Abstract
Heavy metal ions in wastewater endanger ecology and human health, requiring cost-effective treatments. This study innovatively converts abandoned basalt fibers (BFs) into high-performance adsorbents (BFSN) via NaOH etching and chelation with nitrilotriacetic acid (NTA)/carboxymethyl starch (CMS), introducing target functional groups. Characterizations (XPS, FTIR, zeta potential) reveal Cu2+/Pb2+ adsorption mechanisms: -COO− chelation, N-containing group ion exchange, and electrostatic adsorption. Kinetics fit a pseudo-first-order model (R2 > 0.98) and isotherms fit the Langmuir model, confirming monolayer chemisorption. BFSN has excellent thermal stability (≤2% mass loss at 800 °C) and post-adsorption integrity (≈0.11% mass loss post-loading). Waste-derived BFSN, cheaper than commercial adsorbents, has strong economic viability. This “waste-to-value” approach offers efficient, sustainable large-scale heavy metal wastewater remediation, advancing waste utilization and ecological restoration in water treatment.
1. Introduction
Water pollution control remains a global priority for environmental and public health. Pollution sources are categorized as non-point (diffuse origins [1,2]) or point sources (identifiable discharges like industrial effluents [3]). Industrial wastewater, generated as a byproduct of anthropogenic activities, has been persistently identified as a major anthropogenic pollutant capable of exerting long-term deleterious effects on aquatic ecosystems. This wastewater poses significant treatment challenges due to complex compositions (e.g., long-chain organic compounds [4], heavy metals like Cu2+ and Pb2+ [5], nitrate/phosphate [6], dyes [7], pharmaceutical residues [8], and persistent organic pollutants [9]), extreme pH levels, and toxic residuals [10]. Mineral processing wastewater generated at mining sites constitutes a major source of environmental pollution, with mineral processing wastewater constituting approximately 10% of total discharge [11].
The substantial discharge volume of mineral processing wastewater necessitates advanced treatment solutions. While conventional techniques—including natural sedimentation [12], flocculation-enhanced clarification [13], and membrane filtration—effectively remove suspended solids [14], the persistent presence of heavy metal ions (e.g., Cu2+, Pb2+, Cd2+) presents critical treatment barriers. These metal ions, originating from complex ore matrices and multi-stage processing, exhibit strong affinity for dissolved organic ligands, forming stable complexes that resist conventional precipitation methods. The recalcitrant nature of these metal–organic complexes, coupled with their potential for bioaccumulation, constitutes the principal obstacle to achieving closed-loop water reuse in mineral processing operations.
Adsorption has emerged as a promising remediation approach due to its efficiency, cost-effectiveness, and environmental compatibility [15]. The appeal of this method is further enhanced by its straightforward equipment configuration and process design, coupled with its robust adaptability in the effective removal of heavy metal ions. The development of novel adsorbents has emerged as a global research priority. This means that recent investigations have focused on high-porosity nanostructured materials, including graphene [16], fullerenes [17], graphitic carbon nitride [18], and metal–organic frameworks (MOFs) [19], as potential replacements for conventional adsorbents like activated carbon in heavy metal removal from wastewater [20]. However, their practical implementation remains constrained by limitations in adsorption capacity, recyclability, and cost-effectiveness.
Industrial solid wastes provide viable, sustainable alternatives, exemplified by Liupanshui’s annual production of approximately 50,000 tons of waste basalt fiber. The fiber’s manufacturing process is fundamentally constrained by slow crystallization of multicomponent structures (primarily SiO2-Al2O3-FeO/Fe2O3) and inherent melt inhomogeneity, which collectively constitute the primary causes of waste generation [21]. While conventional modification techniques face process optimization challenges [22], chelating modification has emerged as a promising solution. Functionalization with chelating agents such as nitrilotriacetic acid (NTA) and carboxymethyl starch (CMS) introduces active binding sites that significantly enhance heavy metal adsorption capacity through mechanisms including ion exchange and complexation. This approach not only improves adsorption efficiency but also maintains environmental sustainability by utilizing waste-derived materials.
The purpose of this study was to evaluate the treatment effect of a chelating-modified waste basalt fiber on heavy metal ions in mineral wastewater. Comprehensive characterization (XPS, SEM, FTIR, XRD, Zeta potential) evaluates adsorption kinetics and mechanisms. The research contributes to sustainable wastewater management through waste valorization, aligning with circular economy principles in mineral processing industries.
2. Materials and Methods
2.1. Materials
Basalt fiber (BF) was collected from the Liupanshui region of Guizhou Province, China. The received BF was ground into powder using a planetary ball mill and soaked in acetone for 24 h. The processed samples were subjected to washing with deionized water and drying in the oven. Acetone (C3H6O, analytically pure) was provided by the Huatong Chemical Reagent Factory (Guiyang, China). Sodium hydroxide (NaOH, analytically pure) was provided by Jiangchuan Chemical Co., Ltd. (Chongqing, China). Trisodium nitrilotriacetate (C6H8NNa3O7, chemical purity ≥98%) and carboxymethyl starch [C10H19O8Na]n were offered by Acme Biochemical Co., Ltd. (Shanghai, China). Deionized water (resistivity ≥18 mΩ·cm) was adopted.
2.2. Modification of BF
Several NaOH solutions with a pH of 13 were prepared. Precisely 2 g of the cleaned basalt fiber was weighed and immersed in one of the NaOH solutions. Under constant temperature conditions of 40 °C, the solution was agitated for 12 and 20 h. The surface NaOH was carefully removed, and the fibers were rinsed thoroughly. Drying was then carried out in the 60 °C oven before storing the fibers for subsequent utilization. The CMS solutions ranging from 0.5% to 2% and NTA solutions ranging from 1% to 4% were prepared. The uniform mixing of these solutions ensured their suitability as surface-modifying agents for fiber modification. The experimentally determined optimal concentrations of carboxymethyl starch (CMS) and nitrilotriacetic acid (NTA) were 0.5% and 1%, respectively. Subsequently, following etching treatment with an alkaline solution, the fiber samples were immersed in a modifying agent solution prepared using the aforementioned optimal concentrations of CMS and NTA. At an ambient temperature of 25 °C, the solutions were uniformly agitated for 12 h. The samples were then air-dried for 1 h, followed by thorough rinsing with copious amounts of deionized water to remove any residual modifying agents. Drying in a 60 °C oven completed the preparation of the modified basalt fiber material (BFSN).
2.3. Preparation of Lead Ions (Pb (II)) Solutions and Copper Ions (Cu (II)) Solutions
In the laboratory, 6 mg solid reagents Cu (II) sulfate (CuSO4) and lead nitrate (Pb(NO3)2) were accurately weighed using an analytical balance. The powders were transferred into a beaker with a small amount of deionized water to facilitate dissolution. The complete dissolution was followed by transferring the solution to a 100 mL volumetric flask, bringing it to volume with deionized water. Proper mixing was achieved by inverting and shaking the flask to ensure homogeneity of the solution. Labeling and storage were performed in a light-protected environment at room temperature.
