Abstract
The Podgórze uranium deposit is located near Kowary in the Sudetes Mountains, SW Poland. The mine is located in the Karkonosze-Izera block, largely comprising Cambrian to Devonian metamorphic rocks intruded by the Variscan Karkonosze granite. Uranyl minerals from the Podgórze mine can be divided into three assemblages. The first one is associated with heavily ventilated mining galleries. The next assemblage is related to the outflow of water from fissures in the walls of the mine galleries. The last assemblage appears in the mine dump, where there is increased activity of other weathering products. The main purpose of this paper is to determine the mineralogical characteristics of uranyl minerals from the abandoned Podgórze uranium mine and reconstruct the physicochemical crystallization conditions based on the concentrations of rare earth elements (REEs) in these minerals. The results of thermodynamic modeling show that the aqueous species of uranyl ion in the mine water are represented by UO2HAsO4 (aq), UO2CO3(OH)3−, UO2CO3 (aq), and UO2OH+. The content of REEs and their distribution patterns were used to determine the crystallization conditions of uranyl minerals. Uranyl carbonates and arsenates have generally low concentrations of REEs compared to uranyl silicates, phosphates, and hydroxides, which have higher concentrations. The differences in REE concentration patterns may be related with the oxidizing nature of water circulating in the subsurface part of the deposit.
1. Introduction
Uranium can be easily released into the environment during the oxidation of uraninite [1,2]. This process starts with the decomposition of primary mineralization in polymetallic deposits with the oxidation of unstable phases, such as ferric sulfides, in aerobic conditions [3]. The rapid decomposition of sulfides results in a sharp and quick drop in pH, which accelerates the decomposition of barren minerals. Moreover, faster degradation of the primary mineralization is often accelerated by bacterial activity [4,5]. As a result, the water in weathering environments is enriched in various components, and some are considered dangerous and toxic to the environment [6]. In such zones, various secondary minerals can crystallize. In low-pH conditions, uraninite is unstable and uranium is easily leached. During the oxidation process, uranium changes the oxidation state from 4+ to 6+ and occurs in the water mostly as a uranyl cation, UO22+. In this speciation, uranium can migrate over considerably long distances. In the presence of different cations and anions, it can precipitate very quickly as secondary uranium minerals. For this reason, weathering zones may contain a large variety of secondary uranium minerals [7]. The main purpose of this paper is to determine the mineralogical characteristics of uranyl minerals from the abandoned Podgórze uranium mine and reconstruct the physicochemical crystallization conditions based on the concentrations of rare earth elements (REEs) in these minerals.
The Podgórze uranium deposit is located near Kowary in the Sudetes Mountains, SW Poland (Figure 1). The mine is located in the Karkonosze-Izera block, largely comprising Cambrian to Devonian metamorphic rocks intruded by the Variscan Karkonosze granite [8,9]. The uranium ore is developed within gneisses intercalated with graphitic and micaceous schists. The genesis of the vine-hosted-type uranium deposit is connected to the activity of hydrothermal fluids generated by the Karkonosze granite. Hydrothermal fluids emanating along faults from the Karkonosze Granite are inferred to have formed the primary ore, represented by uraninite, hematite, pyrite, chalcopyrite, galena, arsenopyrite, calcite, baryte, and fluorite [10,11,12,13]. The Podgórze mine operated in 1950–1958, and, during this period, 194 tons of uranium ore were extracted.
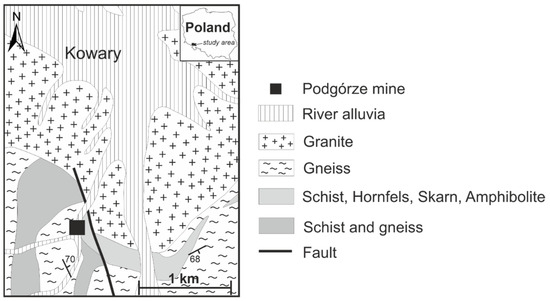
Figure 1.
Geological sketch map of Podgórze mine area (based on https://mapy.geoportal.gov.pl/imap/Imgp_2.html (accessed on 25 February 2022); [14]).
2. Materials and Methods
The samples were collected from adits 19 and 19a of the Podgórze uranium mine (Figure 2) and from a mine dump near the entrance of adit 17. Uranyl minerals collected from adits 19 and 19a were collected up to 1 km deeper into the mine.
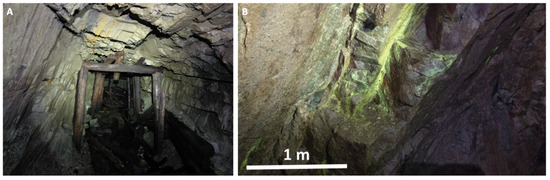
Figure 2.
(A) Abandoned mine gallery in Podgórze mine; (B) sampling site with secondary uranyl minerals in adit 19 of the mine.
Diffraction studies were carried out using a PanAlytical X’Pert PRO MPD powder diffractometer with the Debye–Scherrera–Hull (DSH) method. The registration was made using a Co Kα lamp at 40 kV and 40 mA in the range of 5.0131 to 77.9691 2Θ with steps of 0.0260 2Θ. The results were interpreted using X’Pert Plus HighScore software with access to ICDD PDF-2 (RDB 2008) database. Energy-dispersive analysis (EDS) and SEM imaging were carried out using an FE-SIGMA VP electron microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) with two EDS detectors (Quantax XFlash 6|10, Bruker Nano GmbH, Berlin, Germany). Small fragments of samples were placed on the aluminum mount with carbon conductive tape and coated with a 20 nm layer of carbon by a vacuum coater (Quorum 150T ES, Quorum Technologies, East Sussex, United Kingdom). Analyses were done with 120 μm aperture and 20 kV acceleration voltage. The working distance was 7.8 mm. The microarea chemical analysis (EPMA) of samples was done by using a CAMECA CX-100 electron microprobe (Camcor Inc, Burlington, NC, USA). The standards, analytical lines of each analyzed element, analytical crystals used, and detection limits are presented in Supplementary Table S1. The analyses were carried out at an accelerating voltage of 15 kV and a beam voltage of 10 nA. The beam diameter was 2 and 5 μm for delicate crystals. The RO-PHI-ZAF model was used for the analysis. The amount of crystalized water was calculated from stoichiometry of minerals because the small amounts of mineral samples were insufficient to perform thermogravimetric analysis. The amount of carbonate ion was also calculated from the stoichiometry of minerals.
