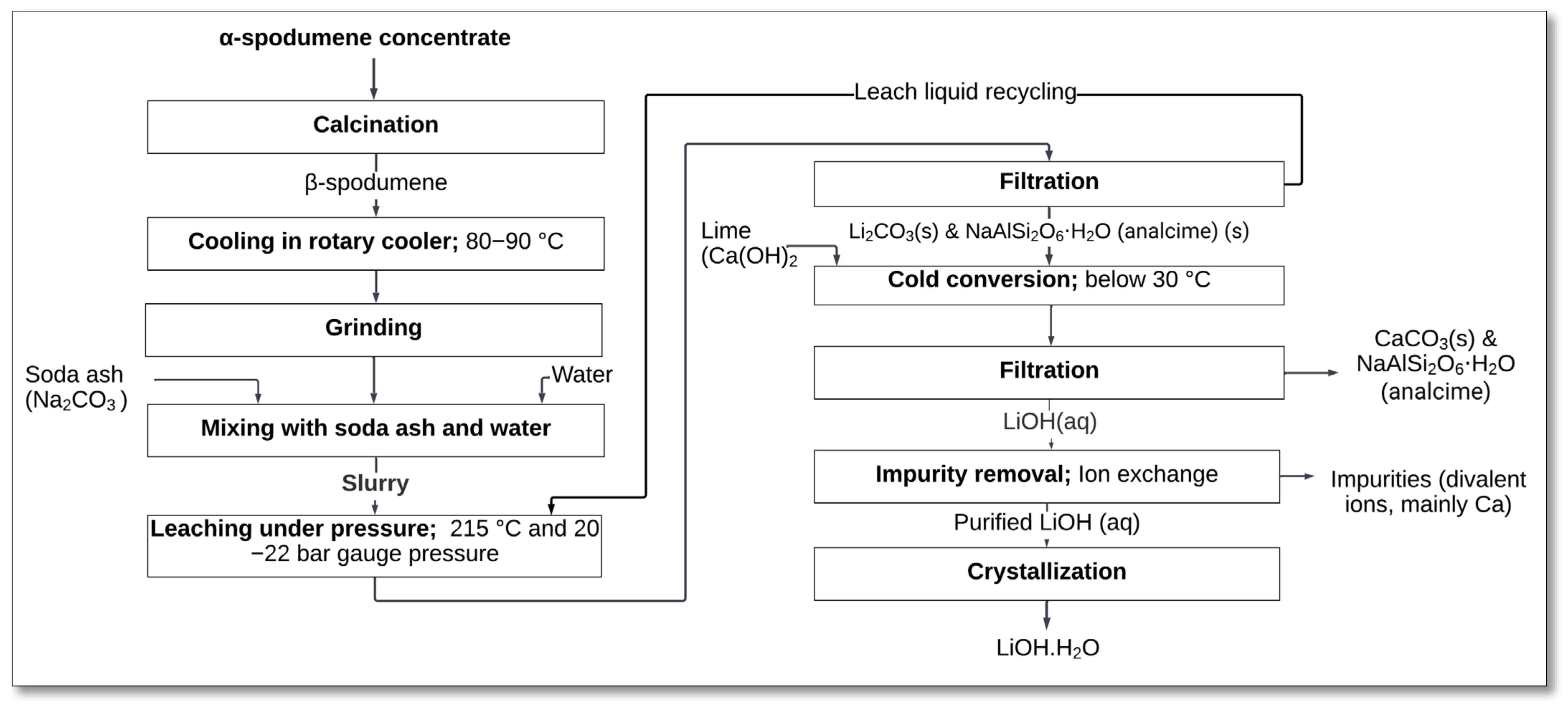
The process begins with the calcination of α-spodumene to convert it into β-spodumene, the more reactive phase. After cooling and grinding, the β-spodumene is mixed with soda ash and water to form a slurry. The mixture is then subjected to high-pressure leaching at 215 °C and 20–22 bar pressure to extract lithium into the leach solution (Equation (40)). This stage is efficient, as it avoids the use of strong acids or sulfates, which are common in conventional processes.
Once the leaching step is completed, the leach solution undergoes filtration to remove solid residues, including analcime. A cold conversion step follows, where lime (Ca(OH)
2) is added. A subsequent filtration process separates the residue, which consists mainly of analcime and calcium carbonate, from the PLS. The remaining impurities, primarily divalent ions such as calcium, are removed through ion exchange, ensuring the production of purified lithium hydroxide. The purified solution is then crystallized and dried to produce battery-grade lithium hydroxide monohydrate (
Figure 23) [
22].
The Metso-Outotec process offers several advantages. It avoids the use of acidic reagents and produces inert and neutral residues, simplifying waste management. Contaminant elements such as Fe, Al, Mg, and Ca exhibit extremely low solubility in the leach solution, minimizing the need for additional impurity removal steps. Furthermore, the process efficiently generates battery-grade lithium hydroxide with a streamlined and rapid throughput, making it suitable for large-scale industrial applications [
22]. Additionally, the analcime tailings produced in this process are not only inert but also valuable, as they can be utilized in the glass and ceramic industries, adding economic value and reducing waste. However, the process does require precise control of high-pressure and -temperature conditions to achieve optimal lithium recovery.
