1. Introduction
Carbonate precipitation is a natural or engineered process in which bivalent ions such as calcium (Ca
2+) or magnesium (Mg
2+) react with carbonate (CO
32−) or bicarbonate (HCO
3−) ions to form solid carbonate minerals, for example, calcite (CaCO
3), dolomite (CaMg(CO
3)
2), and related compounds [
1]. Two widely studied approaches are abiotic CO
2-induced carbonate precipitation (CICP) and microbially induced carbonate precipitation (MICP) [
1]. Abiotic CO
2-induced precipitation is a chemical method in which gaseous or dissolved CO
2 reacts with an alkaline solution, such as NaOH, forming carbonic acid that dissociates into carbonate species. These carbonate ions then combine with metal ions, sourced from salts like CaCl
2 or MgCl
2 or from industrial wastes, to form carbonate minerals. This method offers several advantages, including the potential utilization of captured industrial CO
2, contributing to net-zero carbon targets [
2], production of high-purity minerals [
3], and controlled crystallization under optimized pH and temperature [
4]. However, it also presents limitations, such as the requirement for high pH and the use of a strong base, energy inputs, and lack of a protective organic matrix, which makes the precipitated carbonates more susceptible to acid dissolution [
5].
Conversely, MICP is a biologically driven method that involves the use of microbes to produce enzymes that catalyze reactions leading to carbonate mineral precipitation. The most commonly used microorganisms for MICP are urease-producing bacteria, such as
Sporosarcina pasteurii, which hydrolyze urea to increase local pH and carbonate concentration, resulting in CaCO
3 precipitation. This approach offers advantages such as operation under ambient conditions, enhanced acid-resistance due to embedding in extracellular polymeric substances (EPS), and self-regulating mineral formation. Nonetheless, MICP also faces challenges, including the need for microbial cultivation, potential ammonia release, relatively slower reaction rates, and scalability constraints for large-scale carbonate mineral production [
6,
7,
8,
9].
To address limitations, algae have emerged as a promising alternative biological agent for carbonate precipitation, offering an eco-friendly and sustainable approach to carbon sequestration and biomineralization [
10]. During photosynthesis, algae consume CO
2 and increase local pH, promoting the conversion of dissolved inorganic carbon into carbonate (CO
32−) and bicarbonate (HCO
3−) ions. In the presence of bivalent ions (e.g., Ca
2+, Mg
2+), these species precipitate as solid carbonate minerals such as calcite or aragonite. This biologically driven process, known as algae-induced carbonate precipitation, can occur in both freshwater and marine environments. The advantages of using microalgae include natural CO
2 uptake, low energy requirements, the potential for large-scale cultivation, and the co-benefit of biomass production for biofuels, animal feed or nutrient recovery creating opportunities for integrated, circular approaches [
11,
12,
13,
14]. Additionally, algae can grow on non-arable land and in wastewater, offering potential benefits for integrated carbon capture and water treatment systems. Their photosynthetic activity not only drives carbonate mineralization but also contributes oxygen to the environment, enhancing ecological compatibility. Microalgae can also improve mineral durability by secreting extracellular polymeric substances (EPS) that bind and protect crystals [
15]. However, limitations remain, including the dependence on light and nutrients and the need for optimal environmental conditions to maintain microalgal growth, the relatively slow precipitation rates, and difficulties in controlling mineral morphology [
16]. Despite these constraints, algae-based carbonate precipitation holds significant potential for sustainable carbon capture and sequestration, biomaterial production, and environmental remediation.
Although research on MICP has largely focused on bacterial systems, algae-mediated processes offer a complementary or alternative pathway, with the added benefit of photosynthetic CO
2 capture. Like bacterial MICP, carbonate minerals generated by algae can improve soil stability by filling pore spaces and binding soil particles [
6,
8,
9]. Thus, algal systems could support biocementation-based soil improvement, with fewer chemical byproducts and broader environmental compatibility.
This study presents one of the first direct comparisons between algae-induced carbonate precipitation and abiotic CO2-induced chemical precipitation. The aim is to perform a novel comparative study assessing their relative performance in terms of carbonate yield, mineral type, form and stability, and acid durability under controlled laboratory conditions. This novel comparison addresses a critical gap in the biomineralization literature and highlights the distinctive contributions of photosynthesis and EPS in shaping mineral outcomes compared to the abiotic counterparts. The particular perspective is the potential of algae for soil stabilization by biocementation applications. Although an experimental study of algae-biocemented soils is beyond the scope of this paper, the findings provide a foundation for future studies in bio-based geotechnical engineering.
2. Materials and Methods
2.1. Alga Selection and Cultivation
The cyanobacterial strain used in this study was Synechococcus elongatus (CCAP 1479/1A), a freshwater cyanobacterium obtained from the Culture Collection of Algae and Protozoa (CCAP), UK. According to the suppliers’ information, it is a unicellular, non-axenic (“bacteria present”), and non-pathogenic cyanobacterium strain (Hazard level 1), isolated in 1940 from a garden pool in Cambridge, England, UK.