The purity of separated crystals for REE analysis was tested using SEM-EDS and XRD. About 10 mg of each sample was weighed into a PTFE beaker and dissolved in a stepwise manner using a heated bath. First, 3 mL of 65% HNO3 was heated at 95 °C, then 1 mL of 40% HF was added to the residuum at 95 °C. For HF neutralization, a solution of 1 g/mL boric acid was added. Next, the acid mixture was evaporated to the state of wet solid in 280 °C. After this, 2.5 mL of 65% HClO4 was added and evaporation was repeated. Remaining wet solid was dissolved in 10 mL of 10% HCl and filled to a final volume of 50 mL. Trace and ultra-trace elements were determined on the Element II double-focusing magnetic sector field ICP-MS (Thermo Scientific, Waltham, MA, USA). The measurement parameters were set up according to the manufacturer’s recommendations (double-pass spray chamber, PFA concentric nebulizer with flow rate of 50 µL/s, Ni cones, 1200 W RF power, sampling time 1 min), and the instrument was tuned to achieve the highest sensitivity concurrently with acceptably low oxide formation. Before being injected into the plasma, samples were in-line admixed with an indium internal standard solution for correction of instrumental drift. The system was calibrated using a multi-element calibration solution with commercially available single elemental standards (CPAchem, Bogomilovo, Bulgaria) and multi-elemental standard (CertiPUR, Darmstadt, Germany) for REE using blank and 4 calibration points with up to 10 ppb of each element. The REEs were quantified in low-resolution (LR) setting.
Uranium speciation was calculated using Geochemist’s Workbench community edition software. Modeling of uranium speciation was performed using the combined MINTEQ and 190329g0 thermodynamic database from the Radionuclide Migration Research Group of the Japan Atomic Energy Agency (JAEA, Ibaraki, Japan). Some aquatic uranyl species were manually added to the database. The thermodynamic data of selected uranyl species were taken from the Update on the Chemical Thermodynamics of Uranium, Neptunium, Plutonium, Americium, and Technetium [15]. The conductivity USGS database was used for electrical conductivity calculations.
3. Uranyl Minerals from Podgórze Mine
3.1. Uranyl Arsenates
The most common uranyl minerals in Podgórze are arsenates. The XRD results showed the presence of uranospinite (Ca(UO2)(AsO4)2·10H2O), nováčekite-II (Mg(UO2)2(AsO4)2·10H2O), heinrichite (Ba(UO2)2(AsO4)2·10H2O), and zeunerite (Cu(UO2)2(AsO4)2·12H2O) (Figure 3). The calculated unit cell parameters of uranyl arsenates are presented in Table 1. Uranyl arsenates can be divided into two groups. The first one consists of uranospinite, nováčekite-II, and natrouranospinite. These minerals form crusts near water seepage from cracks and fissures in the walls of mine galleries. The mineral accumulation can reach a size of 1 m and is composed of crystals up to 1 mm in size. The second group consists of uranospinite, zeunerite, and heinrichite. They were found in the mining waste dump of adit 17. These minerals form small efflorescences consisting of lamellar crystals barely visible to the naked eye.
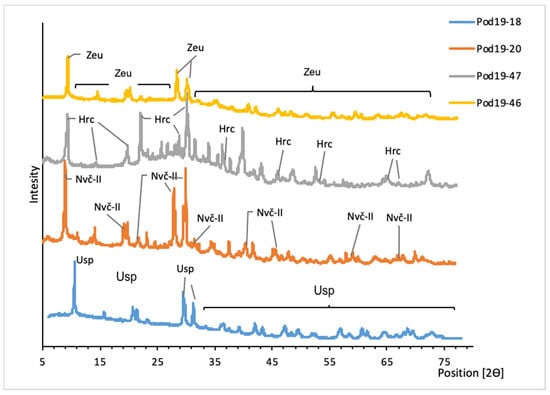
Figure 3.
XRD patterns of uranyl arsenates from Podgórze mine. Usp, uranospinite; Nvč-II, nováčekite-II; Hrc, heinrichite; Zeu, zeunerite.

Table 1.
Unit cell parameters of uranyl arsenates from Podgórze mine.
SEM-EDS also confirmed the occurrence of natrouranospinite ((Na2,Ca)(UO2)2(AsO4)2·5H2O) (Figure 4C). Uranospinite and nováčekite-II crystals were also observed by SEM as very small plate crystals (Figure 4A,B). Natrouranospinite formed similar crystals to uranospinite (Figure 4C). Heinrichite is much rarer than uranospinite and nováčekite-II and was found only in the heap.
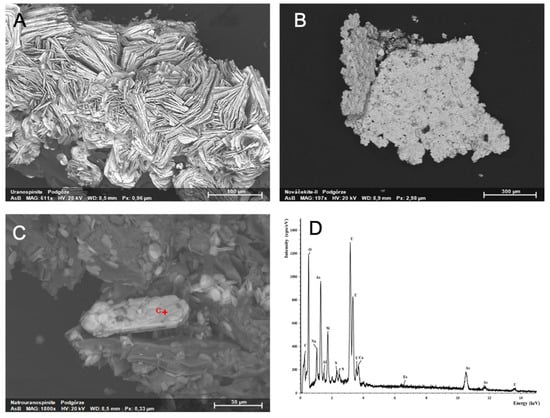
Figure 4.
Uranyl arsenates from Podgórze mine: (A) uranospinite, (B) nováčekite-II, (C) natrouranospinite, and (D) EDS spectrum of natrouranospinite.
The EPMA chemical analysis results for uranospinite are presented in Table 2. The results show a small substitution of K for Ca and SiO43− and PO43− for AsO43−. One uranospinite sample revealed a significant potassium substitution.

Table 2.
Chemical composition of uranospinite by EPMA.