The successful adoption of the Metso-Outotec process by the Keliber Project, scheduled for commercial launch in Finland in 2025, highlights its potential as a sustainable and efficient method for lithium extraction. Its implementation marks a significant step forward in meeting the growing global demand for lithium products.
5.1.3. NaCl
The NaCl high-pressure leaching method, also known as the analcime process, represents a novel approach for extracting lithium from β-spodumene. Highlighted by Alhadad et al. [
19], this process emphasizes the dissolution of β-spodumene and the formation of analcime as a byproduct (
Figure 24). In this process, α-spodumene is first converted to β-spodumene through calcination at 1100 °C for 2 h in a muffle furnace. The leaching stage involved dissolving 20 g of NaCl and 0.5 g of NaOH in 200 mL of distilled water, resulting in a solution with an initial pH of 12.9. The leaching reaction occurred at 200 °C and 15.8 bar with stirring at 600 rpm for 1 h. During this step, sodium ions (Na
+) from the leaching solution replaced lithium ions (Li
+) in β-spodumene, allowing lithium to dissolve into the solution and promoting the formation of analcime as a byproduct, as shown in Equation (41).
Key findings of this process highlight the critical role of alkaline conditions in lithium extraction from β-spodumene. Without pH adjustment, lithium ions (Li
+) in β-spodumene exchange with sodium ions (Na
+), leading to the formation of hydrogen keatite (HAlSi
2O
6) as a byproduct. However, when NaOH was added, β-spodumene dissolved more efficiently, facilitating the precipitation of analcime (NaAlSi
2O
6·H
2O). Under highly alkaline conditions (pH > 11), aluminum and silicon dissolve uniformly, promoting the rapid recrystallization of analcime from the dissolved Al, Na, and Si, with lithium being replaced by sodium during the dissolution–precipitation process rather than through ion exchange. This mechanism accelerated lithium extraction and increased its concentration in the leach solution. As a result, lithium recovery rates were remarkably high, reaching 93% for high-grade spodumene (7% Li
2O) and 92.9% for low-grade spodumene (5.65% Li
2O). Furthermore, this process produced a leach liquor with significantly reduced impurity levels compared to sulfuric acid-based methods, improving both selectivity and efficiency in lithium recovery [
19].
Additionally, the analcime process, like some other lithium extraction methods, allows for the production of various lithium compounds, including lithium carbonate, lithium hydroxide, lithium chloride (LiCl), and lithium phosphate (Li
3PO
4), depending on market demand. The byproduct analcime holds potential for applications in zeolite production, adding further economic value [
19]. It is also used in the glass and ceramic industries, as well as in wastewater treatment for ion exchange, catalysis, and as a precursor for advanced adsorbent materials. Additionally, its thermal stability and porous structure make it suitable for applications in gas separation and molecular sieving.
This method claims sustainability and selectivity, presenting a compelling alternative to traditional sulfuric acid methods. The process flow and outcomes, including the formation of analcime, are illustrated in
