One-Step Synthesis of Iron and Titanium-Based Compounds Using Black Mineral Sands and Oxalic Acid under Subcritical Water Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Iron and Titanium-Based Compounds under sCW Conditions
2.3. Characterization
3. Results
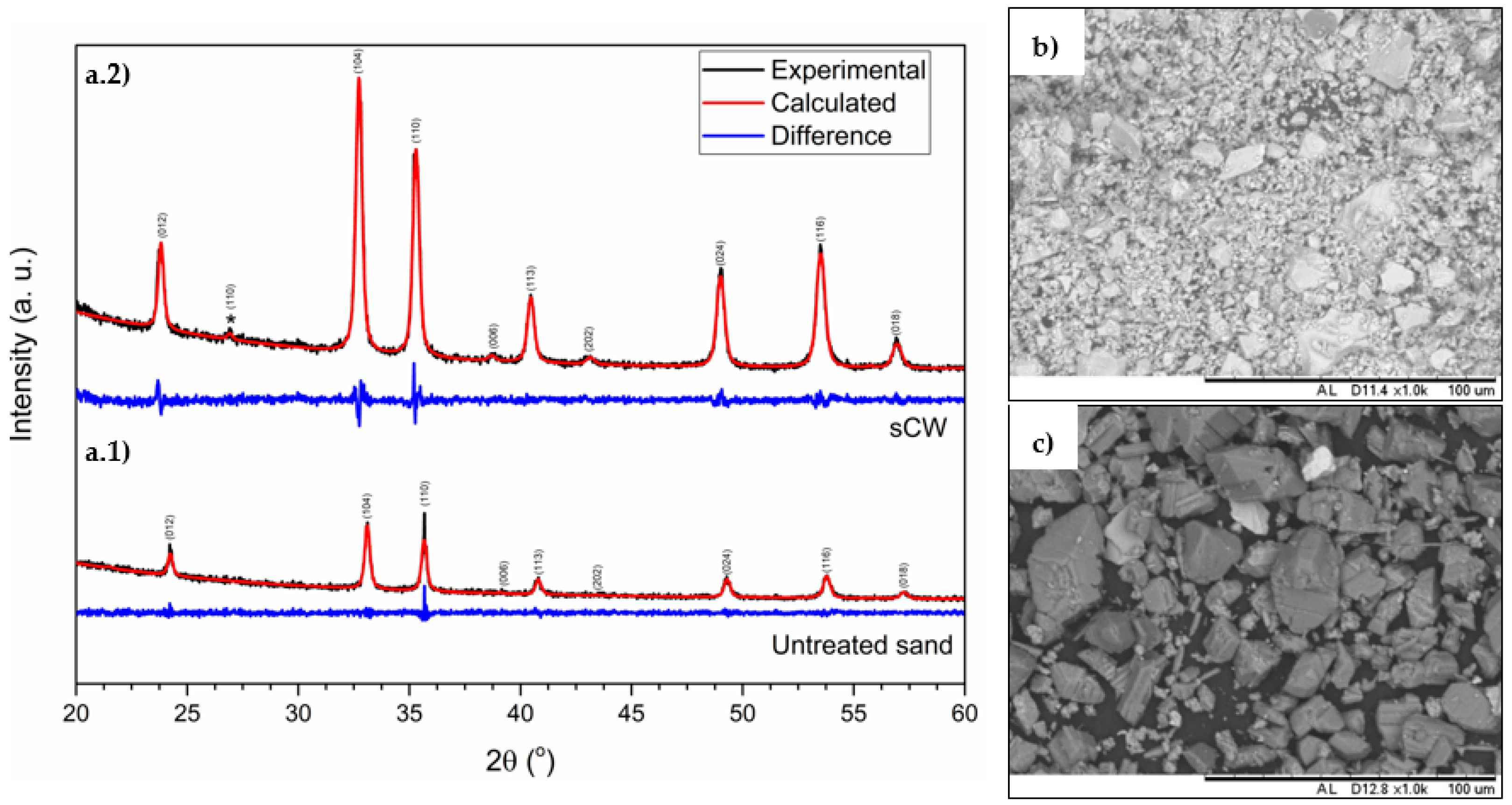
3.1. Synthesis of Iron and Titanium-Based Compounds under sCW Conditions
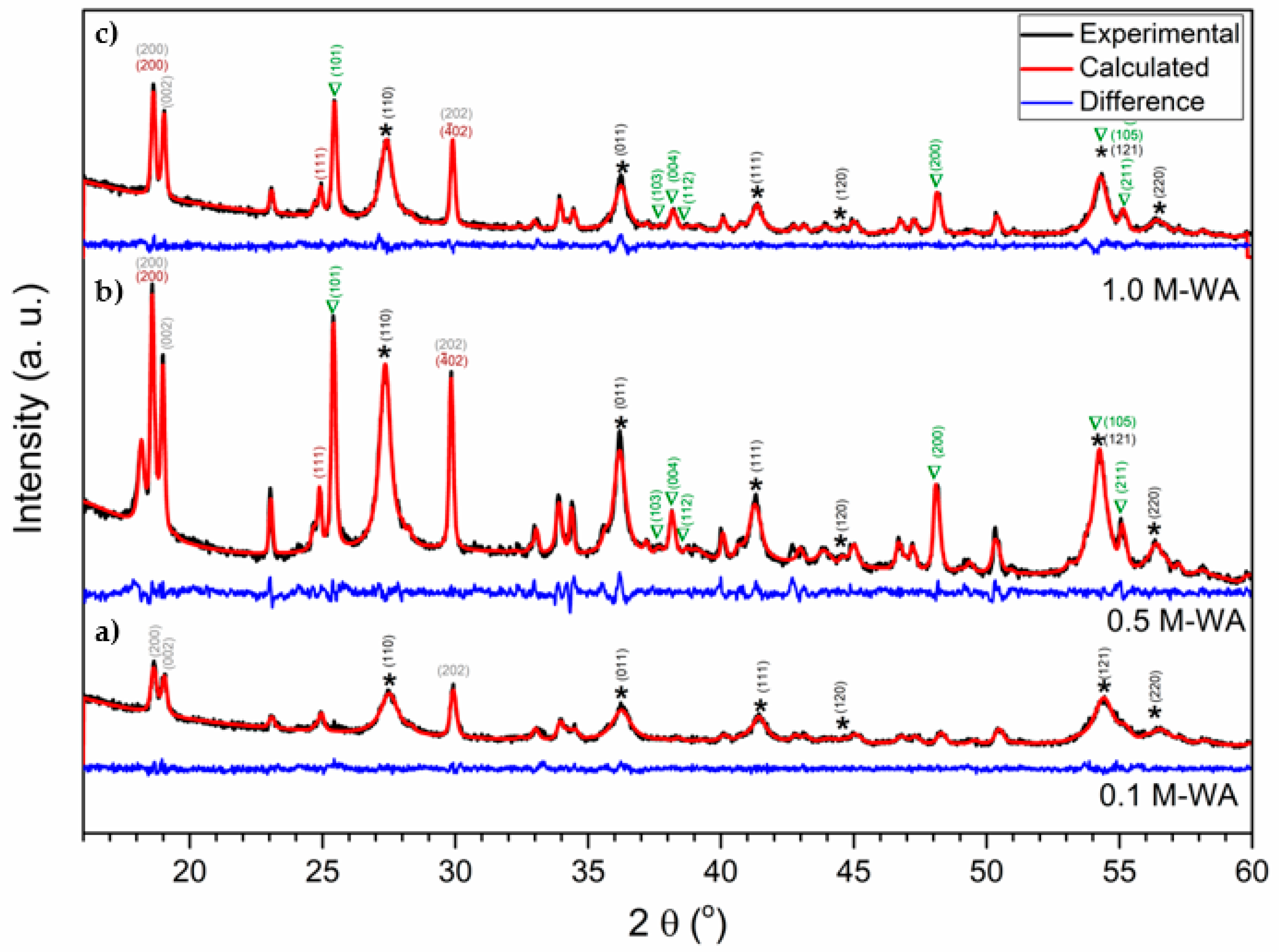
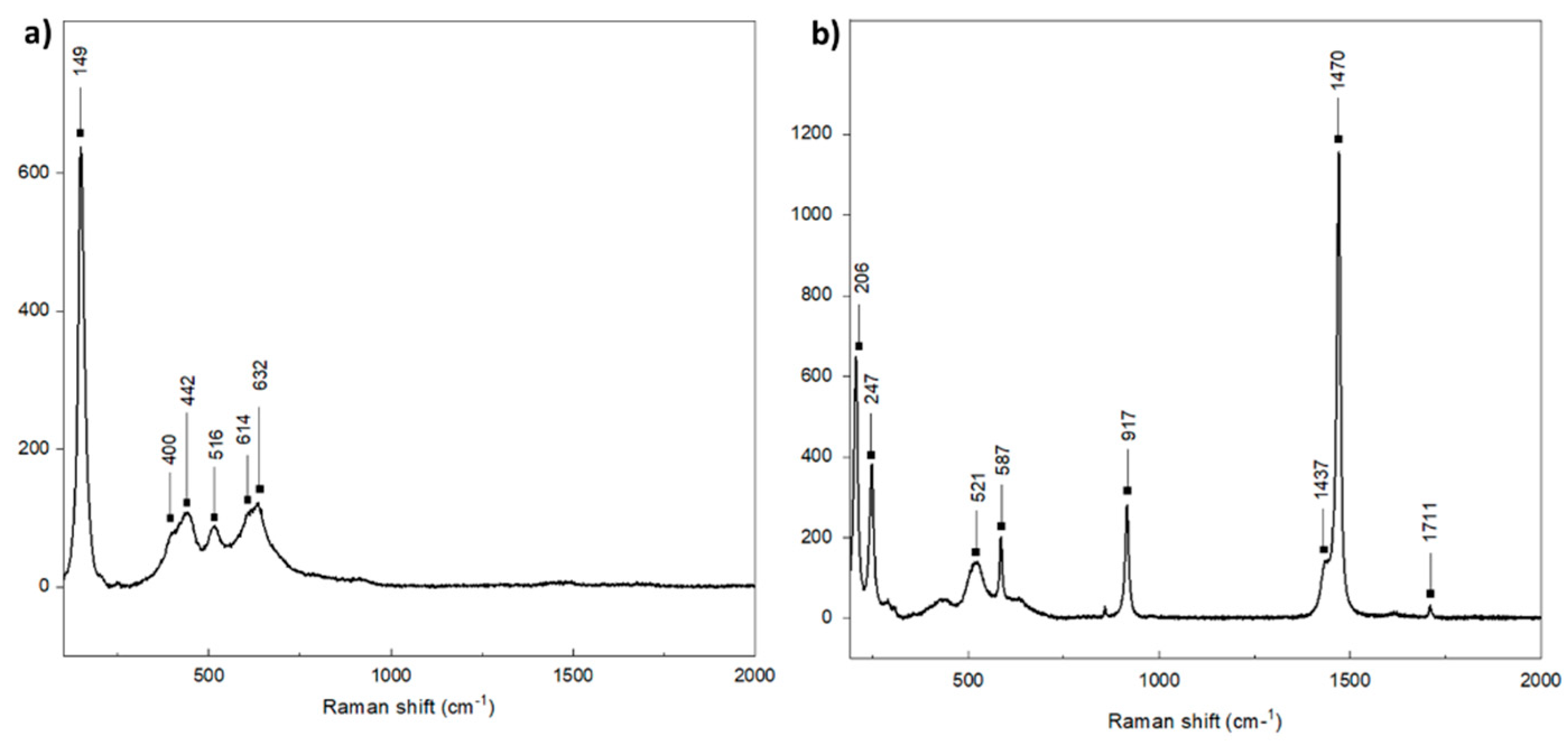
3.2. Characterization of the Obtained Iron and Titanium-Based Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farjana, S.H.; Huda, N.; Mahmud, M.A.P. Life-Cycle environmental impact assessment of mineral industries. IOP Conf. Ser. Mater. Sci. Eng. 2018, 351, 012016. [Google Scholar] [CrossRef]
- Filippou, D.; Hudon, G. Minerals, slags, and other feedstock for the production of titanium metal. In Extractive Metallurgy of Titanium: Conventional and Recent Advances in Extraction and Production of Titanium Metal; Fang, Z., Froes, H., Zhang, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 19–45. [Google Scholar] [CrossRef]
- Venugopal, R.; Sharma, T.; Saxena, V.; Mandre, N. (Eds.) International Seminar on Mineral Processing Technology (MPT-2005); Tata McGraw-Hill Education: New Delhi, India, 2005. [Google Scholar]
- Habashi, F. A New Process to Upgrade Ilmenite to Synthetic Rutile. COM 2014, 53rd Annual Conference of Metallurgists, Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Gupta, A.K.; Aula, M.; Pihlasalo, J.; Mäkelä, P.; Huttula, M.; Fabritius, T. Preparation of Synthetic Titania Slag Relevant to the Industrial Smelting Process Using an Induction Furnace. Appl. Sci. 2021, 11, 1153. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Paramore, J.D.; Sun, P.; Chandran, K.S.R.; Zhang, Y.; Xia, Y.; Cao, F.; Koopman, M.; Free, M. Powder metallurgy of titanium–past, present, and future. Int. Mater. Rev. 2018, 63, 407–459. [Google Scholar] [CrossRef]
