From Waste to Sustainable Resource: Linking Phyllite Parent Rock Mineralogy to Suitability of Manufactured Sand for Concrete Construction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Research on the Maternal Lithology of Phyllite Derived from COPPER Mines
3.1.1. Analysis of Physical and Mechanical Properties of Phyllite from Copper Mines
3.1.2. Process Mineralogical Characterization of Phyllite from Copper Mines
- (1)
- Mineral composition characteristics
- (2)
- Characteristics of elemental distribution
- (3)
- Particle size and shape characteristics
3.2. Research on Properties of Manufactured Sand Derived from Phyllite
3.2.1. Study on Particles and Gradation of Manufactured Sand Derived from Phyllite
3.2.2. Study on the Harmful Substance Content in Phyllite-Derived Manufactured Sand
3.2.3. Study on Alkali Aggregate Reaction and Radioactive Energy of Phyllite-Derived Manufactured Sand
4. Conclusions
- (1)
- The phyllite waste rock is predominantly composed of mica and quartz, with minor amounts of pyrite and chlorite. This composition enables its use in producing medium-grade manufactured sand. The acicular and flaky particle content of phyllite was measured at 5.2%, which falls below the 10% limit specified by the national standard. The MB value of 1.3 and the stone powder content of 9% meet the requirements for Class III and Class II, respectively.
- (2)
- Key physical properties (solidity, crushing index, apparent density, loose packing density, and porosity index) of the phyllite manufactured sand comply with the national standard GB14684-2022, exhibiting favorable mechanical characteristics for use in construction.
- (3)
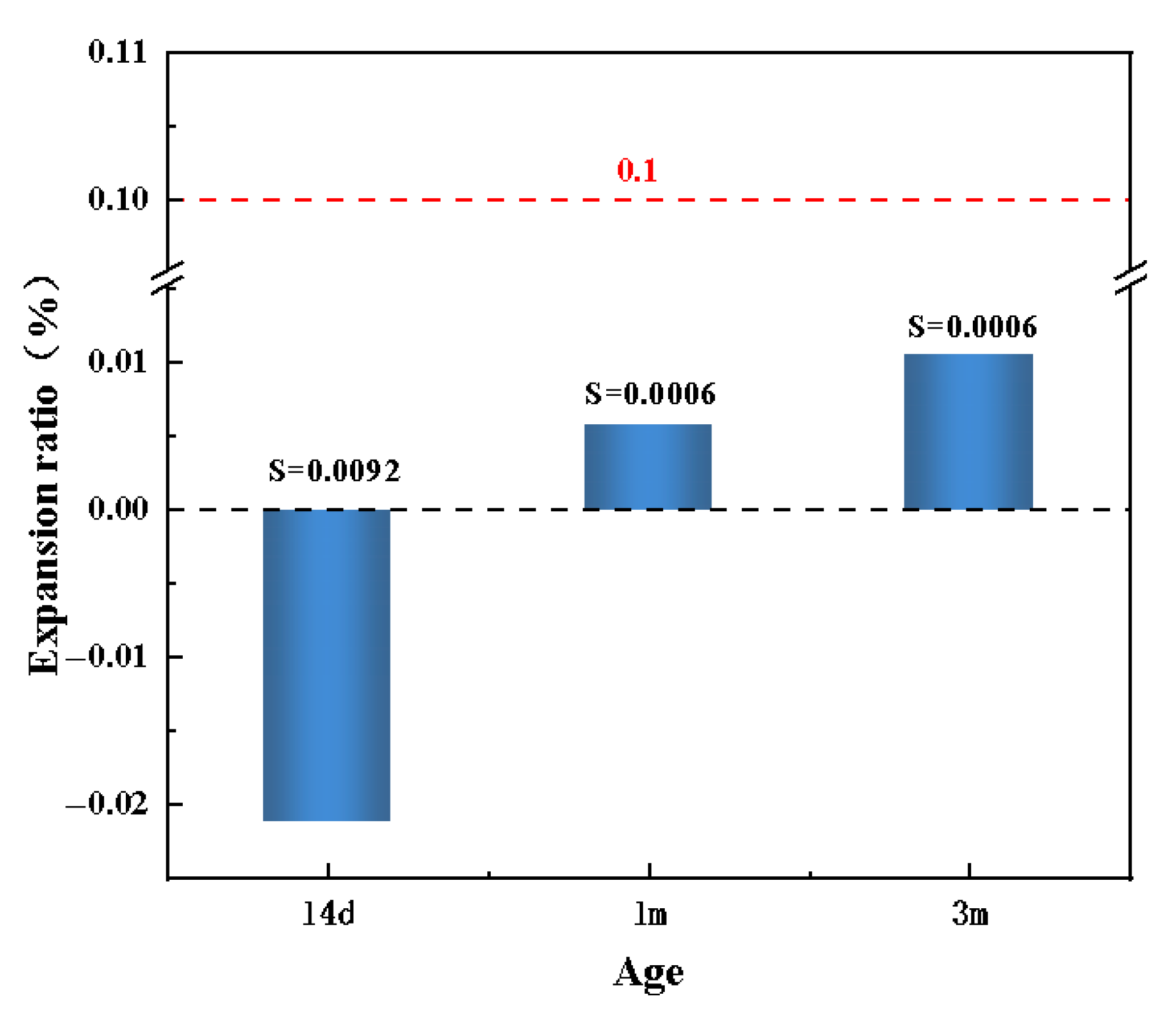
- Concrete prepared with the phyllite manufactured sand exhibited an expansion rate below 0.1% after three months of ASR test, indicating no detectable alkali-silica reaction risk. Moreover, the radioactive energy indicators of the material are within the defined limits, indicating no radiation hazard.
- (4)
- To prepare high-quality manufactured sand from phyllite waste rock on an industrial scale, the combined beneficiation process should be optimized to remove flaky and low-strength and sulfide-containing minerals. Moreover, the long-term performance of concrete prepared with phyllite manufactured sand is recommended to fully mitigate any potential risk for corrosion, and validate its long-term durability properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vriens, B.; Plante, B.; Seigneur, N.; Jamieson, H. Mine Waste Rock: Insights for Sustainable Hydrogeochemical Management. Minerals 2020, 10, 728. [Google Scholar] [CrossRef]
