Abstract
Copper polymetallic sulfide ore from the Asgat copper polymetallic deposit in Mongolia has been found to contain a high grade of antimony and silver in addition to copper. In this research, flotation experiments using sodium butyl xanthate (SBX), ammonium dibutyl dithiophosphate (ADD), isopropyl ethyl thionocarbamate (Z-200), and their mixtures were conducted on a sample from the deposit under natural pH conditions. The results of the flotation tests indicate that optimal conditions were achieved with a feeding of 92% −0.074 mm (92% finer than 0.074 mm), sodium silicate dosage of 800 g/t, sodium humate dosage of 300 g/t, sodium sulfite dosage of 300 g/t, and collectors of 60 g/t of ADD and 60 g/t of Z-200. The closed-circuit flotation tests showed that the recovery of copper, antimony, and silver from the ADD/Z-200 combination was 8.13%, 5.41%, and 9.26% higher than that form the single Z-200 while reducing the reagent cost by 12.75%.
1. Introduction
Copper polymetallic sulfide ores are significant carriers of copper, zinc, lead, and even other rare and precious metals, such as gold and silver [1,2,3,4]. Flotation is undoubtedly the most competitive separation method. However, due to complex lattice structures and the similarity of their surface properties to those of sulfide gangue minerals, these ores pose significant challenges to the Cu-S flotation process within the industry [5,6,7]. Generally, the high-alkali lime technique remains an acknowledged and effective method for copper flotation [8]. However, the addition of large amounts of lime not only depresses sulfide gangue minerals, but also limits the flotation of precious metals such as silver [9,10,11,12,13]. Therefore, the study of the flotation of copper polymetallic sulfide ores under conditions of low alkalinity, or even neutrality, is of great importance, in which the development of novel selective collectors or combinations is highly anticipated.
The inherent problem of the flotation of multiple complex ores stems from the varying surface and interface properties of the diverse mineral compositions; therefore, it is not difficult to understand that the series of problems associated with single-collector flotation, such as high reagent consumption and poor flotation performance. In fact, the initial practice of using combined collectors to address flotation issues in copper sulfide ore can be traced back to the 1950s [14]. Meanwhile, many of the collectors developed subsequently were also combinations, such as No. 25 dithiophosphate collectors. As posited by Buckley A.N. et al. [15], the term “synergism” is defined as the recovery of a specific mineral from an ore using mixed-collector concentrations that are considerably lower than those of either individual collector needed to achieve a similar recovery and grade. In other words, at comparable concentrations, the recovery, grade, and/or rate would be enhanced with specific mixed collectors as compared to the use of individual collectors.
Given to their excellence performance in applications, the potential interactions of mixed collectors, as well as their adsorption on mineral surfaces, have been studied. Lee K. et al. [16] found that using n-octyl hydroxamates (AM28, produced by Ausmelt Limited) in conjunction with conventional sulfide collectors can effectively recover copper sulfides and oxides simultaneously. Furthermore, the copper recovery from blended ore using a mixture of collectors was shown to be superior to the recovery obtained using only xanthate after controlled potential sulfidization. Roy S. et al. [17] conducted a comparative analysis of the flotation performance of copper smelter slag using sodium isopropyl xanthate (SIPX), sodium diethyl dithiophosphate (DTP), alkyl hydroxamate, and their mixtures. Their findings indicate that DTP and alkyl hydroxamate functioned as co-collectors with SIPX, facilitating the recovery of coarse interlocked copper-bearing particles. Additionally, these compounds exhibited a substantial impact on the behavior of the froth phase. The feasibility of selective sulfide flotation using sodium iso-butyl xanthate (SIBX), n-butoxycarbonyl-O-n-butyl thionocarbamate (BBT), disecondary butyl dithiophosphate (DBD), and their mixtures as collectors was evaluated with the complex copper–silver–gold minerals from northern Norway [18]. The results show that a blend of xanthate and thionocarbamate at a ratio of 1:3 exhibited the optimal degree of copper recovery, with percentages of 29% and 98%, respectively. Furthermore, a substantial enhancement in gold and silver recoveries/grades was observed in the presence of collector mixtures. Guner M.K. et al. [19] found that incorporating a diverse array of collectors within the blend enhances flotation performance, as indicated by metrics such as recovery, kinetics, and selectivity. A comparative analysis of collector mixtures revealed that AEROPHINE® 3422, a blend of isopropyl ethyl thionocarbamate (IPETC) and dithiophosphinate (DTPi), exhibited superior performance in both copper and silver flotation when compared to the NAX mixture (a blend of sodium isobutyl xanthate (SIBX) and sodium ethyl xanthate (SEX)). Liu Y. et al. [20] investigated the synergistic effect of sodium diisobutyl dithiophosphinate (Aerophine 3418A, produced by Solvay Limited)/ammonium-dibutyl dithiophosphate (DTP) mixed collector in the flotation of chalcopyrite. The results show that the adsorption quantity of the Aerophine 3418A/DTP mixed collector is larger than that of the single collector on the chalcopyrite surface, thereby promoting the adsorption of adjacent collector molecules.