- Jardim, P.M.; Mancic, L.; Marinkovic, B.A.; Milosevic, O.; Rizzo, F. Nax−yHyTi2−xFexO4·nH2O nanosheets with lepidocrocite-like layered structure synthesized by hydrothermal treatment of ilmenite sand. Cent. Eur. J. Chem. 2011, 9, 415–421. [Google Scholar] [CrossRef]
- Costa, A.M.L.M.; Marinkovic, B.A.; Suguihiro, N.M.; Smith, D.J.; Costa, M.E.H.M.; Paciornik, S. Materials Characterization Fe-doped nanostructured titanates synthesized in a single step route. Mater. Charact. 2015, 99, 150–159. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Glushenkov, A.M. Titanium Oxide Nanorods Extracted From Ilmenite Sands. Cryst. Growth Des. 2009, 9, 1240–1244. [Google Scholar] [CrossRef]
- Simpraditpan, A.; Wirunmongkol, T.; Pavasupree, S. Simple hydrothermal preparation of nanofibers from a natural ilmenite mineral. Ceram. Int. 2013, 39, 2497–2502. [Google Scholar] [CrossRef]
- Tao, T.; Glushenkov, A.M.; Liu, H.; Liu, Z.; Dai, X.J.; Chen, H.; Ringer, S.P.; Chen, Y. Ilmenite FeTiO3 Nanoflowers and Their Pseudocapacitance. J. Phys. Chem. C 2011, 35, 17297–17302. [Google Scholar] [CrossRef]
- Lagos, K.J.; Marinkovic, B.A.; Debut, A.; Vizuete, K.; Guerrero, V.H.; Pardo, E.; Pontón, P.I. Towards Iron-Titanium Oxide Nanostructures from Ecuadorian Black Mineral Sands. Minerals 2021, 11, 122. [Google Scholar] [CrossRef]
- Gao, F.; Nie, Z.; Yang, D.; Sun, B.; Liu, Y.; Gong, X.; Wang, Z. Environmental impacts analysis of titanium sponge production using Kroll process in China. J. Clean. Prod. 2018, 174, 771–779. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Feng, W.; Chen, X.; Zhang, L.; Tu, G. A Clean Process to Prepare High-Quality Acid-Soluble Titanium Slag from Titanium Middling Ore. Minerals 2019, 9, 460. [Google Scholar] [CrossRef]
- Blesa, M.A.; Marinovich, H.A.; Baumgartner, E.C.; Maroto, A.J.G. Mechanism of Dissolution of Magnetite by Oxalic Acid-Ferrous Ion Solutions. Inorg. Chem. 1987, 26, 3713–3717. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schindler, P.W. Photochemical dissolution of goethite in acid/oxalate solution. Clays Clay Miner. 1987, 35, 347–352. [Google Scholar] [CrossRef]
