Natural Hydrogen Generation from Phanerozoic Sedimentary Siderite
Abstract
1. Natural Hydrogen Generation
2. Materials and Methods
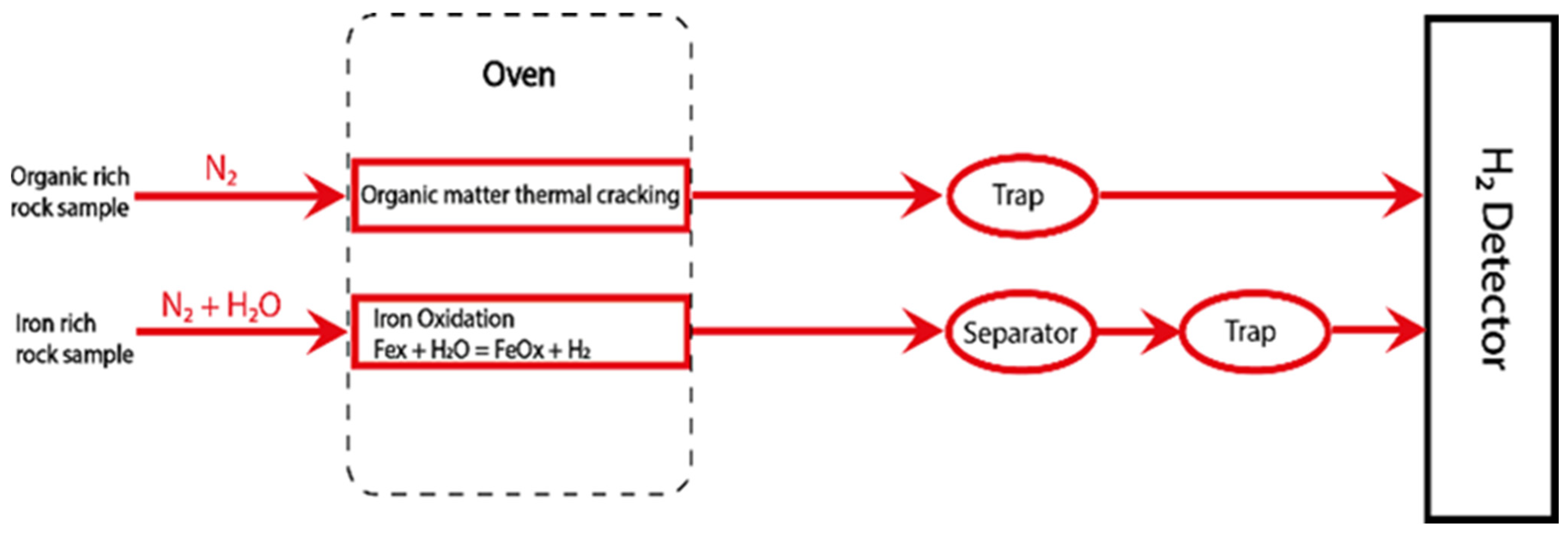
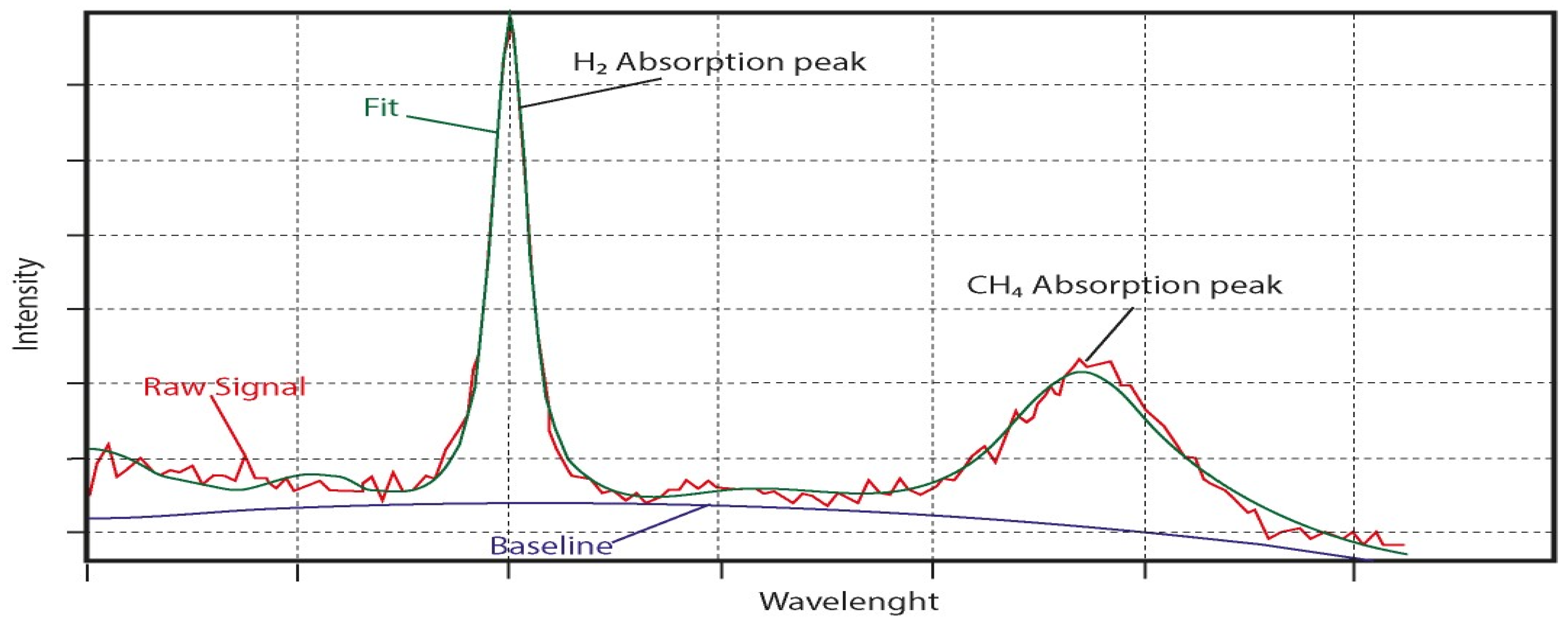
2.1. Main Analytical Process and Technical Settings
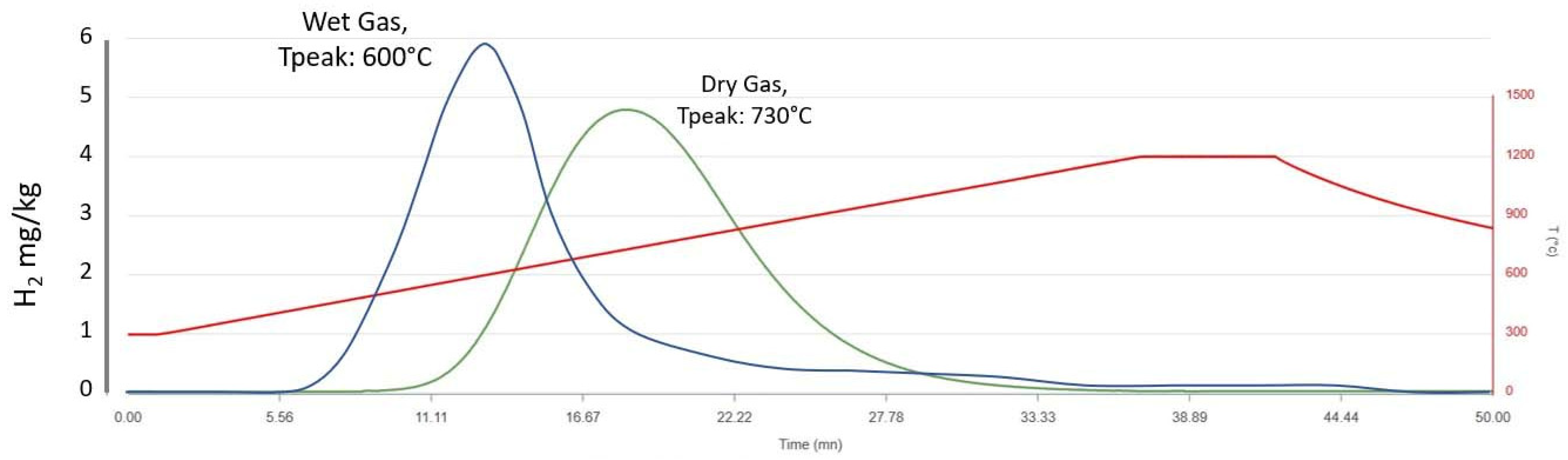
2.1.1. Dry Carrier Gas Mode (DG)
2.1.2. Wet Carrier Gas Mode (WG)
2.1.3. Limitation
2.2. Material
3. Siderite in Sedimentary Basin
3.1. Siderite Deposit
- Shallow Marine Environments and Freshwater: Coastal areas, continental shelf margins, deltas, lakes, and peat bogs.
- Sediments Rich in Organic Matter: The breakdown of organic matter by bacteria consumes oxygen and produces carbon dioxide (CO2).
3.2. The Llanos Basin Short Geological Setting
3.3. Hydrogen in the Llanos
4. Results_1: H2 Yields of the Siderite Beds
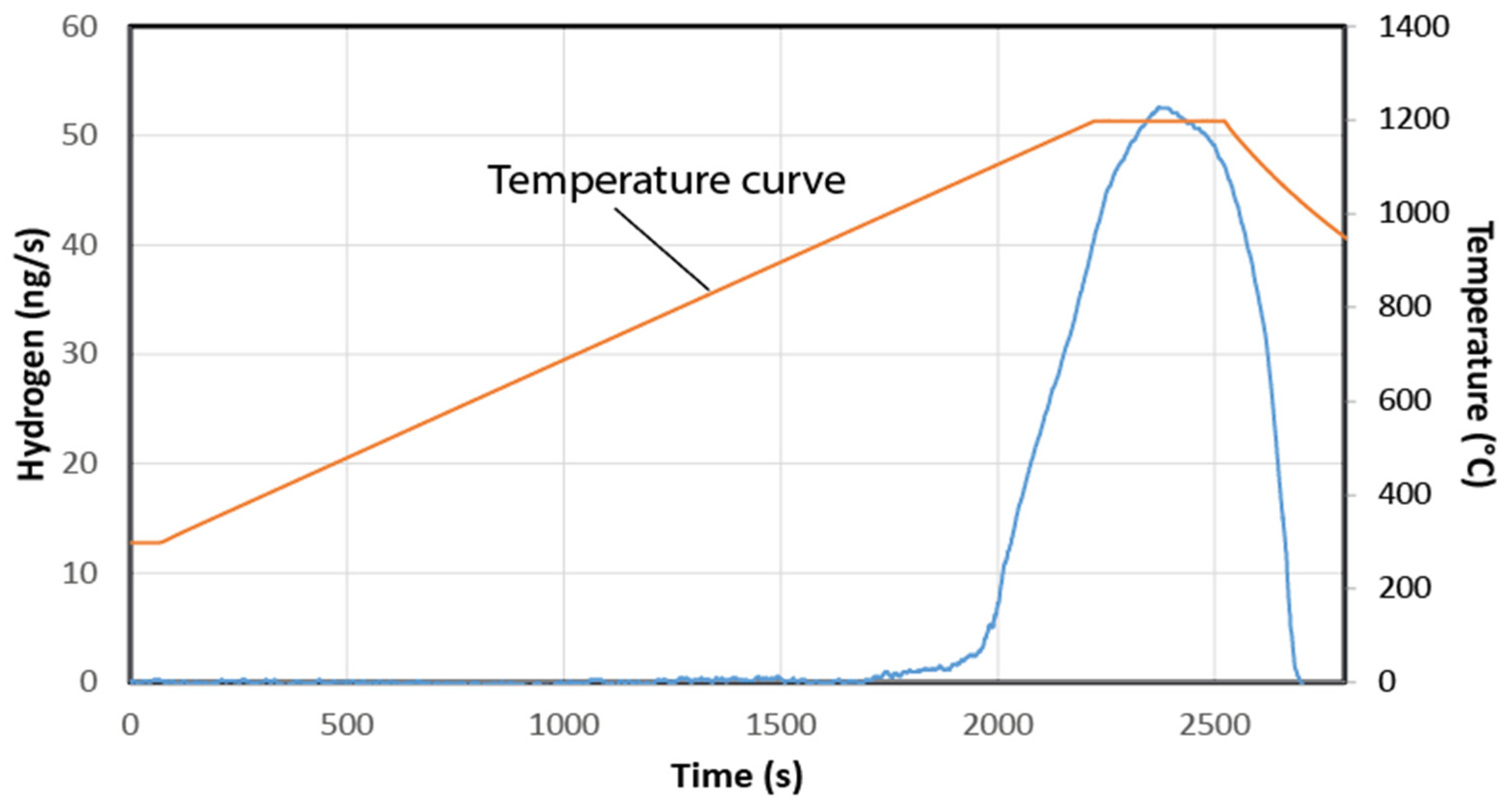
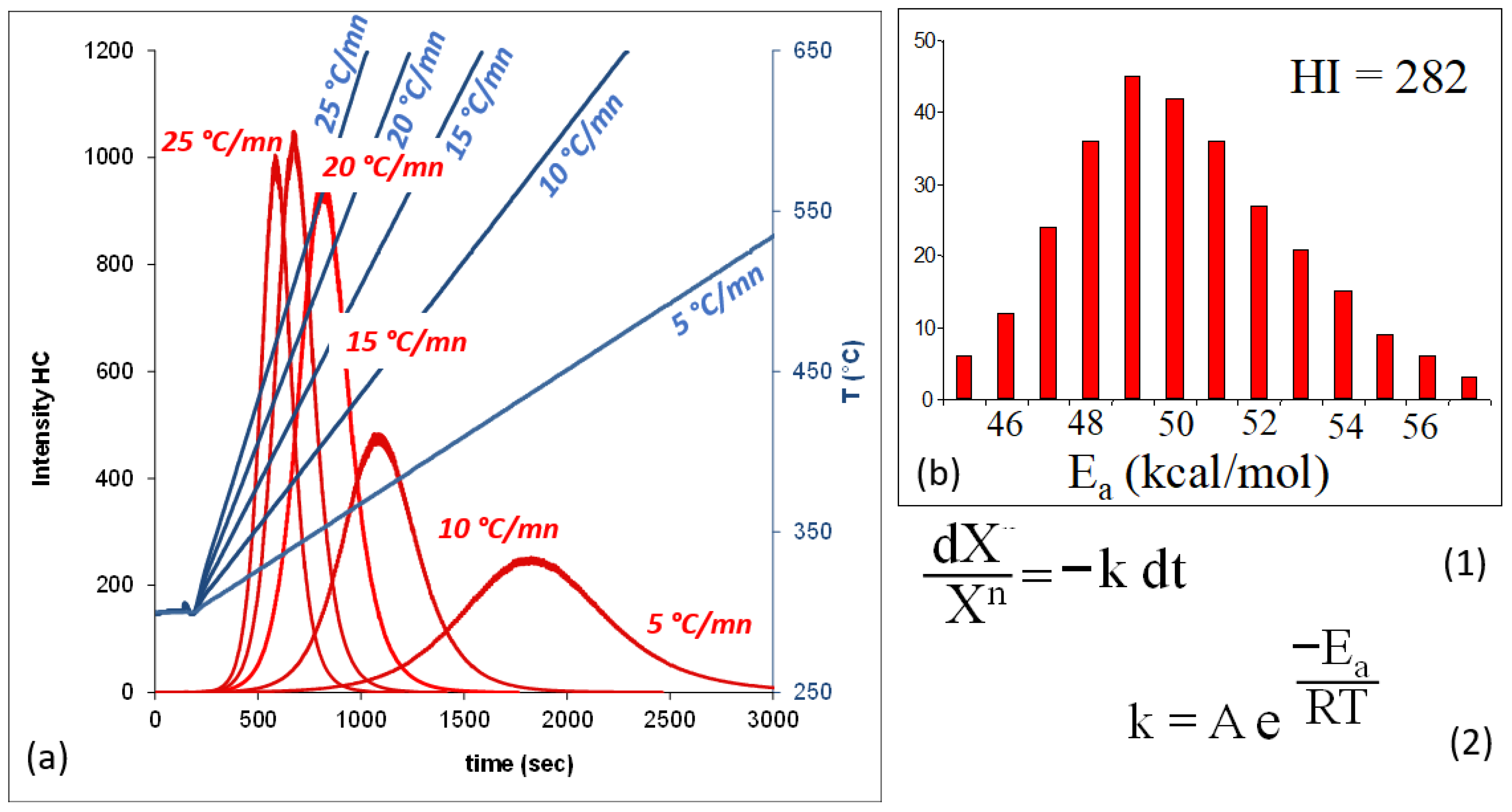
4.1. Pyrolysis of the Samples
4.2. H2 Generation from the Studied Samples
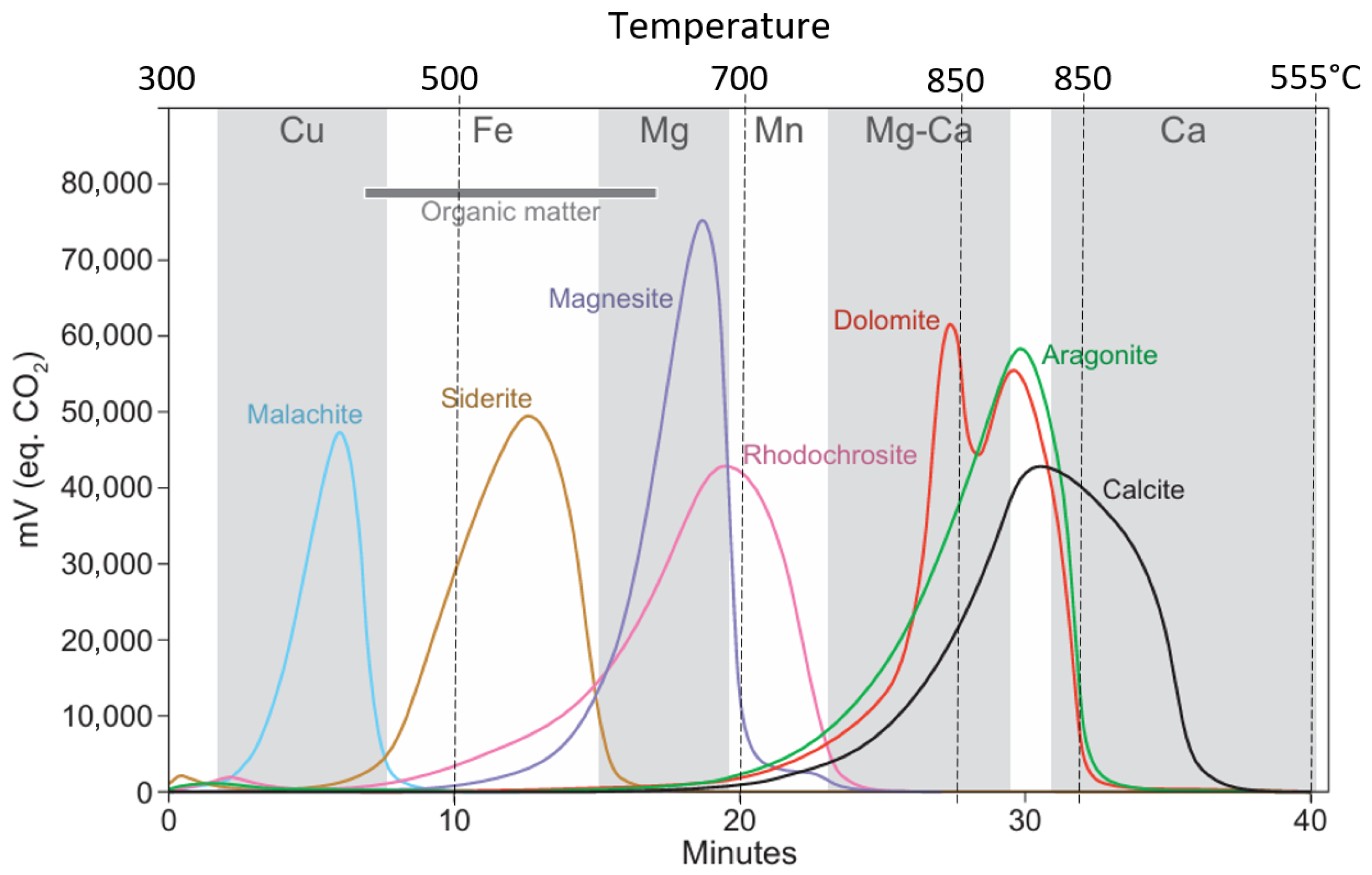
5. Results_2: Siderite Evolution Versus Temperature
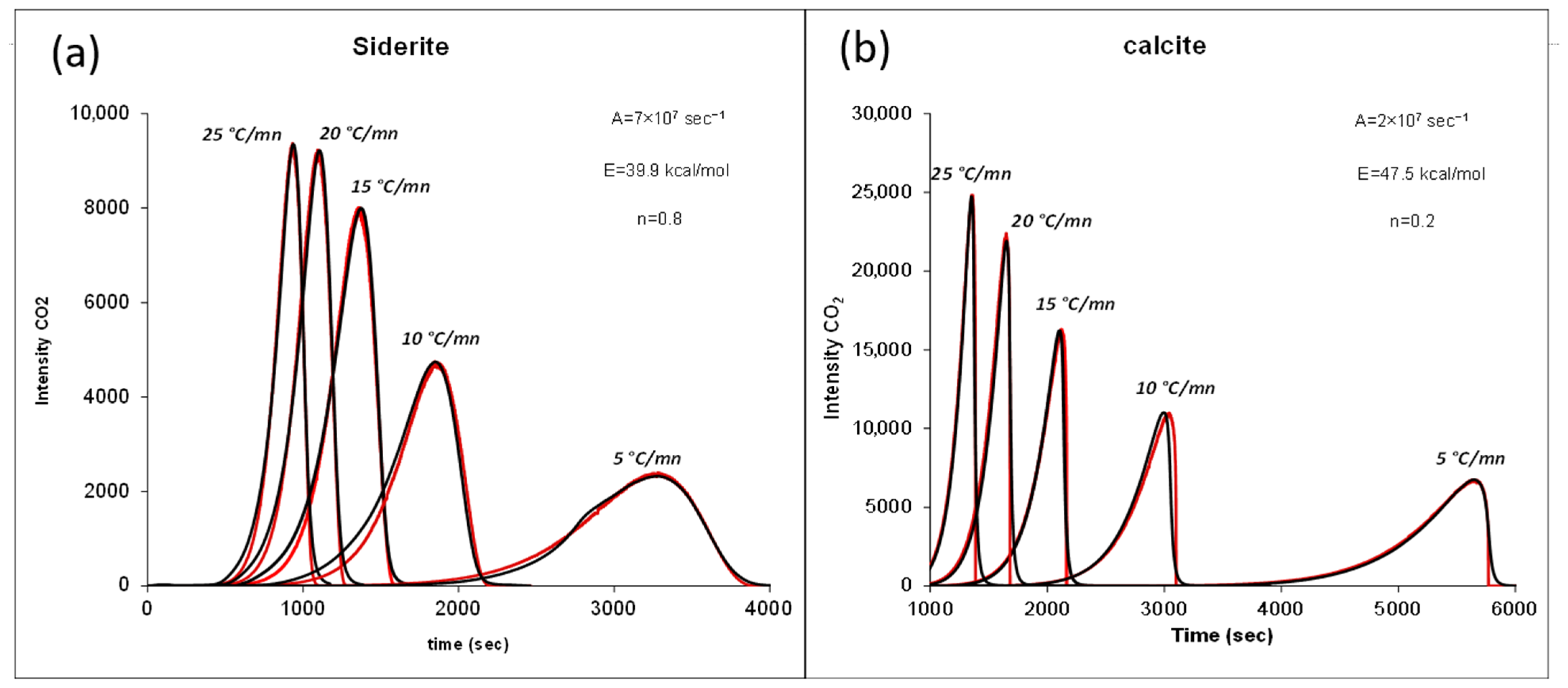
- Kinetic of the Thermal Decomposition of Siderite
6. Discussion
6.1. Potential of Siderite for the Generation of Hydrogen
6.2. Open vs. Closed System
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SR | Source rock for hydrocarbon |
| GR | Generating rock for H2 |
| RE | Rock-Eval Pyrolyzer |
| HC | Hydrocarbon |
| OM | Organic Matter |
References
- Klein, F.; Tarnas, J.D.; Bach, W. Abiotic Sources of Molecular Hydrogen on Earth. Elements 2020, 16, 19–24. [Google Scholar] [CrossRef]
- Lévy, D.; Roche, V.; Pasquet, G.; Combaudon, V.; Geymond, U.; Loiseau, K.; Moretti, I. Natural H2 Exploration: Tools and Workflows to Characterize a Play. Sci. Technol. Energy Transit. 2023, 78, 27. [Google Scholar] [CrossRef]
- Lin, L.-H.; Slater, G.F.; Sherwood Lollar, B.; Lacrampe-Couloume, G.; Onstott, T.C. The Yield and Isotopic Composition of Radiolytic H2, a Potential Energy Source for the Deep Subsurface Biosphere. Geochim. Cosmochim. Acta 2005, 69, 893–903. [Google Scholar] [CrossRef]