- Zhang, X.; Song, S.; Liu, F.; Liu, J.; Wu, R. Review on the Present Situation of Preparing Sand Aggregate from Mine Waste Rock. Fly Ash Compr. Util. 2023, 37, 55–63. [Google Scholar] [CrossRef]
- Wu, J.; Jing, H.; Yin, Q.; Yu, L.; Meng, B.; Li, S. Strength prediction model considering material, ultrasonic and stress of cemented waste rock backfill for recycling gangue. J. Clean. Prod. 2020, 276, 123189. [Google Scholar] [CrossRef]
- Zdravković, A.; Cvetković, V.; Šarić, K.; Pačevski, A.; Rosić, A.; Erić, S. Waste rocks and medieval slag as sources of environmental pollution in the area of the Pb-Zn Mine Rudnik (Serbia). J. Geochem. Explor. 2020, 218, 106629. [Google Scholar] [CrossRef]
- Wang, H.; Sun, G.; Sui, T. Landslide mechanism of waste rock dump on a soft gently dipping foundation: A case study in China. Environ. Earth Sci. 2021, 80, 200. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, X.; Liu, B.; Nan, Y. A Qualitative Study of the Critical Conditions for the Initiation of Mine Waste Debris Flows. Water 2020, 12, 1536. [Google Scholar] [CrossRef]
- Shao, L. Geological disaster prevention and control and resource protection in mineral resource exploitation region. Int. J. Low-Carbon Technol. 2019, 14, 142–146. [Google Scholar] [CrossRef]
- Zhuang, S.; Aurora, T.; Chen, R.; Ye, C. Trends, challenges, and mitigation strategies for the use of sand and gravel resources in China. J. East China Norm. Univ. (Nat. Sci.) 2022, 2022, 137–147. [Google Scholar] [CrossRef]
- S.K., K.; Singh, S.K.; Chourasia, A. Alternative fine aggregates in production of sustainable concrete—A review. J. Clean. Prod. 2020, 268, 122089. [Google Scholar] [CrossRef]
- Yao, H.; Cai, L.; Liu, W.; Qin, W.; Jiao, F.; Yang, C. Current Status and Development of Comprehensive Utilization of Waste Rock in Metal Mines in China. Chin. J. Nonferrous Met. 2021, 31, 1649–1660. [Google Scholar] [CrossRef]
- Liu, W.; Chen, M.; Mao, Y.; Zhang, H.; Shen, Y.; Liu, W. Influence of Crushing Method on the Performance Parameters of Iron Ore Waste Rock Preparation of Manufactured Sand. China Min. Mag. 2023, 32, 118–127. [Google Scholar] [CrossRef]