2.4. Characterization
An X’Pert PRO diffractometer (PXRD) was used to assess the crystallinity. The surface morphology and microstructure of BF were examined via SEM (S-4800, Hitachi Corporation, Tokyo, Japan). The chemical composition of both BF and modified Basalt Fiber (BFSN) was characterized by FTIR (AVATAR 360, Madison and Nicolet, Madison, WI, USA) within the wavenumber range between 400 and 4000 cm−1. Further chemical analysis was performed using XPS (ESCALAB™ 250Xi, Thermo Fisher Scientific, Waltham, MA, USA) with monochromatic Al Kα radiation. Thermo gravimetric analysis (TGA) was conducted under a nitrogen atmosphere from 30 °C to 800 °C at a 10 °C/min heating rate using a ThermMax 500 (Thermo Cahn, Fitchburg, WI, USA). A Zetasizer Nano ZS instrument (Malvern, UK) was utilized to document the zeta potential information for the synthesized samples at room temperature.
2.5. Batch Adsorption Experiments
An adsorption test was conducted on the samples, with each sample consisting of 50 mL of turbid water containing 60 mg/L Cu2+ and 60 mg/L Pb2+, along with a mineral processing agent as an interfering substance. These experiments were performed at 20°, 30°, and 40 °C and a pH of 3~8, which were typical of industrial wastewater conditions. The samples were agitated in an incubator shaker at 120 rpm. Adsorption experiments were conducted for 60 min to reach equilibrium. The concentration of the residual aqueous phase (Ce, mM) was determined using titration methods. The metal adsorbed per unit mass of adsorbent at equilibrium (Qe, mg/g) was determined by applying Equation (1).
where Qe (mg/g) represents the amount of adsorption; m (g) denotes the mass of the adopted adsorbent; V (L) is the solution volume; and Ce and C0 (mg/L) are the equilibrium and initial concentrations of the metal ion M (II), respectively. Each adsorption test was carried out in triplicate, and the average results are reported as the final values.
3. Results and Discussion
3.1. Characterizations
3.1.1. Analysis of Surface Structure and Morphology Changes
To determine the phase composition of basalt fibers (BFs) and assess their corrosion behavior in sodium hydroxide (NaOH), X-ray diffraction (XRD) analysis was conducted on pristine BF and samples subjected to alkaline corrosion for varying durations. As shown in Figure 1a, the XRD pattern of pristine BF exhibited a broad halo at 20°–35°, characteristic of an amorphous silicate glass structure comprising oxides (e.g., SiO2, Al2O3, Fe2O3, CaO, MgO) [23]. After NaOH treatment, the intensity of this amorphous halo significantly decreased, with concurrent attenuation of minor peaks near 40°–45° and 65°. This indicates severe disruption of the glass network structure, leading to enhanced structural disordering [24]. Notably, extending corrosion time from 12 h to 20 h induced only marginal changes in peak intensity, suggesting saturation of the alkaline etching effect [25].
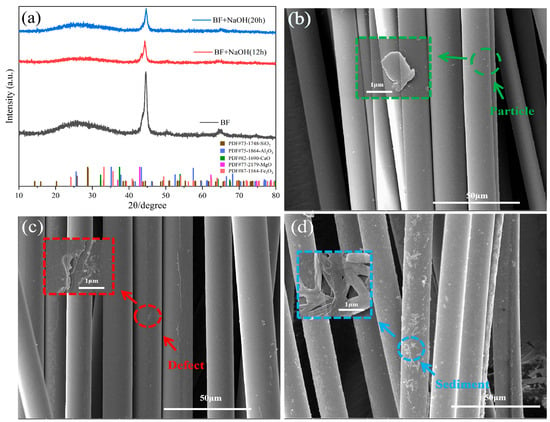
Figure 1.
(a) XRD analysis of basalt fiber under different modification conditions, SEM images of (b) original BF, (c) original BF after sodium hydroxide treatment, and (d) BFSN.
Complementary SEM imaging (Figure 1b–d) revealed morphological evolution induced by corrosion. Pristine BF displayed smooth surfaces with sporadic particulate features (Figure 1b). Alkaline exposure generated severe surface defects (e.g., micron-scale pits and cracks) and sparsely distributed granular deposits, markedly increasing surface roughness (Figure 1c). These defects likely originated from dissolution of Si–O–Si bonds, while the deposits may correspond to silanol groups (≡Si–OH) formed during network degradation. Subsequent co-treatment with NTA and CMS further promoted the adhesion of sediment layers (Figure 1d), thereby augmenting surface-active sites.
In conclusion, NaOH corrosion generates micro/noon-scale surface defects (SEM) and disrupts the glass network (XRD), which synergistically enhance physical adsorption sites and facilitate chemical functionalization. The subsequent NTA/CMS treatment amplifies surface reactivity, substantially improving the adsorption capacity of BF.
3.1.2. FTIR Analysis
To investigate the chemical structural changes of basalt fiber (BF) under different modification treatments, Fourier transform infrared spectroscopy (FTIR) was employed to characterize the samples before and after activation modification (Figure 2). The FTIR spectra of pristine BF exhibited a broad band in the 3600−3200 cm−1 range, with characteristic peaks arising from hydrogen-bonded O−H stretching vibrations of adsorbed water or silanol groups (−Si−OH) [26]. In the low-wavenumber region, absorption bands at 1407 cm−1 and 837 cm−1 correspond to the asymmetric stretching vibration of Si–O–Al bonds and the stretching vibration of Si−O bonds, respectively, confirming the basic chemical structure of BF [26,27].
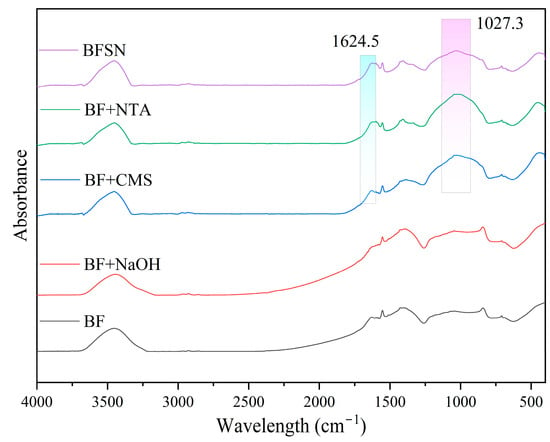
Figure 2.
FTIR spectra of before and after BF modification.
Notably, the FTIR spectrum of NaOH-treated BF (BF + NaOH) reveals negligible alterations in key characteristic bands: the Si-O-Al asymmetric stretching vibration at 1407 cm−1, the Si-O stretching vibration at 837 cm−1, and the broad O-H stretching envelope (3600–3200 cm−1) remain essentially unchanged. This confirms that NaOH etching predominantly modifies the physical morphology of the BF surface, exerting minimal impact on its core chemical structure.