Figure 24.
5.1.4. Nitric Acid
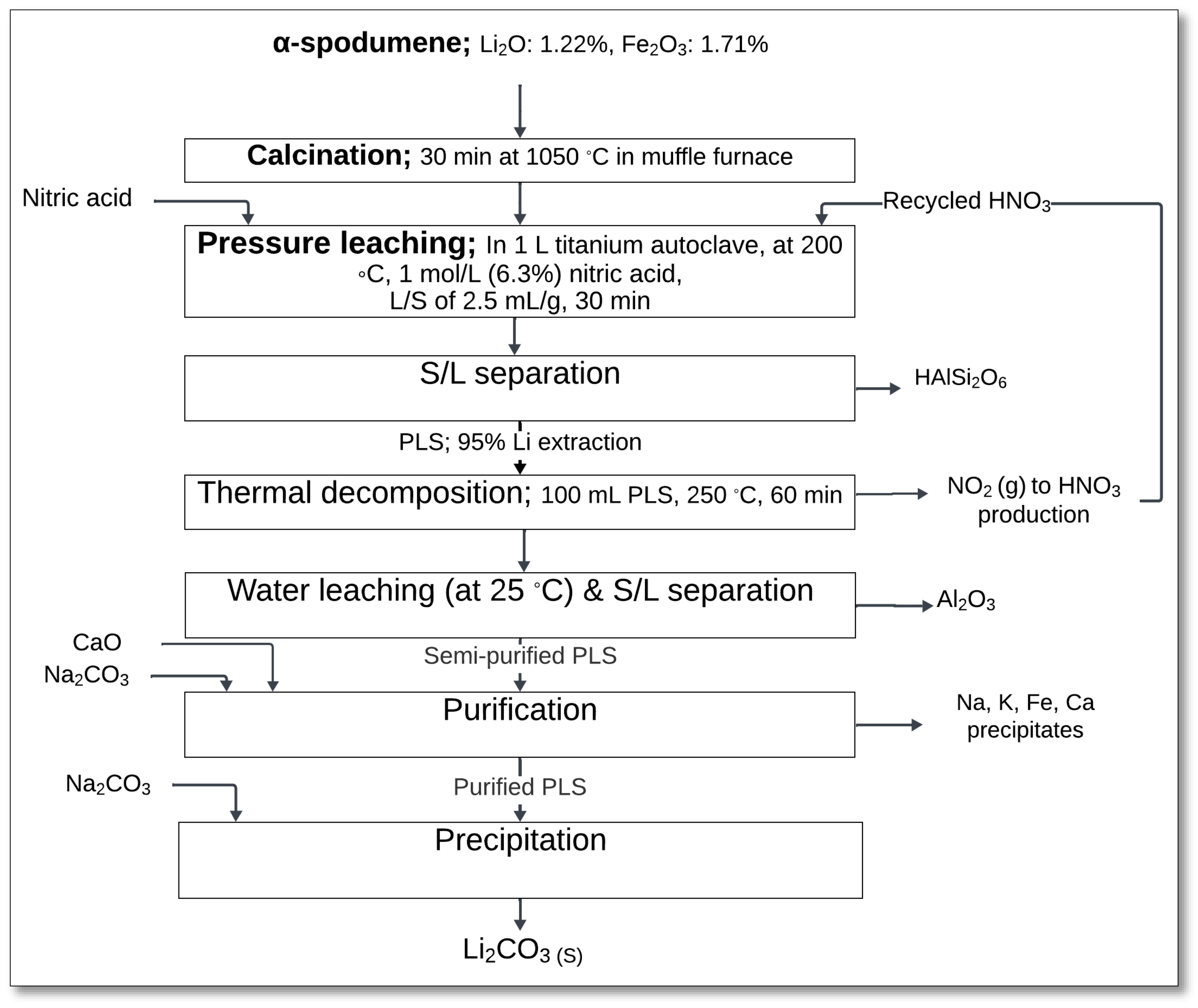
The use of nitric acid for lithium extraction from silicate minerals has been previously patented by Hunwick [
74]. The patented process involves mixing the silicate mineral with nitric acid, followed by a leaching process under controlled conditions to extract lithium into an aqueous phase as lithium nitrate, while minimizing the dissolution of non-lithium components.
More recently, Zhou et al. [
23] proposed a high-pressure leaching method using nitric acid, focusing on reducing reagent consumption, minimizing impurities, and recycling nitric acid to achieve a sustainable and economically viable process. The process started with the calcination of α-spodumene at 1050 °C for 30 min in a muffle furnace to convert it into β-spodumene (
Figure 25). During the leaching step, β-spodumene reacted with a 1 mol/L (6.3%) nitric acid solution in a 1 L titanium autoclave at 200 °C, using a liquid-to-solid (L/S) ratio of 2.5 mL/g for 30 min. This reaction produced lithium nitrate (LiNO
3) in solution and hydrogen keatite (HAlSi
2O
6) as a solid byproduct, as shown in Equation (42) [
23].
The method achieved an impressive lithium recovery rate of 95% from the PLS. After solid–liquid separation, the PLS underwent thermal decomposition at 250 °C for 60 min. During this step, lithium nitrate, which decomposes at a higher temperature of 600 °C, remains in the solution, while aluminum nitrate and other impurities in the residue decompose at a lower temperature of 200 °C (Equation (43)). This decomposition released nitrogen oxides (NO
x), which are subsequently recycled to regenerate nitric acid in a closed-loop system (Equations (44) and (45)). This recycling step enhanced reagent efficiency and significantly reduced waste [
23].
The process includes a purification step to remove impurities such as Na, K, Fe, and Ca, followed by the precipitation of high-purity battery-grade lithium carbonate suitable for battery production (Equation (46)).
While the integration of nitric acid recycling and efficient impurity management enhances the sustainability and environmental friendliness of the process, a notable byproduct is hydrogen keatite (HAlSi
2O
6), which is classified as an acidic tailing requiring proper handling and neutralization to mitigate potential environmental impacts. Therefore, while the method demonstrated significant advantages, careful management and further investigation into beneficial uses of its byproducts are essential for addressing its environmental challenges. As illustrated in
Figure 25, the nitric acid leaching method demonstrated a high degree of selectivity for lithium extraction while minimizing the dissolution of impurities.
5.1.5. Carbonic Acid
Carbonic acid high-pressure leaching is a method designed to extract lithium from β-spodumene using CO
2 gas and water to form carbonic acid under an elevated temperature and pressure, as per Equations (47) and (48).
This process, detailed by Alhadad et al. [
20], offers environmental advantages, including the use of near-neutral leach liquor, the utilization of waste CO
2, and reliance on inexpensive reactants.
