Nature of Pigments in Orange and Purple Coloured Chinese Freshwater Cultured Pearls: Insights from Experimental Raman Spectroscopy and DFT Calculations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Colour Characterisation of the Pearls
3.2. Characterisation of Pigments in Pearls by Raman Spectroscopy
3.3. Theoretical Analysis of Pigments and Colour Origin of Pearls
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarratt, K.; Moses, T.; Akamatsu, S. Characteristics of Nuclei in Chinese Freshwater Cultured Pearls. Gems Gemol. 2000, 36, 98–109. [Google Scholar] [CrossRef]
- Kiefert, L.; Moreno, D.M.; Arizmendi, E.; Hnni, H.A.; Elen, S. Cutured Pearls from the Gulf of California, Mexico. Gems Gemol. 2004, 40, 26–38. [Google Scholar] [CrossRef]
- Karampelas, S.; Fritsch, E.; Mevellec, J.Y.; Gauthier, J.P.; Sklavounos, S.; Soldatos, T. Determination by Raman Scattering of the Nature of Pigments in Cultured Freshwater Pearls from the Mollusk Hyriopsis cumingi. J. Raman Spectrosc. 2007, 38, 217–230. [Google Scholar] [CrossRef]
- Fiske, D.; Shepherd, J. Continuity and Change in Chinese Freshwater Pearl Culture. Gems Gemol. 2007, 43, 138–145. [Google Scholar] [CrossRef]
- Akamatsu, S.; Zansheng, L.T.; Moses, T.M.; Scarratt, K. The Current Status of Chinese Freshwater Cultured Pearls. Gems Gemol. 2001, 37, 96–113. [Google Scholar] [CrossRef]
- Zhang, G.; Xie, X.; Wang, Y. Raman spectra of Carotenoid in the Nacre of Hyriopsis cumingii (Lea) Shell. Acta Mineral. Sin. 2001, 21, 389–392. (In Chinese) [Google Scholar] [CrossRef]