3.2. Uranyl Silicates
Uranyl silicates are represented by uranophane, uranophane-β (both Ca(UO2)2(SiO3OH)2·5H2O), and sklodowskite (Mg(UO2)2(SiO3OH)2·6H2O) (Figure 5). The calculated unit cell parameters of uranyl silicates are shown in Table 3.
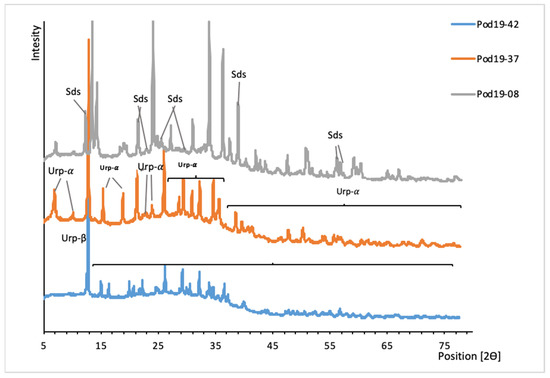
Figure 5.
XRD patterns of uranyl silicates from Podgórze mine. Urp-β, uranophane-β; Urp-α, uranophane; Sds, sklodowskite.

Table 3.
Unit cells parameters of uranyl silicates from Podgórze mine.
They appeared in fissure and cracks that developed vertical to the adits in the deeper parts of the mine. The arrangement of these cracks and fissures suggests that they were formed during tectonic activity in this area. Uranophane-β and sklodowskite were found only in these tectonic zones. However, they did not crystalize there during the tectonic events. Uranophane is the most abundant uranyl silicate there. Characteristic well-formed needle uranophane and uranophane-β crystals can reach 0.5 cm in length (Figure 6). They form aggregates with a size of approximately 10 cm.
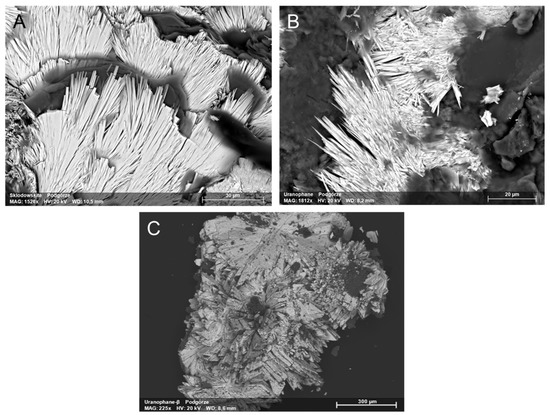
Figure 6.
Uranyl silicates from Podgórze mine: (A) sklodowskite, (B) uranophane, and (C) uranophane-β.
The chemical composition of uranophane and uranophane-β (indistinguishable with EPMA) is shown in Table 4. Uranyl silicates from the Podgórze mine do not differ in chemical composition from those described in the literature [16,17,18].

Table 4.
Chemical composition of uranophanes from Podgórze.
3.3. Uranyl Phosphates
The occurrence of autunite (Ca(UO2)2(PO4)2·10-12H2O) and metauranocircite-I (Ba(UO2)2(PO4)2·7H2O) was confirmed by XRD (Figure 7). The calculated unit cell parameters are presented in Table 5.
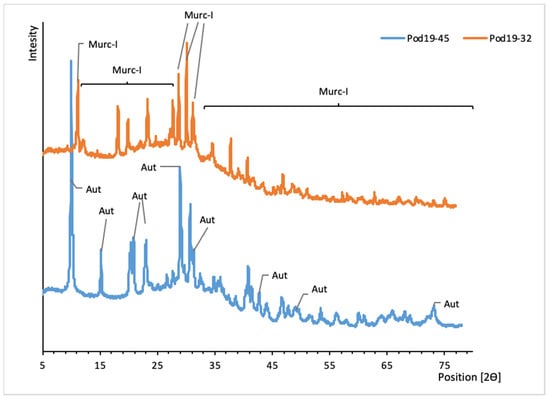
Figure 7.
XRD patterns of uranyl phosphates from Podgórze mine. Aut, autunite; Murc-I, metauranocircite.

Table 5.
Unit cell parameters of uranyl phosphates from Podgórze mine.
They were found only at a heap close to adit 17 as aggregates and crusts up to a few millimeters in size. The individual crystals of uranyl phosphates were small, with a size of a few hundred micrometers. They were visible under high magnification only (Figure 8). The crystals have tabular habits and are rather poorly preserved.

Figure 8.
Aggregates of tabular autunite crystals (SEM image).
3.4. Uranyl Carbonates
Liebigite (Ca2(UO2)(CO3)3·11H2O) and schröckingerite (NaCa3(UO2)(CO3)3(SO4)F·10H2O) were found in the Podgórze mine (Figure 9). The calculated unit cell parameters of liebigite and schröckingerite are presented in Table 6. Uranyl carbonates occurred in highly ventilated mine gallerias. They crystallize in the bottom parts of adits, especially in places where small heaps were formed from collapsed mine wall galleries, which consisted of very finely crushed rock material. Liebigite occurs as well-formed crystals ranging from a few millimeters up to one centimeter (Figure 10B). In comparison to liebigite, schröckingerite forms smaller aggregates (up to a few millimeters in size). They are composed of small platy crystals with a size of approximately 10 micrometers (Figure 10A). The chemical analyses of uranyl carbonates are presented in Table 7. Only small substitutions of Mg, Mn, Cu, and Fe for Ca and Si for CO3 were detected. Additionally, K substitution for Na was found.
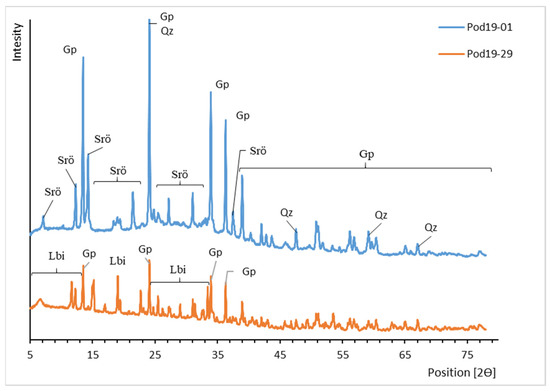
Figure 9.