In contrast, NTA and CMS treatments—both individually and synergistically (NTA-CMS)—induce distinct spectral changes. A prominent new peak emerges at 1624 cm−1, unequivocally assigned to the asymmetric stretching vibration of deprotonated carboxylate groups (−COO−) [28,29,30]. The absorption feature observed at ~1027 cm−1 likely arises from overlapping vibrational modes: primarily the C-O-C ether bond stretching characteristic of CMS and potentially contributions from C-O bonds or the C-N stretching vibration of NTA [31,32]. Notably, in the NTA-CMS spectrum, the peak at 1024 cm−1 signifies the co-contribution of C-O-C/C-O groups (from CMS) and C-N stretching (from NTA) within the functionalized surface layer.
Collectively, FTIR analysis substantiates that NaOH activation serves as a crucial physical pre-treatment, enhancing BF’s receptiveness to subsequent chemical modification. Leveraging this activated surface, the synergistic NTA-CMS system facilitates the stable anchoring of -COO− groups (evidenced by the 1624 cm−1 peak) alongside other functionalities (e.g., ethers, amines), thereby potentially constructing abundant active sites for targeted metal ion binding.
3.1.3. TGA and Zeta Potential Analysis
Thermogravimetric analysis (TGA) was employed to systematically evaluate the thermal stability and compositional changes of the materials during dynamic heating [32]. The experiments were conducted in a nitrogen-protected atmosphere with a heating rate of 10 °C/min and a temperature scanning range from 30 °C to 800 °C. As shown in Figure 3a, the virgin basalt fiber (BF) demonstrated remarkable thermal stability, with a mass loss of less than 2%. After acetone treatment, the mass loss of BF further decreased to below 1% (Figure 3b), fully validating the effective removal of organic impurities from the surface, which is consistent with previous literature reports [23,26]. For the modified basalt fiber (BFSN), the total mass loss was approximately 0.11% (Figure 3c), and this minor mass loss could be ascribed to the decomposition of NTA amine groups and/or the partial thermal decomposition of CMS carboxyl groups. Notably, the TGA curves of BFSN after adsorbing Cu and Pb ions (Figure 3d) exhibited a lower mass loss than the initial BFSN, strongly indicating that the modified material maintained the thermal stability of its backbone structure during the ion adsorption process. This confirms that the grafting of functional groups did not compromise the high-temperature resistance of the substrate material.
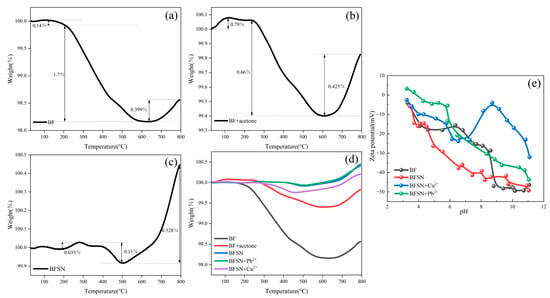
Figure 3.
TGA thermograms of the as-received BF (a), acetone-washed BF (b), the modified BFSN (c), and the summary comparison chart (d). Zeta potential of BF, BFSN, and after adsorption of copper and lead ions (e).
The Zeta potential test revealed the variation trend of the BFSN surface charge state with pH (Figure 3e), which directly influences the adsorption behavior of heavy metal ions [33]. Comparing the Zeta potentials of BF and BFSN in a neutral pH environment (pH 6–8), a significant decrease from −20 mV to −35 mV was observed, clearly indicating the successful grafting of anionic groups such as -COO− onto the surface of BF. In a strongly acidic environment, the Zeta potentials of both BF and BFSN remained stable around 5 mV, which was due to the accumulation of H+ ions resulting from the substantial adsorption of H+ on the material surface. Under strongly alkaline conditions, the Zeta potentials of the two materials dropped below −40 mV, attributed to the enrichment of a large amount of OH− around BF. In the pH range of 3–11, the potential of BFSN after Pb2+ adsorption increased by approximately 10 mV, a significant shift that strongly confirms the formation of coordination complexes between Pb2+ and surface functional groups, such as -COO− forming monodentate or bidentate complexes with Pb2+ [34]. In contrast, the potential of BFSN after Cu2+ adsorption showed a large positive shift in an alkaline environment (reaching up to +45 mV at pH = 8), indicating that the adsorption capacity of BFSN for Cu2+ increased significantly with the increase in pH. It is presumed that this is because Cu2+ forms complexes with hydroxyl groups under alkaline conditions (e.g., the formation of Cu(OH)+ and Cu(OH)2) [35], enhancing the adsorption on the BFSN surface through surface precipitation or stronger bonding. The correlation between Zeta potential and adsorption capacity demonstrated that the grafted carboxyl and amino groups on the surface of BFSN significantly enhanced the selective adsorption of heavy metal ions by effectively regulating electrostatic interactions and coordination ability, especially for Cu2+ under alkaline conditions.
3.1.4. XPS Results
X-ray photoelectron spectroscopy is considered a powerful tool to determine the surface properties of adsorbents before and after adsorbate removal.
In the high-resolution XPS analysis, the C 1s spectrum of raw material BF displayed two distinct peaks (Figure 4a): a dominant peak at ~284.80 eV assigned to C-C/C-H bonds (attributed to atmospheric carbon contamination) and a secondary peak at ~285.97 eV, presumably from surface additives or impurities [36]. After modification, the C 1s spectrum of BFSN resolves into three characteristic peaks: the C-C peak shifts slightly from 284.80 to 284.78 eV, a change within instrumental calibration variations [37]; the 285.97 eV peak shifts to 286.46 eV, corresponding to C-O/C-O-C bonds from oxygenated functional groups (alcohols and ethers) introduced by CMS [38,39]; and a new peak at 288.70 eV emerges, assigned to carbonyl (C=O) groups from -COO− in NTA/CMS and amide (-N-C=O) in NTA [38,39]. Upon Cu2+ adsorption, the 288.70 eV peak shifts significantly, indicating strong coordination between -COO− and Cu2+ [40], whereas Pb2+ adsorption induces negligible shifts, suggesting weak -COO−-Pb2+ interaction. Corroboration with other elemental signals is needed to clarify reactions with additional functional groups.
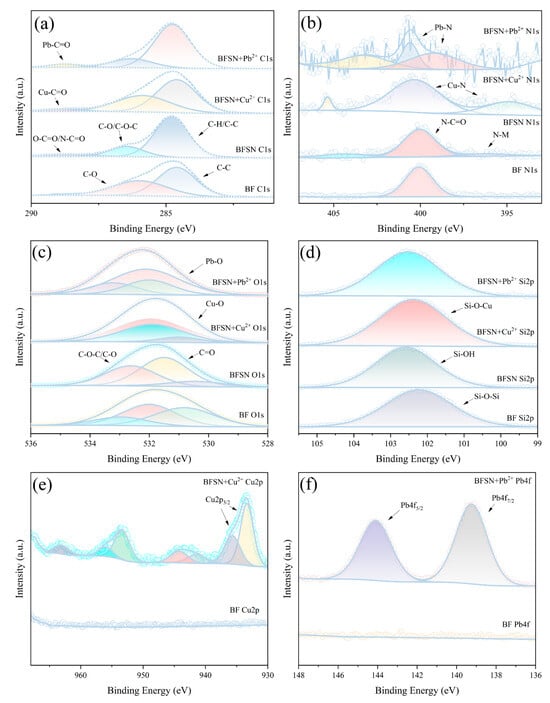
Figure 4.