- Yang, M.; Guo, S.; Shi, L.; Wang, W. Study on Compositions and Colouring Mechanism of Freshwater Cultured Pearls. J. Gems Gemol. 2004, 6, 10–13. (In Chinese) [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, B. Research on Relationship between Colour and Raman Spectrum of Freshwater Cultured Pearl. J. Gems Gemol. 2001, 3, 17–20. (In Chinese) [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, G. In situ Resonance Raman Spectra of Organic Pigments in Freshwater Cultured Pearls. Spectrosc. Spect. Anal. 2004, 26, 78–80. (In Chinese) [Google Scholar]
- Qin, Z.; Ma, H.; Mu, S.; Tong, Z. Research on Relationship between color and Raman Spectrum of Freshwater Cultured Pearl of Good Quality. Acta Mineral. Sin. 2007, 27, 73–76. (In Chinese) [Google Scholar] [CrossRef]
- Urmos, J.; Sharma, S.K.; Mackenzie, F.T. Characterization of Some Biogenic Carbonates with Raman Spectroscopy. Am. Mineral. 1991, 76, 641–646. [Google Scholar]
- Soldati, A.L.; Jacob, D.E.; Wehrmeister, U.; Hger, T.; Hofmeister, W. Micro-Raman Spectroscopy of Pigments Contained in Different Calcium Carbonate Polymorphs from Freshwater Cultured Pearls. J. Raman Spectrosc. 2008, 39, 525–536. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bersani, D.; Csermely, D.; Lottici, P.P. The Nature of the Pigments in Corals and Pearls: A Contribution from Raman Spectroscopy. Spectrosc. Lett. 2011, 44, 453–458. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.; Mao, J.; Han, X. Study of Coloration Mechanism of Cultured Freshwater Pearls from Mollusk Hyriopsis cumingii. J. Appl. Spectrosc. 2014, 81, 97–101. [Google Scholar] [CrossRef]