- Deng, B.; Wang, B.; Su, S.; Ding, S.; Sun, W. Recovery of iron from pyrolusite leaching slag by a lab-scale circulation process of oxalic acid leaching and ultraviolet irradiation. Metals 2018, 8, 8. [Google Scholar] [CrossRef]
- Panias, D.; Taxiarchou, M.; Paspaliaris, I.; Kontopoulos, A. Mechanisms of dissolution of iron oxides in aqueous oxalic acid solutions. Hydrometallurgy 1996, 42, 257–265. [Google Scholar] [CrossRef]
- Jonglertjunya, W.; Rubcumintara, T. Titanium and iron dissolutions from ilmenite by acid leaching and microbiological oxidation techniques. Asia-Pac. J. Chem. Eng. 2013, 8, 323–330. [Google Scholar] [CrossRef]
- Panias, D.; Taxiarchou, M.; Douni, I.; Paspaliaris, I.; Kontopoulos, A. Dissolution of hematite in acidic oxalate solutions: The effect of ferrous ions addition. Hydrometallurgy 1996, 43, 219–230. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Valdivieso-Ramírez, C.S. Pressurized fluid systems: Phytochemical production from biomass. J. Supercrit. Fluids 2015, 96, 228–244. [Google Scholar] [CrossRef]
- Galkin, A.A.; Lunin, V. V Subcritical and supercritical water: A universal medium for chemical reactions. Russ. Chem. Rev. 2005, 74, 21. [Google Scholar] [CrossRef]
- High Pressure Acid Leach. Available online: https://www.calderaengineering.com/industries-served/high-pressure-acid-leach-and-pressure-oxidation/high-pressure-acid-leach (accessed on 12 February 2022).
- Valdivieso-Ramírez, C.S.; Sanchez, J.-E.; Ganzle, M.; Temelli, F.; Saldaña, M.D.A. Carboxylic acid-catalysed hydrolysis of polygalacturonic acid in subcritical water media. J. Supercrit. Fluids 2021, 169, 105103. [Google Scholar] [CrossRef]
- Valdivieso-Ramirez, C.S.; Temelli, F.; Saldaña, M.D.A. Carboxylic acid-catalysed hydrolysis of rhamnogalacturonan in subcritical water media. J. Supercrit. Fluids 2021, 175, 105268. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Chen, M.C.; Koh, P.W.; Ponnusamy, V.K.; Lee, S.L. Titanium dioxide and other nanomaterials based antimicrobial additives in functional paints and coatings: Review. Prog. Org. Coatings 2022, 163, 106660. [Google Scholar] [CrossRef]