- Zhang, F. Experimental Research on Resource Utilization of Iron Tailings Waste Rock. Gansu Metall. 2021, 43, 8–10,13. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, L.; Chen, Z.; Miao, C.; Zhang, C.; Fan, T.; Zhang, J.; Qian, X. Utilization of solid waste from tunnel excavation as manufactured sand with different lithology and pre-washing process for preparation of eco-friendly ultra-high performance concretes: Properties and microstructural analysis. J. Build. Eng. 2024, 82, 108252. [Google Scholar] [CrossRef]
- Song, S.; Cheng, C.; Yang, N. Influence of manufactured sand lithology on mortar and concrete performance. Concrete 2019, 9, 67–70. [Google Scholar] [CrossRef]
- Li, P.; Zhou, S.; Zhang, J.; Pan, A.; Chai, S.; Gong, Y.; Hu, J. Effects of high temperature on pore structure and mechanical properties of phyllite. Case Stud. Therm. Eng. 2025, 73, 106504. [Google Scholar] [CrossRef]
- Adom-Asamoah, M.; Afrifa, R.O. A study of concrete properties using phyllite as coarse aggregates. Mater. Des. 2010, 31, 4561–4566. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Wang, C.; Xue, K.; Chen, G.; Liu, P. Road performance of ordinary Portland cement improvement of strongly weathered phyllite filler. Constr. Build. Mater. 2022, 350, 128801. [Google Scholar] [CrossRef]
- GB/T 14684-2022; Sand for Construction. Standards Press of China: Beijing, China, 2022.
- GB/T 14685-2022; Pebbles and Crushed Stone for Construction. Standards Press of China: Beijing, China, 2022.
- GB/T 50266-2013; Standard Test Methods for Engineering Rocks. China Planning Press: Beijing, China, 2013.
- GB 6566-2010; Radionuclide Limit for Building Materials. Standards Press of China: Beijing, China, 2010.
- Ansari, T.; Kainthola, A.; Singh, K.H.; Singh, T.N.; Sazid, M. Geotechnical and micro-structural characteristics of phyllite derived soil; implications for slope stability, Lesser Himalaya, Uttarakhand, India. Catena 2021, 196, 104906. [Google Scholar] [CrossRef]
- Faesal, A.; Aminuddin, M.I.K.A.; Ubaidillah, A.S. Host rock petrology, hydrothermal alteration characteristics & ore mineralogy of porphyry copper-gold deposit, Brambang, Lombok, West Nusa Tenggara Indonesia. Mater. Today Proc. 2022, 66, 3071–3076. [Google Scholar] [CrossRef]
- Chaparro, M.A.E.; Gnanasaravanan, S.; Rajkumar, P. Trace and major minerals of (natural and manufactured) sand: The importance of manufactured sand for construction purposes and the preservation of rivers. Innov. Infrastruct. Solut. 2021, 6, 52. [Google Scholar] [CrossRef]
- Tuzingila, R.M.; Kong, L.; Koy Kasongo, R. A review on experimental techniques and their applications in the effects of mineral content on geomechanical properties of reservoir shale rock. Rock Mech. Bull. 2024, 3, 100110. [Google Scholar] [CrossRef]
