Abstract
Xanthates are by far the most widely used collectors in the froth flotation beneficiation of sulfide ores. However, the xanthate production process suffers from low yield, low productivity, long reaction time and environmental pollution. To address these issues, an effective method was developed for the synthesis of xanthates using phase transfer catalyst. Sodium isobutyl xanthate was synthesized from isobutyl alcohol ((CH3)2CHCH2OH), sodium hydroxide (NaOH) and carbon disulfide (CS2) with dichloromethane (CH2Cl2) as solvent and cetyltrimethylammonium bromide (CTAB), cetyltrimethylammonium chloride (CTAC), tetrabutylammonium bromide (TBAB) and tetrabutylammonium chloride (TBAC) as phase transfer catalyst. The compound was characterized by elemental analysis, infrared spectrum, 1H NMR and 13C NMR. The influencing factors on the content and yield of sodium isobutyl xanthate including phase transfer catalyst type, phase transfer catalyst dosage and reaction time were studied by single-factor experiments. The influencing factors on the product purity and yield including reaction temperature, solvent volume, material ratio and rotating speed were studied by orthogonal experiments. The results showed that when the amount of TBAC was 3.0%malcohol, the reaction temperature was 35 °C, the solvent volume was 3.5 Valcohol, the rotating speed was 180 rpm, the reaction time was 4 h and the material ratio was n[(CH3)2CHCH2OH]:n(NaOH):n(CS2) = 1:1:1.10, the product yield could be up to 86.66% and the product purity reached 82.56%.
1. Introduction
Sulfide ores are main mineral resource of non-ferrous metals and are widely used to extract elements such as copper, lead, zinc, molybdenum and nickel [1,2,3,4,5,6]. Demond for non-ferrous metals is growing in response to continuous advances in science and technology [7,8]. Currently, most non-ferrous metals are extracted from sulfide mineral, which is recovered from sulfide ores by flotation [9,10,11].
Typically, xanthates are used as sulfide mineral collectors [12,13,14,15]. Research into the synthesis technology of xanthates is important for improving the quality and reducing the cost [16]. There are various synthesis methods of xanthates, the difference lies in the proportion and sequence of the addition, the type of medium or solvent, the reaction equipment and the stirring method. The synthesis methods mainly include: direct synthesis method, crystallization method, solvent method and wet alkali method. Solvent synthesis of xanthates does not require an alkali-making system, the sodium hydroxide added in the synthesis process is easy to disperse, the solvent can remove the reaction heat in time and the conversion rate of alcohol is high [17]. However, there have been few studies that examine the involvement of phase transfer catalysts in the synthesis process.
Compared to other available collectors such as dithiocarbamate, xanthates are the most widely used as flotation reagent, the maximum consumption of collectors and the most economical sulfide ores collectors [18,19]; a medium-sized producer in China can sell 30,000 tons per year domestically and internationally [20]. Xanthates (R-O-CSSMe) have been shown to have weaker selectivity and better collectivity for sulfide minerals than dithiophosphate [21,22,23,24]. The xanthate molecule is composed of a non-polar group and a polar group. The non-polar group plays a hydrophobic role, and the polar group plays a role of immobilization [25,26]. This research has led to reduce the reaction time and improve the quality of xanthates such as ethyl xanthate, butyl xanthate and pentyl xanthate as collectors for flotation recovery of sulfide minerals.
Synthesis of xanthates is a complex physical and chemical process in which the phase transfer catalyst plays an essential role [27,28,29,30]. Adding the phase transfer catalyst is a useful approach for the longer reaction time of xanthate. Other synthesis methods have been proven to be inadequate, they take longer reaction time to obtain the same product quality [31]. There have been a number of investigations of synthesis processes for xanthates by adding the phase transfer catalysis [32]. In each case conditions, including a suitable phase transfer catalyst, reaction temperature, material ratio, solvent volume and reaction time, have been established for the synthesis of the desired product and depression of unwanted product.