- Zgonnik, V. The Occurrence and Geoscience of Natural Hydrogen: A Comprehensive Review. Earth-Sci. Rev. 2020, 203, 103140. [Google Scholar] [CrossRef]
- Arrouvel, C.; Prinzhofer, A. Genesis of Natural Hydrogen: New Insights from Thermodynamic Simulations. Int. J. Hydrogen Energy 2021, 46, 18780–18794. [Google Scholar] [CrossRef]
- Jacquemet, N.; Prinzhofer, A. The Association of Natural Hydrogen and Nitrogen: The Ammonium Clue? Int. J. Hydrogen Energy 2024, 50, 161–174. [Google Scholar] [CrossRef]
- Geymond, U.; Briolet, T.; Combaudon, V.; Sissmann, O.; Martinez, I.; Duttine, M.; Moretti, I. Reassessing the Role of Magnetite during Natural Hydrogen Generation. Front. Earth Sci. 2023, 11, 1169356. [Google Scholar] [CrossRef]
- Geymond, U.; Ramanaidou, E.; Lévy, D.; Ouaya, A.; Moretti, I. Can Weathering of Banded Iron Formations Generate Natural Hydrogen? Evidence from Australia, Brazil and South Africa. Minerals 2022, 12, 163. [Google Scholar] [CrossRef]
- Milesi, V.; Guyot, F.; Brunet, F.; Richard, L.; Recham, N.; Benedetti, M.; Dairou, J.; Prinzhofer, A. Formation of CO2, H2 and Condensed Carbon from Siderite Dissolution in the 200–300 °C Range and at 50MPa. Geochim. Cosmochim. Acta 2015, 154, 201–211. [Google Scholar] [CrossRef]
- Milesi, V.; Prinzhofer, A.; Guyot, F.; Brunet, F.; Richard, L.; Dairou, J.; Benedetti, M. Unconventional Generation of Hydrocarbons in Petroleum Basin: The Role of Siderite/Water Interface. Mineral. Mag. 2013, 77, 1661–1817. [Google Scholar]
- Li, X.; Krooss, B.M.; Weniger, P.; Littke, R. Molecular Hydrogen (H2) and Light Hydrocarbon Gases Generation from Marine and Lacustrine Source Rocks during Closed-System Laboratory Pyrolysis Experiments. J. Anal. Appl. Pyrolysis 2017, 126, 275–287. [Google Scholar] [CrossRef]
- Hirose, T.; Kawagucci, S.; Suzuki, K. Mechanoradical H2 Generation during Simulated Faulting: Implications for an Earthquake-Driven Subsurface Biosphere. Geophys. Res. Lett. 2011, 38, 1–5. [Google Scholar] [CrossRef]
- Lefeuvre, N.; Truche, L.; Donzé, F.-V.; Vandenborre, J.; Gaucher, E.C.; Magnin, V. The Contribution of Mechanoradical Reactions to Crustal Hydrogen Generation. Earth Planet. Sci. Lett. 2025, 660, 119363. [Google Scholar] [CrossRef]