- Karampelas, S.; Fritsch, E.; Makhlooq, F.; Mohamed, F.; Al-Alawi, A. Raman Spectroscopy of Natural and Cultured Pearls and Pearl Producing Mollusc Shells. J. Raman Spectrosc. 2020, 51, 1813–1821. [Google Scholar] [CrossRef]
- Kupka, T.; Buczek, A.; Broda, M.A.; Szostak, R.; Lin, H.M.; Fan, L.W.; Wrzalik, R.; Stobiński, L. Modeling Red Coral (Corallium rubrum) and African Snail (Helixia aspersa) Shell Pigments: Raman Spectroscopy versus DFT Studies. J. Raman Spectrosc. 2016, 47, 908–916. [Google Scholar] [CrossRef]
- Finkel’shtein, E.I. Modern Methods of Analysis of Carotenoids (Review). Pharm. Chem. J. 2016, 50, 96–107. [Google Scholar] [CrossRef]
- Williams, S.T.; Ito, S.; Wakamatsu, K.; Goral, T.; Edwards, N.P.; Wogelius, R.A.; Henkel, T.; De Oliveira, L.F.C.; Maia, L.F.; Strekopytov, S.; et al. Identification of Shell Colour Pigments in Marine Snails Clanculus pharaonius and C. margaritarius (Trochoidea; Gastropoda). PLoS ONE 2016, 11, e0156664. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bersani, D.; Mantovan, S.; Lottici, P.P. Micro-Raman Investigation of Pigments and Carbonate Phases in Corals and Molluscan Shells. Eur. J. Mineral. 2014, 25, 845–853. [Google Scholar] [CrossRef]
- Barnard, W.; De Waal, D. Raman Investigation of Pigmentary Molecules in the Molluscan Biogenic Matrix. J. Raman Spectrosc. 2006, 37, 342–352. [Google Scholar] [CrossRef]
- Stemmer, K.; Nehrke, G. The Distribution of Polyenes in the Shell of Arctica Islandica from North Atlantic Localities: A Confocal Raman Microscopy Study. J. Molluscan Stud. 2014, 80, 365–370. [Google Scholar] [CrossRef]