In our study, we used CTAB, CTAC, TBAB and TBAC as phase transfer catalyst. We investigated the influence of the type and dosage of phase transfer catalyst and reaction time on the synthesis process. Further, we studied the internal connection and influence of reaction temperature, solvent volume, rotation speed and material ratio in the synthesis process of sodium isobutyl xanthate by the orthogonal experiment method and matrix analysis method.
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
Analytical-grade isobutyl alcohol ((CH3)2CHCH2OH), carbon disulfide (CS2) and sodium hydroxide (NaOH) were used to synthesize sodium isobutyl xanthate. Analytical-grade CTAB, CTAC, TBAB and TBAC are used as phase transfer catalyst.
2.1.2. Instruments
The four-necked flask, water bath, spiral condenser, rotary evaporator, thermometer and electronic balance were used as synthetic instruments. The Bruker Advance III 400 (Karlsruhe, Germany) nuclear magnetic resonance spectrometer (TMS was internal standard, CDCl3 was solvent), Perkin Elmer Frontier (Waltham, MA, USA) transform infrared spectrometer (KBr tablet) and Elemantar Vario Unicube (Hanau, Germany) elemental analyzer were used as characterization instruments.
2.1.3. Synthesis Mechanism
The (CH3)2CHCH2OH, NaOH and CS2 were used as raw materials to synthesize sodium isobutyl xanthate, the reaction could be written as follows:
(CH3)2CHCH2OH + NaOH → (CH3)2CHCH2ONa + H2O
(CH3)2CHCH2ONa + CS2 → (CH3)2CHCH2OCSSNa
(CH3)2CHCH2OH + NaOH + CS2 → (CH3)2CHCH2OCSSNa + H2O + Q
The reaction, which was exothermic, for the synthesis of sodium isobutyl xanthate was very fast. During the synthesis process, if the temperature was too high, some side reactions would occur, as shown in the following formulas:
6NaOH + 3CS2 → Na2CS3 + Na2CO3 + H2O
(CH3)2CHCH2OCSSNa + 2NaOH → NaOCOSNa + NaHS + (CH3)2CHCH2OH
The orange-red by-product generated by the side reaction affected the quality of sodium isobutyl xanthate.
In the synthesis process of sodium isobutyl xanthate, firstly (CH3)2CHCH2OH reacted with NaOH to form (CH3)2CHCH2ONa on the surface of granular sodium hydroxide; (CH3)2CHCH2ONa continued to collide with CS2 at the solid-liquid phase interface to react, reacting to produce (CH3)2CHCH2OCSSNa. (CH3)2CHCH2ONa had a low solubility in isobutyl alcohol and was insoluble in dichloromethane. The speed of peeling (CH3)2CHCH2ONa from the surface of the granular sodium hydroxide was slow, resulting in a longer reaction time. The kinetic study results showed that the process of ion pairs entering the organic phase from the solid surface was the key to the reaction speed in the solid-liquid phase reaction. After the phase transfer catalyst was added, ion exchange was carried out at the interface between the solid phase and the liquid phase. (CH3)2CHCH2O− was brought into the liquid phase, which would greatly increase the chance of collision between (CH3)2CHCH2O− and CS2, and increase the nucleophilic reaction rate, increasing the chemical reaction speed of the entire reaction process.
2.2. Methods
2.2.1. Synthesis Method
Into a 500 mL four-neck flask equipped with an electric stirrer, a spiral condenser and a thermometer, a certain amount of dichloromethane was added, the isobutyl alcohol and carbon disulfide according to a certain molar ratio was added, and the phase transfer catalyst according to a certain mass ratio was added. The four-necked flask was placed in a water bath at a set temperature, starting the stirrer to stir, adding a certain amount of granular sodium hydroxide in batches. After reacting for a certain time at a certain temperature, a yellow slurry could be obtained. After the completion of the reaction, the obtained slurry was rotary evaporated under reduced pressure with a rotary evaporator for 40 min at room temperature to obtain a dry product of sodium isobutyl xanthate.