- Mosquera-Rivera, J.E.; Jiménez-Vergara, J.M.; Betancourt-Flórez, R.E.; Vargas-Jiménez, C.A.; Ball, P.J. Evaluating and Revealing Statistical Patterns and Database Performance in Hydrogen Exploration. Interpretation 2025, 13, SA29–SA46. [Google Scholar] [CrossRef]
- Lollar, B.S.; Onstott, T.C.; Lacrampe-Couloume, G.; Ballentine, C.J. The Contribution of the Precambrian Continental Lithosphere to Global H2 Production. Nature 2014, 516, 379–382. [Google Scholar] [CrossRef]
- Lefeuvre, N.; Thomas, E.; Truche, L.; Donzé, F.; Cros, T.; Dupuy, J.; Pinzon-Rincon, L.; Rigollet, C. Characterizing Natural Hydrogen Occurrences in the Paris Basin From Historical Drilling Records. Geochem. Geophys. Geosyst. 2024, 25, e2024GC011501. [Google Scholar] [CrossRef]
- Boreham, C.J.; Edwards, D.S.; Feitz, A.J.; Murray, A.P.; Mahlstedt, N.; Horsfield, B. Modelling of Hydrogen Gas Generation from Overmature Organic Matter in the Cooper Basin, Australia. APPEA J. 2023, 63, S351–S356. [Google Scholar] [CrossRef]
- Horsfield, B.; Mahlstedt, N.; Weniger, P.; Misch, D.; Vranjes-Wessely, S.; Han, S.; Wang, C. Molecular Hydrogen from Organic Sources in the Deep Songliao Basin, P.R. China. Int. J. Hydrogen Energy 2022, 47, 16750–16774. [Google Scholar] [CrossRef]
- Patiño, C.; Piedrahita, D.; Colorado, E.; Aristizabal, K.; Moretti, I. Natural H2 Transfer in Soil: Insights from Soil Gas Measurements at Varying Depths. Geosciences 2024, 14, 296. [Google Scholar] [CrossRef]
- Patiño, C.; Strąpoć, D.; Torres, O.; Mullins, O.; Bustos, U.; Bermudez, O.; López, A.; Trujillo, M.; Morales, H. First Downhole Sampling of a Natural Hydrogen Reservoir in Colombia. Front. Energy Res. 2024, 12, 1443269. [Google Scholar] [CrossRef]
- Vayssaire, A.; Abdallah, H.; Hermoza, W.; Figari, E. Regional Study and Petroleum System Modeling of the Eastern Llanos Basin. AAPG Search Discov. 2014, 9. [Google Scholar]
- Gonzalez-Penagos, F.; Moretti, I.; Guichet, X. Fluid Flow Modeling in the Llanos Basin, Colombia. AAPG Mem. 2017, 114, 191–217. [Google Scholar]