The Asgat mega copper polymetallic deposit, located in the northern side of the Mongolian Altai Mountains, is one of the largest copper–silver ore districts in Central Asia [21]. The data shows that the copper and silver reserves in this region are estimated to be as high as 73,000 and 5000 tons, respectively, and the major mineralogical phases identified include tetrahedrite (Cu12Sb4S13), freibergite ((Cu, Fe, Zn, Ag)12Sb4S13), stromeyerite (AgCuS), and chalcopyrite (CuFeS2) [1,22,23]. In the research reported here, the mixture of ammonium dibuyldithiophosphate (ADD) and O-isopropyl-N-ethylthionocarbamate (Z-200) was introduced as a selective collector for the flotation of copper polymetallic sulfide ore from the Asgat mega copper polymetallic deposit under neutral conditions. It is expected to provide a low-cost solution for the efficient recovery of complex copper polymetallic mineral resources while also providing a valuable case for the development of innovative collectors for refractory sulfide minerals.
2. Materials and Methods
2.1. Minerals
The representative sample used in this work was obtained from the Asgat mega silver polymetallic deposit located in the Bayan Olgii province (Mongolia). The raw ore was first crushed into 100% −2 mm and then mixed for further utilization. Chemical analyses (Table 1) revealed that the copper, antimony, and silver are of high recovery value, with the corresponding contents reaching 1.93%, 1.22%, and 592 g/t, respectively. Mineralogical analysis (Figure 1) showed that copper was mainly concentrated in tetrahedrite, followed by chalcopyrite, while antimony and silver were primarily distributed in tetrahedrite. Pyrite and arsenopyrite were identified as the most significant gangue minerals that limited the recovery of the above desired metal elements. Other non-sulfide gangues included hematite, quartz, feldspar, and so on. As seen in Figure 1a, tetrahedrite is characterized by its coarse grain, typically present at centimeter-scale size in the ore, and localized fissuring. Chalcopyrite (Figure 1b,c) is observed as inclusions or beads within the tetrahedrite, in addition to some coarse particles. Pyrite and arsenopyrite are the main iron-bearing minerals. The former (Figure 1a) mainly distributes as inclusions in chalcopyrite or on the margins of tetrahedrite, while arsenopyrite (Figure 1c) is dispersed at the chalcopyrite margins, and the majority of the arsenopyrite particles within the aggregates exhibit a discernible hexagonal biconical autoclave crystallization pattern.

Table 1.
Multi-composition of the polymetallic ore.
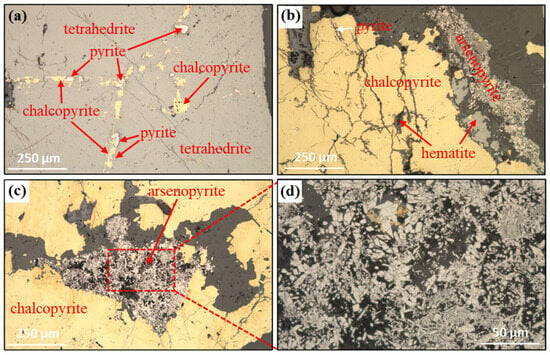
Figure 1.
Microscopic lithology observation results. (a) tetrahedrite, (b) chalcopyrite, (c) intergrowth structure of chalcopyrite and arsenopyrite, (d) local magnification of arsenopyrite.
2.2. Reagents
Sodium butyl xanthate (SBX, Hunan Mingzhu Flotation Reagents Co., Ltd., Zhuzhou, China), ammonium dibutyl dithiophosphate (ADD, Hunan Mingzhu Flotation Reagents Co., Ltd., Zhuzhou, China), and isopropyl ethyl thionocarbamate (Z-200, Hunan Mingzhu Flotation Reagents Co., Ltd., Zhuzhou, China), were used as the collector, sodium silicate (modulus: 2.3) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), sodium humate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and sodium sulfite (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were applied as the depressant, and pinitol oil was used as a frother. Among these reagents, pinitol oil (Hunan Mingzhu Flotation Reagents Co., Ltd., Zhuzhou, China) and Z-200 were utilized without further preparation, while others were prepared using tap water at the required concentration. The chemical solution was freshly prepared on a daily basis prior to experiments. All chemical reagents were of industrial grade, and tap water from Inner Mongolia Academy of Science and Technology (IMAST) was utilized in all flotation tests.
2.3. Flotation Tests
The flotation tests were carried out using a laboratory-scale XFG-II flotation machine (Wuhan Exploration Machinery Co., Ltd., Wuhan, China) with a 1.5 L cell. The air flow rate was kept at 1.5 m3/h, and the impeller speed was adjusted to 1750 rpm. First, the feeding of 500 g grounded sample was added into the cell, and the pulp density was adjusted to 30% solid under natural pH conditions (pH = 6.5~7.5). Then, the chemical reagents, namely the depressant, collector, and frother, were added in sequence. The slurry was stirred for 2 min for each type of flotation reagent, after which a flotation process was initiated and continued for another 4 min. The floated products and unfloated tailings were collected separately, and their dry weights were later recorded and analyzed, respectively. Each condition was repeated three times, and standard deviations were calculated in accordance with the experimental data.