2.2.2. Experiment Method
Firstly, the appropriate phase transfer catalyst type and amount were selected in the catalyst experiment; then the orthogonal experiments were performed on the amount of solvent, material ratio, reaction temperature and rotating speed to optimize the process parameters (the factors and levels investigated by the orthogonal experiments were shown in Table 1, the L9(34) orthogonal table was designed according to the parameters in Table 1 and 9 groups of orthogonal experiments were carried out, each group of orthogonal experiments was repeated 3 times and the results were averaged); finally, a single factor experiment of reaction time was carried out to systematically study the changes of xanthate content, yield and free alkali content in the presence of phase transfer catalyst.

Table 1.
Synthesizing orthogonal factor level.
2.2.3. Content Analysis of Sodium Isobutyl Xanthate
The method of titrating xanthate using sodium rosate as indicator and lead acetate standard solution was to calculate the content of xanthate as follows:
w1 was the content of sodium isobutyl xanthate, %; c1 was the concentration of lead acetate standard solution, mol/L; V1 was the volume of lead acetate standard solution consumed during titration, mL; M1 was the molecular weight of sodium isobutyl xanthate, g/mol; m1 was the sample weight, g.
2.2.4. Content Analysis of Free Alkali
The method of titrating sodium hydroxide using phenolphthalein as indicator and acetic acid standard solution was to calculate the content of free alkali in the product as follows:
w2 was the content of free alkali in the product, %; c2 was the concentration of acetic acid standard solution, mol/L; V2 was the volume of acetic acid standard solution consumed during titration, mL; M2 was the molecular weight of sodium hydroxide, g/mol; m2 was the sample weight, g.
2.2.5. Multi-Target Weight Analysis
The commonly used method of multi-index orthogonal experiment is to take the average value of each index weight and adopt the average weighting method, use the test results to convert the multi-index test problem into a single-index test problem, and then use the single-index analysis method for comprehensive selection of schemes. This method ignores the differences and importance of various indicators, and the analysis results are inevitably remote. In order to solve the problem including huge calculated amount and confirming the weights unreasonably which exist in multi-target orthogonal test method, multi-target orthogonal test design was optimized by matrix analysis.
An orthogonal experiment was designed and a three-layer structure model was established based on its data structure. As shown in Table 2, the first layer was the investigation index layer, the second layer was the factor layer and the third layer was the horizontal layer. The index layer matrix M, the factor layer matrix T, the horizontal layer matrix S and the weight matrix w were established that affected the experimental index value [33,34].

Table 2.
Data structural of multi-target orthogonal test.
In the above matrix,, was , which was the ratio of the index value of the first level of factor A to the total index value of all levels of factor A; Si was , which was the ratio of the range of factor A to the total range of all factors. The value of the product of the two could not only reflect the degree of influence of the first level of factor A on the index value, it could also reflect the magnitude of the extreme difference of factor A, as well as other factors and levels. Through calculation, the weight of the influence of each factor and level on the test results could be obtained. According to the weight, the optimal plan and the priority order of the influencing factors could be obtained.
3. Results and Discussion
3.1. The Influence of the Type of Phase Transfer Catalyst on the Synthesis Process
The amount of phase transfer catalyst was 3% malkohol, the reaction temperature was 25 °C, the solvent volume was 3.5 Valcohol, the rotating speed was 120 rpm, the material ratio was n[(CH3)2CHCH2OH]:n(NaOH):n(CS2) = 1:1:1.05, the reaction time was 4 h, and the rotating speed was 120 rpm. Figure 1 shows that the content and yield of sodium isobutyl xanthate in the product are relatively low, and the content of free alkali is relatively high without the addition of catalyst. After adding the catalysts CTAC, CTAB, TBAB and TBAC, the content and yield of sodium isobutyl xanthate in the product are increased, and the content of free alkali is reduced. Among the four catalysts, TBAC has the greatest impact on product quality, the content and yield of sodium xanthate are relatively highest, and the content of free alkali is relatively lowest. TBAC has excellent properties of molecular symmetry and anion hydrophilicity, the ion exchange effect is relatively good, which greatly increases the chance of collision between (CH3)2CHCH2O− and CS2, and the product purity is relatively high. The results show that the presence of the catalyst optimizes the effect of the reaction under certain conditions.