- Fanrdon, J. World Encyclopedia of Minerals and Gemstones: Exploration and Identification; China Machine Press: Beijing, China, 2014; ISBN 9787111482178. [Google Scholar]
- Chen, C. Mineral and Rock Atlas; Phoenix Science Press: Nanjing, China, 2017; ISBN 9787553765747. [Google Scholar]
- Yao, L.; Shuo, Z.; Jun, L. Analysis of the synergistic effect of particle size of compound mineral admixtures on the hydration properties and porosity of Portland cement under low-temperature conditions. Mater. Today Commun. 2022, 33, 104372. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, M.; Liu, Y.; Chen, L.; Chen, X. Research on expansion characteristics of circulating fluidized bed boiler bottom slag as concrete fine aggregate. J. Build. Eng. 2025, 101, 111953. [Google Scholar] [CrossRef]
- Pillai, A.G.; Gali, M.L. Engineering benefits of replacing natural sand with manufactured sand in landfill construction. Sci. Rep. 2023, 13, 6444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Z.; Chen, X. Frost Durability and Strength of Concrete Prepared with Crushed Sand of Different Characteristics. Adv. Mater. Sci. Eng. 2016, 2016, 2580542. [Google Scholar] [CrossRef]
- Zhou, H.; Ge, C.; Chen, Y.; Song, X. Study on performance and fractal characteristics of high-strength manufactured sand concrete with different MB values. Front. Earth Sci. 2023, 11, 1140038. [Google Scholar] [CrossRef]


 muscovite (1%–2% Fe)
muscovite (1%–2% Fe)  quartz
quartz  quartz (or 5%–8% Al/Fe)
quartz (or 5%–8% Al/Fe)  chlorite
chlorite  biotite
biotite  pyrite
pyrite  calcite (or 1% Si/Al/Fe/Mn/Ti)
calcite (or 1% Si/Al/Fe/Mn/Ti)  unidentified phase
unidentified phase  mica + rutile
mica + rutile  apatite
apatite  calcite (2%–5% Si/Al)
calcite (2%–5% Si/Al)  chalcopyrite
chalcopyrite  ferrodolomite
ferrodolomite  apatite (8%Si/2%Al)
apatite (8%Si/2%Al)  monazite
monazite  Holes.
Holes.
 muscovite (1%–2% Fe)
muscovite (1%–2% Fe)  quartz
quartz  quartz (or 5%–8% Al/Fe)
quartz (or 5%–8% Al/Fe)  chlorite
chlorite  biotite
biotite  pyrite
pyrite  calcite (or 1% Si/Al/Fe/Mn/Ti)
calcite (or 1% Si/Al/Fe/Mn/Ti)  unidentified phase
unidentified phase  mica + rutile
mica + rutile  apatite
apatite  calcite (2%–5% Si/Al)
calcite (2%–5% Si/Al)  chalcopyrite
chalcopyrite  ferrodolomite
ferrodolomite  apatite (8%Si/2%Al)
apatite (8%Si/2%Al)  monazite
monazite  Holes.