- Almeida, L.A.; Habran, M.; Carvalho, R.D.S.; da Costa, M.E.H.M.; Cremona, M.; Silva, B.C.; Krambrock, K.; Pandoli, O.G.; Morgado, E.; Marinkovic, B.A. The influence of calcination temperature on photocatalytic activity of TiO2-acetylacetone charge transfer complex towards degradation of Nox under visible light. Catalysts 2020, 10, 1463. [Google Scholar] [CrossRef]
- Balmer, M.E.; Sulzberger, B. Atrazine degradation in irradiated iron/oxalate systems: Effects of pH and oxalate. Environ. Sci. Technol. 1999, 33, 2418–2424. [Google Scholar] [CrossRef]
- Conte, L.O.; Schenone, A.V.; Alfano, O.M. Photo-Fenton degradation of the herbicide 2,4-in aqueous medium at pH conditions close to neutrality. J. Environ. Manag. 2016, 170, 60–69. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Xiong, S.; Chen, S.; Yin, X.; Wang, L.; Wang, H. The attractive efficiency contributed by the in-situ reactivation of ferrous oxalate in heterogeneous Fenton process. Appl. Surf. Sci. 2019, 467–468, 185–192. [Google Scholar] [CrossRef]
- Chauhan, R.; Dinesh, G.K.; Alawa, B.; Chakma, S. A critical analysis of sono-hybrid advanced oxidation process of ferrioxalate system for degradation of recalcitrant pollutants. Chemosphere 2021, 277, 130324. [Google Scholar] [CrossRef]
- Gu, H.; Hargreaves, J.S.J.; Jiang, J.Q.; Rico, J.L. Potential Routes to Obtain Value-Added Iron-Containing Compounds from Red Mud. J. Sustain. Metall. 2017, 3, 561–569. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ikeda, T.; Mitamura, T.; Kakizaki, K.; Hiratsuka, N. Preparation of magnetic particles by the pyrolysis of ferrous oxalate in various atmospheres. Funtai Oyobi Fummatsu Yakin/J. Japan Soc. Powder Powder Metall. 1996, 43, 89–94. [Google Scholar] [CrossRef][Green Version]
- Jia, Y.; Ni, J.; Fang, F. Thermal decomposition of ferrous oxalate nanowires to porous nanowire-like α-Fe2O3 with a good gas-sensing properties. Mater. Sci. Semicond. Process. 2021, 125, 105650. [Google Scholar] [CrossRef]