The method involves sequential leaching steps in a pressurized autoclave at 200 °C and 100 bar (
Figure 26). Each stage lasts one hour, with a L/S (liquid-to-solid) ratio of 50 and an initial water pH of 6.9–7.1. Lithium ions (Li
+) are extracted, while β-spodumene partially dissolves, forming aluminum hydroxide [Al(OH)
3] and silica phases in the residue (Equation (49)). After six sequential steps, the process achieved a cumulative lithium recovery of 75%, while a single-stage leach yielded only 12.6%, underscoring the need for extended multi-step digestion to improve recovery [
20].
Despite its environmentally friendly features, the process has limitations. At the weakly acidic pH, protons lack the activity needed to penetrate deeply into β-spodumene to exchange Li
+ ions effectively. Instead, protons primarily adsorb onto the mineral’s surface, restricting lithium extraction to partial dissolution. Additionally, the batch process requires significant time—potentially hundreds of hours—to achieve recoveries similar to those of other methods [
20].
Implementing this process at an industrial scale presents further challenges. These include recycling substantial water volumes, mitigating the formation of silica coatings that hinder dissolution, and addressing the dilution of lithium solutions. Furthermore, the slow hydrolysis of the Li-depleted sublayer and industrial hesitancy toward high-pressure treatments must also be considered.
While carbonic acid leaching provides clear environmental benefits, including CO2 utilization and the avoidance of strong acids, its low recovery rates, prolonged digestion times, and operational challenges limit its broader adoption in the lithium extraction industry.
5.2. Atmospheric Leaching
5.2.1. Phosphoric Acid
Atmospheric leaching using phosphoric acid is a potential alternative to traditional sulfuric acid leaching for extracting lithium from β-spodumene. This method, as investigated by Paris et al. [
24], aims to reduce the environmental and safety risks associated with spodumene processing by employing a less hazardous and less energy-intensive reagent.
The process begins with the calcination of α-spodumene at 1100 °C for 4 h to convert it into the β-phase (
Figure 27). The leaching step involved treating β-spodumene with an 8 M phosphoric acid solution at 100 °C for 8 h, using a liquid-to-solid (L/S) ratio of 10 mL/g in 15 mL digestion tubes. The reaction achieved over 40% lithium leaching efficiency, forming aluminum silicate as the tailing [
24].
Despite its advantages, including the use of a less hazardous reagent, the lithium extraction efficiency of this method is significantly lower than traditional sulfuric acid leaching, which typically achieves >95% extraction. However, phosphoric acid leaching offers a safer and more environmentally friendly alternative, especially for operations prioritizing reduced chemical hazards and energy consumption.
While phosphoric acid leaching shows promise as a safer and more sustainable method, its comparatively lower lithium extraction efficiency presents a key limitation. Further research is required to enhance its performance and evaluate its scalability for industrial applications.
5.2.2. Atmospheric Leaching with HF
Atmospheric leaching using hydrofluoric acid (HF) has been explored for lithium extraction from β-spodumene, as described by Rosales et al. [
75], where fluoride-based compounds such as cryolithionite (Li
3Na
3Al
2F
12), sodium hexafluorosilicate (Na
2SiF
6), and cryolite (Na
3AlF
6) were synthesized as byproducts. More recently, Rosales et al. [
25] proposed an alternative method leveraging the high reactivity of HF to dissolve β-spodumene efficiently, achieving high lithium recovery rates. Despite its effectiveness, this method presents challenges due to the corrosive nature of HF, requiring careful handling and specialized equipment.
The process begins with the calcination of α-spodumene at 1100 °C to convert it into the β-phase (
Figure 28). The HF leaching stage was conducted in a 500 mL PVC vessel under atmospheric pressure at 75 °C for 20 min. A solid-to-liquid ratio of 1.82% (
w/
v) and a HF concentration of 7% (
v/
v) were maintained, while stirring at 330 rpm. During this step, β-spodumene reacted with HF to produce a PLS containing lithium fluoride (LiF) along with dissolved aluminum and silicon species [
25], as per Equation (50).
Following leaching, aluminum and silicon were precipitated as Na
3AlF
6 (cryolite) and Na
2SiF
6 (sodium fluorosilicate) by the addition of sodium hydroxide (Equation (51)). These byproducts have potential applications in various industries, offering a pathway for waste valorization.
Lithium was then recovered from the PLS through carbonation with CO
2, precipitating lithium carbonate with a purity of 98.3% and a lithium recovery rate of 90%, as demonstrated by Equations (52)–(54) [
25].
While this method demonstrated high lithium recovery and produced high-purity lithium carbonate, the use of HF introduces significant disadvantages. HF is highly corrosive, posing substantial risks to both operators and equipment. Specialized materials, such as PVC or PTFE, are required for containment, increasing operational costs. Moreover, the hazardous nature of HF necessitates stringent safety protocols and regulatory compliance, further complicating its industrial adoption.
In summary, atmospheric HF leaching offers an efficient method for lithium extraction, with high recovery and purity. However, its reliance on HF presents major environmental, safety, and economic challenges that must be addressed for broader implementation.
5.3. Chlorination with Chlorine Gas
Chlorination with chlorine gas is a promising alternative method for extracting lithium from β-spodumene. Barbosa et al. [
17] investigated this process, which involves roasting β-spodumene with pure chlorine gas in a fixed-bed reactor. This method allows lithium recovery as lithium chloride while leaving aluminum and silicon in the solid residue.