XPS spectra of BF and BFSN composites: (a) C1s spectra comparison of BF, BFSN, and BFSN after Cu2+/Pb2+ adsorption. (b) N1s spectra evolution of BF, BFSN, and BFSN post Cu2+/Pb2+ adsorption. (c) O1s spectral shifts in BF, BFSN, and BFSN following heavy metal adsorption. (d) Si2p binding energy changes in BF, BFSN, and BFSN after metal ion capture. (e) Cu2p spectra of BF and BFSN-Cu2+ adsorption complex. (f) Pb4f spectra of BF and BFSN-Pb2+ adsorption system.
The N 1s spectrum of BFSN features a primary peak at 400.3 eV (Figure 4b), arising from amide (-N-C=O) groups introduced by NTA. A minor peak at 399.2 eV post-modification is attributed to tertiary amine nitrogen (-N(CH2COO−)3) coordination with alkali-etched BF surface metal ions (N-M) [40]. Following Cu2+/Pb2+ adsorption, both 400.3 eV and 399.2 eV peaks shift, confirming coordination of metal ions with amide groups and ion-exchange reactions with surface N-M bonds. Analysis of C 1s and N 1s signals initially reveals functional group introduction and metal ion coordination, with O 1s, Si 2p, etc., pending further mechanistic exploration.
The O 1s spectrum of BFSN (Figure 4c) exhibits a main peak at 531.8 eV, assigned to carboxylate oxygen (-COO−) from CMS/NTA and amide oxygen (-N-C=O) from NTA, and a secondary peak at 532.5 eV from C-O/C-O-C bonds in CMS [40]. Cu2+/Pb2+ adsorption induces significant shifts in the 531.8 eV peak, confirming chelation between -COO−/-N-C=O and metal ions, whereas the 532.5 eV peak shifts marginally, indicating weak C-O/C-O-C coordination. In-depth Si 2p analysis is required for comprehensive mechanism resolution.
The Si 2p spectrum (Figure 4d) shows a shift from 102.24 eV in BF to 102.59 eV in BFSN, resulting from NaOH-disrupted Si-O-Al and Si-O-Si bonds forming Si-OH groups [41]. Upon Cu2+ adsorption, the peak shifts to 102.38 eV, attributed to Si-OH coordination forming Si-O-Cu bonds [42]. Pb2+ adsorption elicits negligible Si 2p shifts, implying weak Si-OH-Pb2+ interaction or preferential Pb2+ reaction with other functional groups. This clarifies silicon-related group roles in metal ion adsorption, warranting subsequent Cu 2p/Pb 4f analysis for adsorbed ion chemical states.
The Cu 2p high-resolution spectrum (Figure 4e) shows characteristic peaks at ~954 eV (2p1/2) and ~935 eV (2p3/2), confirming divalent Cu2+. The 2p3/2 peak resolves into 933.38 eV (Cu-O) and 935.88 eV (-COO−-Cu2+ coordination), with satellite peaks at 943.88 eV and 963.18 eV, further validating Cu2+ [40,42]. The Pb 4f spectrum (Figure 4f) exhibits peaks at 139.24 eV (4f7/2) and 144.10 eV (4f5/2), reflecting altered Pb2+ chemical environments due to coordination with amide and carboxylate groups, forming lead oxides, alongside potential weak Si-OH-Pb2+ interaction and electron transfer effects [43].
In summary, systematic XPS analysis of the C 1s, N 1s, O 1s, Si 2p, Cu 2p, and Pb 4f spectra comprehensively reveals the functional group transformations of BFSN and its coordination mechanisms with Cu2+ and Pb2+. We conclude that the adsorption of Cu2+ by BFSN induces changes in the electronic valence states of almost all non-metallic elements, whereas the adsorption of Pb2+ by BFSN triggers valence state changes exclusively in N and O elements. This work thereby provides a theoretical basis for optimizing the adsorption properties of such materials.
3.2. Adsorption Performance and Mechanism Analysis
3.2.1. Effect of Contact Time, Temperature, and pH
Adsorption kinetic behavior is a critical parameter for evaluating the practical efficacy of adsorbents, as it focuses on elucidating the variation of adsorption capacity with contact time and determining the equilibrium time. Specifically, the adsorption of Cu2+ and Pb2+ by BFSN exhibited distinct stages (Figure 5a). Within the first 50 min, the adsorption capacities of both ions increased rapidly, a phenomenon directly attributed to the abundance of active sites on the adsorbent surface (e.g., surface adsorption sites provided by the porous structure). The ample availability of these sites at this stage furnished a thermodynamic driving force for the rapid binding of the ions. When the contact time exceeded 50 min, the adsorption rate slowed and eventually reached equilibrium due to the saturation of available active sites, indicating that the system approached adsorption equilibrium.
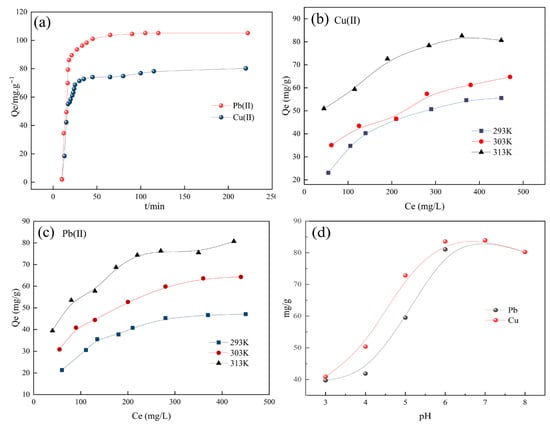
Figure 5.
(a) Impact of contact duration on the Cu (II) and Pb (II) adsorption on BFSN, (b) impact of temperature on the Cu (II) adsorption to BFSN, (c) impact of temperature on the Pb(II) adsorption to BFSN, (d) impact of pH on the adsorption abilities of both ions by BFSN at 25 °C.
To investigate the thermodynamic properties of the adsorption process, the effect of temperature on BFSN’s adsorption performance was further examined over the range of 293–313 K (20–40 °C). The results presented in Figure 5b,c revealed that the equilibrium adsorption capacities (Qe) of both Cu2+ and Pb2+ followed a trend of “rapid increase followed by stabilization” with increasing equilibrium concentration (Ce), a characteristic consistent with typical adsorption isotherm behavior. Specifically, at low concentrations, Qe increased significantly with rising solute supply, as the adsorption sites remained unsaturated. As the concentration increased to a certain threshold, the active sites were gradually occupied, causing the growth rate of Qe to slow until adsorption equilibrium was achieved.
Notably, increasing temperature significantly promoted the adsorption process, with the adsorption capacities of both ions following the order 313 K > 303 K > 293 K, indicating that the adsorption process was endothermic. Specifically, the increment in Cu2+ adsorption capacity at 313 K was more pronounced, potentially attributed to its hydration radius, charge distribution characteristics, or the higher temperature sensitivity of chemical interactions (e.g., chelation, ion exchange) between Cu2+ and the adsorbent surface. Moreover, the adsorption capacity of Pb2+ was higher than that of Cu2+ at all tested temperatures under identical conditions, suggesting a stronger affinity of BFSN for Pb2+.