- Geymond, U.; Truche, L.; Sissmann, O.; Kubániová, D.; Recham, N.; Martinez, I. Mineralogical Changes and H2 Generation Yield During Hydrothermal Alteration of a Magnetite-Siderite Assemblage. JGR Solid Earth 2025, 130, e2024JB030724. [Google Scholar] [CrossRef]
- Behar, F.; Beaumont, V.; De B. Penteado, H.L. Rock-Eval 6 Technology: Performances and Developments. Oil Gas Sci. Technol. —Rev. IFP 2001, 56, 111–134. [Google Scholar] [CrossRef]
- Moretti, I.; Bouton, N.; Ammouial, J.; Carrillo Ramirez, A. The H2 Potential of the Colombian Coals in Natural Conditions. Int. J. Hydrogen Energy 2024, 77, 1443–1456. [Google Scholar] [CrossRef]
- Milesi, V.; Prinzhofer, A.; Guyot, F.; Benedetti, M.; Rodrigues, R. Contribution of Siderite–Water Interaction for the Unconventional Generation of Hydrocarbon Gases in the Solimões Basin, North-West Brazil. Mar. Pet. Geol. 2016, 71, 168–182. [Google Scholar] [CrossRef]
- Pillot, D.; Deville, E.; Prinzhofer, A. Identification and Quantification of Carbonates Species Using Rock-Eval Pyrolysis. Oil Gas Sci. Technol. 2014, 69, 341–349. [Google Scholar] [CrossRef]
- Armenteros, I. Chapter 2 Diagenesis of Carbonates in Continental Settings. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 62, pp. 61–151. ISBN 978-0-444-53526-9. [Google Scholar]
- Kamgaing, T. Précipitation de carbonates de cations divalents dans les systèmes lacustres: Intérêt, état des connaissances des mécanismes et suggestions (Revue critique de la littérature). Rev. Sci. L’eau 2015, 28, 81–102. [Google Scholar] [CrossRef][Green Version]
- Bernard, A.; Symonds, R.B. The Significance of Siderite in the Sediments from Lake Nyos, Cameroon. J. Volcanol. Geotherm. Res. 1989, 39, 187–194. [Google Scholar] [CrossRef]
- Perrier, V.; Charbonnier, S. The Montceau-Les-Mines Lagerstätte (Late Carboniferous, France). Comptes Rendus Palevol 2014, 13, 353–367. [Google Scholar] [CrossRef]
- Mora, A.; Reyes-Harker, A.; Rodriguez, G.; Tesón, E.; Ramirez-Arias, J.C.; Parra, M.; Caballero, V.; Mora, J.P.; Quintero, I.; Valencia, V.; et al. Inversion Tectonics under Increasing Rates of Shortening and Sedimentation: Cenozoic Example from the Eastern Cordillera of Colombia. SP 2013, 377, 411–442. [Google Scholar] [CrossRef]