The cyanobacterium was cultivated and maintained in BG11 medium for blue-green algae [
17], which contained the following components (g/L): NaNO
3—1.5, K
2HPO
4—0.031, MgSO
4·7H
2O—0.036, CaCl
2·2H
2O—0.036, citric acid—0.005, ferric ammonium citrate—0.006, EDTA (disodium salt)—0.001, and Na
2CO
3—0.02. In addition, 1.0 mL of a trace metal mix was added per liter of BG11 medium, prepared from a stock solution containing H
3BO
3—2.86 g, MnCl
2·4H
2O—1.81 g, ZnSO
4·7H
2O—0.222 g, NaMoO
4·2H
2O—0.39 g, CuSO
4·5H
2O—0.079 g, and Co(NO
3)
2·H
2O—49.4 mg. Cultures were incubated for 8 days under controlled laboratory conditions, with cool and warm white fluorescent lighting (151–216 lux), a temperature of 20–22 °C, and a 16 h light/8 h dark cycle to promote optimal growth, i.e., OD
750 = 1.5. The applied light intensity was according to guidelines provided by the bacterial culture repository (CCAP, Scotland, UK), for the freshwater cyanobacterial strain used, which could bleach if exposed to the intense light lux (according to the repository guidelines). During the cyanobacterial growth, sterile conditions were followed. The ingredient stocks used to prepare BG11 medium were filter-sterilized and transferred to an autoclaved distilled water prior to culture inoculation to ensure contamination-free growth.
Figure 1 shows the micro and macro structure of the growing algae colony in this study. As illustrated in
Figure 1a, the microstructure of algae, captured through a microscope, reveals cell-level characteristics with green eukaryotic cells arranged in colonies. This view represents the microscopic scale, where individual cells are visible. In
Figure 1b, the macrostructure of algae is shown at the scale visible to the naked eye in a Petri dish, displaying the overall appearance, spatial distribution, and clumping of algal biomass. This reflects the large-scale form and organization of the culture.
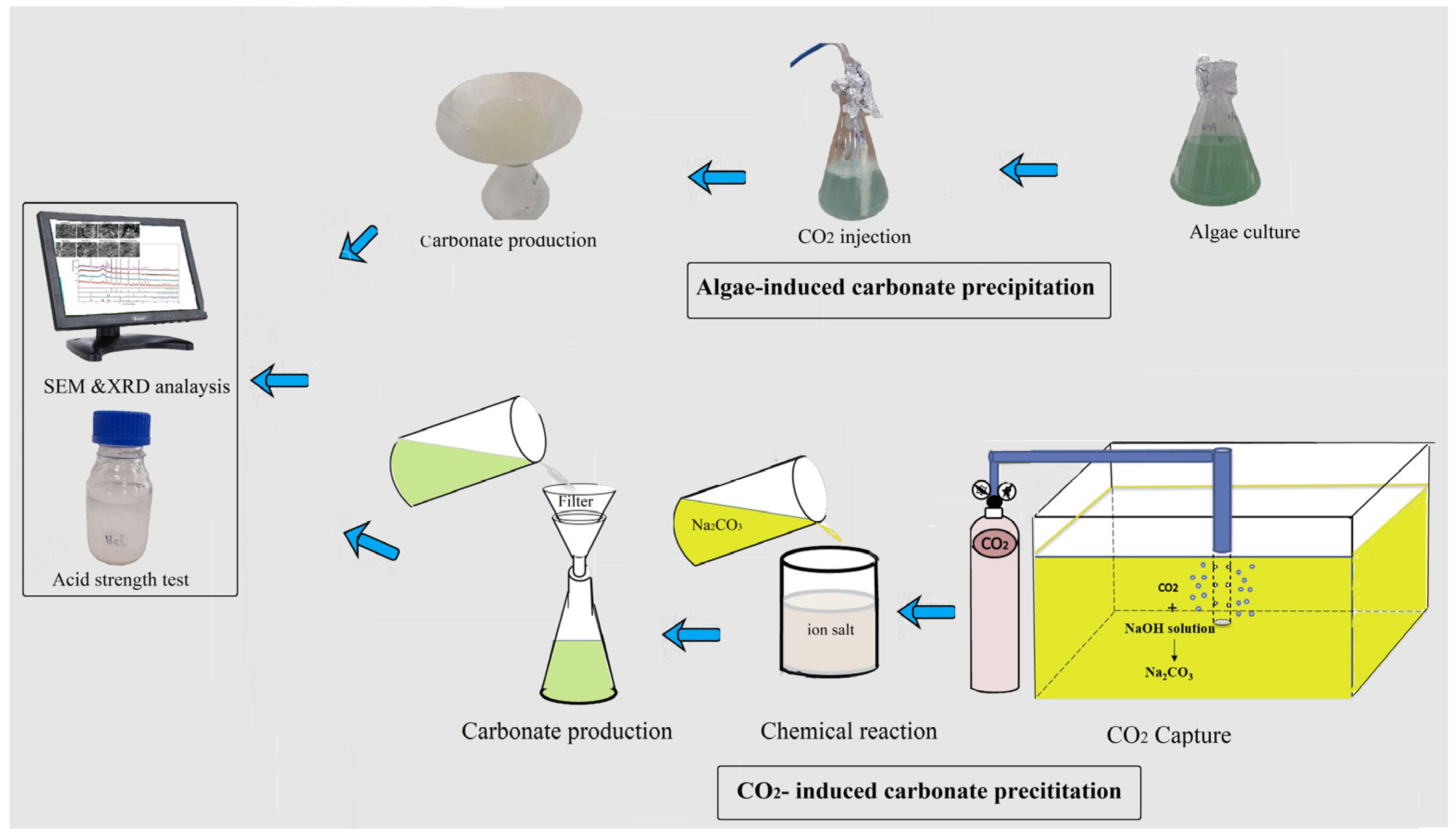
2.2. Biogenic and Abiotic Carbonate Precipitation
In this research, carbonate formation was achieved through both biological and chemical (abiotic) processes. For the biological process, microalgae absorb CO
2 through photosynthesis, converting carbon dioxide into biomass, while also contributing to bicarbonate (HCO
3−) accumulation in the medium. For this process, CO
2 gas was continuously injected into the algal culture broth at a rate of 0.2 L/min for three days to enhance photosynthetic activity. The CO
2 dissolved in water and formed carbonic acid (H
2CO
3), which partially dissociates into bicarbonate and protons, as shown in Equation (1):
For the abiotic process, CO
2 was injected into a 1 molar NaOH solution at the same rate. In this high-pH environment, CO
2 first reacts with NaOH to form sodium bicarbonate (NaHCO
3) (Equation (2)), which can further react with excess NaOH to produce sodium carbonate (Na
2CO
3) (Equation (3)). In aqueous solution, Na
2CO
3 dissociates into sodium ions and carbonate ions (CO
32−) (Equation (4)), which will serve as the reactive species for mineral precipitation:
To induce carbonate mineral precipitation, the carbonate or bicarbonate ions generated via either process were then reacted with metal chloride solutions to induce mineral precipitation. One-molar solutions of FeCl
2, CaCl
2, and MgCl
2 were prepared and reacted with the carbonate-containing solutions to form ferrous, calcium, and magnesium carbonates, respectively. In some cases, a 1:1 mixture of CaCl
2 and MgCl
2 resulted in the formation of dolomite. The relevant precipitation reactions are shown in Equations (5)–(8) below:
The physicochemical properties of the materials used to induce carbonate precipitation are shown in
Table 1.