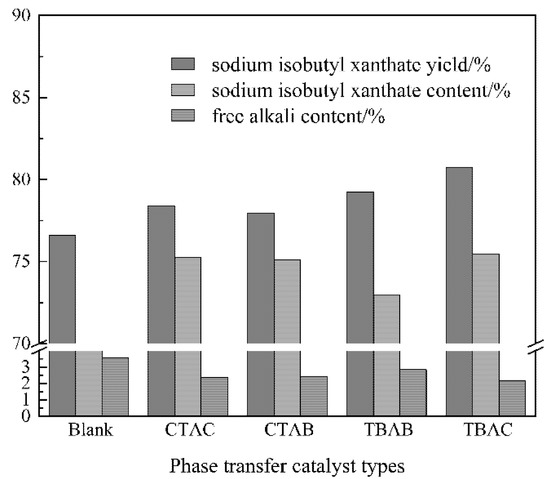
Figure 1.
The effect of catalyst types on the content and yield of sodium isobutyl xanthate.
3.2. The Influence of the Dosage of Phase Transfer Catalyst on the Synthesis Process
The type of phase transfer catalyst was 3% malkohol, the reaction temperature was 25 °C, the solvent volume was 3.5 Valcohol, the rotating speed was 120 rpm, the material ratio was n[(CH3)2CHCH2OH]:n(NaOH):n(CS2) = 1:1:1.05, and the reaction time was 4 h, and the rotating speed was 120 rpm. Figure 2 shows that the content and yield of sodium isobutyl xanthate in the product are increasing, and the content of free alkali is decreasing with the increase in the amount of catalyst. When the amount of TBAC reaches 3.0%malcohol, the content and yield of sodium isobutyl xanthate in the product are relatively minimal. As the amount of TBAC increases, the chance of (CH3)2CHCH2O− colliding with CS2 gradually increases. When the amount of TBAC exceeds 3%, the ion exchange reaches equilibrium and the product purity changes smoothly. The results show that the appropriate amount of catalyst optimizes the effect of the reaction under certain conditions.
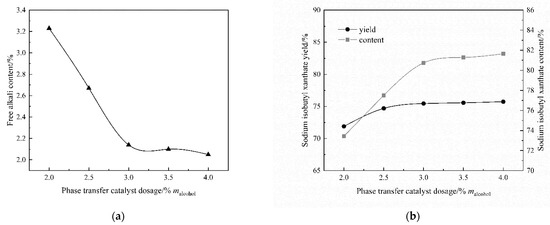
Figure 2.
The effect of catalyst dosage: (a) The content of free alkali; (b) The content and yield of sodium isobutyl xanthate.
3.3. Orthogonal Test
Orthogonal experiments mainly investigated the influence of solvent dosage, material ratio, reaction temperature, rotating speed and other factors on the synthesis process of sodium isobutyl xanthate. There were 3 levels of each influencing factor in the experiment, and the factor level table was shown in Table 1. The required quality indicators were the content of sodium isobutyl xanthate (the higher the better), the yield of sodium isobutyl xanthate (the higher the better) and the content of free alkali (the lower the better). The test was a four-factor three-level experiment, so the L9(34) orthogonal table was selected.
Table 3, which is the intuitive analysis of the experimental results, shows that the optimal solution is A2B2C2D3 for the content of sodium isobutyl xanthate; the optimal solution is A1B3C3D2 for the yield of sodium isobutyl xanthate; the optimal solution is A2B2C3D2 for the content of free alkali. Which one of these three options to choose, usually needs to be solved by the comprehensive balance method, comprehensive scoring method and drawing method. If the matrix analysis method is used to calculate the weight matrices of the three inspection indicators that affect the test results, the optimal solution can be quickly obtained.

Table 3.
Orthogonal experimental results.
