Advances in Analytical Techniques and Applications in Exploration, Mining, Extraction, and Metallurgical Studies of Rare Earth Elements
Abstract
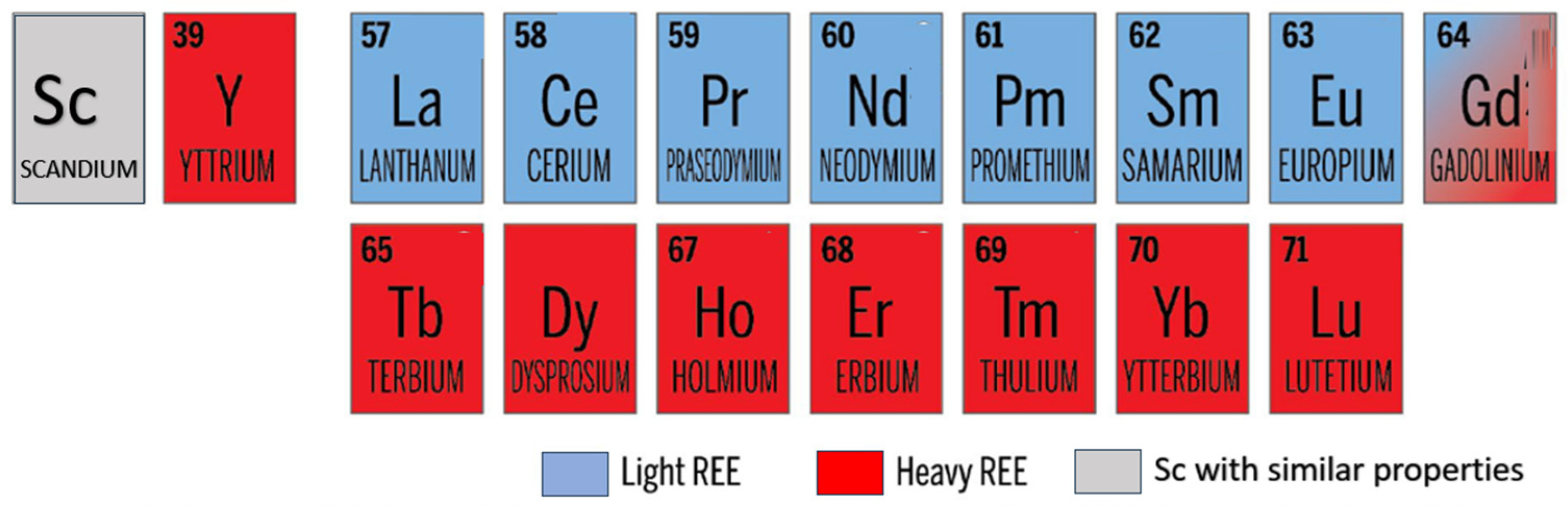
1. Introduction
| Source | ∑ REE |
|---|---|
| Earth’s crust | 150 to 220 µg/g |
| REE ore | 0.1%–10% |
| Surface and groundwater | 0.1–100 pg/g |
| Geothermal fluids | Up to 21.76 µg/g |
| Acid mine drainage | 1–1000 ng/g |
| Coal and pre-combustion by-products | 10–1000 µg/g |
| E-waste | ~600 µg/g |
| Coal ash | 10–1000 µg/g |
| Ferromanganese crust from the Indian Ocean | 1727 to 2511 μg/g |
| Laterites | 0.021 to 0.099 wt% |
| Red mud | 0.23 to 0.38 wt% |
| Phosphorites | up to 0.5 wt% |
| Bauxite mine waste ponds | 1900 to 2600 µg/g |
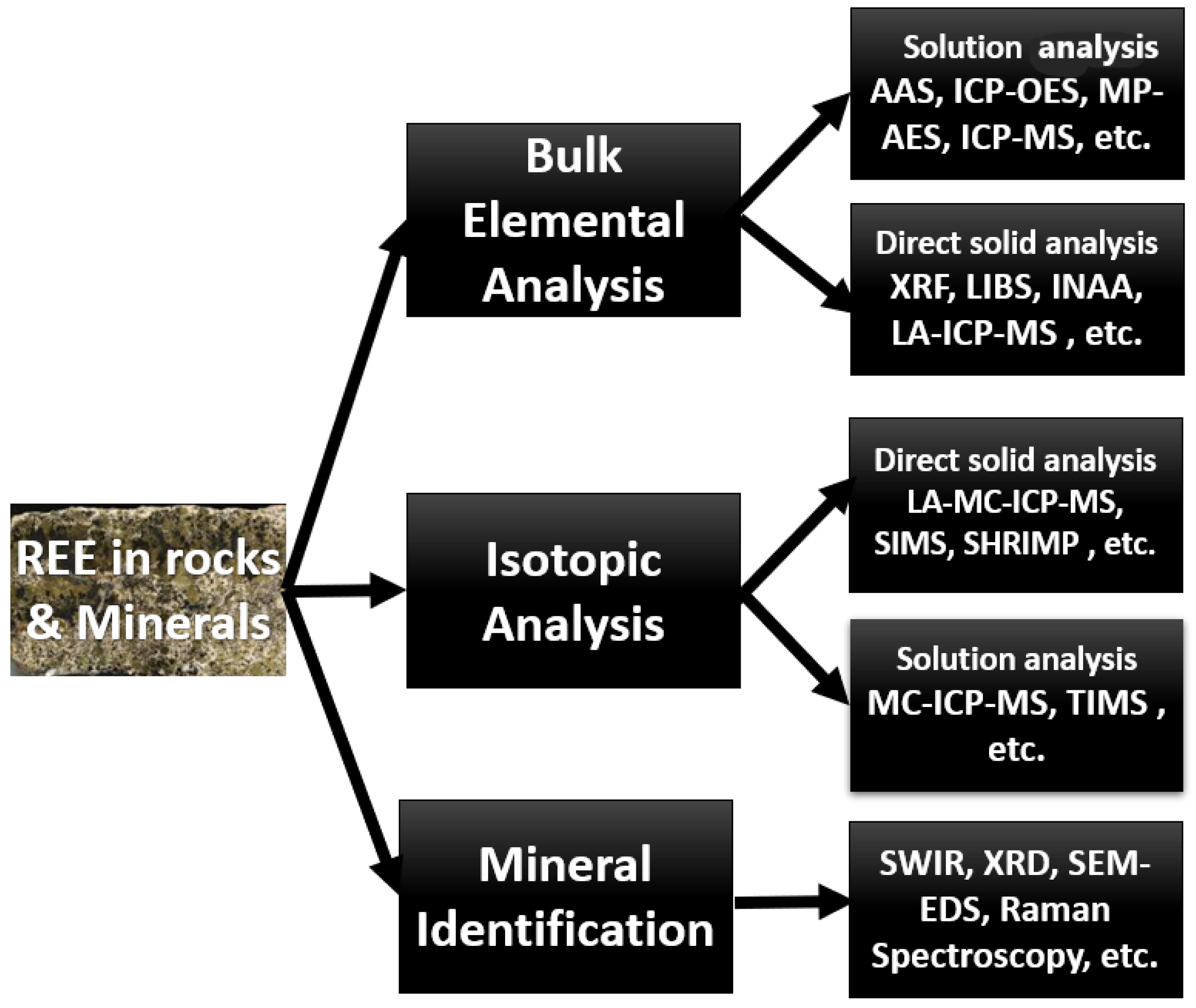
2. Instrumental Analytical Techniques
2.1. UV/Vis Spectrophotometry
2.2. X-ray Fluorescence Spectrometry (Both WD-XRF and ED-XRF)
| Element | ED-XRF Value (µg/g) | Certified Value |
|---|---|---|
| La | 10.78 | 11.23 |
| Ce | 26.44 | 25.65 |
| Nd | 9.59 | 9.45 |
| Sm | 1.72 | 1.69 |
| Y | 3.14 | 3.10 |
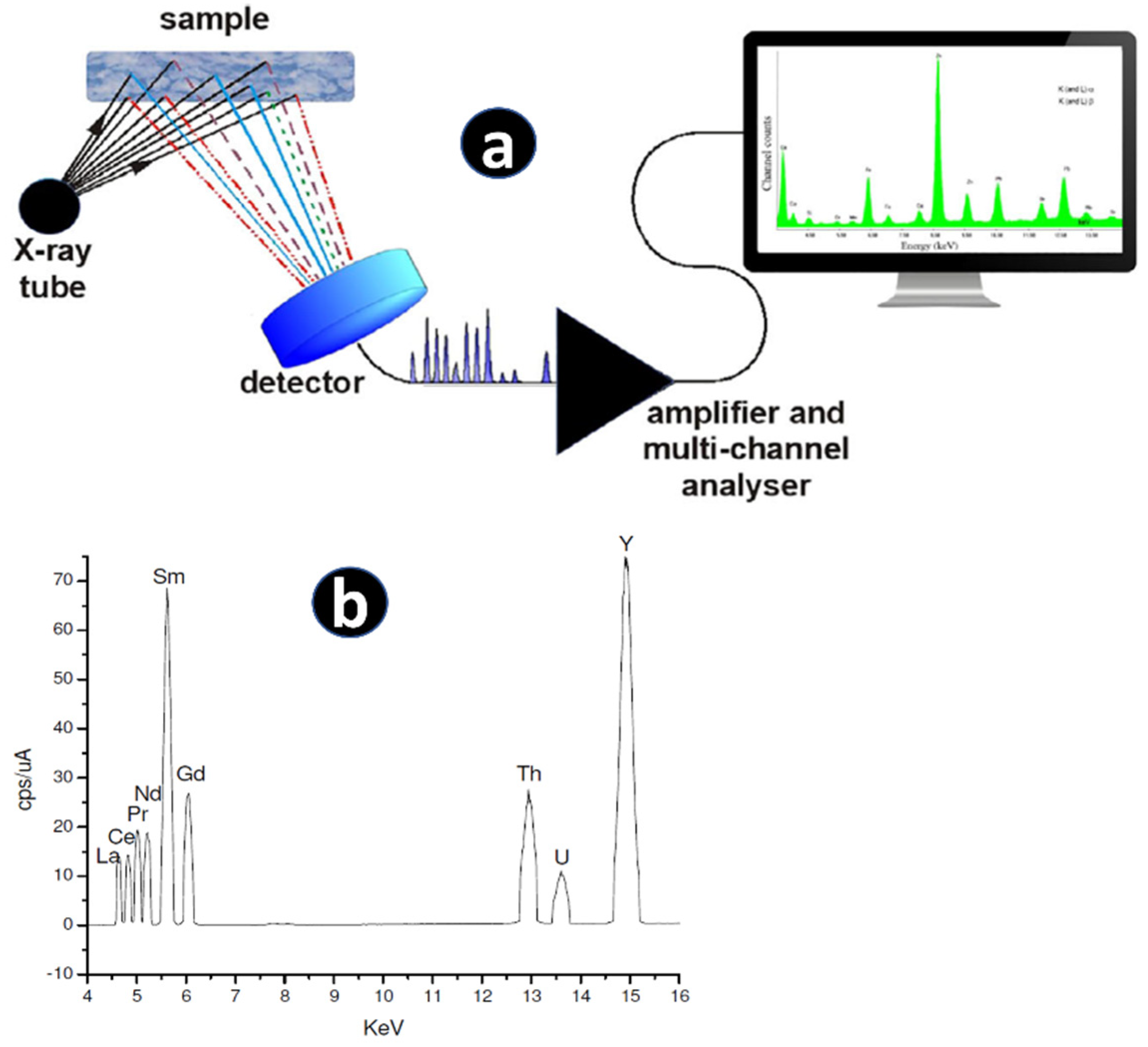
| Nature of Material | Analytes | Sample Preparation/Decomposition Method | Analytical Technique | Remarks | Reference |
|---|---|---|---|---|---|
| REE-bearing rock and soil samples | La, Ce, Nd and Y | Pressed pellets of homogenized soil samples | LIBS | Portable LIB spectrometers are useful in the exploration of new REE deposits | [33] |
| Lunar meteorites | REE | Directly ablating the sample | LIBS | Information on the constituents in sample drawn from spectral details | [34] |
| Waste Sm–Co magnets | REE and several other major, minor, and trace elements | Microwave digestion procedure using HNO3, H2SO4, HCl, and HF | ICP-MS and ICP-OES | Recoveries were between 99%–100% and RSD was <5% | [35] |
| Rocks | REE | Low dilution glass beads made with a sample to lithium borate ratio (1:1), heated twice at 1200 °C with agitation | XRF | Using this method, Y, La, Ce, Pr, Nd, Sm, Gd, Dy, and several other elements were determined in rhyolitic and granitic rocks | [36] |
| Surface waters and sediments of the Mgoua watershed, Cameroon | REE | Acidified water samples analyzed directly. Sediments were dissolved using a mixture of acids before analysis | ICP-MS | REE concentrations in waters of 0.11 to 6.60 ng/mL and 282.12 to 727.67 µg/g in sediments | [37] |
| JCp-1 (coral) and JCt-1 (giant clam) CRMs | REE | Two methods: (i) simple dissolution by HCl, and (ii) HF + HNO3 + HClO4 digestion and a further fusion process with Na2CO3 and H3BO3 in a Pt-crucible | ID-ICP-MS | No significant differences in REE results were found between the two decomposition methods | [38] |
| Sedimentary cores from Laguna Mar Chiquita, Argentina | La, Ce, Nd, Sm, Eu, Tb, Yb & Lu | 200 mg of sediment samples in polyethylene bags were irradiated | INAA | Global REE averages show higher REE contents in clastic than in chemical sediments | [39] |
| Phosphate rocks from Egypt and Saudi Arabia | REE | 30 g aliquots encapsulated in a polyethylene vial and irradiated | INAA | Choice of the nuclear reaction, irradiation and decay times, and of the proper gamma radiation are important | [40] |
| Brazilian geological CRMs | REE | For each sample, one CRM was simultaneously processed in exactly the same way | INAA | Geological CRMs GB-1 and BB-1 provided new trace element data | [41] |
| Sediments of Bouregreg river, Morocco | REE | 100 mg sample of CRMs were irradiated for about 7 h | INAA | INAA offers good sensitivity and selectivity for the analysis of sediments | [42] |
| Apatite mineral | La, Ce, Pr, Nd, Sm, Eu, Gd, and Dy | About 25 mg digested in 25 mL HNO3 and 6 mL HCl. Then, 1 mL of the solution was pipetted onto a Millipore membrane filter (1.2 mm pore size) and dried under an IR heater at 50 °C | WD-XRF | Determination in emission–transmission method. Precisions are ~3% RSD with comparable accuracies | [43] |
| Uranium oxide | Eu, Nd, and Yb | Sample powders were encapsulated in clear tape and analyzed directly | pLIBS | REE constituents in sub-percent levels detected | [44] |
| Alabaster rocks (crystalline CaCO3) | Sc, Lu, Ce, Sm, La, Yb, and Eu | 100 mg powder in polyethylene capsules irradiated | INAA | Technique is useful for geochemical and mineral exploration studies | [45] |
| Fluids from deep-sea hydrothermal vents | REE | REE are isolated from other elements on miniature cation-exchange columns | ICP-MS | ID-TIMS results compare favorably with |CP-MS results and are accurate at the 6% (2a) level | [46] |
| Natural carbonates | REE | Samples dissolved in HNO3 | ICP-MS | The carbonate REE-related studies are useful in climate change, paleoceanography, and environmental research | [47] |
| Drilling subsamples of 50–100 mg analyzed directly | LA-ICP-MS and LA-HR-ICP-MS |