2.3. SEM and XRD Analysis
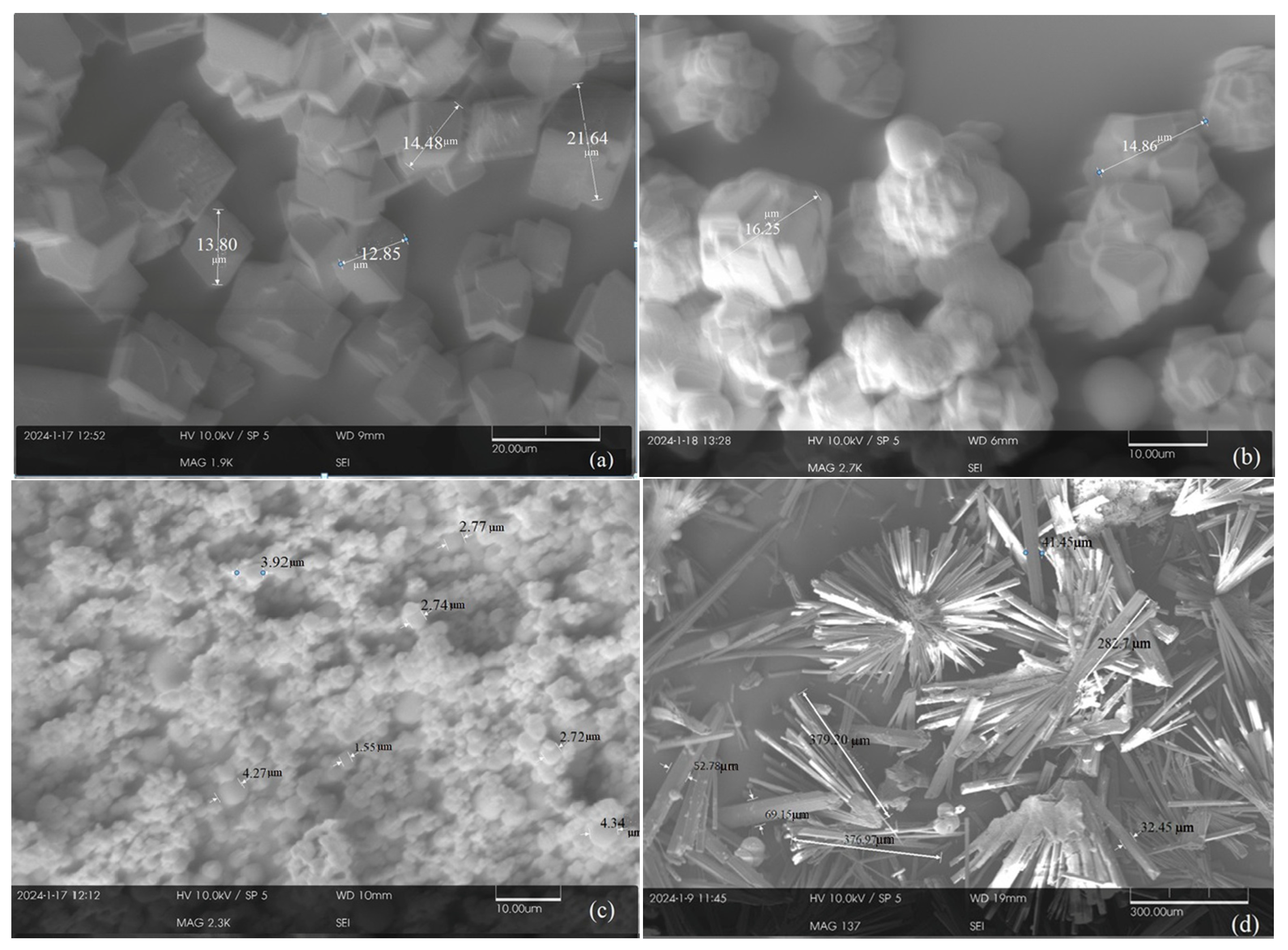
Microstructural analysis of selected specimens was carried out using Scanning Electron Microscopy (SEM) (Tescan Vega3, Libušina třída 21, 623 00 Brno, Czech Republic) and X-ray Diffraction (XRD). The powdered samples were gold-coated to ensure conductivity for SEM observation. An acceleration voltage of 15 kV was used during SEM imaging. For XRD analysis, the crystalline phases formed by carbonate minerals were identified using Cu Kα radiation (λ = 1.5406 Å), with settings of 30 kV and 30 mA.