- Lagos, K.J.; Marinkovic, B.A.; Dosen, A.; Guamán, M.V.; Guerrero, V.H.; Pardo, E.; Pontón, P.I. Data on phase and chemical compositions of black sands from “El Ostional” beach situated in Mompiche, Ecuador. Data Br. 2020, 32, 106214. [Google Scholar] [CrossRef] [PubMed]
- Crossey, L.J. Thermal degradation of aqueous oxalate species. Geochim. Cosmochim. Acta 1991, 55, 1515–1527. [Google Scholar] [CrossRef]
- Martinez-Monteagudo, S.I.; Saldaña, M.D. Chemical Reactions in Food Systems at High Hydrostatic Pressure. Food Eng. Rev. 2014, 6, 105–126. [Google Scholar] [CrossRef]
- Van Eldik, R.; Asano, T.; Le Noble, W.J. Activation and Reaction Volumes in Solution. 2. Chem. Rev. 1989, 89, 549–688. [Google Scholar] [CrossRef]
- Chen, B.; Hoffmann, R.; Cammi, R. The Effect of Pressure on Organic Reactions in Fluids—A New Theoretical Perspective. Angew. Chemie—Int. Ed. 2017, 56, 11126–11142. [Google Scholar] [CrossRef]
- Demazeau, G. High Pressure and Chemical Bonding in Materials Chemistry. ChemInform 2006, 61b, 799–807. [Google Scholar] [CrossRef]
- Brown, N.E.; Navrotsky, A.; Nord, G.L.; Banerjee, S.K. Hematite-ilmenite (Fe2O3-FeTiO3) solid solutions: Determinations of Fe-Ti order from magnetic properties. Am. Mineral. 1993, 78, 941–951. [Google Scholar]
- Cao, G.; Yi, N. Quantitative Analysis of Anatase-Rutile Mixtures by Raman Spectroscopy. ChemistrySelect 2020, 5, 11530–11533. [Google Scholar] [CrossRef]
- Frost, R.L. Raman spectroscopy of natural oxalates. Anal. Chim. Acta 2004, 517, 207–214. [Google Scholar] [CrossRef]
- Molnár, Á. Acids and Acid Catalysis-Homogeneous. In Encyclopedia of Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Jung, Y.H.; Kim, K.H. Acidic Pretreatment. In Pretreatment of Biomass: Processes and Technologies; Ashok, P., Sangeeta, N., Paramenswaran, B., Christian, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 27–50. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical Water as Reaction Environment: Fundamentals of Hydrothermal Biomass Transformation. Chem. Sus. Chem. 2011, 4, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.C.; Gateman, S.M.; Stephens, L.I.; Lacasse, R.; Schulz, R.; Mauzeroll, J. Pourbaix Diagrams as a Simple Route to First Principles Corrosion Simulation. J. Electrochem. Soc. 2019, 166, C3186–C3192. [Google Scholar] [CrossRef]