The process started with the calcination of α-spodumene at 1180 °C for 2 h, converting it to the more reactive β-phase (
Figure 29). The β-spodumene was then roasted in a chlorine gas atmosphere at temperatures ranging from 1000 to 1100 °C for up to 150 min. Lithium in the spodumene structure reacted with chlorine to form LiCl, which volatilized during the roasting process. The remaining solid residue primarily consisted of mullite (Al
6Si
2O
13) and cristobalite (SiO
2), indicating that aluminum and silicon did not form chlorides under these conditions [
17].
A key advantage of this method is its high lithium recovery rate, with complete extraction achievable under optimal conditions (1100 °C and 150 min). The use of chlorine gas also allows for the selective chlorination of lithium, minimizing the formation of unwanted byproducts. However, the process has significant limitations. Chlorine gas is highly toxic and corrosive, requiring stringent safety measures and specialized equipment for containment and handling. Additionally, the need for precise temperature control and recycling of chlorine gas adds complexity and cost to the process.
Despite these challenges, chlorination with chlorine gas offers an efficient pathway for lithium extraction from spodumene, particularly in scenarios where the formation of high-purity lithium chloride is desired. Future work should focus on optimizing reaction conditions, improving chlorine recycling systems, and addressing the environmental and safety risks associated with chlorine handling.
5.4. Salt Roasting
Salt roasting is a viable alternative method for extracting lithium from β-spodumene by replacing lithium ions (Li
+) with alkali metal ions such as Na
+ or Ca
2+. The process involves roasting β-spodumene with salts such as Na
2CO
3, NaCl, NaF, or CaCl
2 at high temperatures, followed by water or sulfuric acid leaching and precipitation of lithium carbonate. Studies by Barbosa et al. [
26], dos Santos et al. [
27], Rosales et al. [
28], and Grasso et al. [
29] have demonstrated the efficiency of this approach, exploring various salt combinations to optimize lithium extraction. The process begins with the calcination of α-spodumene to β-spodumene to enhance its reactivity. The β-spodumene is then roasted with salts under specific conditions to facilitate the exchange of Li
+ ions for Na
+ or Ca
2+ ions.
Different salts and roasting conditions yielded varying efficiencies (
Figure 30). Roasting with NaF at 600 °C for 1 h achieved 90% lithium recovery, following the reaction given in Equation (55) [
28]:
Similarly, roasting with a mixture of Na
2CO
3 and NaCl at 650 °C for 2 h achieved 71% lithium recovery, as described by Equation (56) [
27]:
For Na
2CO
3 alone, roasting at 400 °C for 10 h yielded 70% lithium recovery. Calcium chloride (CaCl
2) roasting at 900 °C for 2 h demonstrated the highest efficiency, with 90.2% lithium recovery, facilitated by the reaction of Equation (57) [
26]:
The salt roasting method offers a few advantages, including relatively simple reaction conditions and reduced reliance on strong acids. Additionally, salts like NaCl promote the formation of highly soluble lithium chloride (LiCl), simplifying downstream processing steps. However, this method has notable drawbacks. The requirement for multiple calcination stages increases energy consumption, while the use of salts such as NaF and CaCl2 can severely corrode equipment, necessitating the use of expensive, corrosion-resistant materials. Large quantities of Na+ or Ca2+ introduced into the leach liquor may affect the quality of the lithium product, requiring additional purification steps.
Despite these challenges, the salt roasting method holds potential for lithium extraction due to its adaptability to various salt reagents. However, industrial implementation must address issues such as equipment corrosion, energy efficiency, and byproduct management. Future research should focus on optimizing reaction conditions and exploring the economic feasibility of this method.
Salt roasting using sodium hydroxide (NaOH) is another promising approach for lithium extraction from β-spodumene. Grasso et al. [
76] explored this method, which combines mechanochemical activation and thermal treatment to achieve high lithium recovery while minimizing the use of water and harsh chemicals. This approach achieved a lithium carbonate recovery rate of 91.2%.
The process began with the calcination of α-spodumene at 1100 °C for 2 h, converting it to the β-phase (
Figure 31). A mechanochemical activation step followed, in which β-spodumene was mixed with solid NaOH in a 1:1 molar ratio. The mixture underwent planetary ball milling for 1 h in air, and creating agglomerates with particle sizes ranging from 10 to 160 μm. This step enhanced chemical reactivity by introducing surface and bulk defects in the material. The activated mixture was then subjected to thermal treatment at 300 °C for 5 h in static air. The reaction during thermal treatment is presented in Equation (58).
The resulting product underwent water leaching with deionized water for 30 min at 60 °C to dissolve lithium compounds, leaving behind NaAlSi
2O
6 as the residue. The PLS was filtered and dried to produce high-purity lithium carbonate [
76].