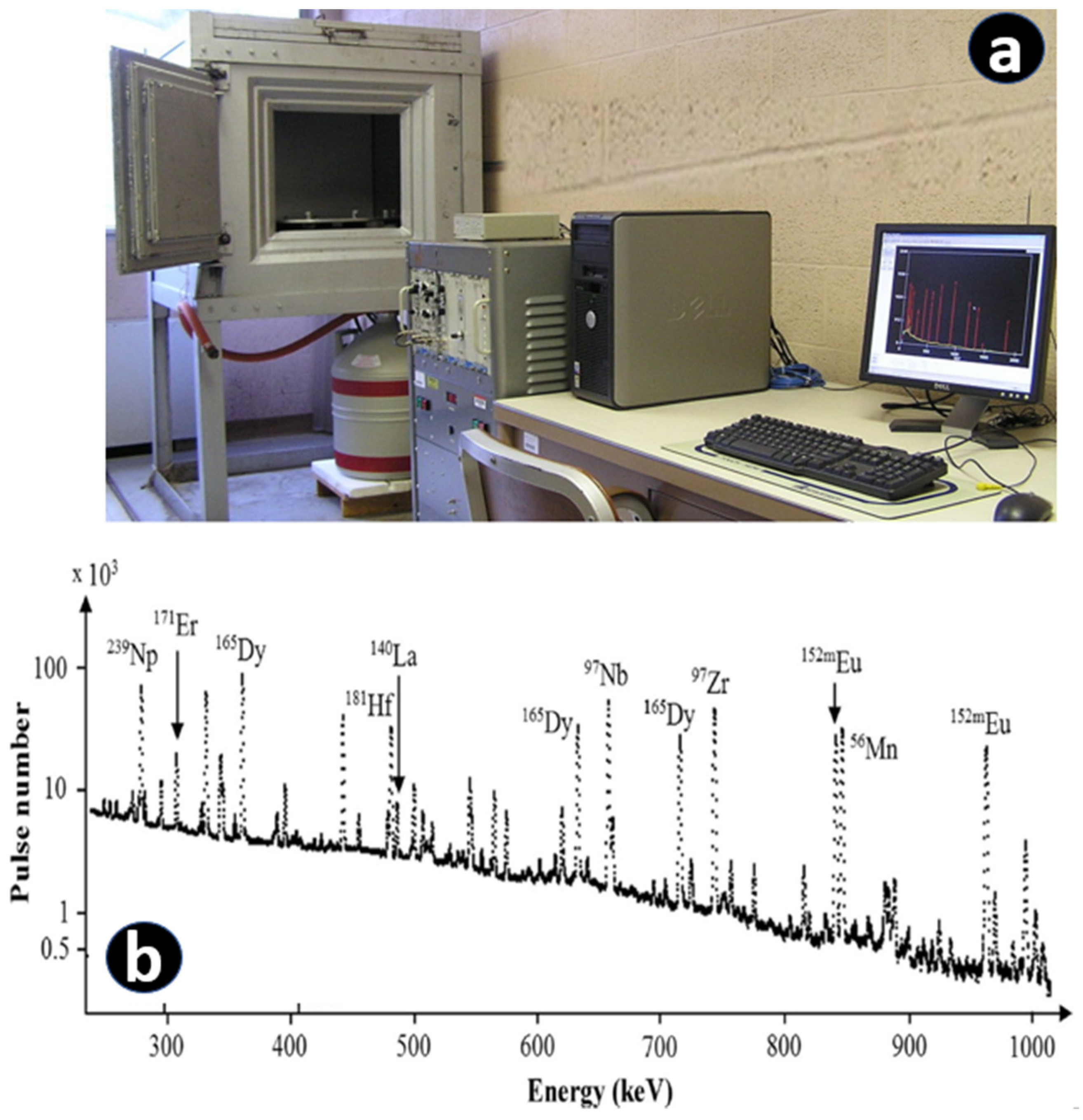
2.3. Instrumental Neutron Activation Analysis (INAA)
2.4. Indirect Measurement of REEs by the Radiometric Method
2.5. Atomic Absorption Spectrometry (Both Flame-AAS and GF-AAS)
2.6. Microwave Plasma Atomic Emission Spectrometry (MP-AES)
2.7. Inductively Coupled Plasma–Optical Emission Spectrometry (ICP-OES)
2.8. Laser-Induced Breakdown Spectroscopy (LIBS)
2.9. Inductively Coupled Plasma–Mass Spectrometry Techniques (All Forms of ICP-MS, ICP-MS/MS, ICP-TOF-MS, HR-ICP-MS, MH-ICP-MS, and MC-ICP-MS with Both Solution and Direct Solid Sampling by Laser Ablation)
2.9.1. Inductively Coupled Mass Spectrometry (ICP-MS)
2.9.2. ICP-Tandem Mass Spectrometry (ICP-MS/MS)
2.9.3. ICP-TOF-MS
2.9.4. Magnetic Sector or High-Resolution ICP-MS (HR-ICP-MS)
2.9.5. MH-ICP-MS
3. Isotopic Studies
3.1. Multi-Collector ICP-MS (MC-ICP-MS)
3.2. Thermal Ionization Mass Spectrometry (TIMS)
3.3. Sensitive High-Resolution Ion Micro Probe (SHRIMP)
4. Mineralogical Studies and In Situ Analytical Techniques
4.1. X-ray Diffractometry (XRD)
4.2. Electron Probe Micro Analyzer (EPMA)
4.3. Ion Microprobe (SIMS)
4.4. Scanning Electron Microprobe (SEM-EDS)
5. Laser Ablation ICP-MS (LA-ICP-MS)
5.1. LA-ICP-MS/MS
5.2. Laser Ablation Split Stream (LASS) Technique
6. Portable Miniatured Analytical Techniques
6.1. Portable XRF (pXRF or µXRF)
6.2. pLIBS
6.3. pXRD
6.4. Portable Raman Spectrometer
6.5. Fourier Transform Infrared (FTIR) Spectrometry
7. Hyperspectral Remote Sensing Techniques (Handheld, Drone, and Satellite-Based)
8. Electrochemical Methods and Biosensors for the Detection of REEs
9. Miscellaneous Analytical Techniques
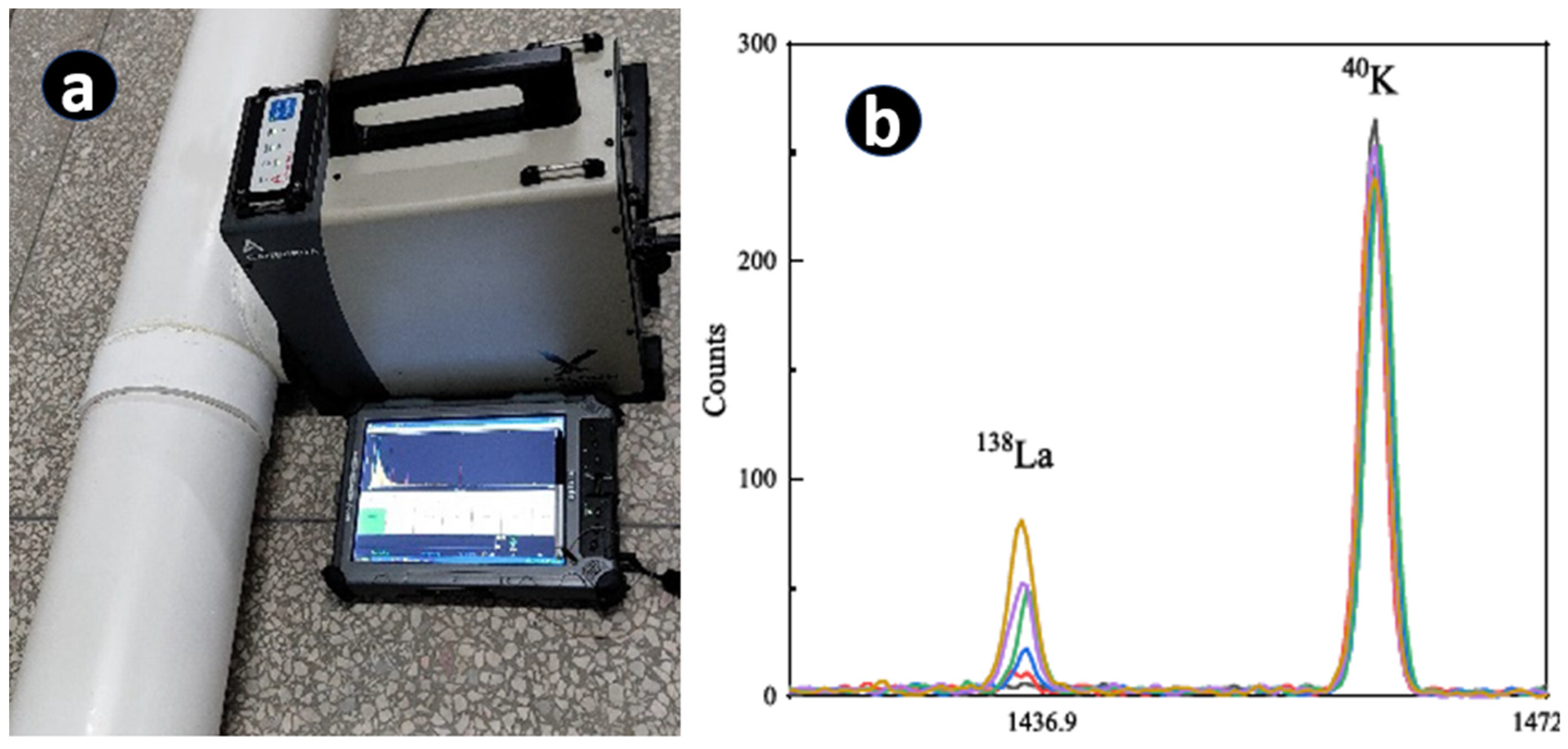
10. Analysis of La, and a Continuous Stream of Data Collection by In Situ Gamma Spectrometry during an Industrial Extraction Process
11. Comparison of Different Analytical Techniques for REE Analysis
12. Sample Preparation Methods for REE Studies (Acid, Fusion, and Microwave)
12.1. Acid Dissolution Methods
12.2. Fusion Dissolution Methods
12.3. Microwave, Ultrasound-Assisted, High-Pressure Digestion, and Infrared Heating Methods
13. Quality Assurance and Quality Control during Analysis
14. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogelj, J.; Geden, O.; Cowie, A.; Reisinger, A. Net-zero emissions targets are vague: Three ways to fix. Nature 2021, 591, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Combating Climate Change and Global Warming for a Sustainable Living in Harmony with Nature. J. Geogr. Res. 2023, 6, 1–17. [Google Scholar] [CrossRef]
- Balaram, V. Recent trends in the instrumental analysis of rare earth elements in geological and industrial materials. Trends Anal. Chem. 1996, 15, 475–486. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.K.; Ghosh, S.; Mondal, A.; Das, D.K. Role of rare earth elements as provenance indicator in coal seams: A case study from IB-River Coalfield, Orissa. Indian Miner. 2006, 60, 171–180. [Google Scholar]
- Drobniak, A.; Mastalerz, M. Rare Earth Elements—A brief overview: Indiana Geological and Water Survey. Indiana J. Earth Sci. 2022, 4. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2018; U.S. Geological Survey: Reston, VA, USA, 2018; pp. 132–133. [CrossRef]
- European Commission. Critical Materials for Strategic Technologies and Sectors in the EU—A Foresight Study; European Commission: Brussels, Belgium, 2020; p. 100. [Google Scholar]
- Fedele, L.; Plant, J.A.; De Vivo, B.; Lima, A. The rare earth element distribution over Europe: Geogenic and anthropogenic sources. Geochem. Explor. Environ. Anal. 2008, 8, 3–18. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Borsato, N.W.; Hoeijmakers, H.J.; Prinoth, B.; Thorsbro, B.; Forsberg, R.; Kitzmann, D.; Jones, K.; Heng, K. The Mantis Network III: Expanding the limits of chemical searches within ultra-hot Jupiters-New detections of Ca, V, Ti, Cr, Ni, Sr, Ba, and Tb in KELT-9 b. Astron. Astrophys. 2023, 673, A158. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B.; French, D.; Hower, J.C.; Graham, I.T.; Zhao, F. Modes of occurrence of elements in coal: A critical evaluation. Earth-Sci. Rev. 2021, 222, 103815. [Google Scholar] [CrossRef]
- Wagh, A.S.; Pinnock, W.R. Occurrence of scandium and rare earth elements in Jamaican bauxite waste. Econ. Geol. 1987, 82, 757–761. [Google Scholar] [CrossRef]
- Cocker, M.D. Lateritic supergene rare earth element (REE) deposits. In Arizona Geological Survey, Special Paper 9; Chapter # 4, 1–20; Arizona Geological Survey: Phoenix, AZ, USA, 2012. [Google Scholar]
- Lister, T.E.; Diaz, L.A.; Clark, G.G.; Keller, P. Process Development for the Recovery of Critical Materials from Electronic Waste. United States 2016. Available online: https://www.osti.gov/biblio/1358185 (accessed on 27 July 2023).