XRD patterns of uranyl carbonates from Podgórze mine. Lbi, liebigite; Srö, schröckingerite; Qz, quartz; Gp, gypsum.

Table 6.
Unit cell parameters of uranyl carbonates from Podgórze mine.

Figure 10.
Clusters of uranyl carbonates from Podgórze mine: (A) schröckingerite; (B) liebigite.

Table 7.
Chemical composition of uranyl carbonates from Podgórze mine (EPMA).
3.5. Uranyl Sulfates
The XRD analyses confirmed the occurrence of rabejacite (Ca(UO2)4(SO4)2(OH)6· 6H2O) and zippeite (K3(UO2)4(SO4)2O3(OH)·3H2O) in the Podgórze mine (Figure 11). The calculated unit cell parameters of uranyl sulfates are presented in Table 8. Uranyl sulfates occur as small efflorescences on the surface of quartz, calcite, or gypsum, which coexisted in the same places as uranyl carbonates. Rabejacite forms small aggregates up to a few nanometers (Figure 12).
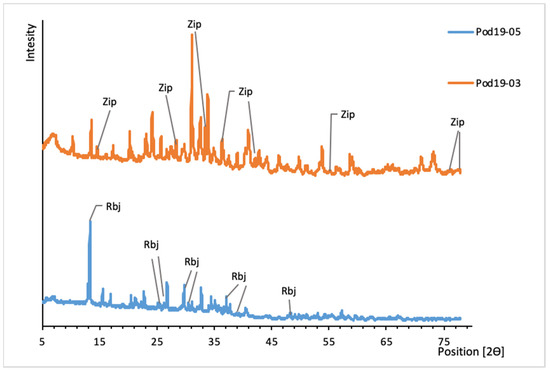
Figure 11.
XRD patterns of uranyl sulfates from Podgórze mine. Rbj, rabejacite; Zip, zippeite.

Table 8.
Unit cell parameters of uranyl sulfates from Podgórze mine.
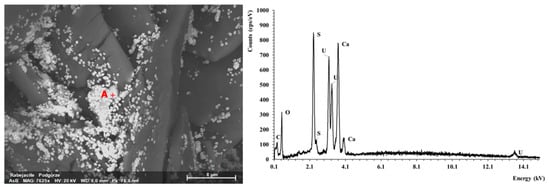
Figure 12.
SEM photo of rabejacite aggregates with EDS spectrum (Point A).
3.6. Uranyl Oxide-Hydroxides
The XRD analyses confirmed the presence of schoepite ((UO2)8O2(OH)12·12H2O) and metaschoepite ((UO2)8O2(OH)12·10H2O) (Figure 13). The calculated unit cell parameters of uranyl oxide-hydroxides are presented in Table 9. Uranyl oxide-hydroxides create a thin film on the surface of weathered uraninite, which was found in the corridor of 19 adit.
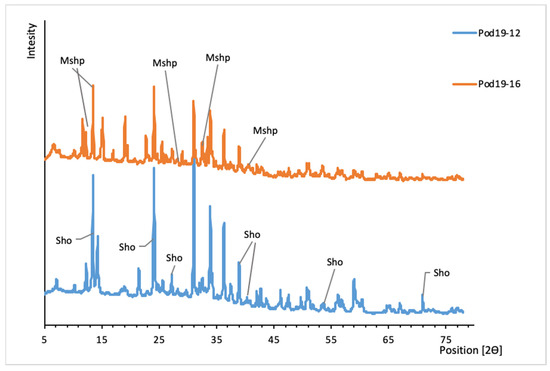
Figure 13.
XRD patterns of uranyl oxide-hydroxides from Podgórze mine. Sho, schoepite; Mshp, metaschoepite.

Table 9.
Unit cell parameters of uranyl oxide-hydroxides from Podgórze mine.
4. Rare Earth Elements in Secondary Uranium Minerals in Podgórze Mine
The main sources of REEs in the Podgórze area may be primary uraninite [19] and co-occurring fluorite. Both minerals were formed from low-temperature hydrothermal solutions enriched in REEs. A similar observation was made by Göb et al. [20,21]. Another source of REEs could be hosting rocks in which the deposit is located [22,23,24,25]. Weathering processes can release REEs contained in primary minerals and surrounding rocks. These elements can then be incorporated into secondary uranium minerals. The REE concentrations in uranyl minerals from the Podgórze mine are presented in Table 10. Uranyl carbonates and arsenates generally have low REE concentrations, in contrast to uranyl silicates, phosphates, and hydroxides, which have higher concentrations. The REE content in the surrounding rocks and secondary uranium mineralization was normalized to post-Archean Australian shale (PAAS) (Supplementary Table S2, Figure 14B) [26]. After averaging the results and normalizing the REE content to PAAS, it was clear that uranyl silicates and phosphates showed very strong HREE positive anomalies (Figure 14A). The uranyl minerals can be divided into two groups according to REE content: one group is represented by schoepite, schröckengerite, liebigite, nováčekite-II, uranospinie, zeunnerite, and heinrichite (Figure 14C), which revealed a positive Eu anomaly and Ce depletion, and the other group is represented by autunite, uranophane, and uranpohane-β, which showed depletion in lanthanum and cerium (Figure 14D). Moreover, the uranophane and autunite showed the depletion in Eu.

Table 10.
Trace element concentrations in secondary uranium minerals from Podgórze area.
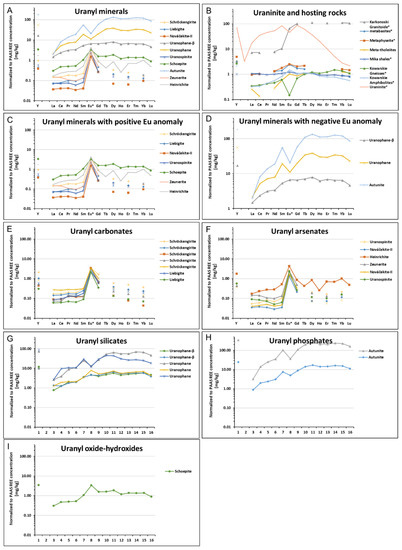
Figure 14.