Holes.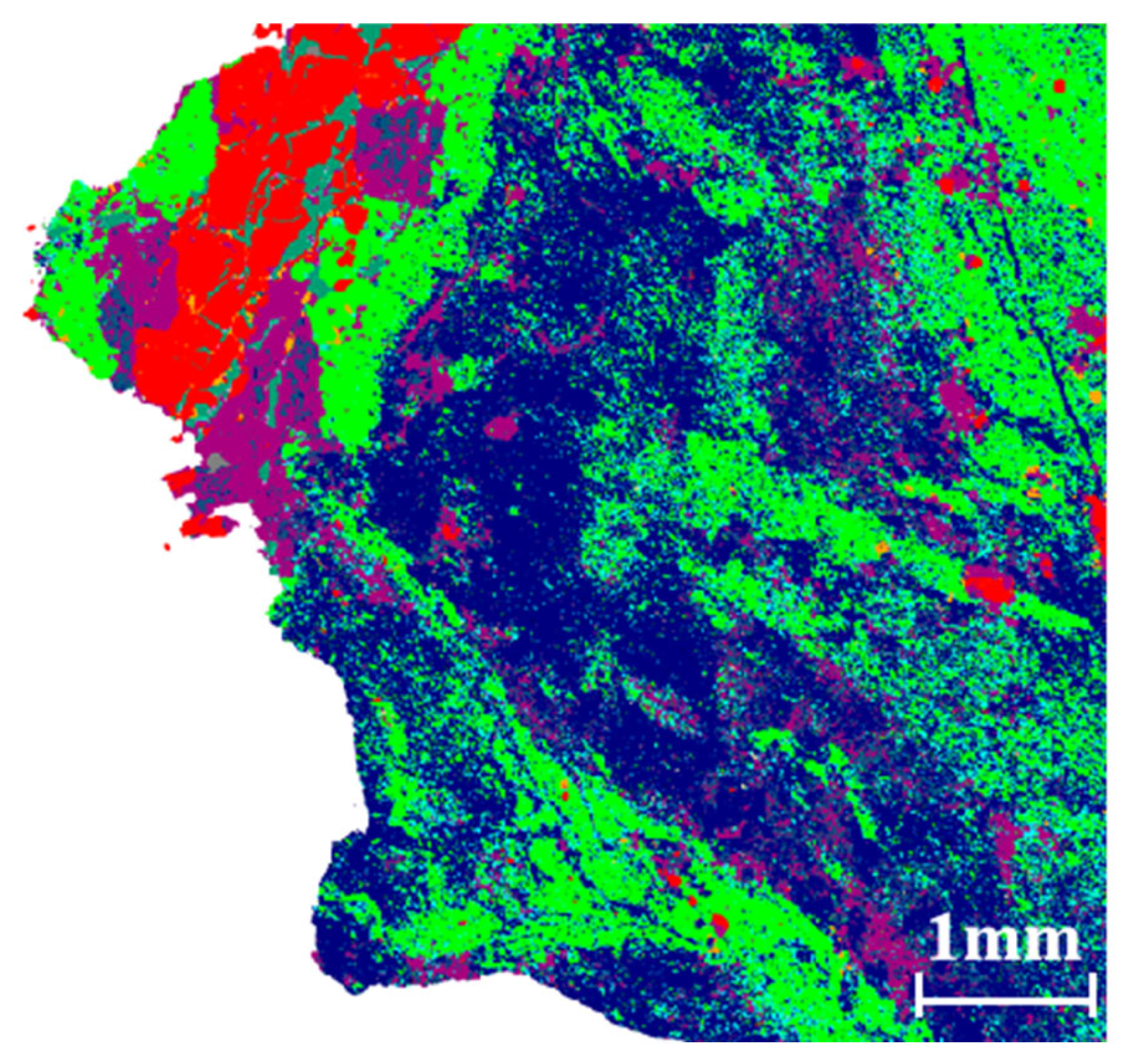








| Type | CaO | SiO2 | Al2O3 | MgO | S | Fe2O3 | K2O | Others |
|---|---|---|---|---|---|---|---|---|
| Phyllite | 3.81 | 56.71 | 20.39 | 4.95 | 1.12 | 3.94 | 5.56 | 3.52 |
| P·O 42.5 | 61.29 | 19.49 | 4.16 | 3.17 | 1.22 | 2.99 | 0.78 | 6.90 |
| Parallel to Foliation | Strength (MPa) | Elasticity Modulus (GPa) | Shear Modulus (GPa) | Poisson Ratio |
|---|---|---|---|---|
| Sample1 | 83.8 | 42.98 | 17.47 | 0.23 |
| Sample2 | 83.5 | 21.2 | 9.06 | 0.17 |
| Sample3 | 93.4 | 28.82 | 12.53 | 0.15 |
| Average | 86.9 | 25.01 | 10.8 | 0.16 |
| Standard deviation | 5.631 | 5.388 | 2.454 | 0.042 |
| Perpendicular to Foliation | Strength (MPa) | Elasticity Modulus (GPa) | Shear Modulus (GPa) | Poisson Ratio |
|---|---|---|---|---|
| Sample1 | 71.6 | 32.28 | 13.34 | 0.21 |
| Sample2 | 77.4 | 31.25 | 12.6 | 0.24 |
| Sample3 | 74.5 | 30.11 | 11.95 | 0.26 |
| Average | 74.4 | 31.21 | 12.63 | 0.24 |
| Standard deviation | 2.751 | 0.806 | 0.695 | 0.042 |
| Number | Minerals | Density (g/cm3) | Hardness (Mohs) |
|---|---|---|---|
| 1 | Muscovite | 2.77–2.88 | 2.5–4.0 |
| 2 | Quartz | 2.65 | 7 |
| 3 | Chlorite | 2.6–3.3 | 2.0–3.0 |
| 4 | Biotite | 2.7–3.4 | 2.5–3.0 |
| 5 | Pyrite | 5 | 6.0–6.5 |
| 6 | Rutile | 4.23 | 6.0–6.5 |
| 7 | Apatite | 3.1–3.2 | 5 |
| 8 | Chalcopyrite | 4.3–4.4 | 3.5–4.0 |
| 9 | Ferrodolomite | 2.97 | 3.5–4.0 |
| 10 | Monazite | 4.9–5.3 | 5–5.5 |
| 11 | Calcite | 2.71 | 3 |
| Item | Standard Limit | Measured Value |
|---|---|---|
| Acicular and flaky particle content | Class I < 10% | 5.20% |
| MB value | Class I < 0.5 Class II < 1.0 Class III ≤ 1.4 | 1.30 |
| Stone powder | Class I < 5% Class II < 10% | 9.0% |
| Item | Standard Limit | Measured Value |
|---|---|---|
| Solidity (%) | <10 | 1.3 |
| Crushing index (%) | <30 | 25.3 |