- Robert, Z.; Andrew, F.; Mark, R.; Jim, P. Maximizing REE Recovery in Geothermal Systems; University of California: Davis, CA, USA; University of Oregon: Eugene, OR, USA, 2018. [Google Scholar] [CrossRef]
- Hartzler, D.; Bhatt, C.; Jain, J.; McIntyre, D.L. Evaluating laser induced breakdown spectroscopy sensor technology for rapid source characterization of rare earth elements. J. Energy Resour. Technol. 2019, 141, 070704. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Element Deposits-Sources, and Exploration Strategies. J. Geol. Soc. India 2022, 98, 1210–1216. [Google Scholar] [CrossRef]
- Valetich, M.; Zivak, D.; Spandler, C.; Degeling, H.; Grigorescu, M. REE enrichment of phosphorites: An example of the Cambrian Georgina Basin of Australia. Chem. Geol. 2022, 588, 120654. [Google Scholar] [CrossRef]
- Cheatham, M.M.; Sangrey, W.F.; White, W.M. Sources of error in external calibration ICP-MS analysis of geological samples and an improved non-linear drift correction procedure. Spectrochim. Acta 1993, 48B, E467–E506. [Google Scholar] [CrossRef]
- Pu, Q.; Liu, P.; Hu, Z.; Su, Z. Spectrophotometric determination of the sum of rare earth elements by flow-injection on-line preconcentration with a novel aminophosphonic–carboxylic acid resin. Anal. Lett. 2002, 35, 1401–1414. [Google Scholar] [CrossRef]
- Saputra, H.A.; Anggraeni, A.; Mutalib, A.; Bahti, H.H. Development of a Fast Simultaneous Analysis Method for Determination of Middle Rare-Earth Elements in Monazite Samples. J. Kim. Sains Dan Apl. 2021, 24, 177–184. [Google Scholar] [CrossRef]
- Potts, P.J. X-ray fluorescence analysis. In Geochemistry. Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- De Vito, I.E.; Olsina, R.A.; Masi, A.N. Enrichment method for trace amounts of rare earth elements using chemofiltration and XRF determination. Fresenius’ J. Anal. Chem. 2000, 368, 392–396. [Google Scholar] [CrossRef]
- Juras, S.J.; Hickson, C.J.; Horsky, S.J.; Godwin, C.I.; Mathews, W.H. A practical method for the analysis of rare-earth elements in geological samples by graphite furnace atomic absorption and X-ray fluorescence. Chem. Geol. 1987, 64, 143–148. [Google Scholar] [CrossRef]
- Wu, W.; Xu, T.; Hao, Q.; Wang, Q.; Zhang, S.; Zhao, C. Applications of X-ray fluorescence analysis of rare earths in China. J. Rare Earths 2010, 28, 30–36. [Google Scholar] [CrossRef]
- De Pauw, E.; Tack, P.; Lindner, M.; Ashauer, A.; Garrevoet, J.; Vekemans, B.; Falkenberg, G.; Brenker, F.E.; Vincze, L. Highly Sensitive Nondestructive Rare Earth Element Detection by Means of Wavelength-Dispersive X-ray Fluorescence Spectroscopy Enabled by an Energy Dispersive pn-Charge-Coupled-Device Detector. Anal. Chem. 2020, 92, 1106–1113. [Google Scholar] [CrossRef]
- Adeti, P.J.; Amoako, G.; Tandoh, J.B.; Gyampo, O.; Ahiamadjie, H.; Amable, A.S.K.; Kansaana, C.; Annan, R.A.T.; Bamford, A. Rare-earth element comparative analysis in chosen geological samples using nuclear-related analytical techniques. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2023, 540, 122–128. [Google Scholar] [CrossRef]
- Sarker, S.K.; Bruckard, W.; Haque, N.; Roychand, R.; Bhuiyan, M.; Pramanik, B.K. Characterization of a carbonatite-derived mining tailing for the assessment of rare earth potential. Process Saf. Environ. Prot. 2023, 173, 154–162. [Google Scholar] [CrossRef]
- Yao, M.; Wang, D.; Zhao, M. Element Analysis Based on Energy-Dispersive X-Ray Fluorescence. Adv. Mater. Sci. Eng. 2015, 2015, 290593. [Google Scholar] [CrossRef]
- Kurniawati, S.; Santoso, M.; Lestiani, D.D.; Adventini, N.; Yatu, W.; Syahfitri, N. Analytical Capabilities of EDXRF for Determination of Rare Earth Elements. Indones. J. Nucl. Sci. Technol. 2021, 22, 1–9. [Google Scholar] [CrossRef]
- Taam, I.; Jesus, C.S.; Mantovano, J.L.; Gante, V. Quantitative Analysis or Rare Earths By X-Ray Fluorescence Spectrometry. In Proceedings of the International Nuclear Atlantic Conference-INAC 2013, Recife, Brazil, 24–29 November 2013; Associação Brasileira De Energia Nuclear–ABEN: Recife, PE, Brazil, 2013. ISBN 978-85-1-05-2. [Google Scholar]
- Balaram, V. Current and emerging analytical techniques for geochemical and geochronological studies. Geol. Jour. 2021, 56, 2300–2359. [Google Scholar] [CrossRef]
- Rethfeldt, N.; Brinkmann, P.; Riebe, D.; Beitz, T.; Köllner, N.; Altenberger, U.; Löhmannsröben, H.-G. Detection of Rare Earth Elements in Minerals and Soils by Laser-Induced Breakdown Spectroscopy (LIBS) Using Interval PLS. Minerals 2021, 11, 1379. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Ananthachar, A.; Choudhari, K.S.; George, S.D.; Chidangil, S.; Unnikrishnan, V.K. Laser-Induced Breakdown Spectroscopy (LIBS) for the Detection of Rare Earth Elements (REEs) in Meteorites. Minerals 2023, 13, 182. [Google Scholar] [CrossRef]
- Korotkova, N.A.; Baranovskaya, V.B.; Petrova, K.V. Microwave Digestion and ICP-MS Determination of Major and Trace Elements in Waste Sm-Co Magnets. Metals 2022, 12, 1308. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakamura, T. X-ray Fluorescence Analysis of Rare Earth Elements in Rocks Using Low Dilution Glass Beads. Anal. Sci. 2005, 21, 815–822. [Google Scholar] [CrossRef]
- Ndjama, J.; Mafany, G.; Ndondo, R.G.N.; Belmond, B.E.; Bessa, A.Z.E. Rare earth elements in surface waters and sediments of the Mgoua watershed, south western Cameroon. Arab. J. Geosci. 2022, 15, 1001. [Google Scholar] [CrossRef]
- Tanaka, T.; Lee, S.-G.; Kim, T.; Han, S.; Lee, H.M.; Lee, J.I. Precise determination of 14 REEs in GSJ/AIST geochemical reference materials JCp-1 (coral) and JCt-1 (giant clam) using isotope dilution ICP-quadrupole mass spectrometry. Geochem. J. 2017, 51, 75–79. [Google Scholar] [CrossRef]
- Oliveira, S.M.B.; Larizzatti, F.E.; Fávaro, D.I.T.; Moreira, S.R.D.; Mazzilli, B.P.; Piovano, E.L. Rare earth element patterns in lake sediments as studied by neutron activation analysis. J. Radioanal. Nucl. Chem. 2003, 258, 531–535. [Google Scholar] [CrossRef]
- Awad, H.; Zakaly, H.M.H.; El-Taher, A.; Sebak, M. Determination of lanthanides in phosphate rocks by instrumental neutron activation analysis. In June 2022 AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2022; Volume 2466, p. 050002. [Google Scholar] [CrossRef]
- Figuelredo, A.M.G.; Marques, L.S. Determination of rare earths and other trace elements in the Brazilian geological standards, BB-1 and GB-1 by neutron activation analysis. Geochim. Bras. 1989, 3, 1–8. [Google Scholar]
- Bounouira, H.; Choukri, A.; Elmoursli, R.C.; Hakam, O.; Chakiri, S. Distribution of the rare earth elements in the sediments of the Bouregreg river (Morocco) using the instrumental neutron activation analysis (INAA). J. Appl. Sci. Environ. Manag. 2007, 11, 57–60. [Google Scholar] [CrossRef][Green Version]
- Sitko, R.; Zawisza, B.; Czaja, M. Fundamental parameters method for determination of rare earth elements in apatites by wavelength-dispersive X-ray fluorescence spectrometry. J. Anal. At. Spectrom. 2005, 20, 741. [Google Scholar] [CrossRef]
- Manard, B.T.; Wylie, E.M.; Willson, S.P. Analysis of Rare Earth Elements in Uranium Using Handheld Laser-Induced Breakdown Spectroscopy (HH LIBS). Appl. Spectrosc. 2018, 72, 1653–1660. [Google Scholar] [CrossRef]
- Alamoudi, Z.; El-Taher, A. Application of Nuclear Analytical Techniques in Elemental Characterization of Wadi El-Nakhil Alabaster, Central Eastern Desert, Egypt. Sci. Technol. Nucl. Install. 2016, 2016, 2892863. [Google Scholar] [CrossRef]
- Klinkhammer, G.; German, C.R.; Elderfield, H.; Greaves, M.J.; Mitra, A. Rare earth elements in hydrothermal fluids and plume particulates by inductively coupled plasma mass spectrometry. Mar. Chem. 1994, 45, 179–186. [Google Scholar] [CrossRef]
- Wu, C.C. Advanced and Applied Studies on Ultra-Trace Rare Earth Elements (REEs) in Carbonates Using SN-ICPMS and LA-ICPMS. Ph.D. Thesis, National Taiwan University, Taipei, Taiwan, 2021; pp. 1–74. [Google Scholar]
- Vukotić, P. Determination of rare earth elements in bauxites by instrumental neutron activation analysis. J. Radioanal. Chem. 1983, 78, 105–115. [Google Scholar] [CrossRef]
- Baidya, T.K.; Mondal, S.K.; Balaram, V.; Parthasarathi, R.; Verma, R.; Mathur, P.K. PGE-Ag-Au mineralisations in a Cu-Fe-Ni sulphide-rich breccia zone of the Precambrian Nuasahi ultramafic-mafic complex, Orissa, India. J. Geol. Soc. India 1999, 54, e473–e482. [Google Scholar]
- Silachyov, I. Zircon concentrate analysis for sixteen rare earth elements by the complex of nuclear analytical methods. J. Radioanal. Nucl. Chem. 2023, 332, 2017–2026. [Google Scholar] [CrossRef]
- Ravisankar, R.; Manikandan, E.; Dheenathayalu, M.; Rao, B.; Seshadreesan, N.P.; Nair, K.G.M. Determination and distribution of rare earth elements in beach rock samples using instrumental neutron activation analysis (INAA). Nucl. Instrum. Methods Phys. Res. B 2006, 251, 496–500. [Google Scholar] [CrossRef]
- Krishnan, K.; Saion, E. Distributions of Rare Earth Element (REE) in Mangrove Surface Sediment by Nuclear Technique. Int. J. 2022, 2022, 1–7. [Google Scholar]
- Ahmed, M.E.; Bounouira, H.; Abbo, M.A.; Amsil, H.; Didi, A.; Aarab, I. Utilizing the k0-IAEA program to determine rare earth elements in soil samples from gold-mining areas in Sudan. J. Radioanal. Nucl. Chem. 2023, 332, 9. [Google Scholar] [CrossRef]
- Kin, F.D.; Prudêncio, M.I.; Gouveia, M.Â.; Magnusson, E. Determination of Rare Earth Elements in Geological Reference Materials: A Comparative Study by INAA and ICP-MS. Geostand. Geoanalytical Res. 1999, 23, 47–58. [Google Scholar] [CrossRef]
- Heinz-Günter, S. Neutron Activation Analysis of the Rare Earth Elements (REE)—With Emphasis on Geological Materials. Phys. Sci. Rev. 2016, 1, 20160062. [Google Scholar] [CrossRef]
- El-Taher, A. Nuclear Analytical Techniques for Detection of Rare Earth Elements. J. Rad. Nucl. Appl. 2018, 3, 53–64. [Google Scholar] [CrossRef]
- Ghannadpour, S.S.; Hezarkhani, A. Prospecting rare earth elements (REEs) using radiation measurement: Case study of Baghak mine, Central Sangan iron ore mine, NE of Iran. Env. Earth Sci. 2022, 81, 363. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, W.; Liu, J.; Liang, X.; Yuan, W.; Ouyang, Q.; Liu, S.; Gok, C.; Wang, J.; Song, G. Preliminary Screening of Soils Natural Radioactivity and Metal(loid) Content in a Decommissioned Rare Earth Elements Processing Plant, Guangdong, China. Int. J. Environ. Res. Public Health 2022, 19, 14566. [Google Scholar] [CrossRef]
- Mohanty, S.; Khan, R.; Tamim, U.; Adak, S.; Bhunia, G.S.; Sengupta, D. Geochemical and Radionuclide studies of sediments as tracers for enrichment of beach and alluvial placers along the eastern coast of India. Reg. Stud. Mar. Sci. 2023, 63, 103003. [Google Scholar] [CrossRef]
- Walsh, A. The application of atomic absorption spectra to chemical analysis. Spectrochim. Acta 1955, 7, 108–117. [Google Scholar] [CrossRef]