2.4. Scanning Electron Microscopy and Energy Dispersive X-Ray Spectroscopy (SEM-EDS) Analysis
A HITACHI UHR FE-SEM SU8220 (Hitachi, Tokyo, Japan) field emission environmental SEM was employed to study the adsorption characteristics. Images and spectra were collected at 15 kV accelerating voltage and a probe current of approximately 700 nA. Elements were determined using standardless energy dispersive X-ray analysis.
2.5. Particle Size Measurement
The particle size of the mineral samples was measured using a Malvern 3000 laser particle size analyzer (Malvern, Worcestershire, UK). Each sample was dried, and then a 10 g sample and 100 mL of deionized water were placed in a beaker. After stirring, the sample was poured into the sample tank of the instrument. After ultrasonic treatment for 2 min, the particle size distribution was automatically measured. The average value was obtained by automatic measurement three times.
3. Results and Discussion
3.1. Effect of Grinding Fineness on Flotation
Achieving mineral liberation through moderate grinding is essential for the separation of the desired minerals from gangues. However, overgrinding not only results in an increase consumption of energy and equipment (i.e., grinding steel), but also exacerbates the environment of flotation [8]. The mesh of grind test on copper polymetallic sulfide ore was conducted using a rougher flotation with 800 g/t of sodium silicate, 300 g/t of sodium humate, 300 g/t of sodium sulfite, ADD/Z-200 50 + 50 g/t, and 20 g/t of pinitol oil. The relationship between grinding fineness and the concentrate grade and recovery is shown in Figure 2.
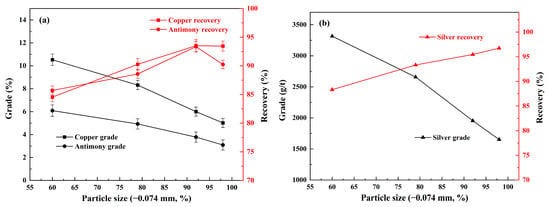
Figure 2.
Effect of grinding fineness on the flotation of copper, antimony (a), and silver (b) under conditions of 800 g/t of sodium silicate, 300 g/t of sodium humate, 300 g/t of sodium sulfite, ADD/Z-200 50 + 50 g/t, and 20 g/t of pinitol oil.
Figure 2 shows the effect of grinding fineness on the flotation of copper, antimony, and silver. As demonstrated in Figure 2, the grade of copper, antimony, and silver in the concentrate decreased as the grinding fineness increased, particularly when the grinding went to a fineness of −0.074 mm exceed 80%. Meanwhile, as the particle size was further reduced, the liberation of the desired minerals increased, which resulted in a significant increase in the recovery of the corresponding three kinds of elements. For example, the recovery of copper, antimony, and silver in the concentrate was 84.5%, 85.7%, and 88.3% with a fineness of −0.074 mm 60%, while that of copper, antimony, and silver in the concentrate was increased up to 93.5%, 93.4%, and 95.5% when the grinding went to a fineness of −0.074 mm 92%. However, the grade and recovery of antimony decreased sharply when the grinding fineness exceeded 92% (−0.074 mm). This might be attributed to the severe overgrinding of the minerals, which significantly increases the agglomeration effect of the mineral particles, thereby substantially heightening the difficulty of activation [24,25].
3.2. Effect of Collector on Flotation
SBX, ADD, and Z-200 are collectors that are commonly used for the flotation of copper sulfides minerals in industry. Herein, we compared the above three kinds of collectors and their mixtures on the flotation of copper polymetallic sulfide ore from the Asgat mega copper polymetallic deposit. The flotation conditions were set as follows: a grinding fineness of −0.074 mm 92%, 800 g/t of sodium silicate, 300 g/t of sodium humate, 300 g/t of sodium sulfite, and 20 g/t of pinitol oil. The usage for individual SBX, ADD, and Z-200 was 120 g/t, and that for SBX/Z-200 and ADD/Z-200 was 60 + 60 g/t, respectively.
Figure 3 shows the flotation of copper, antimony, and silver by using SBX, ADD, Z-200, and their mixtures. The results indicate that the five types of collectors exhibited powerful performances in the flotation of copper, antimony, and silver. Compared to SBX and ADD, Z-200 demonstrated enhanced selectivity in the flotation of the refractory sulfide minerals, particularly in the recovery of silver. This might be attributed to the mildly binding interactions facilitated by the C=S bond within the ester-based molecular structure of Z-200 on the sulfide surfaces [26]. As seen in Figure 3, a concentrate of 1950 g/t silver was achieved in the Z-200 flotation, which was 50 g/t and 30 g/t higher than that of SBX and ADD, respectively. However, the recovery of copper and antimony within the Z-200 flotation was significantly lower than the other two systems. This finding seems support the hypothesis that the distinctiveness of a single collector’s molecular composition makes it difficult to balance both selectivity and collection capacity. By comparison, the SBX/Z-200 and ADD/Z-200 combinations exhibited superior performance in the recovery of the above three types of metallic elements, which indicates the significantly synergistic effect of these systems. The most outstanding performance was achieved through the combination of ADD/Z-200 and the concentrate containing 6.27% copper, 4.0% antimony, and 1963 g/t silver, with the corresponding recovery of 93.0%, 91.3%, and 95.4%, respectively.