The first inspection index was the content of isobutyl xanthate sodium. By the matrix analysis method, the calculation process of the weight matrix w1 as follows (the other two indicators were similar):
The total weight matrix of the indicators examined in this orthogonal experiment was the average of the weight matrices of the three indicator values. The calculation process was as follows:
Figure 3 shows that the three indicators of sodium isobutyl xanthate content, sodium isobutyl xanthate yield and free alkali content affect the synthesis process as follows: C > A > D > B; factors A2, B2, C3 and D2 have the largest weights. The optimal solution of the orthogonal experiment is A2B2C3D2, that is, the amount of TBAC is 3% malcohol, the volume of methylene chloride is 3.5 Valcohol, the material ratio is n[(CH3)2CHCH2OH]:n[NaOH]:n[CS2] = 1:1:1.10, the reaction temperature is 35 °C, and the rotating speed is 180 rpm, the best synthesis effect can be obtained.
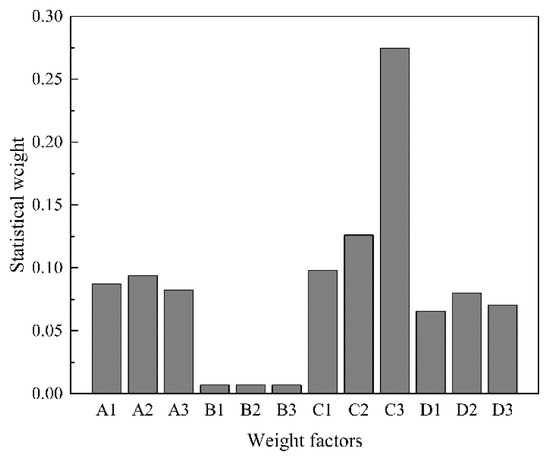
Figure 3.
Comprehensive statistical weight of each weight factor.
3.4. The Influence of the Time on the Synthesis Process
The amount of TBAC was 3.0% malcohol, the reaction temperature was 35 °C, the solvent volume was 3.5 Valcohol, the rotating speed was 180 rpm, reaction time was 4 h and the material ratio was n[(CH3)2CHCH2OH]:n(NaOH):n(CS2) = 1:1:1.10. Figure 4 shows that the content and yield of sodium isobutyl xanthate in the product present an overall upward trend with the reaction time increases, and the content of free alkali presents an overall downward trend; after the reaction time reaches 4 h, the content and yield of sodium isobutyl xanthate in the product present an overall gentle downward trend, and the overall change of free alkali content present a stable trend. In the heterogeneous catalytic synthesis system of sodium isobutyl xanthate, when the reaction time exceeds 4 h, the reaction is close to equilibrium, side reactions gradually intensify and the product purity slightly decreases. The results show that the optimal reaction time optimizes the effect of the synthesis under certain conditions.
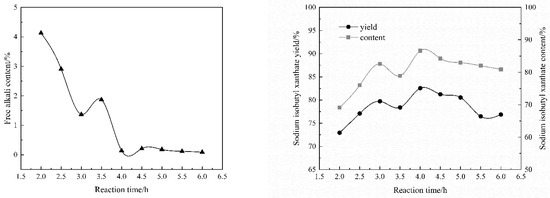
Figure 4.
The effect of reaction time: (a) The content of free alkali; (b) The content and yield of sodium isobutyl xanthate.
3.5. Repetitive Experiment
Under suitable synthetic process conditions, the amount of TBAC was 3% malcohol, the solvent volume was 3.5 Valcohol, the material ratio was n[(CH3)2CHCH2OH]:n(NaOH):n(CS2) = 1:1:1.10, the reaction temperature was 35 °C, the rotating speed was 180 rpm, the reaction time was 4 h and the reproducibility experiment was performed. Table 4 shows that the results of the 4 verification experiments are similar, indicating that the phase transfer catalyst used in the synthesis of sodium isobutyl xanthate has good reproducibility and strong operability.

Table 4.
Reproducible experimental results.