- L’vov, B.V. A continuum source vs. line source on the way toward absolute graphite furnace atomic absorption spectrometry, Spectrochimica. Acta Part B At. Spectrosc. 1999, 54, 1637–1646. [Google Scholar] [CrossRef]
- Balaram, V.; Sunder Raju, P.V.; Ramesh, S.L.; Anjaiah, K.V.; Dasaram, B.; Manikyamba, C.; Ram Mohan, M.; Sarma, D.S. Rapid partial dissolution method in combination with atomic absorption spectroscopy techniques for use in geochemical exploration. At. Spectrosc. 1999, 20, 155–160. [Google Scholar]
- Hammer, M.R. A magnetically excited microwave plasma source for atomic emission spectroscopy with performance approaching that of the inductively coupled plasma. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 456–464. [Google Scholar] [CrossRef]
- Balaram, V. Microwave plasma atomic emission spectrometry (MPAES) and its applications: A critical review. Microchem. J. 2020, 159, 18. [Google Scholar] [CrossRef]
- Balaram, V.; Dharmendra, V.; Roy, P.; Taylor, C.; Kar, P.; Raju, A.K.; Krishnaiah, A. Determination of Precious Metals in Rocks and Ores by Microwave Plasma-Atomic Emission Spectrometry (MP-AES) for Geochemical Prospecting. Curr. Sci. 2013, 104, 1207–1215. [Google Scholar]
- Balaram, V.; Dharmendra, V.; Roy, P.; Taylor, C.; Kamala, C.T.; Satyanarayanan, M.; Kar, P.; Subramanyam, K.S.V.; Raju, A.K.; Krishnaiah, A. Analysis of Geochemical Samples by Microwave Plasma-AES. At. Spectrosc. 2014, 35, 65–78. [Google Scholar]
- Kamala, C.T.; Balaram, V.; Dharmendra, V.; Roy, P.; Satyanarayanan, M.; Subramanyam, K.S.V. Application of Microwave Plasma Atomic Emission Spectrometry (MP-AES) for Environmental Monitoring of Industrially Contaminated sites in Hyderabad City. Environ. Monit. Assess. 2014, 186, 7097–7113. [Google Scholar] [CrossRef]
- Helmeczi, E.; Wang, Y.; Brindle, I.D. A novel methodology for rapid digestion of rare earth element ores and determination by microwave plasma-atomic emission spectrometry and dynamic reaction cell-inductively coupled plasma-mass spectrometry. Talanta 2016, 160, 521–527. [Google Scholar] [CrossRef]
- Varbanova, E.; Stefanova, V. A comparative study of inductively coupled plasma optical emission spectrometry and microwave plasma atomic emission spectrometry for the direct determination of lanthanides in water and environmental samples. Ecol. Saf. 2015, 9, 362–374. [Google Scholar]
- Greenfield, S.; Jones, I.L.I.; Berry, C.T. High pressure plasmas as spectroscopic emission sources. Analyst 1964, 89, 713–720. [Google Scholar] [CrossRef]
- Wendt, R.H.; Fassel, V. Inductively-coupled plasma spectrometric excitation source. Anal. Chem. 1965, 37, 920–922. [Google Scholar]
- Balaram, V.; Anjaiah, K.V.; Reddy, M.R.P. A comparative study o the trace and rare earth element analysis o an Indian Polymetallic Nodule Reference Sample by Inductively Coupled Plasma Atomic Emission Spectrometry and Inductively Coupled Plasma Mass Spectrometry. Analyst 1995, 120, 1401–1406. [Google Scholar] [CrossRef]
- Kumar, N.S.; Dharmendra, V.; Sreenivasulu, V.; Asif, M.; Balaram, V. Separation and Preconcentration of Pb and Cd in Water Samples using 3-(2-hydroxyphenyl)-1H-1,2,4-triazole-5(4H)-thione (HTT) and their Determination by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES). Metals 2017, 7, 240. [Google Scholar] [CrossRef]
- Makombe, M.; van der Horst, C.; Silwana, B.; Iwuoha, E.; Somerset, V. Optimization of Parameters for Spectroscopic Analysis of Rare Earth Elements in Sediment Samples; Chapter 3; INTECH, Open Science: London, UK, 2017. [Google Scholar] [CrossRef]
- Gorbatenko, A.A.; Revina, E.I. A review of instrumental methods for determination of rare earth elements. Inorg. Mater. 2015, 51, 1375–1388. [Google Scholar] [CrossRef]
- Pradhan, S.R.; Ambade, B. Extractive separation of rare earth elements and their determination by inductively coupled plasma optical emission spectrometry in geological samples. J. Anal. At. Spectrom. 2020, 35, 1395–1404. [Google Scholar] [CrossRef]
- Nóbrega, J.; Schiavo, D.; Amaral, C.; Barros, J.; Mogueira, A.R.; Virgilio, A.; Machado, R. Determination of rare earth elements in geological and agricultural samples by ICP-OES. Spectroscopy 2017, 32, 32–36. [Google Scholar]
- Tupaz, C.A.J.; Gregorio, C.G.C.; Arcilla, C. Determination of Scandium (Sc), Yttrium (Y), and Rare-Earth Elements (REEs) in Mafic and Ultramafic Rock Powder by a Modified and Validated Digestion Protocol and Inductively Coupled Plasma—Mass Spectrometry (ICP-MS). Anal. Lett. 2022, 56, 932–943. [Google Scholar] [CrossRef]
- Zhu, Y. Determination of rare earth elements in seawater samples by inductively coupled plasma tandem quadrupole mass spectrometry after coprecipitation with magnesium hydroxide. Talanta 2020, 209, 120536. [Google Scholar] [CrossRef]
- Robinson, P.; Townsend, A.T.; Yu, Z.; Münker, C. Determination of Scandium, Yttrium and Rare Earth Elements in Rocks by High Resolution Inductively Coupled Plasma-Mass Spectrometry. Geostand. Geoanalytical Res. 1999, 23, 31–46. [Google Scholar] [CrossRef]
- Bauchle, M.; Ludecke, T.; Rabieh, S.; Calnek, K.; Bromage, T.G. Quantification of 71 detected elements from Li to U for aqueous samples by simultaneous-inductively coupled plasma-mass spectrometry. RSC Adv. 2018, 8, 37008–37020. [Google Scholar] [CrossRef] [PubMed]
- Leitzke, F.P.; Wegner, A.C.; Porcher, C.C.; Vieira, N.I.M.; Berndt, J.; Klemme, J.; Conceição, R.V. Whole-rock trace element analyses via LA-ICP-MS in glasses produced by sodium borate flux fusion. Braz. J. Geol. 2021, 51, 2. [Google Scholar] [CrossRef]
- Hirose, F.; Itoh, S.; Okochi, H. Determination of Rare-earth Elements in Metallic La, Pr, Nd, Gd and Tb by Glow Discharge Mass Spectrometry. Tetsu—Hagane 1991, 77, 598–604. [Google Scholar] [CrossRef]
- Pedarnig, J.D.; Trautner, S.; Grünberger, S.; Giannakaris, N.; Eschlböck-Fuchs, S.; Hofstadler, J. Review of Element Analysis of Industrial Materials by In-Line Laser—Induced Breakdown Spectroscopy (LIBS). Appl. Sci. 2021, 11, 9274. [Google Scholar] [CrossRef]
- Harmon, R.S.; Senesi, G.S. Laser-Induced Breakdown Spectroscopy—A geochemical tool for the 21st century. Appl. Geochem. 2021, 128, 104929. [Google Scholar] [CrossRef]
- Alamelu, D.; Sarkar, A.; Aggarwal, S.K. Laser-induced breakdown spectroscopy for simultaneous determination of Sm, Eu and Gd in aqueous solution. Talanta 2008, 77, 256–261. [Google Scholar] [CrossRef]
- Abedin, K.M.; Haider, A.F.M.Y.; Rony, M.A.; Khan, Z.H. Identification of multiple rare earths and associated elements in raw monazite sands by laser-induced breakdown spectroscopy. Opt. Laser Technol. 2011, 43, 45–49. [Google Scholar] [CrossRef]
- Bhatt, C.R.; Jain, J.C.; Goueguel, C.L.; McIntyre, D.L.; Singh, J.P. Determination of Rare Earth Elements in Geological Samples Using Laser-Induced Breakdown Spectroscopy (LIBS). Appl. Spectrosc. 2017, 72, 114–121. [Google Scholar] [CrossRef]
- Unnikrishnan, V.K.; Nayak, R.; Devangad, P.; Tamboli, M.M.; Santhosh, C.; Kumar, G.A.; Sardar, D.K. Calibration based laser-induced breakdown spectroscopy (LIBS) for quantitative analysis of doped rare earth elements in phosphors. Mater. Lett. 2013, 107, 322–324. [Google Scholar] [CrossRef]
- Long, J.; Song, W.R.; Hou, Z.Y.; Wang, Z. A data selection method for matrix effects and uncertainty reduction for laser-induced breakdown spectroscopy. Plasma Sci. Technol. 2023, 25, 075501. [Google Scholar] [CrossRef]
- Haider, A.F.M.Y.; Khan, Z.H. Identification of multiple rare earths and other associated elements in zircon by laser-induced breakdown spectroscopy. J. Bangladesh Acad. Sci. 2020, 44, 59–68. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, J.; Jiang, J.; Zhou, Z.; Ye, S. Automatic coal-rock recognition by laser-induced breakdown spectroscopy combined with an artificial neutral network. Spectroscopy 2023, 38, 23–28. [Google Scholar]
- Gaft, M.; Raichlin, Y.; Pelascini, F.; Panzer, G.; Motto Ros, V. Imaging rare-earth elements in minerals by laser-induced plasma spectroscopy: Molecular emission and plasma-induced luminescence. Spectrochim. Acta Part B At. Spectrosc. 2019, 151, 12–19. [Google Scholar] [CrossRef]
- Afgan, M.S.; Hou, Z.; Song, W.; Liu, J.; Song, Y.; Gu, W.; Wang, Z. On the Spectral Identification and Wavelength Dependence of Rare-Earth Ore Emission by Laser-Induced Breakdown Spectroscopy. Chemosensors 2022, 10, 350. [Google Scholar] [CrossRef]
- Houk, R.S.; Fassel, V.A.; Flesch, G.D.; Svec, H.J.; Gray, A.L.; Taylor, C.E. Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements. Anal. Chem. 1980, 52, 2283–2289. [Google Scholar] [CrossRef]
- Balaram, V. Strategies to overcome interferences in elemental and isotopic geochemical studies by quadrupole ICP-MS: A critical evaluation of the recent developments. Rapid Commun. Mass Spectrom. 2021, 35, e9065. [Google Scholar] [CrossRef]
- Atsunori, N.; Ran, K.; Atsuyuki, O. Multi-element analysis of geological samples using ICP-MS equipped with integrated sample introduction and aerosol dilution systems. Bull. Geol. Surv. Jpn. 2023, 74, 71–85. [Google Scholar]
- Mnculwane, H.T. Rare Earth Elements Determination by Inductively Coupled Plasma Mass Spectrometry after Alkaline Fusion Preparation. Analytica 2022, 3, 135–143. [Google Scholar] [CrossRef]
- Veerasamy, N.; Sahoo, S.K.; Murugan, R.; Kasar, S.; Inoue, K.; Fukushi, M.; Natarajan, T. ICP-MS Measurement of Trace and Rare Earth Elements in Beach Placer-Deposit Soils of Odisha, East Coast of India, to Estimate Natural Enhancement of Elements in the Environment. Molecules 2021, 26, 7510. [Google Scholar] [CrossRef]
- Lin, R.; Bank, T.L.; Roth, E.A.; Granite, E.J.; Soong, Y. Organic and inorganic associations of rare earth elements in central Appalachian coal. Int. J. Coal Geol. 2017, 179, 295–301. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Ramzan, M.; Kifle, D.; Wibetoe, G. A simple separation system for elimination of molecular interferences for purity determination of europium and ytterbium oxides by HPLC-ICP-MS. J. Anal. At. Spectrom. 2020, 35, 2594–2599. [Google Scholar] [CrossRef]
- Liu, W.; An, Y.; Qu, Q.; Li, P.; Zhang, L.; Li, C.; Wei, S.; Zhou, H.; Chen, J. An efficient method for separation of REEs from Ba for accurate determination of REEs contents in Ba-rich samples by ICP-MS. J. Anal. At. Spectrom. 2023, 38, 449–456. [Google Scholar] [CrossRef]
- Wysocka, I. Determination of rare earth elements concentrations in natural waters—A review of ICP-MS measurement approaches. Talanta 2021, 221, 121636. [Google Scholar] [CrossRef] [PubMed]
- Palozzi, J.; Bailey, J.G.; Tran, Q.A.; Stanger, R. A characterization of rare earth elements in coal ash generated during the utilization of Australian coals. Int. J. Coal Prep. Util. 2023, 1–30. [Google Scholar] [CrossRef]
- El-Taher, A.; Ashry, A.; Ene, A.; Almeshari, M.; Zakaly, H. Determination of phosphate rock mines signatures using XRF and ICP-MS elemental analysis techniques: Radionuclides, oxides, rare earth and trace elements. Rom. Rep. Phys. 2023, 75, 701. [Google Scholar]