- Thompson, C.M.; North, E.W.; Kennedy, V.S.; White, S.N. Classifying Bivalve Larvae Using Shell Pigments Identified by Raman Spectroscopy. Anal. Bioanal. Chem. 2015, 407, 3591–3604. [Google Scholar] [CrossRef] [PubMed]
- Kantha, S.S. Carotenoids of Edible Molluscs: A Review. J. Food Biochem. 1989, 13, 429–442. [Google Scholar] [CrossRef]
- Llansola-Portoles, M.J.; Pascal, A.A.; Robert, B. Electronic and Vibrational Properties of Carotenoids: From in Vitro to in Vivo. J. R. Soc. Interface 2017, 14, 20170504. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.S.; Ri, J.R.; Schleusener, J.; Lademann, J.; Darvin, M.E. The Non-Homogenous Distribution and Aggregation of Carotenoids in the Stratum Corneum Correlates with the Organization of Intercellular Lipids in Vivo. Exp. Dermatol. 2019, 28, 1237–1243. [Google Scholar] [CrossRef]
- de Oliveira, V.E.; Castro, H.V.; Edwards, H.G.M.; de Oliveiraa, L.F.C. Carotenes and Carotenoids in Natural Biological Samples: A Raman Spectroscopic Analysis. J. Raman Spectrosc. 2010, 41, 642–650. [Google Scholar] [CrossRef]
- Maia, L.F.; Fernandes, R.F.; Lobo-Hajdu, G.; De Oliveira, L.F.C. Conjugated Polyenes as Chemical Probes of Life Signature: Use of Raman Spectroscopy to Differentiate Polyenic Pigments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 1–11. [Google Scholar] [CrossRef]
- Merlin, J.C.; Delé-Dubois, M.L. Resonance Raman Characterization of Polyacetylenic Pigments in the Calcareous Skeleton. Comp. Biochem. Physiol. Part B Biochem. 1986, 84, 97–103. [Google Scholar] [CrossRef]