This method consumes minimal water, which would be beneficial for regions with limited water resources. However, the process requires precise control of reaction parameters, including temperature and mechanochemical activation, to achieve high recovery rates. Additionally, it involves energy-intensive two-stage high-temperature operations, which can increase overall energy consumption and operational costs. In summary, salt roasting with NaOH offers an efficient and sustainable pathway for lithium extraction, with a recovery rate exceeding 90%. Future studies could focus on further optimizing the mechanochemical activation step to enhance efficiency and cost-effectiveness.
6. Discussion
The extraction of lithium from spodumene concentrates has traditionally relied on sulfuric acid roasting, a commercially proven method. However, this route presents several challenges, including high energy consumption, the production of environmentally harmful acidic tailings, and the generation of complex purification demands. In response, numerous alternative processes have been developed and refined to overcome these limitations, offering pathways toward more sustainable, efficient, and environmentally friendly lithium production.
6.1. Lithium Extraction from α-Spodumene
Salt Roasting: Several salt roasting strategies have been evaluated for lithium extraction from α-spodumene. Carbonate-assisted calcination using sodium carbonate (Na
2CO
3) at 850 °C followed by sulfuric acid leaching achieved lithium recoveries up to 99.8% [
30], while a salt roasting reaction between Na
2CO
3 and spodumene at 750 °C followed by water washing obtained 90.4% lithium recovery without acid leaching [
31]. Roasting with sodium sulfate (Na
2SO
4) combined with calcium oxide (CaO) lowered the activation temperature to approximately 900 °C and yielded a lithium recovery of 95.45% through direct water leaching [
33]. Mechanical activation of spodumene–Na
2SO
4 mixtures followed by calcination at 1000 °C also improved lithium extraction, reaching approximately 92% recovery [
34]. Salt roasting approaches using Na
2CO
3, Na
2SO
4, NaOH, and KOH at temperatures from 315 to 1000 °C, depending on the specific reagent, followed by water and sulfuric acid leaching, demonstrated a total lithium recovery around 88% for NaOH roasting, with water leaching alone achieving about 71%, while other reagents exhibited lower efficiencies [
40]. Fluoride-based additives such as sodium fluoride (NaF) [
35], potassium fluoride (KF) [
39], and ammonium bifluoride (NH
4HF
2) [
42] have also been employed to reduce roasting temperatures. NH
4HF
2 allows nearly complete lithium extraction (up to 96%) at around 157 °C, although fluoride-assisted processes raise environmental and residue management concerns.
Chlorine- and fluoride-based salts, despite their high performance in salt roasting processes [
35,
38,
39,
40], present significant challenges, including the need for corrosion-resistant equipment, work safety concerns, and the complex environmental management of residues and effluents. In comparison, sulfate, carbonate, and hydroxide salts are associated with fewer operational issues. Sodium hydroxide roasting has demonstrated high lithium recovery at relatively low temperatures (~320 °C) compared to other sodium salts [
40]. This can be attributed to the alkali reaction promoted by the hydroxide, which enhances silicate solubilization, as well as the smaller ionic radius of sodium (a monovalent cation), which likely facilitates diffusion during solid-state reactions.
On the other hand, lithium recoveries above 95% have been achieved through carbonate roasting at temperatures exceeding 900 °C [
33]. Na
2SO
4–CaO roasting has shown promising performance in terms of lithium recovery and water leaching, without the need for acidic or basic reagents [
33].
At roasting temperatures approaching 1100 °C, energy consumption becomes comparable to that of conventional α- to β-spodumene conversion. Although potential energy savings may still be achieved with salt roasting, the chemical efficiency of the process must exceed that of conventional methods to justify large-scale implementation. Mechanical activation prior to roasting appears to offer limited benefit, as higher recoveries have been obtained through direct roasting at 1000–1200 °C without activation [
43], or at 900 °C using CaO and Na
2SO
4 [
33], making the added value of activation difficult to justify [
34].
Pretreatment–atmospheric leaching: Pretreatment combined with atmospheric leaching has been explored as a lower-energy alternative to conventional calcination and sulfuric acid digestion. Mechanical activation of spodumene with sodium fluoride (NaF) followed by atmospheric leaching with dilute sulfuric acid achieved a lithium recovery of about 81% [
45]. Alkaline decomposition methods, using NaOH [
46,
47] or KOH [
48], have also been studied: NaOH pretreatment followed by acid leaching achieved 78.7% lithium recovery, while KOH pretreatment achieved a higher recovery of 84.1%. These methods reduce energy requirements by avoiding high-temperature phase transformation and enable lithium extraction at moderate conditions. However, challenges remain related to silica gel formation during leaching, impurity control, and relatively modest lithium recoveries compared to roasting-based methods.
Direct high-pressure leaching: High-pressure NaOH leaching demonstrated lithium recoveries up to 95.8% when performed at 250 °C with concentrated NaOH solutions [
49]. The process benefits from low energy consumption and generates byproducts like hydroxysodalite and calcium silicate, although sodium contamination must be managed during purification. High-pressure leaching with combined NaOH and CaO achieved lithium extraction efficiencies of around 93% by forming lithium phosphate intermediates, but it involved harsh reaction conditions and large reagent dosages [
50]. Atmospheric leaching with hydrofluoric acid and sulfuric acid mixtures achieved lithium extraction efficiencies of 96% under mild temperature and pressure conditions [
51,
52]. However, the use of HF raises significant safety and environmental concerns, requiring specialized equipment and careful waste management. An optimized two-stage thermal treatment process has been proposed to minimize fluorine residue and improve lithium recovery to about 87.35% [
53]. Despite the high recovery rates, challenges remain with fluoride management and downstream purification steps.