- Krasavtseva, E.; Sandimirov, S.; Elizarova, I.; Makarov, D. Assessment of Trace and Rare Earth Elements Pollution in Water Bodies in the Area of Rare Metal Enterprise Influence: A Case Study—Kola Subarctic. Water 2022, 14, 3406. [Google Scholar] [CrossRef]
- Xin, W.C.; Zhu, Z.G.; Song, X.Y.; Zhu, A.M.; Zhang, D.L. On pretreatment method for the determination of rare earth elements in deep sea REY-rich sediments by inductively coupled plasma-mass spectrometry. Mar. Geol. Front. 2022, 38, 92–96. [Google Scholar] [CrossRef]
- Li, H.; Tong, R.; Guo, W.; Xu, Q.; Tao, D.; Lai, Y.; Jina, L.; Hu, S. Development of a fully automatic separation system coupled with online ICP-MS for measuring rare earth elements in seawater. RSC Adv. 2022, 12, 24003. [Google Scholar] [CrossRef]
- Wysocka, I.A.; Kurzawa, D.K.; Porowski, A. Development and validation of seaFAST-ICP-QMS method for determination of rare earth elements total concentrations in natural mineral waters. Food Chem. 2022, 388, 133008. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Huang, K.; Wang, Z. Multielemental Determination of Rare Earth Elements in Seawater by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) After Matrix Separation and Pre-concentration with Crab Shell Particles. Front. Environ. Sci. 2021, 9, 781–996. [Google Scholar] [CrossRef]
- Balaram, V. Inductively Coupled Plasma-Tandem Mass Spectrometry (ICP-MS/MS) and Its Applications. J. ISAS 2022, 1, 1–26. [Google Scholar] [CrossRef]
- Zhu, Y. Determination of Rare Earth Elements by Inductively Coupled Plasma–Tandem Quadrupole Mass Spectrometry with Nitrous Oxide as the Reaction Gas. Front. Chem. 2022, 10, 912938. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Thoss, V.; Ribeiro Guevara, S.; Urgast, D.; Raab, A.; Mastrolitti, S.; Feldmann, J. Assessing rare earth elements in quartz rich geological samples. Appl. Radiat. Isot. 2016, 107, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, S.T.; Prohaska, T.; Irrgeher, J. Characterisation of gas cell reactions for 70+ elements using N2O for ICP tandem mass spectrometry measurements. J. Anal. At. Spectrom. 2023, 38, 1135–1145. [Google Scholar] [CrossRef]
- Ntiharirizwa, S.; Boulvais, P.; Poujol, M.; Branquet, Y.; Morelli, C.; Ntungwanayo, J.; Midende, G. Geology and U-Th-Pb Dating of the Gakara REE Deposit, Burundi. Minerals 2018, 8, 394. [Google Scholar] [CrossRef]
- Myers, P.; Li, G.; Yang, P.; Hieftje, G.M. An inductively coupled plasma-time-of-flight mass spectrometer for elemental analysis. Part I: Optimization and characteristics. J. Am. Soc. Mass Spectrom. 1994, 5, 1008–1016. [Google Scholar] [CrossRef]
- Mahoney, P.P.; Ray, S.J.; Hieftje, G.M.; Li, G. Continuum background reduction in orthogonal-acceleration time-of-flight mass spectrometry with continuous ion source. J. Am. Soc. Mass Spectrom. 1997, 125, 125–131. [Google Scholar] [CrossRef]
- Balaram, V.; Satyanarayanan, M.; Murthy, P.K.; Mohapatra, C.; Prasad, K.L. Quantitative multi-element analysis of cobalt crust from Afanasy-Nikitin seamount in the North Central Indian Ocean by inductively coupled plasma time-of-flight mass spectrometry. MAPAN-J. Metrol. Soc. India 2013, 28, 63–77. [Google Scholar]
- Dick, D.; Wegner, A.; Gabrielli, P.; Ruth, U.; Barbante, C.; Kriews, M. Rare earth elements determined in Antarctic ice by inductively coupled plasma—Time of flight, quadrupole and sector field-mass spectrometry: An inter-comparison study. Anal. Chim. Acta 2008, 621, 140–147. [Google Scholar] [CrossRef]
- Nakazato, M.; Asanuma, H.; Niki, S.; Iwano, H.; Hirata, T. Depth-Profiling Determinations of Rare Earth Element Abundances and U-Pb Ages from Zircon Crystals Using Sensitivity-Enhanced Inductively Coupled Plasma-Time of Flight-Mass Spectrometry. Geostand. Geoanalytical Res. 2022, 46, 603–620. [Google Scholar] [CrossRef]
- Peng, J.; Li, D.; Hollings, P.; Fu, Y.; Sun, X. Visualization of critical metals in marine nodules by rapid and high-resolution LA-ICP-TOF-MS mapping. Ore Geol. Rev. 2023, 154, 105342. [Google Scholar] [CrossRef]
- Chew, D.; Drost, K.; Marsh, H.; Petrus, J.A. LA-ICP-MS imaging in the geosciences and its applications to geochronology. Chem. Geol. 2021, 559, 119917. [Google Scholar] [CrossRef]
- Bradshaw, N.; Hall, E.F.H.; Sanderson, N.E. Inductively coupled plasma as an ion source for high-resolution mass spectrometry. J. Anal. At. Spectrom. 1989, 4, 801–803. [Google Scholar] [CrossRef]
- Satyanarayanan, M.; Balaram, V.; Sawant, S.S.; Subramanyam, K.S.V.; Krishna, V.; Dasaram, B.; Manikyamba, C. Rapid determination of REE, PGE and other trace elements in geological and environmental materials by HR-ICP-MS 2018. At. Spectrosc. 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Thomas, R. A Beginner’s Guide to ICP-MS Part VII: Mass Separation Devices—Double-Focusing Magnetic-Sector Technology. Spectroscopy 2001, 16, 22–27. [Google Scholar]
- Charles, C.; Barrat, J.A.; Pelleter, E. Trace element determinations in Fe–Mn oxides by high resolution ICP-MS after Tm addition. Talanta 2021, 233, 122446. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V.; Roy, P.; Subramanyam, K.S.V.; Durai, L.; Mohan, M.R.; Satyanarayanan, M.; Vani, K. REE geochemistry of seawater from Afanasy-Nikitin seamount in the eastern equatorial Indian Ocean by high resolution inductively coupled plasma mass spectrometry. Indian J. Geo-Mar. Sci. 2015, 44, 339–347. [Google Scholar]
- Gao, J.; Lv, D.; van Loon, A.T.; Hower, J.C.; Raji, M.; Yang, Y.; Ren, Z.; Wang, Y.; Zhang, Z. Reconstruction of provenance and tectonic setting of the Middle Jurrasic Yan’an Formation (Ordos Basin, North China) by analysis of major, trace and rare earth elements in the coals. Ore Geol. Rev. 2022, 151, 105218. [Google Scholar] [CrossRef]
- Pedreira, W.R.; Sarkis, J.E.S.; Rodrigues, C.; Queirozb, C.A.D.T.; Abrao, A. Determination of trace amounts of rare earth elements in highly pure praseodymium oxide by double focusing inductively coupled plasma mass spectrometry and high-performance liquid chromatography. J. Alloys Compd. 2001, 323–324, 49–52. [Google Scholar] [CrossRef]
- Nath, B.N.; Balaram, V.; Sudhakar, M.; Pluger, W.L. Rare earth element geochemistry of ferromanganese deposits from the Indian Ocean. Mar. Chem. 1992, 38, 185–208. [Google Scholar] [CrossRef]
- Soto-Jiménez, M.F.; Martinez-Salcido, A.I.; Morton-Bermea, O.; Ochoa-Izaguirre, M.J. Lanthanoid analysis in seawater by seaFAST-SP3™ system in off-line mode and magnetic sector high-resolution inductively coupled plasma source mass spectrometer. MethodsX 2022, 9, 101625. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Nakano, K.; Shikamori, Y.; Itoh, A. Direct determination of rare earth elements in natural water samples by inductively coupled plasma tandem quadrupole mass spectrometry with oxygen as the reaction gas for separating spectral interferences. Spectrochim. Acta Part B At. Spectrosc. 2021, 179, 106100. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Greig, A.; Collerson, K.D.; Kamber, B.S. Direct quantification of rare earth element concentrations in natural waters by ICP-MS. Appl. Geochem. 2006, 21, 839–848. [Google Scholar] [CrossRef]
- Chung, C.-H.; Brenner, I.; You, C.F. Comparison of micro-concentric and membrane desolvation sample introduction systems for determination of low rare earth element concentrations in surface and subsurface waters using sector field inductively coupled plasma mass spectrometry, Spectrochim. Acta Part B 2009, 64, 849–856. [Google Scholar] [CrossRef]
- Rousseau, T.C.C.; Sonke, J.E.; Chmeleff, F.; Candaudap, J.; Lacan, F.; Boaventura, G.; Seyler, P.; Jeandel, C. Rare earth element analysis in natural waters by multiple isotope dilution–sector field ICP-MS. J. Anal. At. Spectrom. 2013, 28, 573–584. [Google Scholar] [CrossRef]
- Yeghicheyan, D.; Carignan, J.; Valladon, M.; Coz, M.B.; Cornec, F.L.; Castrec-Rouelle, M.; Serrat, E. A Compilation of Silicon and Thirty-one Trace Elements Measured in the Natural River Water Reference Material SLRS-4 (NRC-CNRC). Geostand. Geoanalytical Res. 2001, 25, 465–474. [Google Scholar] [CrossRef]
- Balaram, V.; Copia, L.; Kumar, U.S.; Miller, J.; Chidambaram, S. Pollution of Water Resources, Causes, Application of ICP-MS Techniques in Hydrological Studies, Monitoring, and Management. Geosyst. Geoenviron. 2023, 2, 100210. [Google Scholar] [CrossRef]
- Rabieh, S.; Bayaraa, O.; Romeo, E.; Amosa, P.; Calnek, K.; Idaghdour, Y.; Ochsenkühn, M.A.; Amin, S.A.; Goldstein, G.; Bromage, T.G. MH-ICP-MS Analysis of the Freshwater and Saltwater Environmental Resources of Upolu Island, Samoa. Molecules 2020, 25, 4871. [Google Scholar] [CrossRef]
- Walder, A.J.; Freedman, P.A. Communication. Isotopic ratio measurement using a double focusing magnetic sector mass analyzer with an inductively coupled plasma as an ion source. J. Anal. At. Spectrom. 1992, 7, 571. [Google Scholar] [CrossRef]
- Balaram, V.; Rahaman, W.; Roy, P. Recent Advances in MC-ICPMS Applications in the Earth, Environmental Sciences: Challenges and Solution. Geosyst. Geoenviron. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Bai, J.-H.; Liu, F.; Zhang, Z.-F.; Ma, J.-L.; Zhang, L.; Liu, Y.-F.; Zhong, S.-X.; Wei, G.-J. Simultaneous measurement stable and radiogenic Nd isotopic compositions by MC-ICP-MS with a single-step chromatographic extraction technique. J. Anal. At. Spectrom. 2021, 36, 2695–2703. [Google Scholar] [CrossRef]
- Bai, J.H.; Lin, M.; Zhong, S.X.; Deng, Y.N.; Zhang, L.; Kai, L.; Wu, H.; Ma, J.; Wei, G. High intermediate precision Sm isotope measurements in geological samples by MC-ICP-MS. Anal. At. Spectrom. 2023, 38, 629–637. [Google Scholar] [CrossRef]
- Lee, S.G.; Ko, K.S. Development of an analytical method for accurate and precise determination of rare earth element concentrations in geological. materials using an MC-ICP-MS and group separation. Front. Chem. 2023, 10, 906160. [Google Scholar] [CrossRef]
- Kent, A.J.R.; Jacobsen, B.; Peate, D.W.; Waight, T.E.; Baker, J.A. Isotope Dilution MC-ICP-MS Rare Earth Element Analysis of Geochemical Reference Materials NIST SRM 6 10, NIST SRM 6 12, NIS T SRM 6 14, BHVO-2G, BHVO-2, BCR-2G, JB-2, WS-E, W-2, AGV-1 and AGV-2. Geostand. Geoanalytical Res. 2004, 28, 417–429. [Google Scholar] [CrossRef]
- Pourmand, A.; Dauphas, N.; Ireland, T.J. A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI-chondrite and Post-Archean Australian Shale (PAAS) abundances. Chem. Geol. 2012, 291, 38–54. [Google Scholar] [CrossRef]
- Baker, J.; Waight, T.; Ulfbeck, D. Rapid and highly reproducible analysis of rare earth elements by multiple-collector inductively coupled plasma mass spectrometry. Geochim. Cosmochim. Acta 2002, 66, 3635–3646. [Google Scholar] [CrossRef]
- Yang, X.; Kozar, D.; Gorski, D.; Marchese, A.; Pagnotti, J.; Sutterlin, R.; Rezaee, M.; Klima, M.S.; Pisupati, S.V. Using yttrium as an indicator to estimate total rare earth element concentration: A case study of anthracite-associated clays from northeastern Pennsylvania. Int. J. Coal. Sci. Technol. 2020, 7, 652–661. [Google Scholar] [CrossRef]