3.6. Product Characterization Analysis
The measured value of the elemental analysis of the product (%, calculated value of C2H9OS2Na): C 32.81 (34.89), H 5.04 (5.27), S 35.17 (37.24).
1H NMR (400 MHz, CDCl3), δ: 4.23 (d, 2H, CH2), 2.18–2.09 (m, 1H, CH), 0.88 (s, 6H, CH3). 13C NMR (400 MHz, CDCl3), δ: 229.8 (s, O-C(=S)-S), 79.6 (s, O-CH2), 26.0 (s, CH) and 19.2 (s, CH3).
The infrared spectrum of the product was measured with KBr tablet in the range of 400~4000 cm−1. Figure 5 shows that the weak peak at 630.57 cm−1 is the vibration peak of C-S; the peak at 1061.83 cm−1 is the absorption peak of C=S; the peak at 1111.95 cm−1 is the stretching peak of C-O-C; the peak at 1371.94 cm−1 is the absorption peak of CH3; the peak at 1464.25 cm−1 is the absorption peak of CH2; the peak at 2873.00 cm−1 is the stretching vibration peak of CH3; the peak at 2960.26 cm−1 is the stretching vibration peak of CH3.
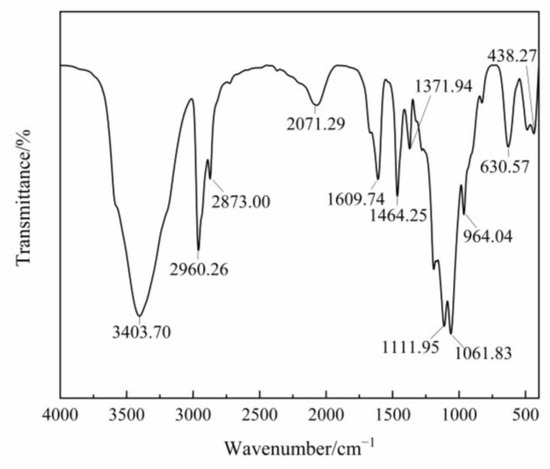
Figure 5.
The infrared spectrum of the product.
The above detections and structural characterizations confirmed that the obtained compound was the target product.
4. Conclusions
The experimental results of the phase transfer catalyst showed that the addition of the phase transfer catalyst accelerated the reaction speed and shortened the reaction time during the synthesis of sodium isobutyl xanthate under certain conditions. When the amount of TBAC was 3% malcohol, the catalytic effect was the best, the xanthate content and yield were the highest and the free alkali content was the least.
Orthogonal experimental matrix analysis results showed that the order of the degree of influence of each factor was: reaction temperature > solvent volume > rotating speed > material ratio. The appropriate process conditions for TBAC to participate in the synthesis of sodium isobutyl xanthate were: the volume of the dichloromethane was 3.5 Valcohol, the material ratio was n[(CH3)2CHCH2OH]:n(NaOH):n(CS2) = 1:1:1.10, the synthesis time was 4 h, the synthesis temperature was 35 °C and the reaction speed was 180 rpm.
The results of repeated experiments showed that the content of sodium isobutyl xanthate in the product was up to 82.53%, the yield reached 86.32% and the content of free alkali was less than 0.15%. It was a qualified product. This synthesis process of sodium isobutyl xanthate did not require a refrigeration system, only used cooling water below 15 °C, so the synthesis process had low power consumption; and the addition of a phase transfer catalyst shortened the reaction time, saved energy and reduced the production cost of sodium isobutyl xanthate.