In addition, the pH value is a critical factor that modulates the adsorption capacity of BFSN toward Cu2+ and Pb2+, as it directly influences the protonation degree of surface functional groups on the adsorbent and the speciation of metal ions in solution. The variation pattern of BFSN’s adsorption performance with solution pH is illustrated in Figure 5d. Within the pH range of 3.0–8.0, the adsorption capacities of both ions exhibited a monotonically increasing trend with the rise in pH. The maximum adsorption capacities were achieved at pH 6.5, reaching approximately 82.5 mg/g for Cu2+ and 80 mg/g for Pb2+, respectively. However, a further increase in pH beyond 6.5 led to a decrease in adsorption capacities, which can be attributed to the formation of hydroxide precipitates by Cu2+ and Pb2+ in the solution under such alkaline conditions.
3.2.2. Adsorption Isotherms
In the present study, four well-established adsorption models, namely, Langmuir (Equation (1)) [44], Freundlich (Equation (3)) [45], Temkin (Equation (5)) [46], and Dubinin–Radushkevich (D–R) (Equation (7)) [47], were employed to analyze the equilibrium adsorption data of Pb(II) and Cu(II) ions. The Langmuir model is predicated on the assumption that adsorption proceeds via a physical process, characterized by monolayer adsorption with a defined maximum adsorption capacity. Key postulates of this model include a constant adsorption energy, an energetically homogeneous adsorbent surface, and the absence of interactions between adjacent adsorbed molecules. Accordingly, the heat of adsorption remains constant across the range of fractional surface coverage. Furthermore, all adsorption events follow an identical mechanism, resulting in a uniform adsorbed structure. The general form of the Langmuir isotherm equation is as follows [48]:
The linear form of Equation (1) can be written as follows:
Herein, Ce (mg/L) denotes the equilibrium concentration of metal ions; qe (mg/g) represents the amount of heavy metal ions adsorbed per unit mass of adsorbent at equilibrium; qm (mg/g) refers to the maximum quantity of metal ions per unit mass of adsorbent required to form a complete monolayer; and KL (L·mg−1) is the Langmuir constant associated with the affinity of binding sites.
The Freundlich equation is widely employed for describing isothermal adsorption processes and is predicated on adsorption onto heterogeneous surfaces. The model is expressed by the following equation [49]:
The linear form can be written as follows:
Herein, n is the intensity parameter, and KF [(mg/g)/(mg/L)−n] refers to the Freundlich capacity factor of the adsorbent.
The Temkin model is a notable isothermal adsorption model in multiphase catalysis, which characterizes the chemical adsorption of gases onto solid surfaces. The expression for the Temkin model is as follows [50]:
The linear form can be written as follows:
where R is the gas constant (8.314 (J/(mol·K)), T is the temperature (K), bt is Temkin’s constant (J·g/mol2)), and Kt is Temkin’s isotherm constant (L/g).
The D–R adsorption model is more general than the Langmuir model and is used to show the adsorption method through a Gaussian energy distribution on a heterogeneous surface. The common form of the D–R isotherm model equation is stated as follows [51]:
The linear form can be written as follows:
Herein, qD denotes the maximum adsorption capacity (mmol·g−1); ε is the Polanyi potential (defined as ε = RT ln(1 + 1/Ce)); BD is the activity coefficient associated with the adsorption free energy (mol2·J−2); T is the absolute temperature (K); and R is the gas constant (8.314 J·mol−1·K−1).
The adsorption parameters of Cu2+ and Pb2+ onto BFSN materials derived from different isothermal models are summarized in Table 1, with the fitting results of the four adsorption isotherm data depicted in Figure 6a–f. The correlation coefficients (R2) indicate that the model fitting quality follows the order of Langmuir > Freundlich > Temkin > Dubinin–Radushkevich, which suggests that monolayer adsorption predominantly occurs on the adsorbent surface and confirms that the adsorption of Cu2+ and Pb2+ by BFSN is predominantly chemisorptive in nature.

Table 1.
Parameters for the adsorption of both ions on BFSN.
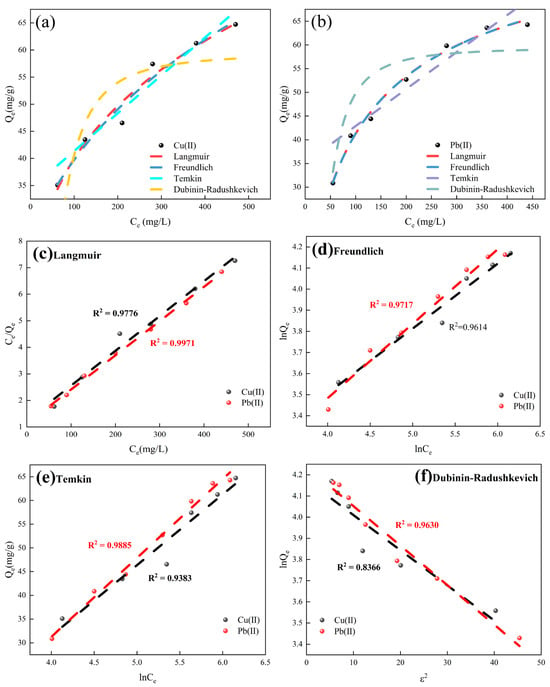
Figure 6.
(a) Adsorption isotherms of BFSN adsorbing copper ions, (b) Adsorption isotherms of BFSN adsorbing lead ions, (c) Langmuir adsorption isotherm, (d) Freundlich adsorption isotherm, (e) Temkin adsorption isotherm, and (f) Dubinin–Radushkevich adsorption isotherm.
In the Langmuir isotherm model, KL is the Langmuir parameter (units: L·mM−1), and C0 denotes the initial concentration (units: mM). A is the dimensionless separation factor (also referred to as the equilibrium parameter), and RL is defined as RL = 1/(1 + KL·C0). RL characterizes the adsorption behavior: irreversible adsorption when RL = 0, favorable adsorption when 0 < RL < 1, and unfavorable adsorption when RL > 1 [52].
Data in the table reveal that for KL values of 0.0105 and 0.0115, the corresponding RL values fall within the range of 0 to 1, confirming that BFSN is an effective adsorbent for the removal of these two ions from wastewater.
3.2.3. Adsorption Kinetics
To elucidate the adsorption mechanism between Cu2+, Pb2+, and BFSN, and to assess key factors such as adsorption rate, equilibrium adsorption time, and rate-controlling steps, two kinetic models were employed: namely, the pseudo-first-order (PFO, Equation (9)) and pseudo-second-order (PSO, Equation (10)) models [53,54].
Figure 7a,b depict the kinetic data along with their corresponding fits using the two proposed kinetic models. Detailed parameters of these models are summarized in Table 2.