REE distribution patterns: (A) all uranyl minerals, (B) uraninite and surrounding rocks *, (C) first group of uranyl minerals, (D) second group of uranyl minerals, (E) uranyl carbonates, (F) uranyl arsenates, (G) uranyl silicates, (H) uranyl phosphates, and (I) uranyl oxide-hydroxides. * Literature data.
5. Thermodynamic Modeling of Uranyl Mineral Stability
The chemical composition of mine water, taken from a paper by Goliáš et al. [27], was used for thermodynamic modeling (Table 11). Calculated aqueous species of uranium in the mine water are presented in Supplementary Table S3. The results of our calculations show that the aqueous species of uranyl ions in the mine water are represented by UO2HAsO4 (aq), UO2CO3(OH)3−, UO2CO3 (aq), and UO2OH+. Moreover, the mineral saturation states show good correlation with the mineral composition of the weathering zone in the Podgórze mine (Supplementary Table S4). The obtained phase stability diagrams indicate that the schröckingerite phase would crystallize in a carbonate-anion-containing environment (Figure 15A). If fluorides or sulfates are lacking in the environment, liebigite would crystallize (Figure 15B). In the absence of carbonates, dissolved silica may play the main role in controlling phase crystallization (Figure 15C). In circumstances where dissolved silica, fluorides, or sulfates are missing and the activity of carbonates is low, liebigite may crystallize. Moreover, the low activity of carbonates and higher activity of sulfates and fluorides may cause the crystallization of schröckeringite (Figure 15D). Otherwise, uranophanes may crystalize (Figure 15E). Higher activity of arsenates in the environment will cause uranospinite crystallization (Figure 15F).

Table 11.
Physicochemical parameters of water used for thermodynamic modeling *.
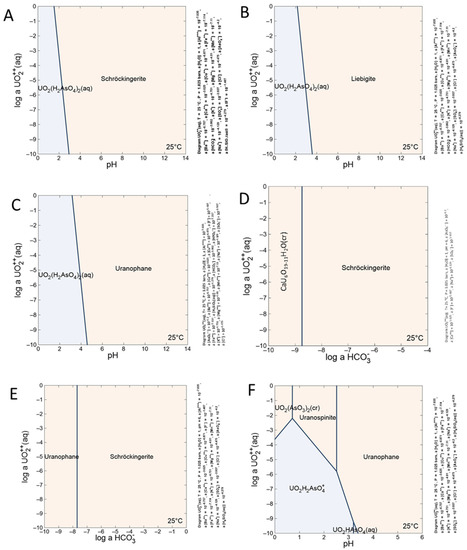
Figure 15.
Stability fields of selected uranyl minerals from Podgórze mine. (A,B) Uranyl ion activity versus pH for schröckingerite and uranophane, respectively. (C,D) Stability fields of uranospinite–schröckingerite and uranophane–schröckingerite, respectively, depending on UO22+, HCO3−, and UO2(H2AsO4)2 (aq) activity. (E,F) Dependence on uranyl activity and pH of liebigite and uranospinite, respectively.
6. Discussion
The oxidation of uraninite in the Podgórze mine led to the formation of various secondary uranyl minerals. As a result of field observations, several characteristic assemblages of secondary uranium minerals could be distinguished in the study area. Based on the literature data, it can be assumed that the decomposition of uraninite begins at a slightly acidic pH of ≈5.7 and partial pressure of CO2 of 3.87 × 10−6 atm [1,28,29]. At the beginning, uraninite decomposes into schoepite and metaschoepite. These phases coat the uraninite aggregates. Similar observations were made by Plášil regarding other uranium deposits [1]. However, uranyl oxide-hydroxides are unstable at pH < 6 and begin to slowly dissolve, releasing uranyl ions into the solution. At this point, uranyl-carbonate complexes such as uranyl mono-, di-, and tricarbonate [28,29,30] take on the most important role. In the first assemblage of uranyl minerals in the Podgórze mine, the most common speciation of uranyl carbonate is probably uranyl tricarbonate. This is indicated by the presence of liebigite. The crystallization of this mineral requires higher activity of carbonate ions. Furthermore, it indicates that the water could have had a pH of 5 to 8 and low activity of sulfate and fluoride ions. In these conditions, liebigite is the most stable phase. It is noteworthy that liebigite crystallizes only in heavily ventilated mining galleries. Under these conditions, the solution is concentrated and the solubility index of liebigite is exceeded. In some parts of the Podgórze weathering zone, the activity of sulfate ions increases due to sulfide oxidation. In such conditions, schröckingerite may crystallize. Its presence also indicates an increased fluoride content in the solution. Moreover, the coexistence of liebigite and schröckingerite was sometimes observed, which may suggest the mixing of pore water of different compositions (Figure 16A).
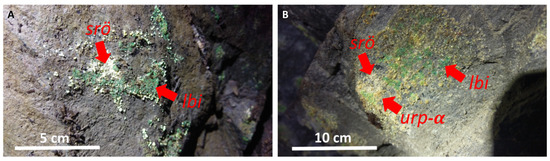
Figure 16.
Uranyl mineral assemblage from Podgórze mine: (A) liebigite (lbi) and schröckingerite (shö); (B) liebigite (lbi), schröckingerite (shö), and uranyl silicates (urp-α).
Uranyl carbonate minerals are sometimes accompanied by uranyl sulfates (zippeite and rabejacite). Their presence proves a further increase in sulfate ion activity in the environment and indicates a decrease in pH to 3. In such conditions, the uranyl sulfate complexes are stable [30,31]. Crystallization of uranyl sulfates in Menzenschwand, Wittichen in the Schwarzwald, and the Bohemian Massif, SE Germany, was observed at similar pH values [20,21,32]. Moreover, sulfate crystallization may be also related to the depletion of carbonate ion, which prevents stronger acidification of solution. The formation of uranyl silicates (uranophane, uranophane-β, sklodowskite) may be connected to the next stage of development of described paragenesis (Figure 16B); they had an HREE distribution similar to uranyl carbonates. However, a depletion in Eu was observed in uranyl silicates. This depletion suggests the crystallization of uranyl silicates from solutions that may have changed their redox potential during migration because Eu is sensitive to changes in redox potential and is removed from the solution in the reducing environment [33].