- Villagómez, D.; Spikings, R. Thermochronology and Tectonics of the Central and Western Cordilleras of Colombia: Early Cretaceous–Tertiary Evolution of the Northern Andes. Lithos 2013, 160–161, 228–249. [Google Scholar] [CrossRef]
- Jaramillo, C.; Romero, I.; D’Apolito, C.; Bayona, G.; Duarte, E.; Louwye, S.; Escobar, J.; Luque, J.; Carrillo-Briceño, J.D.; Zapata, V.; et al. Miocene Flooding Events of Western Amazonia. Sci. Adv. 2017, 3, e1601693. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.; Leon, S.; Jaramillo, J.S.; Schmitt, A.K.; Mejia, D.; Arenas, J.C. Cretaceous Record from a Mariana– to an Andean–Type Margin in the Central Cordillera of the Colombian Andes; The Geology of Colombia; Servicio Geológico Colombiano: Bogotá, Colombia, 2020. [Google Scholar]
- Horton, B.K.; Parra, M.; Mora, A. Construction of the Eastern Cordillera of Colombia: Insights from the Sedimentary Record; Servicio Geológico Colombiano: Bogotá, Colombia, 2019; p. 32. [Google Scholar]
- Sarmiento, L.F. Llanos Basin, Geology and Hydrocarbon Potential; Petroleum Geology of Colombia; Agencia Nacional de los Hydrocarburos: Bogota, Colombia, 2011; Volume 9. [Google Scholar]
- Cooper, M.A.; Addison, F.T.; Alvarez, R.; Hayward, A.B.; Howe, S.; Pulham, A.J.; Taborda, A. Basin Development and Tectonic History of the Llanos Basin, Colombia. In Petroleum Basins of South America; American Association of Petroleum Geologists: Tulsa, OK, USA, 1995; ISBN 978-1-62981-083-6. [Google Scholar]
- Fajardo, A.; Rojas, L.; Christancho, J. Definición del Modelo Estratigráfico en el Intervalo Cretáceo Superior a Mioceno Medio en la Cuenca Llanos Orientales y Piedemonte Llanero; Informe Final ICP-ECOPETROL: Santander, Colombia, 2000; p. 114. [Google Scholar]
- Reyes-Harker, A.; Ruiz-Valdivieso, C.F.; Mora, A.; Ramírez-Arias, J.C.; Rodriguez, G.; De La Parra, F.; Caballero, V.; Parra, M.; Moreno, N.; Horton, B.K.; et al. Hyperspectral Imaging for the Determination of Bitumen Content in Athabasca Oil Sands Core Samples. Bulletin 2015, 99, 1407–1453. [Google Scholar] [CrossRef]