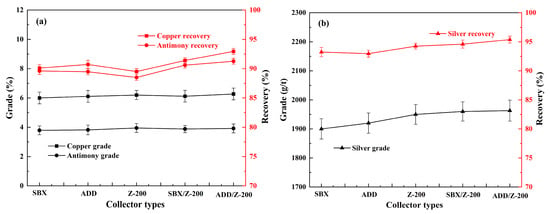
Figure 3.
Effect of SBX, ADD, and Z-200 on the flotation of copper, antimony (a), and silver (b) under conditions of the grinding fineness of −0.074 mm 92%, 800 g/t of sodium silicate, 300 g/t of sodium humate, 300 g/t of sodium sulfite, and 20 g/t of pinitol oil.
Based on the above research, we further investigated the effect of the ratio of ADD and Z-200 on the flotation of refractory copper polymetallic sulfide minerals. The conditions were set as follows: the grinding fineness of −0.074 mm 92%, 800 g/t of sodium silicate, 300 g/t of sodium humate, 300 g/t of sodium sulfite, and 20 g/t of pinitol oil; and the results are shown in Figure 4.
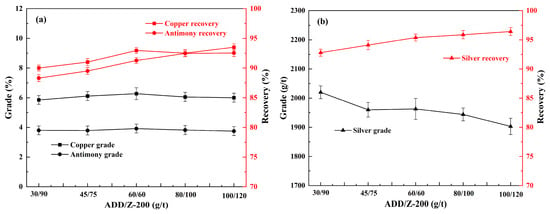
Figure 4.
Effect of ADD/Z-200 on the flotation of copper, antimony (a), and silver (b) under conditions of the grinding fineness of −0.074 mm 92%, 800 g/t of sodium silicate, 300 g/t of sodium humate, 300 g/t of sodium sulfite, and 20 g/t of pinitol oil.
As seen in Figure 4, the grade and recovery of copper and antimony in the concentrate both exhibited a steady upward trend as the ratio of ADD/Z-200 gradually changed from 30:90 to 60:60; meanwhile, the recovery of silver demonstrated a marked increase, despite its grade appearing to decrease. When the usage of ADD and Z-200 were both 60 g/t, the grade and recovery of the three types of metallic elements in the concentrate yielded satisfactory results, with the recovery of copper, antimony, and silver being 94.0%, 93.1%, and 95.3%, respectively. As the usage of ADD and Z-200 further increased, the recovery of these three types of elements in the concentrate did not change significantly. Given the grade and recovery of the three types of elements, as well as the cost of the reagents, the using of ADD and Z-200 was 60 g/t and 60 g/t.
3.3. Effect of Sodium Silicate on Flotation
Sodium silicate is a commonly used adjusting reagent in sulfide flotation, especially for refractory copper sulfide minerals. The prevailing perspective is that the SiO32− and H+ ions dissociated from sodium silicate can adsorb onto the surfaces of minerals, which causes positively charged minerals to become negatively charged, thereby enhancing their interactions with anionic collectors [27,28]. Simultaneously, it can also induce electrostatic repulsion towards fine negatively charged minerals, inhibiting their agglomeration [29]. In this study, the effect of sodium silicate on flotation was investigated using 300 g/t of sodium humate, 300 g/t of sodium sulfite, ADD/Z-200 60 + 60 g/t, and 20 g/t of pinitol oil. The relationship between sodium silicate and the concentrate grade and recovery is shown in Figure 5.
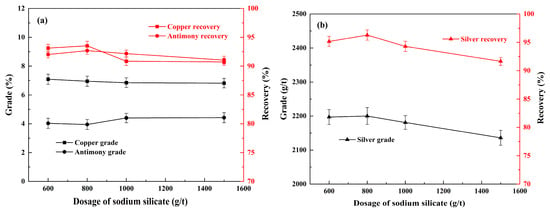
Figure 5.
Effect of sodium silicate on the flotation of copper, antimony (a), and silver (b) under conditions of 300 g/t of sodium humate, 300 g/t of sodium sulfite, ADD/Z-200 60 + 60 g/t, and 20 g/t of pinitol oil.
Figure 5 shows the effect of sodium silicate on the flotation of copper polymetallic sulfide ores. As can be seen in Figure 5a, a concentrate of 7.09% copper and 4.03% antimony was achieved with the corresponding recovery of 93.1% and 91.9%, respectively, when the usage of sodium silicate was 600 g/t. With the usage increased to 800 g/t, the content of copper and antimony in the concentrate decreased slightly; however, their recoveries were increased to 93.8% and 92.9%, respectively. As shown in Figure 5b, there was a substantial enhancement in the grade and recovery of silver, with an increase from 2187 g/t and 94.9% to 2200 g/t and 96.4%, respectively, following the adjustment in sodium silicate usage from 600 g/t to 800 g/t. Thus, as indicated by Xia et al. [29], sodium silicate promotes the selectivity of value-added sulfides against gangue minerals, resulting in both grade and recovery improvements. However, when the dosage of sodium silicate was increased to 1000 g/t, a significant inhibitory was observed, resulting in a substantial decrease in the grade and recovery of the three types of metallic elements. Given the reagent cost and the product of the concentrate, the dosage of sodium silicate was determined to be 800 g/t.