- Fritsch, E.; Karampelas, S. Comment on: Determination of Canthaxanthin in the Red Coral (Corallium rubrum) from Marseille by HPLC Combined with UV and MS Detection (Cvejic et al. Mar Biol 152:855-862, 2007). Mar. Biol. 2008, 154, 929–930. [Google Scholar] [CrossRef]
- Peebles, B.A.; Gordon, K.C.; Smith, A.M.; Smith, G.P.S. First Record of Carotenoid Pigments and Indications of Unusual Shell Structure in Chiton Valves. J. Molluscan Stud. 2017, 83, 476–480. [Google Scholar] [CrossRef]
- Brambilla, L.; Tommasini, M.; Zerbi, G.; Stradi, R. Raman Spectroscopy of Polyconjugated Molecules with Electronic and Mechanical Confinement: The Spectrum of Corallium rubrum. J. Raman Spectrosc. 2012, 43, 1449–1458. [Google Scholar] [CrossRef]
- Darvin, M.E.; Meinke, M.C.; Sterry, W.; Lademann, J. Optical Methods for Noninvasive Determination of Carotenoids in Human and Animal Skin. J. Biomed. Opt. 2013, 18, 061230. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Recent Progress in Structural Studies of Carotenoids in Animals and Plants. Arch. Biochem. Biophys. 2009, 483, 191–195. [Google Scholar] [CrossRef]
- McGraw, K.J.; Nogare, M.C. Carotenoid Pigments and the Selectivity of Psittacofulvin-Based Coloration Systems in Parrots. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2004, 138, 229–233. [Google Scholar] [CrossRef]
- Vershinin, A. Biological Functions of Carotenoids—Diversity and Evolution. BioFactors 1999, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V.; Zanoni, B.; Zaniboni, A. Effect of Water Activity on Carotenoid Degradation in Dehydrated Carrots. Food Chem. 2007, 104, 1705–1711. [Google Scholar] [CrossRef]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; De Oliveira, L.F.C. Raman Spectra of Carotenoids in Natural Products. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Macernis, M.; Sulskus, J.; Malickaja, S.; Robert, B.; Valkunas, L. Resonance Raman Spectra and Electronic Transitions in Carotenoids: A Density Functional Theory Study. J. Phys. Chem. A 2014, 118, 1817–1825. [Google Scholar] [CrossRef]
- Saito, S.; Tasumi, M.; Eugster, C.H. Resonance Raman Spectra (5800–40 cm−1) of All-trans and 15-cis Isomers of Β-carotene in the Solid State and in Solution. Measurements with Various Laser Lines from Ultraviolet to Red. J. Raman Spectrosc. 1983, 14, 299–309. [Google Scholar] [CrossRef]
- Saito, S.; Tasumi, M. Normal-coordinate Analysis of Retinal Isomers and Assignments of Raman and Infrared Bands. J. Raman Spectrosc. 1983, 14, 236–245. [Google Scholar] [CrossRef]
- Okamoto, H.; Saito, S.; Hamaguchi, H.-O.; Tasumi, M.; Eugster, C.H. Resonance Raman Spectra and Excitation Profiles of Tetradesmethyl-β-Carotene. J. Raman Spectrosc. 1984, 15, 331–335. [Google Scholar] [CrossRef]
- Hoskins, L.C.; Alexander, V. Determination of Carotenoid Concentrations in Marine Phytoplankton by Resonance Raman Spectrometry. Anal. Chem. 1977, 49, 695–697. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Scott, A.P.; Radom, L. Harmonic Vibrational Frequencies: An Evaluation of Hartree-Fock, Møller-Plesset, Quadratic Configuration Interaction, Density Functional Theory, and Semiempirical Scale Factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Kashinski, D.O.; Chase, G.M.; Nelson, R.G.; Di Nallo, O.E.; Scales, A.N.; Vanderley, D.L.; Byrd, E.F.C. Harmonic Vibrational