Overall, direct leaching strategies, especially high-pressure NaOH leaching, offer promising lithium recoveries while avoiding complete thermal transformation of α-spodumene. However, careful control of reagent use, impurity management, and safety considerations remain essential for industrial implementation.
Microwave-assisted calcination: Microwave-assisted calcination has shown potential to reduce the high energy consumption associated with conventional spodumene processing [
55,
56]. Lithium recoveries of up to 97% have been achieved using hybrid microwave systems [
56], while also lowering impurity dissolution during leaching. However, the need for susceptors [
56] and careful process control to avoid localized overheating, along with challenges in scaling up microwave systems [
55,
56], remain key limitations for industrial adoption. As an example, microwave rotary kilns face several industrial-scale challenges that further complicate the adoption of microwave-assisted calcination. These include difficulties in achieving uniform heating, controlling temperature profiles, and maintaining stable process conditions. Issues such as material sticking, thermal degradation, and uneven energy distribution can reduce process efficiency. In addition, the high capital and maintenance costs, along with safety concerns related to high-frequency electromagnetic exposure, present further barriers to large-scale implementation [
77].
Bioleaching: Bioleaching approaches using heterotrophic fungi, heterotrophic bacteria, and autotrophic bacteria such as
Acidithiobacillus ferrooxidans have shown limited lithium extraction efficiencies for α-spodumene, with maximum recoveries reaching only 3.43% [
57,
58,
59]. Although fungal leaching generally provided faster kinetics than bacterial routes, overall lithium recoveries remained low, requiring further optimization for industrial application.
6.2. Lithium Extraction from β-Spodumene
High-pressure leaching: High-pressure leaching methods utilize a variety of leaching agents, including sodium sulfate, sodium carbonate, sodium chloride, nitric acid, and carbonic acid, to achieve lithium extraction under elevated temperature and pressure conditions. Sodium sulfate leaching [
18] demonstrated lithium recovery efficiencies of 90.7–93.3% with the added advantage of producing inert analcime tailings, which hold economic potential for applications in the glass and ceramic industries. Similarly, sodium carbonate leaching [
21] achieved over 94% recovery with high-purity lithium carbonate production, making it one of the most efficient high-pressure leaching methods.
In addition to other sodium-based systems, sodium chloride high-pressure leaching [
19] introduces an alternative process in which lithium is selectively extracted as LiCl, achieving a recovery of about 93%, while aluminum and silica phases remain in the residue. Nitric acid high-pressure leaching [
23] achieved over 95% lithium recovery through efficient leaching, followed by a thermal decomposition stage that regenerates nitric acid in a closed-loop system, minimizing reagent loss. While this method is environmentally advantageous, the corrosive nature of nitric acid necessitates careful handling and specialized equipment. Additionally, the resulting tailings are acidic, posing a potential environmental challenge that must be addressed. Carbonic acid high-pressure leaching [
20] offers a more sustainable approach by utilizing CO
2 gas to generate a near-neutral leach liquor. Although its cumulative lithium recovery reached 75%, the process suffers from slow kinetics and limited single-stage efficiency (12.6%) [
20].
The Metso-Outotec lithium hydroxide process is a notable industrial example of high-pressure leaching, scheduled for implementation at Finland’s Keliber Project in 2025. By combining sodium carbonate leaching with precise control of temperature (215 °C) and pressure (20–22 bar), this process produces battery-grade lithium hydroxide while avoiding the use of strong acids and generating environmentally inert analcime tailings [
22,
72]. Its successful deployment would confirm the scalability and economic viability of high-pressure leaching as a sustainable alternative to sulfuric acid digestion.
Atmospheric leaching: While atmospheric leaching methods, such as phosphoric acid [
24] and HF leaching [
25], can reduce energy consumption, they typically achieve lower lithium recovery rates. Phosphoric acid leaching, for instance, achieved ~40% recovery only but is safer and less aggressive than sulfuric acid. In contrast, HF leaching achieved much higher recoveries (~90%) but poses safety risks and equipment corrosion issues. Similarly, chlorination roasting methods [
17] offer selective lithium recovery but introduce handling challenges due to the toxic and corrosive nature of chlorine gas.
Salt roasting: Salt roasting processes, including CaCl
2 [
26], NaF [
28], Na
2CO
3 [
29], and NaOH [
41] roasting, achieved high lithium recoveries of 70%–90% while avoiding strong acids. However, these processes require two-stage roasting: an initial calcination step to convert α-spodumene to β-spodumene, followed by a salt roasting stage. This dual-stage heating significantly increases energy consumption and operational costs, reducing their industrial attractiveness. Additionally, challenges such as equipment corrosion (e.g., when using NaF or CaCl
2) and the introduction of impurities into the product further limit their large-scale adoption.