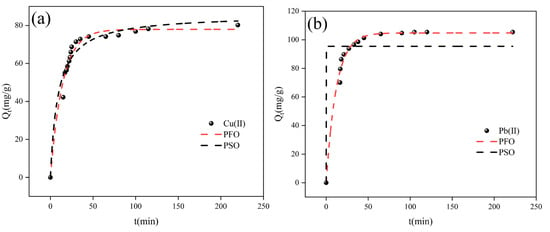
Figure 7.
(a) PFO and PSO kinetics of BFSN adsorbing copper ions, and (b) PFO and PSO kinetics of BFSN adsorbing lead.

Table 2.
PFO and PSO parameters for the adsorption of both ions at BFSN.
The pseudo-first-order (PFO, R2 = 0.98) and pseudo-second-order (PSO, R2 = 0.95) kinetic models both exhibited good fitting for Cu2+ adsorption onto BFSN, suggesting a synergistic effect of physical and chemical adsorption in the process. In contrast, for Pb2+ adsorption onto BFSN, the PFO model showed significantly better fitting (R2 = 0.99) than the PSO model (R2 = 0.84), indicating that the adsorption process was predominantly governed by physical adsorption.
Additionally, the adsorption capacities of Cu2+ and Pb2+ calculated by the PFO model (qe(cal) = 77.93 mg/g and 104.79 mg/g, respectively) were in good agreement with the experimental values (qe(exp) = 80.25 mg/g and 105.38 mg/g, respectively). These results further confirm the reliability of the pseudo-first-order (PFO) kinetic model.
3.2.4. Comparison with Other Adsorbents
To comprehensively evaluate the adsorption performance of BFSN and contextualize its advantages within the existing literature, a comparative analysis of its maximum adsorption capacities (Qm) for Cu2+ and Pb2+ with those of other reported adsorbents is presented in Table 3, alongside key influencing factors (e.g., pH) and adsorption mechanisms.

Table 3.
Comparison of Pb2+ and Cu2+ adsorption capacity by BFSN with other adsorbents.
As summarized in Table 3, the Qm of BFSN for Cu2+ (80.25 mg/g) significantly surpasses that of C-phenylcalix[4]pyrogallolarene (8.140 mg/g), natural zeolite (NZ) (7.98 mg/g), chitosan silica gel composite (1.33 mg/g), and polymer submicron spheres (26.9 mg/g) and even exceeds that of clay (37.26 mg/g). For Pb2+, although BFSN’s Qm (105.38 mg/g) is lower than that of clay (152 mg/g), it remains much higher than the values of C-phenylcalix[4]pyrogallolarene (60.97 mg/g), chitosan silica gel composite (3.82 mg/g), NZ (75.4 mg/g), and polymer submicron spheres (25.3 mg/g). Compared with clay, BFSN also demonstrates a significant advantage in terms of adsorption capacity.
BFSN’s superior adsorption performance for Cu2+ and Pb2+ lies in its unique synergistic dual mechanism (chelation + ion exchange)—a distinction from most single-mechanism adsorbents in the literature. Chelation occurs via BFSN’s CMS/NTA-introduced -COO− and -N-C=O groups, forming stable rings with metal ions for high binding specificity. Concurrently, ion exchange between solution metal ions and BFSN’s surface cations (e.g., Na+, K+) boosts accessible sites, addressing limitations of single-pathway alternatives (e.g., NZ’s [56] pH-sensitive ion exchange; polymer spheres’ [59] limited imprinted sites).
Notably, BFSN shows higher affinity for Cu2+ (evidenced by superior Qₘ) than Pb2+, as Cu2+ forms stronger bonds with -COO−/-N-C=O, while Pb2+ interacts weakly, possibly favoring other sites. Overall, BFSN’s pH-stable dual mechanism and single optimized pH (6.5) enhance practicality, with future studies solidifying its potential as a cost-effective heavy metal adsorbent.
4. Conclusions
Herein, chemically chelate-modified basalt fiber (BFSN) exhibited excellent adsorption performance toward heavy metals in wastewater. To elucidate their physicochemical properties, both pristine and modified BFSN were characterized using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), zeta potential measurements, scanning electron microscopy (SEM), and Fourier-transform infrared spectroscopy (FTIR). Characterization results demonstrated that the modified BFSN possessed not only reduced reagent costs but also distinct functional advantages. Adsorption experiments conducted under varying time intervals and environmental conditions revealed that the superior adsorption capacity of modified BFSN toward Pb2+ and Cu2+ arose from a triple synergistic mechanism, involving electrostatic attraction, ion exchange, and chelation. Kinetic analysis indicated that the adsorption process was well described by the pseudo-first-order (PFO) model, whereas isotherm studies demonstrated that the Langmuir isotherm model effectively characterized the adsorption behavior, with maximum adsorption capacities of 105.38 mg/g for Pb (II) and 80.25 mg/g for Cu (II).
Compared with other reported adsorbents, modified BFSN not only featured lower economic costs and considerable adsorption capacity but also exhibited great application potential owing to its unique chelate modification. In summary, modified BFSN emerges as an environmentally benign and economically viable novel adsorbent, owing to its superior adsorption capacity and cost-effectiveness.