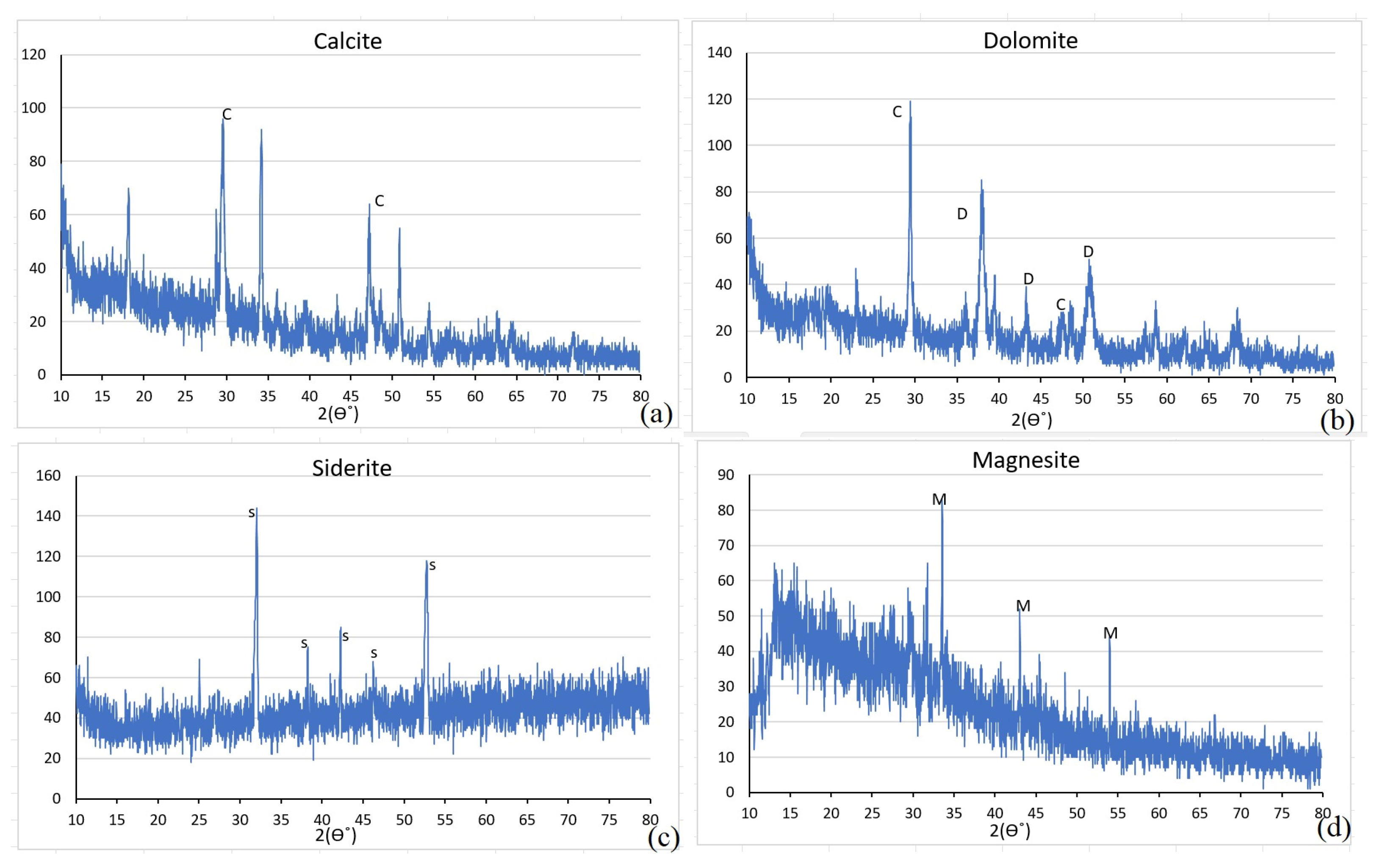
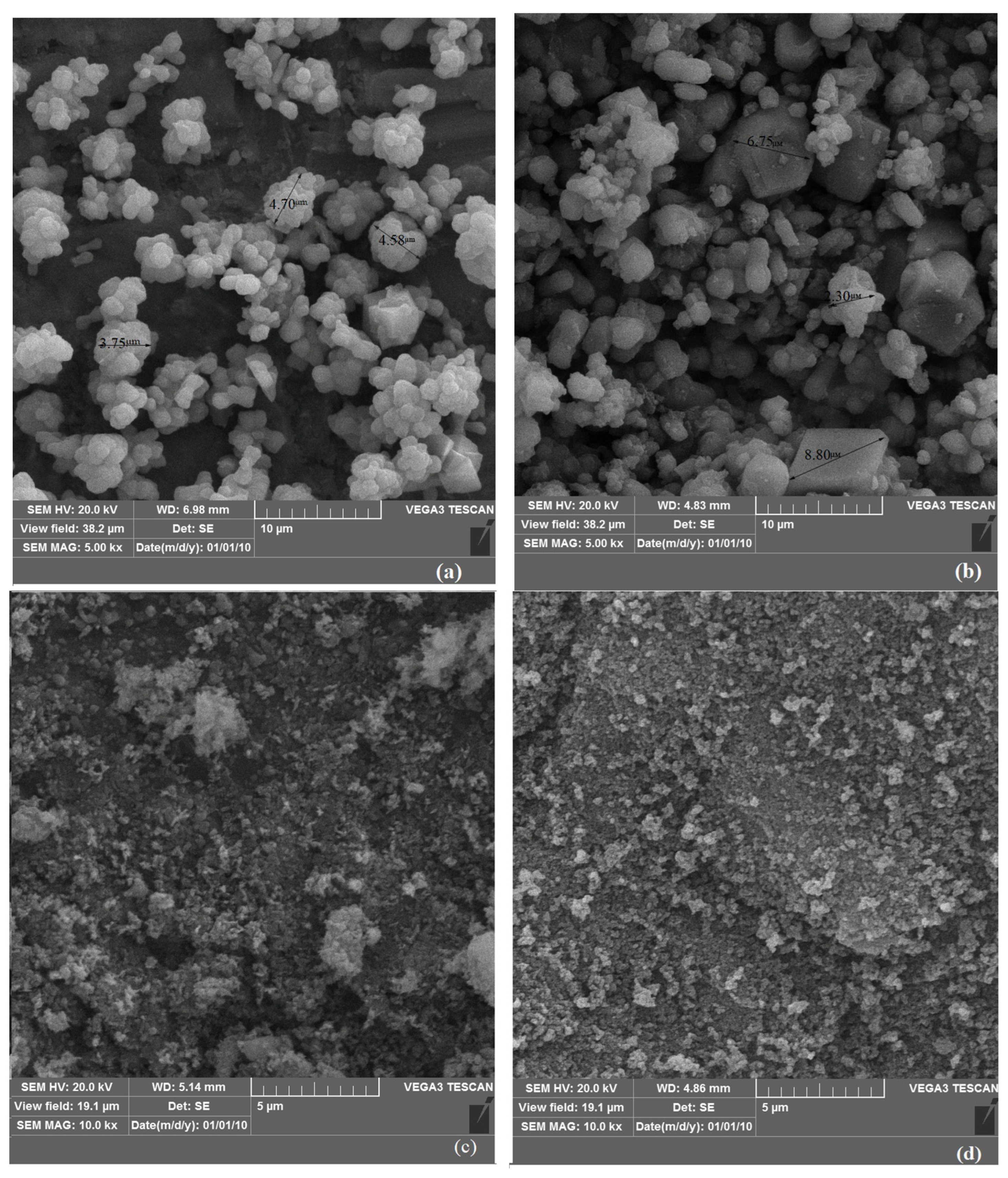
All carbonate minerals produced by algae-induced process as well as the abiotic process were analyzed using both SEM and XRD to characterize their structure and identify mineral types. To investigate the crystal structure, SEM analysis was performed to observe the size, shape, and organization of the minerals, and XRD was used to identify the specific mineral phases produced. To quantify the proportion of carbonate minerals, the XRD data were analyzed using Profex (5.4.1) software, which is capable of determining the relative quantities of different mineral phases.
2.4. Durability Testing
To assess the chemical stability of carbonate minerals, acid immersion testing was conducted by monitoring the mass loss of the samples according to ASTM D5744 [
18]. This test evaluates the resistance of carbonate materials to chemical breakdown (dissolution or degradation) under acidic conditions, simulating environments such as acid rain, industrial emissions, or acidic soils. Chemical durability is a key factor influencing the long-term performance of carbonate-based materials, particularly in construction and environmental remediation applications where stability is critical.
For the purposes of this test, 2 g of oven-dried carbonate minerals were immersed in 100 mL of 0.5 M HCl solution, maintaining a consistent solid-to-liquid ratio (2 g/100 mL). The samples were kept in a shaking incubator at 20–25 °C temperature for 24 h. Subsequently, the mass loss of the samples was recorded to quantify dissolution by calculating the percent mass loss at the end of the 24 h period of exposure to HCl. All tests were repeated three times.
Figure 2 presents a schematic diagram outlining all steps of the experimental procedure used in this study for the biological process.
4. Discussion
4.1. Carbonate Mineralization Pathway in Microalgae