Frequencies: Approximate Global Scaling Factors for TPSS, M06, and M11 Functional Families Using Several Common Basis Sets. J. Phys. Chem. A 2017, 121, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Schügerl, F.B.; Kuzmany, H. Optical Modes of Trans-Polyacetylene. J. Chem. Phys. 1981, 74, 953–958. [Google Scholar] [CrossRef]
- Schaffer, H.E.; Chance, R.R.; Silbey, R.J.; Knoll, K.; Schrock, R.R. Conjugation Length Dependence of Raman Scattering in a Series of Linear Polyenes: Implications for Polyacetylene. J. Chem. Phys. 1991, 94, 4161–4170. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An Sp-Hybridized All-Carboatomic Ring, Cyclo[18]Carbon: Electronic Structure, Electronic Spectrum, and Optical Nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]

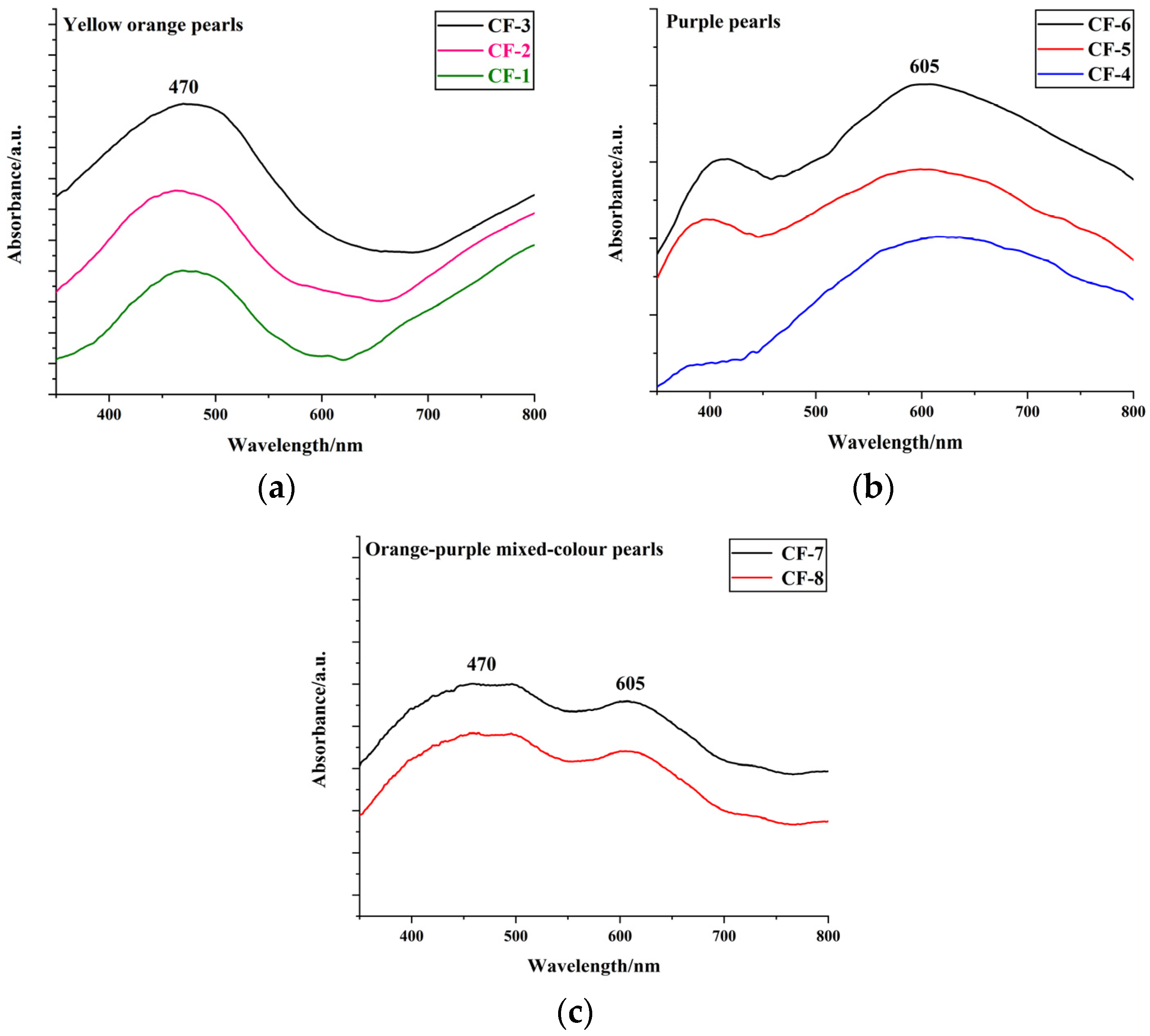
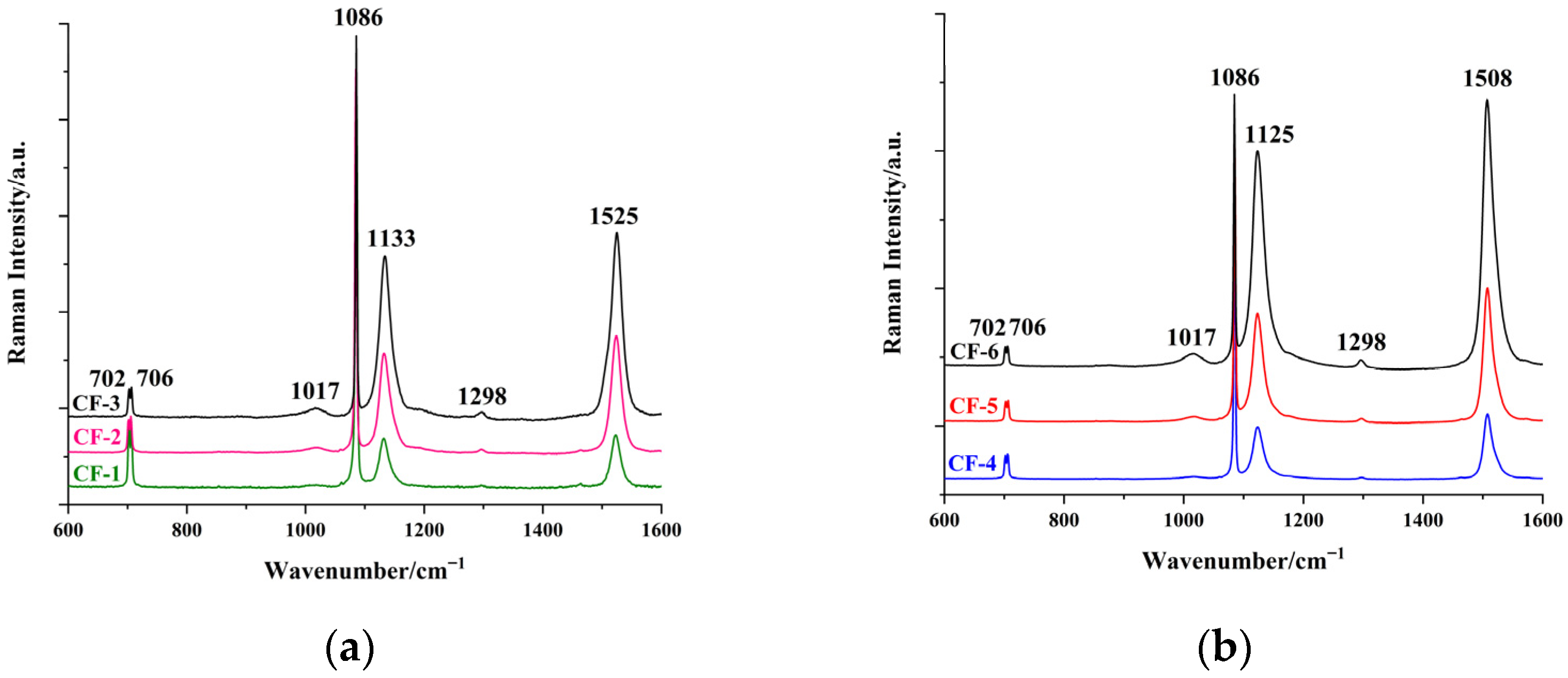

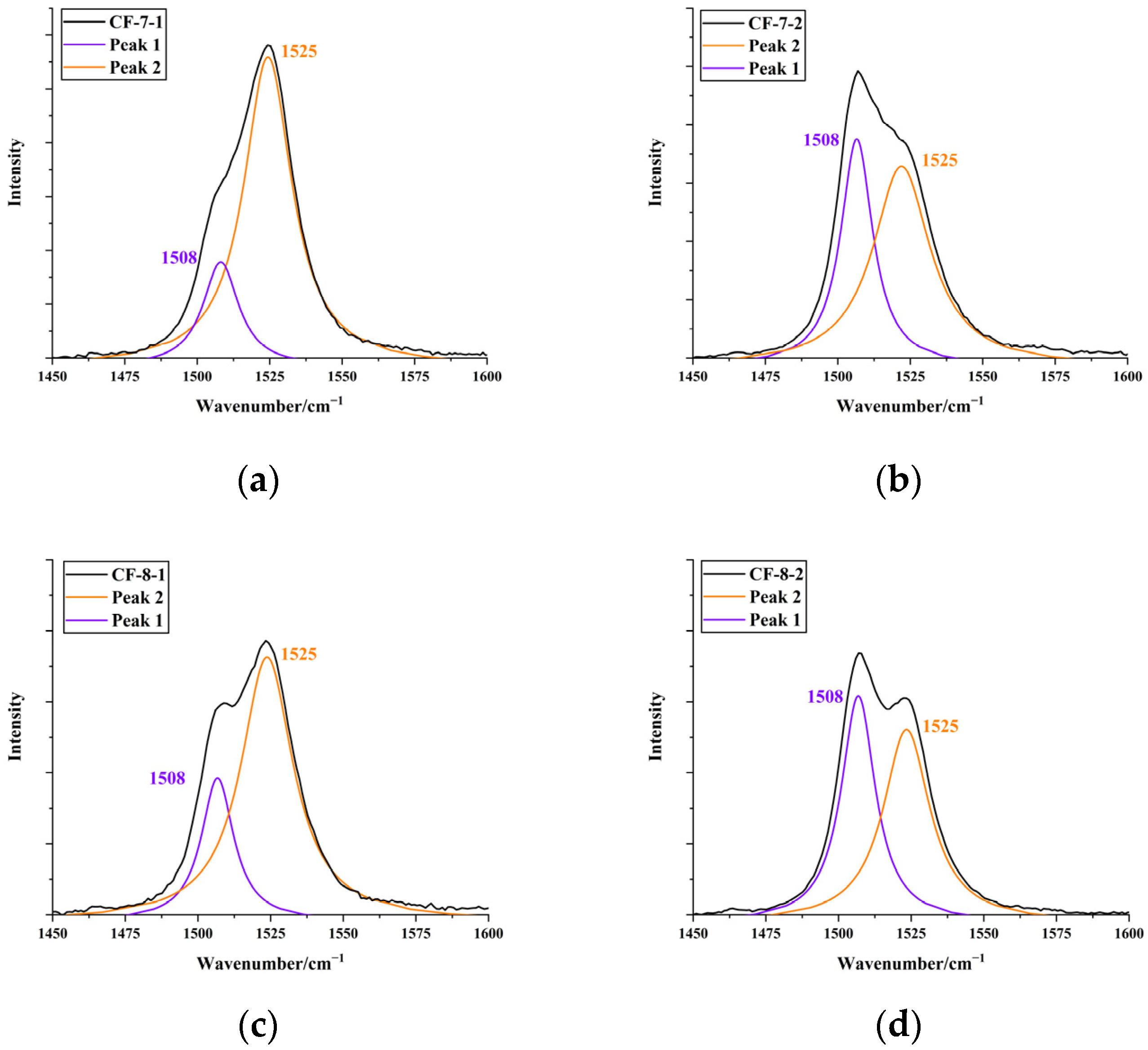
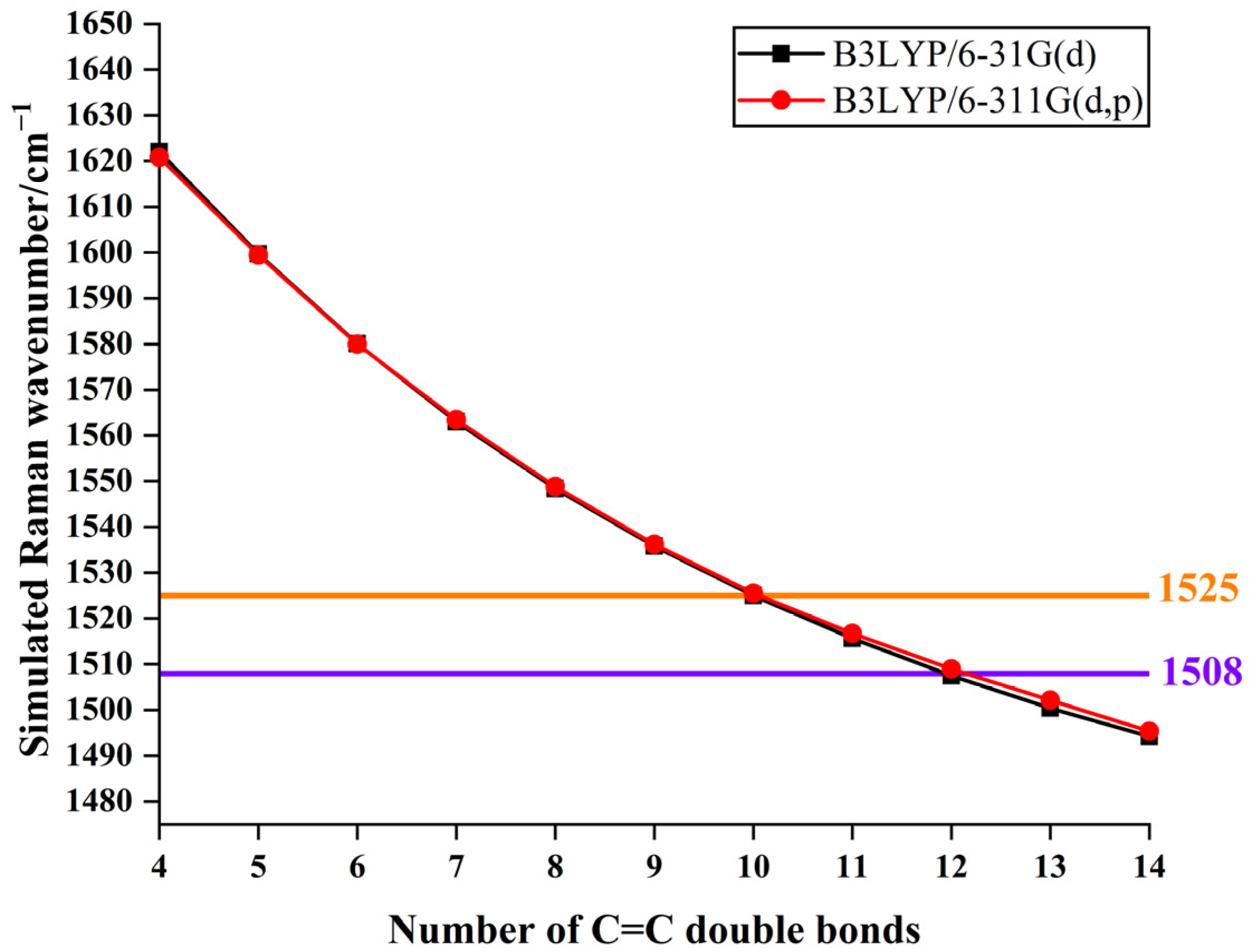
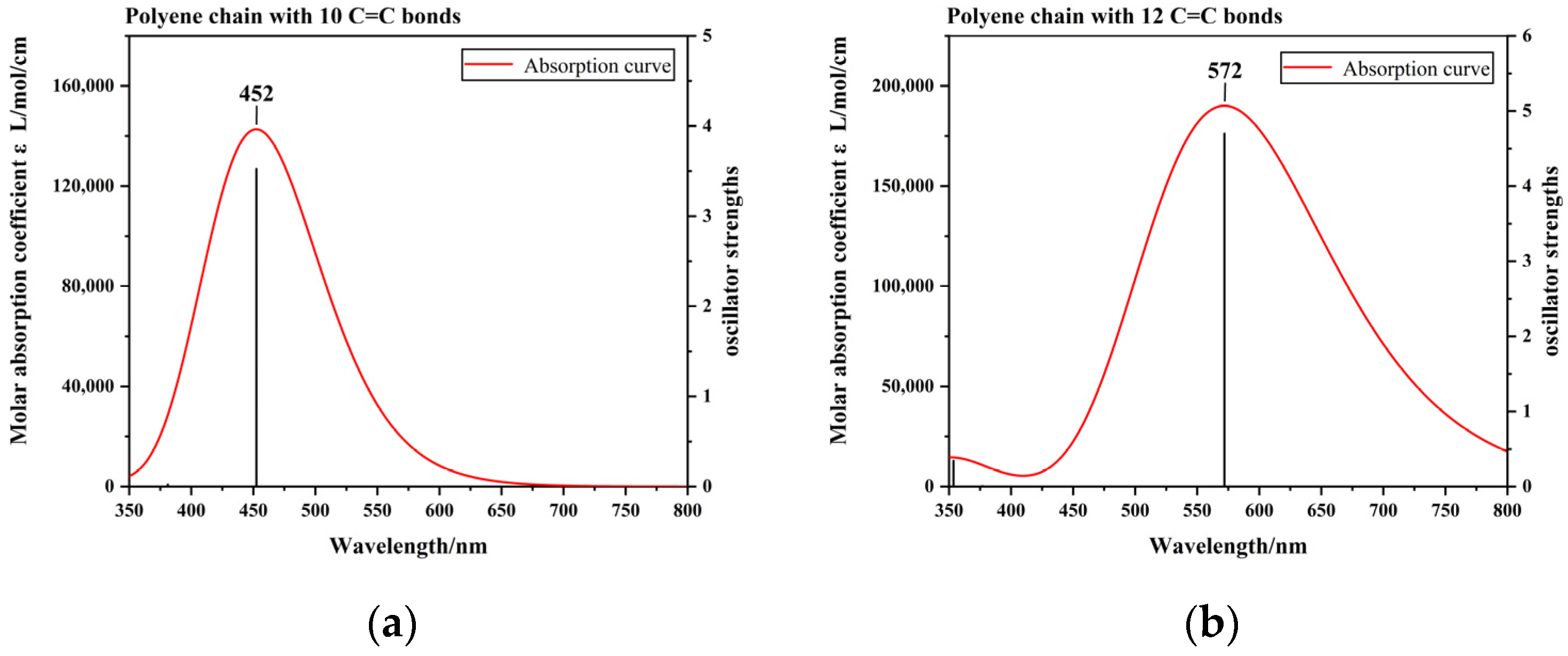

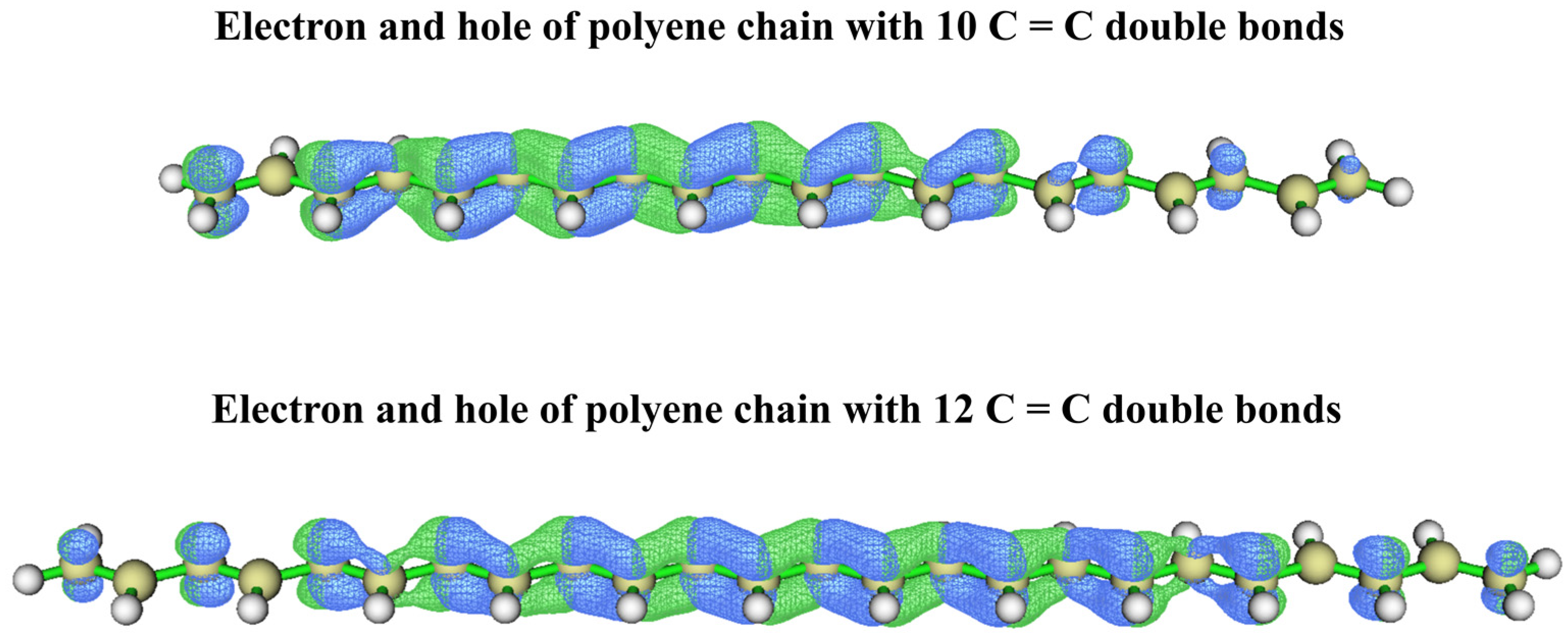
| Sample | Colour | Integral Intensity of UV–Vis Absorbance Band at 470 nm (Boundary: 350 nm to 600 nm) |
|---|---|---|
| CF-1 | Yellow | 10,770 |
| CF-2 | orange | 15,060 |
| CF-3 | Intense orange | 17,440 |
| Sample | Colour | Integral Intensity of UV–Vis Absorbance Band at 605 nm (Boundary: 450 nm to 800 nm) |
|---|---|---|
| CF-4 | Slight purple | 16,540 |
| CF-5 | Purple | 18,600 |
| CF-6 | Intense purple | 20,410 |
| Sample | Colour | Integral Intensity of UV–Vis Absorbance Band at 470 nm (Boundary: 350 to 600 nm) | Integral Intensity of Raman Band at 1525 cm−1 (Boundary: 1450 to 1600 cm−1) |