Uranophane-β and uranophane also differed in their niobium content. Higher Nb content has been found in uranophane (Table 9). This would suggest that they crystallized from solution with different Nb concentrations due to its low mobility. Uranophane with elevated Nb content probably crystalized in the vicinity of weathered uraninite enriched with this element (Table 9). The proportions of LREE and HREE contents in uranyl silicates can be used to estimate their crystallization conditions [34]. The Ce depletion and europium enrichment compared to uraninite may suggest that uranyl silicates were formed in pH higher than 5.9 [35]. This is also confirmed by the results of thermodynamic modeling. The positive Ce anomaly also suggests that uranyl silicates crystallize in slightly acidic and locally anaerobic conditions [36].
The next assemblage of secondary uranium minerals is related to the outflow of water from fissures in the walls of the mine galleries (Figure 2B). In these places, uranyl arsenates, such as uranospinite, nováčekite-II, and natrouranospinite, were found (Figure 17). The chemical composition of this solution was strongly modified by local processes of arsenic ore oxidation. Arsenates in the Podgórze mainly come from the decomposition of arsenopyrite. Its decay begins at low pH and it took place even faster than pyrite decomposition. The decomposition of arsenopyrite leads to the formation of mostly scorodite. It starts to rapidly dissolve at higher pH values (close to neutral or higher) [37]. However, we did not find this mineral in the Podgórze. An additional source of arsenic in the weathering zone may also be pyrite itself, which, according to the literature data, can contain up to 19% of arsenic [38]. Moreover, the results of thermodynamic modeling show that uranyl arsenates are stable under these conditions. These minerals show strong depletion in both LREE and HREE (Figure 14C), which can be explained by the oxidizing nature of water circulating in the subsurface part of the deposit. A strong positive europium anomaly was observed in uranyl arsenates. This anomaly also indicates the oxidizing nature of the solutions from which arsenates crystallize [33,39,40].

Figure 17.
Example of second paragenesis from Podgórze mine.
Another assemblage of uranyl minerals appeared in the mine dump in the Podgórze area. Arsenates (uranospinite, heinrichite, and zeunerite) and phosphates (autunite and metauranocircite) were found there. Uranyl phosphates and arsenates contain higher concentrations of HREE than LREE (Figure 14C,D). As in the case of the assemblages described above, the REE concentrations in arsenates and phosphates collected from the heap confirm the distinctly oxidizing nature of their crystallization environment.
Similar uranyl minerals occur in close proximity to the study area in the Medvědín deposit in the Czech Republic, which is located in the same deposit type [18]. The differences between these two deposits may be related to the composition of primary mineralization. There are larger amounts of arsenic ores in the Podgórze mine, resulting in a higher abundance of uranyl arsenates. On the contrary, higher phosphate ion concentration and activity are observed in Medvědín. Besides, in the Medvědín deposit, REE supergene mineralization was found. The occurrence of agardite-(Y), churchite-(Y), and an unnamed phase (Pb(Ce,REE)3(PO4)3(OH)2·nH2O) was recognized there [18]. The REE and Y distribution patterns showed similar trends to the uranyl arsenates and phosphates from the Podgórze mine, with depletion in HREE and enrichment in Y and Sm. The REE and Y concentration patterns in secondary minerals from these two deposits may suggest similar sources of REE.
The decomposition of primary ore mineralization in Podgórze releases a major number of elements into the environment, some of which are toxic. In the Podgórze deposit, elements such As and U are released into the environment [27]. However, the toxic metals’ halo in the groundwater system around the Podgórze mine was not a subject of research. It may be assumed that the major role in the controlling of immobilization of these elements may be the crystallization of uranyl minerals, but it needs to be investigated further.
7. Conclusions
Uranyl minerals from the Podgórze mine can be divided into three assemblages. The first one is associated with heavily ventilated mining galleries, where liebigite, schröckingerite, uranophane, uranophane-β, sklodowskite, zippeite, and rabejacite occur. The presence of uranyl sulfates (zippeite and rabejacite) depends on the increasing activity of sulfate ion due to local sulfide oxidation. The coexistence of liebigite and schröckingerite may suggest the mixing of pore water of different compositions. The next assemblage is related to the outflow of water from fissures in the walls of the mine galleries, where uranospinite, nováčekite-II, and natrouranospinite occur. The last assemblage appears in the mine dump, where there is increased activity of other weathering products. Arsenates (uranospinite, heinrichite, and zeunerite) and phosphates (autunite and metauranocircite) have been found there.
The results of thermodynamic modeling show that the aqueous species of uranyl ion in the mine water are represented by UO2HAsO4 (aq), UO2CO3(OH)3−, UO2CO3 (aq), and UO2OH+. In the first assemblage of uranyl minerals, the most common speciation of uranyl carbonate was probably uranyl tricarbonate, as indicated by the presence of liebigite. Crystallization of this mineral requires higher activity of carbonate ions.
Primary uraninite may be the main source of REEs in the Podgórze area. Uranyl carbonates and arsenates have generally low concentrations of REEs compared to uranyl silicates, phosphates, and hydroxides, which have higher concentrations. Uranyl silicates and phosphates show very strong positive HREE anomalies. According to REE content, uranyl minerals can be divided into two groups. The first group is represented by schoepite, schröckengerite, liebigite, nováčekite-II, uranospinie, zeunnerite, and heinrichite. These minerals reveal a positive Eu anomaly and Ce depletion. The second group is represented by autunite, uranophane, and uranpohane-β, which showed depletion in lanthanum and cerium. Uranospinite, nováčekite-II, and natrouranospinite from the second assemblage showed strong depletion in both LREE and HREE. Uranyl phosphates and arsenates from the mine dump contained higher concentrations of HREE than LREE. As in the case of the assemblages described above, the REE concentrations in arsenates and phosphates from the heap confirm the distinctly oxidizing nature of their crystallization environment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/min12030307/s1, Table S1: The standards, analytical lines of each analyzed element, used analytical crystals, and detection limits for the electron probe analysis. Table S2: REE concentrations in secondary uranium minerals from Podgórze mine. Table S3: REE concentrations after PAAS normalization and chondrite normalization. Table S4: Uranium aqueous species from waters at Podgórze mine. Table S5: Mineral saturation states of water from Podgórze mine.