This comparison highlights that microalgal precipitation, as a biogenic process, produces carbonate minerals that differ notably in crystal size, shape, and mineral type compared to abiotically precipitated carbonates. These differences may arise from the unique microenvironments created by algal metabolism, particularly through pH shifts, EPS secretion, and selective ion interactions, which can lower the kinetic barriers to mineral formation compared to purely abiotic conditions. For instance, the results showed that the biogenic process mediated by algae led to dolomite formation in a Ca/Mg-rich environment, whereas under the same conditions, the abiotic processes produced high-Mg calcite.
Similarly,
S. pasteurii precipitates carbonate minerals with morphologies distinct from abiotic CO
2 reactions [
1], including spherical vaterite (CaCO
3), botryoidal dolomite (CaMg(CO
3)
2), spherical siderite (FeCO
3), and needle-shaped nesquehonite (MgCO
3·3H
2O), with particle sizes comparable to or slightly exceeding those formed by algae.
The mechanisms behind carbonate precipitation by microalgae and bacteria are fundamentally different. Although both systems involve biological surfaces serving as nucleation sites, they operate through distinct biochemical and structural processes.
S. pasteurii induces active enzymatic precipitation via urease activity, with extracellular polymeric substances (EPS) playing a critical role in ion binding and crystal nucleation. Conversely, microalgae promote passive, surface-mediated mineralization driven by environmental changes, primarily through photosynthetic CO
2 uptake that raises local pH. In MICP, precipitation typically occurs on bacterial cell surfaces or within the biofilm matrix, whereas in algae, the negatively charged cell walls—rich in functional groups such as carboxyl and hydroxyl—serve as passive nucleation sites [
19,
20].
Algal biomineralization is unique compared to bacterial MICP and chemical precipitation because it directly couples photosynthetic CO2 uptake with carbonate formation, avoiding the use of urea and the release of ammonium byproducts typical of bacterial processes. Unlike purely chemical precipitation, which relies on external alkalinity and yields limited mineral phases, algae generate localized microenvironments on their cell surfaces that provide a more environmentally sustainable pathway for CO2 sequestration and soil stabilization.