|---|---|---|---|
| CF-1 | Yellow | 10,770 | 4.371 |
| CF-2 | Orange | 15,060 | 8.124 |
| CF-3 | Intense orange | 17,440 | 14.63 |
| Sample | Colour | Integral Intensity of UV–Vis Absorbance Band at 605 nm (Boundary: 450 to 800 nm) | Integral Intensity of Raman Band at 1508 cm−1 (Boundary: 1450 to 1600 cm−1) |
|---|---|---|---|
| CF-4 | Slight purple | 16,540 | 5.965 |
| CF-5 | Purple | 18,600 | 12.62 |
| CF-6 | Intense purple | 20,410 | 28.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yu, J.; Zhang, C.; Ye, X.; Shen, A.H. Nature of Pigments in Orange and Purple Coloured Chinese Freshwater Cultured Pearls: Insights from Experimental Raman Spectroscopy and DFT Calculations. Minerals 2023, 13, 959. https://doi.org/10.3390/min13070959
Chen C, Yu J, Zhang C, Ye X, Shen AH. Nature of Pigments in Orange and Purple Coloured Chinese Freshwater Cultured Pearls: Insights from Experimental Raman Spectroscopy and DFT Calculations. Minerals. 2023; 13(7):959. https://doi.org/10.3390/min13070959
Chicago/Turabian StyleChen, Chaoyang, Jing Yu, Chuting Zhang, Xu Ye, and Andy H. Shen. 2023. "Nature of Pigments in Orange and Purple Coloured Chinese Freshwater Cultured Pearls: Insights from Experimental Raman Spectroscopy and DFT Calculations" Minerals 13, no. 7: 959. https://doi.org/10.3390/min13070959
APA StyleChen, C., Yu, J., Zhang, C., Ye, X., & Shen, A. H. (2023). Nature of Pigments in Orange and Purple Coloured Chinese Freshwater Cultured Pearls: Insights from Experimental Raman Spectroscopy and DFT Calculations. Minerals, 13(7), 959. https://doi.org/10.3390/min13070959