- Moretti, I.; Mora, C.A.; Zamora, W.; Valendia, M.; Rodriguez, G.; Mayorga, M. Llanos N-S Petroleum System Variation (Colombia). In Proceedings of the AAPG Extended Abstract, AAPG Annual Convention and Exhibition, Denver, CO, USA, 7–10 June 2009; p. 6. [Google Scholar]
- Wolaver, B.D.; Coogan, J.C.; Horton, B.K.; Bermudez, L.S.; Sun, A.Y.; Wawrzyniec, T.F.; Zhang, T.; Shanahan, T.M.; Dunlap, D.B.; Costley, R.A.; et al. Structural and Hydrogeologic Evolution of the Putumayo Basin and Adjacent Fold-Thrust Belt, Colombia. Bulletin 2015, 99, 1893–1927. [Google Scholar] [CrossRef]
- Pasquet, G.; Loiseau, K.; Diatta, M.; Firpo, G.; Swire, P.; Amey, A.; Burckhart, T.; Moretti, I. Natural Hydrogen in the Northern Semail Ophiolite: A Case Study in the Emirate of Ras Al Khaimah, UAE. Int. J. Hydrogen Energy 2024, 110, 787–796. [Google Scholar] [CrossRef]
- Moretti, I.; Geymond, U.; Pasquet, G.; Aimar, L.; Rabaute, A. Natural Hydrogen Emanations in Namibia: Field Acquisition and Vegetation Indexes from Multispectral Satellite Image Analysis. Int. J. Hydrogen Energy 2022, 47, 35588–35607. [Google Scholar] [CrossRef]
- Roche, V.; Geymond, U.; Boka-Mene, M.; Delcourt, N.; Portier, E.; Revillon, S.; Moretti, I. A New Continental Hydrogen Play in Damara Belt (Namibia). Sci. Rep. 2024, 14, 11655. [Google Scholar] [CrossRef]
- Zgonnik, V.; Beaumont, V.; Larin, N.; Pillot, D.; Deville, E. Diffused Flow of Molecular Hydrogen through the Western Hajar Mountains, Northern Oman. Arab. J. Geosci. 2019, 12, 71. [Google Scholar] [CrossRef]
- Dueñas, H. Neoproterozoic Records of the Llanos Orientales Basin, Colombia; Servicio Geológico Colombiano: Bogotá, Colombia, 2019. [Google Scholar]
- Baudin, F.; Bouton, N.; Wattripont, A.; Carrier, X. Carbonates Thermal Decomposition Kinetics and Their Implications in Using Rock-Eval® Analysis for Carbonates Identification and Quantification. Sci. Technol. Energy Transit. 2023, 78, 38. [Google Scholar] [CrossRef]
- Gonzalez Penagos, F.; Moretti, I.; Guichet, X. Formation Water Flow and Diagenesis in the Colombian Llanos Foreland Basin. First Break 2011, 29, 51–58. [Google Scholar] [CrossRef]
- McCollom, T.M.; Donaldson, C. Generation of Hydrogen and Methane during Experimental Low-Temperature Reaction of Ultramafic Rocks with Water. Astrobiology 2016, 16, 389–406. [Google Scholar] [CrossRef]