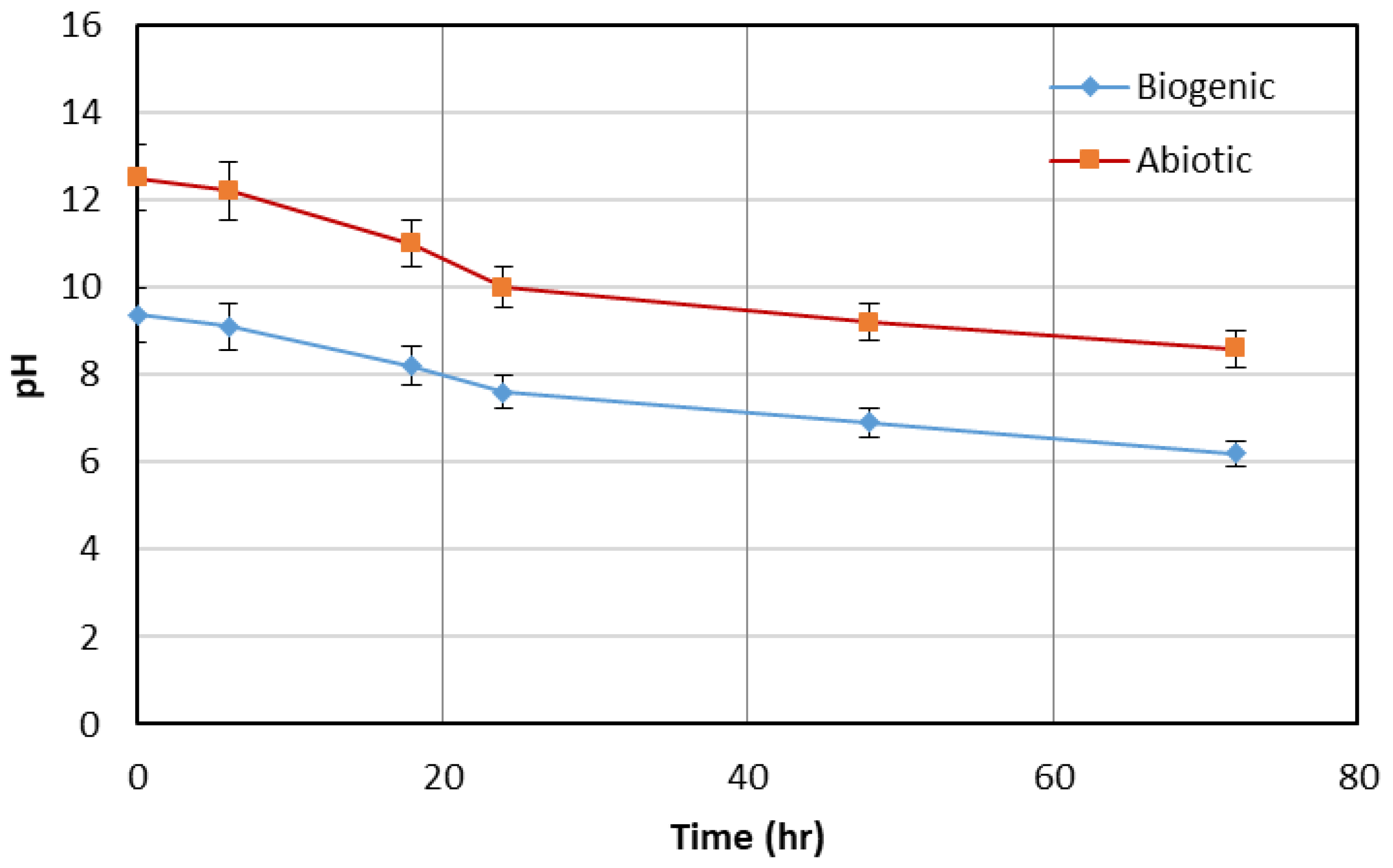
Variations in environmental factors such as temperature, pH, nutrient medium, and light intensity are expected to influence the extent and rate of algae-induced carbonate precipitation. For instance, higher light intensity generally enhances photosynthesis, leading to greater CO2 uptake and a higher pH, which favors carbonate mineral formation. Similarly, an alkaline pH environment accelerates carbonate supersaturation and precipitation. Nutrient composition influences algal growth rate and metabolic activity, thereby affecting the production of alkalinity and nucleation sites. Temperature controls enzymatic activity and solubility of carbonate minerals, with moderate increases often promoting precipitation up to a tolerance limit of the strain. Overall, varying these conditions could either enhance or suppress carbonate formation depending on the balance between algal metabolism and mineral solubility.
4.2. Practical Considerations for Soil Stabilization Applications
This study demonstrated carbonate precipitation via CO
2 capture driven by microalgae and compared it to abiotic chemical processes, with reference to bacterial MICP with
S. pasteurii primarily as a benchmark, given that bacterial results were reported in previous work [
1]. The distinct mechanisms and outcomes of carbonate precipitation via microalgae,
S. pasteurii, and abiotic processes each offer different advantages and limitations for potential soil stabilization by biocementation applications at industrial scale (
Table 2). The study was based on a selected local strain, adapted to the UK ecosystem, with the perspective of using it for soil biocementation in the area from which it was isolated. Additional strains, including those adapted to seawater environments would be required for further generalization for applications in different locations and environments.