3.4. Effect of Sodium Humate on Flotation
Sodium humate has been demonstrated to possess a robust inhibitory effect on iron sulfide and oxide minerals, such as hematite, magnetite, and pyrite, which can effectively reduce the recovery of iron-bearing minerals during the flotation process [30,31,32]. The effect of sodium humate on the flotation of copper, antimony, and silver was investigated with 800 g/t of sodium silicate, 300 g/t of sodium sulfite, ADD/Z-200 60 + 60 g/t, and 20 g/t of pinitol oil. The relationship between sodium humate and the concentrate grade and recovery is shown in Figure 6.
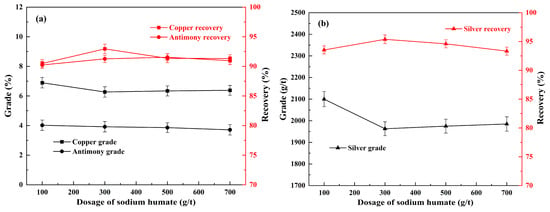
Figure 6.
Effect of sodium humate on the flotation of copper, antimony (a), and silver (b) under conditions of 800 g/t of sodium silicate, 300 g/t of sodium sulfite, ADD/Z-200 60 + 60 g/t, and 20 g/t of pinitol oil.
Figure 6 shows the effect of sodium humate on the flotation of copper polymetallic sulfide minerals. As seen in Figure 6a, an increase in the usage of sodium humate from 100 g/t to 300 g/t resulted in an enhancement of the recovery of copper and antimony in the concentrate from 90.4% and 90.2% to 93.0% and 91.3%, respectively, although there were marginal reductions in their grades, 0.63% and 0.10%, respectively. Similar trends (Figure 6b) were found in the curves of silver, and its recovery increased from 93.5% to 95.4% as the usage of sodium humate increased from 100 g/t to 300 g/t. Thus, it can be concluded that the incorporation of sodium humate positively influences the flotation process of copper sulfide ores. However, the poor selectivity of common organic macromolecular depressant is also quite apparent. As displayed Figure 6b, when the usage of sodium humate further increased to 700 g/t, the recovery of silver decreased to 93.3%. Therefore, the dosage of sodium humate was determined to be 300 g/t.
3.5. Effect of Sodium Sulfite Dosage on Flotation
As a common sulfide depressant, sodium sulfite has been proved to form a hydrophilic film on the surfaces of sphalerite- and iron-bearing sulfide minerals, which can effectively hinder the subsequent adsorption of the collector on these mineral surfaces [33,34]. However, controversy persists regarding copper-bearing sulfide ores. Some reports indicate that the presence of significant quantities of sodium sulfite, either independently or in conjunction with lime, have been observed to exert a substantial inhibitory effect on the flotation of chalcopyrite [35]. However, it has been observed that the addition of low doses not only fails to inhibit the flotation process, but also effectively eliminates the micro-oxidized hydrophilic film that accumulates on the minerals’ surfaces [36,37]. In this study, sodium sulfide was utilized as a regulator to investigate the flotation process, and the flotation conditions were set as follows: a grinding fineness of −0.074 mm 92%, 800 g/t of sodium silicate, 300 g/t of sodium humate, ADD/Z-200 60 + 60 g/t, and 20 g/t of pinitol oil; and the results are shown in Figure 7.
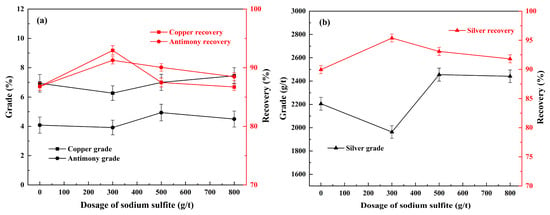
Figure 7.
Effect of sodium sulfite on the flotation of copper, antimony (a), and silver (b) under conditions of grinding fineness of −0.074 mm 92%, 800 g/t of sodium silicate, 300 g/t of sodium humate, ADD/Z-200 60 + 60 g/t, and 20 g/t of pinitol oil.
As shown in Figure 7, the grade and recovery of copper, antimony, and silver were achieved only by 6.94%, 4.08% and 2206 g/t, and 86.8%, 86.8% and 90.0%, respectively, without the addition of sodium sulfite. When the usage of sodium sulfite increased to 300 g/t, the grade of copper, antimony, and silver decreased to varying degrees, but the recovery of the corresponding elements reached as high as 93.0%, 91.3%, and 95.4%, respectively. This phenomenon may be attributed to the activation effect of sodium sulfite on the Cu-bearing sulfide surfaces. However, when the usage was increased from 300 g/t to 500 g/t, the grade of the three metallic elements in the concentrate improved slightly. Nevertheless, the corresponding recovery decreased by 5.5%, 1.2%, and 2.3%, respectively, compared to that of 300 g/t. As the sodium sulfide dosage increased further to 800 g/t, this trend became even more pronounced, which corroborates the above reports that low doses of sodium sulfide can activate sulfide copper ores, whereas high doses inhibit flotation. Therefore, the optimized usage for sodium sulfite was 300 g/t, with a recovery of 93.0% copper, 91.3% antimony, and 95.4% silver achieved in the concentrate.