Author Contributions
M.D.S. analyzed the data and wrote the manuscript. M.D.S. conducted XRD, SEM-EDS, and EPMA analyses. J.P. and R.S. contributed to writing the article. All authors approved the manuscript. J.R. and Š.M. conducted the ICP-MS measurements and conducted sample dissolution for these measurements. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the European Union within the European Regional Development Fund, through an Innovative Economy grant (POIG.02.02.00-00-025/09). Analytical procedures were performed thanks to institutional project RVO67985831.
Acknowledgments
The authors would like to thank M. Kałaska and P. Sierpień for their help with field work. The authors would also like to thank P. Guzik from Kopalnia Podgórze–Podziemna Trasa Turystyczna (Podgórze Mine—Underground Tourist Route) for enabling sampling for the research. Analytical procedures were performed thanks institutional project RVO67985831.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Plášil, J. Oxidation-Hydration Weathering of Uraninite: The Current State-of-Knowledge. J. Geosci. 2014, 59, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Ohnuki, T.; Isobe, H.; Sato, T. Mobility of Uranium during Weathering. Am. Mineral. 1997, 82, 888–899. [Google Scholar] [CrossRef]
- Sato, M. Oxidation of Sulfide Ore Bodies; 1, Geochemical Environments in Terms of Eh and pH. Econ. Geol. 1960, 55, 928–961. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Panova, E.G.; Lessovaia, S.N. (Eds.) Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature; Lecture Notes in Earth System Sciences; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-21613-9. [Google Scholar]
- Samuels, T.; Bryce, C.; Landenmark, H.; Marie-Loudon, C.; Nicholson, N.; Stevens, A.H.; Cockell, C. Microbial Weathering of Minerals and Rocks in Natural Environments. In Biogeochemical Cycles: Ecological Drivers and Environmental Impact; Dontsova, K., Balogh-Brunstad, Z., Le Roux, G., Eds.; Geophysical Monograph Series; Wiley: Hoboken, NJ, USA, 2020; pp. 59–79. ISBN 978-1-119-41330-1. [Google Scholar]
- Gupta, D.K.; Walther, C. (Eds.) Uranium in Plants and the Environment; Radionuclides and Heavy Metals in the Environment; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-14960-4. [Google Scholar]
- Burns, P.C.; Finch, R.J. (Eds.) Uranium: Mineralogy, Geochemistry, and the Environment; Reviews in Mineralogy & Geochemistry; De Gruyter: Berlin, Germany, 1999; Volume 38. [Google Scholar]
- Mochnacka, K.; Oberc-Dziedzic, T.; Mayer, W.; Pieczka, A. Ore Mineralization Related to Geological Evolution of the Karkonosze-Izera Massif (the Sudetes, Poland)—Towards a Model. Ore Geol. Rev. 2015, 64, 215–238. [Google Scholar] [CrossRef]
- Teisseyre, J.H. Skały Metamorficzne Rudaw Janowickich i Grzbietu Lasockiego. Geol. Sudet. 1973, 8, 8–111. [Google Scholar]
- Kaczmarek, L. Uranium Potential of the Karkonosze Granite and Its Eastern Envelope; Unpublished Industrial Report; Archive of the AGH University of Science and Technology: Cracow, Poland, 1959; pp. 15–48. (In Polish) [Google Scholar]
- Zimnoch, E. Gites Métamorphises des Minerais de Fer Dans les Sudetes Compares au Point de Vue de Structure aux Autres Gisements Analogues. Geol. Sudet. 1967, 3, 251–296, (In Polish, French Summary). [Google Scholar]
- Mochnacka, K. The Geology of the Polymetallic Deposit at Kowary (Lower Silesia). Pr. Geol. PAN 1967, 40, 7–73, (In Polish, English Summary). [Google Scholar]
- Mochnacka, K. Ore Minerals of the Polymetallic Deposit at Kowary (Lower Silesia). Pr. Mineral. PAN 1966, 4, 7–71, (In Polish, English Summary). [Google Scholar]
- Pacuła, J.; Cwojdziński, S.; Ihnatowicz, A.; Kozdrój, W. Szczegółowa Mapa Geologiczna Polski 1:50 000. Ark. Kowary (832); Państwowy Instytut Geologiczny—Państwowy Instytut Badawczy: Warsaw, Poland, 2017. [Google Scholar]
- Guillaumont, R.; Fanghänel, T.; Neck, V.; Fuger, J.; Palmer, D.A.; Grenthe, I.; Rand, M.H. Update on the Chemical Thermodynamics of Uranium, Neptunium, Plutonium, Americium and Technetium; Mompean, F.J., Illemassene, M., Domenech-Orti, C., Ben Said, K., Eds.; Chemical Thermodynamics; Elsevier: Amsterdam, The Netherlands; OECD Nuclear Energy Agency: Paris, France, 2003; ISBN 978-0-444-51401-1. [Google Scholar]
- Mamane Mamadou, M.; Cathelineau, M.; Deloule, E.; Schmitt, R.; Brouand, M. Cenozoic Oxidation Episodes in West Africa at the Origin of the in Situ Supergene Mineral Redistribution of the Primary Uranium Orebodies (Imouraren Deposit, Tim Mersoï Basin, Northern Niger). Miner. Depos. 2020, 55, 1333–1352. [Google Scholar] [CrossRef]
- Baik, M.-H.; Cho, H.-R. Roles of Uranyl Silicate Minerals in the Long-Term Mobility of Uranium in Fractured Granite. J. Radioanal. Nucl. Chem. 2021, 331, 451–459. [Google Scholar] [CrossRef]
- Plášil, J.; Sejkora, J.; Čejka, J.; Škoda, R.; Goliáš, V. Supergene Mineralization of the Medvědín Uranium Deposit, Krkonoše Mountains, Czech Republic. J. Geosci. 2012, 54, 15–56. [Google Scholar] [CrossRef] [Green Version]