Microalgae-driven precipitation presents a sustainable, low-energy pathway that captures CO
2 directly through photosynthesis and produces a diverse array of carbonate minerals, including magnesium- and iron-rich types [
21]. This mineral diversity may offer ecological benefits, such as improved soil mineralogy and durability, and material advantages for biocementation by possibly enhancing mechanical properties or resistance to environmental degradation. However, microalgae precipitation is comparatively slow and requires controlled environmental conditions (light, temperature), which pose scalability challenges. Additional practical barriers include harvesting algae biomass and efficient recovery of precipitated minerals, which are critical steps for industrial deployment but remain underexplored. In comparison, ureolytic MICP with
S. pasteurii, is recognized for rapid and controllable carbonate formation through urease activity, making it effective for applications like soil stabilization and biocementation [
20]. However, it also has its own serious limitations for industrial deployment as it produces environmentally unfriendly ammonia byproducts.
As for abiotic CO
2-induced precipitation, while fast and highly controllable, often requires significant energy input, chemical additives, or pressurized CO
2, which can reduce its sustainability unless integrated with waste reuse or carbon capture technologies [
22,
23].
Among the three carbonate precipitation methods, the microalgae approach offers the most renewable and environmentally sustainable option overall. It relies primarily on natural sunlight, directly captured atmospheric or industrial CO2, and basic nutrients—many of which can be sourced from wastewater—making it both low-cost and resource-efficient. Unlike abiotic carbonate precipitation, it does not require energy-intensive conditions such as high-pressure CO2 or chemical additives, nor does it produce harmful byproducts such as ammonia, as seen in ureolytic bacteria MICP methods. Any concerns about potential excess nutrient discharge to water bodies -particularly in open systems -can be addressed through careful nutrient management and containment strategies. Although its slower precipitation rate and dependence on environmental conditions such as light and temperature may present scalability challenges, microalgae-based carbonate deposition remains especially promising for the production of diverse carbonate minerals with a low-carbon footprint in circular resource use frameworks. Beyond soil stabilization applications, these systems can also support the production of biofertilizers, health products, biofuels (from algal biomass), serve as feedstock for bio-based products and contribute to wastewater treatment. In addition to mineral formation, the remaining algal biomass itself may hold residual value. Although not explored experimentally in this study, reusing biomass after carbonate precipitation could be explored as a way of further enhancing the sustainability and economic feasibility of algae-based biocementation and CO2 capture systems. Future work could evaluate the quality, safety, and functionality of post-precipitation algal biomass to determine its reuse potential.
4.3. Contribution to Sustainable Development Goals (SDGs)
Algae-induced carbonate precipitation offers a nature-based solution to environmental challenges by capturing atmospheric or industrial CO
2 and converting it into stable minerals (
Figure 9), thereby contributing to SDG 13 (Climate Action) [
24,
25,
26]. Through its integration with industrial CO
2 sources and wastewater treatment systems, it also advances SDG 12 (Responsible Consumption and Production) by promoting circular resource use. AICP facilitates carbon sequestration through stable carbonate formation. Microalgae capture CO
2 from flue gases or ambient air, converting it into precipitated carbonates. This biologically driven mineralization provides a low-energy, scalable pathway for mitigating CO
2 emissions. While not explored experimentally in this study, the potential reuse of algal biomass, for example as biofertilizer, feedstock, or bioenergy, represents a promising avenue for enhancing circular economy outcomes. These applications are prospective, and further work is required to demonstrate their feasibility.
In marine environments, algal carbonate precipitation can buffer ocean acidification and support reef-like structures, contributing to SDG 14 (Life Below Water). On land, its application in soil stabilization [
27] and land rehabilitation [
28] addresses SDG 15 (Life on Land). The development of bio-based construction materials using algae [
29,
30] also supports SDG 8 & 9 (Economic Growth & Industry, Innovation and Infrastructure) by introducing sustainable alternatives to traditional cement and building processes. Furthermore, when cultivated for biofuel production [
31,
32], algae contribute to SDG 7 (Affordable and Clean Energy) by providing renewable energy sources. Additionally, algal systems can assist in wastewater treatment, nutrient recovery and high-value products from wastewater [
33,
34], supporting SDG 6 (Clean Water and Sanitation) by reducing freshwater demand and enabling resource recovery. Algae thus offer the dual benefit of water purification and mineral production. Overall, algae-induced carbonate precipitation represents a multidisciplinary approach that contributes to climate resilience, sustainable resource use, and ecological protection across diverse environmental and economic sectors.
4.4. Future Directions
Algae-induced carbonate mineralization has promising applications in carbon sequestration, eco-friendly building materials, and environmental remediation, including contaminated water treatment and mine tailings stabilization. These benefits highlight its potential for climate mitigation and sustainable resource management.
However, scaling up the process poses challenges [
31,
32]. Maintaining algal culture viability, controlling mineral composition and morphology, and ensuring economic feasibility are key considerations. Several focused studies have been dedicated to these challenges in the literature. Whereas it is beyond the scope of this paper to make comparative costs or sustainability assessments of the different processes, the findings of previous dedicated studies on challenges for industrial upscaling of algae/cyanobacteria applications (see, e.g., [
35]) identified large-scale cultivation, growth rate, and production costs as the biggest constraints, with a major underlying challenge being contamination, and the fact that more axenic growth conditions also impact the cultivation costs. Generally, proper upscaling of algae/cyanobacteria is relatively more costly compared to heterotrophic bacteria [
35]; however, the fact that algae/cyanobacteria can produce CO
2-based products could partly offset cost unbalance, if premiums were charged for this feature. Moreover, major recent developments were made, in systems for contamination-free cultivation or high-density cultivation [
35] giving promise that those challenges can be overcome with future research, aiming to optimize cultivation and mineralization conditions, achieve consistent product quality, and develop cost-effective strategies for large-scale implementation. Overall, although the cyanobacteria research community recognizes the challenges in utilizing cyanobacteria industrially, they also acknowledge their enormous potential once these challenges are overcome [
35].