Author Contributions
Z.L.: Investigation, methodology, writing—original draft. C.Z.: Project administration, conceptualization, writing—review and editing. H.Z.: Formal analysis, conceptualization. C.W.: Validation, formal analysis. P.C.: Formal analysis, data curation. P.Z.: Supervision, visualization. W.D.: Methodology, software. S.W.: Funding acquisition, methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Innovation Team Foundation of Education Department of Guizhou Province (qian jiao ji [2023]087), the Young Talents Foundation of Education Department of Guizhou Province (qian jiao he KY zi [2022]052) and the Scientific and Technological Development Project of Liupanshui (52020-2023-0-2-8), and the Fund of Liupanshui Normal University (LPSSYQNPY202102).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Takyi, R.; El Mahrad, B.; Nunoo, F.K.E.; Adade, R.; ElHadary, M.; Essandoh, J. Adaptive Management of Environmental Challenges in West African Coastal Lagoons. Sci. Total Environ. 2022, 838, 156234. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, S.C. Best Practices for Solar Water Production Technologies. Nat. Sustain. 2022, 5, 554–556. [Google Scholar] [CrossRef]
- Wang, P.; Yao, J.; Wang, G.; Hao, F.; Shrestha, S.; Xue, B.; Xie, G.; Peng, Y. Exploring the Application of Artificial Intelligence Technology for Identification of Water Pollution Characteristics and Tracing the Source of Water Quality Pollutants. Sci. Total Environ. 2019, 693, 133440. [Google Scholar] [CrossRef]
- Méndez-López, M.; Jiménez-Morillo, N.T.; Fonseca, F.; De Figueiredo, T.; Parente-Sendín, A.; Alonso-Vega, F.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C. Mercury Mobilization in Shrubland after a Prescribed Fire in NE Portugal: Insight on Soil Organic Matter Composition and Different Aggregate Size. Sci. Total Environ. 2023, 904, 167532. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of Copper Ions from Wastewater: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef]
- Di Pippo, F.; Crognale, S.; Levantesi, C.; Vitanza, L.; Sighicelli, M.; Pietrelli, L.; Di Vito, S.; Amalfitano, S.; Rossetti, S. Plastisphere in Lake Waters: Microbial Diversity, Biofilm Structure, and Potential Implications for Freshwater Ecosystems. Environ. Pollut. 2022, 310, 119876. [Google Scholar] [CrossRef] [PubMed]
- Kallawar, G.A.; Bhanvase, B.A. A Review on Existing and Emerging Approaches for Textile Wastewater Treatments: Challenges and Future Perspectives. Environ. Sci. Pollut. Res. 2023, 31, 1748–1789. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, R.; Liu, Y.; Guo, X.; Cheng, J.; Hu, Y.; Chen, Y. Co-Construction of Oxygen Doping and van Der Walls Heterojunction in O-CB/ZnIn2S4 Promoting Photocatalytic Production and Activation of H2O2 for the Degradation of Antibiotics. J. Hazard. Mater. 2023, 459, 132187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, N.; Zhi, Y.; Zhang, X.; Wang, G.; Chen, Y. Sulfur Oxidation Process: A Neglected Contributor to Minimize P Release during Sediment Microbial Fuel Cell Operation. Chem. Eng. J. 2022, 449, 137845. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial Wastewater Treatment: Current Trends, Bottlenecks, and Best Practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Mao, G.; Han, Y.; Liu, X.; Crittenden, J.; Huang, N.; Ahmad, U.M. Technology Status and Trends of Industrial Wastewater Treatment: A Patent Analysis. Chemosphere 2022, 288, 132483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of Heavy Metal Removals from Aqueous Solutions by Chemical Precipitation and Characteristics of Precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Duan, J.; Lu, Q.; Chen, R.; Duan, Y.; Wang, L.; Gao, L.; Pan, S. Synthesis of a Novel Flocculant on the Basis of Crosslinked Konjac Glucomannan-Graft-Polyacrylamide-Co-Sodium Xanthate and Its Application in Removal of Cu2+ Ion. Carbohydr. Polym. 2010, 80, 436–441. [Google Scholar] [CrossRef]
- Yue, X.; Yang, Y.; Li, X.; Ren, J.; Zhou, Z.; Zhang, Y.; Yu, H. Effect of Fe-Based Micro-Flocculation Combined with Gravity-Driven Membrane Ultrafiltration on Removal of Aluminum Species during Water Treatment. J. Environ. Chem. Eng. 2021, 9, 106803. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Wu, W.; Ge, H. Preparation of Amino-Silane and Thiosemicarbazide Modified Graphene Oxide Composite for High Uptake Adsorption of Hg(II) and Pb(II). J. Dispers. Sci. Technol. 2025, 46, 1059–1070. [Google Scholar] [CrossRef]
- Sitko, R.; Musielak, M.; Serda, M.; Talik, E.; Gagor, A.; Zawisza, B.; Malecka, M. Graphene Oxide Decorated with Fullerenol Nanoparticles for Highly Efficient Removal of Pb(II) Ions and Ultrasensitive Detection by Total-Reflection X-Ray Fluorescence Spectrometry. Sep. Purif. Technol. 2021, 277, 119450. [Google Scholar] [CrossRef]
- Alvandi, S.; Hosseinifard, M.; Bababmoradi, M. Enhancement of Pb(II) Adsorptive Removal by Incorporation of UiO-66-COOH into the Magnetic Graphitic Carbon Nitride Nanosheets. RSC Adv. 2024, 14, 8990–9002. [Google Scholar] [CrossRef]
- Sharifzadeh, Z.; Razavi, S.A.A.; Morsali, A. Functionalization of Defective Zr-MOFs for Water Decontamination: Mechanistic Insight into the Competitive Roles of −NH2 and −SH Sites in the Removal of Hg(II) Ions. ACS Appl. Mater. Interfaces 2025, 17, 17726–17740. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of Heavy Metal Ions by Various Low-Cost Adsorbents: A Review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Zha, Q.; Huang, Y.; Zheng, W.; Yang, C.; Wu, Z. Novel Iminodiacetic Acid Functionalized Basalt Fiber for Adsorption of Cu (II) Ions in Batch Experiments. J. Dispers. Sci. Technol. 2023, 44, 317–328. [Google Scholar] [CrossRef]
- Shi, F.J. A Study on Structure and Properties of Basalt Fiber. AMM 2012, 238, 17–21. [Google Scholar] [CrossRef]
- Ejenstam, L.; Swerin, A.; Pan, J.; Claesson, P.M. Corrosion Protection by Hydrophobic Silica Particle-Polydimethylsiloxane Composite Coatings. Corros. Sci. 2015, 99, 89–97. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Liang, X.; Granja, J.; Azenha, M.; Ye, G. Internal Curing of Alkali-Activated Slag-Fly Ash Paste with Superabsorbent Polymers. Constr. Build. Mater. 2020, 263, 120985. [Google Scholar] [CrossRef]
- Iorio, M.; Santarelli, M.L.; González-Gaitano, G.; González-Benito, J. Surface Modification and Characterization of Basalt Fibers as Potential Reinforcement of Concretes. Appl. Surf. Sci. 2018, 427, 1248–1256. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, Y.; Randl, N. Investigation on the Alkali Resistance of Basalt Fiber and Its Textile in Different Alkaline Environments. Constr. Build. Mater. 2021, 272, 121670. [Google Scholar] [CrossRef]
- Kumru, O.S.; Liu, J.; Ji, J.A.; Cheng, W.; Wang, Y.J.; Wang, T.; Joshi, S.B.; Middaugh, C.R.; Volkin, D.B. Compatibility, Physical Stability, and Characterization of an IgG4 Monoclonal Antibody After Dilution into Different Intravenous Administration Bags. J. Pharm. Sci. 2012, 101, 3636–3650. [Google Scholar] [CrossRef]