- Li, X.C.; Yang, K.F.; Spandler, C.; Fan, H.R.; Zhou, M.F.; Hao, J.L.; Yang, Y.H. The effect of fluid-aided modification on the Sm-Nd and Th-Pb geochronology of monazite and bastnäsite: Implication for resolving complex isotopic age data in REE ore systems. Geochim. Cosmochim. Acta 2021, 300, 1–24. [Google Scholar] [CrossRef]
- Guerra-Sommer, M.; Cazzulo-Klepzig, M.; Menegat, R.; Formoso, M.L.L.; Basei, M.S.; Barboza, E.G.; Simas, M.W. Geochronological data from the Faxinal coal succession, southern Paraná Basin, Brazil: A preliminary approach combining radiometric U-Pb dating and palynostratigraphy. J. South Am. Earth Sci. 2008, 25, 246–256. [Google Scholar] [CrossRef]
- Chafe, A.N.; Hanchar, J.M.; Fisher, C.; Piccoli, P.M.; Crowley, J.L.; Dimmell, P.M. Direct dating and characterization of the Pope’s Hill REE Deposit, Labrador. In Proceedings of the American Geophysical Union, Fall Meeting 2012, abstract id. V43C-2845, San Francisco, CA, USA, 3–7 December 2012. [Google Scholar]
- Ramesh, R.; Ramanathan, A.; Ramesh, S.; Purvaja, R.; Subramanian, V. Distribution of rare earth elements and heavy metals in the surficial sediments of the Himalayan River system. Geochem. J. 2000, 34, 295–319. [Google Scholar] [CrossRef]
- Natarajan, T.; Inoue, K.; Sahoo, S.K. Rare earth elements geochemistry and 234U/238U, 235U/238U isotope ratios of the Kanyakumari beach placer deposits: Occurrence and provenance. Minerals 2023, 13, 886. [Google Scholar] [CrossRef]
- Compston, W.; Pidgeon, R. Jack Hills, evidence of more very old detrital zircons in Western Australia. Nature 1986, 321, 766–769. [Google Scholar] [CrossRef]
- Sindern, S. Analysis of Rare Earth Elements in Rock and Mineral Samples by ICP-MS and LA-ICP-MS. Phys. Sci. Rev. 2017, 2, 2. [Google Scholar] [CrossRef]
- Campbell, L.S.; Compston, W.; Sircombe, K.N.; Wilkinson, C.C. Zircon from the East Orebody of the Bayan Obo Fe–Nb–REE deposit, China, and SHRIMP ages for carbonatite-related magmatism and REE mineralization events. Contrib. Miner. Petrol. 2014, 168, 1041. [Google Scholar] [CrossRef]
- Bhunia, S.; Rao, N.V.C.; Belyatsky, B.; Talukdar, D.; Pandey, R.; Lehmann, B. U-Pb Zircon SHRIMP dating of the Carbonatite hosted REE deposit (Kamthai), Late Cretaceous polychronous Sarnu Dandali alkaline Complex, NW India: Links to the Plume-related metallogeny and CO2 outgassing at the K-Pg boundary. Gondwana Res. 2022, 112, 116–125. [Google Scholar] [CrossRef]
- Sano, Y.; Terada, K.; Fukuoka, T. High mass resolution ion microprobe analysis of rare earth elements in silicate glass, apatite and zircon: Lack of matrix dependency. Chem. Geol. 2002, 184, 217–230. [Google Scholar] [CrossRef]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Hong, J.; Khan, T.; Li, W.; Khalil, Y.S.; Narejo, A.A.; Rashid, M.U.; Zeb, M.J. SHRIMP U–Pb ages, mineralogy, and geochemistry of carbonatite–alkaline complexes of the Sillai Patti and Koga areas, NW Pakistan: Implications for petrogenesis and REE mineralization. Ore Geol. Rev. 2021, 139, 104547. [Google Scholar] [CrossRef]
- Balaram, V.; Sawant, S.S. Indicator Minerals, Pathfinder Elements, and Portable Analytical Instruments in Mineral Exploration Studies. Minerals 2022, 12, 394. [Google Scholar] [CrossRef]
- Jo, J.; Shin, D. Geochemical characteristics of REE-enriched weathered anorthosite complex in Hadong district, South Korea. Geochem. J. 2023, 57, 13–27. [Google Scholar] [CrossRef]
- Villanova-de-Benavent, C.; Proenza, J.A.; Torró, L.; Aiglsperger, T.; Domènech, C.; Domínguez-Carretero, D.; Llovet, X.; Suñer, P.; Ramírez, A.; Rodríguez, J. REE ultra-rich karst bauxite deposits in the Pedernales Peninsula, Dominican Republic: Mineralogy of REE phosphates and carbonates. Ore Geol. Rev. 2023, 157, 105422. [Google Scholar] [CrossRef]
- Kumar, O.P.; Gopinathan, P.; Naik, A.S.; Subramani, T.; Singh, P.K.; Sharma, A.; Maity, S.; Saha, S. Characterization of lignite deposits of Barmer Basin, Rajasthan: Insights from mineralogical and elemental analysis. Environ. Geochem. Health 2023, 1–23. [Google Scholar] [CrossRef]
- Reed, S.J.B.; Buckley, A. Rare-earth element determination in minerals by electron-probe microanalysis: Application of spectrum synthesis. Mineral. Mag. 1998, 62, 1–8. [Google Scholar]
- Wu, L.; Ma, L.; Huang, G.; Li, J.; Xu, H. Distribution and Speciation of Rare Earth Elements in Coal Fly Ash from the Qianxi Power Plant, Guizhou Province, Southwest China. Minerals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Sano, Y.; Terada, K.; Hidaka, H.; Nishio, Y.; Amakawa, H.; Nozaki, Y. Ion-Microprobe Analysis of Rare Earth Elements in Oceanic Basalt Glass. Anal. Sci. 1999, 15, 743–748. [Google Scholar] [CrossRef]
- Bottazzi, P.; Ottolini, L.; Vannucci, R. SIMS analyses of rare earth elements in natural minerals and glasses: An investigation of structural matrix effects on ion yields. Scanning 1992, 14, 160–168. [Google Scholar] [CrossRef]
- Zinner, E.; Crozaz, G. A method for the quantitative measurement of rare earth elements in the ion microprobe. Int. J. Mass Spectrom. Ion Process. 1986, 69, 17–38. [Google Scholar] [CrossRef]
- Shi, L.; Sano, Y.; Takahata, N.; Koike, M.; Morita, T.; Koyama, Y.; Kagoshima, T.; Li, Y.; Xu, S.; Liu, C. NanoSIMS Analysis of Rare Earth Elements in Silicate Glass and Zircon: Implications for Partition Coefficients. Front. Chem. 2022, 10, 844953. [Google Scholar] [CrossRef]
- Ling, X.X.; Li, Q.L.; Liu, Y.; Yang, Y.H.; Tang, G.Q.; Li, X.H. In situ SIMS Th–Pb dating of bastnaesite: Constraint on the mineralization time of the Himalayan Mianning–Dechang rare earth element deposits. J. Anal. At. Spectrom. 2016, 31, 1680. [Google Scholar] [CrossRef]
- Sahijpal, S.; Marhas, K.K.; Goswami, J.N. Analytical procedures devised for measurement of rare earth element (REE) abundances using a secondary ion mass spectrometer (ion microprobe) are described. Proc. Indian Acad. Sci. (Earth Planet. Sci.) 2003, 112, 485–498. [Google Scholar]
- Singh, S.P.; Balaram, V.; Satyanarayanan, M.; Sarma, D.S.; Subramanyam, K.S.V.; Anjaiah, K.V.; Kharia, A. Platinum group minerals from the Madawara ultramafic–mafic complex, Bundelkhand Massif, Central India: A preliminary note. J. Geol. Soc. India 2011, 78, 281–283. [Google Scholar]
- Pan, J.; Zhang, L.; Wen, Z.; Nie, T.; Zhang, T.; Zhou, C. The Mechanism Study on the Integrated Process of NaOH Treatment and Citric Acid Leaching for Rare Earth Elements Recovery from Coal Fly Ash. J. Environ. Chem. Eng. 2023, 11, 109921. [Google Scholar] [CrossRef]
- Li, X.; Qiao, X.; Chen, D.; Wu, P.; Xie, Y.; Chen, X. Anomalous concentrations of rare earth elements in acid mine drainage and implications for rare earth resources from late Permian coal seams in northern Guizhou. Sci. Total Environ. 2023, 879, 163051. [Google Scholar] [CrossRef]
- Van Rythoven, A.D.; Pfaff, K.; Clark, J.G. Use of QEMSCAN® to characterize oxidized REE ore from the Bear Lodge carbonatite, Wyoming, USA. Ore Energy Resour. Geol. 2020, 2–3, 100005. [Google Scholar] [CrossRef]
- Gray, A.L. Solid sample introduction by laser ablation for inductively coupled plasma source mass spectrometry. Analyst 1985, 110, 551–556. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Jiang, S.-Y.; Chen, W.; Wang, C.Y.; Su, H.-M.; Cao, Y.; Zhang, H.-X.; Li, W.-T. Precise determination of major and trace elements in micrometer-scale ilmenite lamellae in titanomagnetite using LA-ICP-MS technique: Application of regression analysis to time-resolved signals. RSC Adv. 2023, 13, 13303. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Xu, X.; Song, Z.; Dong, X.; Tian, J.; Yang, Y.; She, H.; Xiang, A.; Kang, Y. Sm-Nd dating and REE Composition of scheelite for the Honghuaerji scheelite deposit, Inner Mongolia, Northeast China. Lithos 2016, 261, 307–321. [Google Scholar] [CrossRef]
- Mohanty, S.; Papadopoulos, A.; Petreli, M.; Papadopoulou, L.; Sengupta, D. Geochemical Studies of Detrital Zircon Grains from River Bank and Beach Placers of Coastal Odisha, India. Minerals 2023, 13, 192. [Google Scholar] [CrossRef]
- Jiu, B.; Huang, W.; Spiro, B.; Hao, R.; Mu, N.; Wen, L.; Hao, H. Distribution of Li, Ga, Nb, and REEs in coal as determined by LA-ICP-MS imaging: A case study from Jungar coalfield, Ordos Basin, China. Int. J. Coal Geol. 2023, 267, 104184. [Google Scholar] [CrossRef]
- Chi, G.; Potter, E.G.; Petts, D.C.; Jackson, S.; Chu, H. LA-ICP-MS Mapping of Barren Sandstone from the Proterozoic Athabasca Basin (Canada)—Footprint of U- and REE-Rich Basinal Fluids. Minerals 2022, 12, 733. [Google Scholar] [CrossRef]
- Oostingh, K. Analysis of Rare Earth Element Concentrations in Barite (BaSO4). Master’s Thesis, Department Earth Sciences, Utrecht University, Utrecht, The Netherlands, 2011; pp. 1–96. [Google Scholar]
- Liu, Y.S.; Hu, Z.C.; Li, M.; Gao, S. Applications of LA-ICP-MS in the elemental analyses of geological samples. Chin. Sci. Bull. 2013, 58, 3863–3878. [Google Scholar] [CrossRef]
- Maruyama, S.; Hattori, K.; Hirata, T.; Suzuki, T.; Danhara, T. Simultaneous determination of 58 major and trace elements in volcanic glass shards from the INTAV sample mount using femtosecond laser ablation-inductively coupled plasma-mass spectrometry. Geochem. J. 2016, 50, 403–422. [Google Scholar] [CrossRef]
- Wu, S.T.; Wang, H.; Yang, Y.H.; Niu, J.; Lan, Z.; Zhang, L.L.; Huang, C.; Xie, L.W.; Xu, L.; Yang, J.H.; et al. In situ Lu-Hf geochronology with LA-ICP-MS/MS analysis. J. Anal. At. Spectrom. 2023, 38, 1285–1300. [Google Scholar] [CrossRef]
- Ham-Meert, A.V.; Bolea-Fernandez, E.; Belza, J.; Bevan, D.; Jochum, K.P.; Neuray, B.; Stoll, B.; Vanhaecke, F.; Van Wersch, L. Comparison of Minimally Invasive Inductively Coupled Plasma–Mass Spectrometry Approaches for Strontium Isotopic Analysis of Medieval Stained Glass with Elevated Rubidium and Rare-Earth Element Concentrations. ACS Omega 2021, 6, 18110–18122. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.L.; Gao, S.; Dai, M.N.; Zong, C.L.; Gunther, D.; Fontaine, G.H.; Diwu, C. Simultaneous determinations of U–Pb age, Hf isotopes and trace element com-positions of zircon by excimer laser ablation quadrupole and multiple-collector ICP-MS. Chem. Geol. 2008, 247, 100–118. [Google Scholar] [CrossRef]
- Qian, S.P.; Zhang, L. Simultaneous in situ determination of rare earth element concentrations and Nd isotope ratio in apatite by laser ablation ICP-MS. Geochem. J. 2019, 53, 319–328. [Google Scholar] [CrossRef]
- Kylander-Clark, A.R.C.; Hacker, B.R.; Cottle, J.M. Laser-ablation split-stream ICP petrochronology. Chem. Geol. 2013, 345, 99–112. [Google Scholar] [CrossRef]
- Simandl, G.J.; Fajber, R.; Paradis, S. Portable X-ray fluorescence in the assessment of rare earth element enriched sedimentary phosphate deposits. Geochem. Explor. Environ. Anal. 2014, 14, 161–169. [Google Scholar] [CrossRef]