5. Conclusions
This study explored the use of microalgae for carbonate precipitation via CO2 uptake, comparing the process to abiotically induced CO2 mineralization. The main findings and implications are
Microalgae-driven precipitation successfully produced a variety of carbonate calcite (CaCO3), dolomite (CaMg(CO3)2), siderite (FeCO3), and magnesite (MgCO3), through CO2 uptake and photosynthesis. While the total mineral yield was lower than that achieved via abiotic chemical precipitation, it was comparable to yields typically obtained via bacterial MICP using S. pasteurii.
Morphological and mineralogical analyses (SEM, XRD) revealed clear differences between biogenic and abiotic precipitates, in terms of morphology, size and mineral type. Algae-derived minerals tended to be finer and more varied in form, with notable dolomite formation in conditions where the abiotic pathway favored simpler carbonates. Specifically, calcite appeared as polygonal or agglomerated crystals (3–5 µm), dolomite was rhombohedral (6–8 µm, ~40% of total), and siderite and magnesite formed as fine-grained, granular to spheroidal particles (<0.5 µm).
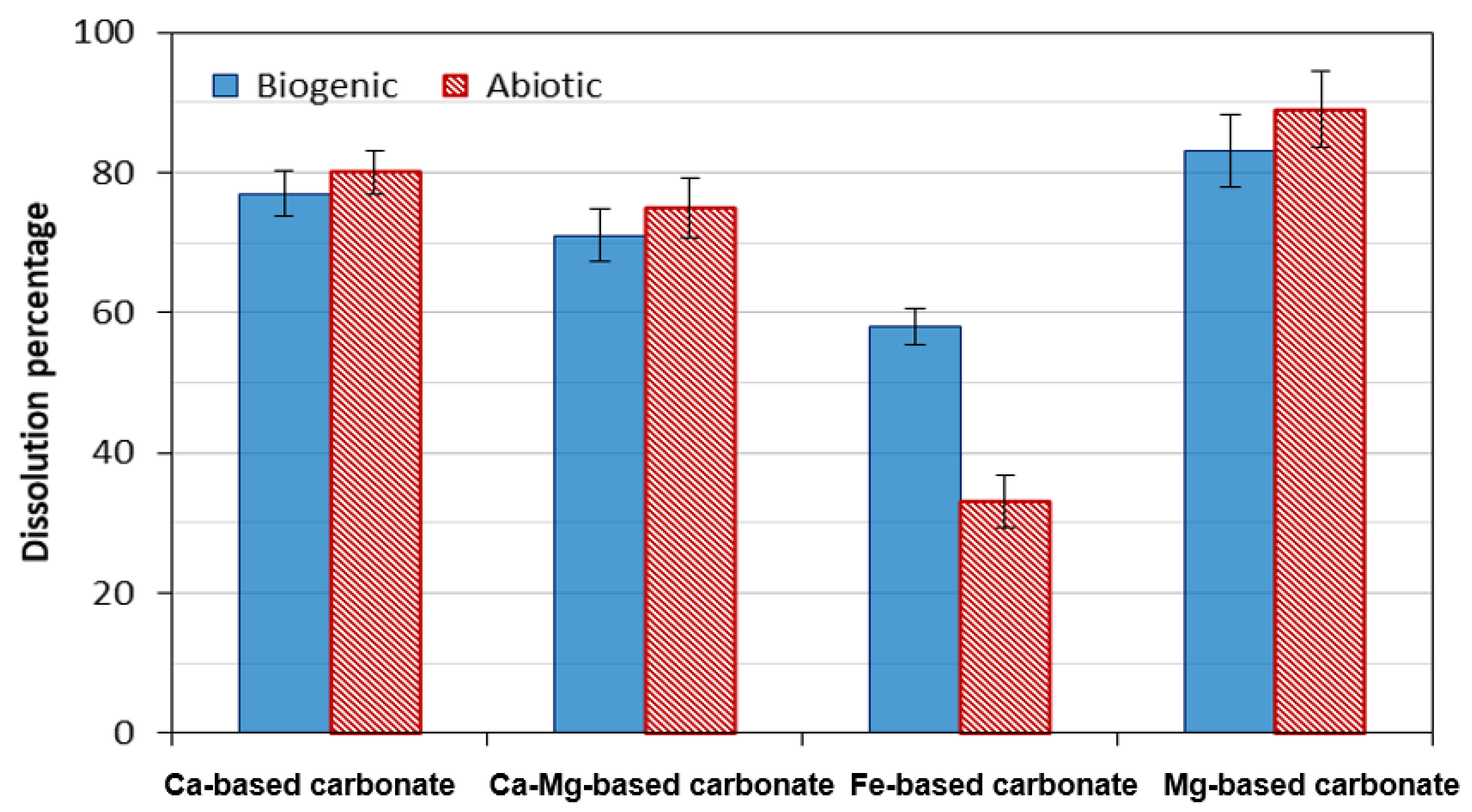
Acid durability tests showed that, except for siderite, algae-induced minerals had overall comparable or improved resistance compared to their counterparts formed by abiotic CO2 consumption. This may reflect microstructure, crystallinity, composition, or the influence of trace biomolecules.
Overall, the results suggest that microalgae-induced mineralization shows promise for sustainable, nature-based soil stabilization with concurrent CO2 sequestration, supporting climate mitigation goals, particularly in applications where slow mineral growth is acceptable and environmental impact minimization is critical. Although scalability and in situ process control remain challenging for in situ soil biocementation, the integration of this process with wastewater reuse, carbon capture, and biomass valorization could offer added environmental and economic benefits. The diversity of mineral products may have possible additional benefits for soil conditioning or environmental remediation. Finally, the potential reuse of algal biomass (for biofertilizers, food, or other green applications) could add value to algae-based systems within circular economy frameworks and would be an interesting direction for future research.