| Apparent density (kg/m3) | >2500 | 2820 |
| Loose packing density (kg/m3) | >1400 | 1740 |
| Porosity (%) | ≤44 | 38.3 |
| Item | Standard Limit | Content |
|---|---|---|
| Mica | ≤2.0% | 1.30% |
| Light substance | ≤1.0% | / |
| Organic matter | up to standard | / |
| Sulfides and sulfates | ≤0.5% | 0.45% |
| Chloride | ≤0.02% | 0.00% |
| Radioactive Energy Test | Value (%) | Standard Limit (%) |
|---|---|---|
| Internal exposure index | 0.15 | IRa ≤ 1.0 |
| External exposure index | 0.47 | Ir ≤ 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Y.; Liu, Z.; Tian, Y.; Yang, A.; Yuan, Q.; Tang, X.; Sun, W.; Zhao, Q.; Wang, M. From Waste to Sustainable Resource: Linking Phyllite Parent Rock Mineralogy to Suitability of Manufactured Sand for Concrete Construction. Minerals 2025, 15, 1098. https://doi.org/10.3390/min15111098
Wang Y, Li Y, Liu Z, Tian Y, Yang A, Yuan Q, Tang X, Sun W, Zhao Q, Wang M. From Waste to Sustainable Resource: Linking Phyllite Parent Rock Mineralogy to Suitability of Manufactured Sand for Concrete Construction. Minerals. 2025; 15(11):1098. https://doi.org/10.3390/min15111098
Chicago/Turabian StyleWang, Yanxiu, Yang Li, Zhengxiang Liu, Yi Tian, Anqi Yang, Qiang Yuan, Xuekun Tang, Wei Sun, Qingchao Zhao, and Mingyuan Wang. 2025. "From Waste to Sustainable Resource: Linking Phyllite Parent Rock Mineralogy to Suitability of Manufactured Sand for Concrete Construction" Minerals 15, no. 11: 1098. https://doi.org/10.3390/min15111098
APA StyleWang, Y., Li, Y., Liu, Z., Tian, Y., Yang, A., Yuan, Q., Tang, X., Sun, W., Zhao, Q., & Wang, M. (2025). From Waste to Sustainable Resource: Linking Phyllite Parent Rock Mineralogy to Suitability of Manufactured Sand for Concrete Construction. Minerals, 15(11), 1098. https://doi.org/10.3390/min15111098