- Fajber, R.; Simandl, G.J. Evaluation of Rare Earth Element-enriched Sedimentary Phosphate Deposits Using Portable X-ray Fluorescence (XRF) Instruments. In Geological Fieldwork 2011; Geological Survey of Canada: Sidney, BC, Canada, 2012; pp. 199–210. [Google Scholar]
- Sukadana, I.G.; Warmada, W.I.; Pratiwi, F.; Harijoko, A.; Adimedha, T.B.; Yogatama, A.W. Elemental Mapping for Characterizing of Thorium and Rare Earth Elements (REE) Bearing Minerals Using μXRF. At. Indones. 2022, 48, 2. [Google Scholar] [CrossRef]
- Gibaga, C.R.L.; Montano, M.O.; Samaniego, J.O.; Tanciongco, A.M.; Quierrez, R.N.M. Comparative Study on Determination of Selected Rare Earth Elements (REEs) in Ion Adsorption Clays Using Handheld LIBS and ICP-MS. Philipp. J. Sci. 2022, 151, 1599–1604. [Google Scholar] [CrossRef]
- Bellie, V.; Gokulraju, R.; Rajasekar, C.; Vinoth, S.; Mohankumar, V.; Gunapriya, B. Laser induced Breakdown Spectroscopy for new product development in mining industry. Mater. Today Proc. 2021, 45, 8157–8161. [Google Scholar] [CrossRef]
- Gerardo, S.; Davletshin, A.R.; Loewy, S.L.; Song, W. From Ashes to Riches: Microscale Phenomena Controlling Rare Earths Recovery from Coal Fly Ash, Environ. Sci. Technol. 2022, 56, 16200–16208. [Google Scholar] [CrossRef]
- Brewer, P.G.; Malby, G.; Pasteris, J.D. Development of a laser Raman spectrometer for deep-ocean science. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2004, 51, 739–753. [Google Scholar] [CrossRef]
- Moroz, T.N.; Edwards, H.G.M.; Zhmodik, S.M. Detection of carbonate, phosphate minerals and cyanobacteria in rock from the Tomtor deposit, Russia, by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119372. [Google Scholar] [CrossRef]
- Ye, X.; Bai, F. Spectral Characteristics, Rare Earth Elements, and Ore-Forming Fluid Constrains on the Origin of Fluorite Deposit in Nanlishu, Jilin Province, China. Minerals 2022, 12, 1195. [Google Scholar] [CrossRef]
- Davletshina, N.; Ermakova, E.; Dolgova, D.; Davletshin, R.; Ivshin, K.; Fedonin, A.; Stoikov, I.; Cherkasov, R. Structure and FT-IR spectroscopic analyses of complexes phosphorylated betaines with rare earth metal ions. Inorganica Chim. Acta 2023, 545, 121245. [Google Scholar] [CrossRef]
- Liu, H.; Yu, T.; Hu, B.; Hou, X.; Zhang, Z.; Liu, X.; Liu, J.; Wang, X.; Zhong, J.; Tan, Z.; et al. UAV-Borne Hyperspectral Imaging Remote Sensing System Based on Acousto-Optic Tunable Filter for Water Quality Monitoring. Remote Sens. 2021, 13, 4069. [Google Scholar] [CrossRef]
- Qasim, M.; Khan, S.D. Detection and Relative Quantification of Neodymium in Sillai Patti Carbonatite Using Decision Tree Classification of the Hyperspectral Data. Sensors 2022, 22, 7537. [Google Scholar] [CrossRef]
- Boesche, N.K.; Rogass, C.; Lubitz, C.; Brell, M.; Herrmann, S.; Mielke, C.; Tonn, S.; Appelt, O.; Altenberger, U.; Kaufmann, H. Hyperspectral REE (Rare Earth Element) Mapping of Outcrops—Applications for Neodymium Detection. Remote Sens. 2015, 7, 5160–5186. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Tangestani, M.H. Potential of Sentinel-2 MSI data in targeting rare earth element (Nd3+) bearing minerals in Esfordi phosphate deposit, Iran. Egypt. J. Remote Sens. Space Sci. 2022, 25, 697–710. [Google Scholar] [CrossRef]
- Booysen, R.; Jackisch, R.; Lorenz, S.; Zimmermann, R.; Kirsch, M.; Nex, P.A.M.; Gloaguen, R. Detection of REEs with lightweight UAV-based hyperspectral imaging. Sci. Rep. 2020, 10, 17450. [Google Scholar] [CrossRef]
- Maia, A.J.; da Silva, Y.J.A.B.; do Nascimento, C.W.A.; Veras, G.; Escobar, M.; Cunha, C.S.M.; da Silva, Y.J.A.B.; Nascimento, R.C.; de Souza, P.L.H. Near-infrared spectroscopy for the prediction of rare earth elements in soils from the largest uranium-phosphate deposit in Brazil using PLS, iPLS, and iSPA-PLS models. Env. Monit Assess. 2020, 192, 675. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, T.; Pan, X. Potential of visible and near-infrared reflectance spectroscopy for the determination of rare earth elements in soil. Geoderma 2017, 306, 120–126. [Google Scholar] [CrossRef]
- Turner, D.J.; Rivard, B.; Groat, L. Visible and short-wave infrared reflectance spectroscopy of selected REE-bearing silicate minerals. Am. Mineral. 2018, 103, 927–943. [Google Scholar] [CrossRef]
- Rocha, D.L.; Maringolo, V.; Araújo, A.N.; Amorim, C.M.P.G.; Montenegro, M.D.C.B.S.M. An overview of Structured Biosensors for Metal Ions Determination. Chemosensors 2021, 9, 324. [Google Scholar] [CrossRef]
- Featherston, E.R.; Issertell, E.J.; Cotruvo, J.A., Jr. Probing Lanmodulin’s Lanthanide Recognition via Sensitized Luminescence Yields a Platform for Quantification of Terbium in Acid Mine Drainage. J. Am. Chem. Soc. 2021, 143, 14287–14299. [Google Scholar] [CrossRef]
- Cruickshank, L.; Officer, S.; Pollard, P.; Prabhu, R.; Stutter, M.; Fernandez, C. Rare elements electrochemistry: The development of a novel electrochemical sensor for the rapid detection of europium in environmental samples using gold electrode modified with 2-pyridinol-1-oxide. Anal. Sci. 2015, 31, 623–627. [Google Scholar] [CrossRef]
- Shehu, G.; Bagudo, I.M. Mineralogical and Structural Analyses of Natural Fluorite from Yantuwaru Mining Site, Nigeria. UMYU Sci. 2023, 2, 43–51. Available online: https://scientifica.umyu.edu.ng/ (accessed on 5 June 2023).
- Xiong, X.; Jiang, T.; Qi, W.; Zuo, J.; Yang, M.; Fei, Q.; Xiao, S.; Yu, A.; Zhu, Z.; Chen, H. Some Rare Earth Elements Analysis by Microwave Plasma Torch Coupled with the Linear Ion Trap Mass Spectrometry. Int. J. Anal. Chem. 2015, 2015, 156509. [Google Scholar] [CrossRef]
- Yuan, L.; Zhou, X.; Cao, Y.; Yan, N.; Peng, L.; Lai, X.; Tao, H.; Jiang, T.; Li, L.; Zhu, Z. Microwave Plasma Torch Mass Spectrometry for some Rare Earth Elements. Arab. J. Chem. 2022, 15, 104379. [Google Scholar] [CrossRef]
- Fayyaz, A.; Ali, R.; Waqas, M.; Liaqat, U.; Ahmad, R.; Umar, Z.A.; Baig, M.A. Analysis of Rare Earth Ores Using Laser-Induced Breakdown Spectroscopy and Laser Ablation Time-of-Flight Mass Spectrometry. Minerals 2023, 13, 787. [Google Scholar] [CrossRef]
- Maia, A.J.; Nascimento, R.C.; da Silva, Y.J.A.B.; Nascimento, C.W.A.D.; Mendes, W.D.S.; Neto, J.G.V.; Filho, J.C.D.A.; Tiecher, T. Near-infrared spectroscopy for prediction of potentially toxic elements in soil and sediments from a semiarid and coastal humid tropical transitional river basin. Microchem. J. 2022, 179, 107544. [Google Scholar] [CrossRef]
- Imashuku, S. Rapid determination of the approximate content of bastnäsite in ores using cathodoluminescence imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122055. [Google Scholar] [CrossRef]
- Duplouy, C. Preliminary Investigation of Rare Earth Elements Ion Exchange on Zeolites. Master’s Thesis, Department of Chemistry, University of Helsinki, Helsinki, Finland, 2016; pp. 1–57. [Google Scholar]
- Borst, A.M.; Smith, M.P.; Finch, A.A.; Estrade, G.; Villanova-de-Benavent, C.; Nason, P.; Marquis, E.; Horsburgh, N.J.; Goodenough, K.M.; Xu, C.; et al. Adsorption of rare earth elements in regolith-hosted clay deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef] [PubMed]
- Obhodaš, J.; Sudac, D.; Meric, I.; Pettersen, H.E.S.; Uroić, M.; Nađ, K.; Valković, V. In-situ measurements of rare earth elements in deep sea sediments using nuclear methods. Sci. Rep. 2018, 8, 4925. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Deep-sea mineral deposits as a source of critical metals for high-and green-technology applications. Miner. Miner. Mater. 2023, 2, 5. [Google Scholar] [CrossRef]
- Stuckman, M.Y.; Lopano, C.L.; Granite, E.J. Distribution and speciation of rare earth elements in coal combustion by-products via synchrotron microscopy and spectroscopy. Int. J. Coal Geol. 2018, 195, 125–138. [Google Scholar] [CrossRef]
- Jochum, K.P.; Seufert, H.M.; Midinet-Best, S.; Rettmann, E.; Schiinberger, K.; Zimmer, M. Multi-element analysis by isotope dilution-spark source mass spectrometry (ID-SSMS). Fresenius J. Anal Chem. 1988, 331, 104–110. [Google Scholar] [CrossRef]
- Zhao, J.; Xing, Y.; Ge, L.; Wang, L.; Li, T.; Zhang, Q.; Wu, H.; Li, W.; Liu, Y. Direct analysis of lanthanum in extraction process by in-situ gamma spectrometry. J. Radioanal. Nucl. Chem. 2022, 331, 3807–3817. [Google Scholar] [CrossRef]
- Shen, S.; Krogstad, E.; Conte, E.; Brown, C. Rapid unseparated rare earth element analyses by isotope dilution multi-collector inductively coupled plasma mass spectrometry (ID-MC-ICP-MS). Int. J. Mass Spectrom. 2022, 471, 116726. [Google Scholar] [CrossRef]
- Folkedahl, B.; Nyberg, C.; Biswas, S.; Zhang, X. Round-Robin Interlaboratory Study on Rare-Earth Elements in 2 U.S.-Based Geologic Materials. Minerals 2023, 13, 944. [Google Scholar] [CrossRef]
- Ardini, F.; Soggia, F.; Rugi, F.; Udisti, R.; Grotti, M. Comparison of inductively coupled plasma spectrometry techniques for the direct determination of rare earth elements in digests from geological samples. Anal. Chim. Acta 2010, 678, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zuma, M.C.; Lakkakula, J.; Mketo, N. Recent trends in sample preparation methods and plasma-based spectrometric techniques for the determination of rare earth elements in geological and fossil fuel samples. Appl. Spectrosc. Rev. 2020, 57, 353–377. [Google Scholar] [CrossRef]
- Balaram, V.; Subramanyam, K.S.V. Sample Preparation for Geochemical Analysis: Strategies and Significance. Adv. Sample Prep. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Xuan, H.; Zi-hui, C.; Shu-chao, Z.; Lei, S. Matrix Separation-Determination of Rare Earth Oxides in Bauxite by Inductively Coupled Plasma-Atomic Emission Spectrometry. Spectrosc. Spectr. Anal. 2022, 42, 3130–3134. [Google Scholar] [CrossRef]
- Schramm, R. Use of X-ray Fluorescence Analysis for the Determination of Rare Earth Elements. Phys. Sci. Rev. 2016, 1, 20160061. [Google Scholar] [CrossRef]
- Lett, R.E.; Paterson, K. A Comparison of Several Commercially Available Methods for the Geochemical Analysis of Rare Earth, Rare Metal and High Field Strength Elements in Geological Samples. In Geological Fieldwork 2010; British Columbia Geological Survey Paper 2011-1; British Columbia Geological Survey: Victoria, BC, Canada, 2011; pp. 181–188. [Google Scholar]
- Hanchar, J.M.; Finch, R.J.; Hoskin, P.W.O.; Watson, E.B.; Cherniak, D.J.; Mariano, A.N. Rare earth elements in synthetic zircon: Part 1. Synthesis, and rare earth element and phosphorus doping. Am. Mineral. 2001, 86, 5. [Google Scholar] [CrossRef]
- Udayakumar, S.; Baharun, N.; Rezan, S.A. Microwave-Assisted Acid Digestion of Malaysian Monazite for Determination of REEs Using ICP-MS. Key Eng. Mater. 2022, 908, 481–486. [Google Scholar] [CrossRef]
- He, H.; Zhao, X.; Zhang, Y.; Zhao, L.; Hu, R.; Li, L. Determination of rare earth elements in uranium ores by ICP-MS after total dissolution with NH4F and matrix separation with TRU resin. J. Radioanal. Nucl. Chem. 2023, 332, 1909–1916. [Google Scholar] [CrossRef]