Author Contributions
Validation, Z.M. and L.C.; resources, X.W. and Y.G.; writing—original draft preparation, L.C. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, B.; Wu, J.; Dong, Z.L.; Jiang, T.; Li, Q.; Yang, Y.B. Flotation performance, structure–activity relationship and adsorption mechanism of a newly-synthesized collector for copper sulfide minerals in Gacun polymetallic ore. Appl. Surf. Sci. 2021, 551, 149420. [Google Scholar] [CrossRef]
- Peng, Y.J.; Grano, S.; Fornasiero, D.; Ralston, J. Control of grinding conditions in the flotation of galena and its separation from pyrite. Int. J. Miner. Process. 2003, 70, 67–82. [Google Scholar] [CrossRef]
- Zeng, L.M.; Ming, O.L. Flotation process and process mineralogy analysis of certain zinc sulfide ore. Chin. J. Nonferrous Met. 2018, 28, 1866–1875. [Google Scholar]
- Lin, Q.Q.; Gu, G.H.; Wang, H.; Wang, C.Q.; Liu, Y.C.; Fu, J.G.; Zhu, R.F. An effective approach for improving flotation recovery of molybdenite fines from a finely-disseminated molybdenum ore. J. Cent. South Univ. 2018, 25, 1326–1339. [Google Scholar] [CrossRef]
- Gutierrez, L.; Uribe, L.; Hernandez, V.; Vidal, V.; Mendonça, R.T. Assessment of the use of lignosulfonates to separate chalcopyrite and molybdenite by flotation. Powder Technol. 2020, 359, 216–225. [Google Scholar] [CrossRef]
- Huang, K.; Li, Q.W.; Chen, J. Recovery of copper, nickel and cobalt from acidic pressure leaching solutions of low-grade sulfide flotation concentrates. Miner. Eng. 2007, 20, 722–728. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Copper demand, supply, and associated energy use to 2050. Glob. Environ. Chang. 2016, 39, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Golmakani, M.H.; Khaki, J.V.; Babakhani, A. A novel method for direct fabrication of ferromolybdenum using molybdenite via self-propagation high temperature synthesis. Mater. Chem. Phys. 2017, 194, 9–16. [Google Scholar]
- Salazar, J.S.; Pablo, R.; Parada, B. Stibnite froth flotation: A critical review. Miner. Eng. 2021, 163, 160713. [Google Scholar]
- Chimonyo, W.; Fletcher, B.; Peng, Y.J. The effect of oxidized starches on chalcopyrite flotation. Miner. Eng. 2021, 165, 106749. [Google Scholar] [CrossRef]
- Li, S.L.; Gao, L.H.; Wang, J.C.; Zhou, H.P.; Liao, Y.F.; Xing, Y.W.; Gui, X.H.; Cao, Y.J. Polyethylene oxide assisted separation of molybdenite from quartz by flotation. Miner. Eng. 2021, 162, 106765. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.M.; Feng, Q.C.; Liu, Y.B. Activation mechanism of lead ions in the flotation of sulfidized azurite with xanthate as collector. Miner. Eng. 2021, 163, 106809. [Google Scholar] [CrossRef]
- Shen, Y.; Nagaraj, D.R.; Farinato, R.; Somasundaran, P.; Tong, S.G. Xanthate decomposition in ore pulp under flotation conditions: Method development and effects of minerals on decomposition. Miner. Eng. 2019, 131, 198–205. [Google Scholar] [CrossRef]
- Maree, W.; Kloppers, L.; Hangone, G.; Oyekola, O. The effects of mixtures of potassium amyl xanthate (PAX) and isopropyl ethyl thionocarbamate (IPETC) collectors on grade and recovery in the froth flotation of a nickel sulfide ore. S. Afr. J. Chem. Eng. 2017, 24, 116–121. [Google Scholar] [CrossRef]
- Fosu, S.; Skinner, W.; Zanin, M. Detachment of coarse composite sphalerite particles from bubbles in flotation: Influence of xanthate collector type and concentration. Miner. Eng. 2015, 71, 73–84. [Google Scholar] [CrossRef]
- Ma, X.; Zhong, H.; Wang, S.; Hu, Y.; Xiao, J.J. Synthesis of sodium iso-butyl xanthate by solvent method. J. Jiangxi Univ. Sci. Technol. 2012, 33, 1–5. [Google Scholar]