6.3. Future Research Directions
Comparative evaluation of alternative lithium extraction methods shows that each approach presents specific advantages and limitations depending on factors such as energy consumption, reagent requirements, environmental impact, and scalability. Salt roasting, atmospheric leaching, microwave-assisted calcination, and pressure leaching all represent potential pathways, with trade-offs that must be considered for industrial application. To further advance lithium extraction technologies, future research should focus on the following:
Optimization of energy usage: This should focus on reducing the overall energy intensity of lithium extraction processes through improved heat recovery, process integration, and the development of lower-temperature treatment methods.
Reagent recycling: This should focus on developing closed-loop systems to recover and reuse reagents, minimizing environmental impact and operational costs.
Byproduct valorization: This should focus on exploring the potential applications of tailings such as analcime in the glass, ceramic, and construction industries, leveraging its aluminosilicate properties for functional benefits. Analcime can also be utilized in the production of zeolites for ion exchange applications, such as wastewater treatment and heavy metal removal. Its porous structure makes it valuable for catalysis in chemical reactions, pollution control, and industrial filtration processes. Furthermore, analcime has been used as a filler material in plastics and paper, as well as a soil conditioner and fertilizer additive, contributing to improved soil quality [
78,
79,
80].
Scalability studies: These should focus on bridging the gap between laboratory-scale research and industrial applications through pilot-scale validation. The Metso-Outotec lithium hydroxide process, scheduled for implementation at Finland’s Keliber Project in 2025, exemplifies how precise control of conditions and valorization of inert analcime tailings can contribute to the economic viability and environmental sustainability of industrial lithium extraction [
22,
72].
Hybrid approaches: These should focus on combining pretreatment methods (e.g., mechanical activation) with calcination and leaching processes to enhance lithium recovery while reducing energy consumption.
Advancement of bioleaching kinetics and scale-up methodologies: This should focus on enhancing the efficiency and speed of microbial processes to eventually make bioleaching a more viable industrial alternative.
While energy consumption is a crucial consideration in assessing lithium extraction methods, the majority of reviewed studies lack detailed quantitative data in this regard, hindering direct comparisons. This highlights the importance of future research efforts systematically reporting energy metrics to allow more accurate evaluations of both the economic viability and environmental impact of different extraction approaches.
Incomplete reporting of process outputs: Many studies do not provide sufficient details regarding the composition of the pregnant leach solution (PLS) and the nature or performance of subsequent purification steps. This lack of standardized information makes it difficult to compare processes and assess trade-offs across different extraction approaches. Future research should prioritize comprehensive reporting to facilitate meaningful evaluations and technology selection.
Continued innovation in these areas is critical to achieving economically and environmentally sustainable lithium supply chains to meet the rapidly growing demand for lithium-ion batteries and related technologies.
7. Conclusions
This review systematically evaluated emerging alternatives to sulfuric acid digestion for lithium extraction from both α- and β-spodumene. The conventional sulfuric acid route, although efficient, faces major challenges such as high energy consumption, the production of acidic waste, and complex downstream purification requirements. In response, several innovative methods have been developed, aiming to reduce environmental impacts, improve process efficiency, and enhance the overall sustainability of spodumene processing.
For α-spodumene, salt roasting with sodium sulfate and calcium oxide emerged as a promising route, enabling high lithium recoveries (>95%) under milder thermal conditions and allowing water-based leaching without aggressive acids. Other alternatives, such as fluoride-assisted roasting, pretreatment–atmospheric leaching, and direct high-pressure alkaline leaching, show potential but introduce specific challenges related to reagent consumption, silica gel formation, fluoride management, or operational complexity. Microwave-assisted calcination may offer significant energy savings and improved lithium recoveries, but industrial scalability remains under development. Bioleaching, despite being environmentally benign, is currently limited by slow kinetics and very low extraction efficiencies.
For β-spodumene, high-pressure leaching processes using sodium sulfate, sodium carbonate, and sodium chloride demonstrated high lithium recovery rates (typically above 90%), with the added advantage of producing environmentally inert residues such as analcime. Nitric acid leaching introduced a closed-loop approach with efficient reagent recycling, though requiring careful management of acidic tailings. Carbonic acid leaching offers a greener alternative but suffers from slower kinetics and lower extraction efficiencies. Atmospheric leaching and salt roasting approaches represent viable options but face challenges related to energy requirements, equipment corrosion, and product purity.
Comparative evaluation reveals that no single method is universally superior. Instead, each alternative presents specific trade-offs between lithium recovery, reagent use, operational conditions, environmental impact, and economic viability. Continued innovation focusing on energy optimization, reagent recycling, byproduct valorization, and scalability will be critical for advancing the next generation of sustainable lithium extraction technologies.
Ultimately, the transition from sulfuric acid digestion to more sustainable processing routes will play a vital role in supporting the growing global demand for lithium, particularly for battery applications, while minimizing the environmental footprint of lithium production.