- Lélias, M.A.; Van Gestel, J.; Maugé, F.; Van Veen, J.A.R. Effect of NTA Addition on the Formation, Structure and Activity of the Active Phase of Cobalt–Molybdenum Sulfide Hydrotreating Catalysts. Catal. Today 2008, 130, 109–116. [Google Scholar] [CrossRef]
- Tudorachi, N.; Chiriac, A.P.; Nita, L.E.; Mustata, F.; Diaconu, A.; Balan, V.; Rusu, A.; Lisa, G. Studies on the Nanocomposites Based on Carboxymethyl Starch-g-Lactic Acid-Co-Glycolic Acid Copolymer and Magnetite. J. Therm. Anal. Calorim. 2018, 131, 1867–1880. [Google Scholar] [CrossRef]
- Al-Saif, F.A.; Al-Humaidi, J.Y.; Binjawhar, D.N.; Refat, M.S. Six New Palladium(II) Mixed Ligand Complexes of 2-, 3-, 4-Monosubstituted Derivative of Pyridine Ring with Caffeine Moiety: Synthesis, Spectroscopic, Morphological Structures, Thermal, Antimicrobial and Anticancer Properties. J. Mol. Struct. 2020, 1218, 128547. [Google Scholar] [CrossRef]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental Methods in Chemical Engineering: Thermogravimetric Analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental Methods in Chemical Engineering: Zeta Potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Hu, M.-L.; Morsali, A.; Aboutorabi, L. Lead(II) Carboxylate Supramolecular Compounds: Coordination Modes, Structures and Nano-Structures Aspects. Coord. Chem. Rev. 2011, 255, 2821–2859. [Google Scholar] [CrossRef]
- Jiang, L.; Guan, J.; Zhao, L.; Li, J.; Yang, W. pH-Dependent Aggregation of Citrate-Capped Au Nanoparticles Induced by Cu2+ Ions: The Competition Effect of Hydroxyl Groups with the Carboxyl Groups. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 216–220. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Zhou, Y.; Xu, X.; Zhan, J. Influence of Air Plasma Modification Power on Surface Properties of Basalt Fibers and Basalt/Poly(Butylene Succinate) Adhesion. Appl. Surf. Sci. 2023, 630, 157416. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of Binding Energy Positions with C1s for XPS Results. J. Wuhan Univ. Technol. Mat. Sci. Edit. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the Robustness of Adventitious Carbon for Charge Referencing (Correction) Purposes in XPS Analysis: Insights from a Multi-User Facility Data Review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Celikci, N.; Shaikiyeva, N.; Moldobaev, M.; Kemelov, K.; Iskakova, J.; Dolaz, M. Synthesis, Characterization, and Investigation of Coating Properties of Carboxymethyl Acorn Starch (CMAS). Starch Stärke 2023, 75, 286. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Du, M. Dendrimer-Assisted Boronate Affinity Cellulose Foams for the Efficient and Selective Separation of Glycoproteins. Carbohydr. Polym. 2021, 265, 118082. [Google Scholar] [CrossRef]
- Rimsza, J.M.; Jones, R.E.; Criscenti, L.J. Interaction of NaOH Solutions with Silica Surfaces. J. Colloid Interface Sci. 2018, 516, 128–137. [Google Scholar] [CrossRef]
- Semenova, A.; Giles, L.W.; Vidallon, M.L.P.; Follink, B.; Brown, P.L.; Tabor, R.F. Copper-Binding Properties of Polyethylenimine–Silica Nanocomposite Particles. Langmuir 2022, 38, 10585–10600. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wu, D.; Yuan, D.; Ge, H.; Wu, X. PbO2 Materials for Electrochemical Environmental Engineering: A Review on Synthesis and Applications. Sci. Total Environ. 2023, 855, 158880. [Google Scholar] [CrossRef]
- Konicki, W.; Aleksandrzak, M.; Moszyński, D.; Mijowska, E. Adsorption of Anionic Azo-Dyes from Aqueous Solutions onto Graphene Oxide: Equilibrium, Kinetic and Thermodynamic Studies. J. Colloid Interface Sci. 2017, 496, 188–200. [Google Scholar] [CrossRef]
- Vigdorowitsch, M.; Pchelintsev, A.; Tsygankova, L.; Tanygina, E. Freundlich Isotherm: An Adsorption Model Complete Framework. Appl. Sci. 2021, 11, 8078. [Google Scholar] [CrossRef]
- Pursell, C.J.; Hartshorn, H.; Ward, T.; Chandler, B.D.; Boccuzzi, F. Application of the Temkin Model to the Adsorption of CO on Gold. J. Phys. Chem. C 2011, 115, 23880–23892. [Google Scholar] [CrossRef]
- Pinto, M.L.; Mestre, A.S.; Carvalho, A.P.; Pires, J. Comparison of Methods to Obtain Micropore Size Distributions of Carbonaceous Materials from CO2 Adsorption Based on the Dubinin−Radushkevich Isotherm. Ind. Eng. Chem. Res. 2010, 49, 4726–4730. [Google Scholar] [CrossRef]
- Van Thang, V.; Nguyen, N.T.D.; Nguyen, P.L.; Phung, T.V.B. Effective Adsorption of Methyl Orange from Aqueous Solution Using MOFs Nanocomposites UiO-66-NH2 /GO@PVA. ACS Omega 2025, 10, 40162–40173. [Google Scholar] [CrossRef] [PubMed]
- Tsatsis, D.E.; Valta, K.A.; Vlyssides, A.G.; Economides, D.G. Assessment of the Impact of Toner Composition, Printing Processes and Pulping Conditions on the Deinking of Office Waste Paper. J. Environ. Chem. Eng. 2019, 7, 103258. [Google Scholar] [CrossRef]
- Kaya, N.; Uzun, Z.Y. Experimental and Modeling Studies on the Removal of Bromocresol Green from Aqueous Solutions by Using Pine Cone-Derived Activated Biochar. Biomass Convers. Bioref. 2024, 14, 30667–30691. [Google Scholar] [CrossRef]
- Golubyatnikov, O.; Akulinin, E. Application of the Dubinin–Radushkevich–Astakhov Equation to Calculate Gases Isotherms on Zeolite Adsorbents (on Example of H2, CO2, CO, CH4, N2 Adsorption on 13X and 5A). Sep. Sci. Technol. 2022, 57, 2871–2884. [Google Scholar] [CrossRef]
- Shu, D.; Feng, F.; Han, H.; Ma, Z. Prominent Adsorption Performance of Amino-Functionalized Ultra-Light Graphene Aerogel for Methyl Orange and Amaranth. Chem. Eng. J. 2017, 324, 1–9. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Kinetics of Solute Adsorption at Solid/Solution Interfaces: A Theoretical Development of the Empirical Pseudo-First and Pseudo-Second Order Kinetic Rate Equations, Based on Applying the Statistical Rate Theory of Interfacial Transport. J. Phys. Chem. B 2006, 110, 16514–16525. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Jumina; Priastomo, Y.; Setiawan, H.R.; Mutmainah; Kurniawan, Y.S.; Ohto, K. Simultaneous Removal of Lead(II), Chromium(III), and Copper(II) Heavy Metal Ions through an Adsorption Process Using C-Phenylcalix[4]Pyrogallolarene Material. J. Environ. Chem. Eng. 2020, 8, 103971. [Google Scholar] [CrossRef]
- Perić, J.; Trgo, M.; Vukojević Medvidović, N. Removal of Zinc, Copper and Lead by Natural Zeolite—A Comparison of Adsorption Isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef]
- Joshi, S.; Srivastava, R.K. Adsorptive Removal of Lead (Pb), Copper (Cu), Nickel (Ni) and Mercury (Hg) Ions from Water Using Chitosan Silica Gel Composite. Environ. Monit. Assess. 2019, 191, 615. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jin, X.; Lu, X.-Q.; Chen, Z. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto Natural Kaolinite Clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, B.; Zhao, P.; Xi, M.; Li, Y. Synthesis of Copper and Lead Ion Imprinted Polymer Submicron Spheres to Remove Cu2+ and Pb2+. J. Inorg. Organomet. Polym. 2021, 31, 4628–4636. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).