- Huang, R.; Sun, W.; Song, M.; Ding, X. Influence of pH on Molecular Hydrogen (H2) Generation and Reaction Rates during Serpentinization of Peridotite and Olivine. Minerals 2019, 9, 661. [Google Scholar] [CrossRef]
- Rezaee, R. Quantifying Natural Hydrogen Generation Rates and Volumetric Potential in Onshore Serpentinization. Geosciences 2025, 15, 112. [Google Scholar] [CrossRef]
- Espitalie, J.; Deroo, G.; Marquis, F. La Pyrolyse Rock-Eval et Ses Applications. Deuxieme Partie. Rev. l’Institut Fr. Pét. 1985, 40, 563–579. [Google Scholar] [CrossRef]
- Behar, F.; Kressmann, S.; Rudkiewicz, J.L.; Vandenbroucke, M. Experimental Simulation in a Confined System and Kinetic Modeling of Kerogen and Oil Cracking. Org. Geochem. 1992, 19, 173–189. [Google Scholar] [CrossRef]
- Burnham, A.K. A Simple Kinetic Model of Petroleum Formation and Cracking; Lawrence livermore Lab: Livermore, CA, USA, 1989; p. 11. [Google Scholar]
- Lewan, M.D. Evaluation of Petroleum Generation by Hydrous Pyrolysis Experimentation. Philos. Trans. 1985, 315, 123–134. [Google Scholar]
- Leila, M.; Lévy, D.; Battani, A.; Piccardi, L.; Šegvić, B.; Badurina, L.; Pasquet, G.; Combaudon, V.; Moretti, I. Origin of Continuous Hydrogen Flux in Gas Manifestations at the Larderello Geothermal Field, Central Italy. Chem. Geol. 2021, 585, 120564. [Google Scholar] [CrossRef]
- McCollom, T.M.; Klein, F.; Robbins, M.; Moskowitz, B.; Berquó, T.S.; Jöns, N.; Bach, W.; Templeton, A. Temperature Trends for Reaction Rates, Hydrogen Generation, and Partitioning of Iron during Experimental Serpentinization of Olivine. Geochim. Cosmochim. Acta 2016, 181, 175–200. [Google Scholar] [CrossRef]
- Miller, H.M.; Mayhew, L.E.; Ellison, E.T.; Kelemen, P.; Kubo, M.; Templeton, A.S. Low Temperature Hydrogen Production during Experimental Hydration of Partially-Serpentinized Dunite. Geochim. Cosmochim. Acta 2017, 209, 161–183. [Google Scholar] [CrossRef]



| Sample | Wells | Depth (Feet) | Temperature BHT (°C) | Gradient °C/km | Estimated Temperature |
|---|---|---|---|---|---|
| 1 | 1 | 3277.25 | 68.3 | 41.7 | 66.7 |