3.6. Open-Circle Flotation
An open-circuit flotation process consisting of one rougher, three cleanings, and two scavengings was design according to the above investigation, and the flowsheet and results are shown in Figure 8 and Table 2. As shown in Table 2, the initial grade of copper, antimony, and silver in the feeding was 1.93%, 1.20%, and 591 g/t, which was very close to the results of the multi-element chemical analysis mentioned above. The findings indicate that the combination of ADD/Z-200 exhibited excellent selectivity in the flotation of the copper polymetallic sulfide ore under natural pH conditions. The grade of copper, antimony, and silver achieved in the concentrate was 33.45%, 18.25%, and 10,968 g/t, with a corresponding recovery of 58.7%, 51.3%, and 62.7%. However, due to the severe oxidation that has occurred in the stored mineral samples, it has become impossible to conduct further microstructural or mineralogical analyses (e.g., scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS) or mineral liberation analyzer (MLA)) on the aforementioned samples.
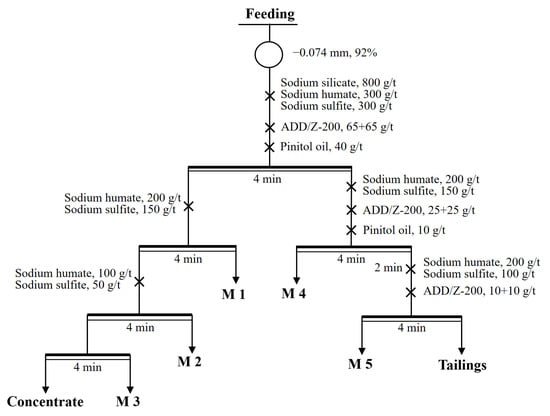
Figure 8.
Flow sheet of open-circuit flotation.

Table 2.
Results of the open-circuit flotation.
3.7. Closed-Circuit Flotation Test
To further confirm the effect of the ADD/Z-200 combination in the flotation of copper polymetallic sulfide ore, closed-circuit flotation tests between ADD/Z-200 combination (Figure 9) and single Z-200 (Figure 10) were conducted, and the results are shown in Table 3 and Table 4. As seen in Table 3, the concentrate obtained from the ADD/Z-200 combination contained 30.30% copper, 19.20% antimony, and 9538 g/t silver, with a recovery of 92.7%, 93.0%, and 95.1%, respectively. In comparison, the grade of copper, antimony, and silver in the concentrate obtained from single Z-200 was found to be 2.43%, 2.53%, and 732 g/t higher than that of the ADD/Z-200 combination. However, the recovery for these three types of metal elements were only 84.6%, 87.6%, and 86.3%, respectively. As illustrated in Table 4, the reagent cost per ton of ore processed for the ADD/Z-200 combination was 8.4 CNY, representing a decrease of 12.75% compared to that of the single Z-200.
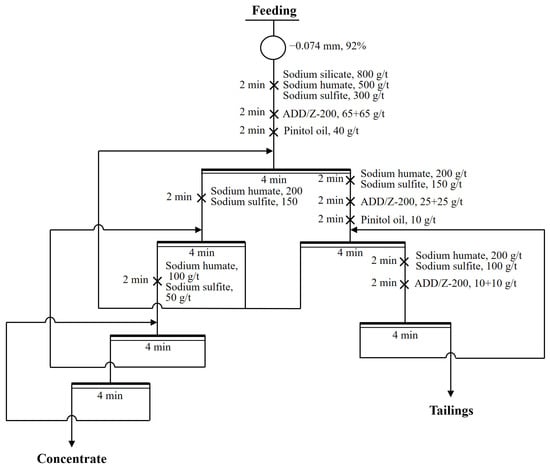
Figure 9.
Flow sheet of closed-circuit flotation with ADD/Z-200.
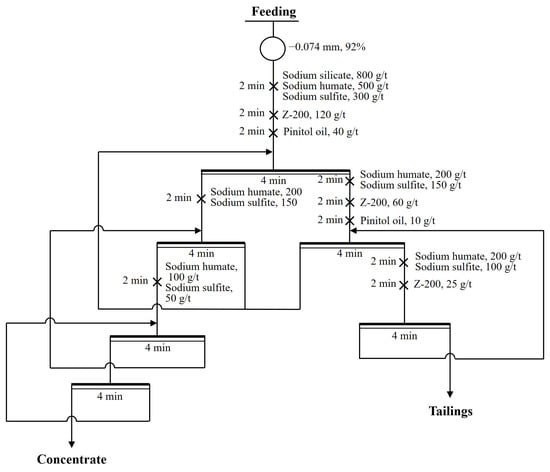
Figure 10.
Flow sheet of closed-circuit flotation with Z-200.

Table 3.
Results of the closed-circuit flotation.

Table 4.
Results of cost accounting for closed-circuit flotation.
4. Conclusions
Flotation experiments using sodium butyl xanthate (SBX), ammonium dibutyl dithiophosphate (ADD), isopropyl ethyl thionocarbamate (Z-200), and their mixtures was performed on the sample from the Asgat copolymetallic deposit under natural pH conditions. The findings indicate that fine grinding was essential for mineral liberation, with the suitable grinding fineness determined to be 92% −0.074 mm. The optimal flotation conditions were achieved with sodium silicate dosage of 800 g/t, sodium humate dosage of 300 g/t, sodium sulfite dosage of 300 g/t, and collectors of 60 g/t of ADD and 60 g/t of Z-200. The results of the closed-circuit flotation tests indicated that the concentrate obtained from the ADD/Z-200 combination contained 30.30% copper, 19.20% antimony, and 9538 g/t silver, with a recovery of 92.7%, 93.0%, and 95.1%, respectively, while the recovery of the above three types of metal elements from the single Z-200 was only 84.6%, 87.6%, and 86.3%, respectively. Meanwhile, the reagent cost per ton of ore processed for the ADD/Z-200 combination was 12.75% lower than that of the single Z-200.