- Frimmel, H.E.; Schedel, S.; Brätz, H. Uraninite Chemistry as Forensic Tool for Provenance Analysis. Appl. Geochem. 2014, 48, 104–121. [Google Scholar] [CrossRef]
- Gob, S.; Guhring, J.-E.; Bau, M.; Markl, G. Remobilization of U and REE and the Formation of Secondary Minerals in Oxidized U Deposits. Am. Mineral. 2013, 98, 530–548. [Google Scholar] [CrossRef]
- Gob, S.; Wenzel, T.; Bau, M.; Jacob, D.E.; Loges, A.; Markl, G. The Redistribution of Rare-Earth Elements in Secondary Minerals of Hydrothermal Veins, Schwarzwald, Southwestern Germany. Can. Mineral. 2011, 49, 1305–1333. [Google Scholar] [CrossRef]
- Winchester, J.A.; Floyd, P.A.; Chocyk, M.; Horbowy, K.; Kozdroj, W. Geochemistry and Tectonic Environment of Ordovician Meta-Igneous Rocks in the Rudawy Janowickie Complex, SW Poland. J. Geol. Soc. 1995, 152, 105–115. [Google Scholar] [CrossRef]
- Oberc-Dziedzic, T.; Pin, C.; Kryza, R. Early Palaeozoic Crustal Melting in an Extensional Setting: Petrological and Sm-Nd Evidence from the Izera Granite-Gneisses, Polish Sudetes. Int. J. Earth Sci. 2005, 94, 354–368. [Google Scholar] [CrossRef]
- Oberc-Dziedzic, T.; Kryza, R.; Mochnacka, K.; Larionov, A. Ordovician Passive Continental Margin Magmatism in the Central-European Variscides: U-Pb Zircon Data from the SE Part of the Karkonosze-Izera Massif, Sudetes, SW Poland. Int. J. Earth Sci. 2010, 99, 27–46. [Google Scholar] [CrossRef]
- Oberc-Dziedzic, T.; Kryza, R.; Pin, C.; Mochnacka, K.; Larionov, A. The Orthogneiss and Schist Complex of the Karkonosze-Izera Massif (Sudetes, SW Poland): U-Pb SHRIMP Zircon Ages, Nd-Isotope Systematics and Protoliths. Geol. Sudet. 2009, 41, 3–24. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution: An Examination of the Geochemecial Record Preserved in Sedimentary Rocks; Geoscience Texts; Blackwell: Oxford, UK, 1985; ISBN 978-0-632-01148-3. [Google Scholar]
- Goliáš, V.; Przylibski, T.A.; Lipanský, T.; Dohnal, J.; Miśta, W.; Nowakowski, R.; Tejnecký, V.; Mokrá, Z.; Vávrová, J.; Šimon, J.; et al. Prameny radioaktivních minerálních vod na území Kowary—Horní Malá Úpa. Opera Corcon. 2010, 75–90. Available online: https://www.proquest.com/docview/870631298 (accessed on 21 June 2021).
- Omel’yanenko, B.I.; Petrov, V.A.; Poluektov, V.V. Behavior of Uranium under Conditions of Interaction of Rocks and Ores with Subsurface Water. Geol. Ore Depos. 2007, 49, 378–391. [Google Scholar] [CrossRef]
- Jokelainen, L.; Markovaara-Koivisto, M.; Read, D.; Lindberg, A.; Siitari-Kauppi, M.; Hellmuth, K.-H. Understanding Uranium Behaviour at the Askola Uranium Mineralization. Radiochim. Acta 2010, 98, 743–747. [Google Scholar] [CrossRef]
- Langmuir, D. Uranium Solution-Mineral Equilibria at Low Temperatures with Applications to Sedimentary Ore Deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Riedel, T.; Kübeck, C. Uranium in Groundwater—A Synopsis Based on a Large Hydrogeochemical Data Set. Water Res. 2018, 129, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Dill, H.G.; Gerdes, A.; Weber, B. Age and Mineralogy of Supergene Uranium Minerals—Tools to Unravel Geomorphological and Palaeohydrological Processes in Granitic Terrains (Bohemian Massif, SE Germany). Geomorphology 2010, 117, 44–65. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium Redox Equilibria in Aqueous Solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Lee, S.-G.; Lee, D.-H.; Kim, Y.; Chae, B.-G.; Kim, W.-Y.; Woo, N.-C. Rare Earth Elements as Indicators of Groundwater Environment Changes in a Fractured Rock System: Evidence from Fracture-Filling Calcite. Appl. Geochem. 2003, 18, 135–143. [Google Scholar] [CrossRef]
- Inguaggiato, C.; Censi, P.; Zuddas, P.; Londoño, J.M.; Chacón, Z.; Alzate, D.; Brusca, L.; D’Alessandro, W. Geochemistry of REE, Zr and Hf in a Wide Range of PH and Water Composition: The Nevado Del Ruiz Volcano-Hydrothermal System (Colombia). Chem. Geol. 2015, 417, 125–133. [Google Scholar] [CrossRef]
- Chelnokov, G.A.; Kharitonova, N.A.; Bragin, I.V.; Aseeva, A.V.; Bushkareva, K.Y.; Liamina, L.A. The Geochemistry of Rare Earth Elements in Natural Waters and Secondary Mineral Sediments of Thermal Fields of Kamchatka. Mosc. Univ. Geol. Bull. 2020, 75, 196–204. [Google Scholar] [CrossRef]
- Harvey, M.C.; Schreiber, M.E.; Rimstidt, J.D.; Griffith, M.M. Scorodite Dissolution Kinetics: Implications for Arsenic Release. Environ. Sci. Technol. 2006, 40, 6709–6714. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of Gold in Arsenian Pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Bau, M. Rare-Earth Element Mobility during Hydrothermal and Metamorphic Fluid-Rock Interaction and the Significance of the Oxidation State of Europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Lakshtanov, L.Z.; Stipp, S.L.S. Experimental Study of Europium (III) Coprecipitation with Calcite. Geochim. Cosmochim. Acta 2004, 68, 819–827. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).