| 2 | 1 | 3284.7 | 68.3 | 41.7 | 66.8 |
| 3 | 2 | 3660.66 | 71.1 | 39 | 68.6 |
| 4 | 2 | 3662.33 | 71.1 | 39 | 68.6 |
| 5 | 2 | 3662.5 | 71.1 | 39 | 68.6 |
| 6 | 2 | 3665.5 | 71.1 | 39 | 68.7 |
| 7 | 3 | 3433.42 | 71.1 | 41.7 | 68.7 |
| 8 | 3 | 3435.5 | 71.1 | 41.7 | 68.7 |
| Sample | Siderite (in %) | H2_DG mmol/kg | H2_WT mmol/kg |
|---|---|---|---|
| 1 | 26.86 | 790 | 1624 |
| 2 | 4.15 | 573 | 817 |
| 3 | 9.06 | 295 | 618 |
| 4 | 11.76 | 342 | 982 |
| 5 | 13.98 | 436 | 1007 |
| 6 | 39.49 | 1074 | 3364 |
| 7 | 23.34 | 1312 | 1345 |
| 8 | 26.33 | 572 | 1660 |
| Sample | S1 (mg/g) | S2 (mg/g) | S3 (mg/g) | S3’ (mg/g) | S5 (mg/g) | HI | TOC % | Pyro MINC % | MINC % | Siderite % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.01 | 0.09 | 2.37 | 92.05 | 7.22 | 21 | 0.44 | 2.66 | 2.86 | 26.1 |
| 2 | 0.05 | 0.15 | 0.13 | 14.92 | 0.86 | 47 | 0.31 | 0.43 | 0.45 | 4.3 |
| 3 | 0.01 | 0.02 | 2.45 | 34.53 | 2.95 | 9 | 0.2 | 0.96 | 1.04 | 10.0 |
| 4 | 0.01 | 0.02 | 3.19 | 46.02 | 2.59 | 6 | 0.32 | 1.27 | 1.35 | 13.0 |
| 5 | 0.01 | 0.02 | 3.18 | 54.77 | 2.62 | 5 | 0.33 | 1.52 | 1.6 | 15.4 |
| 6 | 0.01 | 0.01 | 0.83 | 142.43 | 1.86 | 1 | 0.67 | 4.39 | 4.44 | 42.8 |
| 7 | 0.01 | 0 | 1.45 | 89.15 | 1.75 | 0 | 0.3 | 2.62 | 2.67 | 25.75 |
| 8 | 0.01 | 0.01 | 0.68 | 93.91 | 2.34 | 2 | 0.46 | 2.89 | 2.95 | 28.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, I.; Prinzhofer, A.; Ammouial, J.; Bouton, N. Natural Hydrogen Generation from Phanerozoic Sedimentary Siderite. Minerals 2025, 15, 1218. https://doi.org/10.3390/min15111218
Moretti I, Prinzhofer A, Ammouial J, Bouton N. Natural Hydrogen Generation from Phanerozoic Sedimentary Siderite. Minerals. 2025; 15(11):1218. https://doi.org/10.3390/min15111218
Chicago/Turabian StyleMoretti, Isabelle, Alain Prinzhofer, Jérémie Ammouial, and Nicolas Bouton. 2025. "Natural Hydrogen Generation from Phanerozoic Sedimentary Siderite" Minerals 15, no. 11: 1218. https://doi.org/10.3390/min15111218
APA StyleMoretti, I., Prinzhofer, A., Ammouial, J., & Bouton, N. (2025). Natural Hydrogen Generation from Phanerozoic Sedimentary Siderite. Minerals, 15(11), 1218. https://doi.org/10.3390/min15111218