Author Contributions
Methodology, B.Y., Y.L., X.J., O.E., and A.T.; Validation, X.J. and A.T.; Formal analysis, X.J.; Investigation, Y.L., O.E., and A.T.; Resources, Z.B.; Data curation, B.Y.; Writing—original draft, B.Y.; Supervision, Y.L., O.E., and Z.B.; Project administration, Y.L. and Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “National Key R&D Program of China, grant number 2022YFE0210800”, “Industrial Technology Innovation Program of IMAST, grant number 2024RCYJ06002 and 2024RCYJ06003”, and “Natural Science Foundation of Inner Mongolia Autonomous Region of China, grant number 2025QN05074”.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, C.X.; Wen, S.M.; Zhang, J.H.; Wang, C.L.; Liu, J.; Shen, H.Y. Comprehensive recovery of valuable metals from copper polymetallic sulphide ore. Adv. Mater. Res. 2012, 524–527, 957–964. [Google Scholar] [CrossRef]
- Li, G.; Zou, X.; Cheng, H.; Geng, S.; Xiong, X.; Xu, Q.; Zhou, Z.; Lu, X. A novel ammonium chloride roasting approach for high-efficiency co-sulfation of nickel, cobalt, and copper in polymetallic sulfide minerals. Metall. Mater. Trans. B 2020, 51, 2769–2784. [Google Scholar] [CrossRef]
- Qin, S.; Ren, H.; Hu, Y.; Ma, S.; Ding, J.; Lai, F.; Zhang, C.; Shen, C.; Zhao, H. Efficient recovery of Cu, Co, and Au from polymetallic magnetite: Insights into mineral liberation mechanisms and interfacial behavior. J. Environ. Chem. Eng. 2025, 13, 115940. [Google Scholar] [CrossRef]
- Zou, K.; Zhang, Y.; Shen, L.; Shang, H.; Chen, B.; Wen, J.; Zhao, H. Highly efficient resource recovery from polymetallic sulfide ores under neutral–alkaline conditions: Chemical oxidation and coordination leaching. Chem. Eng. J. 2025, 503, 158198. [Google Scholar] [CrossRef]
- Shengo, M.L.; Kime, M.-B.; Mambwe, M.P.; Nyembo, T.K. A review of the beneficiation of copper–cobalt-bearing minerals in the Democratic Republic of Congo. J. Sustain. Min. 2019, 18, 226–246. [Google Scholar] [CrossRef]
- Cui, W.; Chen, J. Insight into mineral flotation fundamentals through the DFT method. Int. J. Min. Sci. Technol. 2021, 31, 983–994. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, J.; Liu, Z.; Chen, J. Selective enhancement of jamesonite flotation using Aerophine 3418A/DDTC mixture. Miner. Eng. 2023, 191, 107934. [Google Scholar] [CrossRef]
- Wills, B.A.; Napier-Munn, T. Wills’ Mineral Processing Technology; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Tolley, W.; Kotlyar, D.; Van Wagoner, R. Fundamental electrochemical studies of sulfide mineral flotation. Miner. Eng. 1996, 9, 603–637. [Google Scholar] [CrossRef]
- Pana, R.J.; William, T.; Junji, K.; Kazutoshi, H.; Yasushi, T.; Atsushi, S. Effect of flotation reagents for upgrading and recovery of Cu and Mo from mine tailing by flotation. Resour. Process. 2011, 58, 14–21. [Google Scholar] [CrossRef]
- Sun, Z.M.; Sun, C.B.; Wang, J.Z.; Yin, W.Z. Optimization and mechanism of gold-bearing sulfide flotation. Rare Met. 2014, 33, 363–368. [Google Scholar] [CrossRef]
- Ndoro, T.O.; Witika, L.K. A review of the flotation of copper minerals. Int. J. Sci. Basic Appl. Res. 2017, 34, 145–165. [Google Scholar]
- Feng, Q.; Yang, W.; Chang, M.; Wen, S.; Liu, D.; Han, G. Advances in depressants for flotation separation of Cu–Fe sulfide minerals at low alkalinity: A critical review. Int. J. Miner. Metall. Mater. 2024, 31, 1–17. [Google Scholar] [CrossRef]
- Plaksin, I.N.; Glembotskii, V.A.; Okolovich, A.M. Simultaneous use of several collector reagents for the intensification of the flotation of pyrite. Izv. Akad. Nauk SSSR Otd. Tekh. Nauk 1952, 51, 405–415. [Google Scholar]
- Buckley, A.N.; Hope, G.A.; Parker, G.K.; Steyn, J.; Woods, R. Mechanism of mixed dithiophosphate and mercaptobenzothiazole collectors for Cu sulfide ore minerals. Miner. Eng. 2017, 109, 80–97. [Google Scholar] [CrossRef]
- Lee, K.; Archibald, D.; McLean, J.; Reuter, M.A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
- Roy, S.; Datta, A.; Rehani, S. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures. Int. J. Miner. Process. 2015, 143, 43–49. [Google Scholar] [CrossRef]
- Dhar, P.; Thornhill, M.; Kota, H.R. Comparison of single and mixed reagent systems for flotation of copper sulphides from Nussir ore. Miner. Eng. 2019, 142, 105930. [Google Scholar] [CrossRef]