- Arnaut, L. Elementary reactions in solution. In Chem. Kinet, 2nd ed.; Arnaut, L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 263–293. [Google Scholar] [CrossRef]
- Lee, S.O.; Tran, T.; Park, Y.Y.; Kim, S.J.; Kim, M.J. Study on the kinetics of iron oxide leaching by oxalic acid. Int. J. Miner. Process. 2006, 80, 144–152. [Google Scholar] [CrossRef]
- Vehmaanperä, P.; Gong, B.; Sit, P.H.L.; Salmimies, R.; Barbiellini, B.; Häkkinen, A. Formation of Humboldtine during the dissolution of hematite in oxalic acid-Density Funtional Theory (DFT) calculations and experimetal verification. Clays Clay Miner. 2021, 69, 1–8. [Google Scholar] [CrossRef]
- Lee, S.O.; Tran, T.; Jung, B.H.; Kim, S.J.; Kim, M.J. Dissolution of iron oxide using oxalic acid. Hydrometallurgy 2007, 87, 91–99. [Google Scholar] [CrossRef]
- Shahien, M.G.; Khedr, M.M.H.; Maurice, A.E.; Farghali, A.A.; Ali, R.A.M. Synthesis of high purity rutile nanoparticles from medium-grade Egyptian natural ilmenite. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 207–213. [Google Scholar] [CrossRef]
- Salmani Nuri, O.; Irannajad, M.; Mehdilo, A. Effect of surface dissolution by oxalic acid on flotation behavior of minerals. J. Mater. Res. Technol. 2019, 8, 2336–2349. [Google Scholar] [CrossRef]
- Omidi, M.H.; Nuri, O.S.; Tavakoli, H.; Omidi, M.H.; Nuri, O.S.; Tavakoli, H. Optimization of Ilmenite Dissolution by Synergistic Effect of Oxalic Acid and Hydrochloric Acid for Preparing Synthetic Rutile. Int. J. Nonferrous Metall. 2018, 7, 25–38. [Google Scholar] [CrossRef][Green Version]
- Corbin, D.R.; Griffin, T.P.; Keith, P.; Hutchenson, K.; Sheng, L.; Shiflett, M.B.; Toradi, C.; Zaher, J.J. Processes for Producing Titanium Dioxide. AU2006352486B2, 28 December 2006. [Google Scholar]