- Kasar, S.; Murugan, R.; Arae, H.; Aono, T.; Sahoo, S.K. A Microwave Digestion Technique for the Analysis of Rare Earth Elements, Thorium and Uranium in Geochemical Certified Reference Materials and Soils by Inductively Coupled Plasma Mass Spectrometry. Molecules 2020, 25, 5178. [Google Scholar] [CrossRef]
- Roy, P.; Balaram, V.; Kumar, A.; Satyanarayanan, M.; Rao, T.G. New REE and Trace Element Data on Two International Kimberlitic Reference Materials by ICP-MS. Geostand. Geoanalytical Res. 2007, 31, 261–273. [Google Scholar] [CrossRef]
- Balaram, V. Microwave dissolution techniques for the analysis of geological materials by ICP-MS. Curr. Sci. 1997, 73, 1019–1023. [Google Scholar]
- Zuma, M.C.; Nomngongo, P.N.; Mketo, N. Simultaneous Determination of REEs in Coal Samples Using the Combination of Microwave-Assisted Ashing and Ultrasound-Assisted Extraction Methods Followed by ICP-OES Analysis. Minerals 2021, 11, 1103. [Google Scholar] [CrossRef]
- Balaram, V.; Satyanarayanan, M. Data Quality in Geochemical Elemental and Isotopic Analysis. Minerals 2022, 12, 999. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Zhou, J.; Yang, J.; Deng, J.; Shao, J.; Zheng, T.-F.; Ke, Y.; Long, T. Preparation of REE-doped NaY(WO4)2 single crystals for quantitative determination of rare earth elements in REE:NaY(WO4)2 laser crystals by LA-ICP-MS. Anal. Methods 2022, 14, 4085–4094. [Google Scholar] [CrossRef] [PubMed]
- Akhmetzhanova, T.F.; Popov, A.M. Direct determination of lanthanides by LIBS in REE-rich ores: Comparison between univariate and DoE based multivariate calibrations with respect to spectral resolution. J. Anal. At. Spectrom. 2022, 37, 2330–2339. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Antweiler, R.; CNordstrom, D.K.; Taylor, H.E. Standard reference water samples for rare earth element determinations. Appl. Geochem. 2001, 16, 231–244. [Google Scholar] [CrossRef]

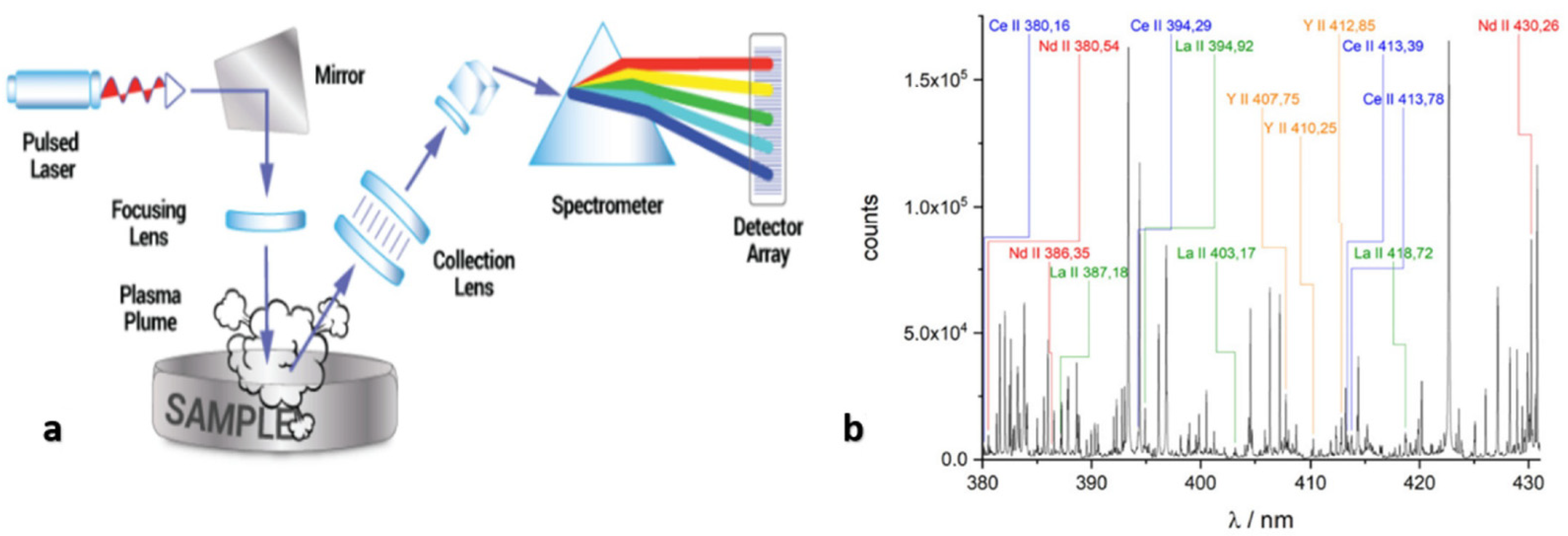
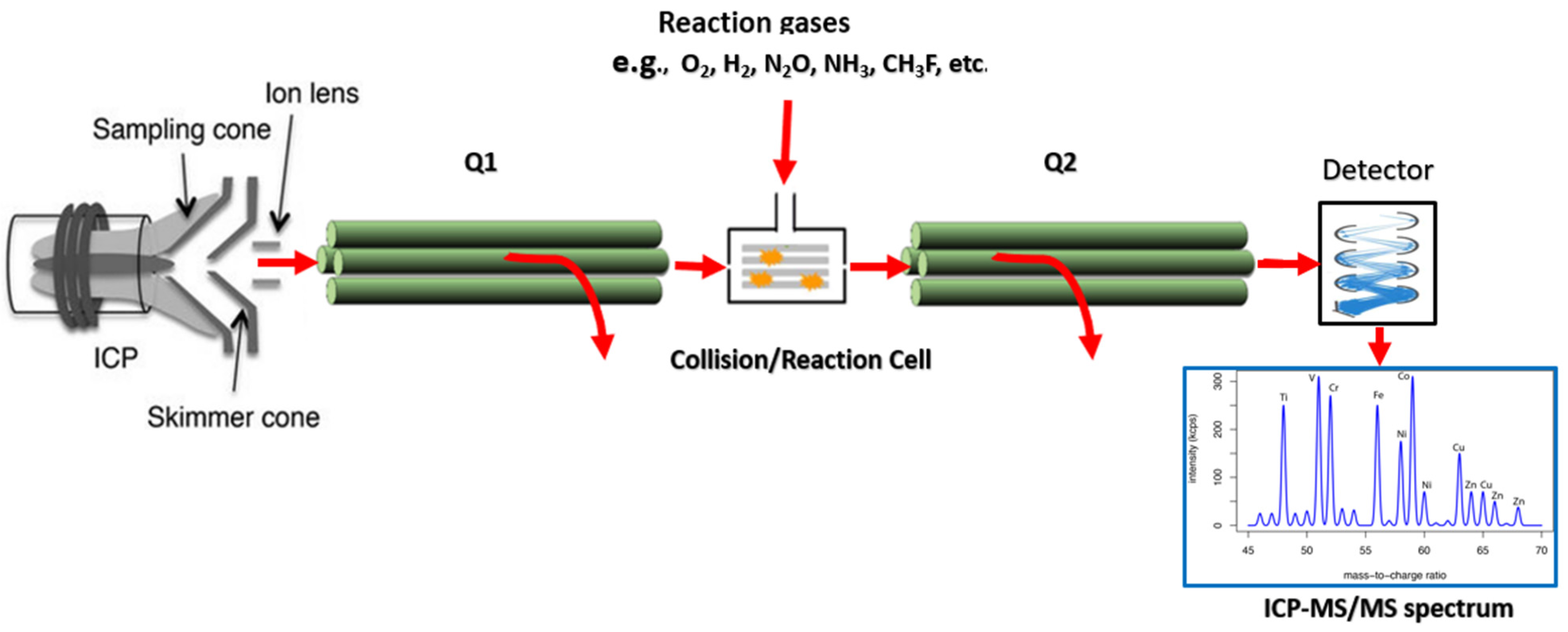
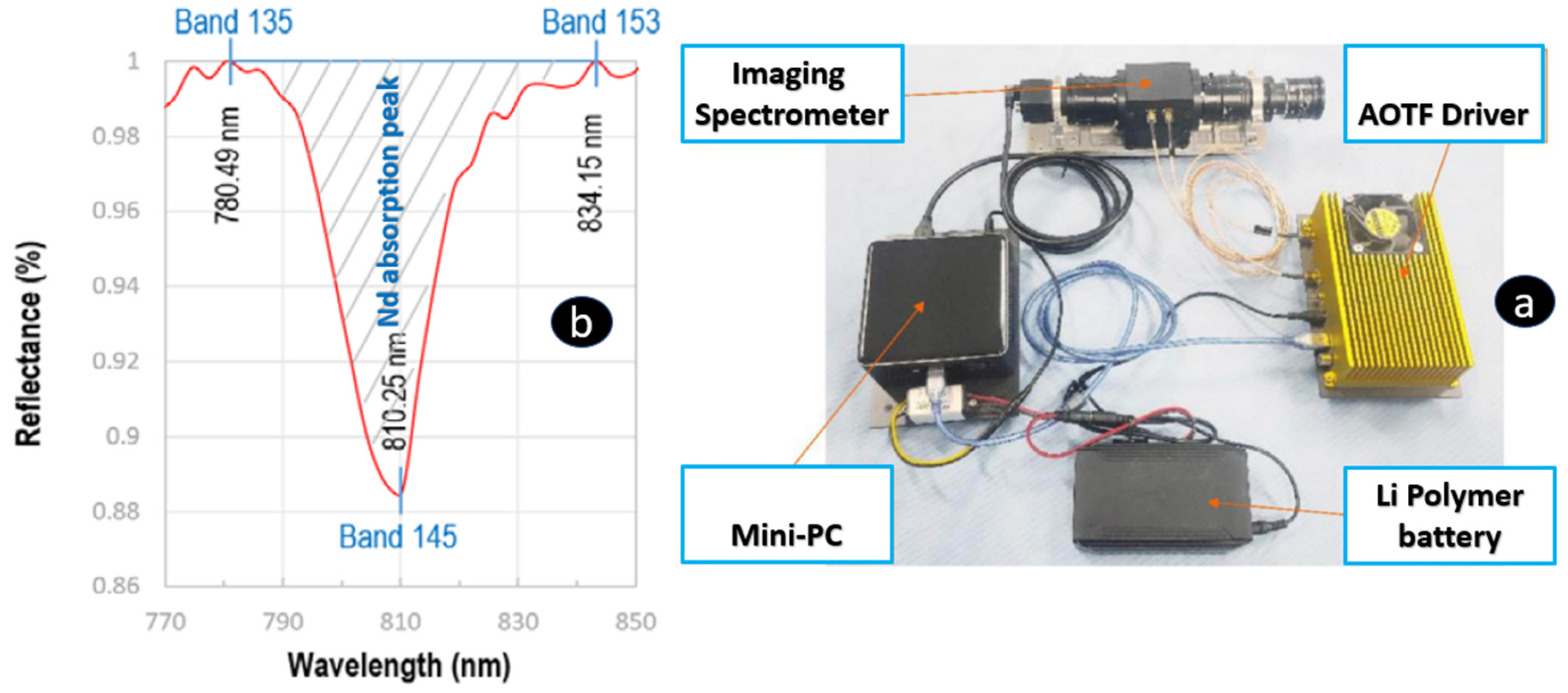
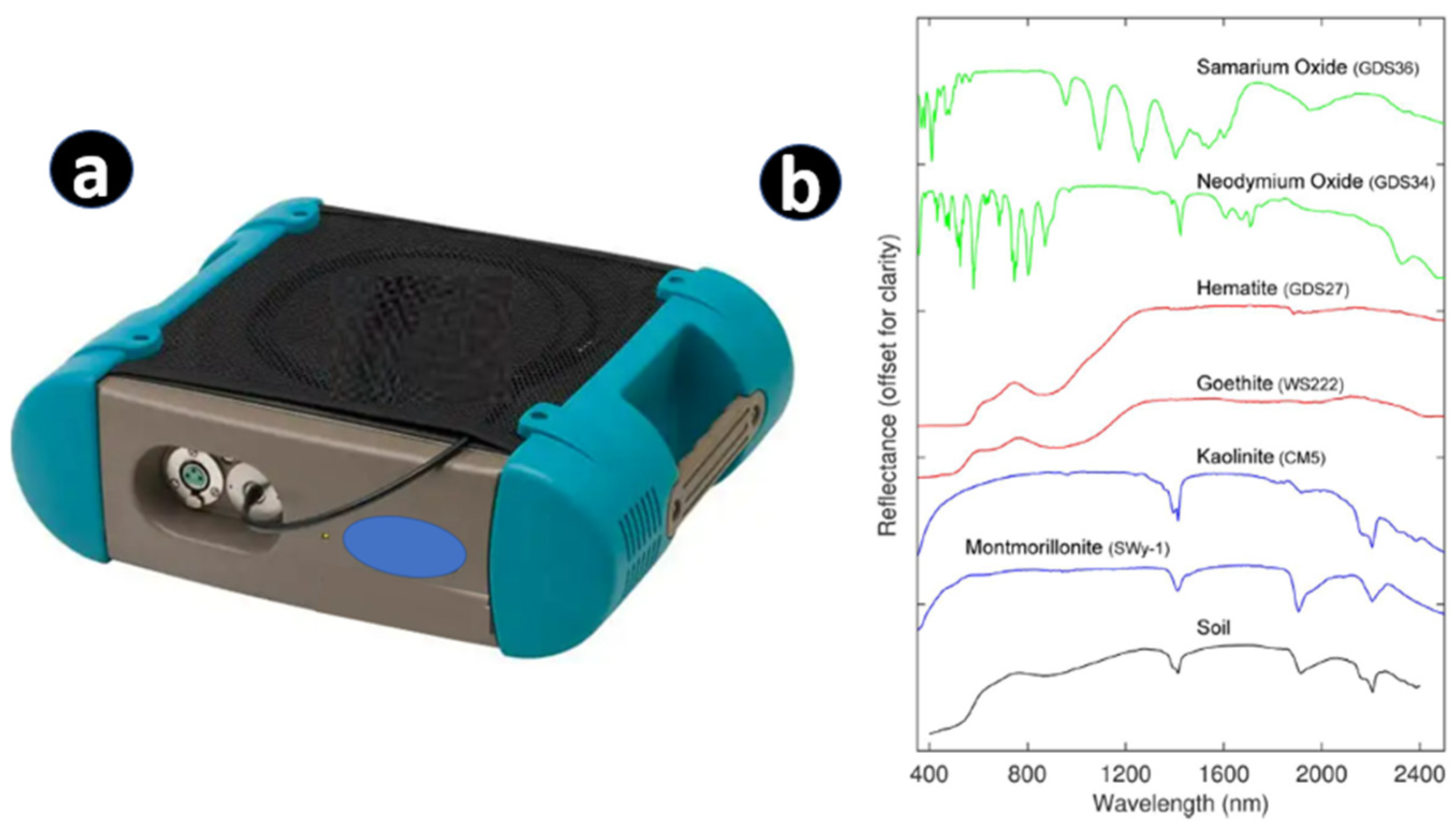
| REE | Concentration (µg/g) | |
|---|---|---|
| INAA Value | Certified Value | |
| La | 264 ± 25 | 260 ± 9 |
| Ce | 460 ± 45 | 463 ± 20 |
| Pr | 47.6 ± 5.7 | 47.1 ± 2.4 |
| Nd | 145 ± 14 | 152 ± 8 |
| Sm | 24.0 ± 2.3 | 23.6 ± 0.4 |
| Eu | 3.64 ± 0.43 | 3.71 ± 0.23 |
| Gd | 23.6 ± 3.0 | 23.6 ± 1.4 |
| Tb | 3.71 ± 0.32 | 3.80 ± 0.23 |
| Dy | 23.7 ± 2.8 | 23.2 ± 0.4 |
| Ho | 4.89 ± 0.45 | 4.81 ± 0.14 |
| Er | 15.4 ± 1.9 | 14.9 ± 0.5 |
| Tm | 2.33 ± 0.21 | 2.31 ± 0.11 |
| Yb | 15.7 ± 1.3 | 14.9 ± 0.4 |
| Lu | 2.30 ± 0.20 | 2.26 ± 0.11 |
| Sc | 6.68 ± 0.50 | 6.10 |
| Y (ED-XRF) | 132 ± 23 | 142 ± 3 |
| REE | ICP-MS [78] (ng/mL) | ICP-MS/MS [79] (pg/mL) | HR-ICP-MS [80] (pg/mL) | MH-ICP-MS [81] (ng/mL) | ICP-OES [74] (µg/mL) | LA-HR-ICP-MS [82] (µg/g) | INAA [54] (µg/g) | LIBS [34] (µg/g) | GD-MS [83] (ng/g) |
|---|---|---|---|---|---|---|---|---|---|
| La | 910 | 0.12 | 0.15 | 0.005 | 1.1 | 0.002 | 0.3 | 160 | 5.6 |
| Ce | 260 | 0.15 | 0.33 | 0.007 | 1.6 | 0.01 | 0.9 | 285 | 1.5 |
| Pr | 3 | 0.16 | 0.09 | <0.001 | 1.2 | 0.003 | - | - | 5.6 |
| Nd | 10 | 0.14 | 1.06 | 0.003 | 2.4 | 0.005 | 0.6 | 414 | 4.5 |
| Sm | 5 | 0.16 | 0.50 | 0.005 | 2.8 | 0.002 | 0.04 | - | 4.6 |
| Eu | 10 | 0.19 | 0.35 | 0.003 | 0.8 | 0.001 | 0.02 | - | 2.0 |
| Gd | 4 | 0.13 | 0.97 | 0.009 | 1.1 | 0.006 | - | - | - |
| Tb | 1 | 0.17 | 0.09 | 0.001 | 2.3 | - | 0.05 | - | - |
| Dy | 5 | 0.08 | 0.16 | 0.006 | 1.4 | 0.006 | - | - | 3.6 |
| Ho | 3 | 0.18 | 0.04 | 0.003 | 0.8 | 0.0009 | - | - | 0.4 |
| Er | 4 | 0.15 | 0.10 | 0.001 | 0.5 | 0.006 | - | - | 0.7 |
| Tm | 2 | 0.15 | 0.05 | 0.001 | 0.5 | - | - | - | 0.3 |
| Yb | 10 | 0.13 | 0.12 | 0.006 | 0.1 | 0.008 | 0.08 | - | - |
| Lu | 1 | 0.15 | 0.05 | 0.002 | 0.1 | 0.001 | 0.03 | - | 1.4 |
| Sc | 60 | - | 17.9 | 0.006 | - | - | - | - | 0.5 |
| Y | 170 | - | 0.38 | 0.003 | 0.1 | 0.003 | - | 227 | 0.6 |
| REE | Na2O2 Fusion/ICP-MS/MS | INAA | Certified Value |
|---|---|---|---|
| La | 26 ± 4 | 29.4 ± 0.8 | 27 ± 2 |
| Ce | 50 ± 8 | 58 ± 3 | 54 ± 6 |
| Pr | 5.7 ± 0.9 | - | - |
| Nd | 21 ± 3 | 28 ± 2 | 26 |
| Sm | 3.9 ± 0.5 | 4.9 ± 0.3 | 4.9 ± 0.2 |
| Eu | 1.2 ± 0.2 | 1.38 ± 0.06 | 1.43 ± 0.12 |
| Gd | 3.7 ± 0.5 | - | - |
| Tb | 0.7 ± 0.1 | 0.67 ± 0.05 | 0.71 ± 0.07 |
| Dy | 3.3 ± 0.4 | 4.8 ± 0.5 | 3.8 ± 0.3 |
| Ho | 0.73 ± 0.15 | - | - |
| Er | 2.0 ± 0.2 | - | - |
| Tm | 0.30 ± 0.06 | - | 0.37 ± 0.04 |
| Yb | 2.1 ± 0.3 | 2.7 ± 0.3 | 2.3 ± 0.2 |
| Lu | 0.31 ± 0.07 | 0.37 ± 0.03 | 0.37 ± 0.4 |
| Sc | - | 9.7 ± 0.3 | - |
| Y | 22 ± 4 | - | 24 ± 3 |
| REE | Nod A-1 (µg/g) | Nod P1 (µg/g) | ||||
|---|---|---|---|---|---|---|
| ICP-MS Value [130] | HR-ICP-MS Value [126] | ICP-MS Value Nath et al., 1992 [130] | HR-ICP-MS Value [126] | |||
| LR | HR | LR | HR | |||
| La | 115 | 111.2 | 110.0 | 105 | 106.4 | 107.1 |
| Ce | 656 | 745 | 740 | 318 | 319 | 321 |
| Pr | 21.7 | 23.85 | 23.79 | 27.5 | 23.85 | 23.79 |
| Nd | 94 | 99.55 | 99.45 | 114 | 132.2 | 134.5 |
| Sm | 20.4 | 21.79 | 21.73 | 27.2 | 31.87 | 32.41 |
| Eu | 6.10 | 5.28 | 5.41 | 7.44 | 7.68 | 7.97 |
| Gd | 23.6 | 24.28 | 24.75 | 33.8 | 30.28 | 30.85 |
| Tb | 4.20 | 3.84 | 3.90 | 4.53 | 4.71 | 4.74 |
| Dy | 25.80 | 23.06 | 22.92 | 25.99 | 26.29 | 27.03 |
| Ho | 5.09 | 4.96 | 5.02 | 4.73 | 5.00 | 5.16 |
| Er | 15.6 | 14.31 | 14.52 | 13.3 | 13.42 | 12.70 |
| Tm | 2.19 | - | - | 1.72 | - | - |
| Yb | 15.40 | 13.48 | 13.69 | 13.26 | 12.70 | 13.08 |
| Lu | 2.21 | 2.08 | 2.14 | 1.75 | 1.82 | 1.93 |
| %RSD | <5.0 | <2.9 | <5.37 | <5.0 | <1.4 | <5.73 |
| Analyte | 1A | 2A | 3A | 4A | 5A | 6A | 7A | 8A | 9A | 10A | 11A | 12A | 13A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Puga Drill Hole | Puga River | Chumathang | Chumathang | Chumathang | Kiagor-Tso Lake | Tso-Morari | Yan River Side | Kalra Nala | Ribil | Sundo Confluence | Indus River | Indus River |
| Type | Spring Water | River Water | Spring Water | River Water | River Water | Lake Water | Lake Water | River Water | River Water | River Water | River Water | River Water | River Water |
| Sc | 23.1 | 5.6 | 19.7 | 5.3 | 3.5 | 1.5 | 4.7 | 1.1 | 1.4 | 4.7 | 2.6 | 5.6 | 4.7 |
| Y | 53.8 | 153.8 | 21.2 | 626.3 | 393.6 | 91.6 | 90.6 | 77.5 | 23.3 | 433.9 | 222.3 | 128.6 | 49.0 |
| La | 46.2 | 303.4 | 27.7 | 1441.9 | 847.1 | 149.5 | 177.5 | 196.4 | 31.6 | 1176.3 | 443.1 | 176.4 | 67.3 |
| Ce | 74.5 | 482.0 | 40.2 | 2273.0 | 1321.6 | 254.7 | 287.3 | 278.8 | 48.9 | 1879.7 | 695.8 | 274.3 | 99.8 |
| Pr | 5.9 | 35.7 | 4.1 | 147.9 | 86.4 | 16.5 | 20.2 | 22.0 | 5.0 | 116.0 | 46.7 | 21.2 | 8.6 |
| Nd | 19.7 | 71.8 | 18.0 | 277.4 | 169.9 | 36.9 | 44.1 | 50.0 | 21.7 | 227.6 | 100.8 | 50.9 | 31.2 |
| Sm | 6.1 | 19.7 | 4.9 | 73.1 | 43.1 | 11.3 | 11.6 | 13.2 | 4.4 | 54.0 | 25.1 | 13.1 | 6.4 |
| Eu | 0.6 | 0.9 | 0.6 | 3.9 | 2.5 | 0.6 | 0.7 | 0.6 | 0.3 | 3.5 | 1.8 | 0.7 | 0.4 |
| Gd | 3.2 | 8.0 | 2.2 | 27.5 | 16.2 | 4.9 | 5.2 | 4.6 | 1.9 | 21.2 | 10.2 | 5.6 | 2.4 |
| Tb | 1.0 | 2.2 | 0.5 | 6.8 | 4.3 | 1.3 | 1.4 | 1.2 | 0.5 | 4.8 | 2.4 | 1.4 | 0.7 |
| Dy | 3.6 | 8.8 | 1.9 | 29.7 | 19.5 | 5.7 | 5.3 | 5.1 | 2.2 | 21.1 | 10.9 | 7.1 | 3.3 |
| Ho | 1.8 | 1.6 | 0.8 | 4.8 | 3.2 | 0.9 | 1.0 | 0.8 | 0.5 | 3.7 | 1.9 | 1.3 | 0.7 |
| Er | 7.4 | 5.2 | 3.2 | 14.3 | 8.5 | 2.5 | 2.4 | 2.4 | 1.3 | 11.3 | 5.6 | 4.1 | 2.5 |
| Tm | 0.5 | 3.4 | 0.4 | 1.5 | 1.1 | 0.5 | 0.5 | 0.4 | 0.3 | 1.4 | 0.8 | 0.5 | 0.4 |
| Yb | 1.7 | 2.4 | 1.4 | 6.1 | 4.1 | 1.8 | 1.7 | 1.3 | 1.1 | 5.6 | 3.2 | 2.0 | 1.3 |
| Lu | 0.6 | 0.9 | 0.4 | 2.8 | 2.0 | 0.7 | 0.7 | 0.5 | 0.4 | 2.6 | 1.3 | 0.8 | 0.4 |
| REE | Concentration (pg/mL) | |||||
|---|---|---|---|---|---|---|
| ICP-MS/MS [132] | ICP-MS [133] | HR-ICP-MS [134] | ID-HR-ICP-MS [135] | Compiled Values [132] | Compiled Value [136] | |
| La | 294.5 ± 3.2 | 302.2 ± 7.3 | 279 ± 12 | 290.3 ± 6.4 | 291 ± 9 | 287 ± 8 |
| Ce | 357.5 ± 3.2 | 378.4 ± 8.2 | 369 ± 15 | 364.1 ± 3.5 | 363 ± 9 | 360 ± 12 |
| Pr | 70.9 ± 0.4 | 73.6 ± 1.5 | 75.4 ± 8.0 | 70.6 ± 2.3 | 71 ± 2.4 | 69.3 ± 1.8 |
| Nd | 274.2 ± 3.2 | 277.4 ± 5.7 | 261 ± 9 | 270.3 ± 2.8 | 271 ± 6 | 269 ± 14 |
| Sm | 58.5 ± 1.9 | 59.3 ± 1.4 | 54.3 ± 5.0 | 57.2 ± 0.3 | 57.6 ± 1.8 | 57.4 ± 2.8 |
| Eu | 8.06 ± 0.41 | 8.09 ± 0.61 | 8.4 ± 0.8 | 8.00 ± 0.7 | 8.44 ± 0.57 | 8.0 ± 0.6 |
| Gd | 33.86 ± 1.46 | 35.13 ± 1.01 | 38.3 ± 6.0 | 33.80 ± 0.36 | 34.2 ± 1.8 | 34.2 ± 2.0 |
| Tb | 4.27 ± 0.20 | 4.50 ± 0.23 | 4.1 ± 0.5 | 4.30 ± 0.12 | 4.32 ± 0.14 | 4.3 ± 0.4 |
| Dy | 22.82 ± 0.75 | 23.91 ± 0.66 | 21.7 ± 3.0 | 23.60 ± 0.16 | 23.6 ± 1.0 | 24.2 ± 1.6 |
| Ho | 4.39 ± 0.19 | 4.86 ± 0.11 | 4.2 ± 0.5 | 4.60 ± 0.18 | 4.66 ± 0.27 | 4.7 ± 0.3 |
| Er | 13.21 ± 0.46 | 13.53 ± 0.70 | 11.4 ± 3.0 | 13.10 ± 0.06 | 13.2 ± 0.8 | 13.4 ± 0.6 |
| Tm | 1.75 ± 0.11 | 1.91 ± 0.04 | 1.8 ± 0.2 | 1.80 ± 0.02 | 1.82 ± 0.08 | 1.7 ± 0.2 |
| Yb | 11.73 ± 0.36 | 12.03 ± 0.51 | 10.6 ± 2.0 | 12.30 ± 0.07 | 12.2 ± 0.7 | 12.0 ± 0.4 |
| Lu | 1.76 ± 0.09 | 1.86 ± 0.11 | 1.7 ± 0.4 | 1.95 ± 0.02 | 1.91 ± 0.10 | 1.9 ± 0.10 |
| REE | Mean Concentrations (ng/mL) |
|---|---|
| La | 0.075 |
| Ce | 0.17 |
| Pr | 0.021 |
| Nd | 0.064 |
| Sm | 0.053 |
| Eu | 0.012 |
| Gd | 0.026 |
| Tb | 0.0083 |
| Dy | 0.017 |
| Ho | 0.012 |
| Er | 0.014 |
| Tm | 0.0025 |
| Yb | 0.025 |
| Lu | 0.0025 |
| Sc | - |
| Y | 0.094 |
| REE | AS3 Zircon (µg/g) | |
|---|---|---|
| Nano SIMS Value | Certified Value | |
| La | 0.250 ± 0.147 | 0.096 ± 0.063 |
| Ce | 11.56 ± 0.362 | 7.69 ± 1.07 |
| Pr | 0.544 ± 0.295 | 0.578 ± 0.173 |
| Nd | 7.34 ± 3.07 | 7.60 ± 2.09 |
| Sm | 12.77 ± 4.45 | 9.21 ± 2.24 |
| Eu | 0.399 ± 0.159 | 0.331 ± 0.073 |
| Gd | 42.7 ± 11.4 | 40.9 ± 8.5 |
| Tb | 15.63 ± 3.65 | 14.94 ± 3.18 |
| Dy | 165.6 ± 34.5 | 168.5 ± 30.0 |
| Ho | 53.2 ± 9.9 | 63.3 ± 10.7 |
| Er | 222.5 ± 45.8 | 261.0 ± 41.7 |
| Tm | 40.5 ± 6.7 | 54.2 ± 8.2 |
| Yb | 332.5 ± 50.8 | 408.6 ± 57.3 |
| Lu | 62.7 ± 10.3 | 89.6 ± 12.2 |
| Selected REE | Average Concentration (µg/g) | |
|---|---|---|
| pLIBS | ICP-MS | |
| La | 54.3 | 53.99 |
| Ce | 99.5 | 94.77 |
| Pr | 13.1 | 13.72 |
| Nd | 36.4 | 52.62 |
| Sm | 15.6 | 11.38 |
| Gd | 7.5 | 10.40 |
| Dy | 6.4 | 9.58 |
| Yb | 8.7 | 4.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaram, V. Advances in Analytical Techniques and Applications in Exploration, Mining, Extraction, and Metallurgical Studies of Rare Earth Elements. Minerals 2023, 13, 1031. https://doi.org/10.3390/min13081031
Balaram V. Advances in Analytical Techniques and Applications in Exploration, Mining, Extraction, and Metallurgical Studies of Rare Earth Elements. Minerals. 2023; 13(8):1031. https://doi.org/10.3390/min13081031
Chicago/Turabian StyleBalaram, V. 2023. "Advances in Analytical Techniques and Applications in Exploration, Mining, Extraction, and Metallurgical Studies of Rare Earth Elements" Minerals 13, no. 8: 1031. https://doi.org/10.3390/min13081031
APA StyleBalaram, V. (2023). Advances in Analytical Techniques and Applications in Exploration, Mining, Extraction, and Metallurgical Studies of Rare Earth Elements. Minerals, 13(8), 1031. https://doi.org/10.3390/min13081031