- Guner, M.K.; Lode, S.; Malafeevskiy, N.; Aasly, K.; Kowalczuk, P. Mixed thiol collector system in the flotation of copper ore using the REFLUX Flotation Cell (RFC). Miner. Eng. 2024, 216, 108843. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Li, Y.; Chen, J. Co-adsorption mechanism of selective Aerophine 3418A/DTP collectors and their industrial application for chalcopyrite flotation. Miner. Eng. 2025, 228, 109304. [Google Scholar] [CrossRef]
- Pavlova, G.G.; Borisenko, A.S. The age of Ag–Sb deposits of Central Asia and their correlation with other types of ore systems and magmatism. Ore Geol. Rev. 2009, 35, 164–185. [Google Scholar] [CrossRef]
- Nyamdelger, S.; Burmaa, G.; Narangarav, T.; Ariunaa, G. Dissolution behaviour of freibergite–tetrahedrite concentrate in acidic dichromate solution. Mong. J. Chem. 2014, 14, 36–40. [Google Scholar] [CrossRef]
- Avirmed, D. Mineral resources of Mongolia as a driving force of the country. Mong. J. Int. Aff. 2021, 22, 61–66. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Leistner, T.; Peuker, U.A.; Rudolph, M. How gangue particle size can affect the recovery of ultrafine and fine particles during froth flotation. Miner. Eng. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Li, Y.; Liu, M.; Liu, Y.; Zhao, C.; Cui, W. Effects of surface spatial structures and electronic properties of chalcopyrite and pyrite on Z-200 selectivity. Miner. Eng. 2025, 163, 106803. [Google Scholar] [CrossRef]
- Tussupbayev, N.K.; Rulyov, N.N.; Kravtchenco, O.V. Microbubble augmented flotation of ultrafine chalcopyrite from quartz mixtures. Trans. Inst. Min. 2016, 125, 5–9. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Li, W.; Zhu, Y. A fundamental study of chalcopyrite flotation in sea water using sodium silicate. Miner. Eng. 2019, 139, 10586. [Google Scholar] [CrossRef]
- Xia, L.; Hart, B.; Sidorkiewicz, V.; Shaw, D. The role of soluble sodium silicate for enhancing flotation selectivity of sulphides: Example from a Cu-sulphide ore. In Proceedings of the First Global Conference on Extractive Metallurgy, Denver, CO, USA, 24–27 February 2019; pp. 2901–2913. [Google Scholar]
- Dong, Z.; Zhi, H.; Li, W.; Man, X.; Yang, X.; Fu, Y.; Liu, J. Study on inhibition effect and mechanism of sodium humate in hematite reverse flotation. Miner. Eng. 2022, 189, 107883. [Google Scholar] [CrossRef]
- Sun, D.; Li, M.; Fu, Y.; Pan, Z.; Cui, R.; Wang, D.; Zhang, M.; Yao, W. Selective separation of chalcopyrite from pyrite using sodium humate: Flotation behavior and adsorption mechanism. ACS Omega 2023, 47, 45129–45136. [Google Scholar] [CrossRef]
- Yuan, Z.; Du, Y.; Meng, Q.; Li, Y. Selective dispersion and separation mechanisms of sodium humate in ilmenite flotation. Sep. Purif. Technol. 2025, 376, 134081. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Miki, H.; Ochi, D.; Aoki, Y.; Ura, K.; Berdakh, D.; Ulmaszoda, A.; Dwitama, E.P.; Sasaki, K.; Hirajima, T. Sodium metabisulfite as a copper depressant in the selective flotation of copper-molybdenum concentrate using seawater. Adv. Powder Technol. 2023, 34, 104258. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, F.; Cui, Y.; Lin, X.; Qin, W.; Wei, Q. Application and electrochemical depression mechanism of sodium sulfite on the flotation separation of galena–pyrite mixed concentrate in Yiliang typical high–sulfur lead–zinc deposit. Geochemistry 2025, 85, 126219. [Google Scholar] [CrossRef]
- Yang, X.; Lai, H.; Wei, X.; Shen, P.; Wang, Y.; Li, M.; Cai, J.; Liu, D. Adsorption mechanism of Na2S2O3 and FeSO4 as a combined depressant for galena in chalcopyrite–galena flotation separation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 706, 135799. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Sun, W.; Li, W.; Dong, Y.; Wang, C. Electrochemical mechanism and flotation of chalcopyrite and galena in the presence of water glass and sodium sulfite. Trans. Nonferrous Met. Soc. China 2020, 30, 1091–1101. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K.; Kuroiwa, S.; Aoki, Y. Effect of Na2SO3 on the floatability of chalcopyrite and enargite. Miner. Eng. 2021, 173, 107222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).