- Truong, Q.D.; Le, T.H.; Liu, J.Y.; Chung, C.C.; Ling, Y.C. Synthesis of TiO2 nanoparticles using novel titanium oxalate complex towards visible light-driven photocatalytic reduction of CO2 to CH3OH. Appl. Catal. A Gen. 2012, 437–438, 28–35. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef]
- García-Fernández, A.J.; Espín, S.; Gómez-Ramírez, P.; Martínez-López, E. Oxalates. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 1, pp. 730–734. [Google Scholar] [CrossRef]
- Swaney, R.; Akhtar, M.; Horn, E.; Lentz, M.; Klungness, J.; Sabourin, M. Oxalic acid pretreatment for mechanical pulping greatly improves paper strength while maintaining scattering power and reducing shives and triglycerides. In 2003 TAPPI Fall Technical Conference: Engineering, Pulping & PCE&I; TAPPI Press: Atlanta, GA, USA, 2003. [Google Scholar]
- Ado, A.; Yahaya, H.; Kwalli, A.A.; Abdulkadir, R. Dyeing of Textiles with Eco-Friendly Natural Dyes: A Review. Int. J. Environ. Monit. Prot. 2015, 1, 76–81. [Google Scholar]
- Veeken, A.H.M.; Hamelers, H.V.M. Removal of heavy metals from sewage sludge by extraction with organic acids. Water Sci. Technol. 1999, 40, 129–136. [Google Scholar] [CrossRef]
- Oustan, S.; Heidari, S.; Neyshabouri, M.R.; Reyhanitabar, A.; Bybordi, A. Removal of heavy metals from a contaminated calcareous soil using oxalic and acetic acids as chelating agents. In Proceedings of the International Conference on Environment Science and Engineering, Bali Island, Indonesia, 1–3 April 2011. [Google Scholar]
- Gaber, S.E.; Rizk, M.S.; Yehia, M.M. Extraction of certain heavy metals from sewage sludge using different types of acids. Biokemistri 2011, 23, 41–48. [Google Scholar]
- Kim, J.O.; Lee, Y.W.; Chung, J. The role of organic acids in the mobilization of heavy metals from soil. KSCE J. Civ. Eng. 2013, 17, 1596–1602. [Google Scholar] [CrossRef]
- Wei, M.; Chen, J.; Wang, X. Removal of arsenic and cadmium with sequential soil washing techniques using Na2EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261. [Google Scholar] [CrossRef]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.G.; Junier, P. Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar] [CrossRef]