- Ma, X. Research on the green synthesis technology of alkyl xanthate collectors. Master’s Thesis, Central South University, Changsha, China, 2013. [Google Scholar]
- Lotter, N.O.; Bradshaw, D.J. The formulation and use of mixed collectors in sulphide flotation. Miner. Eng. 2010, 23, 945–951. [Google Scholar] [CrossRef]
- Lee, K.; Archibald, D.; Mclean, J.; Reuter, M.A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.; Zhong, H. Effective production of sodium isobutyl xanthate using carbon disulfide as a solvent: Reaction kinetics, calorimetry and scale-up. J. Clean. Prod. 2018, 200, 444–453. [Google Scholar] [CrossRef]
- Tijsseling, L.T.; Dehaine, Q.; Rollinson, G.K.; Glass, H.J. Flotation of mixed oxide sulphide copper-cobalt minerals using xanthate, dithiophosphate, thiocarbamate and blended collectors. Miner. Eng. 2019, 138, 246–256. [Google Scholar] [CrossRef]
- Huang, X.P.; Huang, K.H.; Jia, Y.; Wang, S.; Cao, Z.F.; Zhong, H. Investigating the selectivity of a xanthate derivative for the flotation separation of chalcopyrite from pyrite. Chem. Eng. Sci. 2019, 205, 220–229. [Google Scholar] [CrossRef]
- Pålsson, B.I.; Eric, K.S. Computer-assisted calculations of thermodynamic equilibria in sphalerite-xanthate systems. Int. J. Miner. Process. 1989, 26, 223–258. [Google Scholar] [CrossRef]
- Dhar, P.; Thornhill, M.; Kota, H.R. Comparison of single and mixed reagent systems for flotation of copper sulphides from Nussir ore. Miner. Eng. 2019, 142, 105930. [Google Scholar] [CrossRef]
- Cui, W.Y.; Chen, J.H.; Li, Y.Q.; Chen, Y.; Zhao, C.H. Interactions of xanthate molecule with different mineral surfaces: A comparative study of Fe, Pb and Zn sulfide and oxide minerals with coordination chemistry. Miner. Eng. 2020, 159, 106565. [Google Scholar]
- Han, S.; Nguyen, A.V.; Kim, K.; Park, J.K.; You, K. Measurements and analysis of xanthate chain length effect on bubble attachment to galena surfaces. Miner. Eng. 2020, 159, 106651. [Google Scholar] [CrossRef]
- Dehmlow, E.V. Phase-transfer catalyzed two-phase reactions in preparative organic chemistry. Angew. Chem. Int. Edit. 1974, 13, 170–179. [Google Scholar] [CrossRef]
- Herriott, A.W.; Picker, D. Phase transfer catalysis. Evaluation of catalysis. J. Am. Chem. Soc. 1975, 97, 2345–2349. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Stalikas, C.D. Phase-transfer catalysis in analytical chemistry. Anal. Chim. Acta 2005, 550, 1–12. [Google Scholar] [CrossRef]
- Brändström, A. Principles of phase-transfer catalysis by quaternary ammonium salts. Adv. Phys. Org. Chem. 1977, 15, 267–330. [Google Scholar]
- Leung, L.M.; Chan, W.H.; Leung, S.K.; Fung, S.M. Synthesis of aliphatic and aromatic poly (s-dithiocarbonates) using a phase-transfer catalyst. J. Macromol. Sci. Pure Appl. Chem. 1994, 31, 495–505. [Google Scholar] [CrossRef]
- Wu, Y.N.; Feng, W.G.; Wu, Q.W.; Sun, S.Q. Study om phase transfer catalyst used in the synthesis of isoamyl xanthate. Energy Chem. Ind. 2019, 40, 29–32. [Google Scholar]
- Wei, X.L.; Xu, B.J.; Zhao, Q. Optimization design of the stability for the plunger assembly of oil pumps based on multi-target orthogonal test design. J. Hebei Univ. Eng. 2010, 27, 95–99. [Google Scholar]
- Zhao, T.T.; He, C.H.; Tan, M.E.; Sun, Z.H. Optimization in ethanol extraction process of Qianlieshuang Granules based on multi-index weight analysis and single factor-orthogonal experiments. Chem. Bioeng. 2020, 37, 45–50. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).