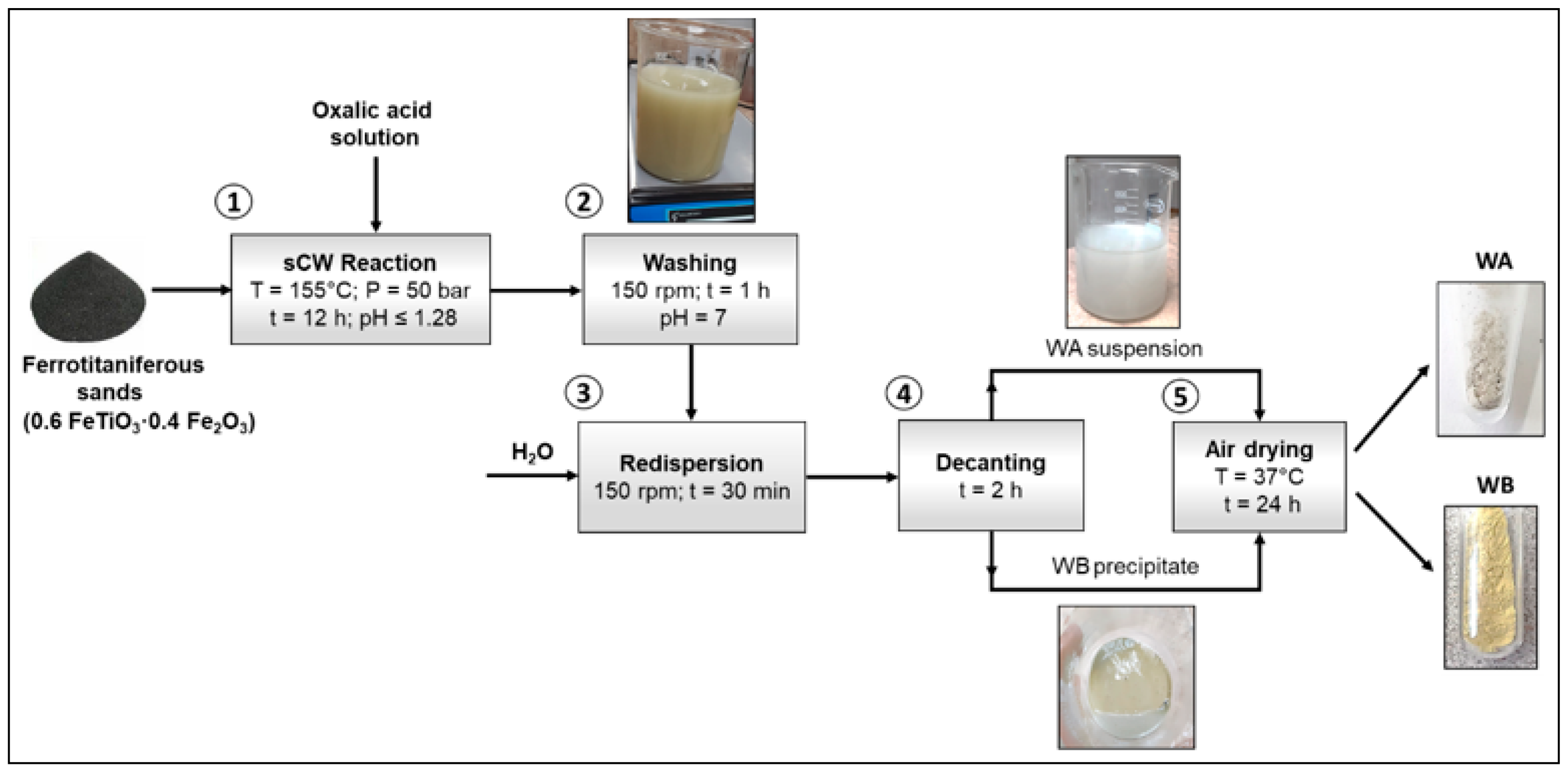
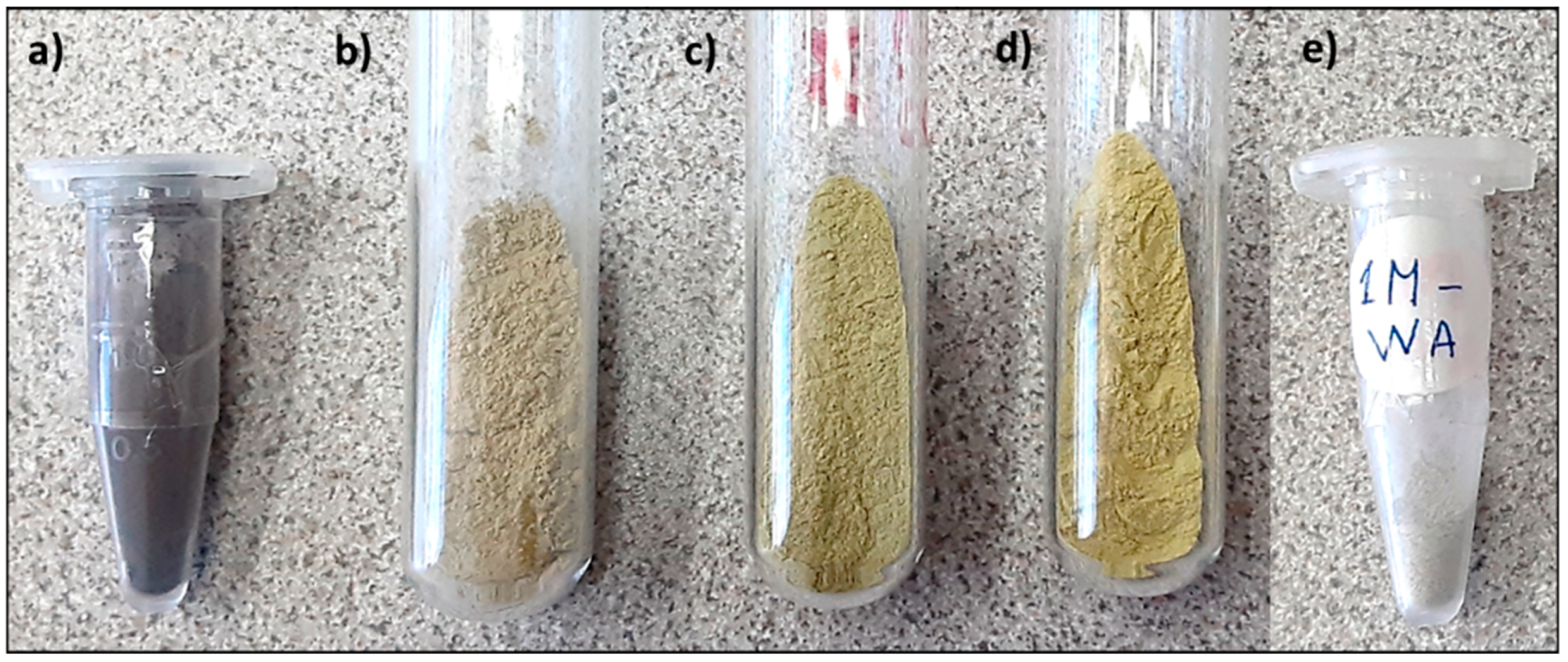


| Oxalic Acid Concentration (M) | Control Sample | Whitish Fraction (WA) | Yellowish Fraction (WB) |
|---|---|---|---|
| 0 (H2O) | sCW | ||
| 0.1 | 0.1 M-WA | 0.1 M-WB | |
| 0.5 | 0.5 M-WA | 0.5 M-WB | |
| 1.0 | 1.0 M-WA | 1.0 M-WB | |
| Oxalic Acid (M) | pH | Ka1 5.6 × 10−2 | Ka2 5.4 × 10−5 | Ionic Strength (M) | Main Crystalline Phases * (WA + WB) |
|---|---|---|---|---|---|
| C2HO4−1 (M) | C2O4−2 (M) | ||||
| 0.1 | 1.28 | 5.2 × 10−2 | 5.39 × 10−5 | 0.052 | Ferrous oxalate, rutile |
| 0.5 | 0.85 | 14.2 × 10−2 | 5.41 × 10−5 | 0.142 | Ferrous oxalate, rutile, anatase |
| 1.0 | 0.68 | 21.0 × 10−2 | 5.41 × 10−5 | 0.210 | Ferrous oxalate, rutile, anatase, hematite |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivieso-Ramírez, C.S.; Pontón, P.I.; Dosen, A.; Marinkovic, B.A.; Guerrero, V.H. One-Step Synthesis of Iron and Titanium-Based Compounds Using Black Mineral Sands and Oxalic Acid under Subcritical Water Conditions. Minerals 2022, 12, 306. https://doi.org/10.3390/min12030306
Valdivieso-Ramírez CS, Pontón PI, Dosen A, Marinkovic BA, Guerrero VH. One-Step Synthesis of Iron and Titanium-Based Compounds Using Black Mineral Sands and Oxalic Acid under Subcritical Water Conditions. Minerals. 2022; 12(3):306. https://doi.org/10.3390/min12030306
Chicago/Turabian StyleValdivieso-Ramírez, Carla S., Patricia I. Pontón, Anja Dosen, Bojan A. Marinkovic, and Victor H. Guerrero. 2022. "One-Step Synthesis of Iron and Titanium-Based Compounds Using Black Mineral Sands and Oxalic Acid under Subcritical Water Conditions" Minerals 12, no. 3: 306. https://doi.org/10.3390/min12030306
APA StyleValdivieso-Ramírez, C. S., Pontón, P. I., Dosen, A., Marinkovic, B. A., & Guerrero, V. H. (2022). One-Step Synthesis of Iron and Titanium-Based Compounds Using Black Mineral Sands and Oxalic Acid under Subcritical Water Conditions. Minerals, 12(3), 306. https://doi.org/10.3390/min12030306







